Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Ra Pharmaceuticals, Inc. | a17-28220_1ex99d1.htm |
| 8-K - 8-K - Ra Pharmaceuticals, Inc. | a17-28220_18k.htm |
Forward Looking Statements This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding the safety, efficacy, regulatory and clinical progress and therapeutic potential of our product candidates, including RA101495 SC, and management’s estimates about the potential size and characteristics for the patient populations that our product candidates are targeting. All such forward-looking statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include the risks that Ra Pharma’s product candidates, including RA101495 SC, will not successfully be developed or commercialized; the risk that interim results as of November 30, 2017 from the Company’s global Phase 2 clinical program evaluating RA101495 SC for the treatment of PNH may not be indicative of final study results; the risk that the interim results from the patient enrollment and dosing to date may not be indicative of the data from the full patient enrollment and dosing planned for such study; as well as the other factors discussed in the “Risk Factors” section in Ra Pharma’s most recently filed Annual Report on Form 10-K, as well as other risks detailed in Ra Pharma’s subsequent filings with the Securities and Exchange Commission. There can be no assurance that the actual results or developments anticipated by Ra Pharma will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Ra Pharma. All information in this presentation is as of November 30, 2017, and Ra Pharma undertakes no duty to update this information unless required by law. 2

Executive Summary Positive Interim Phase 2 Data Provides Foundation for C5 Inhibitor Franchise Lead clinical program: RA101495 SC Potent, synthetic, macrocyclic peptide inhibitor of complement C5 Convenient, self-administered, subcutaneous (SC) dosing Phase 2 program in paroxysmal nocturnal hemoglobinuria (PNH) ongoing (N=29) – – – – – Safe and well-tolerated Near complete complement inhibition observed Primary endpoint met for LDH reduction in eculizumab naïve patients (p=0.002) Successful switching