Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ENTELLUS MEDICAL INC | d425487d8k.htm |
Exhibit 99.1

Entellus Investor
Presentation
August 2017
entellus MEDICAL

Forward Looking Statements
This presentation may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements
generally can be identified by the use of words such as “expect,” “anticipate,” “could”, “may,” “intend,” “will,” “continue,” “outlook,” “guidance,”
“future,” “look forward,” “next steps,” other words of similar meaning and the use of future dates. Forward-looking statements in this presentation include statements about our total addressable market, financial
guidance and future financial performance, the effect of our acquisition of Spirox, Inc. on our business and operating results, product performance and benefits, ability to implement our business model and strategic plan, ability to integrate the
Spirox operations and ability to manage and grow our business, ability to establish and maintain intellectual property protection for our products or avoid claims of infringement, ability to hire and retain key personnel, and expectations about
market trends, along with third-party payor reimbursement and coverage decisions. These forward-looking statements are based on the current expectations of our management and involve known and unknown risks and uncertainties that may cause our
actual results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the dependence of our net sales on our XprESS family of products, future market acceptance and
adoption of our products and adequate levels of coverage or reimbursement for procedures using our products; risks involved in our recent acquisition of Spirox, including the failure to achieve the revenues, cost savings, earnings, growth prospects
and any or other synergies expected from the acquisition or delays in realization thereof; delays and challenges in integrating Spirox’s business and operations; operating costs and business disruption following the acquisition, including
adverse effects on employee retention and on business relationships with third parties, including physicians, providers, distributors and vendors; and issues with customers securing reimbursement for nasal surgery procedures using Spirox’s
LATERA device; our ability to successfully develop and commercialize new ENT products; competition; ability to expand, manage and maintain our direct sales organization and market and sell our products in the United States and internationally; risks
and uncertainties involved in our international operations; the compliance of our products and activities with the laws and regulations of the countries in which they are marketed; failure or delay in obtaining FDA or other regulatory approvals or
the effect of FDA or other regulatory actions; our ability to manage our anticipated growth; risk of product recalls, product liability claims and litigation and inadequate insurance coverage relating thereto; intellectual property disputes; loss of
key suppliers; inadequate capital resources and inability to raise additional financing when needed and on favorable terms. Other factors that may cause actual results to differ materially from current expectations include, among other things, those
described in the section entitled “Risk Factors” and elsewhere in greater detail in our Annual Report on Form 10-K for the year ended
December 31,
2016 filed with the Securities and Exchange Commission on February 22, 2017 and other filings with the SEC including our Quarterly Report on Form 10-Q for the quarter ended June 30, 2017. We undertake no obligation to update or revise any
forward-looking statements, even if subsequent events cause our views to change. This presentation contains statistical data that we obtained from industry publications and reports generated by third parties. Although we believe that the
publications and reports are reliable, we have not independently verified this statistical data.
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL

Note on Non-GAAP Financial Measures
To supplement its consolidated financial statements prepared in accordance with United States generally accepted accounting principles (“GAAP”), Entellus uses certain
non-GAAP financial measures, including adjusted EBITDA and adjusted gross margin. Entellus’s management team believes that the presentation of these measures provides useful information to investors and that these measures may assist investors
in evaluating the company’s operations, period over period. EBITDA is calculated by adding back to net income charges for interest, income taxes and depreciation and amortization expenses. Adjusted EBITDA is calculated by adding back to EBITDA
non-cash stock-based compensation, non-operating income and expense, non-cash contingent consideration adjustments associated with business combinations, non-cash inventory step-up amortization and special charges, including transaction and
integration-related expenses. Entellus’s adjusted gross margin is calculated by excluding the impact of purchase accounting in connection with Entellus’s recent acquisition of Spirox, Inc. With respect to Entellus’s third quarter and
full year 2017 financial guidance regarding non-GAAP adjusted EBITDA and non-GAAP adjusted gross margin in its press release, Entellus cannot provide a quantitative reconciliation to the most directly comparable GAAP measure without unreasonable
effort due to its inability to make accurate projections and estimates related to certain information needed to calculate some of the adjustments as described above. Reconciliations of historical non-GAAP financial measures used in this presentation
to the most comparable GAAP measures can be found on Entellus’s website.
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
Large, unpenetrated market and disease states treated by the same physicians
Transforming
Minimally invasive technologies for better the ENT outcomes
Market
Shifting ENT procedures to cost effective settings
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
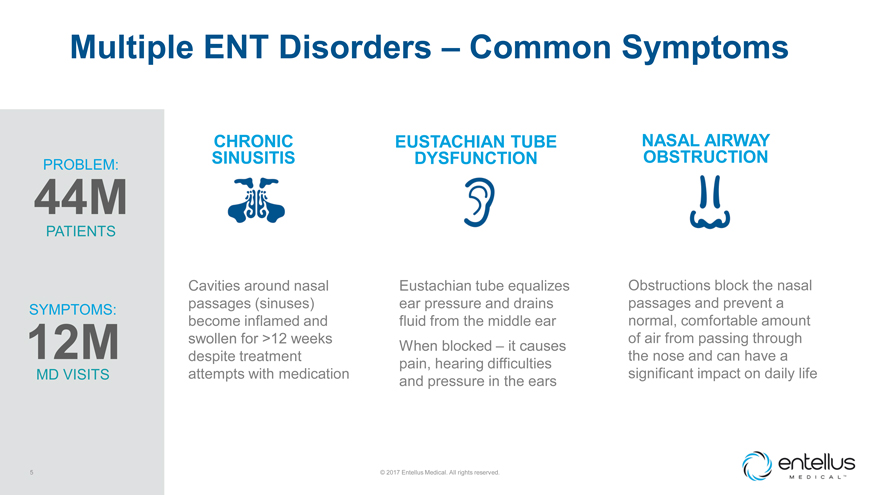
Multiple ENT Disorders – Common Symptoms
PROBLEM: 44M PATIENTS SYMPTOMS: 12M MD VISITS 5 CHRONIC SINUSITIS Cavities around nasal passages (sinuses) become inflamed and swollen for >12 weeks despite treatment attempts
with medication EUSTACHIAN TUBE DYSFUNCTION Eustachian tube equalizes ear pressure and drains fluid from the middle ear When blocked – it causes pain, hearing difficulties and pressure in the ears © 2017 Entellus Medical. All rights
reserved. NASAL AIRWAY OBSTRUCTION Obstructions block the nasal passages and prevent a normal, comfortable amount of air from passing through the nose and can have a significant impact on daily life
entellus MEDICAL
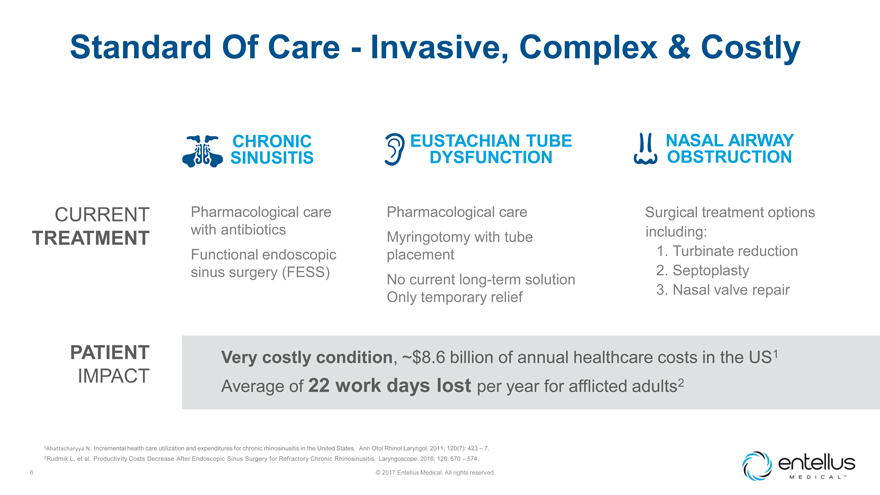
Standard Of Care—Invasive, Complex & Costly
CHRONIC SINUSITIS EUSTACHIAN TUBE DYSFUNCTION NASAL AIRWAY OBSTRUCTION
CURRENT TREATMENT,
Pharmacological care with antibiotics Functional endoscopic sinus surgery (FESS), Pharmacological care Myringotomy with tube placement No current long-term solution Only temporary relief, Surgical treatment options including: 1. 2. 3.,
Turbinate reduction Septoplasty Nasal valve repair
PATIENT
Very costly condition, ~$8.6 billion of annual healthcare costs in the US1
IMPACT
Average of 22 work days lost per year for afflicted adults2
1Ahattacharyya N. Incremental
health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann Otol Rhinol Laryngol. 2011; 120(7): 423 – 7. 2Rudmik L, et al. Productivity Costs Decrease After Endoscopic Sinus Surgery for Refractory Chronic
Rhinosinusitis. Laryngoscope. 2016; 126: 570 – 574.
