Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - AVADEL PHARMACEUTICALS PLC | q32017earningsrelease.htm |
| 8-K - 8-K - AVADEL PHARMACEUTICALS PLC | avadel_8kxq3x2017.htm |

Q3 2017
Earnings Conference Call
September 8, 2017

2
Safe Harbor
This presentation may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and
Section 21E of the Securities Exchange Act of 1934. The words “will,” “may,” “believe,” “expect,” “anticipate,” “estimate,” “project”
and similar expressions, and the negatives thereof, identify forward-looking statements, each of which speaks only as of the date the
statement is made. Although we believe that our forward-looking statements are based on reasonable assumptions within the
bounds of our knowledge of our business and operations, our business is subject to significant risks and as a result there can be no
assurance that actual results of our research, development and commercialization activities and our results of operations will not
differ materially from the results contemplated in such forward-looking statements. These risks include: (i) risks relating to our
license agreement with Serenity Pharmaceuticals, LLC including that our internal analyses may overstate the market opportunity in
the United States for the drug desmopressin acetate (the “Drug”) or we may not effectively exploit such market opportunity, that
significant safety or drug interaction problems could arise with respect to the Drug, that we may not successfully increase awareness
of nocturia and the potential benefits of the Drug, and that the need for management to focus attention on the development and
commercialization of the Drug could cause our ongoing business operations to suffer; and (ii) the other risks, uncertainties and
contingencies described in the Company's filings with the U.S. Securities and Exchange Commission, including our annual report on
Form 10-K for the year ended December 31, 2016, in particular under the captions “Forward-Looking Statements” and “Risk Factors,”
including without limitation: our dependence on a small number of products and customers for the majority of our revenues; the
possibility that our Bloxiverz®,Vazculep® and Akovaz® products, which are not patent protected, could face substantial competition
resulting in a loss of market share or forcing us to reduce the prices we charge for those products; the possibility that we could fail to
successfully complete the research and development for pipeline products we are evaluating for potential application to the FDA
pursuant to our "unapproved-to-approved" strategy, or that competitors could complete the development of such products and
apply for FDA approval of such products before us; the possibility that our products may not reach the commercial market or gain
market acceptance; our need to invest substantial sums in research and development in order to remain competitive; our
dependence on certain single providers for development of several of our drug delivery platforms and products; our dependence on
a limited number of suppliers to manufacture our products and to deliver certain raw materials used in our products; the possibility
that our competitors may develop and market technologies or products that are more effective or safer than ours, or obtain
regulatory approval and market such technologies or products before we do; the challenges in protecting the intellectual property
underlying our drug delivery platforms and other products; and our dependence on key personnel to execute our business plan.
Except as may be required by law, we disclaim any obligation to publicly update any forward-looking statements to reflect events
after the date of this presentation.

3
Strategy Execution
Cash Generation Proprietary Product
Development
Business Development
$39.7 Million Q3 Revenues
$30 Million in Operating
Cash Flow YTD
REST-ON Phase III Trial of
FT218, Micropump® Sodium
Oxybate
In-licensed Noctiva™ on
September 1, 2017
Continued Growth Through Cash Generation, Application of Proprietary Technology and
Business Development
Noctiva is not yet available for prescription
For full prescribing and important safety information please see slide 16 in the appendix

4
Noctiva™
First & Only FDA Approved Product to Treat Nocturia due to Nocturnal Polyuria in Adults
Noctiva:
Proprietary, low-dose (7 – 27x lower than existing
forms), intranasal desmopressin acetate formulation
Condition:
Nocturia due to nocturnal polyuria causes patients to
awaken 2 or more times / night to urinate
Prevalence: ~40 million U.S. patients with nocturia*
Diagnosed:
Independent research & claims data estimate 3 million
patients diagnosed & on some form of treatment *
*Data on file
Noctiva is not yet available for prescription
For full prescribing and important safety information please see slide 16 of the appendix