demonstrated in transfusion-independent patients Encouraging early data from first patients in inadequate responder cohort Phase 2 study recruiting in generalized myasthenia gravis (gMG) Phase 1b renal impairment PK study planned to support development for treatment of atypical hemolytic uremic syndrome (aHUS) and lupus nephritis (LN) Portfolio of C5 inhibitors in pre-clinical development Extended release formulation of RA101495 SC Oral small molecule C5 inhibitor 3
Phase 2 PNH Program Design Goal: Dose-finding study to evaluate the safety, tolerability, preliminary efficacy, pharmacokinetics, and pharmacodynamics of RA101495 SC in patients with PNH Design: Open-label (12 weeks) with long-term extension Global Program addressing 3 PNH populations Eculizumab Naïve –10 patients enrolled; dosing completed Eculizumab Switch –16 patients enrolled; dosing ongoing; recruitment closed Eculizumab Inadequate Responders (US only) –3 patients recently began treatment; dosing ongoing; recruitment closed Dose-Finding Regimen Loading dose: 0.3mg/kg SC on Day 1 Starting dose: 0.1mg/kg SC once daily for the first 2 weeks Up-titration: from the week 2 visit onwards, if LDH is > 1.5xULN, the dose is increased to 0.3mg/kg SC once daily Primary Efficacy Endpoint: Change in lactate dehydrogenase (LDH) from baseline to the mean level from Week 6 to Week 12 4

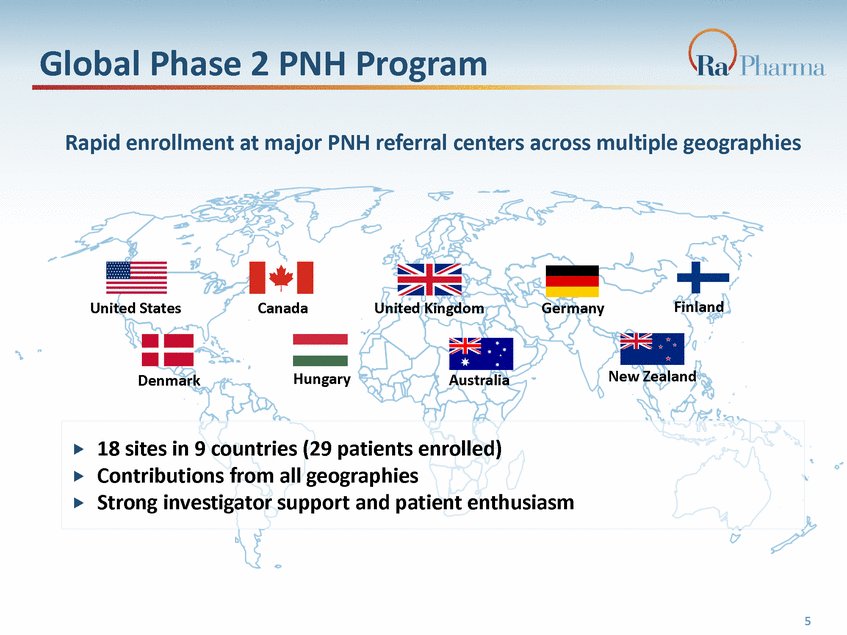
Global Phase 2 PNH Program Rapid enrollment at major PNH referral centers across multiple geographies Finland United States Canada United Kingdom Germany New Zealand Hungary Australia Denmark 5 18 sites in 9 countries (29 patients enrolled) Contributions from all geographies Strong investigator support and patient enthusiasm
Eculizumab Naïve Cohort 6

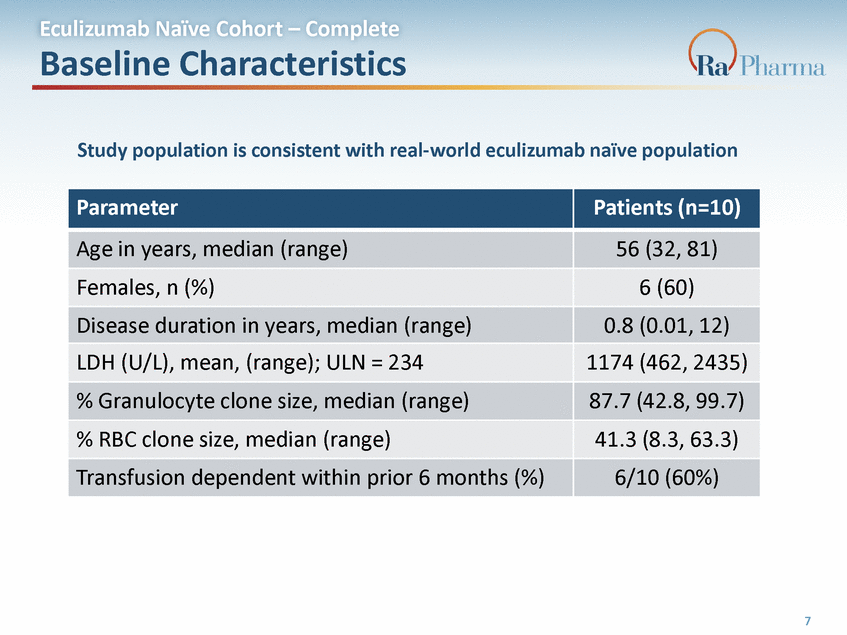
Eculizumab Naïve Cohort – Complete Baseline Characteristics Study population is consistent with real-world eculizumab naïve population 7 Parameter Patients (n=10) Age in years, median (range) 56 (32, 81) Females, n (%) 6 (60) Disease duration in years, median (range) 0.8 (0.01, 12) LDH (U/L), mean, (range); ULN = 234 1174 (462, 2435) % Granulocyte clone size, median (range) 87.7 (42.8, 99.7) % RBC clone size, median (range) 41.3 (8.3, 63.3) Transfusion dependent within prior 6 months (%) 6/10 (60%)
