Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - Nemaura Medical Inc. | ex32x1.htm |
| EX-31.2 - EXHIBIT 31.2 - Nemaura Medical Inc. | ex31x2.htm |
| EX-31.1 - EXHIBIT 31.1 - Nemaura Medical Inc. | ex31x1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
☑ QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended: December 31, 2020
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-38355
| Nemaura Medical Inc. |
| (Exact name of registrant as specified in its charter) |
| NEVADA | 46-5027260 | |||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||
|
57 West 57th Street Manhattan, NY 10019 | ||||
| (Address of Principal Executive Offices) (Zip Code) | ||||
| 646-416-8000 | ||||
| (Registrant’s Telephone Number, Including Area Code) | ||||
| N/A | ||||
| (Former name, former address and former fiscal year, if changed since last report) | ||||
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| Common Stock | NMRD | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer", "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |
|
Non-accelerated filer ☑
|
Smaller reporting company ☑ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☑
The number of shares of common stock, par value $0.001 per share, outstanding as of February 9, 2021 was 22,930,407.
| |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). All statements, other than statements of historical fact, included in this Quarterly Report on Form 10-Q regarding development of our strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management are forward-looking statements. Forward-looking statements may include, but are not limited to, statements about:
- any statements of the plans, strategies and objectives of management for future operations;
- any statements concerning proposed new products, services or developments;
- any statements regarding future economic conditions or performance;
- our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others;
- our estimates regarding the sufficiency of our cash resources and our need for additional funding;
- any statement that our business, financial condition and results of operations may be materially adversely affected by global health epidemics, including the recent COVID-19 pandemic; and
- any statement regarding the effectiveness of our continuous temperature monitoring system to assist with the diagnosis and monitoring of symptoms of COVID-19 or the effectiveness of our continuous lactate monitoring system (CLM) to monitor disease progression in COVID -19 patients.
The words "believe," "anticipate," "design," "estimate," "plan," "predict," "seek," "expect," "intend," "may," "could," "should," "potential," "likely," "projects," "continue," "will," and "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements reflect our current views with respect to future events, are based on assumptions and are subject to risks and uncertainties. We cannot guarantee that we actually will achieve the plans, intentions or expectations expressed in our forward-looking statements and you should not place undue reliance on these statements. There are a number of important factors that could cause our actual results to differ materially from those indicated or implied by forward-looking statements. These factors and the other cautionary statements made in this Quarterly Report on Form 10-Q should be read as being applicable to all related forward-looking statements whenever they appear herein. Except as required by law, we do not assume any obligation to update any forward-looking statement. We disclaim any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
| 2 |
NEMAURA MEDICAL INC.
TABLE OF CONTENTS
| Page | |||
| PART I: FINANCIAL INFORMATION | |||
| ITEM 1 | FINANCIAL STATEMENTS | ||
| Condensed Consolidated Balance Sheets as of December 31, 2020 (unaudited) and March 31, 2020 | 4 | ||
| Condensed Consolidated Statements of Comprehensive Loss for the Three and Nine Months Ended December 31, 2020 and 2019 (unaudited) | 5 | ||
| Condensed Consolidated Statements of Changes in Stockholders’ Equity for the Three Months Ended December 31, 2020 and 2019 (unaudited) and the Nine Months Ended December 31, 2020 and 2019 (unaudited) | 6-7 | ||
| Condensed Consolidated Statements of Cash Flows for the Nine Months Ended December 31, 2020 and 2019 (unaudited) | 8 | ||
| Notes to Condensed Consolidated Financial Statements (unaudited) | 9-13 | ||
| ITEM 2 | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 14-17 | |
| ITEM 3 | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 17 | |
| ITEM 4 | CONTROLS AND PROCEDURES | 17 | |
| PART II: OTHER INFORMATION | 18 | ||
| ITEM 1 | LEGAL PROCEEDINGS | 18 | |
| ITEM 1A | RISK FACTORS | 18 | |
| ITEM 2 | UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS | 18 | |
| ITEM 3 | DEFAULTS UPON SENIOR SECURITIES | 18 | |
| ITEM 4 | MINE SAFETY DISCLOSURES | 18 | |
| ITEM 5 | OTHER INFORMATION | 18 | |
| ITEM 6 | EXHIBITS | 18 | |
| SIGNATURES | 19 | ||
| 3 |
PART I – FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
| NEMAURA MEDICAL INC. |
| Condensed Consolidated Balance Sheets |
As of December 31, 2020 (Unaudited) | As of March 31, 2020
| |||||||
| ($) | ($) | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | 14,959,785 | 106,107 | ||||||
| Prepaid expenses and other receivables | 850,388 | 452,463 | ||||||
| Inventory (raw materials) | 818,236 | 286,309 | ||||||
| Total current assets | 16,628,409 | 844,879 | ||||||
| Other assets: | ||||||||
| Property and equipment, net of accumulated depreciation | 200,260 | 162,064 | ||||||
| Intangible assets, net of accumulated amortization | 810,319 | 213,080 | ||||||
| Total other assets | 1,010,579 | 375,144 | ||||||
| Total assets | 17,638,988 | 1,220,023 | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY / (DEFICIT) | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | 166,698 | 293,608 | ||||||
| Liability due to related parties | 395,923 | 830,093 | ||||||
| Other liabilities and accrued expenses | 76,147 | 168,966 | ||||||
| Notes payable, net of unamortized discount | 3,609,588 | — | ||||||
| Deferred revenue | 102,367 | 93,022 | ||||||
| Total current liabilities | 4,350,723 | 1,385,689 | ||||||
| Non-current portion of notes payable, net of unamortized discount | 1,698,727 | — | ||||||
| Non-current portion of deferred revenue | 1,262,533 | 1,147,278 | ||||||
| Total non-current liabilities | 2,961,260 | 1,147,278 | ||||||
| Total liabilities | 7,311,983 | 2,532,967 | ||||||
| Commitments and contingencies: | ||||||||
| Stockholders’ equity / (deficit): | ||||||||
| Series A convertible preferred stock, $0.001 par value, 200,000 shares authorized; 0 shares issued and outstanding at December 31, 2020 and March 31, 2020, respectively | — | — | ||||||
| Common stock, $0.001 par value, | ||||||||
| 42,000,000 shares authorized and 22,930,407 and 20,850,848 | ||||||||
| shares issued and outstanding at December 31, 2020 and March 31, 2020, respectively | 22,931 | 20,851 | ||||||
| Additional paid-in capital | 31,998,346 | 16,589,272 | ||||||
| Accumulated deficit | (21,714,045 | ) | (17,586,075 | ) | ||||
| Accumulated other comprehensive income / (loss) | 19,773 | (336,992 | ) | |||||
| Total stockholders’ equity / (deficit) | 10,327,005 | (1,312,944 | ) | |||||
| Total liabilities and stockholders’ equity | 17,638,988 | 1,220,023 | ||||||
See notes to the unaudited condensed consolidated financial statements.