6 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
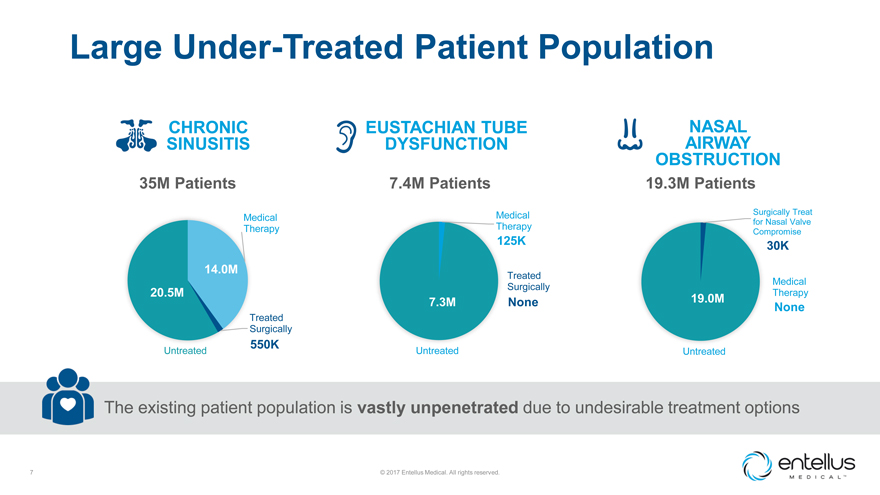
Large Under-Treated Patient Population
CHRONIC EUSTACHIAN TUBE NASAL SINUSITIS DYSFUNCTION AIRWAY
OBSTRUCTION
35M Patients 7.4M Patients 19.3M Patients
Medical Surgically Treat
Medical for Nasal Valve
Therapy Therapy
125K Compromise
30K 14.0M
Treated
Medical Surgically 20.5M Therapy
7.3M None 19.0M
None
Treated Surgically
550K
Untreated Untreated Untreated
The existing patient population is vastly unpenetrated due to
undesirable treatment options
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
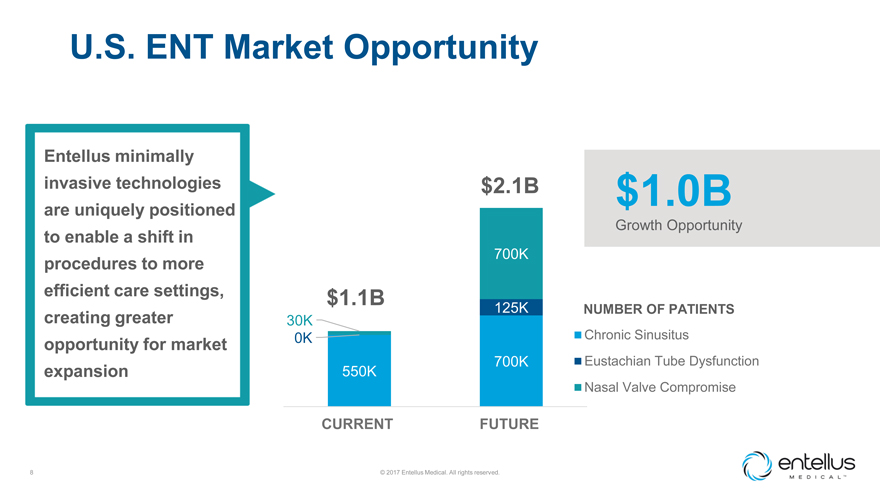
U.S. ENT Market Opportunity
Entellus minimally invasive technologies are uniquely positioned to enable a shift in procedures to more efficient care settings, creating greater opportunity for
market expansion
$2.1B $1.0B
Growth Opportunity
700K
$1.1B
30K 125K NUMBER OF PATIENTS
0K Chronic Sinusitus
700K Eustachian Tube Dysfunction
550K
Nasal Valve Compromise
CURRENT FUTURE
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
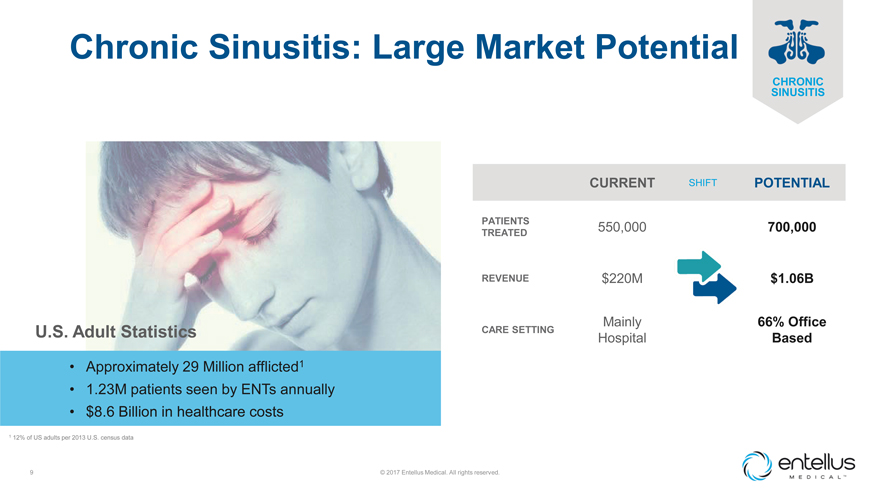
Chronic Sinusitis: Large Market Potential
U.S. Adult Statistics
| • |
|
Approximately 29 Million afflicted1 |
| • |
|
1.23M patients seen by ENTs annually |
| • |
|
$8.6 Billion in healthcare costs |
| 1 | 12% of US adults per 2013 U.S. census data |
CURRENT SHIFT POTENTIAL
PATIENTS 550,000 700,000 TREATED
REVENUE $220M $1.06B
Mainly 66% Office
CARE SETTING
Hospital Based
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
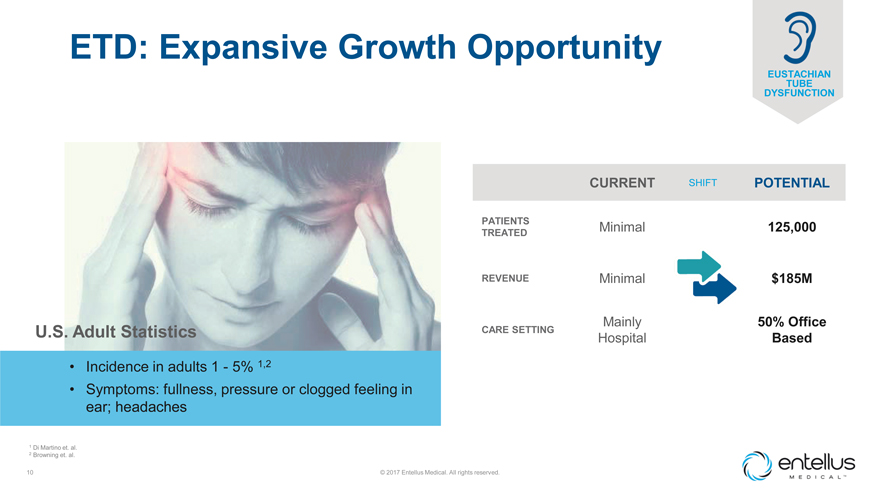
ETD: Expansive Growth Opportunity
EUSTACHIAN TUBE DYSFUNCTION
U.S. Adult Statistics
| • |
|
Incidence in adults 1—5% 1,2 |
| • |
|
Symptoms: fullness, pressure or clogged feeling in ear; headaches |
| 1 | Di Martino et. al. |
| 2 | Browning et. al. |
CURRENT SHIFT POTENTIAL
PATIENTS Minimal 125,000 TREATED
REVENUE Minimal $185M
Mainly 50% Office
CARE SETTING
Hospital Based
10 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
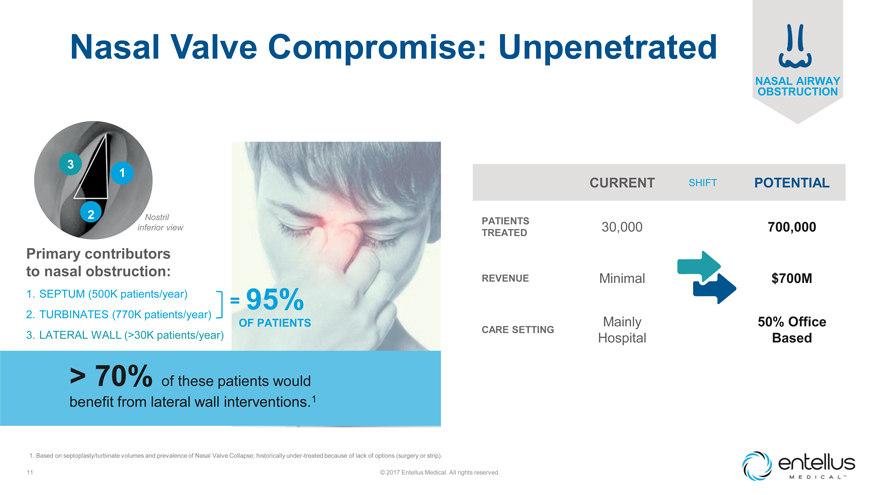
Nasal Valve Compromise: Unpenetrated
3
1
2
Nostril inferior view
Primary contributors to nasal obstruction:
1. SEPTUM
(500K patients/year) = 95%
2. TURBINATES (770K patients/year)
OF PATIENTS
3. LATERAL WALL (>30K patients/year)
> 70% of these patients would
benefit from lateral wall interventions.1
1. Based on septoplasty/turbinate volumes and prevalence of Nasal Valve Collapse; historically under-treated because of
lack of options (surgery or strip).
