Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Asterias Biotherapeutics, Inc. | ex99_1.htm |
| 8-K - 8-K - Asterias Biotherapeutics, Inc. | form8k.htm |
Exhibit 99.2

Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: ASTOctober 2017 14219863Text 04698Text 230237246Text

Statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ filings with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements. Forward-Looking Statements

Disclaimers on Patient Stories AST-OPC1 is a cell-based therapy still under development and the Food and Drug Administration (“FDA”) has not evaluated or approved AST-OPC1 for commercial sale for any use. The company is in the early stages of testing AST-OPC1 in a Phase 1/2a clinical research study for patients with severe cervical spinal cord injuries. The clinical trial process is complex, expensive, and takes time. Approval by FDA of AST-OPC1 for commercial use may take years, require additional studies, and may not occur at all.While the initial results of the patients described in this presentation have been encouraging, results for patients treated with AST-OPC1 will vary by patient. Also, the long-term safety and efficacy results for patients treated with AST-OPC1, including those patients described in this presentation, are still unknown. Future studies are likely necessary to distinguish efficacy due to a patient receiving AST-OPC1 cells from any natural recovery which may occur in the patient over time following the patient’s traumatic injury.Not all patients with spinal cord injuries are eligible to participate in the current study as criteria such as type and timing of injury apply. Additional information on the existing trial, including trial sites and eligibility criteria, can be found at www.clinicaltrials.gov or at www.scistar-study.com.

A cell therapy company with lead programs in: Neurology: Spinal cord injuryImmunotherapy: Lung cancer, AMLFunding Partners:California Institute of Regenerative MedicineCancer Research UK Background Information

Investment Highlights 14219863Text 04698Text 230237246Text Strong Leadership TeamTechnology Platforms with Broad ApplicationSpinal Cord Injury Program Generating Exciting DataCancer Immunotherapy Programs Provide Multiple Shots-on-GoalMultiple Near-Term Data Readouts and other Events Provide Future Potential CatalystsNon-Dilutive Funding Opportunities for ProgramsNear-Term Partnering Opportunities

Strong Leadership Team with Proven Track Record Name Experience Don BaileyChairman Former CEO of Questcor Pharmaceuticals, led Questcor’s corporate turnaround Mike MulroyPresident and CEO Former EVP of Strategic Affairs, CFO and GC of Questcor PharmaceuticalsKey executive during Questcor’s growth and eventual salePrior investment banking experience at Citigroup and Merrill Lynch Ryan ChavezCFO & GC Former General Counsel for Mallinckrodt ARD divisionPart of corporate team at Questcor; held financial positions at GE Katy Spink, Ph.D.EVP, COO Former SVP, Cell Therapy Program Operations at GeronPrior to Geron, Dr. Spink was a management consultant at McKinsey Ed Wirth, M.D., Ph.D.CMO Former CSO of InVivo Therapeutics25 years experience translational research at the University of Chicago, Geron and InVivo Jane Lebkowski, Ph.D.CSO Former CSO of GeronOver 30 years experience in R&D of cell and gene therapies at Geron, Applied Immune Sciences, and Rhône Poulenc Rorer 14219863Text 04698Text 230237246Text
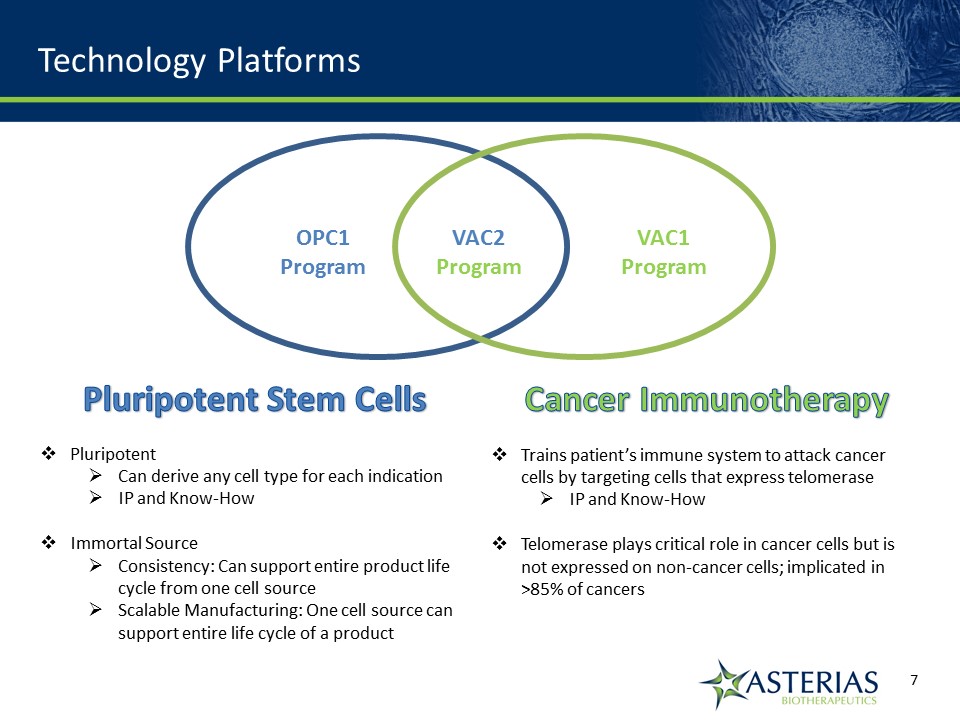
Technology Platforms Pluripotent Stem CellsPluripotentCan derive any cell type for each indicationIP and Know-HowImmortal SourceConsistency: Can support entire product life cycle from one cell sourceScalable Manufacturing: One cell source can support entire life cycle of a product Cancer ImmunotherapyTrains patient’s immune system to attack cancer cells by targeting cells that express telomeraseIP and Know-HowTelomerase plays critical role in cancer cells but is not expressed on non-cancer cells; implicated in >85% of cancers OPC1Program VAC2Program VAC1Program
