Attached files
| file | filename |
|---|---|
| 8-K - 8-K - WEST PHARMACEUTICAL SERVICES INC | form8kinvestorconfseptembe.htm |

West Pharmaceutical Services, Inc.
September 2017

Safe Harbor Statement
Cautionary Statement Under the Private Securities Litigation Reform Act of 1995
This presentation and any accompanying management commentary contain “forward-looking statements” as that term is defined in the Private
Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about product development and operational
performance. Each of these statements is based on preliminary information, and actual results could differ from any preliminary estimates. We
caution investors that the risk factors listed under “Cautionary Statement” in our press releases, as well as those set forth under the caption "Risk
Factors" in our most recent Annual Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or supplemented by
our quarterly reports on Form 10-Q, could cause our actual results to differ materially from those estimated or predicted in the forward-looking
statements. You should evaluate any statement in light of these important factors. Except as required by law or regulation, we undertake no
obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise.
Non-U.S. GAAP Financial Measures
Certain financial measures included in these presentation materials, or which may be referred to in management’s discussion of the Company’s
results and outlook, have not been calculated in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”), and therefore are
referred to as non-GAAP financial measures. Non-GAAP financial measures should not be considered in isolation or as an alternative to such
measures determined in accordance with GAAP. Please refer to “Reconciliation of Non-GAAP Measures” at the end of these materials for more
information.
Trademarks
Registered trademarks used in this report are the property of West Pharmaceutical Services, Inc. or its subsidiaries, in the United States and
other jurisdictions, unless noted otherwise. Daikyo Crystal Zenith® is a registered trademark of, and is licensed from, Daikyo Seiko, Ltd.
2 |

Our Mission
By your side…
Pharmaceutical, biotechnology, generic and medical device
companies trust West and our ability to deliver consistent
high-quality and technologically advanced containment and
delivery solutions
We share the commitment of our customers to improve the
health of patients worldwide
3 |

A 90-Year History
4 |

An Integral Part of the Healthcare Industry
Top 50
Injectable biologics
rely on West and
Daikyo components
~ 41 Billion
Components
manufactured
annually
Top 75
Supplier to the top 75
pharmaceutical & biotech
injectable companies
5 |

$1.5B Net Sales*
By Product
Category
$1.5B Net Sales*
By Geography
Americas
Europe, Middle East, Africa
Asia Pacific
54% 39%
7%
$1.5B Net Sales*
By Market
Group
High-Value Components
Standard Packaging
Delivery Devices
Contract Manufacturing
P
R
OPRI
E
T
A
R
Y
P
R
O
D
U
C
T
S
Pharma
Generics
Biologics
Contract Manufacturing
22%
P
R
OPRI
E
T
A
R
Y
P
R
O
D
U
C
T
S
West Business at a Glance
21%
7%
33%
39%
21%
35%
* Based on full-year 2016 net sales
>2,000 Customers
Largest Customer:
~7% of Net Sales
50 Global Locations
28 Manufacturing Sites
7,300 Employees
22%
6 |

Long-term Strategy
Market Led
and Exceptional
Customer
Experience
Operational
Effectiveness
Product
and Service
Differentiation
Drive
Shareholder
Value
Become the
world leader in
integrated
containment
and delivery of
injectable
medicines
7 |

Addressing Unique Customer Needs
Quality – A High Bar Set By Our Customers
GENERICS
Speed to
market
Efficient
manufacturing
BIOLOGICS
Packaging
solutions for
sensitive
molecules
Self-injection
technologies
PHARMA
Total cost of
ownership
Life cycle
management
Design for
manufacturing
CONTRACT
MANUFACTURING
Quality
manufacturing
Patient Focus
8 |
West solutions:
Integrated containment & delivery
Increasing levels of
customer intimacy
Increasing
value to West
STANDARD
est Differentiated Solutions:
Standard Packaging—Stoppers & Seals
9 |

West solutions:
Integrated containment & delivery
Increasing levels of
customer intimacy
Increasing
value to West
STANDARD
est Differentiated Solutions:
High-Value Components
10 |
STERILIZED
WASHED
COATED