CURRENT SHIFT POTENTIAL
PATIENTS 30,000
700,000
TREATED
REVENUE Minimal $700M
Mainly 50% Office
CARE SETTING
Hospital Based
11 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
Entellus – Improved Treatment Options
Providing less invasive technologies that offer better outcomes in more cost effective settings
CHRONIC SINUSITIS
EUSTACHIAN TUBE DYSFUNCTION
NASAL AIRWAY OBSTRUCTION
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
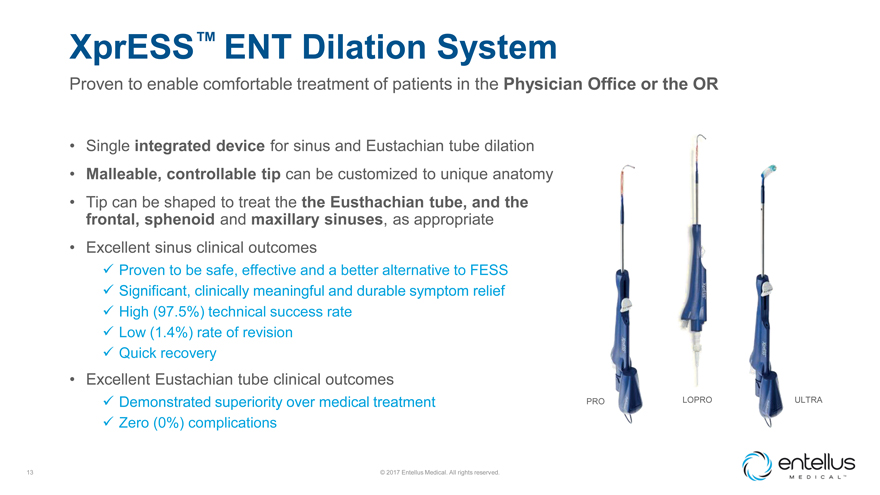
XprESS™ ENT Dilation System
Proven to enable comfortable treatment of patients in the Physician Office or the OR
Single
integrated device for sinus and Eustachian tube dilation
Malleable, controllable tip can be customized to unique anatomy
Tip can be shaped to treat the the Eusthachian tube, and the frontal, sphenoid and maxillary sinuses, as appropriate
Excellent sinus clinical outcomes
? Proven to be safe, effective and a better alternative to
FESS
? Significant, clinically meaningful and durable symptom relief
? High
(97.5%) technical success rate
? Low (1.4%) rate of revision
?
Quick recovery
Excellent Eustachian tube clinical outcomes
? Demonstrated
superiority over medical treatment PRO LOPRO ULTRA
? Zero (0%) complications
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
XprESS Commercial Experience
XprESS ENT Dilation System has been used on more than 150,000 patients
3.6 days 2 days
Saved due to Saved from prescription faster recovery pain medicine exposure
PATIENT
20 minutes
Saved from General Anesthetic exposure
14 © 2017 Entellus Medical. All rights reserved.
Over $300 million in cost savings to the U.S. health care system
NICE
estimates the National Health System in England may save approximately
£7.4 million per year by 2020
entellus MEDICAL
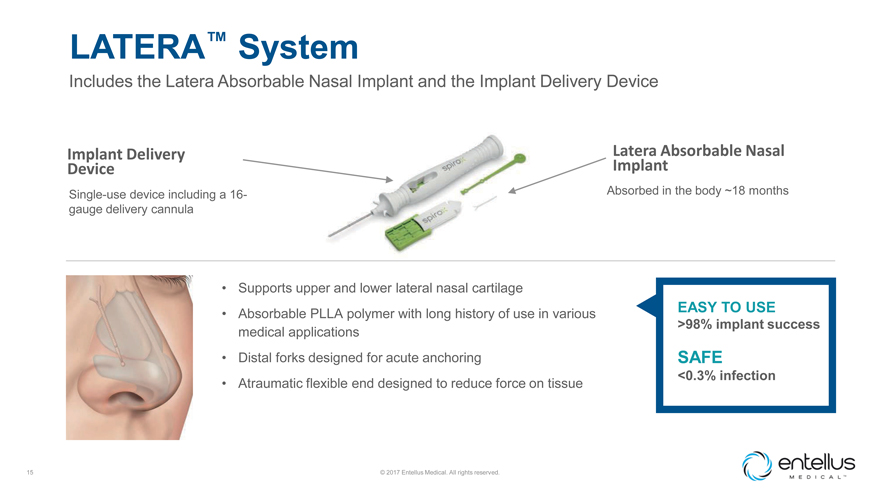
LATERA™ System
Includes the Latera Absorbable Nasal Implant and the Implant Delivery Device
Implant Delivery Device
Single-use device including a 16-gauge delivery
cannula
Latera Absorbable Nasal Implant
Absorbed in the body ~18 months
Supports upper and lower lateral nasal cartilage
Absorbable PLLA polymer with
long history of use in various medical applications
Distal forks designed for acute anchoring
Atraumatic flexible end designed to reduce force on tissue
EASY TO USE
>98% implant success
SAFE
<0.3% infection
© 2017 Entellus Medical. All rights reserved.
15
entellus MEDICAL

LATERA
Experience
500+ Clinicians Using LATERA
5,000+ Cases
Reported
10,000+ Implants Delivered
16 © 2017 Entellus Medical. All rights reserved.