| 4 |
NEMAURA MEDICAL INC. |
| Condensed Consolidated Statements of Comprehensive Loss |
|
(Unaudited) (in Dollars, except Share Amounts) |
| Three Months Ended December 31, | Nine Months Ended December 31, | |||||||||||||||
| 2020 | 2019 | 2020 | 2019 | |||||||||||||
| Revenue: | — | — | — | — | ||||||||||||
| Total revenue | — | — | — | — | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 486,957 | 516,672 | 1,258,549 | 1,535,370 | ||||||||||||
| General and administrative | 581,520 | 542,697 | 1,948,773 | 1,896,230 | ||||||||||||
| Total operating expenses | 1,068,477 | 1,059,369 | 3,207,322 | 3,431,600 | ||||||||||||
| Loss from operations | (1,068,477 | ) | (1,059,369 | ) | (3,207,322 | ) | (3,431,600 | ) | ||||||||
| Interest (expense) / income | (378,220 | ) | — | (920,648 | ) | 3,926 | ||||||||||
| Loss before income tax benefit | (1,446,697 | ) | (1,059,369 | ) | (4,127,970 | ) | (3,427,674 | ) | ||||||||
| Provision for income tax benefit | — | 614,362 | — | 614,362 | ||||||||||||
| Net Loss | (1,446,697 | ) | (445,007 | ) | (4,127,970 | ) | (2,813,312 | ) | ||||||||
| Other comprehensive income / (loss): | ||||||||||||||||
| Foreign currency translation adjustment | 371,275 | (25,834 | ) | 356,765 | (48,986 | ) | ||||||||||
| Comprehensive loss | (1,075,422 | ) | (470,841 | ) | (3,771,205 | ) | (2,862,298 | ) | ||||||||
| Loss per share: | ||||||||||||||||
| Basic and diluted | (0.06 | ) | (0.02 | ) | (0.19 | ) | (0.14 | ) | ||||||||
| Weighted average number of shares outstanding | 22,922,387 | 20,808,050 | 22,068,290 | 20,798,013 | ||||||||||||
See notes to the unaudited condensed consolidated financial statements.
| 5 |
NEMAURA MEDICAL INC.
Condensed Consolidated Statements of Changes in Stockholders’ Equity
Three Months Ended December 31, 2020 and 2019 (Unaudited)
| Common Stock | Additional Paid-in | Accumulated | Accumulated Other Comprehensive Income / | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount ($) | Capital ($) | Deficit ($) | (Loss) ($) | Equity ($) | |||||||||||||||||||
| Balance at September 30, 2020 | 22,893,705 | 22,894 | 31,838,383 | (20,267,348 | ) | (351,502 | ) | 11,242,427 | ||||||||||||||||
| Restricted shares issued as stock-based compensation to consultants and investor relations advisors | 36,702 | 37 | 159,963 | — | — | 160,000 | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | 371,275 | 371,275 | ||||||||||||||||||
| Net loss | — | — | — | (1,446,697 | ) | — | (1,446,697 | ) | ||||||||||||||||
| Balance at December 31, 2020 | 22,930,407 | 22,931 | 31,998,346 | (21,714,045 | ) | 19,773 | 10,327,005 | |||||||||||||||||
| Balance at September 30, 2019 | 20,802,930 | 20,803 | 16,332,734 | (15,794,184 | ) | (363,040 | ) | 196,313 | ||||||||||||||||
| Restricted shares issued as stock-based compensation to consultants and investor relations advisors | 5,000 | 5 | 39,995 | — | — | 40,000 | ||||||||||||||||||
| Reverse split adjustment | 418 | — | — | — | — | — | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (25,834 | ) | (25,834 | ) | ||||||||||||||||
| Net loss | — | — | — | (445,007 | ) | — | (445,007 | ) | ||||||||||||||||
| Balance at December 31, 2019 | 20,808,348 | 20,808 | 16,372,729 | (16,239,191 | ) | (388,874 | ) | (234,528 | ) | |||||||||||||||
See notes to the unaudited condensed consolidated financial statements.
| 6 |
NEMAURA MEDICAL INC.
Condensed Consolidated Statements of Changes in Stockholders’ Equity
Nine Months Ended December 31, 2020 and 2019 (Unaudited)
| Common Stock | Additional Paid-in | Accumulated | Accumulated Other Comprehensive Income / | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount ($) | Capital ($) | Deficit ($) | (Loss) ($) | Equity ($) | |||||||||||||||||||
| Balance at March 31 2020 | 20,850,848 | 20,851 | 16,589,272 | (17,586,075 | ) | (336,992 | ) | (1,312,944 | ) | |||||||||||||||
| Issuance of common shares, net of offering costs of $957,193 | 1,994,924 | 1,995 | 14,791,484 | — | — | 14,793,479 | ||||||||||||||||||
| Restricted shares issued as stock-based compensation to consultants and investor relations advisors | 46,702 | 47 | 223,153 | — | — | 223,200 | ||||||||||||||||||
| Exercise of warrants | 37,933 | 38 | 394,437 | — | — | 394,475 | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | 356,765 | 356,765 | ||||||||||||||||||
| Net loss | — | — | — | (4,127,970 | ) | — | (4,127,970 | ) | ||||||||||||||||
| Balance at December 31, 2020 | 22,930,407 | 22,931 | 31,998,346 | (21,714,045 | ) | 19,773 | 10,327,005 | |||||||||||||||||
| Balance at March 31, 2019 | 20,765,592 | 20,766 | 15,971,905 | (13,425,879 | ) | (339,888 | ) | 2,226,904 | ||||||||||||||||
| Exercise of warrants | 2,500 | 3 | 25,997 | — | — | 26,000 | ||||||||||||||||||
| Issuance of common shares, net of offering costs | 14,338 | 14 | 142,903 | — | — | 142,917 | ||||||||||||||||||
| Restricted shares issued as stock-based compensation to consultants and investor relations advisors | 25,500 | 25 | 231,924 | — | — | 231,949 | ||||||||||||||||||
| Reverse split adjustment | 418 | — | — | — | — | — | ||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | — | (48,986 | ) | (48,986 | ) | ||||||||||||||||
| Net loss | — | — | — | (2,813,312 | ) | — | (2,813,312 | ) | ||||||||||||||||
| Balance at December 31, 2019 | 20,808,348 | 20,808 | 16,372,729 | (16,239,191 | ) | (388,874 | ) | (234,528 | ) | |||||||||||||||
See notes to the unaudited condensed consolidated financial statements.