West solutions:
Integrated containment & delivery
Increasing levels of
customer intimacy
Increasing
value to West
STANDARD
est Differentiated Solutions:
Increasing Quality & Inspection
11 |
STERILIZED
WASHED
COATED
CAMERA
INSPECTED
QUALITY
BY DESIGN

West solutions:
Integrated containment & delivery
ADMINISTERED
SELF
INJECTION
est Differentiated Solutions:
Reconstitution & Self Injection Devices
12 |
Increasing levels of
customer intimacy
Increasing
value to West
CONTAINMENT
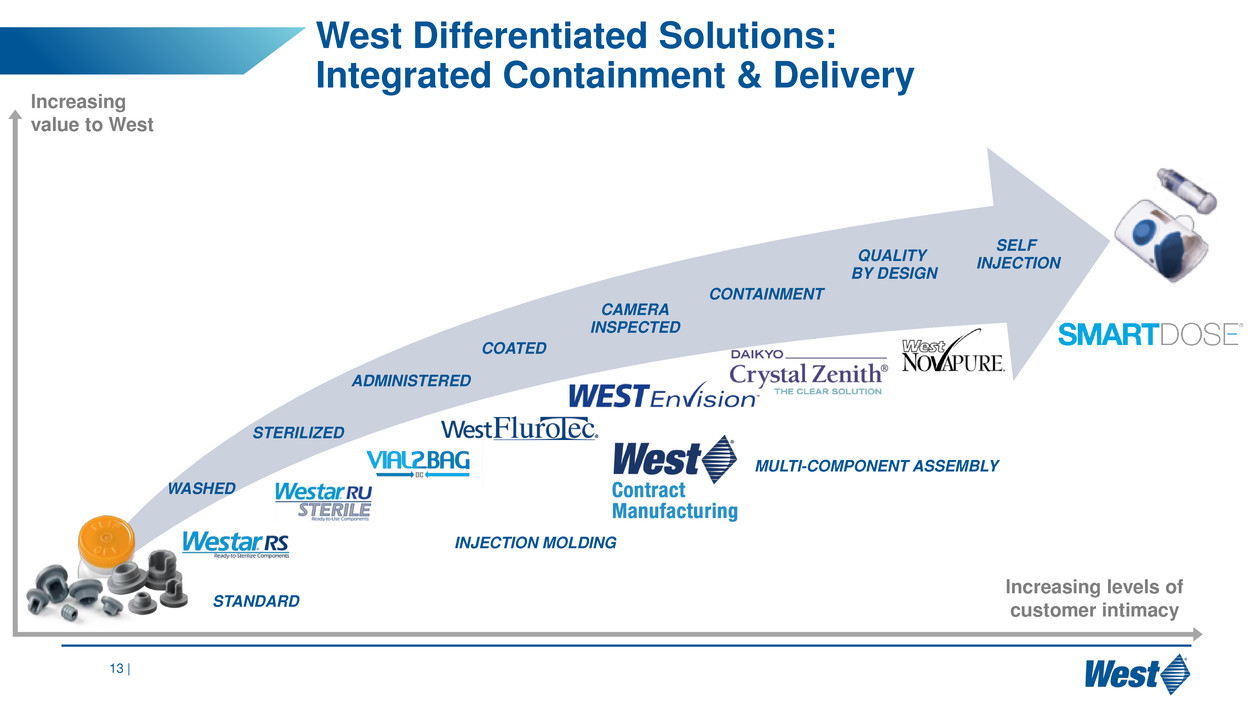
West solutions:
Integrated containment & delivery
STERILIZED
WASHED
COATED
ADMINISTERED
CAMERA
INSPECTED
QUALITY
BY DESIGN
CONTAINMENT
SELF
INJECTION
INJECTION MOLDING
MULTI-COMPONENT ASSEMBLY
STANDARD
est Differentiated Solutions:
Integrated Containment & Delivery
13 |
Increasing levels of
customer intimacy
Increasing
value to West

