Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Asterias Biotherapeutics, Inc. | form8k.htm |

Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE Market: ASTMay 2017 14219863Text 04698Text 230237246Text

Statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ Registration Statement on Form S-3 and Prospectus, as well as its other periodic reports, filed with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements.Asterias has filed with the Securities and Exchange Commission ("SEC") a registration statement (including a prospectus) and has filed or will file with the SEC a prospectus supplement to the prospectus for the offering to which this presentation relates. Before you invest, you should read the prospectus supplement and the accompanying prospectus in that registration statement and the documents incorporated by reference or filed as exhibits to the registration statement for more complete information about Asterias and this offering. You may get these documents and other documents for free by visiting EDGAR on the SEC web site www.sec.gov. Forward-Looking Statements

Disclaimers on Patient Stories AST-OPC1 is a cell-based therapy still under development and the Food and Drug Administration (“FDA”) has not evaluated or approved AST-OPC1 for commercial sale for any use. The company is in the early stages of testing AST-OPC1 in a Phase 1/2a clinical research study for patients with severe cervical spinal cord injuries. The clinical trial process is complex, expensive, and takes time. Approval by FDA of AST-OPC1 for commercial use may take years, require additional studies, and may not occur at all.While the initial results of the patients described in this presentation have been encouraging, results for patients treated with AST-OPC1 will vary by patient. Also, the long-term safety and efficacy results for patients treated with AST-OPC1, including those patients described in this presentation, are still unknown. Future studies are likely necessary to distinguish efficacy due to a patient receiving AST-OPC1 cells from any natural recovery which may occur in the patient over time following the patient’s traumatic injury.Not all patients with spinal cord injuries are eligible to participate in the current study as criteria such as type and timing of injury apply. Additional information on the existing trial, including trial sites and eligibility criteria, can be found at www.clinicaltrials.gov or at www.scistar-study.com.

Strong Leadership Team with Proven Track Record Name Experience Steve CarttPresident and CEO Former COO of Questcor PharmaceuticalsLed Questcor’s 2007-2014 commercial strategy, turnaround and multiple expansionsGrew company revenue from $10 million to $1 billionOver 30 years of experience in pharma and biotech; at Questcor, Elan, ALZA Pharmaceuticals and ALZA Cor Don BaileyChairman of the Board Former CEO of Questcor Pharmaceuticals, led Questcor’s corporate turnaround Jane Lebkowski, Ph.D.President R&D and CSO Former CSO of GeronOver 30 years experience in R&D of cell and gene therapies at Geron, Applied Immune Sciences, and Rhône Poulenc Rorer Katy Spink, Ph.D.COO Former SVP, Cell Therapy Program Operations at GeronPrior to Geron, Dr. Spink was a management consultant at McKinsey Ed Wirth, M.D., Ph.D.CMO Former CSO of InVivo Therapeutics25 years experience translational research at the University of Chicago, Geron and InVivo Ryan ChavezCFO, EVP Finance & GC Former General Counsel for Mallinckrodt ARD divisionAssociate General Counsel at Questcor; financial positions at GE During Mr. Cartt’s and Mr. Bailey’s seven year tenure, Questor’s stock price went from $0.35/share in August 2007 to the acquisition price of $93.58/share in 2014During this period, Questcor’s valuation increased over 200x, from $25 million to the company’s acquisition by Mallinckrodt for $5.8 billion 14219863Text 04698Text 230237246Text

Two Technology Platforms Foundation for Clinical Programs Pluripotent Stem CellsPluripotentCan derive any cell type for each indicationIP and Know-HowImmortal SourceConsistency: Can support entire product life cycle from one cell sourceScalable Manufacturing: One cell source can support entire life cycle of a product Cancer ImmunotherapyTrains patient’s immune system to attack cancer cells by targeting cells that express telomeraseIP and Know-HowTelomerase plays critical role in cancer cells but is not expressed on non-cancer cells; implicated in >95% of cancers OPC1Program VAC2Program VAC1Program

PROGRAM PRECLIN PHASE 1 PHASE 2 PHASE 3 Partners/Funding AST-OPC1*Spinal Cord Injury (subacute) AST-VAC1**Leukemia (AML) Autologous AST-VAC2**Lung Cancer Allogeneic * Potential for application in MS, stroke, Alzheimer’s, ALS** Potential in multiple cancer types/stages as well as in combination with other cancer therapies Phase 1/2a in progress; positive early efficacy data Positive phase 2 dataProcess development in progress Phase 1/2a to be initiated in 2017 Clinical Programs – Development Pipeline 14219863Text 04698Text 230237246Text