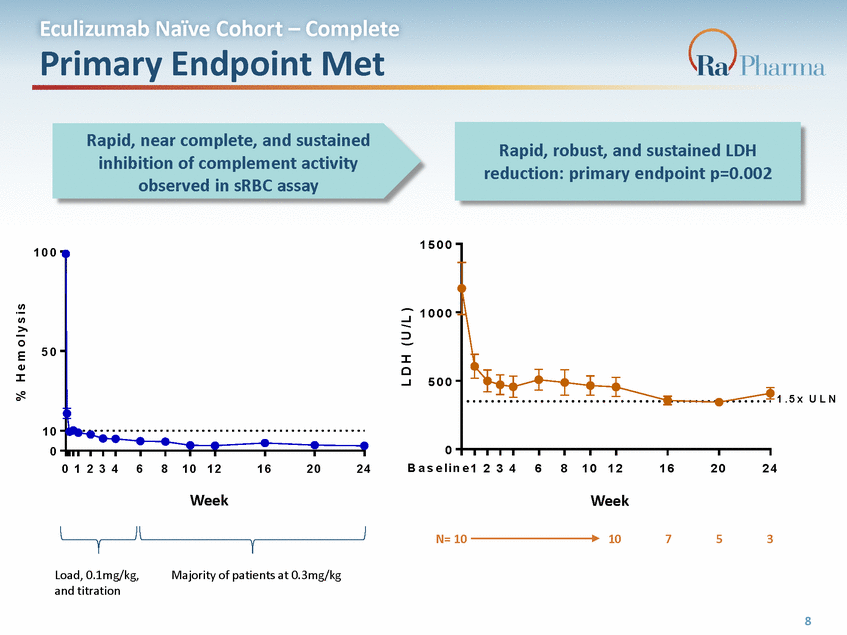
Eculizumab Naïve Cohort – Complete Primary Endpoint Met Rapid, near complete, and sustained inhibition of complement activity observed in sRBC assay 1 5 0 0 1 0 0 1 0 0 0 5 0 5 0 0 1 . 5 x U L N 1 0 0 0 B a s e l i n e 1 0 1 2 3 4 6 8 1 0 1 2 1 6 2 0 2 4 2 3 4 6 8 1 0 1 2 1 6 2 0 2 4 R A 1 0 1 4 9 5 . 2 0 1Week ( W e e k ) R A 1 0 1 4 9 5 . 2 0 1Week ( W e e k ) 10 R A 1 0 1 4 9 5 . 2 0 2 ( W e e k ) R A 1 0 1 4 9 5 . 2 0 2 ( W e e k ) N= 10 7 5 3 0.3mg/kg 1 2 e average of the screening and week 0 data per patient % H e m o l y s i s L D H ( U / L ) Load, 0.1mg/kg,Majority of patients at Week Baseline * 1 2 3 4 6 8 10 12 10 10 10 10 10 10 10 6 hr) and t itration 3 4 6 Patien ts, n 10 10 8 * 10 Baselin 12 e is th 16 20 24 8 0, 9 10 10 10 10 10 10 8 7 2 2 2 Rapid, robust, and sustained LDH reduction: primary endpoint p=0.002
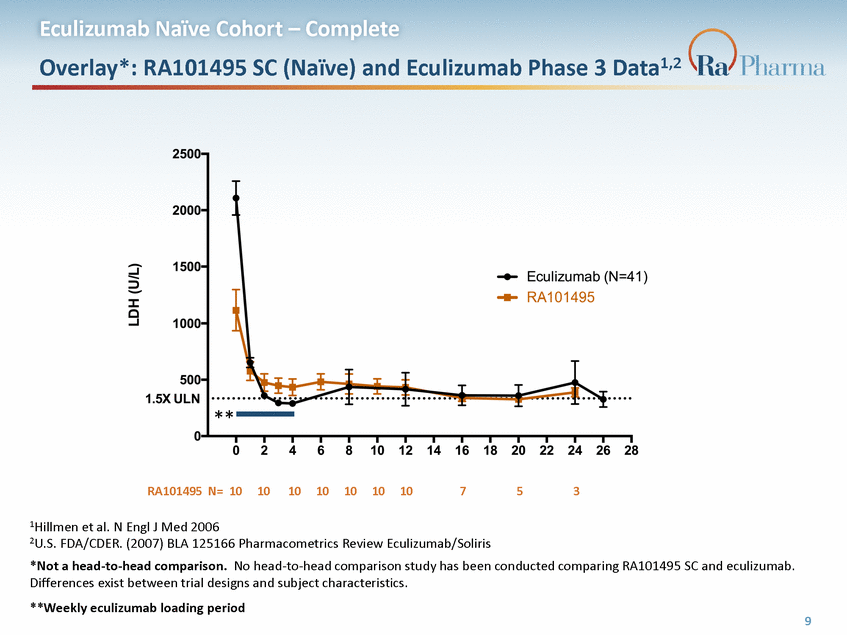
Eculizumab Naïve Cohort – Complete Overlay*: RA101495 SC (Naïve) and Eculizumab Phase 3 Data1,2 ** RA101495 N= 10 10 10 10 10 10 10 7 5 3 1Hillmen et al. N Engl J Med 2006 2U.S. FDA/CDER. (2007) BLA 125166 Pharmacometrics Review Eculizumab/Soliris *Not a head-to-head comparison. No head-to-head comparison study has been conducted comparing RA101495 SC and eculizumab. Differences exist between trial designs and subject characteristics. **Weekly eculizumab loading period 9
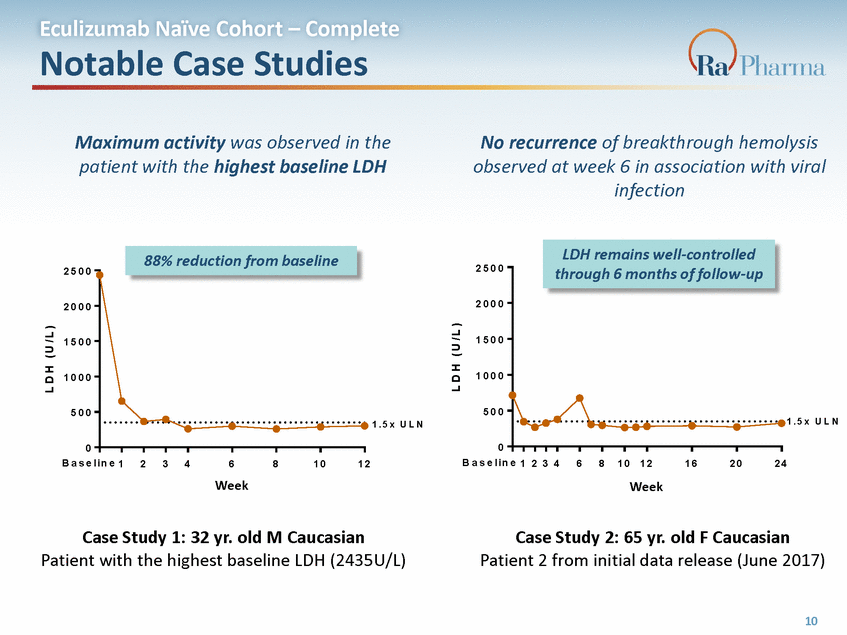
Eculizumab Naïve Cohort – Complete Notable Case Studies Maximum activity was observed in the patient with the highest baseline LDH No recurrence of breakthrough hemolysis observed at week 6 in association with viral infection 2 5 0 0 2 5 0 0 2 0 0 0 2 0 0 0 1 5 0 0 1 5 0 0 1 0 0 0 1 0 0 0 5 0 0 5 0 0 1 . 5 x U L N 1 . 5 x U L N 0 0 B a s e l i n e 1 2 3 4 6 WWeekk 8 1 0 1 2 B a s e l i n e 1 2 3 4 6 8 1 0 1 2 1 6 2 0 2 4 Week R A 1 0 1 4 9 5 . 2 0 1 ( W e e k ) R A 1 0 1 4 9 5 . 2 0 2 ( W e e k ) Case Study 1: 32 yr. old M Caucasian Patient with the highest baseline LDH (2435U/L) Case Study 2: 65 yr. old F Caucasian Patient 2 from initial data release (June 2017) 10 L D H ( U / L ) L D H ( U / L ) 88% reduction from baseline LDH remains well-controlled through 6 months of follow-up