| 7 |
| NEMAURA MEDICAL INC. |
| Condensed Consolidated Statements of Cash Flows |
| (Unaudited) |
| Nine Months Ended December 31, | ||||||||
2020 ($) | 2019 ($) | |||||||
| Cash Flows From Operating Activities: | ||||||||
| Net loss | (4,127,970 | ) | (2,813,312 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 68,310 | 47,550 | ||||||
| Accretion of debt discount | 920,648 | — | ||||||
| Stock-based compensation | 84,000 | 317,664 | ||||||
| Changes in assets and liabilities: | ||||||||
| Prepaid expenses and other receivables | (397,926 | ) | 206,716 | |||||
| Inventory | (531,927 | ) | (186,137 | ) | ||||
| Accounts payable | (126,910 | ) | 21,259 | |||||
| Liability due to related parties | (434,170 | ) | (268,483 | ) | ||||
| Other liabilities and accrued expenses | (92,819 | ) | 110,780 | |||||
| Net cash used in operating activities | (4,638,764 | ) | (2,563,963 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Capitalized patent costs | (48,273 | ) | (50,570 | ) | ||||
| Software development costs in process | (446,455 | ) | — | |||||
| Purchase of property and equipment | (70,547 | ) | (162,615 | ) | ||||
| Net cash used in investing activities | (565,275 | ) | (213,185 | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Costs incurred in relation to equity financing | (957,193 | ) | (9,575 | ) | ||||
| Commission paid on note payable | (325,000 | ) | — | |||||
| Proceeds from issuance of notes | 5,000,000 | — | ||||||
| Proceeds from issuance of common stock in relation to equity financing | 15,750,672 | 152,492 | ||||||
| Proceeds from warrant exercise | 394,475 | 26,000 | ||||||
| Repayments of note payable | (300,000 | ) | — | |||||
| Repayment of insurance financing | (82,555 | ) | (28,207 | ) | ||||
| Net cash provided by financing activities | 19,480,399 | 140,710 | ||||||
| Net increase / (decrease) in cash | 14,276,360 | (2,636,438 | ) | |||||
| Effect of exchange rate changes on cash | 577,318 | (36,563 | ) | |||||
| Cash at beginning of period | 106,107 | 3,740,664 | ||||||
| Cash at end of period | 14,959,785 | 1,067,663 | ||||||
| Supplemental disclosure of non-cash financing activities: | ||||||||
| Gross amount of insurance funded through note payable | — | 132,352 | ||||||
| Licenses acquired through issuance of restricted common stock | 100,000 | — | ||||||
See notes to the unaudited condensed consolidated financial statements.
| 8 |
NEMAURA MEDICAL INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
NOTE 1 – ORGANIZATION AND PRINCIPAL ACTIVITIES
Nemaura Medical Inc. (“Nemaura” or the “Company”), through its operating subsidiaries, performs medical device research and manufacturing of a continuous glucose monitoring system (“CGM”), named sugarBEAT®. The sugarBEAT® device is a non-invasive, wireless device for use by persons with Type I and Type II diabetes and may also be used to screen pre-diabetic patients. The sugarBEAT® device extracts analytes, such as glucose, to the surface of the skin in a non-invasive manner where it is measured using unique sensors and interpreted using a unique algorithm.
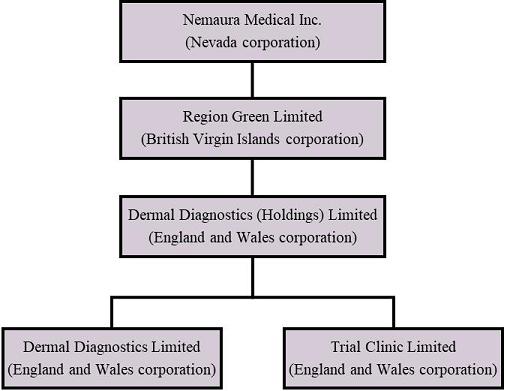
Nemaura is a Nevada holding company organized in 2013. Nemaura owns 100% of Region Green Limited, a British Virgin Islands corporation (“RGL”) formed on December 12, 2013. RGL owns 100% of the stock in Dermal Diagnostic (Holdings) Limited, an England and Wales corporation (“DDHL”) formed on December 11, 2013, which in turn owns 100% of Dermal Diagnostics Limited, an England and Wales corporation formed on January 20, 2009 (“DDL”), and 100% of Trial Clinic Limited, an England and Wales corporation formed on January 12, 2011 (“TCL”).
DDL is a diagnostic medical device company headquartered in Loughborough, Leicestershire, England, and is engaged in the discovery, development and commercialization of diagnostic medical devices. The Company’s initial focus has been on the development of the sugarBEAT® device, which consists of a disposable patch containing a sensor, and a non-disposable miniature wireless transmitter with a re-chargeable power source, which is designed to enable trending or tracking of blood glucose levels. While the Company’s key operations and assets are located in England, the Company has recently commenced commercial operations in the United States.
The following diagram illustrates Nemaura’s corporate structure as of December 31, 2020:
| 9 |
The Company was incorporated in 2013 and has reported recurring losses from operations to date and an accumulated deficit of $21,714,045 as of December 31, 2020. These operations have resulted in the successful completion of clinical programs to support approval of a CE mark (European Union approval of the product) which is managed via an ISO 13485 accredited Quality Management System that is subject to annual audit by the accreditation body (“BSI”); this accreditation was successfully renewed during November 2020. In addition to this, a medical device Premarket Approval (“PMA”) application was submitted to the U.S. Food and Drug Administration (“FDA”) in July 2020; however, we, along with other applicants, have been informed by the FDA that the approval process is currently subject to delays as a result of the FDA’s Center for Devices and Radiological Health (“CDRH”) being actively engaged in responding to the current pandemic caused by COVID-19. According to recent notifications received from the FDA, this has resulted in staff being reallocated to other approval requests associated with COVID-19. As such, the timeline for other, non-COVID-19 related, approvals continues to be adversely impacted. Current guidance provided by the FDA indicates that this staff reallocation is likely to last at least through mid-April, 2021.
