Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SELLAS Life Sciences Group, Inc. | gale-201708088xk.htm |

Galena Biopharma and SELLAS Life Sciences to Combine
AUGUST 8, 2017

GALENA FORWARD LOOKING STATEMENT
Some of the information contained in this presentation may include forward-looking statements about strategy, future operations, future
financial position, prospects, plans and objectives of management of Galena, SELLAS or the combined company. Examples of such statements
include, but are not limited to, statements relating to the structure, timing and completion of the proposed merger; the combined company’s
listing on the NASDAQ Capital Market after closing of the proposed merger; expectations regarding the capitalization, resources and
ownership structure of the combined company; the combined company’s ability to successfully initiate and complete clinical trials; anticipated
milestones; the nature, strategy and focus of the combined company; the development and commercial potential of any product candidates of
the combined company; the executive and board structure of the combined company; and expectations regarding voting by Galena’s and
SELLAS’ stockholders. The combined company may not actually achieve the plans, carry out the intentions or meet the expectations or
projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such
statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ
materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, risks and
uncertainties associated with stockholder approval of and the ability to consummate the proposed merger through the process being
conducted by Galena and SELLAS, the ability to project future cash utilization and reserves needed for contingent future liabilities and business
operations, the availability of sufficient resources of the combined company to meet its business objectives and operational requirements, the
fact that the results of earlier studies and trials may not be predictive of future clinical trial results, the protection and market exclusivity
provided by SELLAS’ intellectual property, risks related to the drug discovery and the regulatory approval process and the impact of
competitive products and technological changes. These forward-looking statements are subject to a number of risks, uncertainties and
assumptions, including those identified under “Risk Factors” in our Annual Report on Form 10-K filed with the SEC on March 15, 2017, and
Quarterly Report on Form 10-Q filed with the SEC on May 10, 2017, in subsequently filed Form 10-Qs and other documents filed with the SEC
and available on our website around this transaction. Actual results may differ materially from those contemplated by these forward-looking
statements. Galena and SELLAS each disclaim any intent or obligation to update these forward-looking statements to reflect events or
circumstances that exist after the date on which they were made.
1

GALENA BIOPHARMA
PRESENTER:
Stephen Ghiglieri
Interim Chief Executive Officer & Chief Financial
Officer
OTHER PARTICIPANTS:
Remy Bernarda, IRC
SVP, Investor Relations & Corporate Communications
Thomas J. Knapp, Esq
Interim General Counsel & Corporate Secretary
SELLAS LIFE SCIENCES
PRESENTER:
Angelos M. Stergiou, MD, SCD H.C.
Chief Executive Officer
3
CONFERENCE CALL PARTICIPANTS

GALENA-SELLAS PROPOSED REVERSE MERGER TRANSACTION
•Follows Galena’s extensive review of strategic alternatives
•All-stock transaction: Galena to acquire all outstanding shares of SELLAS in exchange for newly issued
shares of Galena common stock
•On a pro forma, fully diluted basis*, Galena stockholders will own approximately 32.5% and SELLAS
shareholders will own approximately 67.5% of the combined company upon completion of the
proposed transaction
•Combined company will become SELLAS Life Sciences Group and shares of the combined company’s
common stock are expected to trade on the NASDAQ Capital Market under the ticker symbol SLS
•Transaction has been unanimously approved by the boards of directors of both companies
•Transaction Next Steps
• Galena to file multiple documents with the SEC over the next few months
• Galena shareholder meeting expected in October/November 2017
•Transaction expected to close in 4Q 2017, subject to the approval of the stockholders of Galena and
SELLAS and other closing conditions
4
*Excludes 2,556,851 out-of-the money Galena warrants

