Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - ALBIREO PHARMA, INC. | d401605dex993.htm |
| EX-99.1 - EX-99.1 - ALBIREO PHARMA, INC. | d401605dex991.htm |
| 8-K - FORM 8-K - ALBIREO PHARMA, INC. | d401605d8k.htm |
Exhibit 99.2
PROSPECTUS SUPPLEMENT SUMMARY
Overview
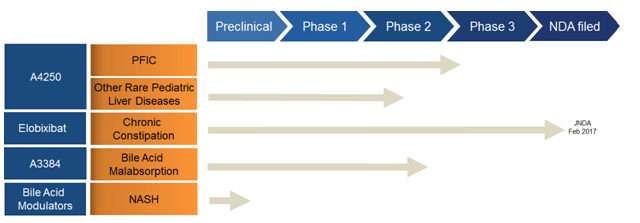
We are a biopharmaceutical company focused on the development and commercialization of novel bile acid modulators to treat orphan pediatric liver diseases and gastrointestinal, or GI, disorders where improper flow or absorption of bile causes serious medical conditions for which there is high unmet need. The initial target indication for our lead product candidate, A4250, is progressive familial intrahepatic cholestasis, or PFIC, a rare, life-threatening genetic disorder affecting young children for which there is currently no approved drug treatment. We have evaluated A4250 in a Phase 2 clinical trial in children with chronic cholestasis that is intended to support a planned Phase 3 clinical trial in patients with PFIC. In addition to PFIC and subject to obtaining additional capital, we plan to conduct future clinical development of A4250 as a treatment for one or more other pediatric cholestatic liver diseases and disorders and we expect to initiate a clinical trial in another such disease or disorder in 2018. Our clinical-stage product candidates in addition to A4250 include elobixibat, for which our licensee has filed a new drug application for approval in Japan to treat chronic constipation, and A3384, which is in development to treat bile acid malabsorption, or BAM. We also have a preclinical program in nonalcoholic steatohepatitis, or NASH.
Bile acids are a component of bile, a key digestive liquid made in the liver, that play a critical role in dietary absorption and the regulation of metabolic processes. Specifically, bile acids are recycled from the small intestine to the liver as part of a process known as enterohepatic circulation. When the flow of bile from the liver stops or is disrupted, a condition known as cholestasis, bile acids accumulate in the liver and in the serum, which is a component of blood. Elevated bile acids in the liver and serum often leads to severe liver damage and other consequences, including itching, or pruritus. A4250 partially inhibits a protein known as ileal sodium dependent bile acid transporter, or IBAT, which is responsible for initiating the recirculation of bile acids from the small intestine to the liver. As an IBAT inhibitor, A4250 acts to reduce bile acids in the liver and serum and to increase bile acids being excreted through the colon. Elobixibat is also an IBAT inhibitor.
A4250 — our lead product candidate for PFIC and potentially other orphan pediatric liver diseases. A4250 is a novel, minimally absorbed, orally administered IBAT inhibitor. Our initial target indication for A4250 is PFIC. We have evaluated A4250 in an open label, dose finding Phase 2 clinical trial in children with chronic cholestasis that is intended to support a planned Phase 3 clinical trial in patients with PFIC. The Phase 2 trial included children with chronic cholestasis caused by any of a number of different liver conditions, including PFIC, biliary atresia, Alagille syndrome, or ALGS, and sclerosing cholangitis. Because it was an open label study, preliminary data are available as of the date of this prospectus supplement. These data showed a reduction in serum bile acids in a substantial majority of patients and improvement in pruritus that was significantly correlated with the reduction in serum bile acids. In addition, A4250 was generally well tolerated in the trial.