AST-OPC1 14219863Text 04698Text 230237246Text
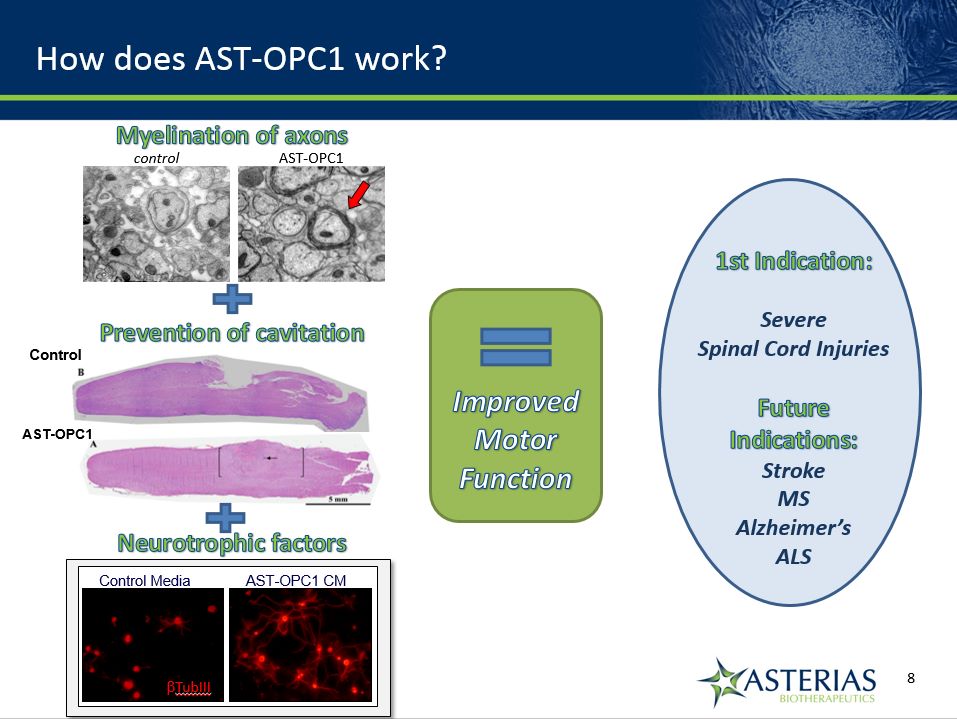
How does AST-OPC1 work? Improved Motor Function 1st Indication:Severe Spinal Cord InjuriesFuture Indications:StrokeMSAlzheimer’sALS Prevention of cavitation Neurotrophic factors Control AST-OPC1 Promote increased neurite outgrowth Control Media AST-OPC1 CM TubIII control AST-OPC1 Myelination of axons

SCI: Significant opportunity exists in helping restore hand/arm/finger motor function and reducing healthcare burden 17,000 new SCI cases in the US annually(1)Primarily affects young/healthy males in their 20s and 30s No currently approved therapiesLifetime direct healthcare costs for a 25 year old patient can reach $5MVery high unemployment rate >4,000 under first label C4-C7 ASIA A/B/C 2016 NSCIC SCI Facts and Figures at a GlanceEstimates by indication calculated based on 2014 NSCIC survey data, adjusted to exclude penetrating and secondary medical injuriesBased on typical pricing for new therapeutics addressing devastating orphan diseases/disorders 5,000 additional patients in remaining cervical and thoracic injuries >$1B >$1B Potential market(3) Target patient estimates(2) ______>$2B

SCi-Star Study in Complete Cervical Spinal Cord Injury (SCI) Dosing complete Currently enrolling Future enrollment Cohort 1 – 2 million3 subjects AIS-ACohorts Cohort 2 – 10 million6 subjects Cohort 3 – 20 million5-8 subjects AIS-BCohorts Cohort 4 – 10 million5-8 subjects Cohort 5 – 20 million5-8 subjects Enrollment completed in August 2015 Enrollment completed in August 2016Six month readout Q1 201712 month readout expected Q3 2017 Enrollment expected to be completed 1H 20176 month data expected Q4 2017 Enrollment expected to be completed 1H 20176 month data expected Q4 2017 Enrollment expected to be completed in 2H 20176 month data expected in 1H 2018 Enrollment Progress/Anticipated Completion
Distinguishing Features of Asterias’ SCI Program I personally have been involved in spinal injury research, transplantation, for 20 years. For the first time ever, we are seeing real, positive results.... We are seeing, we believe, significant improvement.Richard Fessler, MD, PhDProfessor of Neurosurgery, Rush University Medical Center