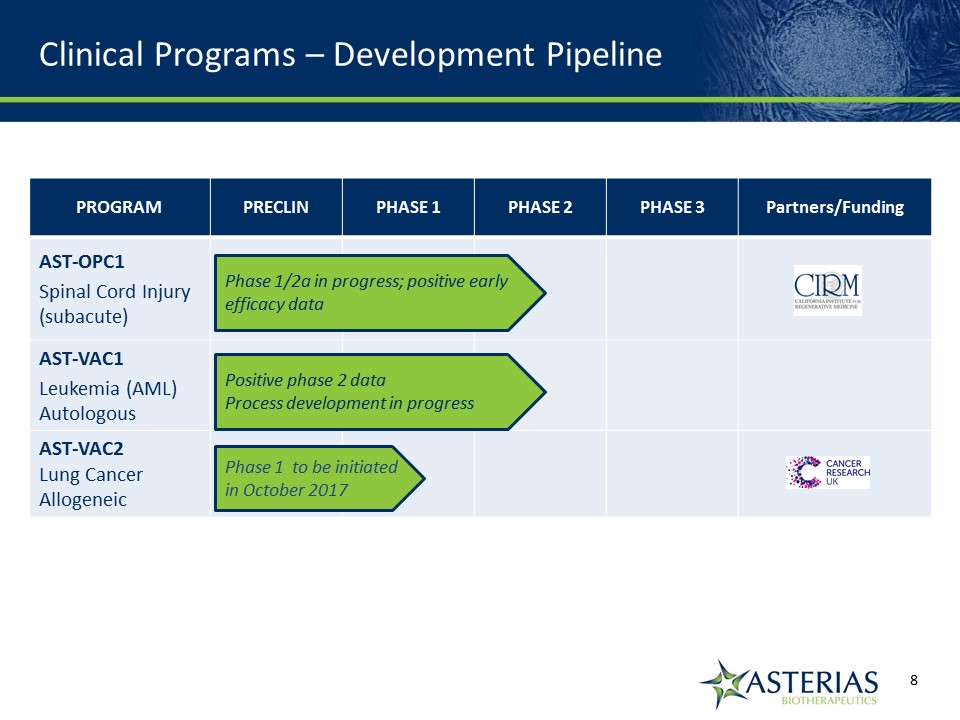
PROGRAM PRECLIN PHASE 1 PHASE 2 PHASE 3 Partners/Funding AST-OPC1Spinal Cord Injury (subacute) AST-VAC1Leukemia (AML) Autologous AST-VAC2Lung Cancer Allogeneic Phase 1/2a in progress; positive early efficacy data Positive phase 2 dataProcess development in progress Phase 1 to be initiated in October 2017 Clinical Programs – Development Pipeline 14219863Text 04698Text 230237246Text

Spinal Cord Injury Program 14219863Text 04698Text 230237246Text

Significant unmet medical need for patients with severe spinal cord injuries (“SCI”) Asterias is conducting a Phase 1/2a study (“the SCiStar study”) in severe cervical SCIMore than 25 patients have been administered AST-OPC1; strong safety profile to dateThe therapeutic goal for AST-OPC1 is to help restore arm, hand and finger function in SCI patients thereby increasing their independence and quality of lifeAST-OPC1 is a cellular therapy utilizing oligodendrocyte progenitor cells (OPCs)OPCs are found in the human body and are precursors to oligodendrocyte cells which have multiple MOAs, including electrical insulation for nerve axons in the form of a myelin sheathAST-OPC1 administers OPCs into the body to supplement the body’s own internal supply of OPCs with a non-patient specific supply of cells AST-OPC1 is made from a well-established, pluripotent embryonic stem cell line originally isolated in the 1990sPrior efficacy updates from the SCiStar study have shown promising initial results; a key open issue for the program has been showing benefit through 12 mos. AST-OPC1 Overview 14219863Text 04698Text 230237246Text
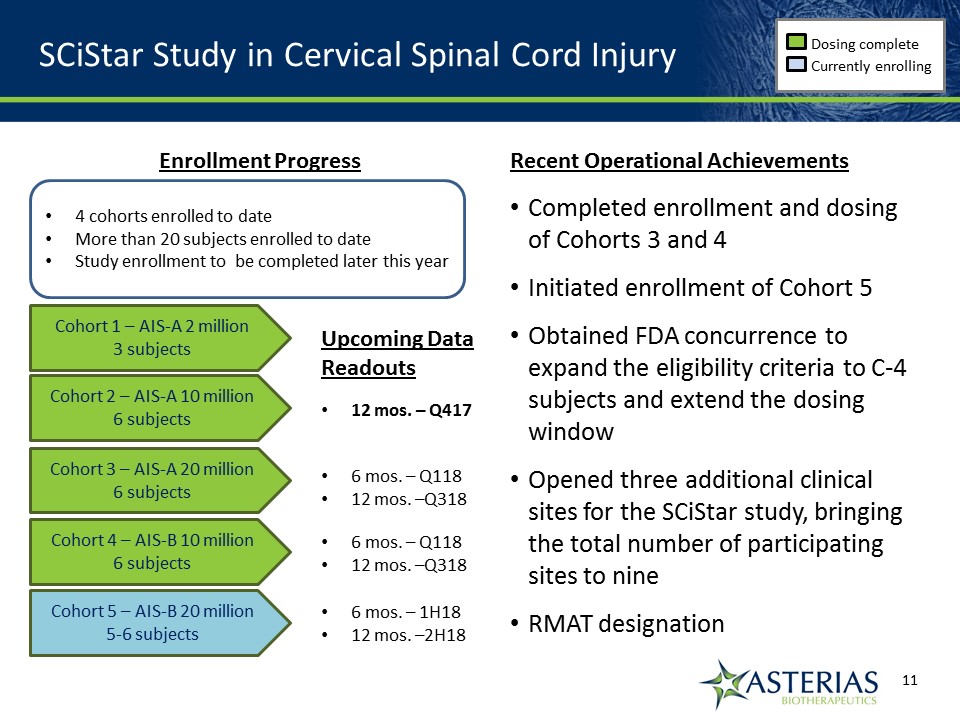
SCiStar Study in Cervical Spinal Cord Injury Dosing complete Currently enrolling Cohort 1 – AIS-A 2 million3 subjects Cohort 2 – AIS-A 10 million6 subjects Cohort 3 – AIS-A 20 million6 subjects Cohort 4 – AIS-B 10 million6 subjects Cohort 5 – AIS-B 20 million5-6 subjects Enrollment Progress Recent Operational AchievementsCompleted enrollment and dosing of Cohorts 3 and 4Initiated enrollment of Cohort 5 Obtained FDA concurrence to expand the eligibility criteria to C-4 subjects and extend the dosing windowOpened three additional clinical sites for the SCiStar study, bringing the total number of participating sites to nineRMAT designation 4 cohorts enrolled to dateMore than 20 subjects enrolled to dateStudy enrollment to be completed later this year Upcoming Data Readouts 12 mos. – Q417 6 mos. – Q11812 mos. –Q318 6 mos. – Q11812 mos. –Q318 6 mos. – 1H1812 mos. –2H18
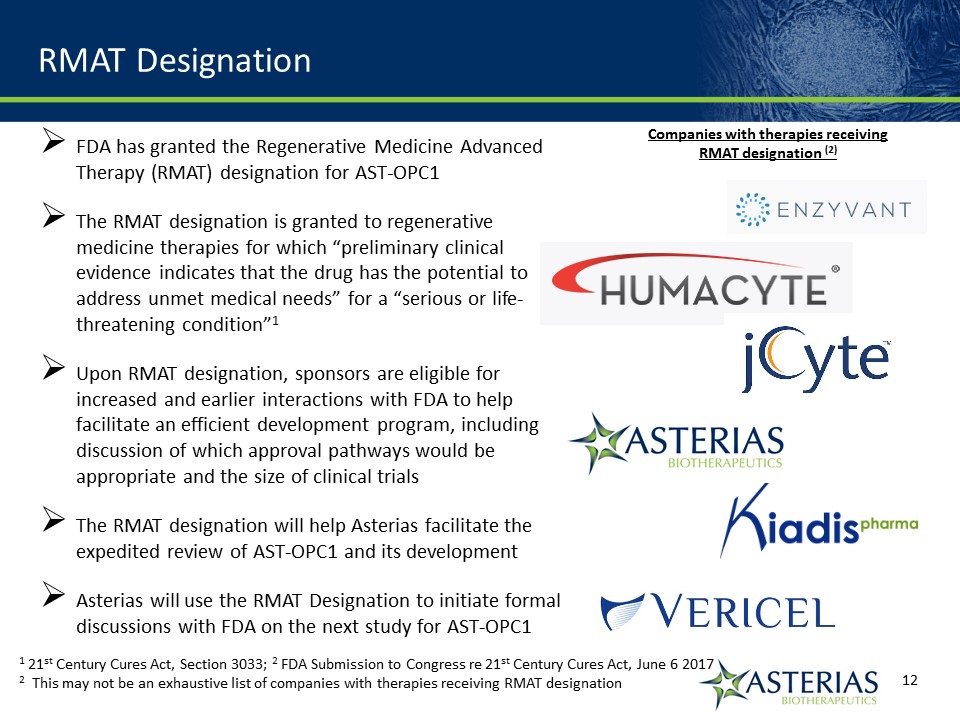
FDA has granted the Regenerative Medicine Advanced Therapy (RMAT) designation for AST-OPC1 The RMAT designation is granted to regenerative medicine therapies for which “preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs” for a “serious or life-threatening condition”1Upon RMAT designation, sponsors are eligible for increased and earlier interactions with FDA to help facilitate an efficient development program, including discussion of which approval pathways would be appropriate and the size of clinical trialsThe RMAT designation will help Asterias facilitate the expedited review of AST-OPC1 and its developmentAsterias will use the RMAT Designation to initiate formal discussions with FDA on the next study for AST-OPC1 RMAT Designation 1 21st Century Cures Act, Section 3033; 2 FDA Submission to Congress re 21st Century Cures Act, June 6 20172 This may not be an exhaustive list of companies with therapies receiving RMAT designation Companies with therapies receiving RMAT designation (2)
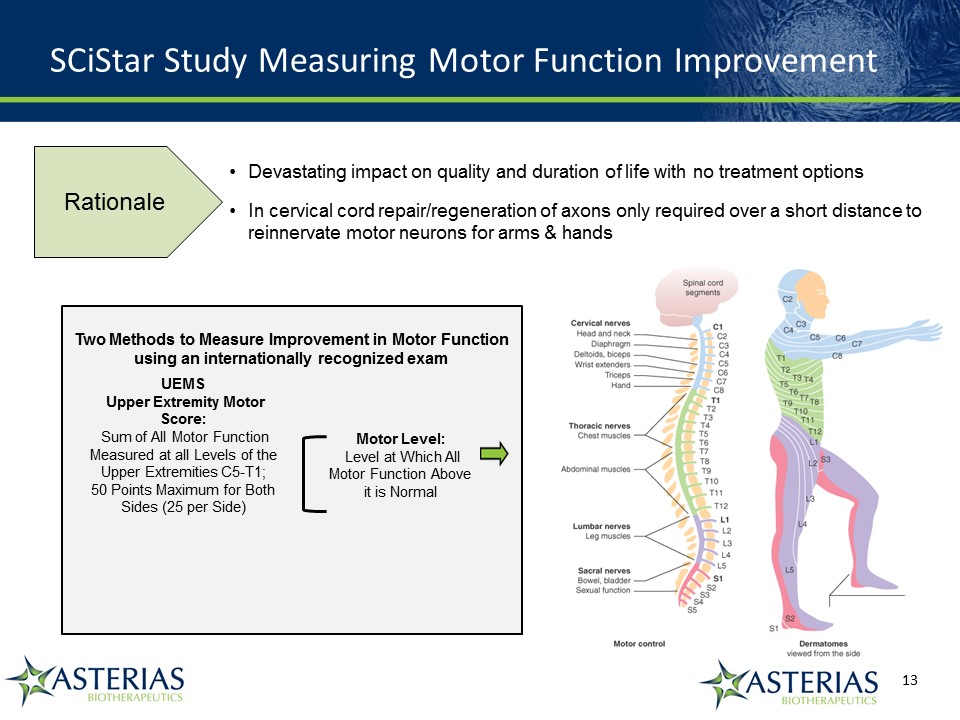