Circles reflect relative size of 2016 net sales
Standard
Packaging
High-Value
Components
0%
5%
10%
15%
20%
0% 30% 60%
2016 Category Gross Margin %
Proprietary
Devices
Contract
Manufacturing
2016 GM 33.2%
5-Yr Sales CAGR 7.4%
Product Net Sales & Margin Growth
2012-2016 compound annual net sales growth rates
(excludes currency)
> High-Value
Components have
driven growth
> Proprietary Devices
present significant
potential growth
opportunity
> Steady Contract
Manufacturing
and Standard
Packaging businesses
5
-Y
e
a
r
CAG
R
14
0%
14 |

Global Operations
Global manufacturing network of 28 plants across U.S.,
Europe, Asia and South America
Increased industry-leading quality metrics
Increased capacity and efficiencies, reducing lead times
and backlog levels for high-value products
Global Supply Chain and Procurement drove lower costs
and increased savings
Completed 60,000 sq ft expansion of Dublin contract
manufacturing site
Planned increases of high-value product capacity with new
facility in Waterford and expansions in Kinston, NC and
Singapore
Dublin contract
manufacturing
15 |

0%
100%
200%
300%
400%
500%
2011 2012 2013 2014 2015 20162012 2013 2014 2015 2016
Net Sales
Sustained, Consistent Growth
2012 2013 2014 2015 2016
Adjusted Diluted EPS*
(Non-GAAP)
Constant Currency
CAGR 6.8%
Comparison of Cumulative
Five-Year Total Return
S&P MidCap
400 Index
West Pharmaceutical
Services, Inc. (WST)
Sources: IR Insight, Company estimates
*Please refer to “Reconciliation of Non-GAAP Measures” at the end of these materials for more information.
Adjusted Diluted EPS
CAGR 12.1%*
$1.38
$2.18
+19%
$1.27
Billion
$1.51
Billion
+9.1%
organic
CAGR
4.5%
16 |