The Company expects to continue to incur losses from operations until revenues are generated through licensing fees or product sales. However, given the completion of the requisite clinical programs, these losses are expected to be reduced over time. Management has entered into licensing agreements with unrelated third parties relating to the United Kingdom, Europe, Qatar and all countries in the Gulf Cooperation Council.
Management has evaluated the expected expenses to be incurred along with its available cash and has determined that the Company has the ability to continue as a going concern for at least one year after the date of issuance of these unaudited condensed consolidated financial statements.
On April 15, 2020, the Company entered into a note purchase agreement resulting in net cash proceeds of $4,675,000, as further described in Note 6. The Company had $14,959,785 of cash at December 31, 2020. The Company believes the cash position as of December 31, 2020, is adequate for our current level of operations through at least February 2022, and for the achievement of certain of our product development milestones. Our plan is to utilize the cash to continue establishing commercial manufacturing operations for the commercial supply of the sugarBEAT® device and patches now that CE mark approval has been received.
NOTE 2 – BASIS OF PRESENTATION
| (a) | Basis of presentation |
The accompanying unaudited condensed consolidated financial statements have been prepared pursuant to the rules and regulations of the U.S. Securities and Exchange Commission (the “SEC”), and do not include all of the information and footnotes required by U.S. generally accepted accounting principles (“U.S. GAAP”) for complete financial statements. However, such information reflects all adjustments consisting of normal recurring accruals which are, in the opinion of management, necessary for a fair statement of the financial condition and results of operations for the interim periods. The results for the three months and nine months ended December 31, 2020 are not indicative of annual results. The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with U.S. GAAP for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X. It is suggested that these unaudited condensed consolidated financial statements be read in conjunction with the consolidated financial statements and the notes thereto included in the Company’s Annual Report on Form 10-K for the year ended March 31, 2020, as filed with the SEC.
The accompanying unaudited condensed consolidated financial statements include the accounts of the Company and the Company’s subsidiaries. References to “we”, “us”, “our”, or the “Company” refer to Nemaura Medical Inc. and its consolidated subsidiaries. The unaudited condensed consolidated financial statements are prepared in accordance with U.S. GAAP, and all significant intercompany balances and transactions have been eliminated in consolidation.
The functional currency for the majority of the Company’s operations is the Great Britain Pound Sterling (“GBP”), and the reporting currency is the U.S. Dollar (“USD”).
| (b) | Changes to significant accounting policies |
The Company adopted the Financial Accounting Standards Board’s Accounting Standards Update ("ASU") No. 2016-02, Leases, as of April 1, 2020 and the impact of adoption of this ASU on the Company’s consolidated financial statements is not significant. There have been no other material changes to our significant accounting policies from those detailed in the Company’s Annual Report on Form 10-K for the year ended March 31, 2020, as filed with the SEC.
(c) Recently adopted accounting pronouncements
The Company continually assesses any new accounting pronouncements to determine their applicability. When it is determined that a new accounting pronouncement affects the Company's financial reporting, the Company undertakes a study to determine the consequences of the change to its consolidated financial statements and assures that there are proper controls in place to ascertain that the Company's consolidated financial statements properly reflect the change.
This Quarterly Report on Form 10-Q does not discuss recent pronouncements that are not anticipated to have a current and/or future impact on the Company, or are unrelated to the Company’s financial condition, results of operations, cash flows or disclosures.
| 10 |
NOTE 3 – LICENSING AGREEMENTS
United Kingdom and the Republic of Ireland, the Channel Islands and the Isle of Man
In March 2014, the Company entered into an Exclusive Marketing Rights Agreement (the “Marketing Rights Agreement”) with an unrelated third party (the “Licensee”), that granted to the Licensee the exclusive right to market and promote the sugarBEAT® device and related patches under its own brand in the United Kingdom and the Republic of Ireland, the Channel Islands and the Isle of Man. The Company received a non-refundable, up-front cash payment of GBP 1,000,000 (approximately $1.365 million and $1.240 million as of December 31, 2020 and March 31, 2020, respectively), which is wholly non-refundable, upon signing the Marketing Rights Agreement.
The Company is in ongoing dialogue with the Licensee about the timing of its plans with respect to its product launch. The current expectation is for this to occur in the quarter ending June 30, 2021. The upfront fees received from the Marketing Rights Agreement have been deferred and will be recorded as income over the term of the Marketing Rights Agreement. Consequently, approximately $102,000 and $93,000 of the deferred revenue has been classified as a current liability as of December 31, 2020 and March 31, 2020, respectively.
NOTE 4 – RELATED PARTY TRANSACTIONS
Nemaura Pharma Limited (“Pharma”), NDM Technologies Limited (“NDM”) and Black and White Health Care Limited (“B&W”) are entities controlled by the Company’s Chief Executive Officer, President, director and majority stockholder, Dewan F.H. Chowdhury.
These unaudited condensed consolidated financial statements are intended to reflect all costs associated with the operations of DDL and TCL. Pharma has a service agreement with DDL to undertake development, manufacture and regulatory approvals under Pharma’s ISO13485 accreditation. In lieu of these services, Pharma invoices DDL on a periodic basis for said services. Services are provided at cost plus a service surcharge amounting to less than 10% of the total costs incurred.
The table below provides a summary of activity between the Company and Pharma and NDM for the nine months ended December 31, 2020 and 2019, and the year ended March 31, 2020. These amounts are unsecured, interest free, and payable on demand.
Nine Months Ended December 31, 2020 (unaudited) ($) | Nine Months Ended December 31, 2019 (unaudited) ($) | Year Ended March 31, 2020
($) | ||||||||||
| Liability due to related parties at beginning of period | 830,093 | 964,679 | 964,679 | |||||||||
| Amounts invoiced by Pharma to DDL, NDM and TCL (1) | 1,859,548 | 1,369,272 | 1,800,517 | |||||||||
| Amounts invoiced by DDL to Pharma | (17,213 | ) | (5,874 | ) | (10,963 | ) | ||||||
| Amounts paid by DDL to Pharma | (2,338,701 | ) | (1,642,019 | ) | (1,897,222 | ) | ||||||
| Foreign exchange differences | 62,196 | 15,970 | (26,918 | ) | ||||||||
| Liability due to related parties at end of period | 395,923 | 702,028 | 830,093 | |||||||||
| (1) | These amounts are primarily incurred as a result of research and development expenses charged to the Company by Pharma. |
The Company routinely reviews its condensed consolidated statements of cash flows presentation of related party transactions for financing or operating classification based on the underlying nature of the item and intended repayment.