SCiStar Study Measuring Motor Function Improvement Devastating impact on quality and duration of life with no treatment optionsIn cervical cord repair/regeneration of axons only required over a short distance to reinnervate motor neurons for arms & hands UEMS Upper Extremity Motor Score: Sum of All Motor Function Measured at all Levels of the Upper Extremities C5-T1; 50 Points Maximum for Both Sides (25 per Side) Motor Level: Level at Which All Motor Function Above it is Normal Two Methods to Measure Improvement in Motor Function using an internationally recognized exam Rationale
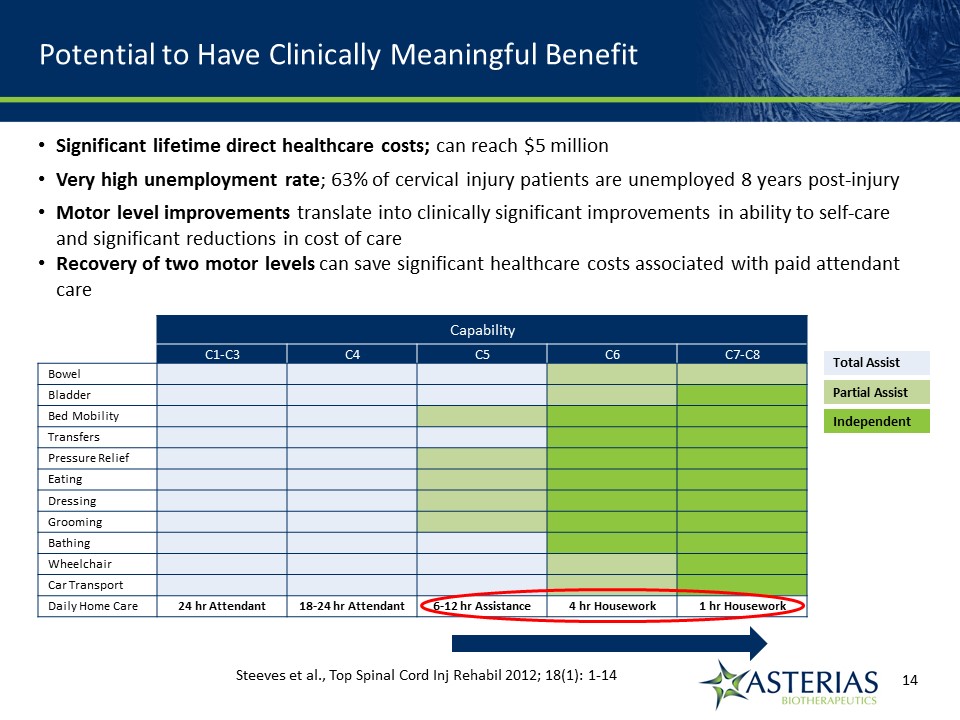
Potential to Have Clinically Meaningful Benefit Capability C1-C3 C4 C5 C6 C7-C8 Bowel Bladder Bed Mobility Transfers Pressure Relief Eating Dressing Grooming Bathing Wheelchair Car Transport Daily Home Care 24 hr Attendant 18-24 hr Attendant 6-12 hr Assistance 4 hr Housework 1 hr Housework Total Assist Partial Assist Independent Steeves et al., Top Spinal Cord Inj Rehabil 2012; 18(1): 1-14 14219863Text 04698Text 230237246Text Significant lifetime direct healthcare costs; can reach $5 millionVery high unemployment rate; 63% of cervical injury patients are unemployed 8 years post-injuryMotor level improvements translate into clinically significant improvements in ability to self-care and significant reductions in cost of careRecovery of two motor levels can save significant healthcare costs associated with paid attendant care
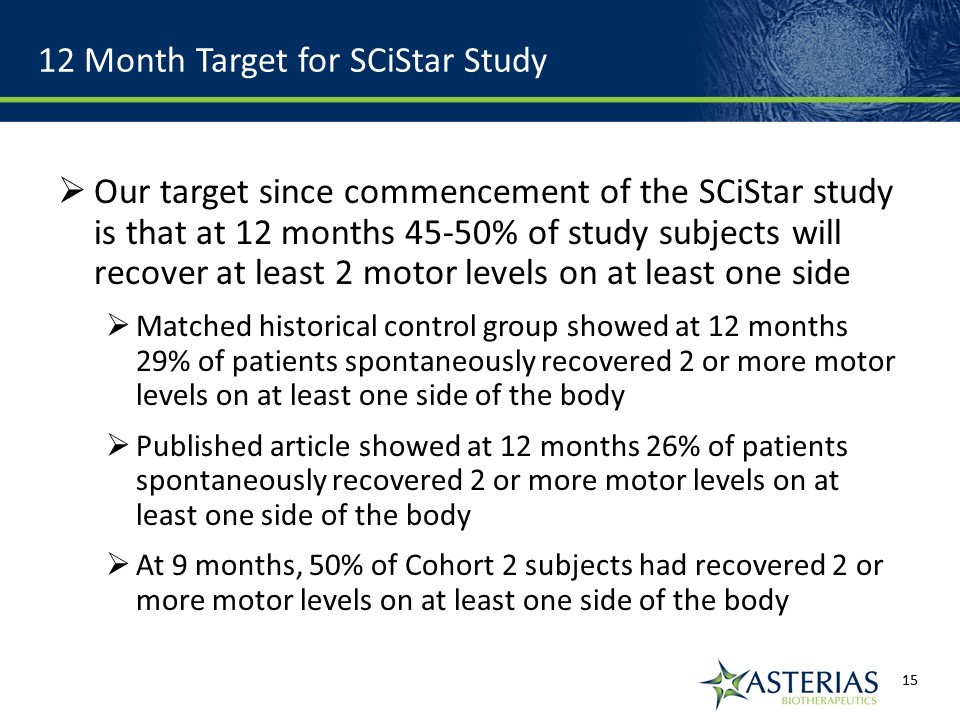
Our target since commencement of the SCiStar study is that at 12 months 45-50% of study subjects will recover at least 2 motor levels on at least one sideMatched historical control group showed at 12 months 29% of patients spontaneously recovered 2 or more motor levels on at least one side of the body Published article showed at 12 months 26% of patients spontaneously recovered 2 or more motor levels on at least one side of the bodyAt 9 months, 50% of Cohort 2 subjects had recovered 2 or more motor levels on at least one side of the body 12 Month Target for SCiStar Study
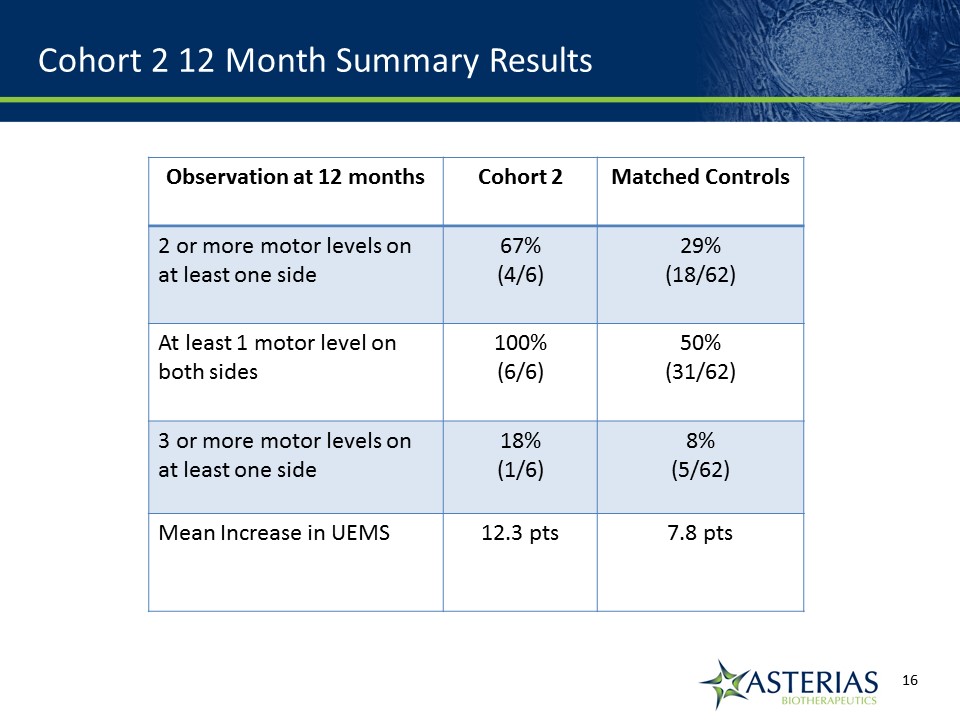
Cohort 2 12 Month Summary Results Observation at 12 months Cohort 2 Matched Controls 2 or more motor levels on at least one side 67%(4/6) 29%(18/62) At least 1 motor level on both sides 100%(6/6) 50%(31/62) 3 or more motor levels on at least one side 18%(1/6) 8%(5/62) Mean Increase in UEMS 12.3 pts 7.8 pts
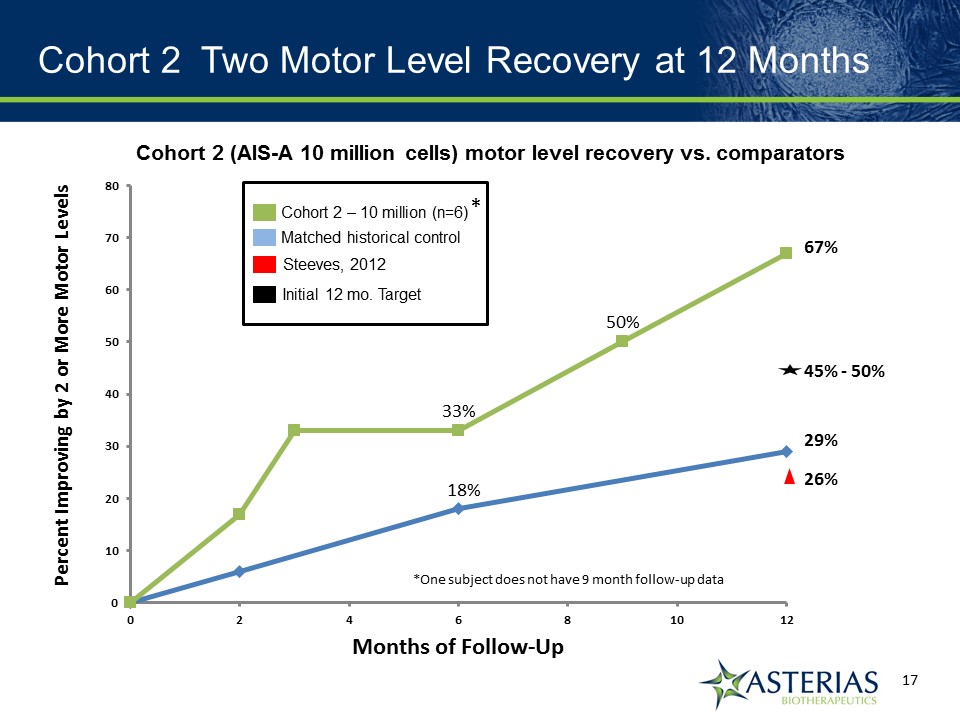
Cohort 2 Two Motor Level Recovery at 12 Months Cohort 2 (AIS-A 10 million cells) motor level recovery vs. comparators Cohort 2 – 10 million (n=6) Matched historical control Steeves, 2012 26% 29% 67% 50% 33% 18% Initial 12 mo. Target 45% - 50% * *One subject does not have 9 month follow-up data
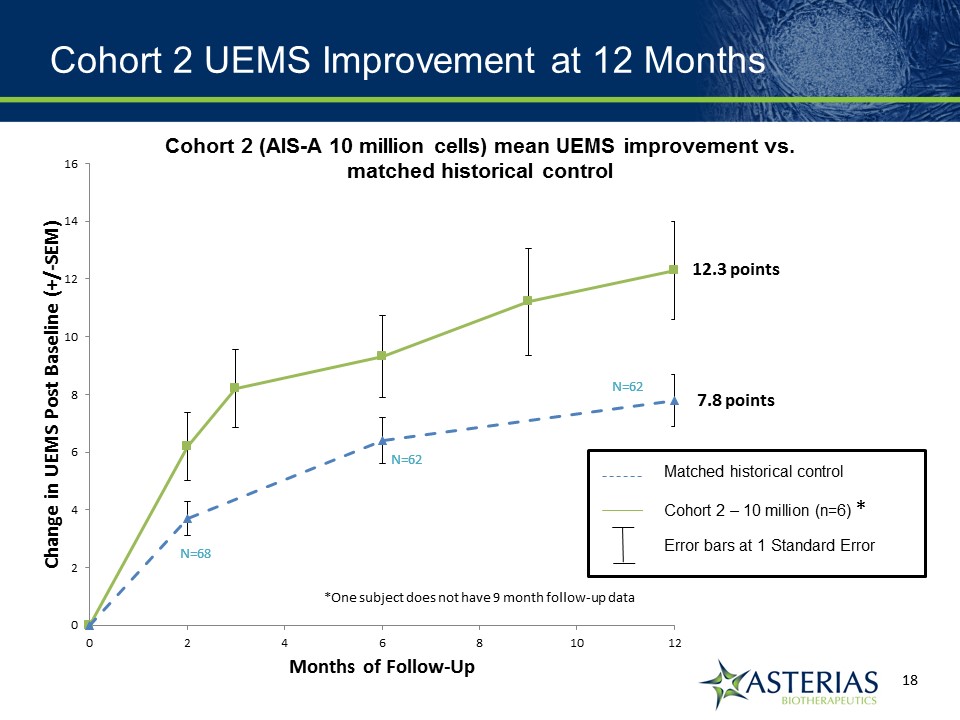
Cohort 2 UEMS Improvement at 12 Months *One subject does not have 9 month follow-up data Matched historical control Cohort 2 – 10 million (n=6) Error bars at 1 Standard Error * 12.3 points 7.8 points Cohort 2 (AIS-A 10 million cells) mean UEMS improvement vs. matched historical control
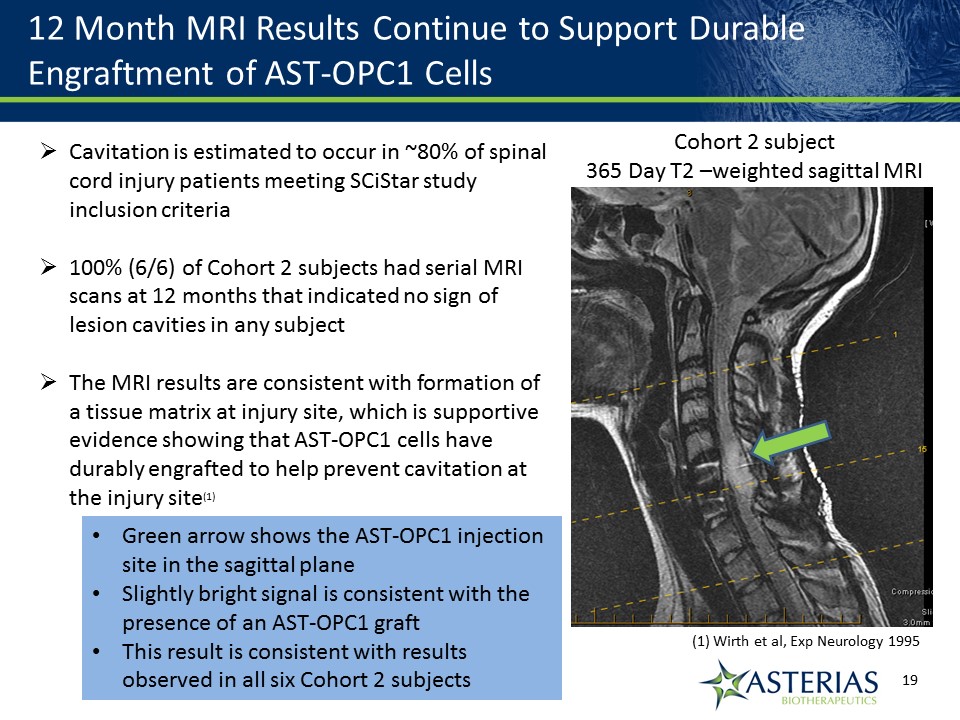
12 Month MRI Results Continue to Support Durable Engraftment of AST-OPC1 Cells Cavitation is estimated to occur in ~80% of spinal cord injury patients meeting SCiStar study inclusion criteria100% (6/6) of Cohort 2 subjects had serial MRI scans at 12 months that indicated no sign of lesion cavities in any subjectThe MRI results are consistent with formation of a tissue matrix at injury site, which is supportive evidence showing that AST-OPC1 cells have durably engrafted to help prevent cavitation at the injury site(1) (1) Wirth et al, Exp Neurology 1995 Cohort 2 subject365 Day T2 –weighted sagittal MRI Green arrow shows the AST-OPC1 injection site in the sagittal planeSlightly bright signal is consistent with the presence of an AST-OPC1 graftThis result is consistent with results observed in all six Cohort 2 subjects

Personal Stories Lucas threw the first pitch at a MLB game on August 13thLucas can now write with a pen, type e-mails at 40 words per minute, use his phone, and feed himself. Kris can now use his hands and fingers to feed himself, drink, text, send e-mails, sign his name and even play video games.