● PFIC. We plan to conduct a Phase 3 clinical trial of A4250 in patients with PFIC, as well as an open label extension study to assess long-term safety and durability of response. We expect the PFIC trial to commence in the second half of 2017. We have chosen PFIC as the lead indication for A4250 because we believe there is an especially strong scientific rationale for the use of an IBAT inhibitor to prevent progressive liver disease caused by PFIC. Our planned Phase 3 PFIC trial, which we expect to commence in the second half of 2017, is designed to be a randomized, double blind, placebo controlled, multicenter, clinical trial designed to enroll 60 patients, with a treatment duration of six months. The primary endpoint for U.S. Food and Drug Administration, or FDA, evaluation, and a key secondary endpoint for European Medicines Agency, or EMA, evaluation, will be an assessment of change in pruritus. The primary endpoint for EMA evaluation, and a key secondary endpoint for FDA evaluation, will be serum bile acid responder rate, where a responder is a patient who achieves a predetermined reduction in serum bile acid levels. The trial will also have several additional secondary endpoints. The planned double blind Phase 3 trial, together with available data from the then-ongoing extension study, are expected to form the primary support for drug approval applications for A4250 in both the United States and European Union for the treatment of patients with PFIC.
The precise prevalence of PFIC is unknown, but PFIC has been estimated to affect between one in every 50,000 to 100,000 children born worldwide. Based on the estimated incidence and need for liver transplant, published birth rates, and estimates of the effect of pediatric liver transplant on life expectancy, we estimate the
1
prevalence of PFIC to be approximately 10,000 patients in major pharmaceutical markets, including approximately 3,200 in the United States and 5,000 in the European Union. There are currently no drugs approved for the treatment of PFIC. First-line treatment for PFIC is typically off-label ursodeoxycholic acid, or UDCA, which is approved in the United States and elsewhere for the treatment of primary biliary cholangitis, or PBC. However, many PFIC patients do not respond well to UDCA, undergo partial external bile diversion, or PEBD, surgery and often require liver transplantation. PEBD surgery is a life-altering and undesirable procedure in which bile is drained outside the body to a stoma bag that must be worn by the patient 24 hours a day. Of all PFIC patients, we believe A4250 will primarily benefit those who have not yet undergone PEBD surgery or liver transplant, as well as those who have had or may have surgery to reverse the PEBD procedure. Accordingly, we estimate the addressable PFIC patient population for A4250 to be approximately 1,200 patients in the United States and approximately 1,900 patients in the European Union.
● Other Indications Under Consideration. We intend to conduct future clinical development of A4250 as a treatment for other pediatric cholestatic liver diseases and disorders in addition to PFIC. These indications may include one or more of ALGS, biliary atresia, sclerosing cholangitis and pediatric cholestatic pruritus.
ALGS is a genetic condition associated with liver, heart, eye and skeletal abnormalities. In particular, ALGS patients have fewer than normal bile ducts inside the liver, which leads to cholestasis and the accumulation of bile and causes scarring in the liver. The prevalence of ALGS has been estimated to be one in 70,000 newborns. There are currently no drugs approved for the treatment of ALGS. Current treatment for ALGS is generally in line with current treatments for PFIC as described above.
Biliary atresia is a partial or total blocking or absence of large bile ducts that causes cholestasis and resulting accumulation of bile that damages the liver. The estimated worldwide incidence of biliary atresia is 18.5 per 100,000 births. There are currently no drugs approved for the treatment of biliary atresia. The current standard of care is a surgery known as the Kasai procedure, or hepatoportoenterostomy, in which the obstructed bile ducts are removed and a section of the small intestine is connected to the liver directly. However, only an estimated 25% of those initially undergoing the Kasai procedure will survive to their twenties without need for liver transplantation.
Sclerosing cholangitis refers to swelling (inflammation), scarring, and destruction of bile ducts inside and outside of the liver. The first symptoms are typically fatigue, itching and jaundice, and many patients with sclerosing cholangitis also suffer from irritable bowel syndrome with diarrhea. The estimated incidence of sclerosing cholangitis is 6.3 cases per 100,000 people. There are currently no drugs approved for the treatment of sclerosing cholangitis. First-line treatment is typically off-label UDCA, although UDCA has not been established to be safe and effective in patients with sclerosing cholangitis in well controlled clinical trials.