93%
Satisfied with Appearance
86%
Satisfied with Breathing
entellus MEDICAL
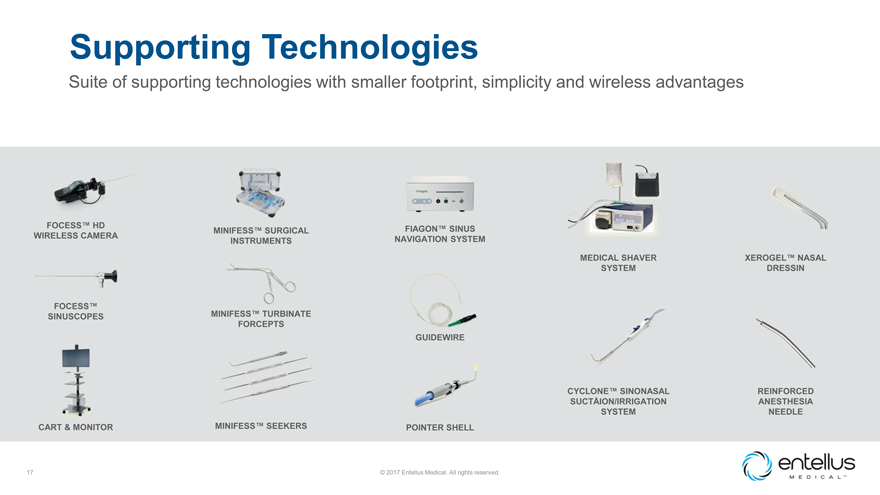
Supporting Technologies
Suite of supporting technologies with smaller footprint, simplicity and wireless advantages
FOCESS™ HD FIAGON™ SINUS MINIFESS™ SURGICAL
WIRELESS CAMERA NAVIGATION SYSTEM
INSTRUMENTS
MEDICAL SHAVER XEROGEL™ NASAL SYSTEM DRESSIN
FOCESS™
MINIFESS™ TURBINATE
SINUSCOPES FORCEPTS
GUIDEWIRE
CYCLONE™ SINONASAL REINFORCED SUCTÅION/IRRIGATION ANESTHESIA
SYSTEM NEEDLE
CART & MONITOR MINIFESS™ SEEKERS POINTER SHELL
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL

Shift to Cost Efficient Settings
$
PATIENTS PHYSICIANS PAYORS
Faster recovery Greater efficiency in office Favorable economics for the
Lower cost for
patients setting healthcare system
High level of patient comfort Familiar design and ease-of- Decreased healthcare utilization, use for physicians migrating
treatment of the same
? Smaller device enables easier symptomatic patient to the office sinus access and procedure can reduce costs by 45%
? Anesthesia approach allows for comfortable office procedures
18 © 2017 Entellus
Medical. All rights reserved.
entellus MEDICAL
|
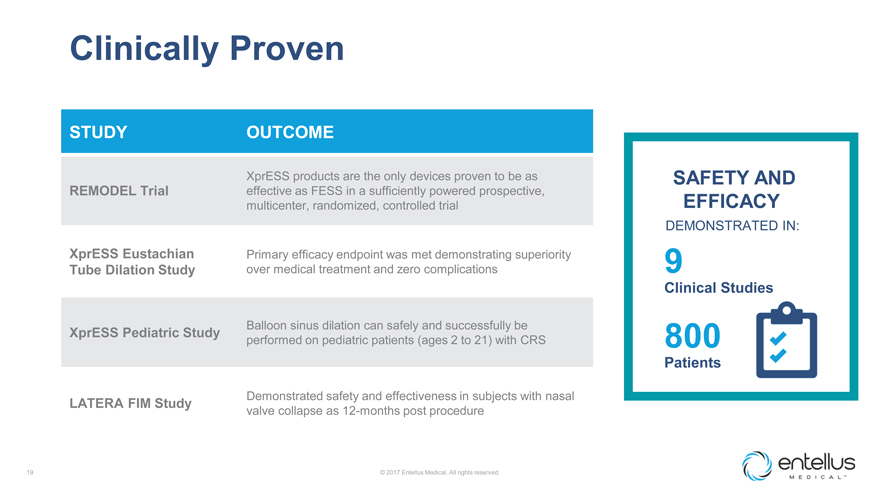
Clinically Proven
STUDY
OUTCOME
REMODEL Trial
XprESS products are the only devices proven to be as effective as FESS in a
sufficiently powered prospective, multicenter, randomized, controlled trial
XprESS Eustachian Tube Dilation Study
Primary efficacy endpoint was met demonstrating superiority over medical treatment and zero complications
XprESS Pediatric Study
Balloon sinus dilation can safely and successfully be performed on
pediatric patients (ages 2 to 21) with CRS
LATERA FIM Study
Demonstrated
safety and effectiveness in subjects with nasal valve collapse as 12-months post procedure
19 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
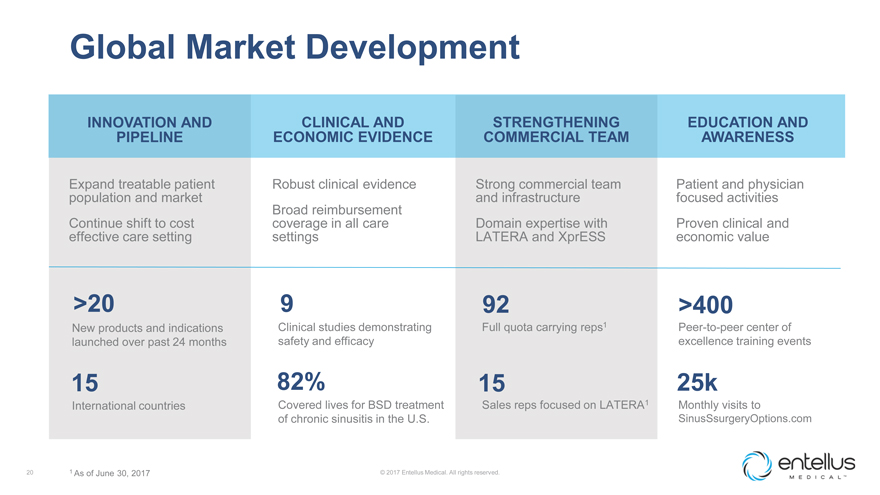
Global Market Development
INNOVATION AND PIPELINE
CLINICAL AND ECONOMIC EVIDENCE
STRENGTHENING COMMERCIAL TEAM
EDUCATION AND AWARENESS
Expand treatable patient population and market
Robust clinical evidence Broad reimbursement
Strong commercial team and infrastructure
Patient and physician focused
activities
Continue shift to cost effective care setting
coverage in all care
settings
Domain expertise with LATERA and XprESS
Proven clinical and economic
value
>20
9
92
>400
New products and indications launched over past 24 months
Clinical studies
demonstrating safety and efficacy
Full quota carrying reps1
Peer-to-peer
center of excellence training events
15
82%
15
25k
International countries
Covered lives for BSD treatment of chronic sinusitis
in the U.S.
