Exhibit 99.1


This presentation includes forward-looking statements that are subject to many risks and uncertainties. These forward-looking statements, such as statements about Nemaura’s short-term and long-term growth strategies, can sometimes be identified by use of terms such as “intend,” “expect,” “plan,” “estimate,” “future,” “strive,” and similar words. These statements involve many risks and uncertainties that may cause actual results to differ from what may be expressed or implied in these statements. These risks are discussed in Nemaura’s filings with the Securities and Exchange Commission (the “Commission”), including the risks identified under the section captioned “Risk Factors” in Nemaura’sQuarterly Report on Form 10-K filed with the Commission on February 09, 2017 and in Nemaura’s Registration Statement on Form S-3 filed with the Commission on March 18, 2016. Nemaura disclaims any obligation to update information contained in these forward-looking statements whether as a result of new information, future events, or otherwise.

Nemaura Medical is a UK-based innovator of unique diagnostic medical devices that have evolved out of our core area of expertise in transdermal drug delivery.
We believe empowering patients to easily monitor key analytes via our proprietary BEAT™ technology will result in better management of multiple chronic health conditions.
We have engineered a highly sensitive skin patch that utilizes state-of-the-art sensors capable of continuously reading any analyte or prescription medicine present in interstitial fluid. Our products are differentiated by being:
Non-Invasive
Needle-Free (replacing finger-stick technology)
Pain-Free
Daily-Disposable
Cost-Effective
Continuously Monitoring (every 5 mins)
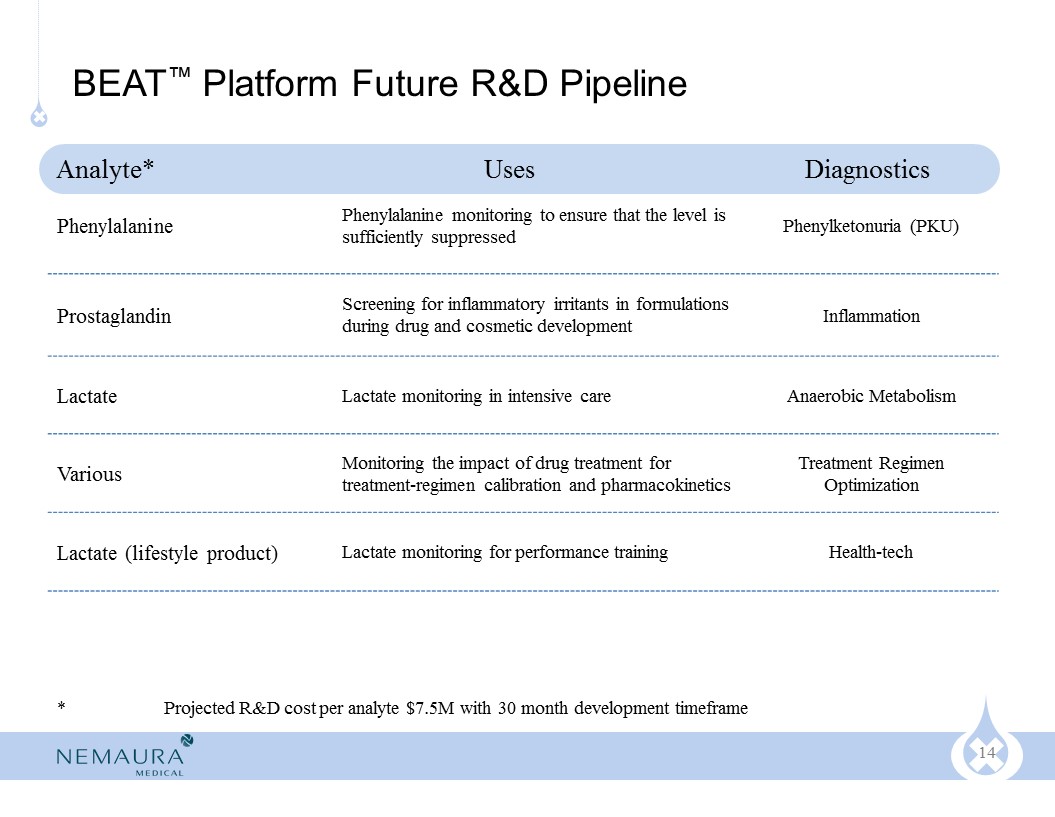
Our pipeline of applications include:
Continuous Glucose Monitoring (CGM) for diabetics
Monitoring new drugs during development for potential inflammatory irritants (prostaglandin)
Amino Acid monitoring for patients with PKU (phenylketonuria)
Oxygen level monitoring for patients in intensive care (oxygen depletion)
Prescription Therapeutic Drug Monitoring (TDM) for numerous molecules
Glucose trending device suitable for pre-diabetic / health-tech market
Lactate trending device for athletic / health-tech market


