Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REGENERON PHARMACEUTICALS, INC. | a18-2403_18k.htm |
| EX-99.2 - EX-99.2 - REGENERON PHARMACEUTICALS, INC. | a18-2403_1ex99d2.htm |
JP Morgan 2018 Leonard S. Schleifer MD, PhD, President & CEO George D. Yancopoulos MD, PhD, President & CSO January 8th, 2018
Safe Harbor Statement circa 1988 2 Regeneron is a risky investment. That we hope will pay off handsomely!

Note regarding FORWARD-LOOKING STATEMENTs and non-gaap financial measures 3 This presentation includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of Regeneron’s products, product candidates, and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, Praluent® (alirocumab) Injection, Dupixent® (dupilumab) Injection, Kevzara® (sarilumab) Injection, cemiplimab, fasinumab, Regeneron’s earlier-stage product candidates, and the use of human genetics in Regeneron’s research programs; the extent to which the results from Regeneron’s research programs or preclinical testing may lead to advancement of product candidates to clinical trials or therapeutic applications; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron’s late-stage product candidates and new indications for marketed products, including without limitation EYLEA, Praluent, Dupixent, Kevzara, cemiplimab, and fasinumab; risks associated with intellectual property of other parties and pending or future litigation relating thereto, including without limitation the patent litigation proceedings relating to Praluent, the ultimate outcome of any such litigation proceeding, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition; the likelihood and timing of achieving any of the anticipated milestones described in this presentation; ongoing regulatory obligations and oversight impacting Regeneron’s marketed products (such as EYLEA, Praluent, Dupixent, and Kevzara), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s products and product candidates; competing drugs and product candidates that may be superior to Regeneron’s products and product candidates; uncertainty of market acceptance and commercial success of Regeneron’s products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, suppliers, or other third parties to perform filling, finishing, packaging, labelling, distribution, and other steps related to Regeneron’s products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the assumptions underlying those projections or guidance; and the potential for any license or collaboration agreement, including Regeneron’s agreements with Sanofi, Bayer, and Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), to be cancelled or terminated without any further product success. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2016 and its Form 10-Q for the quarterly period ended September 30, 2017, including in each case in the section thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise. This presentation uses non-GAAP unreimbursed R&D and non-GAAP SG&A, which are financial measures that are not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). These non-GAAP financial measures are computed by excluding certain non-cash and other items from the related GAAP financial measure. Non-GAAP adjustments also include the income tax effect of reconciling items. The Company makes such adjustments for items the Company does not view as useful in evaluating its operating performance. For example, adjustments may be made for items that fluctuate from period to period based on factors that are not within the Company’s control, such as the Company’s stock price on the dates share-based grants are issued. Management uses these and other non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides forecasts to investors on this basis. Additionally, such non-GAAP measures provide investors with an enhanced understanding of the financial performance of the Company’s core business operations. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company’s non-GAAP financial measures may not be comparable with non-GAAP information provided by other companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP.

The early days ATING NEURONS Founded by physician-scientists 30 years ago, our science-driven approach has resulted in six FDA-approved medicines and numerous product candidates in a range of diseases, including asthma, pain, cancer and infectious diseases. 0 products in development Focused on genetics 0 manufacturing capabilities A small group of people Lots of great ideas 1988 4
THEN & now 1988 2018 5 Still innovating, still focused on genetics ~6,000 people 6 approved medicines, 15 candidates in clinical development, all from our own labs Just getting started
Combining Science and technology to improve patients’ lives 6 Use biology and genetics to identify & validate targets Invent scalable, cutting-edge technology to empower biology Improve patients’ lives Follow the science and unmet need to create a dynamic pipeline Inventing tools and technologies (e.g. VelociSuite, RGC) to overcome bottlenecks in discovering, validating and producing drugs All pipeline programs were discovered at Regeneron Labs 7 out of 13 members of Board of Directors are members of the National Academy of Science Regeneron is committed to consistently and repeatedly bringing new medicines to patients with serious diseases
Fostering a culture of scientific excellence 7 Named #1 top global biopharmaceutical employer FOR THE FIFTH TIME TOP 10 SINCE 2013 2017 300 top Scholars to be announced tomorrow! Over 50% of senior scientists have been with the company for more than a decade

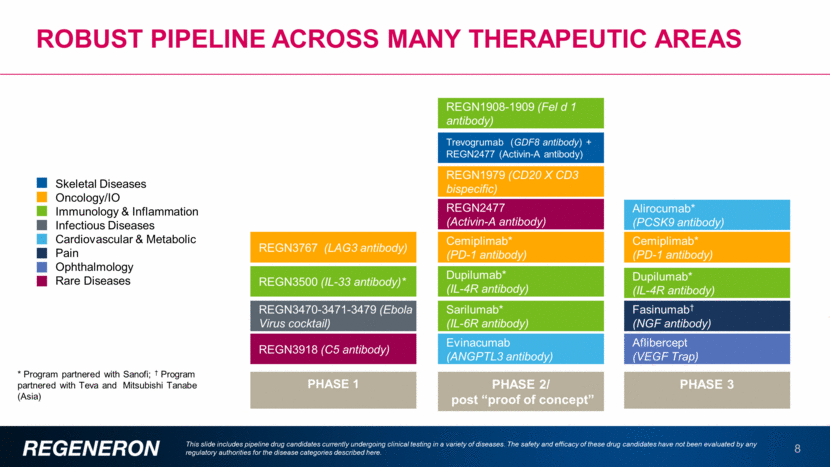
Robust pipeline across many therapeutic areas 8 This slide includes pipeline drug candidates currently undergoing clinical testing in a variety of diseases. The safety and efficacy of these drug candidates have not been evaluated by any regulatory authorities for the disease categories described here. * Program partnered with Sanofi; † Program partnered with Teva and Mitsubishi Tanabe (Asia) PHASE 1 PHASE 2/ post “proof of concept” PHASE 3 REGN1979 (CD20 X CD3 bispecific) Trevogrumab (GDF8 antibody) + REGN2477 (Activin-A antibody) REGN3470-3471-3479 (Ebola Virus cocktail) REGN2477 (Activin-A antibody) REGN3500 (IL-33 antibody)* REGN3767 (LAG3 antibody) REGN1908-1909 (Fel d 1 antibody) Cemiplimab* (PD-1 antibody) Evinacumab (ANGPTL3 antibody) Sarilumab* (IL-6R antibody) Dupilumab* (IL-4R antibody) Cemiplimab* (PD-1 antibody) Fasinumab† (NGF antibody) Aflibercept (VEGF Trap) Dupilumab* (IL-4R antibody) Alirocumab* (PCSK9 antibody) REGN3918 (C5 antibody) Skeletal Diseases Oncology/IO Immunology & Inflammation Infectious Diseases Cardiovascular & Metabolic Pain Ophthalmology Rare Diseases