Sales reps focused on LATERA1
Monthly visits to
SinusSsurgeryOptions.com
20 1 As of June 30, 2017 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
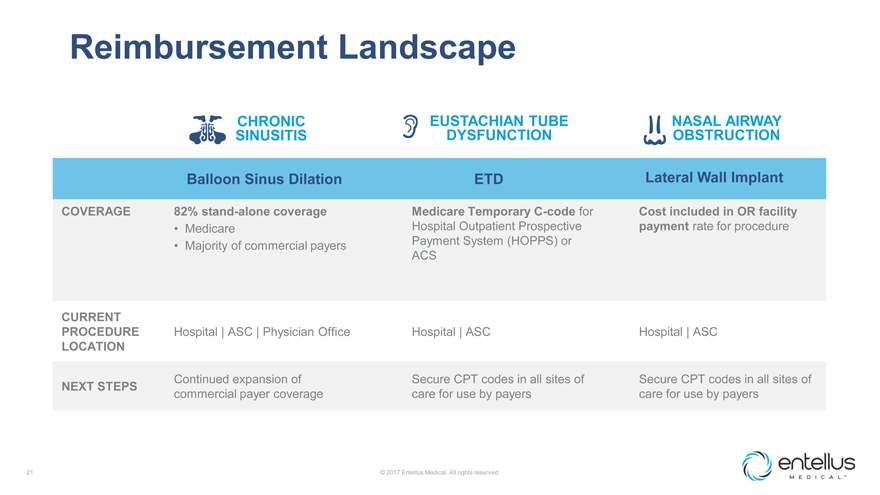
Reimbursement Landscape
CHRONIC SINUSITIS
EUSTACHIAN TUBE DYSFUNCTION
NASAL AIRWAY OBSTRUCTION
Balloon Sinus Dilation
ETD
Lateral Wall Implant
COVERAGE
82% stand-alone coverage
Medicare Temporary C-code for
Cost included in OR facility
Medicare
Hospital Outpatient Prospective
payment rate for procedure
Majority of commercial payers
Payment System (HOPPS) or
ACS
CURRENT PROCEDURE LOCATION
Hospital
ASC
Physician Office
Hospital
ASC
Hospital
ASC
NEXT STEPS
Continued expansion of commercial payer coverage
Secure CPT codes in all sites of care for use by payers
Secure CPT codes in all sites of care
for use by payers
21 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
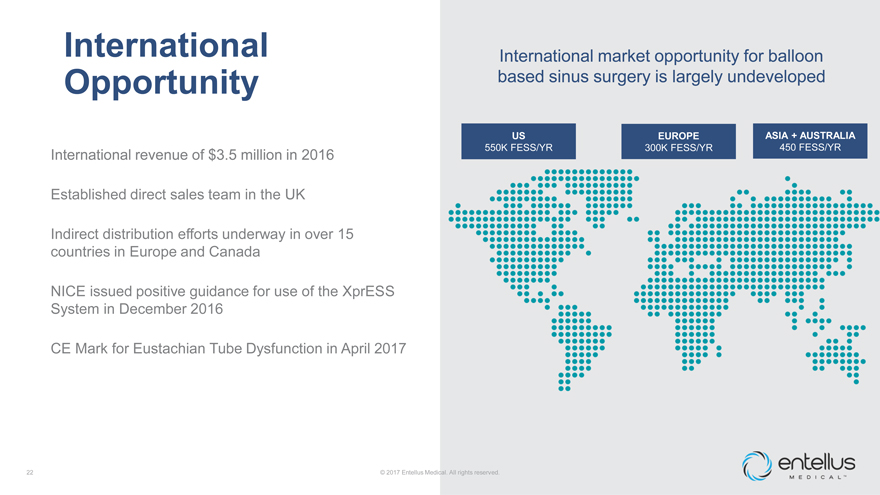
International Opportunity
International revenue of $3.5 million in 2016
Established direct sales team in the UK
Indirect distribution efforts underway in over 15 countries in Europe and Canada
NICE issued positive guidance for use of the XprESS System in December 2016
CE Mark for
Eustachian Tube Dysfunction in April 2017
International market opportunity for balloon based sinus surgery is largely undeveloped
US
EUROPE
ASIA + AUSTRALIA
550K FESS/YR
300K FESS/YR
450 FESS/YR
22 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
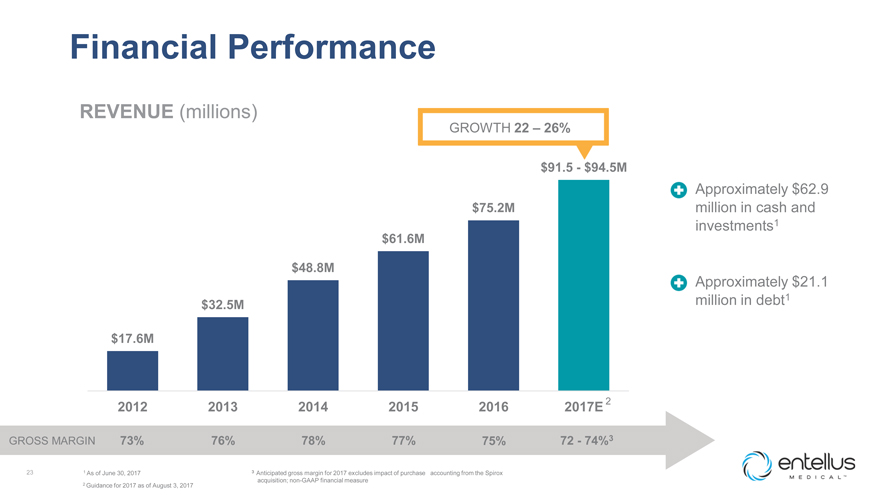
Financial Performance
REVENUE (millions)
GROWTH 22 – 26%
$91.5—$94.5M
Approximately $62.9 $75.2M million in cash and investments1 $61.6M
$48.8M
Approximately $21.1 million in debt1 $32.5M
$17.6M
2012 2013 2014 2015 2016 2017E 2
GROSS MARGIN 73% 76% 78% 77% 75% 72—74%3
23 1 As of June 30, 2017 3 Anticipated
gross margin for 2017 excludes impact of purchase accounting from the Spirox
2 Guidance for 2017 as of August 3, 2017 acquisition; non-GAAP financial measure
entellus MEDICAL

|
2017 Outlook
Total revenue $91.5 – $94.5 million, growth of 22% – 26%1 Expect international sales to be in the mid-single digits as a percentage of total 2017 revenue Gross margin is
expected to be in a range of 72% to 74%2 Sales force expansion
Adding 10-20 full quota-carrying reps in 2017
1 Guidance for 2017 as of August 3, 2017
24 2 Anticipated gross margin for 2017 excludes
impact of purchase accounting from the Spirox acquisition; non-GAAP financial measure
entellus MEDICAL

|
LARGE MARKET
Significant opportunities to shift patients from surgical and medical management options, $2.1 billion opportunity in the U.S.
CLINICAL EVIDENCE
Randomized trial shows Entellus balloon dilation is as effective as sinus
surgery
BETTER OUTCOMES
Provides superior economic outcomes for patients,
physicians and payors
GLOBAL OPPORTUNITY
Attractive OUS markets for growth
and expansion
STRONG POSITION
Well capitalized, strong financial position,
established performance and revenue growth
© 2017 Entellus Medical. All rights reserved.
Investment Highlights
25
entellus MEDICAL

|
Thank You
26 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL

|
Appendix
27 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
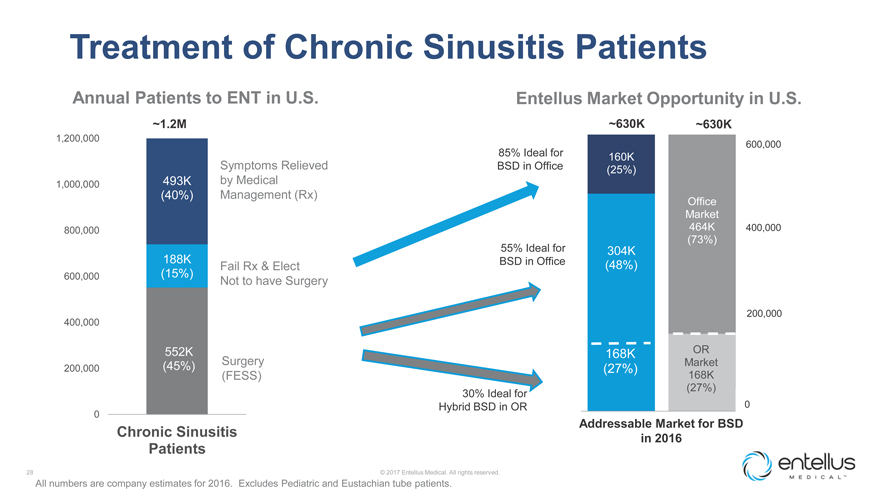
Treatment of Chronic Sinusitis Patients
Annual Patients to ENT in U.S. Entellus Market Opportunity in U.S.
~1.2M ~630K ~630K
1,200,000
600,000
85% Ideal for 160K
Symptoms Relieved BSD in Office (25%)
1,000,000 493K by Medical
(40%) Management (Rx)
Office Market
800,000 464K 400,000
(73%) 55% Ideal for 304K
188K BSD in Office
Fail Rx & Elect (48%)
600,000 (15%)
Not to have Surgery
400,000 200,000
552K 168K OR
(45%) Surgery Market
200,000 (27%)
(FESS) 168K (27%) 30% Ideal for Hybrid BSD in OR 0
0 Addressable Market for BSD
Chronic Sinusitis
in 2016
Patients
28 © 2017 Entellus Medical. All rights reserved.