NOTE 5 – STOCKHOLDERS’ EQUITY
Reverse stock split
The Company was notified by The NASDAQ Stock Market (“NASDAQ”) on July 15, 2019 that the Company no longer met the requirements of NASDAQ Rule 5550(a)(2) requiring listed securities to maintain a minimum closing bid price of $1.00 per share. Thereafter, the Company effected:
| (i) | A reverse split of the Company’s issued and outstanding common stock on a one (1) for ten (10) basis; and |
| (ii) | A decrease in the Company’s authorized number of shares of common stock on the same basis from 420,000,000 shares of common stock to 42,000,000 shares of common stock. |
The reverse stock split and decrease in authorized common stock were effective on December 5, 2019. On December 19, 2019, the Company received confirmation from NASDAQ that the Company had regained compliance with NASDAQ’s minimum bid price rule and the matter is now resolved. Amounts are retroactively restated for all periods presented.
| 11 |
Other equity transactions
On October 19, 2018, the Company entered into an Equity Distribution Agreement (the “Distribution Agreement”) with Maxim Group LLC, as sales agent (“Maxim”), pursuant to which the Company may offer and sell, from time to time, through Maxim, up to $20,000,000 in shares of its common stock. On August 8, 2020, pursuant to the terms of the Distribution Agreement, as amended, between the Company and Maxim, the Company provided notice of termination of the Distribution Agreement, as amended, to Maxim. Accordingly, the Distribution Agreement, as amended, terminated on August 18, 2020.
During the nine month period ended December 31, 2020, for which the Distribution Agreement was active, a total of 408,718 shares were issued, generating gross proceeds of $4,250,676 with associated costs of $127,520.
On July 28, 2020, the Company entered into a placement agency agreement with Kingswood Capital Markets, a division of Benchmark Investments, Inc., with respect to the issuance and sale of an aggregate of 1,586,206 shares of the Company’s common stock, and warrants to purchase up to 793,103 shares of common stock. Each share of common stock and accompanying one-half of a warrant were sold for a combined purchase price of $7.25, for a total deal size of approximately $11.5 million, not including any future proceeds from the exercise of the warrants and before deducting the Placement Agent fees and offering expenses. Each whole warrant is immediately exercisable at a price of $8.00 per share, subject to adjustment in certain circumstances, and will expire five years from the date of issuance. The shares of common stock were offered together with the warrants, but the securities were issued separately and are separately transferable. The closing of the offering took place on July 30, 2020 and the net proceeds from the sale of the common stock and warrants were approximately $10.7 million after deducting the Placement Agent commission and other expenses incurred by the Company as a result of the offering.
During the nine month period ended December 31, 2020, 37,933 warrants were exercised, generating $394,475 in additional funds; no warrants were exercised in the three month period ended December 31, 2020. During the nine month period ended December 31, 2019, 2,500 warrants were exercised generating funds of $26,000, all of which were exercised during the three month period ended June 30, 2019.
At December 31, 2020, there were 940,740 warrants outstanding.
Effective December 18, 2018, the Company issued a unit purchase option to Dawson James Securities, Inc., the then placement agent, to purchase 9,710 shares of common stock and 9,710 warrants. The Company has classified this option as equity. The unit purchase option has a term of three years and an exercise price of $13.00.
Loss per share
The following table sets forth the computation of basic and diluted loss per share for the periods indicated.
| Three months ended December 31, | Nine months ended December 31, | |||||||||||||||
| 2020 | 2019 | 2020 | 2019 | |||||||||||||
| Net loss attributable to common stockholders ($) | (1,446,697 | ) | (445,007 | ) | (4,127,970 | ) | (2,813,312 | ) | ||||||||
| Weighted average basic and diluted shares outstanding | 22,922,387 | 20,808,050 | 22,068,290 | 20,798,013 | ||||||||||||
| Basic and diluted loss per share ($): | (0.06 | ) | (0.02 | ) | (0.19 | ) | (0.14 | ) | ||||||||
Because the Company is in a loss position, the Company excludes warrants outstanding, which are anti-dilutive, from the basic and diluted loss per share calculation.
Basic loss per share is computed by dividing loss available to common stockholders by the weighted average number of common shares outstanding during the period. For the three and nine month periods ended December 31, 2020 and 2019, warrants to purchase one million shares of common stock were anti-dilutive and were excluded from the calculation of diluted loss per share. Additionally, for the three and nine month periods ended December 31, 2020, there were a further 940,740 shares of common stock, respectively, and a unit purchase option to purchase 9,710 shares of common stock that were considered anti-dilutive and also excluded from the calculation of diluted loss per share. For the three and nine month periods ended December 31, 2019, the equivalent number of warrants excluded from this calculation was 185,570 and the unit purchase option was 9,710.
NOTE 6 – NOTES PAYABLE
On April 15, 2020, the Company entered into a note purchase agreement (the “Note Purchase Agreement”) with an unrelated third party (the “Investor”). Pursuant to the terms of the Note Purchase Agreement, the Company agreed to issue and sell to the Investor and the Investor agreed to purchase from the Company, a secured promissory note (the “Secured Note”) in the original principal amount of $6,015,000. In consideration thereof, on April 15, 2020 (the closing date), (i) the Investor (a) paid $1,000,000 in cash, (b) issued to the Company (1) Investor Note #1 in the principal amount of $2,000,000 (“Investor Note #1”), and (2) Investor Note #2 in the principal amount of $2,000,000 (“Investor Note #2” together with Investor Note #1, the “Investor Notes”), and (ii) the Company delivered the Secured Note on behalf of the Company, to the Investor, against delivery of the Purchase Price. For these purposes, the “Purchase Price” means the Investor’s initial cash purchase price, together with the sum of the initial principal amounts of the Investor Notes.
| 12 |
The Secured Note is secured by all patents and related rights and items as defined in the related security agreement within the Secured Note. The Secured Note carries an original issue discount (“OID”) of $1,000,000. In addition, the Company agreed to pay $15,000 to the Investor to cover the Investor’s legal fees, accounting costs, due diligence, monitoring and other transaction costs incurred in connection with the purchase and sale of the Secured Note (the “Transaction Expense Amount”), all of which amount is included in the initial principal balance of the Secured Note. The Purchase Price for the Secured Note is $5,000,000, computed as follows: $6,015,000 original principal balance, less the OID, less the commission expense of $325,000, resulting in cash proceeds of $4,675,000. The debt less the discount will be accreted over the 24-month term of the Secured Note using the effective interest method. The effective interest rate is 34.3%. A monitoring fee equal to 0.833% of the outstanding balance will automatically be added to the outstanding balance on the first day of each month. Accretion for the three and nine month periods ended December 31, 2020 was $378,220 and $920,648, respectively.