Strong pipeline and operational execution in 2017 9 CLINICAL & REGULATORY PROGRESS EYLEA® superior to laser in proliferative diabetic retinopathy study (CLARITY) KEVZARA® approved for rheumatoid arthritis in U.S., EU and Japan DUPIXENT® approved for moderate-to-severe atopic dermatitis in U.S. and EU Dupilumab positive topline data from two Phase 3 asthma studies; sBLA submitted to FDA Dupilumab positive Phase 2 data in eosinophilic esophagitis Cemiplimab positive pivotal data in advanced cutaneous squamous cell carcinoma (cSCC) GDF8 antibody + Activin-A antibody: positive data in muscle program Robust pipeline of 15 molecules in clinical development COMMERCIAL PROGRESS EYLEA U.S. net sales were ~$3.7Bn* Dupixent ongoing launch in atopic dermatitis in the U.S. and EU Cemiplimab (PD-1) launch preparations underway in cSCC ADVANCES IN GENETICS Harnessing human genetics data from Regeneron Genetics Center to advance science, guide development of therapeutics, and improve patient outcomes: >250,000 exomes sequenced CORPORATE PROGRESS Manufacturing facility in Raheen, Ireland, approved by FDA Appellate Court orders a new trial and vacates permanent injunction in ongoing patent case regarding Praluent® Expected 2017 Effective Tax Rate to be 26%-29% (before tax reform adjustment) *Based on fiscal 2017 unaudited, preliminary numbers
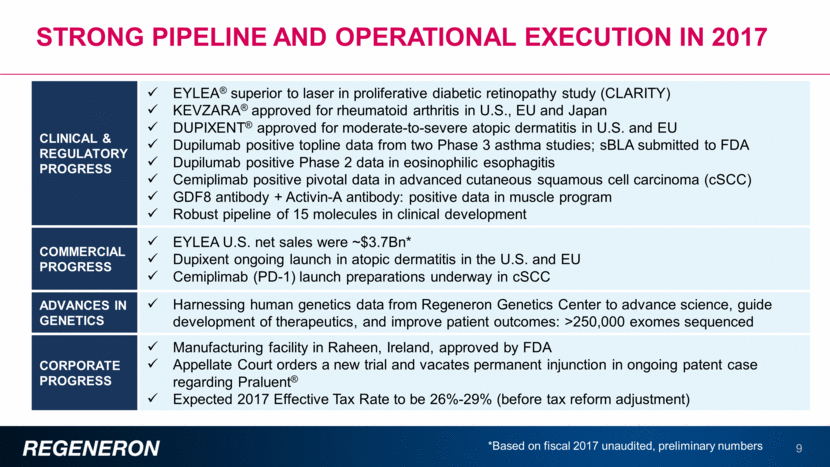
2017: EYLEA® (aflibercept) Injection highlights 10 2017 U.S. EYLEA net sales were ~$3.7Bn* 2017 ex-U.S. EYLEA net sales expected to be in excess of $2Bn*† EYLEA continued to be the market-leading product among FDA-approved anti-VEGF agents for its approved indications EYLEA superior to laser in proliferative diabetic retinopathy study (CLARITY) sBLA filed for Q12W dosing interval for patients with wet-AMD; PDUFA of Aug 11, 2018 PANORAMA Phase 3 study in diabetic retinopathy completed enrollment U.S. Net Sales, $Bn *Unaudited, preliminary numbers, † Outside the United States, EYLEA net product sales comprise sales by Bayer in countries other than Japan and sales by Santen Pharmaceutical Co., Ltd. in Japan under a co-promotion agreement with an affiliate of Bayer * $0.8 $1.4 $1.7 $2.7 $3.3 ~$3.7 2012 2013 2014 2015 2016 2017
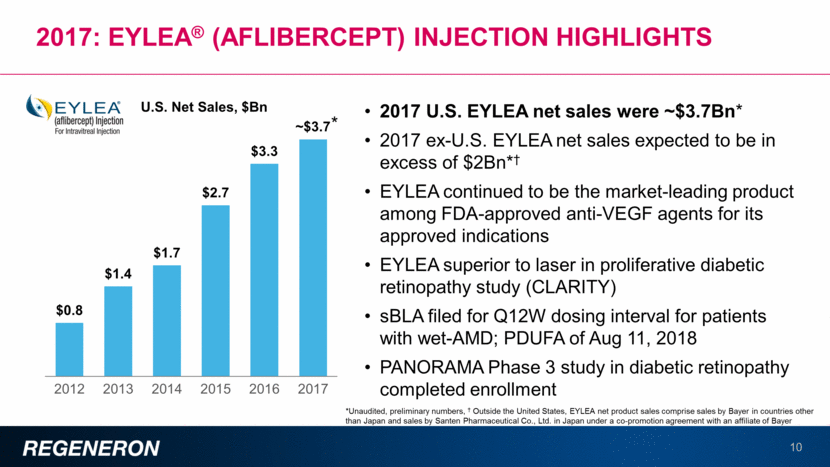
Dupixent®: Launch in atopic dermatitis progressing well 11 Strong prescription trajectory with >8,000 healthcare providers having written a prescription for DUPIXENT On average, ~750 prescriptions/week written On average, ~500 new patients/week are dispensed drug High patient and physician satisfaction — >90% of patients have renewed their prescription Trending ahead of other recent biologic launches in dermatology Weekly TRx since launch Cosentyx Taltz 0 500 1,000 1,500 2,000 2,500 3,000 3,500 4,000 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39
Anticipated CLINICAL & REGULATORY highlights for 2018 12 EYLEA Phase 3 data from diabetic retinopathy study (PANORAMA) EYLEA FDA submission of sBLAs for diabetic retinopathy and pre-filled syringe EYLEA FDA regulatory decision for Q12W dosing in wet-AMD Dupilumab FDA regulatory decision in asthma in 2H18 Dupilumab FDA regulatory submission in pediatric AD (ages 12–17) in 2H18 Dupilumab topline data from two Phase 3 studies in nasal polyps Dupilumab studies initiated in eosinophilic esophagitis, COPD, food and inhaled allergies, co-morbid conditions REGN3500 (IL-33) advance as monotherapy and combination with dupilumab in multiple indications Cemiplimab FDA and EU submission (1Q18) and potential approval (2H18) in cSCC Cemiplimab data presentation at a medical conference Cemiplimab multiple late-stage studies in NSCLC (1st and 2nd line), basal cell carcinoma, cervical cancer Fasinumab readout of first Phase 3 study in osteoarthritis I/O: Advance targets into clinical development, including MUC16XCD3 and BCMA x CD3, and GITR PRALUENT: data from Phase 3 cardiovascular OUTCOMES study in 1Q18 Evinacumab: Initiate a Phase 3 study in HoFH Increased investment to accelerate advances with cemiplimab, dupilumab and REGN3500 (IL-33) Advance other pipeline programs such as Activin-A, GDF8, LAG3, CD20XCD3 Advance 4-6 new molecules into clinical development