All numbers are company estimates for
2016. Excludes Pediatric and Eustachian tube patients.
entellus MEDICAL
|
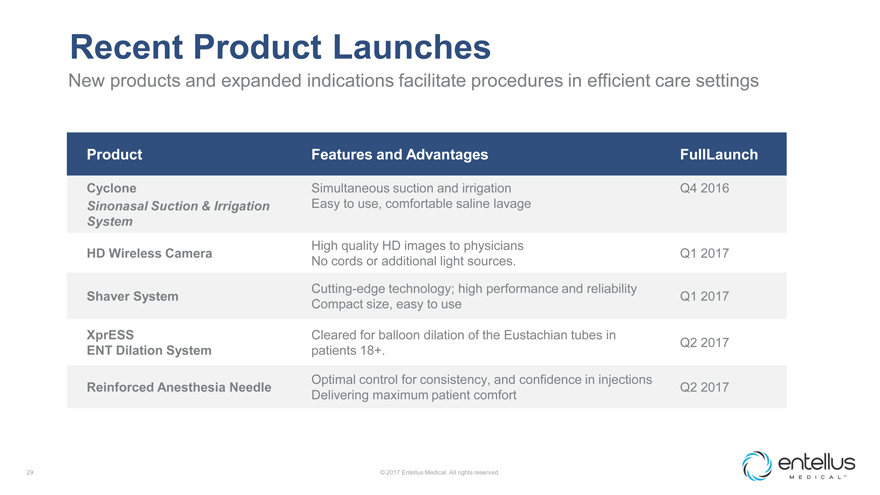
Recent Product Launches
New products and expanded indications facilitate procedures in efficient care settings
Product
Features and Advantages FullLaunch
Cyclone Simultaneous suction and irrigation Q4 2016 Sinonasal Suction & Irrigation Easy to use, comfortable saline
lavage
System
High quality HD images to physicians
HD Wireless Camera Q1 2017 No cords or additional light sources.
Cutting-edge technology; high
performance and reliability
Shaver System Q1 2017 Compact size, easy to use
XprESS Cleared for balloon dilation of the Eustachian tubes in
Q2 2017
ENT Dilation System patients 18+.
Optimal control for consistency, and
confidence in injections
Reinforced Anesthesia Needle Q2 2017 Delivering maximum patient comfort
29 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
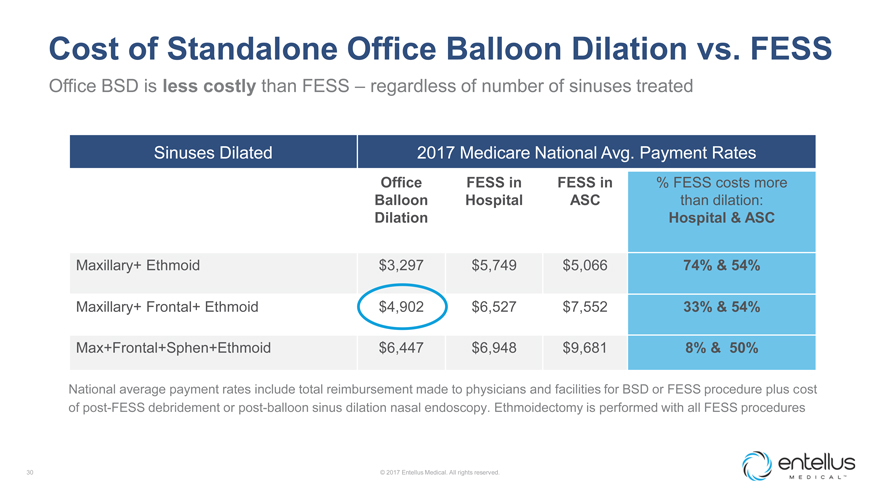
Cost of Standalone Office Balloon Dilation vs. FESS
Office BSD is less costly than FESS – regardless of number of sinuses treated
Sinuses Dilated 2017 Medicare National Avg. Payment Rates
Office FESS in FESS in % FESS costs
more Balloon Hospital ASC than dilation: Dilation Hospital & ASC
Maxillary+ Ethmoid $3,297 $5,749 $5,066 74% & 54% Maxillary+ Frontal+ Ethmoid
$4,902 $6,527 $7,552 33% & 54% Max+Frontal+Sphen+Ethmoid $6,447 $6,948 $9,681 8% & 50%
National average payment rates include total reimbursement
made to physicians and facilities for BSD or FESS procedure plus cost of post-FESS debridement or post-balloon sinus dilation nasal endoscopy. Ethmoidectomy is performed with all FESS procedures
30 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
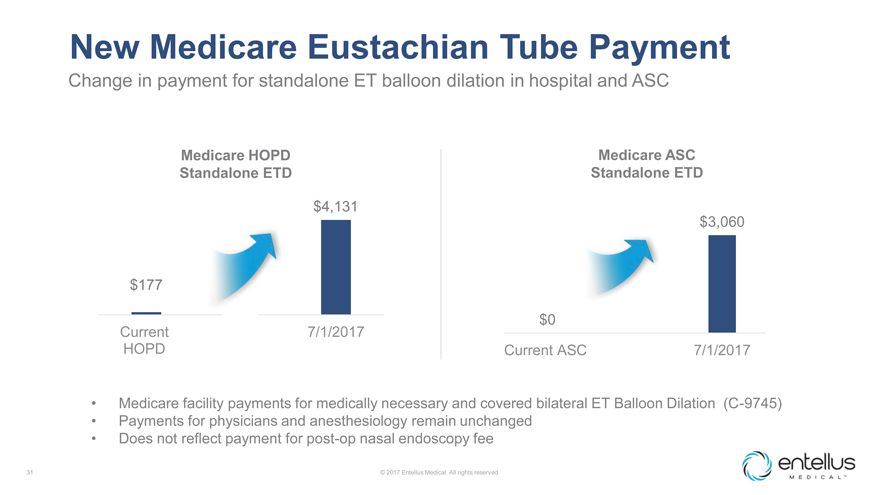
New Medicare Eustachian Tube Payment
Change in payment for standalone ET balloon dilation in hospital and ASC
Medicare HOPD
Medicare ASC Standalone ETD Standalone ETD
$4,131 $3,060
$177
Current 7/1/2017 $0
HOPD Current ASC 7/1/2017
Medicare facility payments for medically necessary and covered bilateral ET Balloon Dilation (C-9745)
Payments for physicians and anesthesiology remain unchanged
Does not reflect payment for
post-op nasal endoscopy fee
31 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
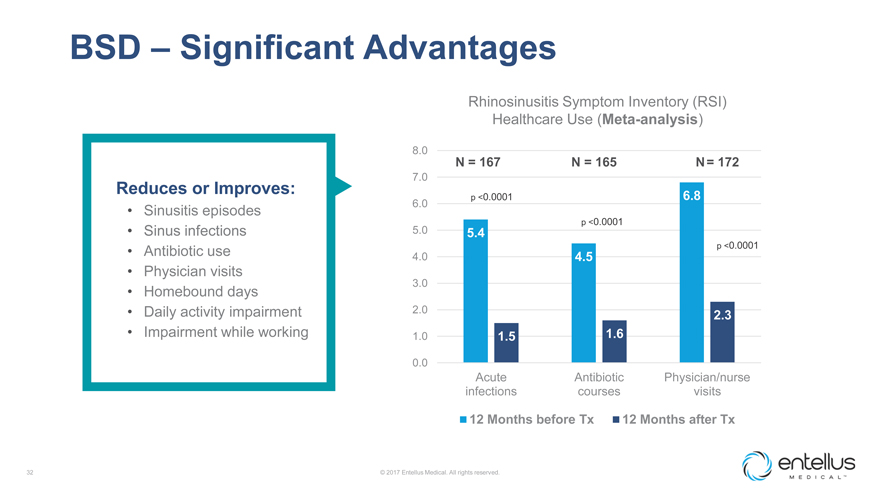
BSD – Significant Advantages
Rhinosinusitis Symptom Inventory (RSI) Healthcare Use (Meta-analysis)
8.0
N = 167 N = 165 N = 172
7.0
Reduces or Improves:
p <0.0001 6.8
6.0
Sinusitis episodes
p <0.0001
Sinus infections 5.0 5.4
Antibiotic use p <0.0001
4.0 4.5
Physician visits
3.0
Homebound days
Daily activity impairment 2.0 2.3
Impairment while working 1.0 1.5 1.6
0.0
Acute Antibiotic Physician/nurse infections courses visits
12 Months before Tx 12 Months after
Tx
32 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL

|
XprESS Pediatric Study Published Nov 2016
Prospective, Multicenter Evaluation of Balloon Sinus Dilation for
Treatment
of Pediatric Chronic Rhinosinusitis
Zachary M. Soler, MD, MSc; Jeffrey S. Rosenbloom, MD; Michael Gutman, MD;
Mark J. Hoy, MD and Shaun A. Nguyen, MD
International Forum of Allergy & Rhinology.