Pediatric cholestatic pruritus refers to pruritus symptoms in children suffering from any disease or condition characterized by chronic cholestasis. Severe pruritus is a debilitating symptom afflicting cholestatic patients and, although pruritus cannot reliably be relieved by scratching, patients often resort to destructive scratching behaviors that can cause bleeding and scarring. Cholestatic liver disease has been estimated to affect approximately one in 2,500 newborns worldwide, and, based on reported rates of pruritus across several different causes of pediatric cholestasis, we estimate pruritus to affect approximately 45% of all children with cholestatic liver disease. There are currently no drugs approved specifically for the treatment of pediatric cholestatic pruritus.
Other Clinical-Stage Product Candidates — Elobixibat and A3384.
● Elobixibat. Elobixibat is another novel, minimally absorbed, orally administered IBAT inhibitor. We have granted commercial rights to elobixibat for the treatment of chronic constipation and other GI diseases in Japan and other select markets in Asia to EA Pharma Co., Ltd. (formerly known as Ajinomoto Pharmaceuticals Co., Ltd.), or EA Pharma, a company formed by a business combination between Ajinomoto Pharmaceuticals and the GI treatment business of Eisai Co., Ltd. Following positive results from a Phase 3 clinical trial conducted by EA Pharma in Japan, we announced in February 2017 that EA Pharma submitted a new drug application to the Japanese Ministry of Health, Labour and Welfare for elobixibat for the treatment of chronic constipation. We expect a decision in the first half of 2018 as to whether EA Pharma’s new drug application will be approved. If elobixibat receives marketing approval in Japan, EA Pharma plans to co-market elobixibat in Japan with another company, Mochida Pharmaceutical Co., Ltd.
2
We have commercial rights to elobixibat in the United States, Europe, China and otherwise outside of the territories licensed to EA Pharma. We are currently evaluating whether we will seek a license or other partnering transaction with a third party for elobixibat in the United States or Europe. Whether or not we elect to seek such a transaction, we do not currently anticipate that we will conduct future clinical trials of elobixibat independently.
● A3384. A3384 is a proprietary formulation of cholestyramine that is designed to release cholestyramine directly in the colon. Subject to obtaining additional capital, we plan to initiate a clinical trial of A3384 as a treatment for BAM in 2018. BAM, which is sometimes also called bile acid diarrhea, occurs when bile acids are not sufficiently reabsorbed in the small intestine, causing elevated levels of bile acids to instead reach the colon and leading to chronic watery diarrhea. Based on a reported estimate of the prevalence of irritable bowel syndrome with diarrhea, or IBS-D, and published third-party studies that suggest approximately one-third of IBS-D patients have BAM, we estimate the prevalence of BAM to be approximately 1.3 million people in the United States and approximately 2.2 million people in the European Union. There are currently no drugs approved for the treatment of BAM.
Cholestyramine, which is approved in some countries in Europe to treat diarrhea associated with certain GI conditions, is commonly prescribed off-label to treat BAM. Cholestyramine is a well characterized bile acid sequestrant, which is also known as a resin. The benefit of cholestyramine has historically been limited both because of poor tolerability and because of its negative effect on absorption of other medications and important fat soluble vitamins. We believe that a better tolerated formulation that is capable of delaying the activity of cholestyramine until it reaches the colon has potential to provide therapeutic benefit for patients with BAM.
In addition to our clinical-stage product candidates, we have a preclinical program in NASH, as well as preclinical compounds that include one or more peroxisome proliferator-activated receptors, or PPAR, agonists and neurokinin, or NK, antagonists.
Our Pipeline
The following table summarizes our most advanced product candidates and programs:
Our Strategy
Our goal is to be a leader in the development and commercialization of novel therapeutics for orphan pediatric cholestatic liver diseases and disorders where there is high unmet medical need, while also leveraging our expertise in bile acid modulation to treat other liver and GI diseases and disorders. To achieve our goal, we intend to pursue the following strategies.
● Rapidly develop A4250 to marketing approval to treat patients with PFIC. It is our objective to conduct a single Phase 3 clinical trial in patients with PFIC that, together with available data from a then-ongoing long-term, open label extension study, forms the primary support for applications for marketing approval of A4250 in both the United States and European Union.