Summary and Next Steps Cohorts 1 and 2 data support:A strong safety profile for AST-OPC1 Encouraging evidence of efficacy amongst the most severely impaired SCI patientsEvidence of dose dependence of efficacyProlonged effects sustained at least a year after administration SCiStar study data and RMAT designation will be used to:Design a randomized, controlled trialSelect dose, key endpoints, and inclusion criteria Evaluate expansion into less severely impaired SCI patients where recovery of lower extremity function may be possible
What’s Next for AST-OPC1 14219863Text 04698Text 230237246Text 1H’18 2H’18 Cohort 3 - 6 month data updateCohort 4 - 6 month data updateSCiStar study 6 month update, including Cohort 5 Cohort 3 - 12 month updateCohort 4 - 12 month updateSCiStar study 12 month update, including Cohort 5 Data Updates Other 2018 Potential Catalysts Regulatory clearance for randomized controlled trial for AST-OPC1Non-dilutive funding to partially offset costs for next trialJapanese partnership

Cancer Immunotherapy 14219863Text 04698Text 230237246Text
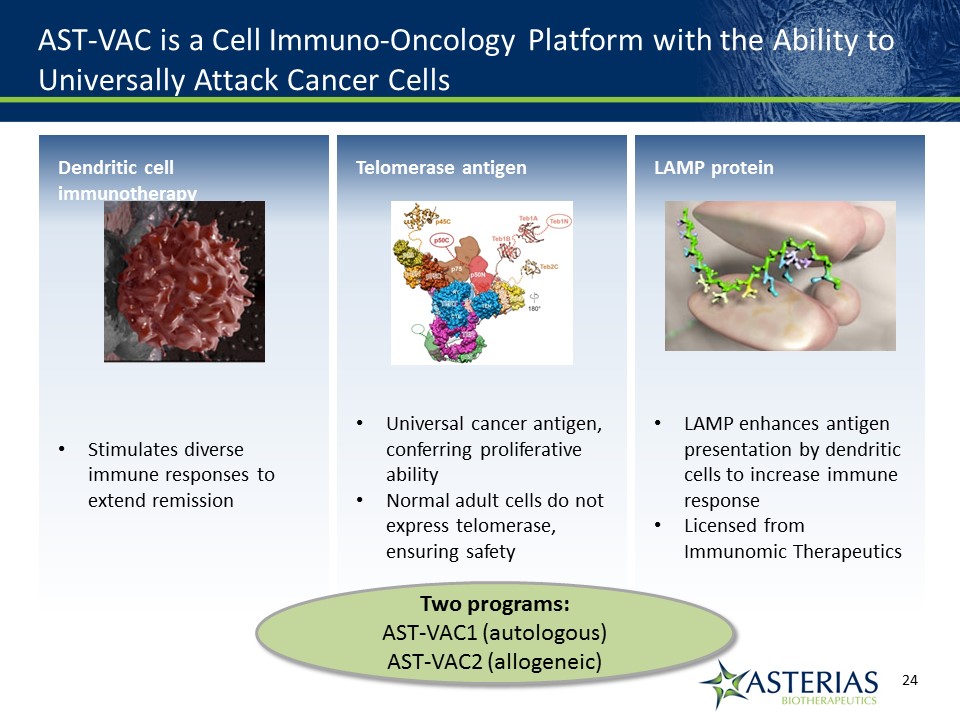
AST-VAC is a Cell Immuno-Oncology Platform with the Ability to Universally Attack Cancer Cells Telomerase antigenUniversal cancer antigen, conferring proliferative abilityNormal adult cells do not express telomerase, ensuring safety Dendritic cell immunotherapyStimulates diverse immune responses to extend remission LAMP proteinLAMP enhances antigen presentation by dendritic cells to increase immune responseLicensed from Immunomic Therapeutics Two programs:AST-VAC1 (autologous)AST-VAC2 (allogeneic)
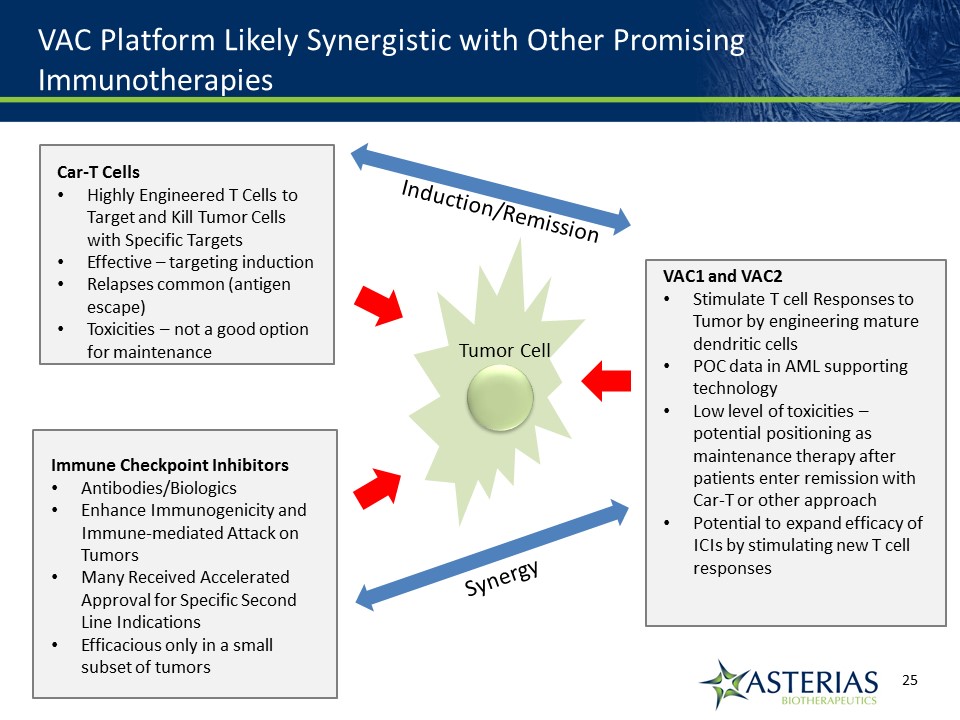
VAC Platform Likely Synergistic with Other Promising Immunotherapies Immune Checkpoint InhibitorsAntibodies/BiologicsEnhance Immunogenicity and Immune-mediated Attack on TumorsMany Received Accelerated Approval for Specific Second Line IndicationsEfficacious only in a small subset of tumors Car-T CellsHighly Engineered T Cells to Target and Kill Tumor Cells with Specific TargetsEffective – targeting inductionRelapses common (antigen escape)Toxicities – not a good option for maintenance VAC1 and VAC2Stimulate T cell Responses to Tumor by engineering mature dendritic cellsPOC data in AML supporting technologyLow level of toxicities – potential positioning as maintenance therapy after patients enter remission with Car-T or other approachPotential to expand efficacy of ICIs by stimulating new T cell responses Tumor Cell Synergy Induction/Remission
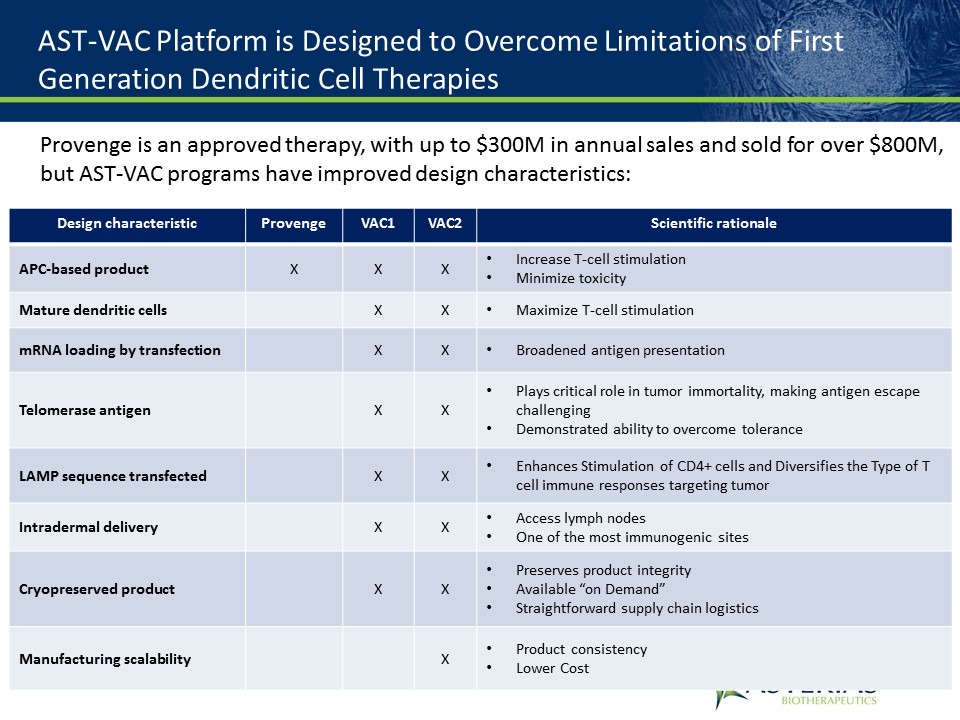