•Focused on developing hematology and oncology therapeutics that address unmet medical needs
•Immunotherapy:
• Lead Asset is NeuVax™ (nelipepimut-S), an immune therapy targeting the HER2 protein
• Three Phase 2 investigator sponsored clinical trials (ISTs) ongoing in breast cancer
• Phase 2b in Combination w/trastuzumab HER2 1+/2+: Complete enrollment expected in
Q2 2017 / Interim Efficacy analysis expected Q1 2018
• Phase 2 in Combination w/trastuzumab HER2 3+: Ongoing
• Phase 2 in Ductal Carcinoma in Situ (DCIS): Screening Patients
• Two immunotherapy assets targeting Folate Binding Protein
• Multiple early stage trials completed in ovarian, endometrial cancer and breast cancer
•Hematology: Phase 3-ready asset with a proprietary, controlled release (CR) formulation of the FDA-
approved drug, anagrelide to reduce elevated platelets in Essential Thrombocythemia (ET)
• 505(b)2 regulatory path allows for abbreviated submission package and potentially faster
approval timelines
5
GALENA OVERVIEW

•Late-stage cancer immunotherapy pipeline
•Galinpepimut-S:
• Innovative properties that differentiate it from other products in this class
• Two Phase 3 ready indications with favorable safety and compelling efficacy (overall survival)
profiles from completed Phase 2 trials
• Overall survival in Phase 2 AML trial was meaningfully longer than predicted
• Phase 3 AML trial design has been reviewed by FDA
• Phase 3 mesothelioma trial design also reviewed by FDA
• EMA & FDA Orphan Drug Designation and FDA Fast Track status for both settings
• Ongoing Phase 2 trial in multiple myeloma, and a nivolumab (OPDIVO®) combination trial in
ovarian cancer
• Expansion of checkpoint combination in hematology and solid tumors planned
•NeuVax
• Multiple Phase 2 ISTs ongoing in breast cancer
• Phase 2b interim efficacy analysis expected in Q1 2018
•Combination trials ongoing with both agents with additional trials planned
•Expansion pipeline provides multiple partnering/strategic opportunities
•Multiple important catalysts throughout 2018 and 2019
6
GALENA/SELLAS SYNERGISTIC DEVELOPMENT PROGRAMS
OPDIVO® is a trademark of Bristol-Myers Squibb Company.

LEADERSHIP TEAM
Name POSITION PRIOR EXPERIENCE / AFFILIATIONS
Angelos M. Stergiou, MD, ScD h.c. Chief Executive Officer Paion AG, Biovest International, Accentia, Analytica, Avanex Life Sciences
Nicholas J. Sarlis, MD, PhD, FACP Chief Medical Officer NIH, MD Anderson, Sanofi, Incyte
William Pollett, ACA, CFA Chief Financial Officer PWC, ACE, Montpelier, Blue Capital
Gregory M. Torre, PhD, JD
Chief Regulatory Officer, &
SVP Technical Operations Pfizer, Accentia, Bristol-Meyers, Sanofi
Martin G. Baum Chief Operating Officer VestIQ, Accentia, SkyePharma, GSK
David Moser, JD VP, Corporate Legal Affairs Accentia, Biovest International
7
PLANNED BOARD OF DIRECTORS
• Angelos Stergiou, MD, ScD h.c., SELLAS CEO
• Representative from SELLAS
• Representative from SELLAS
• New, Independent Director appointed by SELLAS
• New, Independent Director appointed by SELLAS
• New appointment by Galena
• New appointment by Galena