3
● Maximize the benefit and commercial potential of A4250 by expanding development to additional orphan pediatric cholestatic indications. Although we have chosen PFIC as our lead indication for A4250, we also believe A4250 can benefit children suffering from other cholestatic diseases and disorders. We intend to conduct future clinical development of A4250 as a treatment for one or more of these other pediatric cholestatic liver diseases and disorders in addition to PFIC to help address these unmet medical needs and to maximize the commercial potential of A4250.
● Develop the capability to commercialize A4250 to treat orphan pediatric liver diseases, if approved, through a targeted sales force in the United States and Europe and collaborate selectively to commercialize A4250 outside of these regions. If we receive marketing approval in the United States or Europe for A4250 to treat patients with PFIC or any other pediatric cholestatic liver disease or disorder, we plan to build the capabilities to effectively commercialize A4250 in the approved indication(s) in the applicable region. We believe that the required commercial organization would be modest in size and targeted to the relatively small number of specialists in the United States and Europe who treat children with cholestatic liver disease. If we receive marketing approval outside of the United States and Europe for A4250 to treat patients with PFIC or any other pediatric cholestatic liver disease or disorder, we plan to selectively utilize collaboration, distribution and other marketing arrangements with third parties to commercialize A4250 in the approved indication(s) in the regions outside the United States or Europe where we receive approval.
● Collaborate selectively to develop and commercialize product candidates targeting nonorphan indications, potentially including elobixibat, A3384 or any future product candidate to treat NASH. We intend to selectively seek alliances and collaborations to assist us in furthering the development or commercialization of product candidates targeting large primary care markets that must be served by large sales and marketing organizations. These product candidates may include any or all of A3384, any potential future product candidate that arises from our preclinical program in NASH, and elobixibat.
Risks Relating to Our Business
We are a biopharmaceutical company, and our business and ability to execute our business strategy are subject to a number of significant risks of which you should be aware before you decide to buy shares of our common stock. Among these important risks are the following:
| • | We have incurred significant losses since our inception. We expect to continue to incur losses and may never generate profits from operations or maintain profitability. |
| • | Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability. |
| • | We will need substantial additional funding and, in particular, may not have sufficient cash to fund our planned Phase 3 clinical trial of A4250 in patients with PFIC to completion. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts. |
| • | We depend heavily on the success of our lead product candidate, A4250, which we are developing initially for the treatment of patients with PFIC and potentially also for other pediatric cholestatic liver diseases and disorders. We also depend on the success in Japan of our product candidate elobixibat, which our licensee EA Pharma is developing in Japan for the treatment of chronic constipation. If we are unable to commercialize A4250 or experience significant delays in doing so, or if EA Pharma is unable to commercialize elobixibat in Japan or experiences significant delays in doing so, our business will be materially harmed. |
| • | If clinical trials of A4250 or any of our other product candidates fail to demonstrate safety and efficacy to the satisfaction of the FDA or the EMA, or do not otherwise produce favorable results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of the applicable product candidate. |
4
| • | Favorable results seen to date in clinical trials of A4250, including our open label Phase 2 trial of A4250 in patients with cholestatic liver disease, may not be predictive of favorable results in our planned Phase 3 clinical trial of A4250 in patients with PFIC, which is expected to be placebo controlled and involve different doses, treatment duration, number of patients and outcome measures and may have other differences in design or execution. |
| • | We plan to use, for purposes of FDA evaluation, change in pruritus as the primary endpoint in our planned Phase 3 trial in PFIC patients. Because the assessment of pruritus relies on subjective patient or caregiver feedback, it is challenging to evaluate and measure consistently and, for any patient, a self-reported outcome may vary from a caregiver-reported outcome. |
| • | The clinical trial designs, durations, endpoints and outcomes that will ultimately be required to obtain marketing approval of A4250 to treat PFIC patients are uncertain and, in any case, may vary among the FDA, EMA and other regulatory authorities outside of the United States and European Union. Based on feedback that we have received from the FDA and the EMA, we expect both regulatory authorities to place a greater emphasis on the totality of the data from our planned Phase 3 clinical trial, including secondary endpoints, than may generally be expected. As a result, there is risk that, even if the primary endpoint of our planned Phase 3 clinical trial of A4250 for FDA evaluation purposes or for EMA evaluation purposes is met with statistical significance, the applicable regulatory authority may not find the overall results of our planned Phase 3 trial to be sufficient to support marketing approval of A4250 to treat PFIC, a symptom of PFIC such as pruritus or any other indication, and we may never receive marketing approval. Similar risks also apply for A3384, which we are developing as a treatment for BAM. |