AST-VAC Platform is Designed to Overcome Limitations of First Generation Dendritic Cell Therapies Design characteristic Provenge VAC1 VAC2 Scientific rationale APC-based product X X X Increase T-cell stimulationMinimize toxicity Mature dendritic cells X X Maximize T-cell stimulation mRNA loading by transfection X X Broadened antigen presentation Telomerase antigen X X Plays critical role in tumor immortality, making antigen escape challengingDemonstrated ability to overcome tolerance LAMP sequence transfected X X Enhances Stimulation of CD4+ cells and Diversifies the Type of T cell immune responses targeting tumor Intradermal delivery X X Access lymph nodesOne of the most immunogenic sites Cryopreserved product X X Preserves product integrityAvailable “on Demand”Straightforward supply chain logistics Manufacturing scalability X Product consistencyLower Cost Provenge is an approved therapy, with up to $300M in annual sales and sold for over $800M, but AST-VAC programs have improved design characteristics:
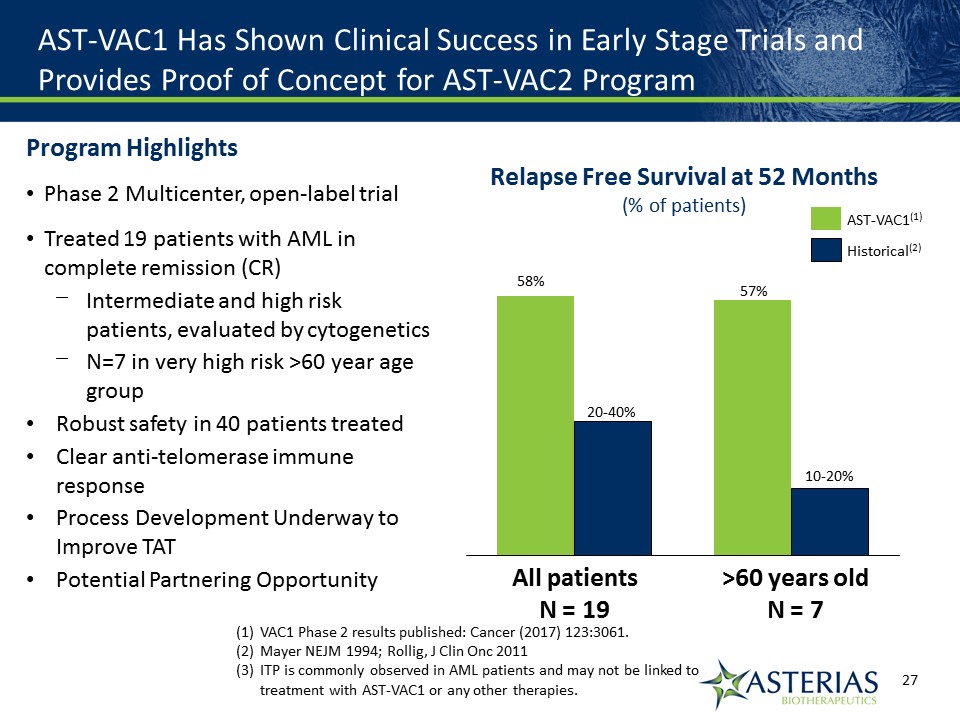
AST-VAC1 Has Shown Clinical Success in Early Stage Trials and Provides Proof of Concept for AST-VAC2 Program 57% 10-20% >60 years oldN = 7 20-40% 58% All patientsN = 19 Relapse Free Survival at 52 Months(% of patients) Historical(2) AST-VAC1(1) VAC1 Phase 2 results published: Cancer (2017) 123:3061.Mayer NEJM 1994; Rollig, J Clin Onc 2011ITP is commonly observed in AML patients and may not be linked to treatment with AST-VAC1 or any other therapies. Program HighlightsPhase 2 Multicenter, open-label trialTreated 19 patients with AML in complete remission (CR)Intermediate and high risk patients, evaluated by cytogeneticsN=7 in very high risk >60 year age groupRobust safety in 40 patients treatedClear anti-telomerase immune responseProcess Development Underway to Improve TATPotential Partnering Opportunity
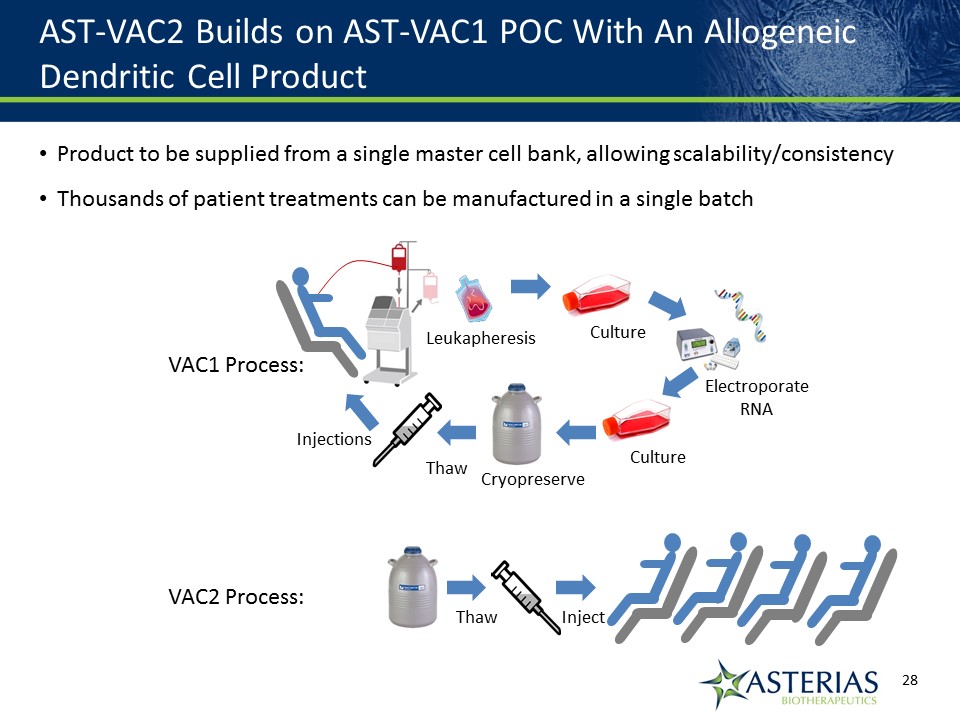
AST-VAC2 Builds on AST-VAC1 POC With An Allogeneic Dendritic Cell Product Product to be supplied from a single master cell bank, allowing scalability/consistencyThousands of patient treatments can be manufactured in a single batch 14219863Text 04698Text 230237246Text Culture ElectroporateRNA Culture Leukapheresis Cryopreserve Thaw Injections Thaw Inject VAC1 Process: VAC2 Process:
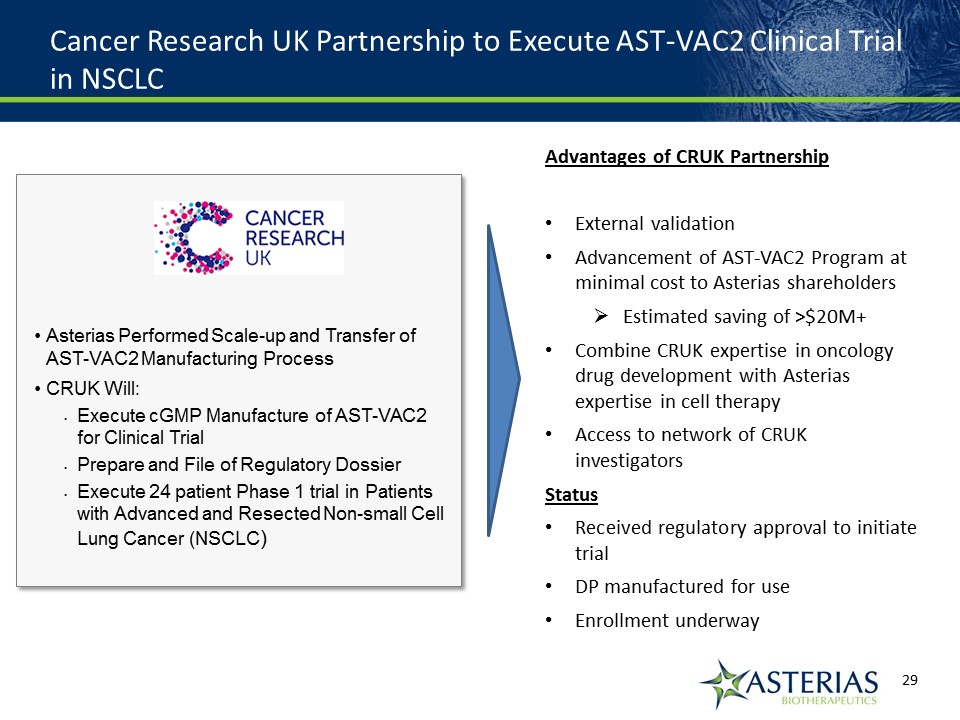
Cancer Research UK Partnership to Execute AST-VAC2 Clinical Trial in NSCLC Asterias Performed Scale-up and Transfer of AST-VAC2 Manufacturing ProcessCRUK Will:Execute cGMP Manufacture of AST-VAC2 for Clinical TrialPrepare and File of Regulatory DossierExecute 24 patient Phase 1 trial in Patients with Advanced and Resected Non-small Cell Lung Cancer (NSCLC) Advantages of CRUK PartnershipExternal validationAdvancement of AST-VAC2 Program at minimal cost to Asterias shareholdersEstimated saving of >$20M+Combine CRUK expertise in oncology drug development with Asterias expertise in cell therapyAccess to network of CRUK investigatorsStatusReceived regulatory approval to initiate trialDP manufactured for useEnrollment underway