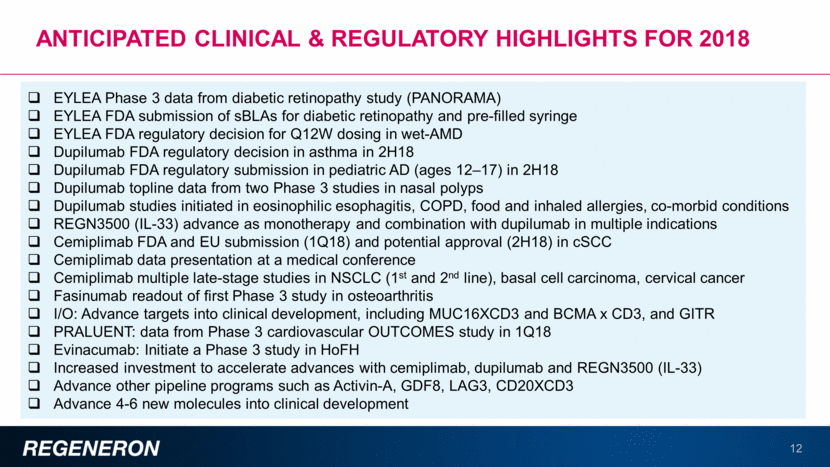
Important pipeline advances expected in 2018 13 Product candidates in clinical development, all discovered at Regeneron Product candidates expected to enter clinical development Regulatory submissions and new/expanded indications Programs in late-stage clinical development 2018 15 4-6 5 17
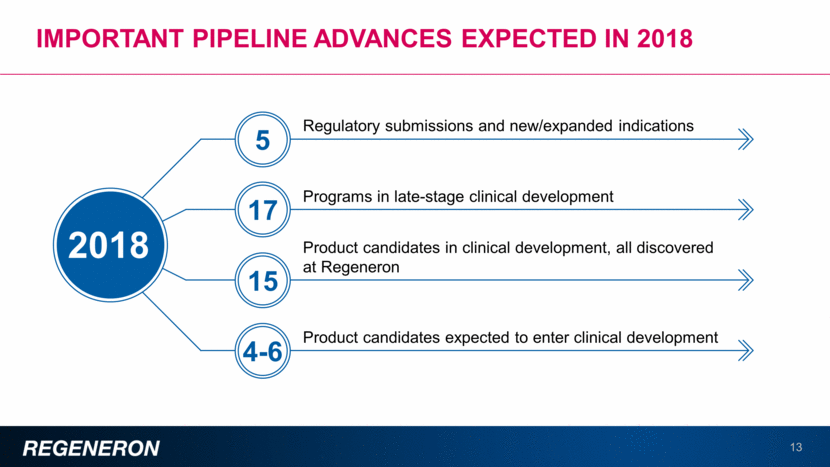
Diabetic eye diseases present important opportunities for eylea 14 Diabetic Retinopathy Diabetic Macular Edema Characterized by vascular abnormalities that can lead to profound vision loss, by causing edema, hemorrhage, or vascular proliferation Characterized by visual loss due to edema or swelling in the most important part of the retina
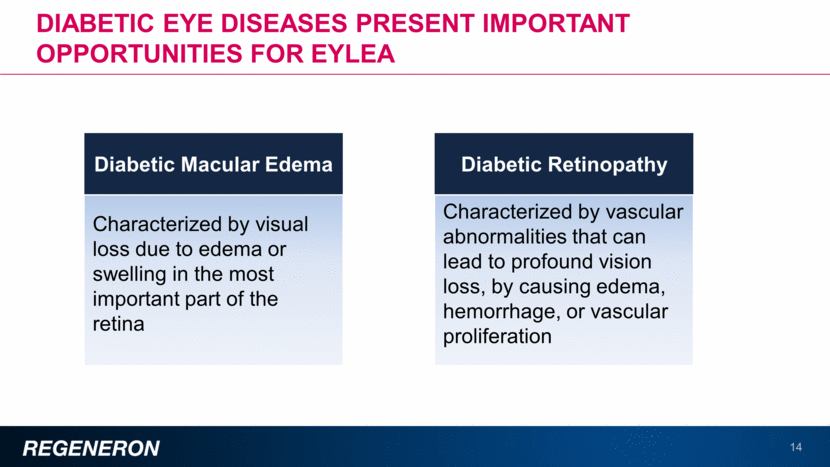
Diabetic macular edema (DME) is an important opportunity for EYLEA 15 1.5 million adults diagnosed with DME ~750,000 patients have clinically significant macular edema ~200,000 patients are treated ~125,000 (8% of diagnosed) patients receive an anti-VEGF therapy Takeaway text Patients with DME continue to be under-diagnosed and under-treated and even when treated, they may receive sub-optimal laser therapy
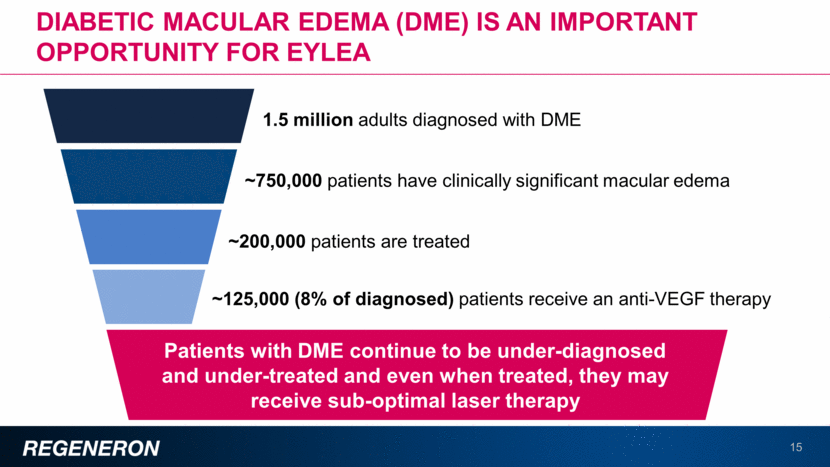
Diabetic retinopathy without dme is an important related opportunity for EYLEA 16 Severe, non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) may result in profound vision loss and represent a potential opportunity for EYLEA The majority of people with PDR are now treated with pan-retinal photocoagulation (laser) therapy, which was inferior to EYLEA in the CLARITY study Phase 3 PANORAMA study in diabetic retinopathy ongoing; topline results expected in 1H18 3.5 million people in the U.S. are diagnosed with diabetic retinopathy without DME1,2 Mild NPDR 46% Moderate NPDR 25% Severe NPDR 16% PDR 13% 1 NHANES 2005-2008, projected to 2012 US population; American Diabetes Association. 2 BioTrends Research Group, Treatment Trends®: Diabetic Retinopathy / Diabetic Macular Edema (US) 2013. About 30% of people with DR present the greatest unmet need
Topline data from 18,000 patient cardiovascular outcomes study expected 1Q18 Regulatory U.S. and EU submissions expected 3Q18 Committed to helping patients receive access to PRALUENT® Praluent®: topline Data from odyssey outcomes expected in 1Q18 17
Launch ongoing in rheumatoid arthritis in U.S. and EU Increased formulary coverage starting January 2018 Studies planned in additional indications Giant Cell Arteritis (U.S. prevalence >228,000)1 Polymyalgia Rheumatica (U.S. prevalence ~711,000)1 Kevzara®: launched in rheumatoid arthritis; Planning pivotal studies in additional indications 18 1Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58(1):26-35
Regeneron and Sanofi Accelerate and Expand Investment for Cemiplimab and Dupilumab Development Programs 19 Investment in cemiplimab to be increased by ~$1 billion Companies will continue to equally fund cemiplimab development The companies will also continue their additional investment in other immuno-oncology programs under their existing Immuno-oncology Discovery Agreement Additional investment in dupilumab and anti-IL-33 (REGN3500) development program To accelerate planned new studies of dupilumab in chronic obstructive pulmonary disease, peanut and grass allergy, and in patients with multiple allergic disorders The additional investment will also accelerate and expand development of REGN3500 with studies expected to be conducted in atopic dermatitis, asthma and chronic obstructive pulmonary disease Funding pursuant to existing antibody collaboration with Sanofi