NOTE 7 – OTHER ITEMS
(a) COVID-19 Pandemic
The outbreak of COVID-19 originating in Wuhan, China, in December 2019 has since rapidly increased its exposure globally. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. We continue to monitor the global outbreak of COVID-19 and are working with our employees, suppliers and other stakeholders to mitigate the risks posed by its spread, COVID-19 is not expected to have any long-term detrimental effect on the Company’s success. While key suppliers have not been accessible throughout the whole period of the outbreak, we have been able to be flexible in our priorities and respond favorably to the challenges faced during the outbreak. We have also seen a surge in the uptake of technologies for remote and patient self-monitoring, which therefore potentially enhances the prospects for the likes of the Company and its CGM product and planned digital healthcare offering.
(b) Management consultancy agreements
During the nine month period ended December 31, 2020, $59,000 in stock-based compensation was shown as expense in relation to a management consulting company. No stock-based compensation was provided during the three month period ended December 31, 2020 for these services.
Total stock-based compensation recognized during the three and nine month periods ended December 31, 2019 was $40,000 and $317,664, respectively.
(c) Investor relations agreements
During the three and nine month periods ended December 31, 2020, Nemaura entered into an agreement with a third party provider of investor relations services for which $25,000 in stock-based compensation was shown as expense in relation to the services received. The contract for services is for a 12 month period, with a similar expense expected to be incurred through stock-based compensation in each quarter of this term.
No stock-based compensation expense was incurred for any similar services for the three or nine month periods ended December 31, 2019.
(d) Subsequent events
On February 8, 2021, the Company entered into an additional note purchase agreement (“Note Purchase Agreement 2”) with an affiliate of the unrelated third party who holds the existing Note Purchase Agreement that was issued dated April 15, 2020 (the “Investor”), see Note 6. Pursuant to the terms of Note Purchase Agreement 2, the Company agreed to issue and sell to the Investor and the Investor agreed to purchase from the Company, a secured promissory note (“Secured Note 2”) in the original principal amount of $24,015,000. In consideration thereof, on February 9, 2021 (the “closing date”), (i) the Investor paid $20,000,000 in cash to the Company, and (ii) the Company delivered Secured Note 2 on behalf of the Company, to the Investor, against the delivery of the Purchase Price. For these purposes, the “Purchase Price” means the Investor’s initial cash purchase price. After adjusting for commission expense of $1,200,000, cash proceeds received were $18,800,000.
Secured Note 2 is secured against all of the Company’s assets owned as of the closing date and extends to any assets acquired at any time that the Company’s obligations under Secured Note 2 are outstanding.
The Company has taken on this additional, non-dilutive funding, to enable the acceleration of future revenue growth.
| 13 |
ITEM 2: MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are a medical technology company developing sugarBEAT®, a non-invasive, affordable, and flexible continuous glucose monitoring system for adjunctive use by persons with diabetes. sugarBEAT® consists of a disposable adhesive skin-patch connected to a rechargeable wireless transmitter that displays glucose readings at regular five minute intervals via a mobile app. sugarBEAT® works by extracting glucose from the skin into a chamber in the patch that is in direct contact with an electrode-based sensor. The transmitter sends the raw data to a mobile app where it is processed by an algorithm and displayed as a glucose reading, with the ability to track and trend the data over days, weeks, and months. While sugarBEAT® requires once per day calibration by the patient using a blood sample obtained by a finger stick, we believe sugarBEAT® will be adopted by non-insulin dependent persons with diabetes alongside insulin-injecting persons with diabetes, who all perform multiple daily finger sticks to manage their disease.
CE approval was granted by the European Notified Body BSI in May 2019, allowing the product to be made available for commercial sale, this approval is subject to an annual review of the underlying ISO 13485 accredited Quality Management System, the accreditation was successfully renewed during November 2020. The Company has commenced a phase 1 launch whereby devices were made available to limited cohorts of users to gauge their feedback so that any fine-tuning could be completed prior to a mass market launch. A larger market launch is due to commence in the UK in the coming months via the UK licensee DB Ethitronix, whereby sugarBEAT® will be available via a number of subscription models the exact nature of which is at the discretion of the UK licensee.
In July 2020, Nemaura filed a PMA application with the FDA to use sugarBEAT® as an adjunct to finger prick testing for blood glucose trending. We, along with other applicants, have been informed by the FDA that the approval process is currently subject to delays as a result of the FDA’s Center for Devices and Radiological Health (“CDRH”) being actively engaged in responding to the current pandemic caused by COVID-19. According to recent notifications received from the FDA, this has resulted in staff being reallocated to other approval requests associated with COVID-19. As such, the timeline for other, non-COVID-19 related, approvals continues to be adversely impacted. Current guidance provided by the FDA indicates that this staff reallocation is likely to last at least through mid-April, 2021.
In addition to this Nemaura has established that proBEATTM which is based on the sugarBEAT® platform, can be classified under the Wellness guidance when it is used according to the FDA Wellness guidance notes, to provide prompts and educate users on factors affecting their blood sugar profiles. Nemaura launched proBEAT™ in the U.S. in December 2020, as part of a diabetes prevention and reversal program branded BEATdiabetes.life. During the quarter ended December 31, 2020, Nemaura licensed a clinically validated weight loss program for the management of diabetes from Healthimation, LLC, which was originally developed at the Joslin Diabetes Center, an affiliate of Harvard Medical School. This program, together with proBEATTM, forms the BEATdiabetes.life program currently being developed for commercialization in the United States.