Second-Quarter 2017 Results
* Excluding the impact from translational changes in foreign exchange
Net sales of $397.6 million, a record high, representing 3.9% organic sales
growth*
‒ Proprietary Products organic sales growth of 2.2%
‒ Contract-Manufactured Products organic sales growth of 10.5%
Gross profit margin decreased year-over-year by 300 basis points and adjusted
operating profit margin by 190 basis points
SmartDose® and Daikyo Crystal Zenith® products – strong double-digit organic
sales growth
Reported diluted EPS of $0.51. Adjusted diluted EPS of $0.66, a 12% increase
over prior-year adjusted diluted EPS. This includes a $0.13 favorable EPS impact
from tax benefits associated with stock-based compensation expense
17 |
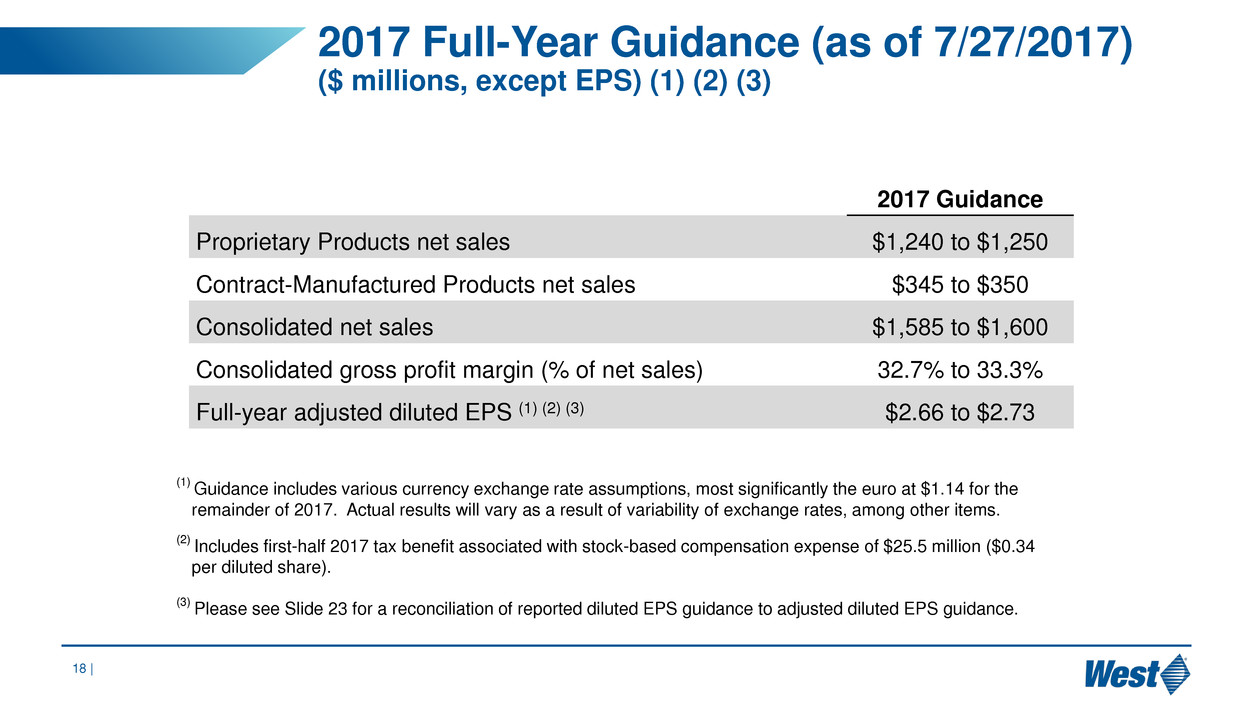
2017 Full-Year Guidance (as of 7/27/2017)
($ millions, except EPS) (1) (2) (3)
(1)
Guidance includes various currency exchange rate assumptions, most significantly the euro at $1.14 for the
remainder of 2017. Actual results will vary as a result of variability of exchange rates, among other items.
(2)
Includes first-half 2017 tax benefit associated with stock-based compensation expense of $25.5 million ($0.34
per diluted share).
(3)
Please see Slide 23 for a reconciliation of reported diluted EPS guidance to adjusted diluted EPS guidance.
2017 Guidance
Proprietary Products net sales $1,240 to $1,250
Contract-Manufactured Products net sales $345 to $350
Consolidated net sales $1,585 to $1,600
Consolidated gross profit margin (% of net sales) 32.7% to 33.3%
Full-year adjusted diluted EPS (1) (2) (3) $2.66 to $2.73
18 |

Building for the Future
Global operations
expanding capacity
to meet growing
customer demand
Market-led
strategy
addressing the
specific needs of
pharmaceutical,
biotechnology,
generic and
medical device
customers
Strong competitive
position
Quality culture
Designed into
regulated
products
Scientific and
technical
expertise
Proprietary
products and
contract
manufacturing
expected to drive
net sales growth
and margin
expansion
Financial
strength to invest
with a strong
balance sheet and
operating
cash flow
Innovations in
integrated
containment and
delivery driving
new products and
services for long-
term growth
Create Value for Customers, Patients, Employees and Shareholders
19 |