5
Hospital Products
*Based on IMS data
For full prescribing information for Bloxiverz, Vazculep and Akovaz, please see slide 17 of the appendix
**AV001 is part of Avadel’s Unapproved Marketed Drug (UMD) strategy, for which it takes currently unapproved products through the FDA approval process
Bloxiverz®
~35% share of neostigmine
market volume in Q3*
Four competing neostigmine
products during the 3rd quarter
Sugammadex has taken ~
50% neostigmine volume*
Akovaz®
~42% share of ephedrine sulfate
market volume in Q3*
Four competing neostigmine
products during the 3rd quarter
More competition expected
in 2018
Vazculep®
~40% share of 1mL vial volume
100% share of 5mL & 10mL vial
volume*
Two competing 1mL formats
More competition expected
in 2018
AV001**
Undisclosed sterile injectable
product
Market value ~$30 - $40 million Filing mid-2018
Hospital Products Accounted for $38 Million of 3Q 2017 Revenues

6
REST-ON Progress
5 of 6 sites active in Canada
24 of 31 sites active in US
16 of 18 sites active in Europe
Additional sites to be added in US and UK
Sodium Oxybate Naïve Criterion Remains High Hurdle During Screening Process
For more details on our clinical trial, please visit www.rethinknarcolepsy.com

7
Non-GAAP Financial Results
*Reconciliations from GAAP to Non-GAAP can be found in the appendix
Three Months Ended
(in 000s) 09/30/17 06/30/17 09/30/16
Sales $ 39,675 $ 47,411 $ 32,087
Cost of products and services sold 3,790 4,561 2,844
Research and development expenses 8,095 6,792 8,143
Selling, general and admin expenses 11,563 12,429 12,740
Intangible asset amortization — — —
Restructuring costs — — —
Operating Expenses 23,448 23,782 23,727
Contingent consideration payments and accruals 7,264 8,516 5,884
Operating income (loss) 8,963 15,113 2,476
Interest and other expense (net) 847 264 226
Other expense - contingent consideration payments and
accruals (963) (1,166) (785)
Income (loss) before income taxes 8,847 14,211 1,917
Income tax provision 5,100 6,046 5,416
Net income (loss) $ 3,747 $ 8,165 $ (3,499)
Diluted earnings (loss) per share $ 0.09 $ 0.19 $ (0.08)
(in $000s, except for per share amounts)

8
GAAP Financial Results
Three Months Ended
(in 000s) 09/30/17 06/30/17 09/30/16
Sales $ 39,675 $ 46,311 $ 32,087
Cost of products and services sold 3,790 4,561 2,844
Research and development expenses 8,095 6,792 8,143
Selling, general and admin expenses 11,563 12,429 12,740
Intangible asset amortization 564 564 3,702
Restructuring costs (549) 1,069 —
Operating Expenses 23,463 25,415 27,429
(Gain)/loss - changes in fair value of related party contingent
consideration (9,906) (13,230) 20,848
Operating income (loss) 26,118 34,126 (16,190)
Interest and other expense (net) 714 501 1,475
Other income (expense) - changes in fair value of related party
payable 768 1,670 (1,828)
Income (loss) before income taxes 27,600 36,297 (16,543)
Income tax provision 5,921 7,370 3,451
Net income (loss) $ 21,679 $ 28,927 $ (19,994)
Diluted earnings (loss) per share $ 0.52 $ 0.68 $ (0.48)
(in $000s, except for per share amounts)

9
Revenues (GAAP)
(in $000's) Q3 2017 Q2 2017 Q3 2016
Q3 2017
vs.
Q2 2017
Q3 2017
vs.
Q3 2016
Bloxiverz $ 9,920 $ 13,719 $ 15,591 $ (3,799) $ (5,671)
Vazculep 9,573 10,154 9,340 (581) 233
Akovaz 18,561 20,912 5,568 (2,351) 12,993
Other 1,093 2,320 841 (1,227) 252
Total product sales and services 39,147 47,105 31,340 (7,958) 7,807
License and research revenue 528 794 747 (266) (219)
Total revenues $ 39,675 $ 47,899 $ 32,087 $ (8,224) $ 7,588

10
Cash Flow Summary
(in $000's) Nine Months Ended September 30,
Total Cash and Marketable Securities 2017 2016
Beginning Balance 154,195 144,802
Operating Cash Flows (excl tax and earnout payments) 73,258 49,636
Earnout/Royalty Payments (29,136) (24,227)
Income Taxes (14,605) (22,200)
Acquisition of Noctiva Asset (52,139) —
Share Repurchases (16,707) —
Capital Spending (533) (1,000)
Other 1,277 2,656
Change in Total (38,585) 4,865
Ending Balance 115,610 149,667