We believe there are additional applications for sugarBEAT® and the underlying BEAT technology platform, which may include:
| · | a web-server accessible by physicians and diabetes professionals to track the condition remotely, thereby reducing healthcare costs and managing the condition more effectively; |
| · | a complete virtual doctor that monitors a person's vital signs and transmits results via the web; |
| · | other patches using the BEAT technology platform to measure alternative analytes, including lactate, uric acid, lithium and drugs. This would be a step-change in the monitoring of conditions, particularly in the hospital setting. Lactate monitoring is currently used to determine the relative fitness of professional athletes and we completed preliminary studies demonstrating the application of the BEAT technology for continuous lactate monitoring; |
| · | a continuous temperature monitoring system which could have various applications, including use for individuals to monitor their temperature in connection with diagnosis and monitoring of symptoms of novel coronavirus (COVID-19); |
| · | monitoring disease progression in COVID-19 patients using continuous lactate monitoring (CLM). |
The Company has experienced recurring losses and negative cash flows from operations. As of December 31, 2020, the Company had cash balances of $14,959,785, working capital of $12,277,686, total stockholders' equity of $10,327,005 and an accumulated deficit of $21,714,045. To date, the Company has in large part relied on equity financing to fund its operations with some third party debt finance having been raised at the start of the current fiscal year. The Company expects to continue to incur losses from operations for the near-term and these losses could be significant as product development, regulatory activities, clinical trials, and other commercial and product development related expenses are incurred.
Management's strategic assessment includes the following potential options:
| · | obtaining further regulatory approval for the sugarBEAT® device in other countries, such as the United States; |
| · | pursuing capital raising opportunities; |
| · | exploring licensing and collaboration opportunities; and |
| · | developing the sugarBEAT® device platform for commercialization for other applications. |
| 14 |
COVID-19 Pandemic
The outbreak of COVID-19 originating in Wuhan, China, in December 2019 has since rapidly increased its exposure globally. On March 11, 2020, the World Health Organization declared the outbreak a pandemic. We continue to monitor the global outbreak of COVID-19 and are working with our employees, suppliers, and other stakeholders to mitigate the risks posed by its spread, COVID-19 is not expected to have any long-term detrimental effect on the Company’s success. While key suppliers have not been accessible throughout the whole period of the outbreak, we have been able to be flexible in our priorities and respond favourably to the challenges faced during the outbreak. We have also seen a surge in the uptake of technologies for remote and patient self-monitoring, which therefore potentially enhances the prospects for the likes of the Company and its CGM product and planned digital healthcare offering.
Results of Operations
Comparative Results for the Nine Months Ended December 31, 2020 and 2019
Revenue
There was no revenue recognized in the nine months ended December 31, 2020 and 2019. In 2014, we received an upfront non-refundable cash payment of GBP 1 million (approximately $1.365 million and $1.240 million as of December 31, 2020 and March 31, 2020, respectively) in connection with an Exclusive Marketing Rights Agreement with an unrelated third party that provides the third party the exclusive right to market and promote the sugarBEAT® device and related patch under its own brand in the United Kingdom and the Republic of Ireland. We have deferred this licensing revenue until sales are due to commence, and we expect to record the revenue as income over an approximately 10-year term from the date sales commence. Although the revenue is deferred at December 31, 2020, the cash payment became immediately available and has been used to fund our operations, including research and development costs associated with successfully obtaining the CE mark approval.
Research and Development Expenses
Research and development expenses were $1,258,549 and $1,535,370 for the nine months ended December 31, 2020 and 2019, respectively. This amount consisted primarily of expenditures on wages and sub-contractor activities incurred for improvements made to the sugarBEAT® device. The decrease of $276,821 is due to a decrease in these costs as the sugarBEAT® product is nearing commercial launch. Moving forward we anticipate that the cost reductions seen to-date in this area will continue as we see a re-balancing across general and administrative expenses driven by the commercial operations of the business become more relevant.
General and Administrative Expenses
General and administrative expenses were $1,948,773 and $1,896,230 for the nine months ended December 31, 2020 and 2019, respectively. These consisted of fees for legal, professional, consultancy, audit services, investor relations, insurance, and wages. We expect general and administrative expenses will increase going forward, as the business transitions to a different structure to support an increased operational base that will encompass an increase in operational functions associated with sales, marketing, customer service, as well as enhancements to other existing functions.
Other Comprehensive Income / (Loss)
For the nine months ended December 31, 2020 and 2019, other comprehensive income / (loss) was $356,765 and ($48,986), respectively. Currently all transactions recorded through other comprehensive income / (loss) arise from fluctuations in the USD: GBP exchange rate and the impact that this has on consolidation of the Company’s non-USD denominated assets and liabilities.
Comparative Results for the Three Months Ended December 31, 2020 and 2019
Revenue
As noted above, the Company is currently pre-revenue. As such, no revenue was recognized in the three months ended December 31, 2020 and 2019.
Research and Development Expenses
Research and development expenses were $486,957 and $516,672 for the three month periods ended December 31, 2020 and 2019, respectively. This continues to be largely composed of expenditures on wages and sub-contractor activities incurred in finalizing the product design for the sugarBEAT® device in order to enable scaling of the production ability.
General and Administrative Expenses
General and administrative expenses were $581,520 and $542,697 for the three month periods ended December 31, 2020 and 2019, respectively. While the cost drivers in this area are largely representative of fees for legal, professional, consultancy, audit services, investor relations, insurance and wages, the increase seen in the quarter is expected to be maintained moving forward as we develop the operational capabilities within the Company to support commercialization.
| 15 |
Other Comprehensive Income / (Loss)
For the three months ended December 31, 2020 and 2019, other comprehensive income / (loss) was $371,275 and ($25,834), respectively. Currently all transactions recorded through other comprehensive income / (loss) arise from fluctuations in the USD: GBP exchange rate and the impact that this has on consolidation of the Company’s non-USD denominated assets and liabilities.
Liquidity and Capital Resources
We have experienced net losses and negative cash flows from operations since our inception. We have sustained cumulative losses of $21,714,045 through December 31, 2020. We have historically financed our operations through the issuances of equity and contributions of services from related entities.
Having reached certain agreed internal product development milestones, the Company undertook various fund-raising activities, as set out in greater detail in Notes 5 and 6 to the condensed consolidated financial statements (unaudited) above, which encompassed both debt and equity in order to ensure that sufficient funding was in place to enable the Company to move forward with the next phase of its commercialization strategy.