These presentation materials use the following financial measures that have not been calculated in accordance with generally accepted accounting principles (GAAP) accepted in the U.S., and
therefore are referred to as non-GAAP financial measures:
Net sales at constant currency (organic sales)
Adjusted operating profit
Adjusted operating profit margin
Adjusted net income
Adjusted income tax expense
Adjusted diluted EPS
Net debt
Total invested capital
Net debt to total invested capital
West believes that these non-GAAP measures of financial results provide useful information to management and investors regarding business trends, results of operations, and the Company’s
overall performance and financial position. Our executive management team uses these financial measures to evaluate the performance of the Company in terms of profitability and efficiency,
to compare operating results to prior periods, to evaluate changes in the operating results of each segment, and to measure and allocate financial resources to our segments. The Company
believes that the use of these non-GAAP financial measures provides an additional tool for investors to use in evaluating ongoing operating results and trends in comparing its financial
measures with other companies.
Our executive management does not consider such non-GAAP measures in isolation or as an alternative to such measures determined in accordance with GAAP. The principal limitation of
these financial measures is that they exclude significant expenses and income that are required by GAAP to be recorded. In addition, they are subject to inherent limitations as they reflect the
exercise of judgment by management about which items are excluded. In order to compensate for these limitations, non-GAAP financial measures are presented in connection with GAAP
results. We urge investors and potential investors to review the reconciliations of our non-GAAP financial measures to the comparable GAAP financial measures, and not to rely on any single
financial measure to evaluate the Company’s business.
Net sales at constant currency translates the current-period reported sales of subsidiaries whose functional currency is other than the U.S. dollar at the applicable foreign exchange rates in
effect during the comparable prior-year period. In calculating adjusted operating profit, adjusted income tax expense, adjusted net income and adjusted diluted EPS, we exclude the impact of
items that are not considered representative of ongoing operations. Such items generally include restructuring and related costs, certain asset impairments, other specifically identified gains or
losses, and discrete income tax items.
Please see “Financial Guidance” and “Non-GAAP Financial Measures” in the Company’s second-quarter 2017 earnings release for further information concerning reconciling items.
Notes to Non-GAAP Financial Measures
For additional details, please see Safe Harbor Statement
20 |

Notes to Non-GAAP Financial Measures
RECONCILIATION OF NON-GAAP MEASURES (UNAUDITED)
21 |
Reconciliation of Reported and Adjusted Operating Profit, Net Income and Diluted EPS
($ million, except EPS data)
Three months ended June 30, 2017
Operating
profit
Income
tax
expense
Net
income
Diluted
EPS
Reported (GAAP) $42.7 $2.9 $38.8 $0.51
Venezuela deconsolidation 11.1 - 11.1 0.15
Adjusted (Non-GAAP) $53.8 $2.9 $49.9 $0.66
Six months ended June 30, 2017
Operating
profit
Income
tax
expense
Net
income
Diluted
EPS
Reported (GAAP) $104.0 $5.1 $99.7 $1.32
Venezuela deconsolidation 11.1 - 11.1 0.15
Adjusted (Non-GAAP) $115.1 $5.1 $110.8 $1.47