Nov, 2016.
[printed version]
Prospective, multicenter, single-arm study of
balloon sinus dilation in 50
pediatric CRS patients (ages 2 to 21 years)
Follow-up conducted at one-, three- and six-months post procedure
33 ©
2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
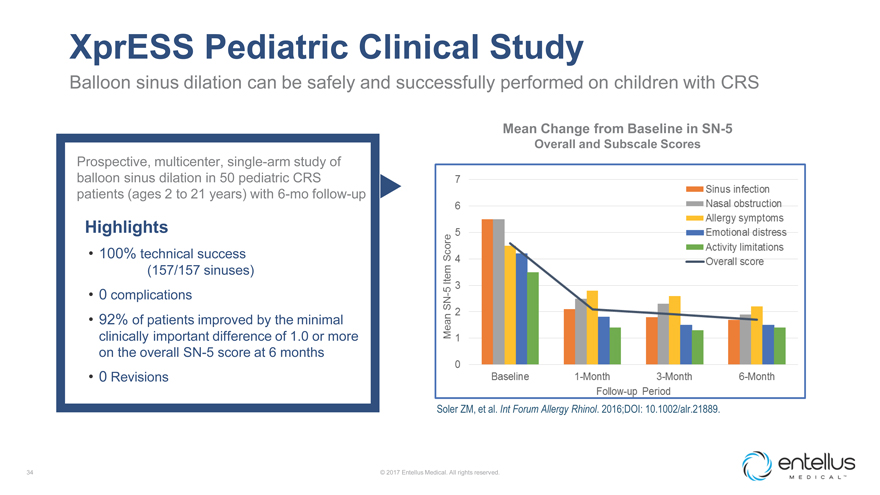
XprESS Pediatric Clinical Study
Balloon sinus dilation can be safely and successfully performed on children with CRS
Mean
Change from Baseline in SN-5
Overall and Subscale Scores
Prospective,
multicenter, single-arm study of balloon sinus dilation in 50 pediatric CRS patients (ages 2 to 21 years) with 6-mo follow-up
Highlights
100% technical success (157/157 sinuses)
0 complications
92% of patients improved by the minimal clinically important difference of 1.0 or more on the overall SN-5 score at 6 months
0 Revisions
Soler ZM, et al. Int Forum Allergy Rhinol. 2016;DOI: 10.1002/alr.21889.
© 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
XprESS Eustachian Tube Dilation Study
Prospective, multicenter, randomized controlled trial
60 ETD patients randomized to balloon
dilation or continued medical therapy
Primary endpoints
? Comparison between
arms in the change from baseline to 6 weeks in mean overall ETDQ-7 score: Balloon dilation was superior to medical therapy (p<0.0001)
? Complication rate = 0%
Nearly 75% of patients were treated in the office under local anesthesia only
Long-term (12-month) follow-up is ongoing
35 © 2017 Entellus Medical.
All rights reserved.
entellus MEDICAL
|
REMODEL Study Published Jan 2016
REMODEL Larger Cohort With Long-term Outcomes and Meta-Analysis of Standalone Balloon Dilation Studies
Rakesh K. Chandra, MD; Robert C. Kern, MD; Jeffrey L. Cutler, MD; Kevin C. Welch, MD; Paul T. Russell, MD
The Laryngoscope. January, 2016. [printed version]
REMODEL randomized trial data on larger
cohort of 135 patients with 1 to 2 years of follow-up
Meta-analysis of 7 standalone balloon dilation studies including 358 patients with follow-up from 6 months to
2 years
36 © 2017 Entellus Medical. All rights reserved.
entellus
MEDICAL
|
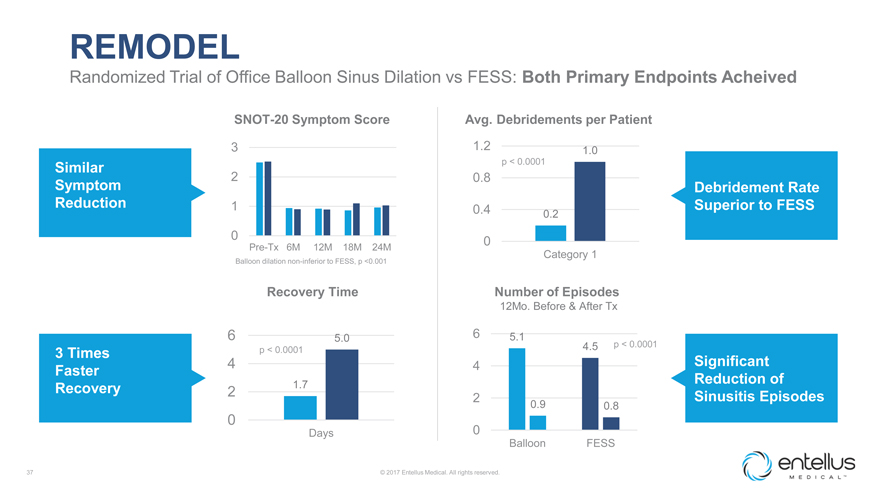
REMODEL
Randomized Trial of Office Balloon Sinus Dilation vs FESS: Both Primary Endpoints Acheived
SNOT-20 Symptom Score Avg. Debridements per Patient
3 1.2 1.0
Similar p < 0.0001
2 0.8
Symptom Debridement Rate Reduction 1 Superior to FESS
0.4 0.2 0 0
Pre-Tx 6M 12M 18M 24M
Category 1
Balloon dilation non-inferior to FESS, p <0.001
Recovery Time Number of Episodes
12Mo. Before & After Tx
6 5.0 6 5.1
4.5 p < 0.0001
3 Times p < 0.0001 Significant
4 4
Faster
1.7 Reduction of Recovery 2
2 Sinusitis Episodes
0.9 0.8
0
Days 0
Balloon FESS
37 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
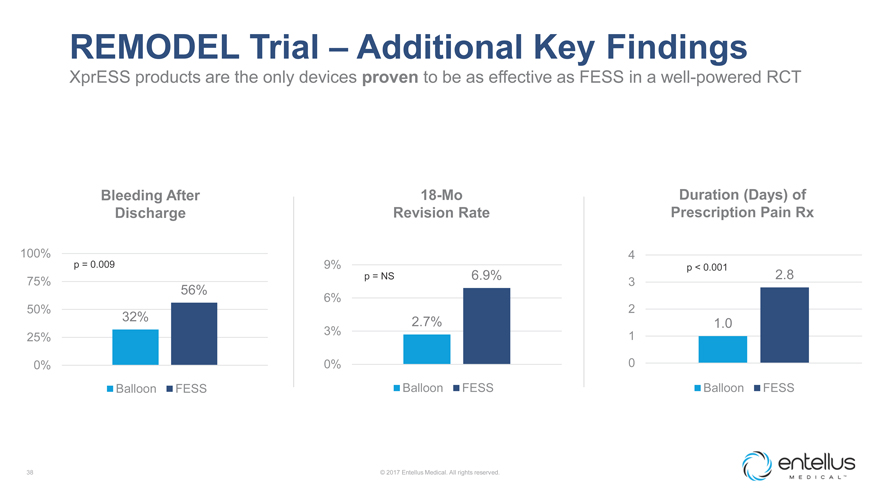
REMODEL Trial – Additional Key Findings
XprESS products are the only devices proven to be as effective as FESS in a well-powered RCT
Bleeding After 18-Mo Duration (Days) of Discharge Revision Rate Prescription Pain Rx
100% 4 p
= 0.009 9% p < 0.001 2.8 75% p = NS 6.9%
3
56%
6% 2 50%
32% 2.7%
1.0
3% 1 25%
0% 0% 0
Balloon FESS Balloon FESS Balloon FESS
38 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
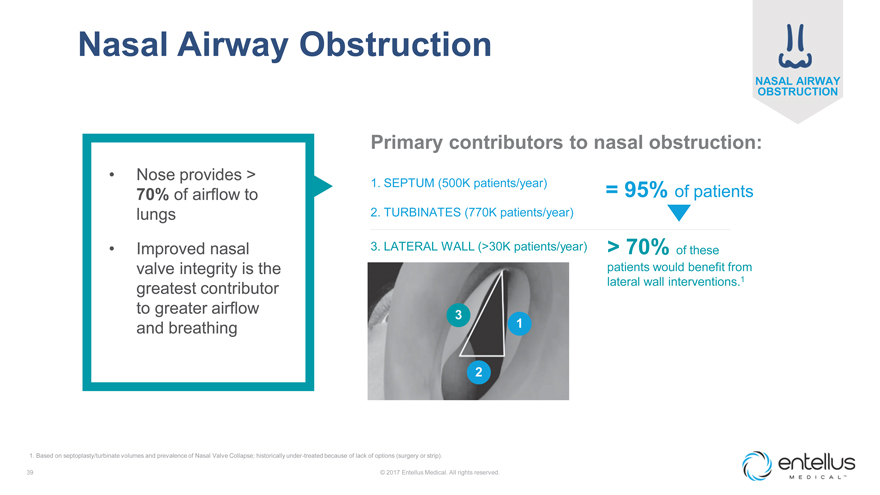
Nasal Airway Obstruction
NASAL AIRWAY OBSTRUCTION
Primary contributors to nasal obstruction:
Nose provides >
1. SEPTUM (500K patients/year)
70% of airflow to = 95% of patients
lungs 2. TURBINATES (770K patients/year)
Improved nasal 3. LATERAL WALL (>30K patients/year) > 70% of these valve integrity is the patients would benefit from lateral wall interventions.1
greatest contributor to greater airflow 3 and breathing 1
2
1. Based on septoplasty/turbinate volumes and prevalence of Nasal Valve Collapse; historically under-treated because of lack of options (surgery or strip).