The BEAT™ patch extracts glucose from below the skin to the surface on to a patch containing a sensor.
The Bluetooth-enabled sensor measures and transmits glucose data to a proprietary application pre-downloaded on user's own smartwatch / smartphone
A proprietary algorithm within the application converts this data to a concentration value
This value is displayed on the smartwatch /smartphone with and can be forwarded on to cloud based health care team
The patch construction and the algorithm that processes the signal to determine glucose levels are our proprietary patented/patent pending technology

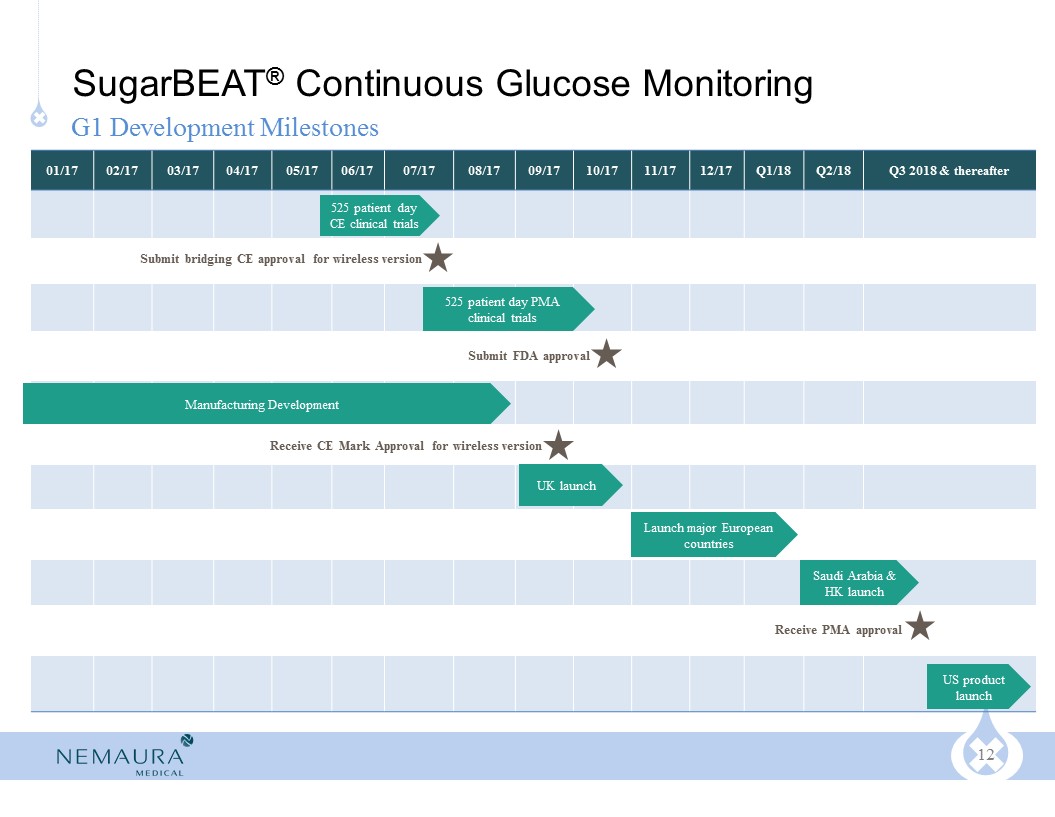
SugarBEAT® G1 commercial launch expected Q3 2017 in UK
CE Mark granted Q1 2016 on predecessor version (watch format)
Bridging CE approval required for wireless version as adjunct device, with CE submission due in Q3 2017 and PMA submission in Q4 2017
Pre-downloaded App on Smartphone, Tablet,
Smartwatch
Providing full color, high-resolution images of the data, rapidly converted using an algorithm, and displayed in various formats including tabular and graphical, with user ability to set alarms for high and low readings
Daily-disposable adhesive skin-patch integrated with Bluetooth-enabled sensor
SugarBEAT G1 applied to the skin, containing (i) reusable Bluetooth-enabled sensor, (ii) disposable battery and (iii) disposable skin-patch.
Combined approximate size 4cm x 3cm x 9mm

vApproximate 30 minutes warm-up period
vSensor measures glucose within extracted interstitial fluid
vSensor records a reading every 5 minutes and transmits via Bluetooth to user’s smart device
vEach disposable skin-patch lasts up to 24 hours
vFinger prick calibration required whenever new patch is affixed
vShelf-life for reusable component expected to be 2 years
SugarBEAT® G1 skin-patch and sensor successfully tested in-house
SugarBEAT® G1 App successfully tested in-house (Medical Device Grade)
SugarBEAT® G1 sensor performance improved, with almost 2 order of magnitude enhancement to detection limit
Additional patent filed in Jan 2017 to cover new sensor features
Improved sensor expected to lead to better MARD in upcoming clinical trials on SugarBEAT® G1, due to commence Q2 2017