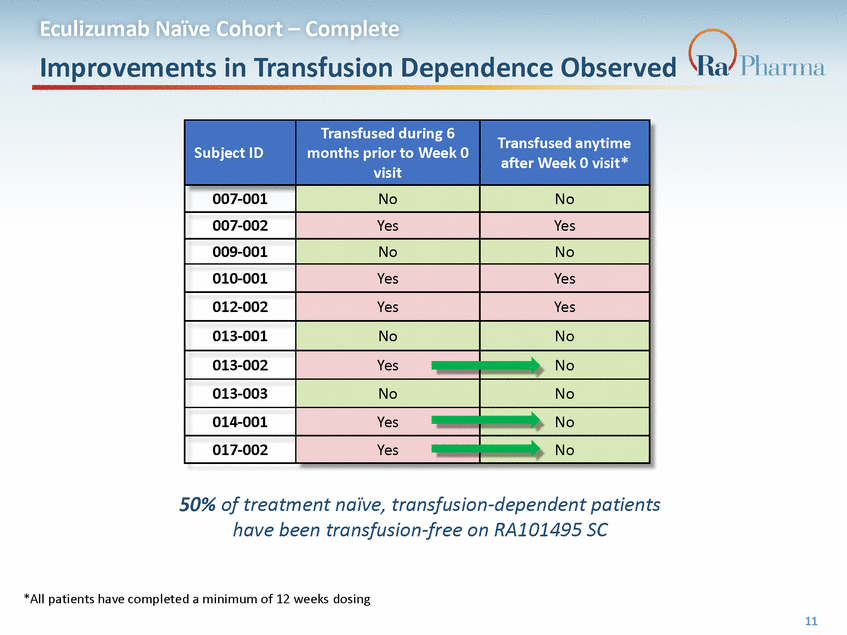
Eculizumab Naïve Cohort – Complete Improvements in Transfusion Dependence Observed after Week 0 visit* 50% of treatment naïve, transfusion-dependent patients have been transfusion-free on RA101495 SC *All patients have completed a minimum of 12 weeks dosing 11 Subject ID Transfused during 6 months prior to Week 0 visit Transfused anytime 007-001 No No 007-002 Yes Yes 009-001 No No 010-001 Yes Yes 012-002 Yes Yes 013-001 No No 013-002 Yes No 013-003 No No 014-001 Yes No 017-002 Yes No
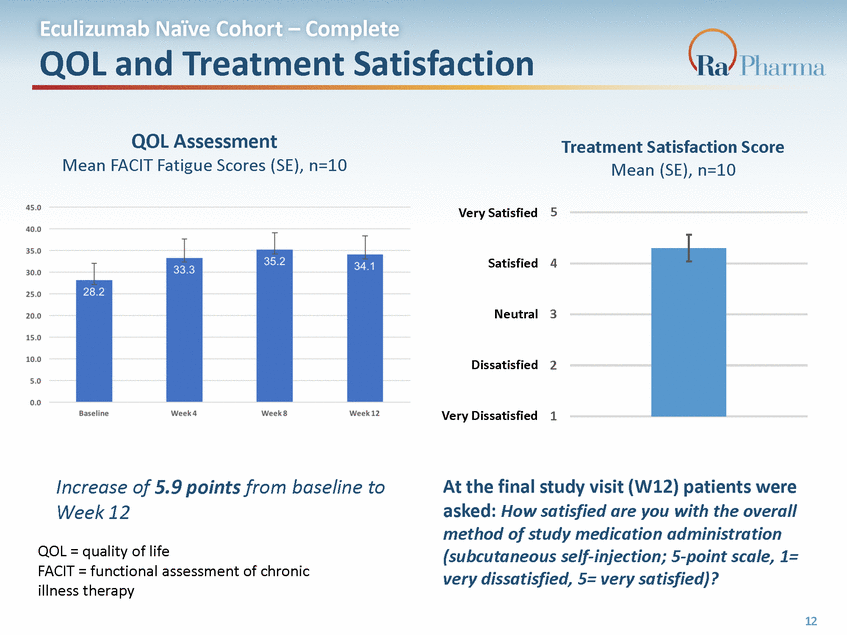
Eculizumab Naïve Cohort – Complete QOL and Treatment Satisfaction QOL Assessment Mean FACIT Fatigue Scores (SE), n=10 Treatment Satisfaction Score Mean (SE), n=10 5 Very Satisfied 4 Satisfied Neutral 3 Dissatisfied 2 Very Dissatisfied 1 Increase of 5.9 points from baseline to Week 12 QOL = quality of life FACIT = functional assessment of chronic illness therapy At the final study visit (W12) patients were asked: How satisfied are you with the overall method of study medication administration (subcutaneous self-injection; 5-point scale, 1= very dissatisfied, 5= very satisfied)? 12
Eculizumab Switch Cohort 13

Eculizumab Switch Cohort – Ongoing Switch Population: Response to Long-Term Eculizumab Therapy Two distinct populations after 3 years of eculizumab treatment 80% 20% Hillmen et al. Br J Hematol 2013 14
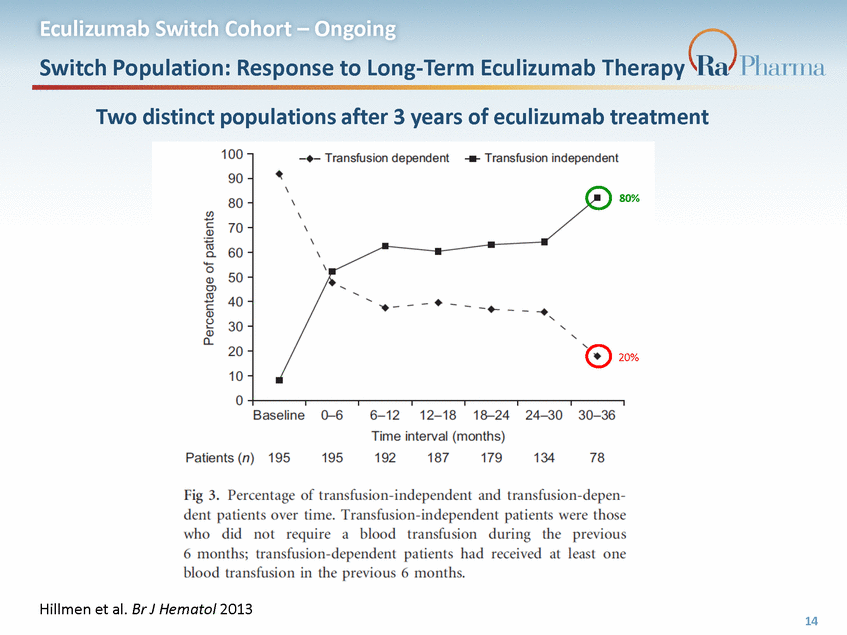
Eculizumab Switch Cohort – Ongoing Baseline Characteristics Study population is overrepresented by poor responders to eculizumab who remain transfusion-dependent on long-term therapy (69% in study vs 20% real-world1) 1Hillmen et al. Br J Hematol 2013 15 Parameter Patients (n=16) Age in years, median (range) 53 (22, 72) Females, n (%) 7 (44) Disease duration in years, median (range) 5.1 (1.8, 36) LDH (U/L), mean, (range; ULN = 234) 287 (222, 542) % Granulocyte clone size, median (range) 97.0 (21.2, 99.8) % RBC clone size, median (range) 66.0 (5.4, 99.0) Eculizumab dose > 900mg, n (%) 4 (25%) Duration of eculizumab treatment in years, median (range) 3.8 (1.0, 12) Transfusion dependent within prior 6 months 11/16 (69%)