SELLAS OVERVIEW
8
•Focused on developing novel immune therapies for cancer
•Lead asset is galinpepimut-S, an immune therapy targeting the Wilms Tumor 1 (WT1) protein
• WT1 - top-ranked by the National Cancer Institute among cancer antigens for immunotherapy
•Technology licensed from Memorial Sloan Kettering Cancer Center (MSKCC)
•Positioned for both the current and future immuno-oncology landscape
• Exhibits strong immune response
• Development ongoing as both monotherapy and combination
•Two Phase 3 ready indications: well tolerated therapy with compelling efficacy profile from completed
Phase 2 trials
• Acute Myeloid Leukemia: Elderly (>60 yrs) - 35.51 months median overall survival, far higher than what is expected
for current care
• Malignant Pleural Mesothelioma: 24.8 months median overall survival vs. 16.6 months (blinded, randomized-
controlled Phase 2)
• EMA & FDA Orphan Drug Designation and FDA Fast Track status
•Ongoing Phase 1/2 studies in two additional indications
• Multiple Myeloma: median progression-free survival: 23.6 months; median overall survival not yet reached
• Ovarian Cancer (combo study with nivolumab (OPDIVO®): initial data expected in 2H 2017
•Additional checkpoint combination studies planned for 2018
OPDIVO® is a trademark of Bristol-Myers Squibb Company.

SELLAS/ GALENA COMBINED DEVELOPMENT PIPELINE
9
GALINPEPIMUT-S (GPS) – DEVELOPMENT PROGRAMS
Ovarian Cancer (comb. w/ Nivolumab - BMS)
PROGRAM PRECLINICAL PHASE 1 PHASE 2 PHASE 3
Multiple Myeloma
Immune Combination Trial
WT1-Lm Product (ADXS)
In-Licensed WT1 Delivery Technology
Ph 2 Study – Ongoing
Ph 1/2 Study – Ongoing
Phase 2a POC Study Planned – 1H: 18
Ph1 FIH/ FPI:
1H:18
IND-enabling studies
Comb w/trastuzumab (HER2 1+/2+)
Comb w/trastuzumab (HER2 3+)
Ductal Carcinoma in Situ
Ph 2 Study Ongoing: Interim analysis expected 1Q:18
Ph 2 Study Ongoing
Ph 2 Study Screening Patients
Chronic Myelogenous Leukemia (CML)** Ph 2 Study - Planned
Essential Thrombocythemia
Anagrelide Controlled Release (CR)
Ph 3 Protocol reviewed by FDA / 505(b)2 Regulatory Pathway confirmed Ph 3 Ready
NeuVax™ (nelipepimut-S) – BREAST CANCER DEVELOPMENT PROGRAMS
Mesothelioma Ph 3 Ready
AML (with Hypomethylating Agent)** Ph 2 Study - Pending
Ph 3 Protocol reviewed by FDA
Multiple Myeloma –Randomized Ph2b** Ph 2b Study Planned – 2018
Acute Myeloid Leukemia Ph3 Ready Ph 3 Protocol reviewed by FDA, >100 sites pre-screened
**In collaboration with multi-center cooperative groups/ clinical trial consortia
NeuVax is a trademark of Galena Biopharma, Inc.

Data shown are based on published literature; list of citations below:
Dupont, 2004; Acs, 2004; Menssen, 2000; Rosenfeld, 2003; Oji, 1999; Al-Hussaini, 2004; Rodeck, 1994; Menssen, 1995; Campbell,
1998; Foster, 2001; Loeb, 2001; Dennis, 2002; Oji, 2002, 2003 and 2004 (multiple publications); Waldstrøm, 2005; Hylander, 2006;
Nakatsuka, 2006; Hosen, 2007; Huang, 1990; Park, 1993; Hosen, 2002
AML: acute myelogenous leukemia
MPM: malignant pleural mesothelioma
Tumor types pursued
by SELLAS’ clinical program
Other tumor types
WT1: TOP-RANKED CANCER ANTIGEN BY THE NATIONAL CANCER INSTITUTE (NCI)*
• Targeting WT1, which was
highest ranked by the National
Cancer Institute as a cancer
antigen for immunotherapy
with the potential to treat 25+
cancers
• Expressed broadly in
hematological malignancies
and solid tumors; not found
appreciably in adult tissues,
which lowers potential off-
target toxicity
* “The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research”* Cheever et.al; Clinical Cancer
Research 2009
10
28/28
33/34 54/56
23/25 41/46
(Positive samples / Total samples)