11
2017 Non - GAAP Guidance
2017 Guidance
Updated Previous
Sales $165M - $175M $165M - $175M
R&D Expense $30M - $35M $30M - $40M
Income Tax Rate 55% - 65% 60% - 70%
Diluted EPS (Adjusted) $0.25 - $0.35 $0.30 - $0.45

12
APPENDIX
13
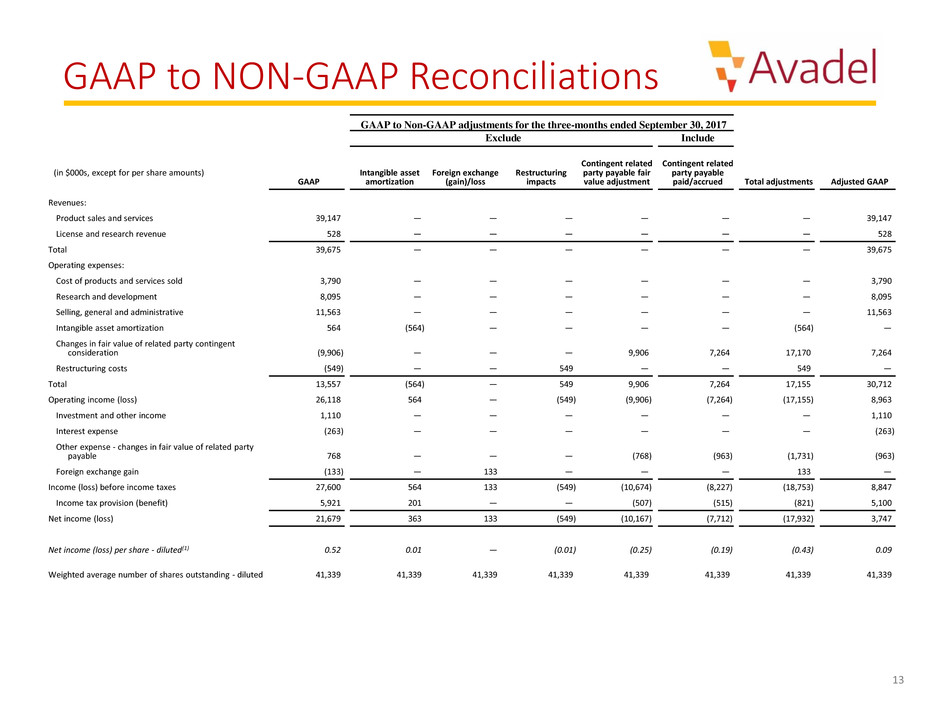
GAAP to NON-GAAP Reconciliations
GAAP to Non-GAAP adjustments for the three-months ended September 30, 2017
Exclude Include
GAAP
Intangible asset
amortization
Foreign exchange
(gain)/loss
Restructuring
impacts
Contingent related
party payable fair
value adjustment
Contingent related
party payable
paid/accrued Total adjustments Adjusted GAAP
Revenues:
Product sales and services 39,147 — — — — — — 39,147
License and research revenue 528 — — — — — — 528
Total 39,675 — — — — — — 39,675
Operating expenses:
Cost of products and services sold 3,790 — — — — — — 3,790
Research and development 8,095 — — — — — — 8,095
Selling, general and administrative 11,563 — — — — — — 11,563
Intangible asset amortization 564 (564) — — — — (564) —
Changes in fair value of related party contingent
consideration (9,906) — — — 9,906 7,264 17,170 7,264
Restructuring costs (549) — — 549 — — 549 —
Total 13,557 (564) — 549 9,906 7,264 17,155 30,712
Operating income (loss) 26,118 564 — (549) (9,906) (7,264) (17,155) 8,963
Investment and other income 1,110 — — — — — — 1,110
Interest expense (263) — — — — — — (263)
Other expense - changes in fair value of related party
payable 768 — — — (768) (963) (1,731) (963)
Foreign exchange gain (133) — 133 — — — 133 —
Income (loss) before income taxes 27,600 564 133 (549) (10,674) (8,227) (18,753) 8,847
Income tax provision (benefit) 5,921 201 — — (507) (515) (821) 5,100
Net income (loss) 21,679 363 133 (549) (10,167) (7,712) (17,932) 3,747
Net income (loss) per share - diluted(1) 0.52 0.01 — (0.01) (0.25) (0.19) (0.43) 0.09
Weighted average number of shares outstanding - diluted 41,339 41,339 41,339 41,339 41,339 41,339 41,339 41,339