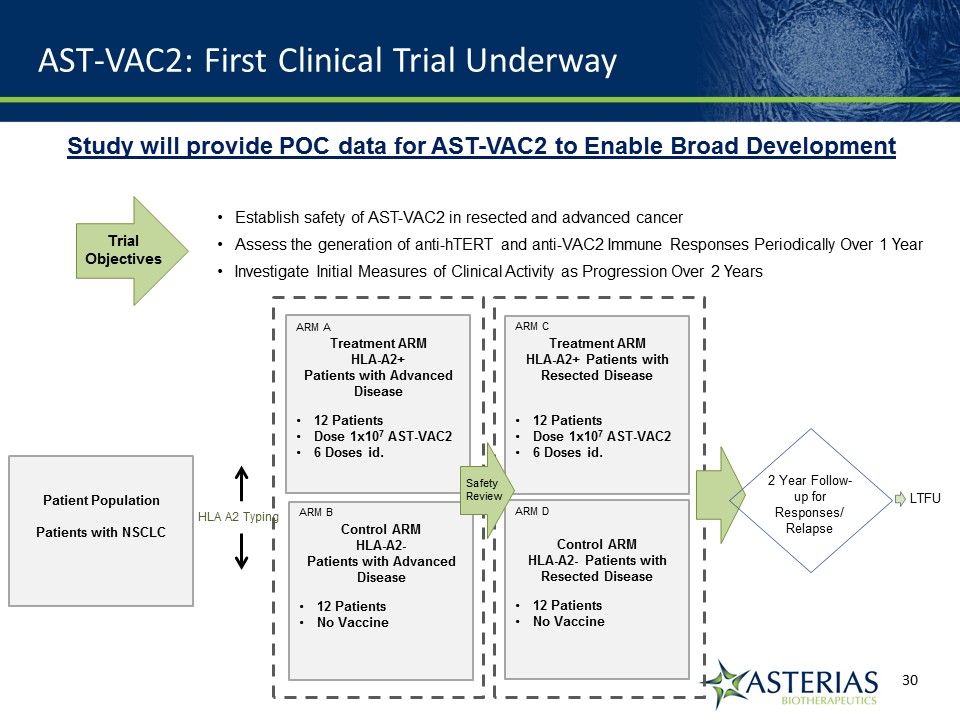
AST-VAC2: First Clinical Trial Underway Patient PopulationPatients with NSCLC Treatment ARMHLA-A2+ Patients with Advanced Disease12 PatientsDose 1x107 AST-VAC26 Doses id. HLA A2 Typing 2 Year Follow-up for Responses/ Relapse Control ARMHLA-A2- Patients with Advanced Disease12 Patients No Vaccine Treatment ARMHLA-A2+ Patients with Resected Disease12 PatientsDose 1x107 AST-VAC26 Doses id. Control ARMHLA-A2- Patients with Resected Disease12 PatientsNo Vaccine ARM A ARM B ARM C ARM D SafetyReview LTFU Establish safety of AST-VAC2 in resected and advanced cancerAssess the generation of anti-hTERT and anti-VAC2 Immune Responses Periodically Over 1 Year Investigate Initial Measures of Clinical Activity as Progression Over 2 Years TrialObjectives Study will provide POC data for AST-VAC2 to Enable Broad Development
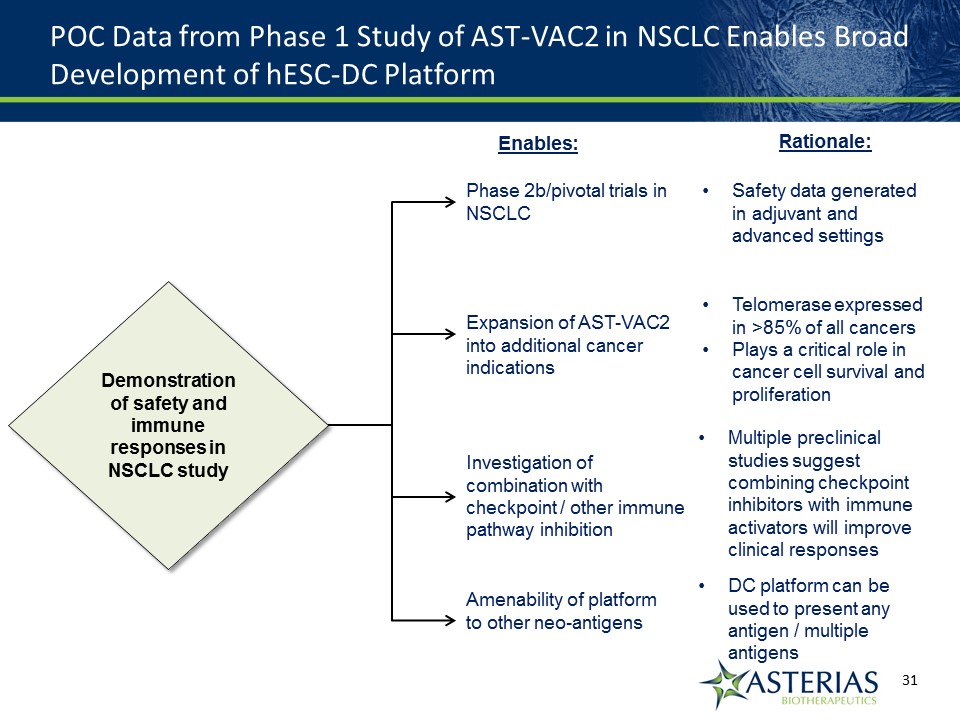
POC Data from Phase 1 Study of AST-VAC2 in NSCLC Enables Broad Development of hESC-DC Platform Demonstration of safety and immune responses in NSCLC study Demonstration of safety and immune responses in NSCLC study Phase 2b/pivotal trials in NSCLC Expansion of AST-VAC2 into additional cancer indications Investigation of combination with checkpoint / other immune pathway inhibition Amenability of platform to other neo-antigens Enables: Rationale: Safety data generated in adjuvant and advanced settings Telomerase expressed in >85% of all cancersPlays a critical role in cancer cell survival and proliferation Multiple preclinical studies suggest combining checkpoint inhibitors with immune activators will improve clinical responses DC platform can be used to present any antigen / multiple antigens

Next Steps 14219863Text 04698Text 230237246Text Program Next Steps VAC1 Identify alternatives to move forward with phase 2/3 study in AML VAC2 2017Sites openedFPI dosed in NSCLC trialFirst safety data generated 2018Further data on safety and immune response Use data to evaluate best approach to broaden platform 2019Data on clinical activity in NSCLCSecond trial/added trial arm as monotherapy or in combination with other therapy

Company Milestones 14219863Text 04698Text 230237246Text 6 month data Cohort 29 month data Cohort 2 Complete enrollment in Cohort 3Complete enrollment Cohort 412 month data Cohort 2Complete trial enrollment 6 month data Cohort 36 month data Cohort 46 month data Cohort 5 12 month data Cohort 312 month data Cohort 412 month data Cohort 5Topline efficacy data, all cohorts OPC1 VAC MHRA clearance for VAC2 trialFPI in VAC2 trial VAC2 interim data VAC2 interim data

Appendix 14219863Text 04698Text 230237246Text
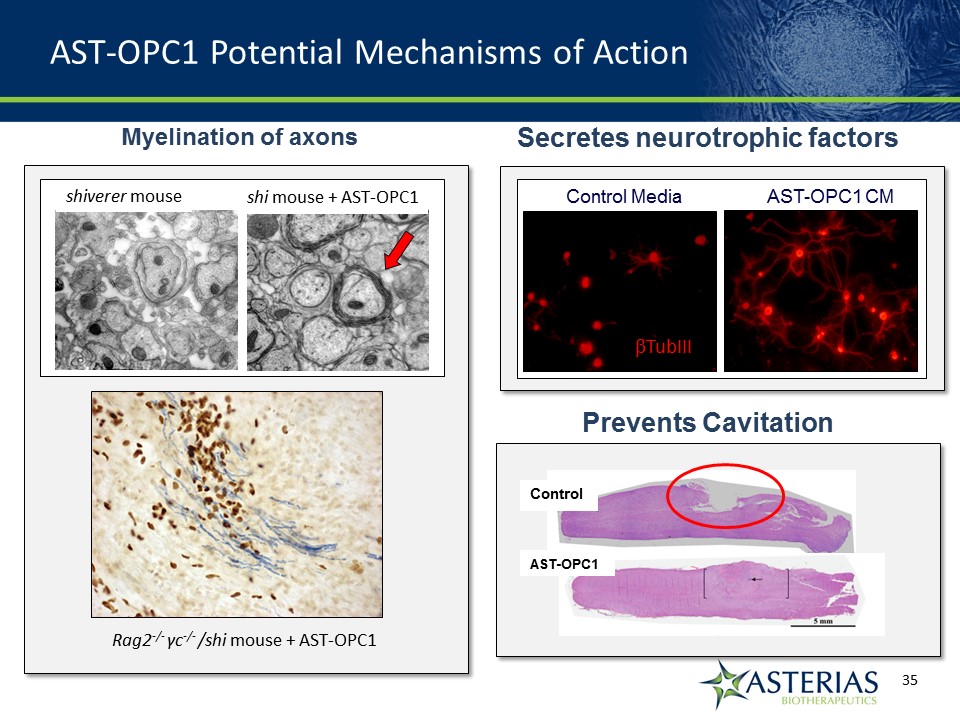