Non-GAAP Unreimbursed R&D Non-GAAP SG&A Sanofi Collaboration Revenue: Reimbursement of Regeneron Commercialization-Related Expenses Effective Tax Rate Capital Expenditures 2018 Financial guidance1 20 As of January 8, 2018. The guidance does not assume the completion of any significant business development transaction that had not been completed as of the date of the guidance. Regeneron does not undertake any obligation to update publicly any financial projection or guidance, whether as a result of new information, future events, or otherwise. $1,230MM - $1,330MM $1,350MM - $1,450MM $450MM - $500MM 15%-19% $420MM - $500MM
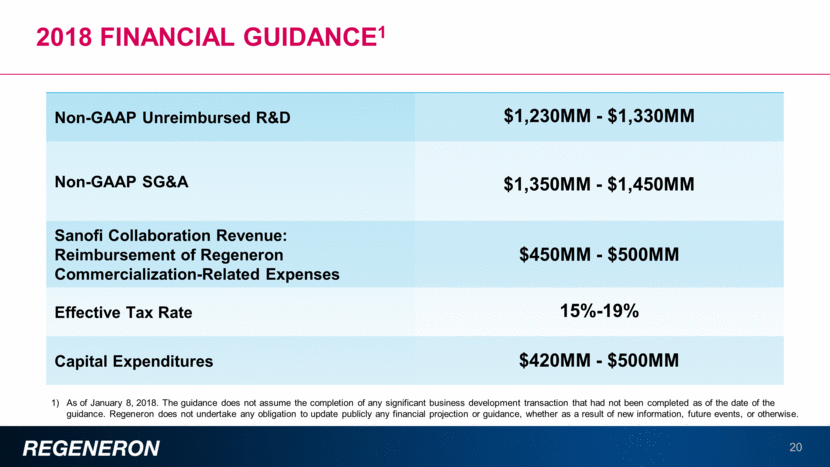
Dupilumab: AN IL-4/IL-13 Blocker with positive data in many allergic diseases 21 Eosinophilic Esophagitis Positive Phase 2 proof-of-concept data reported Phase 3 studies to be initiated in 2018 Approved in U.S. and Europe for atopic dermatitis Studies ongoing in pediatric populations Asthma: Efficacy demonstrated in 3 pivotal studies; sBLA submitted Largest Phase 3 biologic program in asthma Pediatric studies ongoing Nasal Polyps Positive Phase 2 proof-of-concept data reported Both Phase 3 studies fully enrolled Peanut allergy (Planned) and other food allergies Grass allergy (Planned) and other inhaled allergens Potential for future use in other indications COPD (Planned) Studies in patients with multiple allergic disorders (Planned) Dupilumab
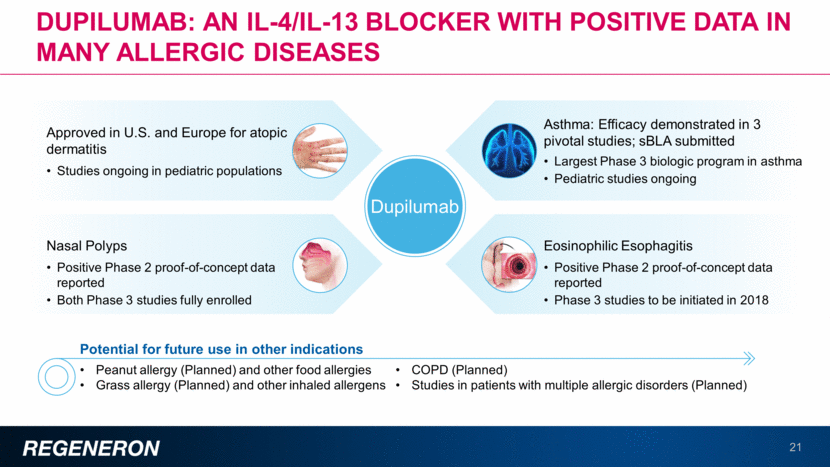
Dupixent is approved for adult patients with moderate-to-severe atopic dermatitis (AD), with average improvement in EASI score of ~70%-80% Prevalence of AD is ~10% of U.S. pediatric population1 1%–2% of these pediatric AD patients have severe disease2,3,4, with up to more than 50% of their skin surface covered with lesions Limited treatment options currently available Three distinct Phase 3 pediatric studies ongoing or planned In children between 12 and 17 years; regulatory submission expected in 2018 In children between 6 and 11 years ongoing In children between 6 mos. and 5 years planned Pediatric atopic dermatitis represents a high disease burden with limited treatment options 22 1Shaw et al., J In Derm, Eczema Prevalence in the United States; Data from the 2003 National Survey of Children’s Health, 2011, 131, 67-73 2Charman CR, Williams HC. Epidemiology. In: Bieber T, Leung DYM, editors. Atopic Dermatitis. New York: Dekker; 2002. pp. 21–42 3Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. British Journal of Dermatology. 1998;139(1):73–6 4 Atopic Eczema in Children: Management of Atopic Eczema in Children from Birth up to the Age of 12 Years. NICE Clinical Guidelines, No. 57. National Collaborating Centre for Women's and Children's Health (UK). London: RCOG Press; 2007 Dec EASI Eczema Area Severity Index
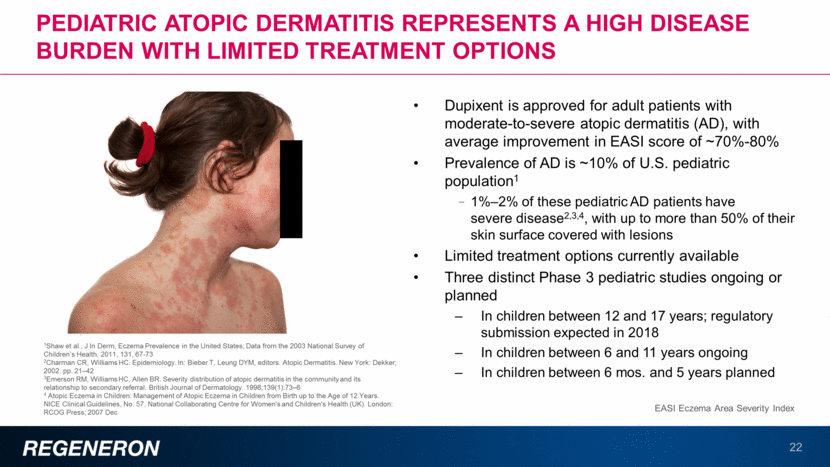
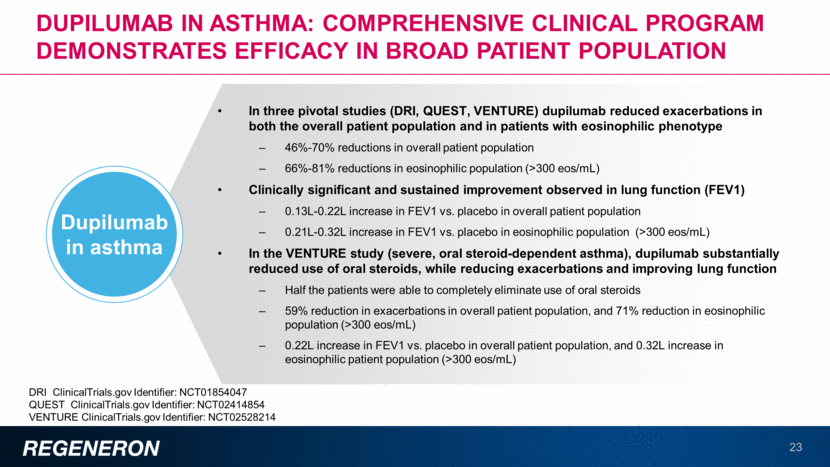