(in $000s, except for per share amounts)
14
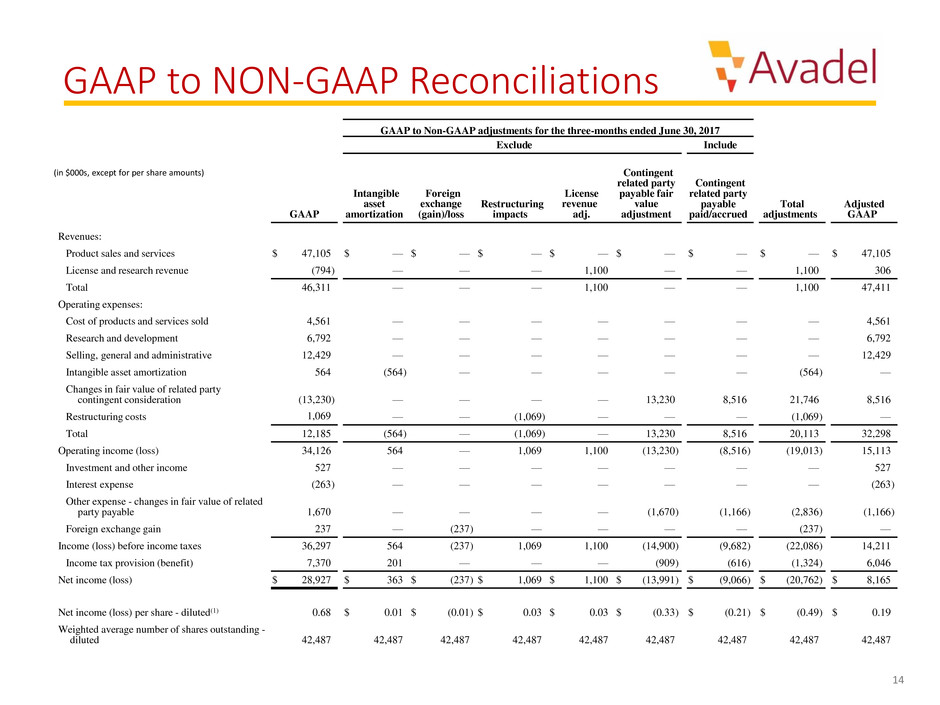
GAAP to NON-GAAP Reconciliations
GAAP to Non-GAAP adjustments for the three-months ended June 30, 2017
Exclude Include
GAAP
Intangible
asset
amortization
Foreign
exchange
(gain)/loss
Restructuring
impacts
License
revenue
adj.
Contingent
related party
payable fair
value
adjustment
Contingent
related party
payable
paid/accrued
Total
adjustments
Adjusted
GAAP
Revenues:
Product sales and services $ 47,105 $ — $ — $ — $ — $ — $ — $ — $ 47,105
License and research revenue (794) — — — 1,100 — — 1,100 306
Total 46,311 — — — 1,100 — — 1,100 47,411
Operating expenses:
Cost of products and services sold 4,561 — — — — — — — 4,561
Research and development 6,792 — — — — — — — 6,792
Selling, general and administrative 12,429 — — — — — — — 12,429
Intangible asset amortization 564 (564) — — — — — (564) —
Changes in fair value of related party
contingent consideration (13,230) — — — — 13,230 8,516 21,746 8,516
Restructuring costs 1,069 — — (1,069) — — — (1,069) —
Total 12,185 (564) — (1,069) — 13,230 8,516 20,113 32,298
Operating income (loss) 34,126 564 — 1,069 1,100 (13,230) (8,516) (19,013) 15,113
Investment and other income 527 — — — — — — — 527
Interest expense (263) — — — — — — — (263)
Other expense - changes in fair value of related
party payable 1,670 — — — — (1,670) (1,166) (2,836) (1,166)
Foreign exchange gain 237 — (237) — — — — (237) —
Income (loss) before income taxes 36,297 564 (237) 1,069 1,100 (14,900) (9,682) (22,086) 14,211
Income tax provision (benefit) 7,370 201 — — — (909) (616) (1,324) 6,046
Net income (loss) $ 28,927 $ 363 $ (237) $ 1,069 $ 1,100 $ (13,991) $ (9,066) $ (20,762) $ 8,165
Net income (loss) per share - diluted(1) 0.68 $ 0.01 $ (0.01) $ 0.03 $ 0.03 $ (0.33) $ (0.21) $ (0.49) $ 0.19
Weighted average number of shares outstanding -
diluted 42,487 42,487 42,487 42,487 42,487 42,487 42,487 42,487 42,487
(in $000s, except for per share amounts)