GALINPEPIMUT-S (GPS): KEY FEATURES
11
is
substantially

GALINPEPIMUT-S: KEY DIFFERENTIATORS
GALINPEPIMUT-S
CR or MRD is the
preferred setting
for treatment as
monotherapy Heteroclitic
peptides
↑ Immune
response
↓ potential for
tolerance
Multivalent
polypeptide
structure drives
differentiated
immuno-
therapeutic
efficacy
Potential
combination
approaches
underway
Potentially
applicable to 25+
cancer types
worldwide and
all HLA types
Cost-effective
manufacturing:
allogeneic, off-
the-shelf vialed
drug; not
patient-specific
Positive early
clinical data on
survival,
tolerability,
safety
12

PRIVATE AND CONFIDENTIAL
•Prolonged median overall survival: 67.6 months (all ages)
•Aggregate population of patients > 60 years (Phase 3 Population): median overall
survival (mOS) = 32.2 months in Phase 1 / 35.5 months in Phase 2
•88% of patients had evidence of immune response by either CD8+ or CD4+ reactivity
to any of the 4 peptides in galinpepimut-S after administration.
•CD4+ responses seen across human leukocyte antigen (HLA)-Class II subtypes
• No discernable effect of HLA allelic type expression on clinical outcomes
•Well tolerated
GALINPEPIMUT-S (GPS); AML PHASE 1 AND 2 CLINICAL PROGRAM
CONCLUSIONS
13

Brayer, 2015
• Independent trial at the Moffit Cancer Center (Tampa, FL) in patients after second
complete remission (CR2)
•AML pts receiving > 2 administrations of galinpepimut-S compared to group of paired
patients in CR2 treated at Moffit Cancer Center during a similar time period
• 10 patients treated with GPS compared to 15 paired patients; median age 74
•Disease-free survival results: 319 vs 131 days
•The overall survival (OS) in galinpepimut-S treated individuals was significantly greater
than the comparator group, 495 vs. 165 days, p = 0.0175
•28% AML patients experienced sustained responses with durations either equaling or
exceeding the duration of first complete remission (CR1)
GALINPEPIMUT-S: PATIENTS IN AML CR2 AT MOFFITT CANCER CENTER
THESE DATA SUPPORT GALINPEPIMUT-S’ EFFECT IN ELDERLY, HEAVILY-TREATED AML PATIENTS
14

INDICATION INTERVENTION MEDIAN AGE (Y)
PATIENT
NUMBERS
2 YR OS (%)
> 60y CR1 1 Chemotherapy 67 471 30
>60y CR1 2 Bone Marrow Transplant 64 191 36
All ages CR1 3 Chemotherapy 54 6283 42
All ages CR1 4 Antibody-Drug Conjugate 57 132 53
All ages CR1 5 Galinpepimut-S 66 32 69
Galinpepimut-S <60 14 79
Galinpepimut-S >60 18 61
1Kahl, 2016; 2McClune, 2010; 3Walter, 2010; ; 4Castaigne, 2012; 5SELLAS, pooled analysis, Data on file
HISTORICAL COMPARISONS:
ALTERNATE THERAPIES VS. GALINPEPIMUT-S (GPS) IN AML CR1
PHASE 2 SURVIVAL WITH GPS IN THE POPULATION PLANNED FOR PHASE 3 TRIAL FAR EXCEEDS EVEN STEM CELL TRANSPLANT
15