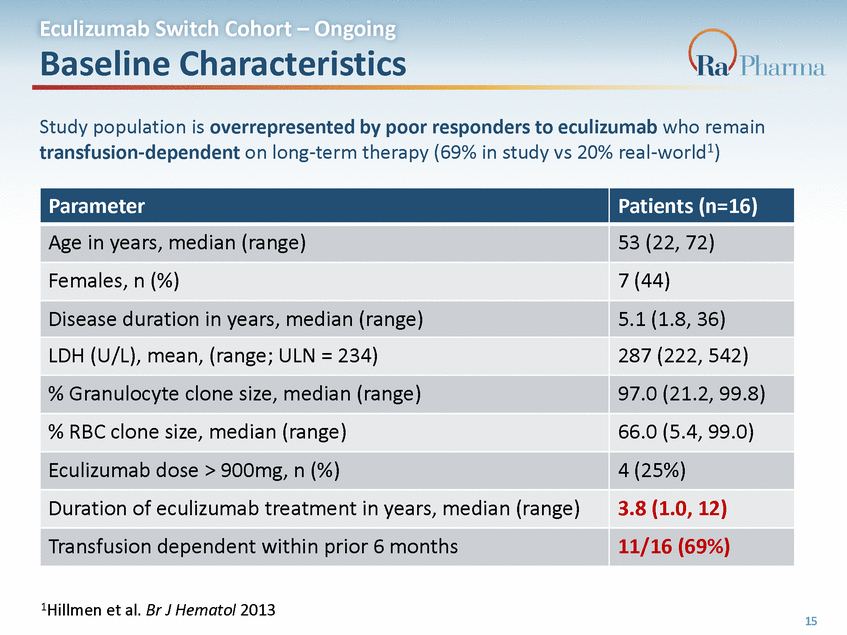
Eculizumab Switch Cohort – Ongoing Near Complete Complement Inhibition Observed Near complete, sustained, and uninterrupted inhibition of complement activity has been observed in sRBC assay during and after eculizumab washout, which exceeded the level of inhibition present at eculizumab trough (Week 0) 1 0 0 3 0 0 % H e m o ly s is E c u li z u m a b ( g / m l) 2 2 5 5 0 1 5 0 7 5 0 0 1 2 3 4 6 8 1 0 1 2 R A 1W0 1e4e9k5 . 2 0 1 ( W e e k ) 16 E c u l i z u m a b ( g / m L ) % H e m o l y s i s
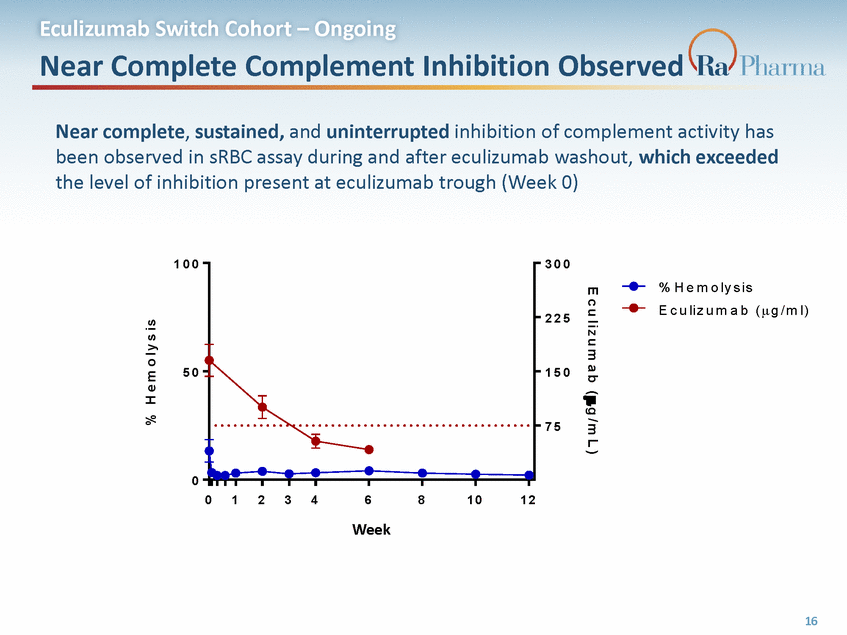
Eculizumab Switch Cohort – Ongoing Robust LDH Control in Transfusion-Independent Patients - In transfusion-independent eculizumab patients (~80% of real-world population on long-term treatment), switching to RA101495 SC has resulted in stable LDH levels and no episodes of breakthrough hemolysis In transfusion-dependent eculizumab patients (~20% of real-world population on long-term treatment), breakthrough hemolysis was observed in 7, all of whom reverted to eculizumab therapy between Week 4 and Week 10 without complications - 1 5 0 0 T r a n s f u s io n D e p e n d e n t T r a n s f u s io n I n d e p e n d e n t 1 0 0 0 5 0 0 1 . 5 x U L N Transfusion-dependent switch patients will be excluded from planned Phase 3 0 B a s e l i n e 1 2 3 4 6 8 Week 1 0 1 2 1 6 R1A0 1 0 1 479 5 . 2 051 R1 A 1 0 1 4 9 5 . 210 2 N= N= 11 5 11 5 5 3 ( W e3 e k ) ( W e e k ) 4 3 3 2 17 L D H ( U / L )
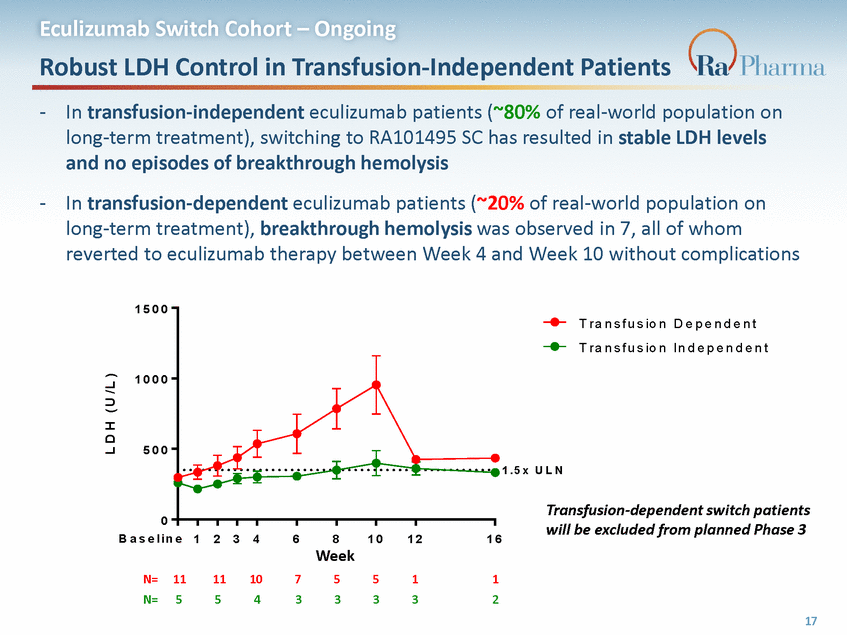
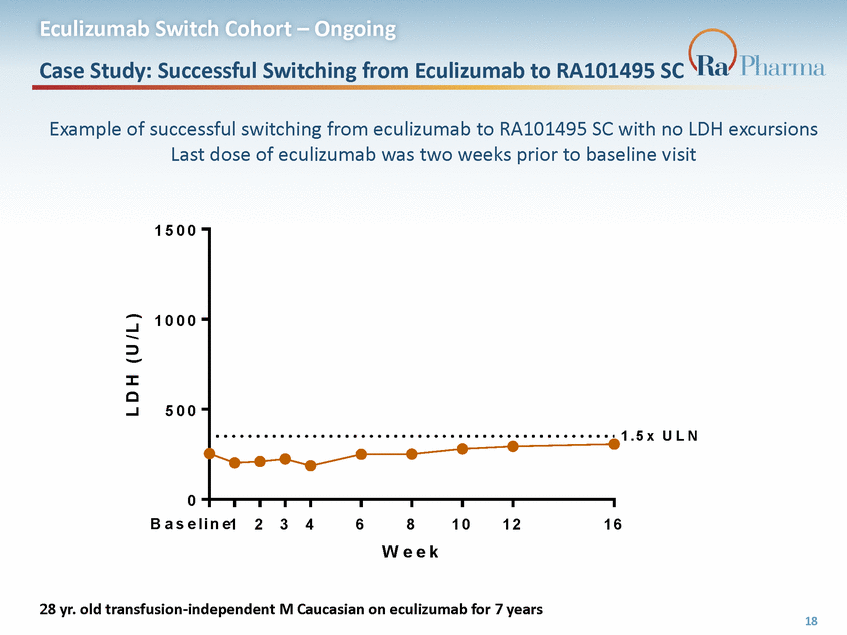
Eculizumab Switch Cohort – Ongoing Case Study: Successful Switching from Eculizumab to RA101495 SC Example of successful switching from eculizumab to RA101495 SC with no LDH excursions Last dose of eculizumab was two weeks prior to baseline visit 1 5 0 0 1 0 0 0 5 0 0 1 . 5 x U L N 0 B a s e l i n e1 2 3 4 6 8 W e e k 1 0 1 2 1 6 28 yr. old transfusion-independent M Caucasian on eculizumab for 7 years 18 L D H ( U / L )
Eculizumab Inadequate Responder Cohort 19