| • | The design of our planned Phase 3 clinical trial of A4250 in patients with PFIC may not conform precisely in all respects to the recommendations or preferences expressed by either the FDA or EMA. |
| • | If we experience delays or difficulties in the enrollment of patients in our planned Phase 3 clinical trial of A4250 in patients with PFIC, our receipt of marketing approval for A4250 could be delayed or prevented. |
| • | If the commercial opportunity in PFIC is smaller than we anticipate, or if A4250 receives approval to treat only a specific subpopulation of patients with PFIC or only a specific symptom of PFIC such as pruritus, our future revenue from A4250 may be adversely affected and our business may suffer. |
| • | If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell A4250 or any of our other current or potential future product candidates, we may not be successful in commercializing the applicable product candidate if it receives marketing approval. |
| • | We face substantial competition, which may result in others discovering, developing or commercializing products to treat our target indications or markets before or more successfully than we do. |
| • | We rely on EA Pharma for the successful development and commercialization of elobixibat to treat chronic constipation in Japan and other select markets in Asia. |
| • | We rely on third parties to conduct our clinical trials and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such clinical trials. |
| • | Use of third parties to manufacture our product candidates may increase the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts. |
| • | If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be adversely affected. |
5
| • | Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business. |
| • | Even if we complete the necessary clinical trials, the marketing approval process is expensive, time consuming and uncertain and may prevent us from obtaining approvals for the commercialization of some or all of our product candidates. If we are not able to obtain, or if there are delays in obtaining, required marketing approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired. |
For additional information about the risks we face, please see the information contained in or incorporated by reference under “Risk Factors” in this prospectus supplement and the accompanying prospectus.
Corporate Information
Prior to November 3, 2016, we were a specialty biopharmaceutical company known as Biodel Inc. that historically had been focused on the development and commercialization of innovative treatments for diabetes. Biodel was originally incorporated in the State of Delaware in December 2003 under the name “Global Positioning Group, Ltd.” and subsequently changed its name to “Biodel Inc.” Albireo Limited was formed in connection with a spinout transaction from AstraZeneca AB in 2008 in which AstraZeneca assigned to Albireo AB all of its rights in and to its portfolio of IBAT inhibitors, including elobixibat and A4250, as well as other programs that are currently at a preclinical stage.
On November 3, 2016, we completed a share exchange transaction, or the Transaction, pursuant to an Amended and Restated Share Exchange Agreement dated July 13, 2016 that we entered into with Albireo Limited and the shareholders and noteholders of Albireo Limited. In the Transaction, each holder of Albireo Limited shares or notes convertible into Albireo Limited shares sold their shares of Albireo Limited for newly issued shares of our common stock. As a result, Albireo Limited became a wholly owned subsidiary of Biodel, Biodel’s corporate name was changed to Albireo Pharma, Inc., the business of Albireo Limited became our business and we became a biopharmaceutical company focused on the development and commercialization of novel bile acid modulators to treat orphan pediatric liver diseases and gastrointestinal disorders.
Our corporate headquarters are located at 10 Post Office Square, Suite 502 South, Boston, Massachusetts 02109 and our telephone number is (857) 254-5555. We also have an office in Gothenburg, Sweden. We maintain a website at www.albireopharma.com, to which we regularly post copies of our press releases as well as additional information about us. The information contained on, or that can be accessed through, our website is not a part of this prospectus supplement or the accompanying prospectus. We have included our website address in this prospectus supplement solely as an inactive textual reference.
Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available free of charge through the investor relations page of our internet website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
All brand names or trademarks appearing in this prospectus supplement and the accompanying prospectus are the property of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this prospectus supplement and the accompanying prospectus is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
6