Dr. Dewan Fazlul Hoque Chowdhury
Chief Executive Officer
Over 18 years' experience in the pharmaceutical and medical device industry and holder of over 50 patents across more than 15 patent families
2009-Present: President, Chief Executive Officer and Board Director in charge of research and development of core technologies, product development, innovation and commercialization; coordinates and oversees legal compliance; development of the company mission; policy and planning
2005- 2009: founder and CEO of Microneedle Technologies and Nemaura Pharma Limited; developed and launched a microneedle device used in skin clinics; responsible for negotiating licensing deals for a transdermal patch to treat Alzheimer's disease currently under FDA review
MSc in Microsystem and nanotechnology, from Cranfield University and PhD in Nanomedicine from Oxford University
Iain Anderson
Chief Financial Officer
Chartered Certified Accountant since 1992
Over twenty years' experience with US-owned businesses, including subsidiaries of Hitachi, TriMas Corporation, Precision Castparts Corporation and Hospira Inc.
Qualified whilst working for the Big Four UK practice of Touche Ross (now Deloitte)
Joined Nemaura Medical Inc in August 2016
Gained an MBA from Loughborough University in 1999
Bashir Timol
Director of Strategy & Corporate Dev
Over 10 years experience as business entrepreneur and angel investor in life-science and bio-tech firms
Led initial angel-consortium that provided start-up funding for Nemaura Medical
2013-Present: Director of Strategy responsible for investor relations and corporate strategies
2007-Present: Director of Nemaura Pharma Ltd
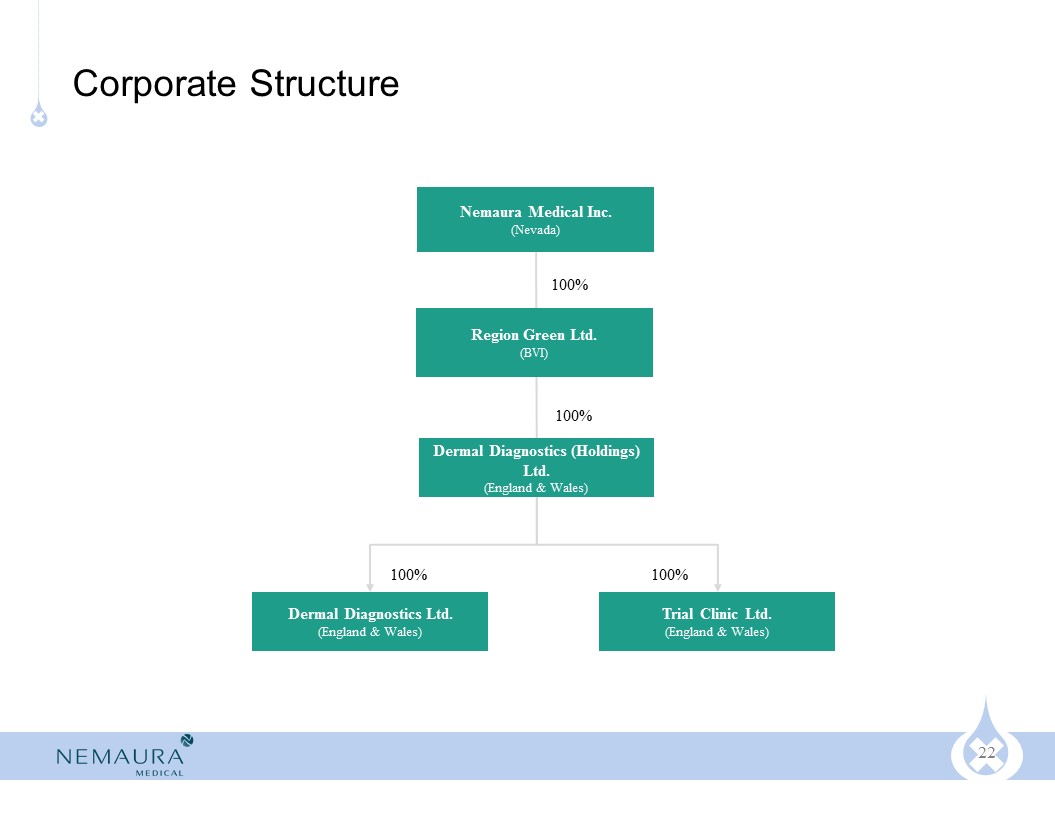
2013-Present: Director of Dermal Diagnostics
2009- Present: Director at SABT 1 Ltd and OneE Group Ltd
Bachelor degree in Economics from the University of Central Lancashire, UK.