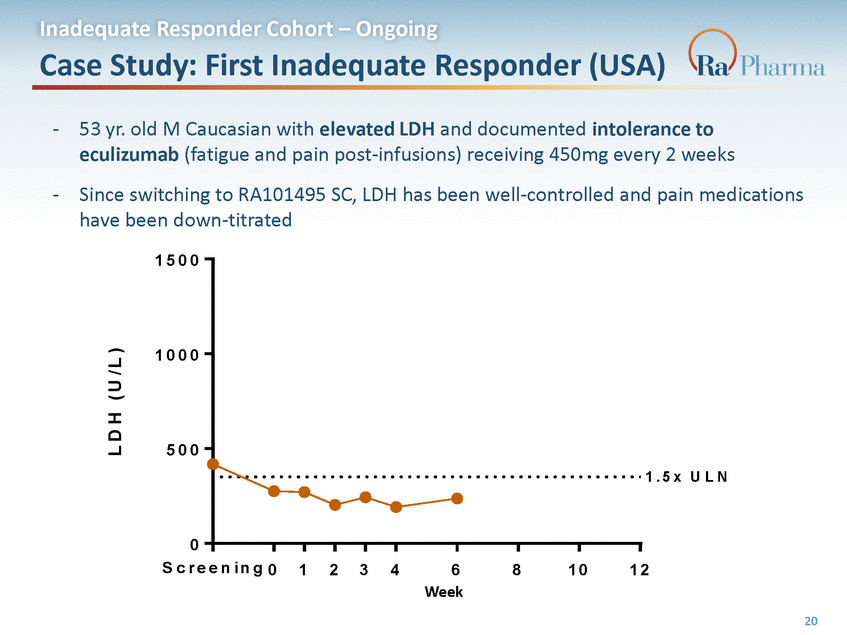
Inadequate Responder Cohort – Ongoing Case Study: First Inadequate Responder (USA) - 53 yr. old M Caucasian with elevated LDH and documented intolerance to eculizumab (fatigue and pain post-infusions) receiving 450mg every 2 weeks Since switching to RA101495 SC, LDH has been well-controlled and pain medications have been down-titrated - 1 5 0 0 1 0 0 0 5 0 0 1 . 5 x U L N 0 S c r e e n i n g 0 1 2 3 4 6 Week 8 1 0 1 2 R A 1 0 1 4 9 5 . 2 0 3 20 L D H ( U / L )
RA101495 SC Phase 2 PNH Program Safety and Tolerability Cumulative RA101495 SC exposure: Over 300 patient-weeks No dosing interruptions, down-titrations, or discontinuations due to tolerability No meningococcal infections observed No thromboembolic events observed ~100% compliance with once daily subcutaneous self-administration at home observed (monitored remotely by smartphone) Majority of adverse events observed deemed unrelated to study drug Most common related adverse event observed has been headache 7 mild (grade 1) ISRs among 3 subjects out of >2,000 self-administered injections 21
RA101495 SC Phase 2 PNH Program Summary Primary endpoint met in naïve cohort (p=0.002) In treatment-naïve patients, LDH reductions, transfusion avoidance, and QOL improvements are similar to observations from published eculizumab Phase 3 data Successful switching observed for transfusion-independent eculizumab patients, which constitutes the vast majority (80%) of patients on long-term eculizumab therapy Encouraging early data from first patient in inadequate responder cohort PK and PD data support 0.3 mg/kg as the preferred dose for Phase 3 No meaningful safety, tolerability, or compliance concerns observed 22

RA101495 SC Potential 1st Line Therapy in PNH Product profile and pricing flexibility provide potential to establish RA101495 SC as 1st line therapy in PNH Designed to expand patient access through convenient, self-administered therapy Leveraging key properties of RA101495 SC to develop an optimal commercial presentation Phase 2/Planned Phase 3 Device Highly stable at room temperature Small volume (~0.5 mL, which can be reduced further) Low viscosity (Vehicle: PBS) Administered by 29-31 gauge needles Compatible with advanced, multi-dose pen technologies BD UltraSafe Plus™ (with pre-filled syringe) Used in more than 50 commercial products 23