Dupilumab in asthma: comprehensive clinical program demonstrates efficacy in broad patient population 23 In three pivotal studies (DRI, QUEST, VENTURE) dupilumab reduced exacerbations in both the overall patient population and in patients with eosinophilic phenotype 46%-70% reductions in overall patient population 66%-81% reductions in eosinophilic population (>300 eos/mL) Clinically significant and sustained improvement observed in lung function (FEV1) 0.13L-0.22L increase in FEV1 vs. placebo in overall patient population 0.21L-0.32L increase in FEV1 vs. placebo in eosinophilic population (>300 eos/mL) In the VENTURE study (severe, oral steroid-dependent asthma), dupilumab substantially reduced use of oral steroids, while reducing exacerbations and improving lung function Half the patients were able to completely eliminate use of oral steroids 59% reduction in exacerbations in overall patient population, and 71% reduction in eosinophilic population (>300 eos/mL) 0.22L increase in FEV1 vs. placebo in overall patient population, and 0.32L increase in eosinophilic patient population (>300 eos/mL) Dupilumab in asthma DRI ClinicalTrials.gov Identifier: NCT01854047 QUEST ClinicalTrials.gov Identifier: NCT02414854 VENTURE ClinicalTrials.gov Identifier: NCT02528214
Dupilumab: Potential in indications beyond atopic dermatitis and asthma 24 Nasal Polyps Positive Phase 2 proof-of-concept data reported Both Phase 3 studies fully enrolled COPD Planning on initiating Phase 3 program in 2018 Eosinophilic Esophagitis Positive Phase 2 proof-of-concept data reported Planning to initiate Phase 3 program in 2018 Eosinophilic esophagitis is thought to result from multiple undefined food allergies Food Allergy Program In pre-clinical studies dupilumab substantially improved and accelerated responses to allergen desensitization Peanut allergy program to be initiated in 2018 in combination with desensitization and as monotherapy Inhaled Allergy Program Grass allergy program to be initiated in 2018 in combination with desensitization
Role of IL-33 in respiratory disease has been validated by genetic associations (Regeneron Genetics Center) Genetic link established to asthma: Common “gain-of-function” (GOF) variants in IL-33 and its receptor increase the risk of asthma Rare “loss-of-function” (LOF) variants in IL-33 decrease risk of eosinophilic asthma by more than 50% Preclinical models have shown REGN3500 can have additive and complementary effects with dupilumab Initial clinical study showed favorable pharmacokinetics and safety profile Expect to initiate* Phase 2 in AD Proof-of-concept in COPD Proof-of-concept in asthma REGN3500 (IL-33) clinical development underway 25 * These studies will include combination arms with dupilumab
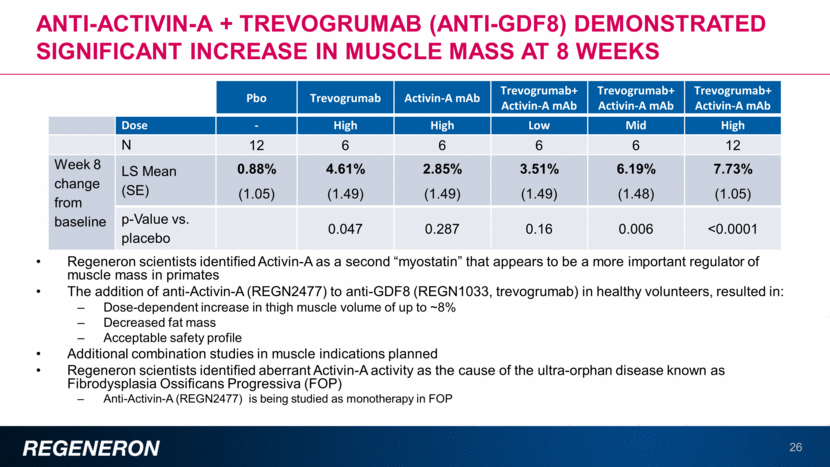
Regeneron scientists identified Activin-A as a second “myostatin” that appears to be a more important regulator of muscle mass in primates The addition of anti-Activin-A (REGN2477) to anti-GDF8 (REGN1033, trevogrumab) in healthy volunteers, resulted in: Dose-dependent increase in thigh muscle volume of up to ~8% Decreased fat mass Acceptable safety profile Additional combination studies in muscle indications planned Regeneron scientists identified aberrant Activin-A activity as the cause of the ultra-orphan disease known as Fibrodysplasia Ossificans Progressiva (FOP) Anti-Activin-A (REGN2477) is being studied as monotherapy in FOP Anti-Activin-A + Trevogrumab (anti-GDF8) demonstrated significant increase in muscle mass at 8 weeks 26 Thigh Muscle Volume, cm3 Pbo Trevogrumab Activin-A mAb Trevogrumab+ Activin-A mAb Trevogrumab+ Activin-A mAb Trevogrumab+ Activin-A mAb Dose - High High Low Mid High N 12 6 6 6 6 12 Week 8 change from baseline LS Mean (SE) 0.88% (1.05) 4.61% (1.49) 2.85% (1.49) 3.51% (1.49) 6.19% (1.48) 7.73% (1.05) p-Value vs. placebo 0.047 0.287 0.16 0.006 <0.0001
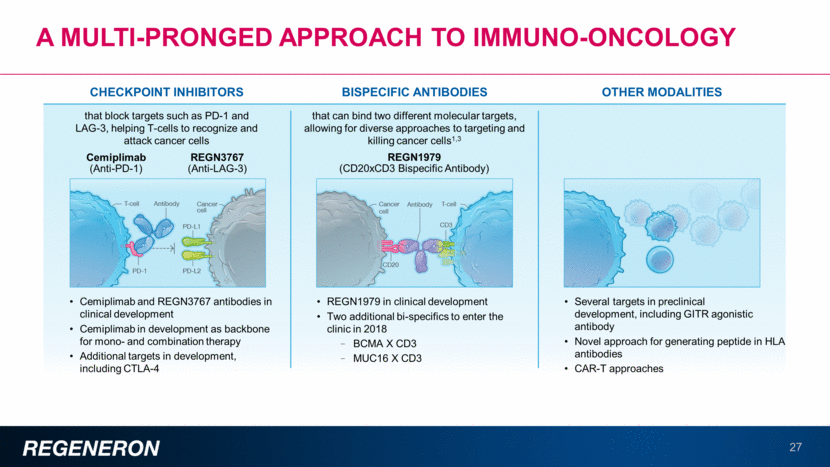