SELLAS PHASE 3 TRIAL DISCUSSED WITH THE FDA
16
Aspects of the study
related to development
and approval-related
items such as study
design, endpoints,
statistical analysis plan,
CMC were agreed upon
with FDA
PLANNED PHASE 3 DOUBLE-BLIND, PLACEBO-CONTROLLED MULTICENTER STUDY IN AML CR1
• N 390 evaluable patients ≥ 60y
• Population Newly diagnosed AML in CR1, ineligible to transplant,
following physician’s choice of chemotherapy
• Randomization 2:1 fashion for vaccine : placebo
• Regimen Up to 16 doses of galinpepimut-S in ~2 years post-remission therapy
• Power 90% power to detect a 45% survival difference (9 vs. 13 months)
• Hazard Ratio and α 0.692 and 1-sided 2.5%
• Study Sites ~180 sites in North America, Europe, Aus/NZ, Asia
• Primary Endpoint Overall survival
• Sec /Exp Endpoints LFS, Safety / MRD, immune response
• Interim Analysis Three pre-planned looks by DSMB (76, 190, 284 deaths)
•Controlled, randomized blinded Phase 2 study with galinpepimut-S after surgery
and other treatment
•Galinpepimut-S increased overall survival (OS) vs. control group
•Median overall survival: 24.8 vs. 16.6 months (HR: 0.51)
•Median overall survival for patients with complete (R0) resections: 39.3 vs. 24.8
months (HR: 0.415)
•Galinpepimut-S induced both CD8+ and CD4+ T-cell activation
•Well tolerated
•Adverse events were mainly low grade reactions at the site of injection
•Successful End of Phase 2 meeting and discussion with U.S. FDA in March 2017
17
GALINPEPIMUT-S: PHASE 2 RANDOMIZED MALIGNANT PLEURAL MESOTHELIOMA (MPM)

18
GALINPEPIMUT-S: PHASE 2 IN MULTIPLE MYELOMA
•Phase 1/2, open-label study of GPS in myeloma patients following stem cell transplant
•Primary objective was to assess T-cell responses 12‒14 weeks after initial GPS
administration
•Secondary objectives included safety, WT1 expression on malignant plasma cells,
proportion of patients with minimal residual disease (MRD), progression-free survival
(PFS), and overall survival (OS)
•Patients were treated for up to 9 months (12 treatments)
•15/18 patients had high-risk cytogenetics and all patients remained at least MRD(+) after
stem cell transplant^
•This type of MM patients typically have much poorer prognosis than lower risk ones
•Clinical results were meaningfully better than prior trials in this patient group
•Median PFS: GPS: 23.6 months*
•Progression-free survival at 12 months: 81%*
•Progression-free survival at 18 months: 62%*
•Overall survival at 18 months: 88%, Median OS not yet reached
*SELLAS & MSKCC, Data on file; Koehne, EBMT 2017 presentation; ^: autologous SCT

•Ovarian cancer trial: galinpepimut-S + nivolumab
• Open-label, non-randomized Phase 1/2 trial
• In patients with recurrent ovarian cancer who are in second or greater clinical remission
following chemotherapy
• Investigator-sponsored trial (galinpepimut-S + nivolumab) in a different cancer type
expected to start in 2018
•Broader immune PD1 checkpoint combinations: planning ongoing
• Confirm immune profile of GPS in combination with PD1 blockade in multiple solid tumor
settings
• Focus on WT1 (+) solid tumors whereby modest activity with monotherapy PD1 blockade
has been seen
• Explore GPS combination in hematological settings
•Continue NeuVax Development to assess upcoming data releases
19
ADDITIONAL SELLAS DEVELOPMENT PROGRAMS
1 Le DT , NEJM 2015; 2 Nakatsuka, Modern Pathol 2006