Major exam used to classify spinal cord injuriesMeasures functional improvement in hands/arms/fingers using upper extremity motor scores (UEMS) and motor levelImprovement in upper extremity motor function translates into improvements in ability to self-care and reduce cost of care ISNCSCI Exam

6 9 12 3 0 6 10 12 4 8 14 2 0 13 Cohort 2 Experienced Greater UEMS Recovery than Matched Historical Control Group Change in UEMS from baseline over time Matched historical control (n=68) 1 Cohort 2 – 10 million (n=6)2 Initial Evidence of a Dose Dependent EffectCohort 1 shows similar UEMS recovery to spontaneous improvement in historical controls, demonstrating safetyMeaningful improvements over historical controls in Cohort 2 1 n=62 at 6 and 12 months2 n=3 at 9 months Error bars at 1 Standard Error Time post baselineMonths Cohort 1 – 2 million (n=3)
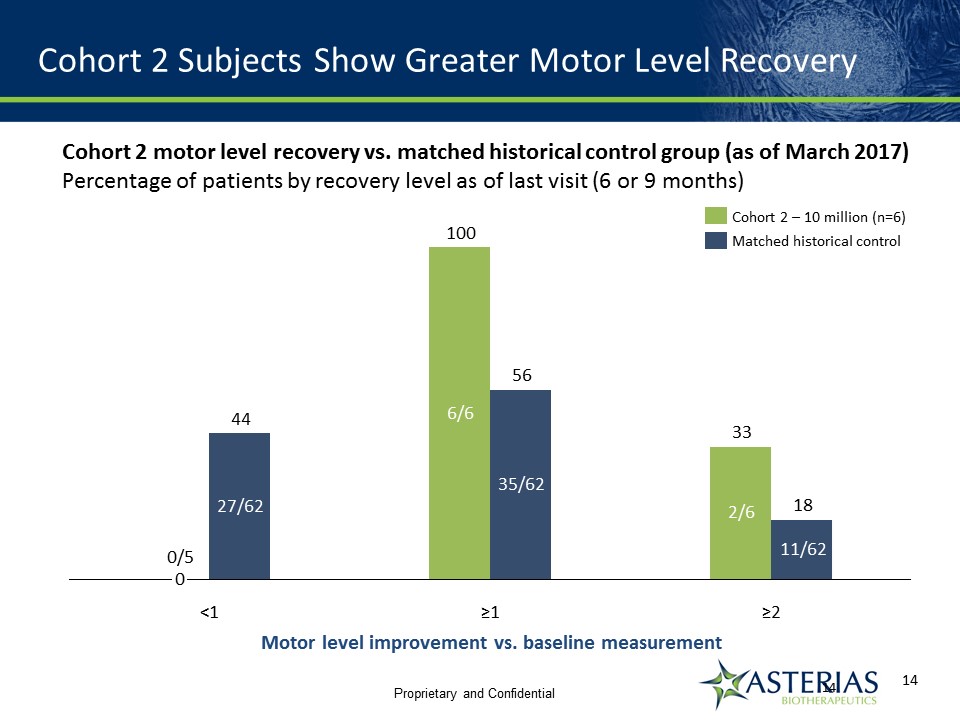
Cohort 2 Subjects Show Greater Motor Level Recovery 18 33 56 ≥1 ≥2 <1 35/62 11/62 2/6 27/62 6/6 0 100 44 0/5 Cohort 2 – 10 million (n=6) Matched historical control Motor level improvement vs. baseline measurement Cohort 2 motor level recovery vs. matched historical control group (as of March 2017)Percentage of patients by recovery level as of last visit (6 or 9 months) 14 Proprietary and Confidential

AST-OPC1 Therapy is Designed to Increase Patient Motor Function and Improve Quality of Life and Ability to Live Independently Capability C1-C3 C4 C5 C6 C7-C8 Bowel Bladder Bed Mobility Transfers Pressure Relief Eating Dressing Grooming Bathing Wheelchair Car Transport Daily Home Care 24 hr Attendant 18-24 hr Attendant 6-12 hr Assistance 4 hr Housework 1 hr Housework Total Assist Partial Assist Independent Steeves et al., Top Spinal Cord Inj Rehabil 2012; 18(1): 1-14 14219863Text 04698Text 230237246Text Lifetime direct healthcare costs for a 25 year old patient can reach $5 millionVery high unemployment rate; 63% of cervical injury patients are unemployed 8 years post-injuryMotor level improvements translate into clinically significant improvements in ability to self-care and significant reductions in cost of care

