Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Ra Pharmaceuticals, Inc. | a17-28220_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Ra Pharmaceuticals, Inc. | a17-28220_1ex99d1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): December 4, 2017
Ra Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
|
DELAWARE |
|
001-37926 |
|
26-2908274 |
|
(State or other jurisdiction |
|
(Commission |
|
(I.R.S. Employer |
|
87 Cambridge Park Drive |
|
02140 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code (617) 401-4060
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 7.01. Regulation FD Disclosure.
On December 4, 2017, Ra Pharmaceuticals, Inc. (the “Company”) issued a press release titled, “Ra Pharmaceuticals Announces Positive Interim Results from Phase 2 Study of RA101495 SC in Paroxysmal Nocturnal Hemoglobinuria.” In addition, the Company held a conference call and presented certain slides (the “Investor Presentation”) to discuss interim results from its ongoing, global Phase 2 clinical program evaluating RA101495 SC for the treatment of paroxysmal nocturnal hemoglobinuria.
The information in this Item 7.01 and Exhibits 99.1 and 99.2 attached hereto shall not be deemed “filed” for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
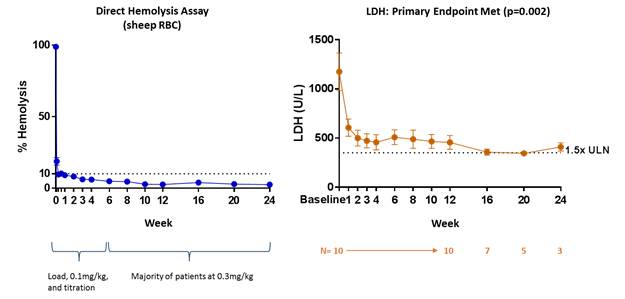
On December 4, 2017, the Company announced positive interim results from its ongoing, global Phase 2 clinical program evaluating RA101495 SC for the treatment of paroxysmal nocturnal hemoglobinuria (“PNH”). The global, dose-finding Phase 2 clinical program was designed to evaluate the safety, tolerability, preliminary efficacy, pharmacokinetics and pharmacodynamics of RA101495 SC in patients with PNH. As of November 30, 2017, a total of 29 patients have been dosed with RA101495 SC in three cohorts. The first cohort, which is referred to as Eculizumab Naïve Cohort, enrolled and completed dosing in 10 patients who have not previously been treated with eculizumab, which are referred to as eculizumab naïve patients. The second cohort, which is referred to as Eculizumab Switch Cohort, enrolled 16 patients for which dosing is ongoing. Such patients are currently treated with eculizumab and are switching over to treatment with RA101495, which are referred to as switch patients. The third cohort enrolled three patients for which dosing is ongoing. Such patients are U.S. based and are inadequate responders to eculizumab and who are also switching over to RA101495, which are referred to as eculizumab inadequate responders. Patients in all three cohorts will be eligible for a long-term extension study following the completion of the initial 12-week studies. The primary efficacy endpoint is change in lactate dehydrogenase (“LDH”), from baseline to the mean level from week 6 to week 12.
RA101495 SC met the primary endpoint in eculizumab naïve patients (n=10). The Company observed a rapid, robust, and sustained reduction in LDH levels compared to baseline (p=0.002) and near complete suppression of complement activity, as depicted in the figure below. 50% of eculizumab naïve patients who required a blood transfusion during the previous six months, who are referred to as transfusion-dependent patients, prior to enrollment, have been transfusion-free since commencing RA101495 SC. Based on preliminary data, meaningful improvements in standard measures of quality of life, as shown by the Functional Assessment of Chronic Illness Therapy fatigue score, have been observed, as well as a high level of patient satisfaction with subcutaneous self-administration based on patient surveys completed to date.
Interim results from the ongoing switch cohort demonstrate near complete, sustained, and uninterrupted inhibition of complement activity during and after eculizumab washout. The LDH response observed to date in switch patients is bimodal based on prior transfusion requirements on eculizumab. In transfusion-independent patients from this cohort (n=5), a population segment representing approximately 80% of patients on long-term eculizumab therapy, switching to RA101495 SC has been successful as indicated by stable LDH levels and no episodes of breakthrough hemolysis. Among difficult to treat eculizumab patients who were transfusion-dependent at baseline (n=11) from this cohort, breakthrough hemolysis occurred after switching in seven patients, who all reverted to eculizumab treatment without complications.
In the U.S.-based cohort of inadequate responders to eculizumab, who have a history of elevated LDH, three patients have been enrolled. LDH stabilization and relief of side effects associated with eculizumab intolerance have been observed in the first patient enrolled in this cohort.
Across all cohorts, no major safety or tolerability concerns have been identified after more than 300 patient weeks of cumulative exposure. The majority of adverse events were deemed unrelated to the study drug and the most frequent study drug-related adverse event to date was headache. No meningococcal infections or thromboembolic events have been observed. Out of more than 2,000 doses administered to date, only seven mild (grade 1) injection site reactions have occurred among three patients. Full compliance with once daily subcutaneous self-administration of RA101495 SC has been observed thus far.
If developed and approved, the Company believes, due to its product profile and pricing flexibility, RA101495 SC has the potential to serve as the natural first-line therapy for newly diagnosed naïve patients with PNH, which the Company estimates to constitute approximately 10% of the patient population, as well as an attractive alternative for transfusion-free patients switching from eculizumab, which the Company estimates to constitute approximately 72% of the patient population. Despite eculizumab sales in 2017 totaling approximately $1.5 billion, the Company believes the majority of patients with PNH have yet to initiate treatment, based on PNH registry data. In developing its commercial presentation, the Company intends to leverage key properties of RA101495 SC, including its stability at room temperature, small volume, low viscosity and resistance to shear forces and compatibility with needles.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit |
|
Description |
|
|
|
|
|
99.1 |
|
Press Release dated December 4, 2017. |
|
99.2 |
|
Investor Presentation dated December 4, 2017. |
EXHIBIT INDEX
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
99.2 |
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
Date: December 8, 2017 |
RA PHARMACEUTICALS, INC. | |
|
|
|
|
|
|
By: |
/s/ David C. Lubner |
|
|
|
David C. Lubner |
|
|
|
Executive Vice President and Chief Financial Officer |