A multi-pronged approach to immuno-oncology 27 Cemiplimab and REGN3767 antibodies in clinical development Cemiplimab in development as backbone for mono- and combination therapy Additional targets in development, including CTLA-4 OTHER MODALITIES BISPECIFIC ANTIBODIES that can bind two different molecular targets, allowing for diverse approaches to targeting and killing cancer cells1,3 REGN1979 (CD20xCD3 Bispecific Antibody) Cemiplimab (Anti-PD-1) CHECKPOINT INHIBITORS that block targets such as PD-1 and LAG-3, helping T-cells to recognize and attack cancer cells REGN3767 (Anti-LAG-3) REGN1979 in clinical development Two additional bi-specifics to enter the clinic in 2018 BCMA X CD3 MUC16 X CD3 Several targets in preclinical development, including GITR agonistic antibody Novel approach for generating peptide in HLA antibodies CAR-T approaches
Phase 2 study results in 82 patients with metastatic and locally advanced disease demonstrated Primary endpoint: 46.3% ORR by independent review 32 of 38 responses ongoing (with ³6 months follow-up) Safety profile generally consistent with approved anti-PD-1 drugs pivotal study in advanced cscc demonstrated high response rate and durable responses 28 Cohort 1: Metastatic (nodal and distant) cSCC Cohort 2: Unresectable locally advanced cSCC Cohort 3: Metastatic (nodal and distal) cSCC Single arm cohorts* Cemiplimab 3 mg/kg every 14 days Cemiplimab 350 mg every 3 weeks U.S. and EU regulatory submissions expected 1Q18 ~1M cases of cSCC annually in the U.S. While most are well-treated with surgery, cSCC results in 3,900 to 8,800 deaths/year in US1 (compared to 9,700 deaths from melanoma) There are no FDA-approved cSCC therapies *Cohorts 2 and 3 are still enrolling patients 1Karia PS et al. J Am Acad Dermatol. 2013;68:957–66
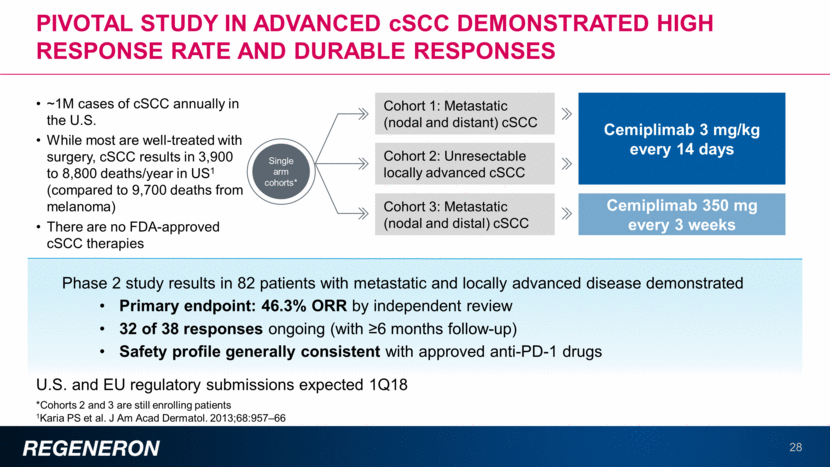
pivotal study in advanced cscc demonstrated high response rate and durable responses 29 U.S. and EU regulatory submissions expected 1Q18 Phase 2 study results in 82 patients with metastatic and locally advanced disease demonstrated Primary endpoint: 46.3% ORR by independent review 32 of 38 responses ongoing (with ³6 months follow-up) Safety profile generally consistent with approved anti-PD-1 drugs Cohort 1: Metastatic (nodal and distant) cSCC Cohort 2: Unresectable locally advanced cSCC Cohort 3: Metastatic (nodal and distal) cSCC Single arm cohorts* Cemiplimab 3 mg/kg every 14 days Cemiplimab 350 mg every 3 weeks ~1M cases of cSCC annually in the U.S. While most are well-treated with surgery, cSCC results in 3,900 to 8,800 deaths/year in US1 (compared to 9,700 deaths from melanoma) There are no FDA-approved cSCC therapies *Cohorts 2 and 3 are still enrolling patients 1Karia PS et al. J Am Acad Dermatol. 2013;68:957–66 (ASCO2017)
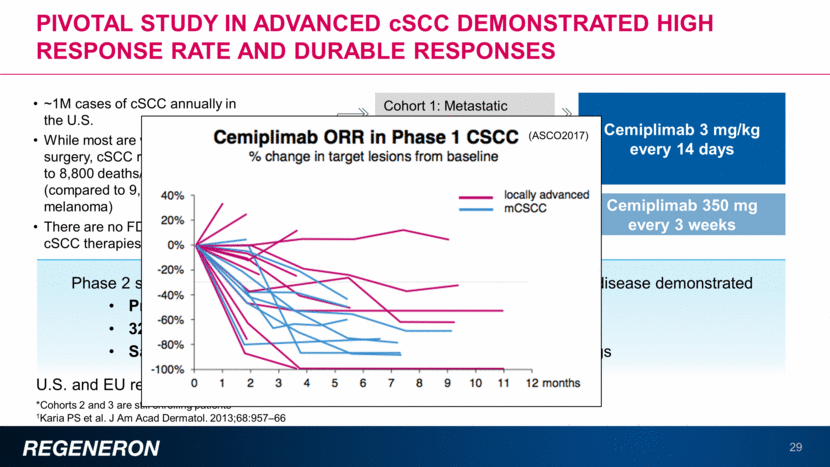
CD20XCD3 Bi-specific (REGN1979): positive data observed in b-cell malignancies 30 FULLY HUMAN, WITH MINIMAL ENGINEERING1 REGN1979 resembles a full-length human antibody, engineered to minimize risk of undesired immune activity and to enhance its stability in the body. DESIGNED FOR SCALE A small change on one of its arms helps separate REGN1979 from other proteins during the purification and scale up process Cancer cell Antibody CD20 CD3 T-cell REGN1979 monotherapy demonstrated response rates of 50% at highest tested doses in heavily pre-treated/Rituxan-refractory NHL1,2 Dose escalation ongoing Manageable safety profile thus far REGN1979 is being tested in combination with cemiplimab (anti-PD-1), which may result in enhanced anti-tumor activity2 1Bannerji R, et al. Presented at the 59th ASH Annual Meeting & Exposition. 2017. Atlanta, GA. 2Topp MS, et al. Presented at the 59th ASH Annual Meeting & Exposition. 2017. Atlanta, GA.