Opportunity to Expand Patient Access with a More Convenient and Cost Naïve patients: “PNH: Majority of patients with PNH have yet to initiate treatment” (Alexion 3Q17 Earnings Presentation, Oct. 26, 2017) PNH Registry data (2017 ASH Abstracts) Effective C5 Inhibitor ~16,000 PNH patients WW Transfusion dependent eculizumab patients (18%) Transfusion independent eculizumab patients (72%) Nearly 2,500 patients in PNH safety database were not receiving eculizumab (July 2016)1 Significant disease burden even among patients with clone size <10%2 Switch patients: Addressable population 80% of long-term eculizumab patients are transfusion independent3 5/5 transfusion-independent patients switching to RA101495 SC have had stable LDH levels and no episodes of breakthrough hemolysis Current accessible RA101495 market Naïve patients (new starts) (10%) Potential for market growth and increased/earlier use in naïve patients based on patient access and convenience WW untreated naïve patients A more convenient and cost-effective C5 inhibitor may expand access for PNH patients 24 ~$1.5B eculizumab PNH market (2017)
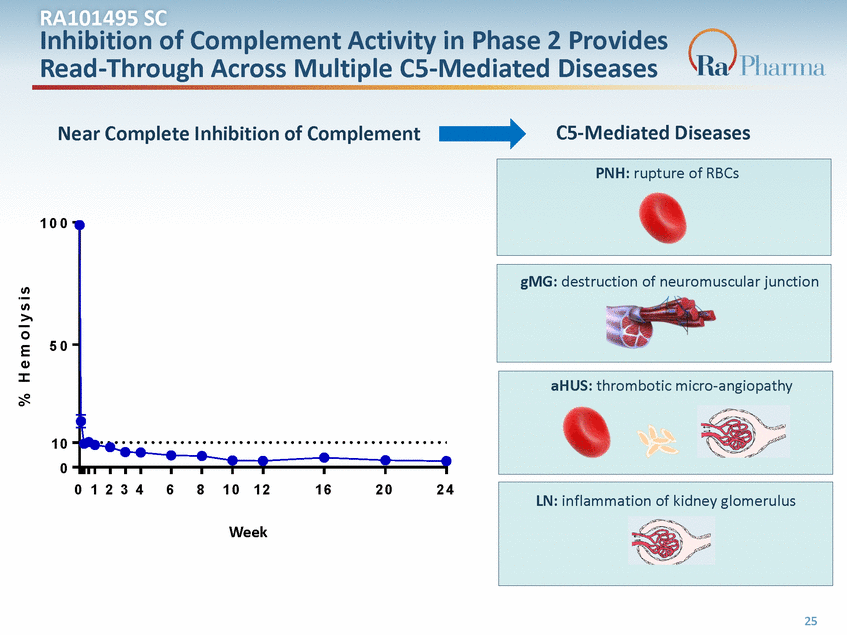
ARltAer1n0at1iv4e9P5atShCway Classical Pathway Lectin Pathway InAhcitibvaitteidobny noonf-sCelof cmellsplemeAncttivaAtecdtbiyvaitntyiboindy-Panhtiagesneco2mpPlerxoesvidesActivated by pathogen su Read-ThrCo3ugh Across Multiple C1q -–MC1er d– iCa1ts ed Diseases Factor D, Factor B C2, C3, C4 C5-Mediated Diseases Near Complete Inhibition ofCC5 omplement Alternative Pathway Classical Pathway Lectin Pathway PANctHiv: artuepdtubryepoaftRhBoCgsen surfaces Activated by non-self cells C5a ProinflammC3atory Activated by antibody-antigen complexes C5b C1q – C1r – C1s 1 0 0 cytokine Factor D, Factor B C2, C3, C4 RA101495 Binds C5 & C5b C5b6 eculizumab Binds C5 C5 gMG: destruction of neuromuscul MAC C5a C5b Proinflammatory 5cy0tokine C6 PNH: rupture of RBC C5b6 C7, C8, eculizumab Binds C5 RA101495 Binds C5 & C5b me MAC Factor targeted by Ra Pharma product candidates 1 0 0 0 1 2 3 4 6 8 1 0 1 2 1 6 2 0 2 4 LN: inflammation of kidney glomerulus R A 1 0 1 4 9 5 . 