Patient Stories (Video Available Upon Request) Lucas can now write with a pen, type e-mails at 30-plus words per minute, use his phone, and feed himself. Kris can now use his hands and fingers to feed himself, drink, text, send e-mails, sign his name and even play video games. To view the video, go to https://www.youtube.com/watch?v=iZ2ycm21CwA

VAC Programs 14219863Text 04698Text 230237246Text
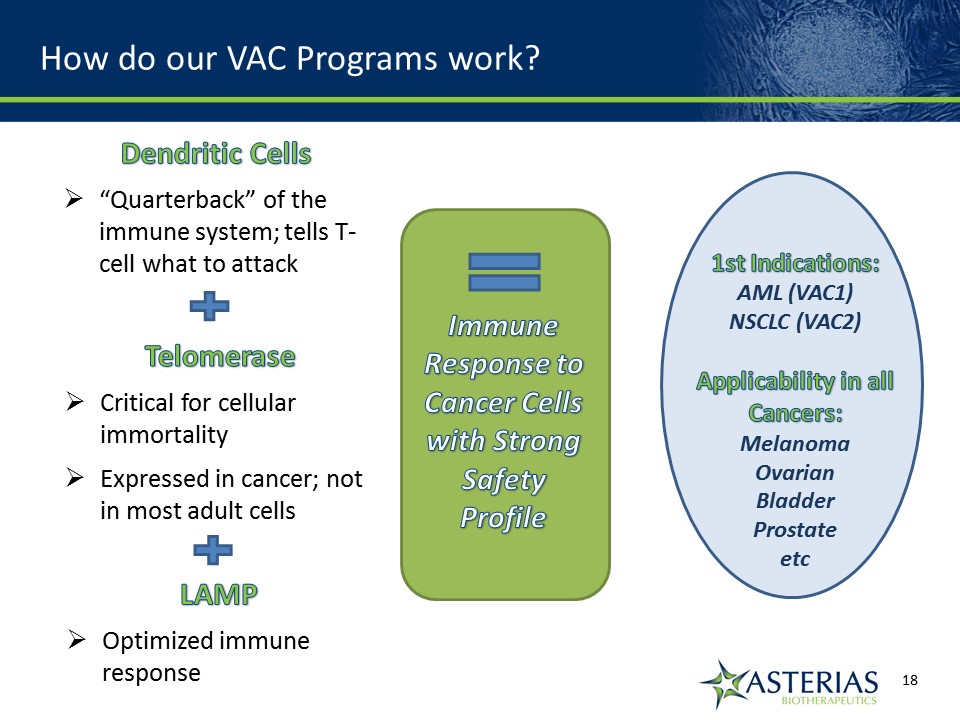
How do our VAC Programs work? Immune Response to Cancer Cellswith Strong Safety Profile Dendritic Cells“Quarterback” of the immune system; tells T-cell what to attack TelomeraseCritical for cellular immortalityExpressed in cancer; not in most adult cells LAMPOptimized immune response 1st Indications:AML (VAC1)NSCLC (VAC2)Applicability in all Cancers:MelanomaOvarianBladderProstateetc

AST-VAC1 and AST-VAC2 Robust safety in 40 patients treatedProlonged Remissions in Phase 2 AML studyProcess Development UnderwayPotential Partnering Opportunity POC confirmed by AST-VAC1 resultsScalable, off-the-shelf allogeneic manufacturing Validated by Cancer Research UK (CRUK) partnership– non-dilutive funding of full cost of trial (estimated $25-$30M savings)Non small cell lung cancer (NSCLC) trial to commence in 2017 AST-VAC1Autologous AST-VAC2Allogeneic

Upcoming Company Milestones 14219863Text 04698Text 230237246Text 6 month data Cohort 2Complete enrollment in Cohorts 3&4 12 month data Cohort 26 month data Cohort 36 month data Cohort 4Complete trial enrollment 12 month data Cohort 312 month data Cohort 46 month data Cohort 5 Topline efficacy data, all cohorts OPC1 VAC Complete VAC1 PDMHRA clearance for VAC2 trial FPI in VAC2 trial VAC2 interim data VAC2 interim data

AST-OPC1Ongoing SCiSTAR Phase 1/2a trial in spinal cord injuryPositive initial efficacy readoutsData readouts throughout 2017 and full enrollment of study expected later this yearAST-VAC1 Ongoing process development effort underwayPhase 2b/3 ready asset following completion of process development activitiesAST-VAC2Phase 1/2a trial in non-small cell lung cancer (NSCLC) to commence in 2017First data readouts expected in 2018CorporateAs of March 31, 2017, Asterias has an estimated $32M in cash and available-for-sale securities to fund operations Summary