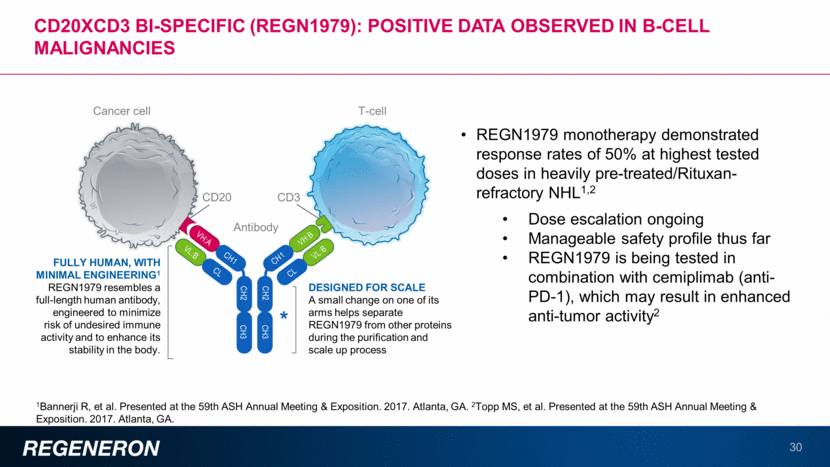
Developing a portfolio of immuno-oncology therapies: Multiple Registration studies underway with cemiplimab 31 All therapies are investigational. Safety and efficacy of these investigational products have not been evaluated by any regulatory authority. TARGET CONDITION/DISEASE STUDY STATUS Advanced CSCC Chemo naïve or experienced, monotherapy cemiplimab File BLA 1Q18 NSCLC ³ 50% PD-L1 1st line monotherapy: cemiplimab vs platinum doublet Ongoing (N=300) NSCLC <50% PD-L1 1st line combos with cemiplimab + vs platinum doublet Ongoing NSCLC ³ PD-L1 1st line combos with cemiplimab + vs pembrolizumab Planned 2nd line NSCLC Phase 2 study with cemiplimab and combos Planned Platinum-refractory cervical cancer Phase 3 study in 2nd line setting with cemiplimab Ongoing Basal cell carcinoma Phase 2 study with cemiplimab Ongoing
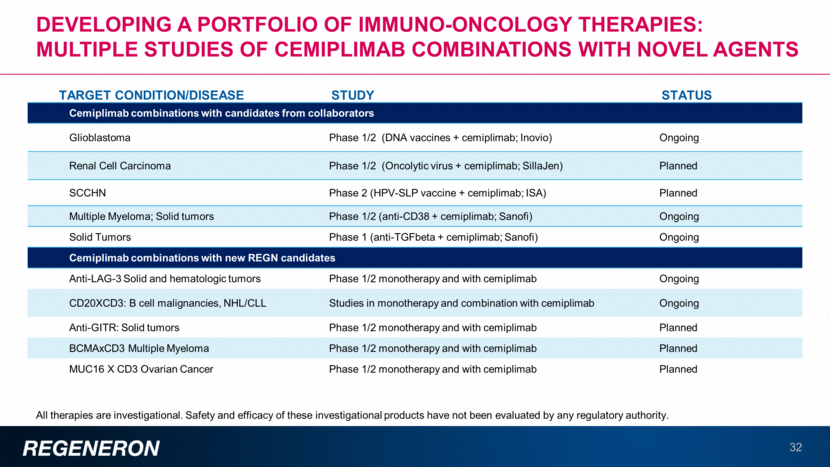
Developing a portfolio of immuno-oncology therapies: Multiple studies OF cemiplimab Combinations with novel agents 32 All therapies are investigational. Safety and efficacy of these investigational products have not been evaluated by any regulatory authority. TARGET CONDITION/DISEASE STUDY STATUS Cemiplimab combinations with candidates from collaborators Glioblastoma Phase 1/2 (DNA vaccines + cemiplimab; Inovio) Ongoing Renal Cell Carcinoma Phase 1/2 (Oncolytic virus + cemiplimab; SillaJen) Planned SCCHN Phase 2 (HPV-SLP vaccine + cemiplimab; ISA) Planned Multiple Myeloma; Solid tumors Phase 1/2 (anti-CD38 + cemiplimab; Sanofi) Ongoing Solid Tumors Phase 1 (anti-TGFbeta + cemiplimab; Sanofi) Ongoing Cemiplimab combinations with new REGN candidates Anti-LAG-3 Solid and hematologic tumors Phase 1/2 monotherapy and with cemiplimab Ongoing CD20XCD3: B cell malignancies, NHL/CLL Studies in monotherapy and combination with cemiplimab Ongoing Anti-GITR: Solid tumors Phase 1/2 monotherapy and with cemiplimab Planned BCMAxCD3 Multiple Myeloma Phase 1/2 monotherapy and with cemiplimab Planned MUC16 X CD3 Ovarian Cancer Phase 1/2 monotherapy and with cemiplimab Planned
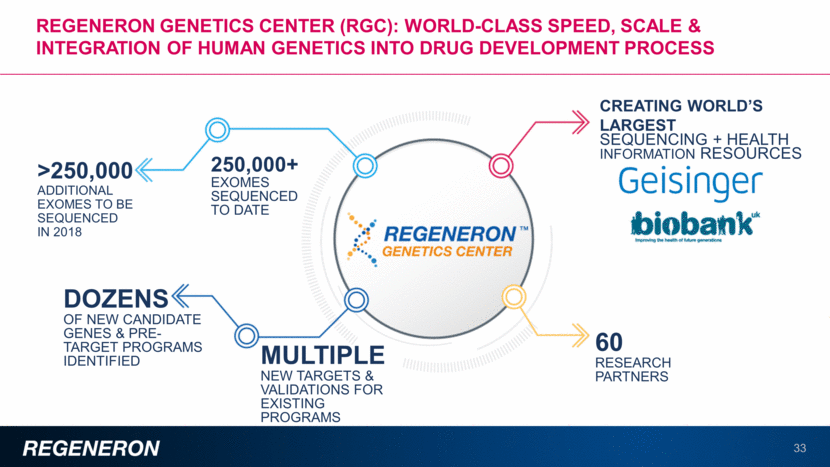
Regeneron genetics center (RGC): WORLD-CLASS speed, scale & integration of Human Genetics into Drug development PROCESS CREATING WORLD’S LARGEST SEQUENCING + HEALTH INFORMATION RESOURCES 60 RESEARCH PARTNERS 250,000+ EXOMES SEQUENCED TO DATE >250,000 ADDITIONAL EXOMES TO BE SEQUENCED IN 2018 MULTIPLE NEW TARGETS & VALIDATIONS FOR EXISTING PROGRAMS DOZENS OF NEW CANDIDATE GENES & PRE-TARGET PROGRAMS IDENTIFIED 33
RGC to sequence exomes from 500,000 people by end of 2019; data will be paired with detailed, de-identified health information All data will be openly available to the global research community Largest database of its kind may have profound impact on human health Additional collaborators expected LEADING A NEW LIFE SCIENCES CONSORTIUM TO BUILD AN Unprecedented, ACCESSIBLE ‘BIG DATA’ RESOURCE 34

35 velociHUM Inventing technologies that address bottlenecks & complement biology A suite of broadly enabling technologies