20
NAME POSITION
David Scheinberg, MD, PhD
Chair, Experimental Therapeutics Center at Memorial Sloan Kettering Cancer Center
(MSKCC)
Jedd D. Wolchok, MD, PhD
Chief, Melanoma & Immunotherapeutics Service at Memorial Sloan Kettering Cancer
Center (MSKCC)
Jeffrey Weber, MD, PhD
Deputy Director of the Perlmutter Cancer Center at the New York Univ. (NYU)-
Langone Cancer Center
Alexander M.M. Eggermont, MD
Director General of Institut Gustave Roussy Cancer Campus Grand Paris, Villejuif,
France
Larry W. Kwak, MD, PhD Asociate Director Cancer Center for the City of Hope National Medical Center
Javier Pinilla-Ibarz, MD
Director of Immunotherapy for Malignant Hematology at the H. Lee Moffitt Cancer
Center
Sattva Neelapu, MD, PhD
Associate Professor, Department of Lymphoma/Myeloma, at MD Anderson Cancer
Center
Guenther Koehne, MD
Director, Cytotherapy Laboratory at Memorial Sloan Kettering Cancer Center
(MSKCC)
SCIENTIFIC ADVISORY BOARD
21
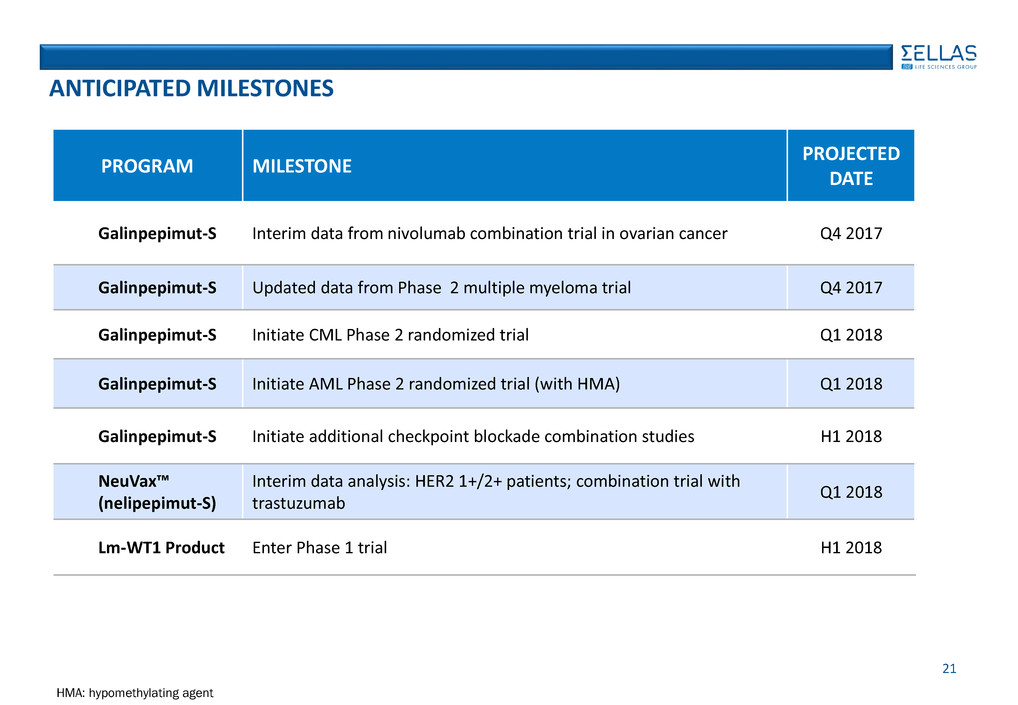
PROGRAM MILESTONE
PROJECTED
DATE
Galinpepimut-S Interim data from nivolumab combination trial in ovarian cancer Q4 2017
Galinpepimut-S Updated data from Phase 2 multiple myeloma trial Q4 2017
Galinpepimut-S Initiate CML Phase 2 randomized trial Q1 2018
Galinpepimut-S Initiate AML Phase 2 randomized trial (with HMA) Q1 2018
Galinpepimut-S Initiate additional checkpoint blockade combination studies H1 2018
NeuVax™
(nelipepimut-S)
Interim data analysis: HER2 1+/2+ patients; combination trial with
trastuzumab
Q1 2018
Lm-WT1 Product Enter Phase 1 trial H1 2018
HMA: hypomethylating agent
ANTICIPATED MILESTONES

AUGUST 8, 2017