AST-OPC1 Potential Mechanisms of Action Secretes neurotrophic factors Promote increased neurite outgrowth Myelination of axons Rag2-/- γc-/- /shi mouse + AST-OPC1 Control Media AST-OPC1 CM shiverer mouse shi mouse + AST-OPC1 TubIII Prevents Cavitation Control AST-OPC1
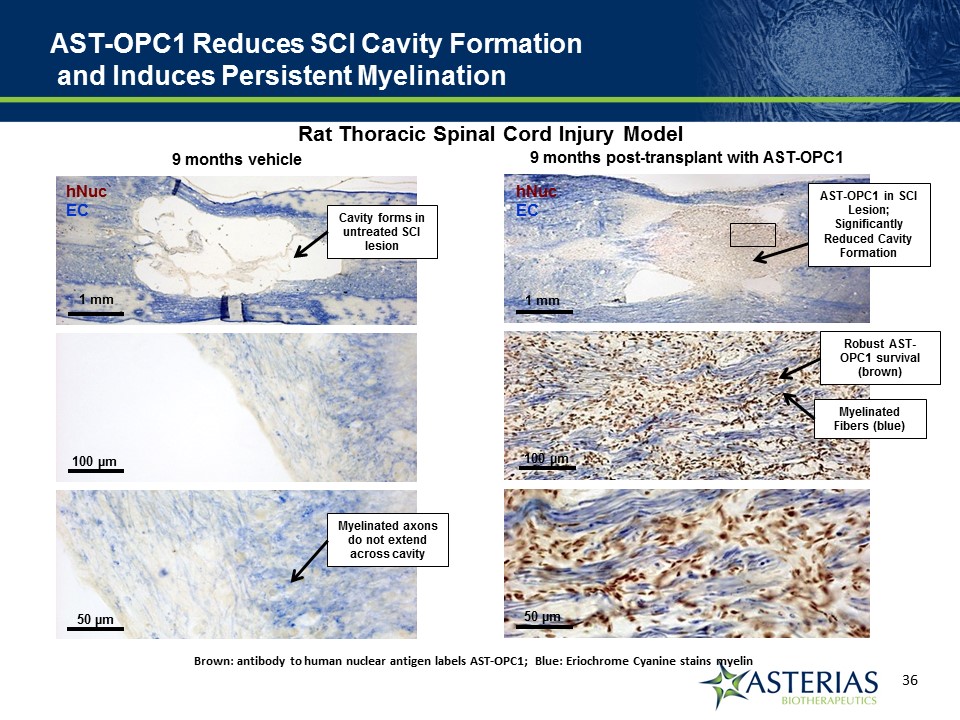
AST-OPC1 Reduces SCI Cavity Formation and Induces Persistent Myelination Brown: antibody to human nuclear antigen labels AST-OPC1; Blue: Eriochrome Cyanine stains myelin 9 months post-transplant with AST-OPC1 hNucEC 1 mm 100 µm 50 µm AST-OPC1 in SCI Lesion; Significantly Reduced Cavity Formation Robust AST-OPC1 survival (brown) Myelinated Fibers (blue) Rat Thoracic Spinal Cord Injury Model 9 months vehicle hNucEC 1 mm 100 µm 50 µm Cavity forms in untreated SCI lesion Myelinated axons do not extend across cavity
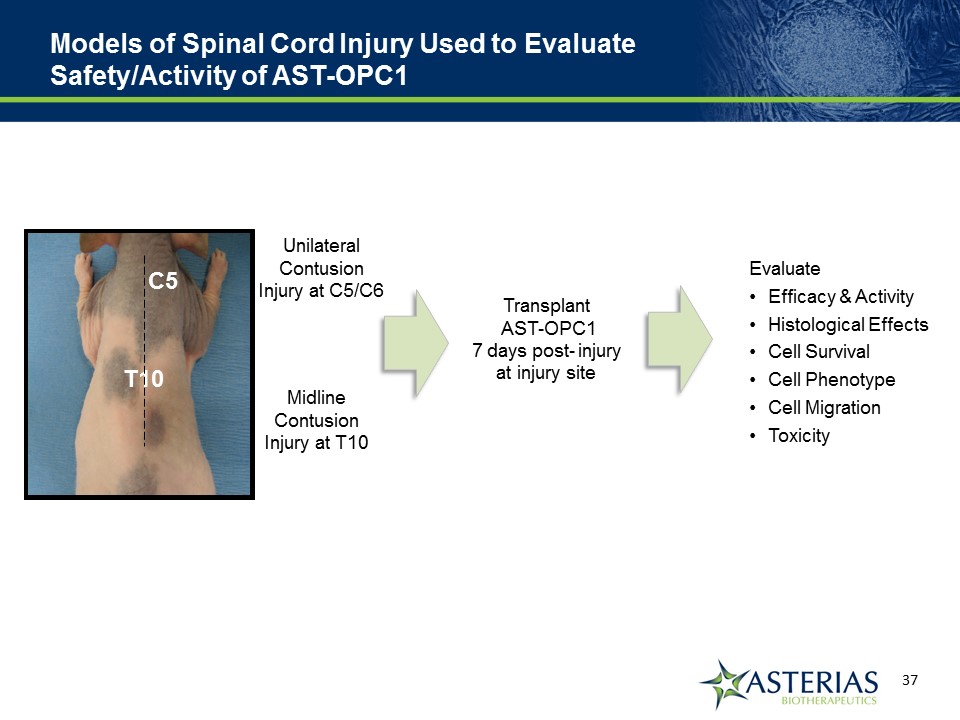
Models of Spinal Cord Injury Used to Evaluate Safety/Activity of AST-OPC1 MidlineContusion Injury at T10 Unilateral ContusionInjury at C5/C6 Thoracic Injury Cervical Injury Transplant AST-OPC1 7 days post- injury at injury site EvaluateEfficacy & ActivityHistological EffectsCell SurvivalCell PhenotypeCell MigrationToxicity T10 C5
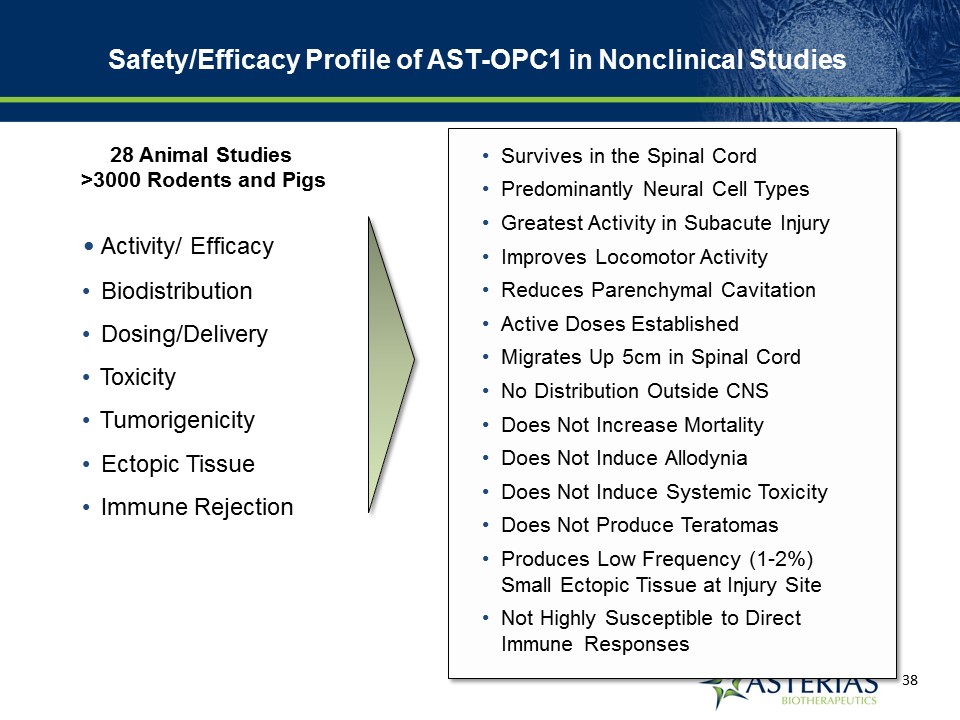
Activity/ Efficacy Biodistribution Dosing/Delivery Toxicity Tumorigenicity Ectopic Tissue Immune Rejection Survives in the Spinal CordPredominantly Neural Cell TypesGreatest Activity in Subacute InjuryImproves Locomotor ActivityReduces Parenchymal CavitationActive Doses EstablishedMigrates Up 5cm in Spinal CordNo Distribution Outside CNSDoes Not Increase MortalityDoes Not Induce AllodyniaDoes Not Induce Systemic ToxicityDoes Not Produce TeratomasProduces Low Frequency (1-2%) Small Ectopic Tissue at Injury Site Not Highly Susceptible to Direct Immune Responses 28 Animal Studies >3000 Rodents and Pigs Safety/Efficacy Profile of AST-OPC1 in Nonclinical Studies
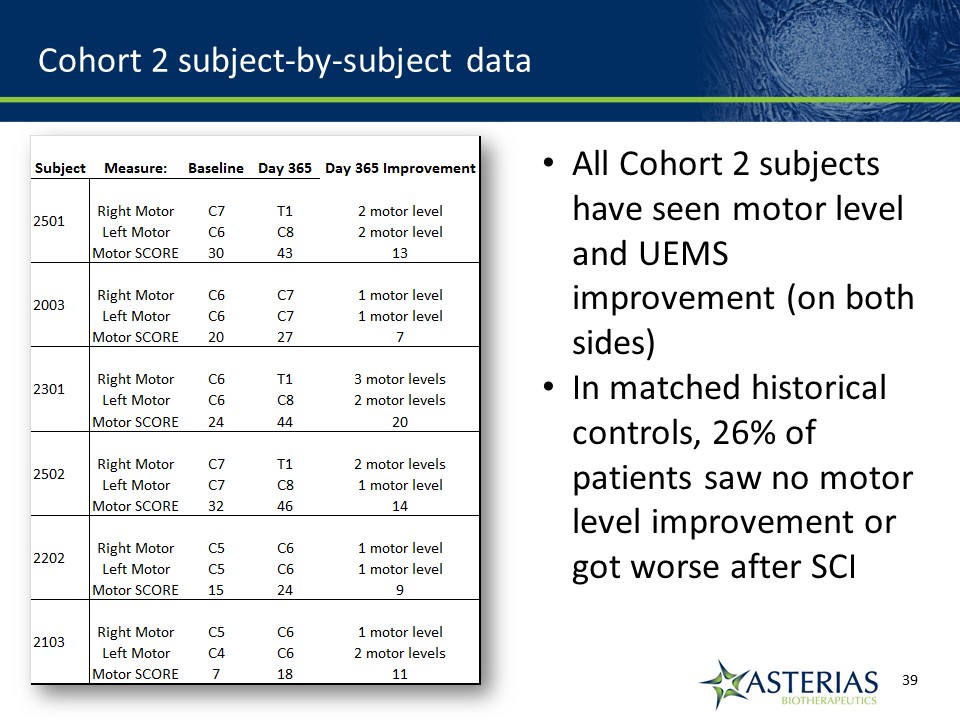
Cohort 2 subject-by-subject data All Cohort 2 subjects have seen motor level and UEMS improvement (on both sides)In matched historical controls, 26% of patients saw no motor level improvement or got worse after SCI
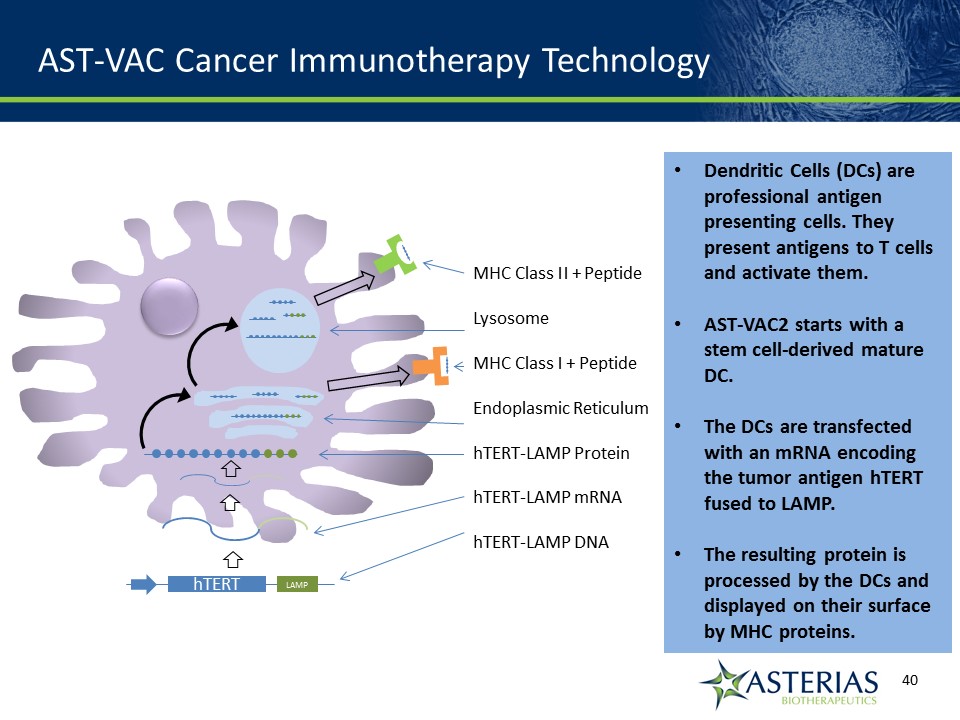
AST-VAC Cancer Immunotherapy Technology Dendritic Cells (DCs) are professional antigen presenting cells. They present antigens to T cells and activate them.AST-VAC2 starts with a stem cell-derived mature DC.The DCs are transfected with an mRNA encoding the tumor antigen hTERT fused to LAMP.The resulting protein is processed by the DCs and displayed on their surface by MHC proteins. hTERT LAMP MHC Class II + PeptideLysosomeMHC Class I + PeptideEndoplasmic ReticulumhTERT-LAMP ProteinhTERT-LAMP mRNAhTERT-LAMP DNA
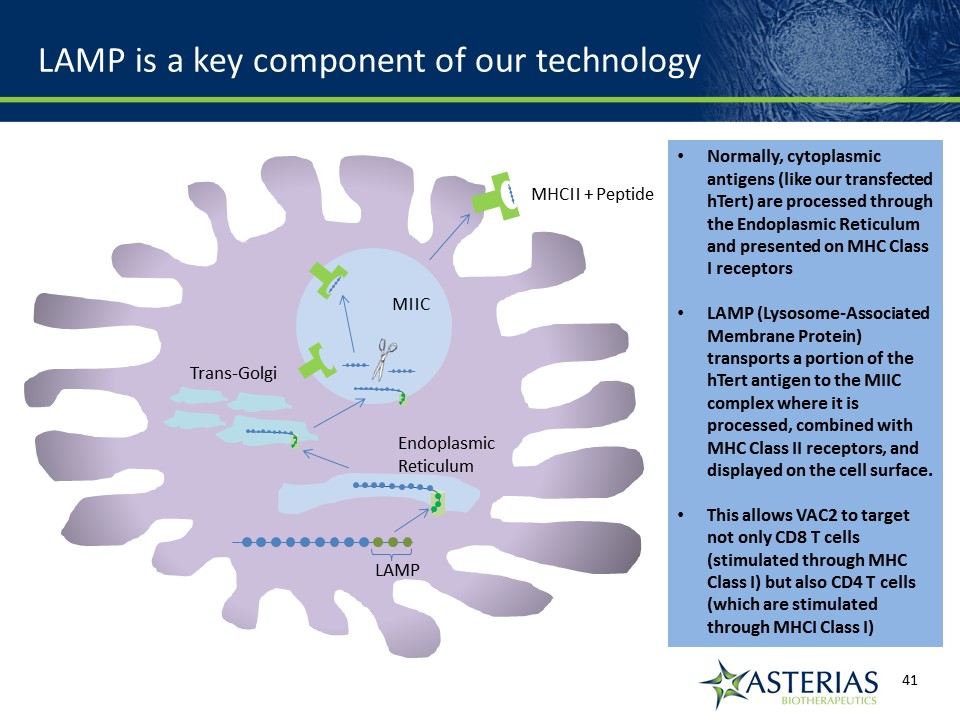