39 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
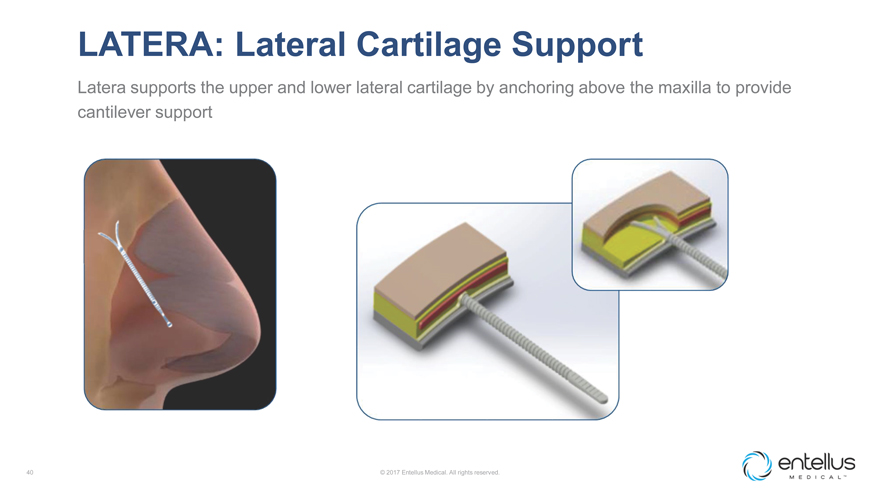
LATERA: Lateral Cartilage Support
Latera supports the upper and lower lateral cartilage by anchoring above the maxilla to provide cantilever support
40 © 2017 Entellus Medical. All rights reserved.
entellus MEDICAL
|
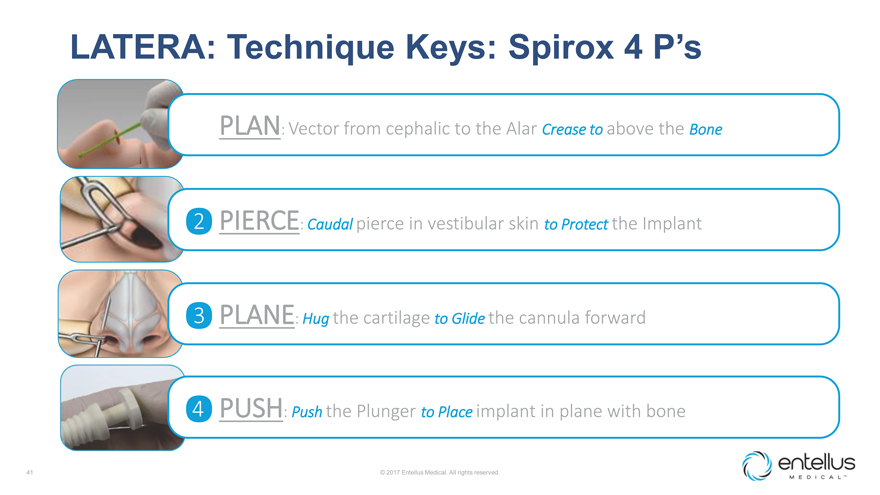
LATERA: Technique Keys: Spirox 4 P’s
? PLAN: Vector from cephalic to the Alar Crease to above the Bone
? PIERCE:
Caudal pierce in vestibular skin to Protect the Implant
? PLANE: Hug the cartilage to Glide the cannula forward
? PUSH: Push the Plunger to Place implant in plane with bone
41 © 2017 Entellus Medical.
All rights reserved.
entellus MEDICAL
|
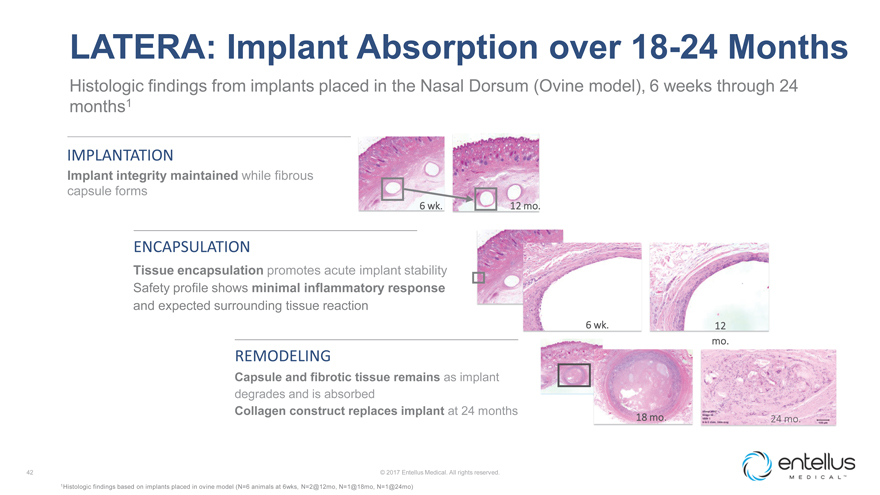
LATERA: Implant Absorption over 18-24 Months
Histologic findings from implants placed in the Nasal Dorsum (Ovine model), 6 weeks through 24 months1
12 mo.
6 wk.
Tissue encapsulation promotes acute implant stability
Safety profile shows minimal
inflammatory responseand expected surrounding tissue reaction
ENCAPSULATION
6
wk.
Implant integrity maintainedwhile fibrous capsule forms
IMPLANTATION
12 mo.
REMODELING
18 mo.
24 mo.
Capsule and fibrotic tissue remainsas implant degrades and is absorbed
Collagen construct
replaces implant at 24 months
1Histologic findings based on implants placed in ovine model (N=6 animals at 6wks, N=2@12mo, N=1@18mo, N=1@24mo)© 2017 Entellus
Medical. All rights reserved. 42
entellus MEDICAL