15
GAAP to NON-GAAP Reconciliations
GAAP to Non-GAAP adjustments for the three-months ended September 30, 2016
Exclude Include
GAAP
Intangible asset
amortization
Foreign exchange
(gain)/loss
Contingent related
party payable fair value
adjustment
Contingent related
party payable
paid/accrued Total adjustments Adjusted GAAP
Revenues:
Product sales and services 31,340 — — — — — 31,340
License and research revenue 747 — — — — — 747
Total 32,087 — — — — — 32,087
Operating expenses:
Cost of products and services sold 2,844 — — — — — 2,844
Research and development 8,143 — — — — — 8,143
Selling, general and administrative 12,740 — — — — — 12,740
Intangible asset amortization 3,702 (3,702) — — — (3,702) —
Changes in fair value of related party
contingent consideration 20,848 — — (20,848) 5,884 (14,964) 5,884
Restructuring costs — — — — — — —
Total 48,277 (3,702) — (20,848) 5,884 (18,666) 29,611
Operating income (loss) (16,190) 3,702 — 20,848 (5,884) 18,666 2,476
Investment and other income 490 — — — — — 490
Interest expense (264) — — — — — (264)
Other expense - changes in fair value of
related party payable (1,828) — — 1,828 (785) 1,043 (785)
Foreign exchange gain 1,249 — (1,249) — — (1,249) —
Income (loss) before income taxes (16,543) 3,702 (1,249) 22,676 (6,669) 18,460 1,917
Income tax provision (benefit) 3,451 1,329 — 1,021 (385) 1,965 5,416
Net income (loss) (19,994) 2,373 (1,249) 21,655 (6,284) 16,495 (3,499)
Net income (loss) per share - diluted(1) (0.48) 0.06 (0.03) 0.53 (0.15) 0.40 (0.08)
Weighted average number of shares outstanding
- diluted 41,241 41,241 41,241 41,241 41,241 41,241 41,241
(in $000s, except for per share amounts)

16
Noctiva™ (desmopressin acetate)
Boxed Warning
WARNING: HYPONATREMIA
NOCTIVA can cause hyponatremia. Severe hyponatremia can be life-threatening, leading to seizures,
coma, respiratory arrest, or death.
NOCTIVA is contraindicated in patients at increased risk of severe hyponatremia, such as patients with
excessive fluid intake, illnesses that can cause fluid or electrolyte imbalances, and in those using loop
diuretics or systemic or inhaled Glucocorticoids.
Ensure serum sodium concentrations are normal before starting or resuming NOCTIVA. Measure
serum sodium within seven days and approximately one month after initiating therapy or increasing the
dose, and periodically during treatment. More frequently monitor serum sodium in patients 65 years of
age and older and in patients at increased risk of hyponatremia.
If hyponatremia occurs, NOCTIVA may need to be temporarily or permanently discontinued.

17
Product & Safety Information
www.bloxiverz.com
www.vazculep.com
www.akovaz.com
Full Prescribing & Safety
Information
www.karbinaler.com
http://www.aciphexsprinkle.com
http://cefaclororal.com
http://flexichamber.com
Akovaz®
Bloxiverz®
Vazculep®
Noctiva™
Karbinal™ER
Aciphex®Sprinkle™
Cefaclor
Flexichamber®