2 0 1Week R A 1 0 1 4 9 5 . 2 0 2 Factor target(eWdebeykR)a Pharma product( Wcaende ikd)ates 1 25 % H e m o l y s i s LN: inflammation of kidney glomerulus aHUS: thrombotic micro-angiopathy C9 LN: inflammation of kidney glo gMG: destruction of neuromuscular junction C7g,MC8G,:Cd9estruction of neuromuscular junction junction C6 PNH: rupture of RBC
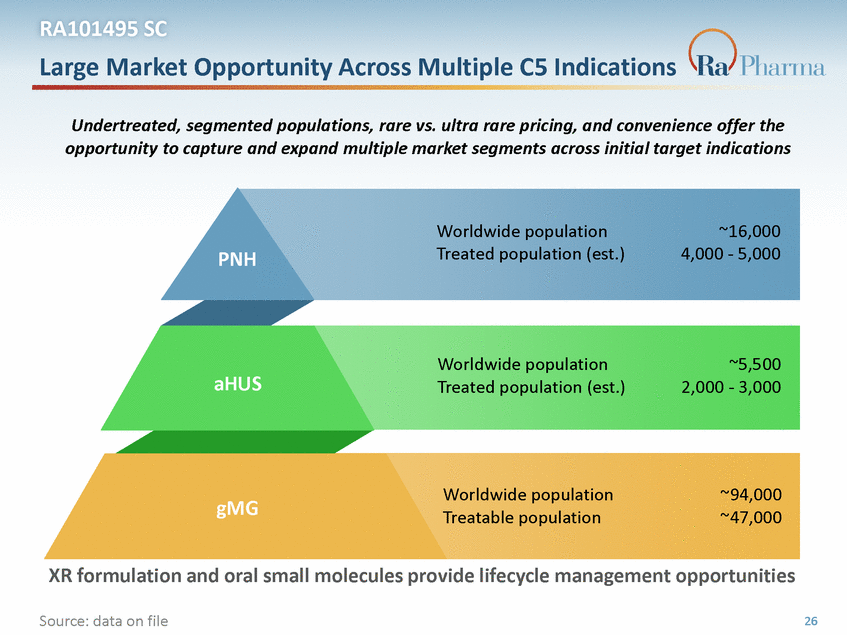
RA101495 SC Large Market Opportunity Across Multiple C5 Indications Undertreated, segmented populations, rare vs. ultra rare pricing, and convenience offer the opportunity to capture and expand multiple market segments across initial target indications Worldwide population Treated population (est.) ~16,000 4,000 - 5,000 PNH Worldwide population Treated population (est.) ~5,500 2,000 - 3,000 aHUS Worldwide population Treatable population ~94,000 ~47,000 gMG XR formulation and oral small molecules provide lifecycle management opportunities Source: data on file 26
Executive Summary Positive Interim Phase 2 Data Provides Foundation for C5 Inhibitor Franchise Lead clinical program: RA101495 SC Potent, synthetic, macrocyclic peptide inhibitor of complement C5 Convenient, self-administered, subcutaneous (SC) dosing Phase 2 program in paroxysmal nocturnal hemoglobinuria (PNH) ongoing (N=29) – – – – – Safe and well-tolerated Near complete complement inhibition observed Primary endpoint met for LDH reduction in eculizumab naïve patients (P=0.002) Successful switching demonstrated in transfusion-independent patients Encouraging early data from first patients in inadequate responder cohort Phase 2 study recruiting in generalized myasthenia gravis (gMG) Phase 1b renal impairment PK study planned to support development for treatment of atypical hemolytic uremic syndrome (aHUS) and lupus nephritis (LN) Portfolio of C5 inhibitors in pre-clinical development Extended release formulation of RA101495 SC Oral small molecule C5 inhibitor 27
Q&A

Contact: Jen Robinson Senior Director, Investor Relations & Corporate Communications jrobinson@rapharma.com