Notes to Non-GAAP Financial Measures
RECONCILIATION OF NON-GAAP MEASURES (UNAUDITED)
22 |
Reconciliation of Net Sales to Net Sales at Constant Currency(1)
($ million, except EPS data)
(1) Net sales at constant currency translates the current-period reported sales of subsidiaries whose functional currency is
other than the U.S. dollar at the applicable foreign exchange rates in effect during the comparable prior-year period.
Three months ended June 30, 2017 Proprietary CM Eliminations Total
Reported net sales (GAAP) $312.8 $84.9 $(0.1) $397.6
Effect of changes in currency translation rates 5.1 0.5 - 5.6
Net sales at constant currency (Non-GAAP)(1) $317.9 $85.4 $(0.1) $403.2
Six months ended June 30, 2017 Proprietary CM Eliminations Total
Reported net sales (GAAP) $621.6 $164.0 $(0.3) $785.3
Effect of changes in currency translation rates 10.3 1.1 - 11.4
Net sales at constant currency (Non-GAAP)(1) $631.9 $165.1 $(0.3) $796.7
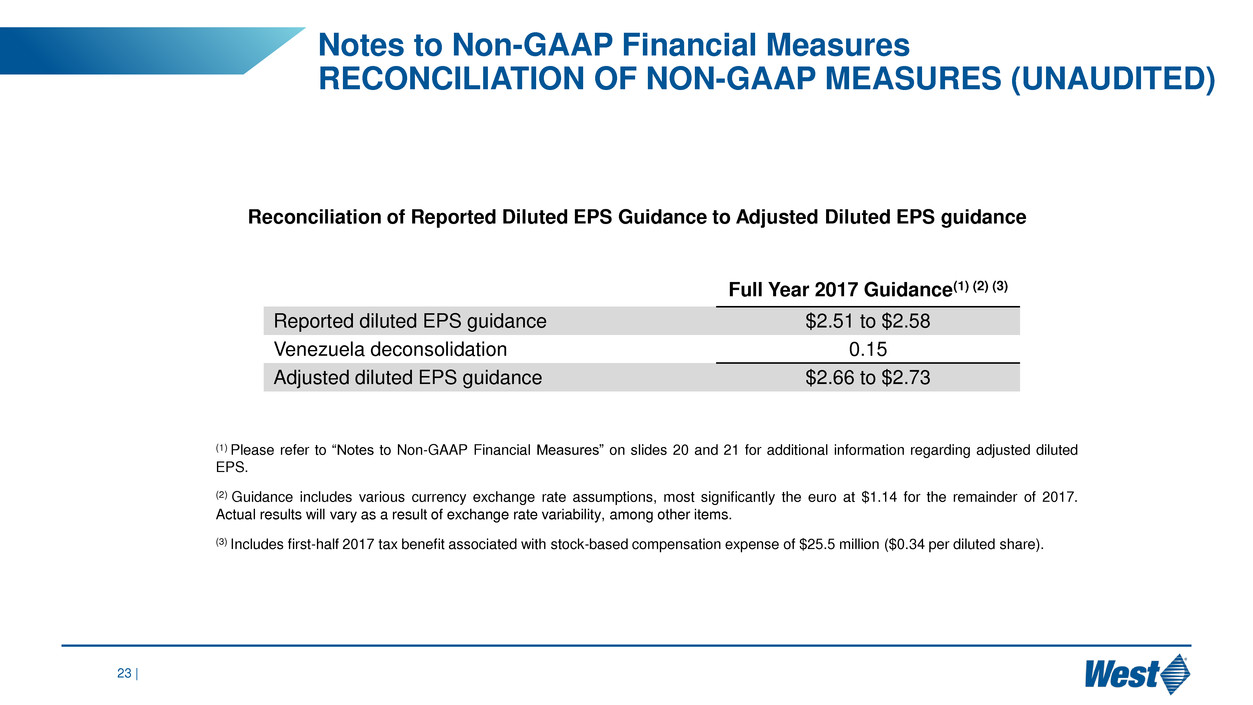
23 |
Reconciliation of Reported Diluted EPS Guidance to Adjusted Diluted EPS guidance
Full Year 2017 Guidance(1) (2) (3)
Reported diluted EPS guidance $2.51 to $2.58
Venezuela deconsolidation 0.15
Adjusted diluted EPS guidance $2.66 to $2.73
(1) Please refer to “Notes to Non-GAAP Financial Measures” on slides 20 and 21 for additional information regarding adjusted diluted
EPS.
(2) Guidance includes various currency exchange rate assumptions, most significantly the euro at $1.14 for the remainder of 2017.
Actual results will vary as a result of exchange rate variability, among other items.
(3) Includes first-half 2017 tax benefit associated with stock-based compensation expense of $25.5 million ($0.34 per diluted share).
Notes to Non-GAAP Financial Measures
RECONCILIATION OF NON-GAAP MEASURES (UNAUDITED)

Notes to Non-GAAP Financial Measures
RECONCILIATION OF NON-GAAP MEASURES (UNAUDITED)
24 |
Reconciliation of Reported and Adjusted Diluted EPS(1)
2016 2015 2014 2013 2012
Diluted EPS Reported (GAAP) $1.91 $1.30 $1.75 $1.57 $1.15
Restructuring and related charges 0.23 - - - 0.05
Venezuela currency devaluation 0.04 - - - -
Pension (curtailment) settlement (0.01) 0.43 - - -
Executive retirement and related costs - 0.09 - - -
License costs - - 0.01 - -
Discrete tax items 0.01 0.01 0.02 0.06 0.03
Acquisition-related contingencies - - - - 0.01
Extinguishment of debt - - - - 0.14
Diluted EPS Adjusted (Non-GAAP) $2.18 $1.83 $1.78 $1.63 $1.38
(1) Please see “Non-GAAP Financial Matters” in the Company’s earnings press releases and the Company’s SEC filings for further
information concerning reconciling items.