LAMP is a key component of our technology Normally, cytoplasmic antigens (like our transfected hTert) are processed through the Endoplasmic Reticulum and presented on MHC Class I receptorsLAMP (Lysosome-Associated Membrane Protein) transports a portion of the hTert antigen to the MIIC complex where it is processed, combined with MHC Class II receptors, and displayed on the cell surface.This allows VAC2 to target not only CD8 T cells (stimulated through MHC Class I) but also CD4 T cells (which are stimulated through MHCI Class I) MIIC EndoplasmicReticulum Trans-Golgi MHCII + Peptide LAMP
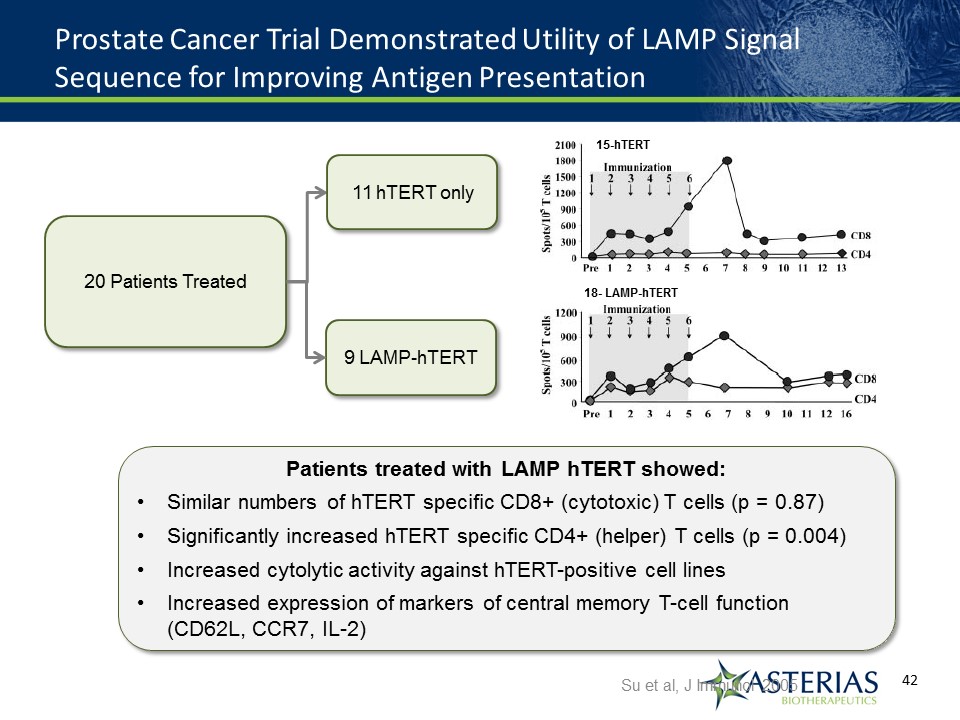
Prostate Cancer Trial Demonstrated Utility of LAMP Signal Sequence for Improving Antigen Presentation Su et al, J Immunol 2005 20 Patients Treated 11 hTERT only 9 LAMP-hTERT Patients treated with LAMP hTERT showed:Similar numbers of hTERT specific CD8+ (cytotoxic) T cells (p = 0.87)Significantly increased hTERT specific CD4+ (helper) T cells (p = 0.004)Increased cytolytic activity against hTERT-positive cell linesIncreased expression of markers of central memory T-cell function (CD62L, CCR7, IL-2) 15-hTERT 18- LAMP-hTERT
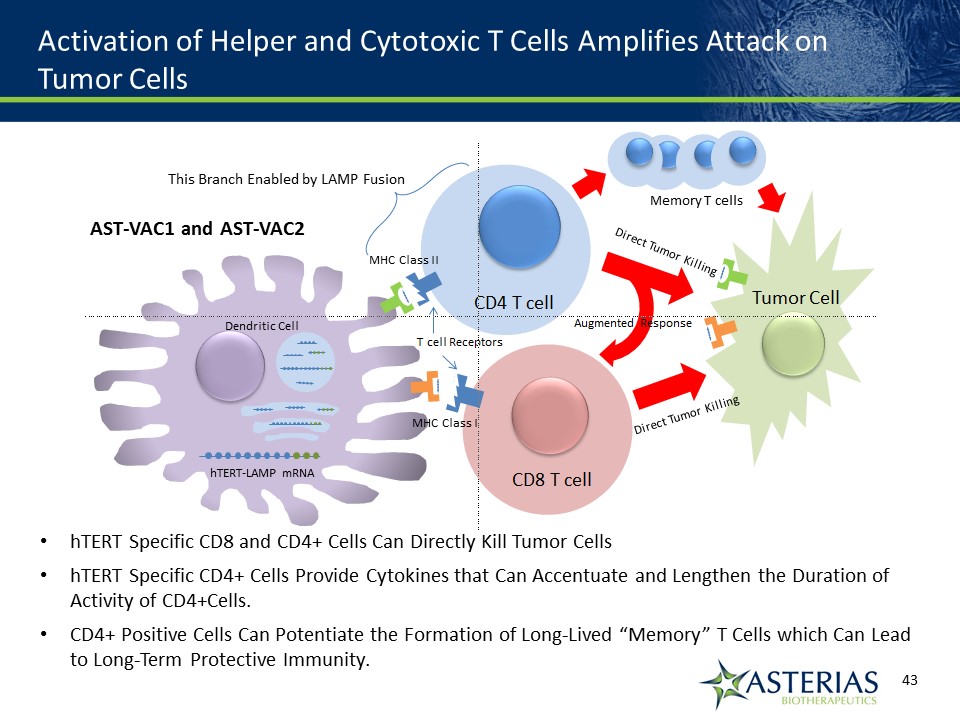
Activation of Helper and Cytotoxic T Cells Amplifies Attack on Tumor Cells hTERT Specific CD8 and CD4+ Cells Can Directly Kill Tumor CellshTERT Specific CD4+ Cells Provide Cytokines that Can Accentuate and Lengthen the Duration of Activity of CD4+Cells. CD4+ Positive Cells Can Potentiate the Formation of Long-Lived “Memory” T Cells which Can Lead to Long-Term Protective Immunity. AST-VAC1 and AST-VAC2 MHC Class II MHC Class I Dendritic Cell hTERT-LAMP mRNA This Branch Enabled by LAMP Fusion Memory T cells
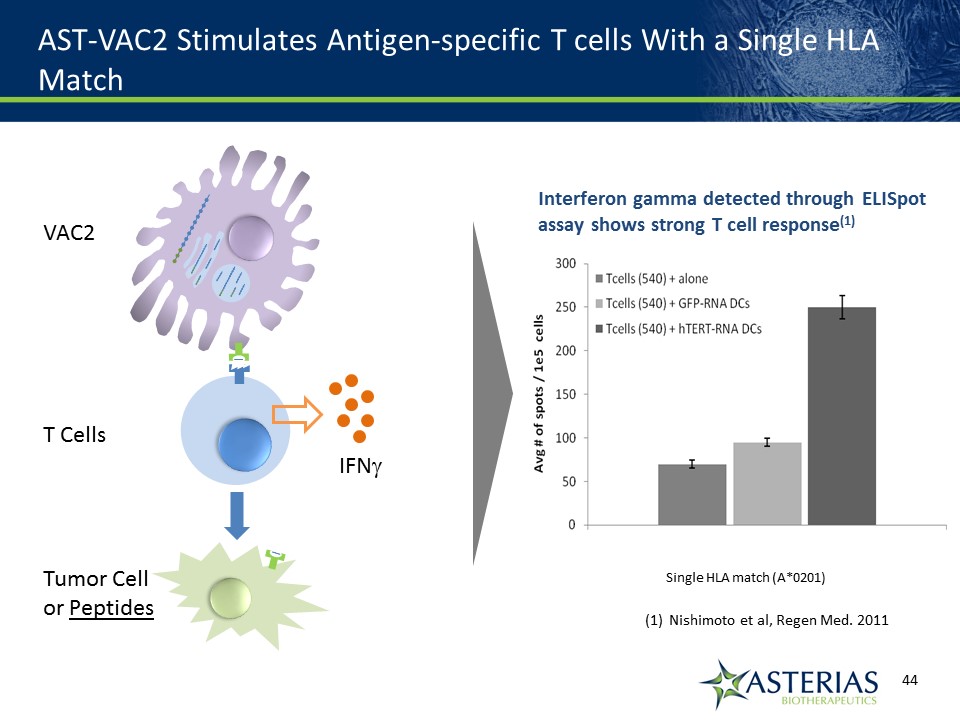
AST-VAC2 Stimulates Antigen-specific T cells With a Single HLA Match Interferon gamma detected through ELISpot assay shows strong T cell response(1) Single HLA match (A*0201) Nishimoto et al, Regen Med. 2011 VAC2T CellsTumor Cellor Peptides IFNg
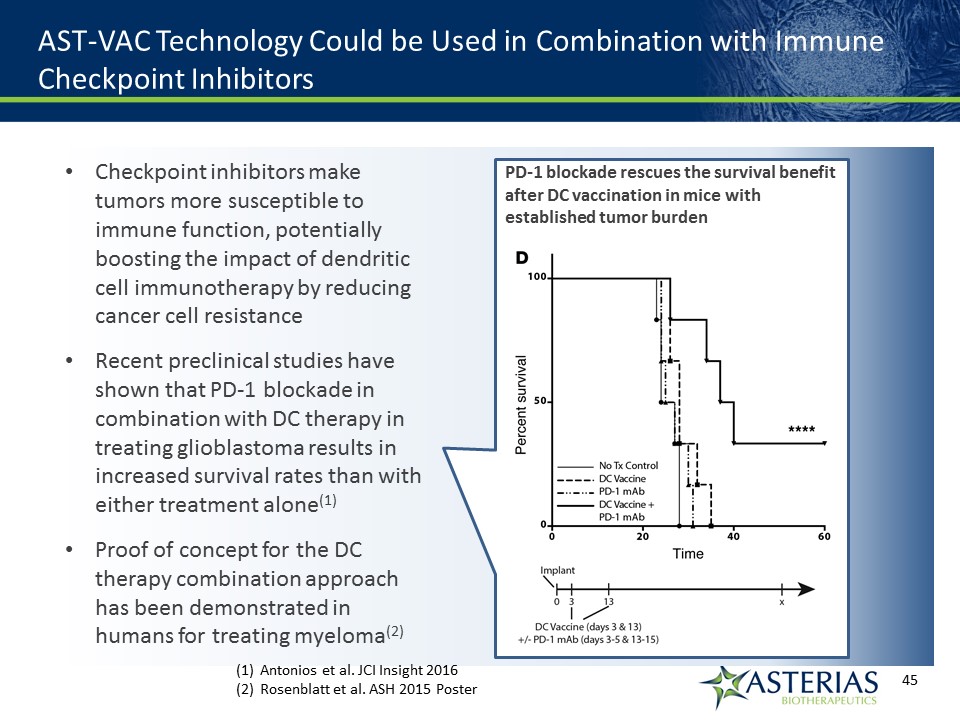
AST-VAC Technology Could be Used in Combination with Immune Checkpoint Inhibitors Checkpoint inhibitors make tumors more susceptible to immune function, potentially boosting the impact of dendritic cell immunotherapy by reducing cancer cell resistanceRecent preclinical studies have shown that PD-1 blockade in combination with DC therapy in treating glioblastoma results in increased survival rates than with either treatment alone(1)Proof of concept for the DC therapy combination approach has been demonstrated in humans for treating myeloma(2) PD-1 blockade rescues the survival benefit after DC vaccination in mice with established tumor burden Antonios et al. JCI Insight 2016Rosenblatt et al. ASH 2015 Poster