As of December 31, 2020, the Company had net working capital of $12,277,686 which included cash balances of $14,959,785. The Company reported a net loss for the three and nine month periods ended December 31, 2020 of $1,446,697 and $4,127,970, respectively. These losses are after taking account of interest charges arising predominantly from the note purchase agreement for the three and nine month periods ended December 31, 2020 interest of $378,220 and $920,648, respectively, was charged to the statement of comprehensive loss.
We believe the cash position as of December 31, 2020, is adequate for our current planned level of operations, through at least February 2022, which encompasses the continued establishment of commercial manufacturing operations for the supply of the sugarBEAT® device and patches in relation to our existing licensing agreements, combined with the steps that the Company initiated in December 2020 to commence establishment of an operational footprint in the United States.
Cash Flows
Net cash used in operating activities for the nine months ended December 31, 2020 was $4,638,764, with the key drivers being: net loss of $4,127,970 which includes non-cash charges of $68,310 in relation to depreciation and amortization, $920,648 in relation to the accretion of debt discount, and $84,000 in stock-based compensation. In addition to this, we saw an increase in prepaid expenses of $397,926 which was largely driven by an increase in the value of our forward purchasing for raw materials with long lead times (to ensure the risk to future production capabilities would be mitigated as a result of key inventory becoming unavailable as the global economy rebounds post-COVID-19); we also incurred upfront cash commitments to acquire fixed assets to support future production development capabilities. An increase of $531,927 was also seen in inventory as the business moved to prepare its capacity to support of the imminent expectation of product launch. The Company also saw reductions in accounts payable of $126,910, as well as decreases in accruals of $92,819 and in the liability due to related parties of $434,170.
Net cash used in operating activities for the nine months ended December 31, 2019 was $2,563,963, with the key drivers being: net loss of $2,813,312, non-cash stock-based compensation of $317,664, a decrease in prepaid expenses of $206,716, an increase of inventory of $186,137, an increase in accruals of $110,780, a decrease in liability due to related parties of $268,483, and an increase in accounts payable of $21,259.
Net cash used in investing activities was $565,275 for the nine months ended December 31, 2020, which reflects $446,455 in software development that is being treated as work-in-progress for the BEATdiabetes.life platform. The Company also spent $48,273 on patent filing costs and $70,547 on the purchase of property and equipment to support future production and sensor development.
For the nine months ended December 31, 2019, net cash used in investing activities was $213,185, which reflected patent filing costs of $50,570 and the purchase of property and equipment of $162,615.
Net cash provided by financing activities for the nine months ended December 31, 2020 was $19,480,399. Shares issued during the period delivered proceeds of $15,750,672 via a combination of the ATM facility and the placement facilitated by Kingswood, with costs directly associated with these activities totalling $957,193. $394,475 was also raised in relation to the exercise of 37,933 warrants and proceeds were also received from the issuance of notes totalling $5,000,000 less commission expense of $325,000 and repayments against debt facilities of $382,555.
For the nine months ended December 31, 2019, net cash provided by financing activities was $140,710. The ATM facility delivered proceeds of $152,492. In addition, $26,000 was raised in relation to the exercise of 25,000 warrants. The Company also incurred direct costs of $9,575 related to the ATM financing. A debt facility was entered into in the three months ended December 31, 2019 to finance an invoice. Repayments during that period totalled $28,207.
| 16 |
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including unrecorded derivative instruments that have or are reasonably likely to have a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Critical Accounting Policies
When we prepare our condensed consolidated financial statements and accompanying notes in conformity with U.S. generally accepted accounting principles (“U.S. GAAP”), we must make estimates and assumptions about future events that affect the amounts we report. Certain of these estimates result from judgements that can be subjective and complex. As a result of that subjectivity and complexity, and because we continuously evaluate these estimates and assumptions based on a variety of factors, actual results could materially differ from our estimates and assumptions if changes in one or more factors require us to make accounting adjustments. We believe our critical accounting policies affect our more significant judgments and estimates used in the preparation of the unaudited condensed consolidated financial statements. During the three and nine months ended December 31, 2020, we have made no material changes or additions with regard to such policies and estimates.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Foreign Exchange Risk
Our foreign currency exposure gives rise to market risk associated with exchange rate movements against the U.S. dollar, our reporting currency. Currently, most of our expenses and cash are denominated in Great Britain Pounds Sterling (“GBP”), with the remaining portion denominated in U.S. dollars. Fluctuations in exchange rates, primarily the U.S. dollar against the GBP, will affect our financial position. At December 31, 2020, the Company held approximately $6.635 million in GBP-denominated bank accounts. Based on this balance, a 1% depreciation of the GBP against the U.S. dollar would cause an approximate $66,350 reduction in cash account balances.
We have not utilized any hedging instruments to mitigate foreign currency risk.
Inflation
Historically, with UK inflation rates having been low in recent years, inflation has not had a significant effect on our business in the UK, the location of the substantial part of our activities.
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Based on their evaluation as of December 31, 2020, the Company’s Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, 2020, the Company’s disclosure controls and procedures (as defined in Rules 13a-15 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) were not effective due to a material weakness in the Company’s internal control over financial reporting.
Changes in Internal Control over Financial Reporting
In connection with the evaluation as of December 31, 2020 the Chief Executive Officer and Chief Financial Officer identified certain changes, as identified in this paragraph, in the Company’s internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting. On September 15, 2020, the Company’s Board of Directors appointed Justin Mclarney to serve as the Company’s Chief Financial Officer. Mr. Mclarney has significant experience building and maintaining accounting and business functions compliant with the Sarbanes-Oxley Act of 2002, as amended. Prior to Mr. Mclarney’s appointment, Mr. Chowdhury, the Company’s Chief Executive Officer, President and member of the Company’s Board of Directors, also served as interim Chief Financial Officer. Following Mr. Mclarney’s appointment, Mr. Chowdhury continues to serve as the Company’s Chief Executive Officer, President and director. In addition, the Company hired Mazars, LLP, to provide third party internal audit support to facilitate the creation and maintenance of an internal control environment to remediate the material weakness identified.
As a result of the onboarding of a permanent Chief Financial Officer, in combination with the on-going work that the Company has been undertaking with the guidance of Mazars, LLP, we believe that our internal control over financial reporting has improved significantly during the quarter ended December 31, 2020. However, the changes and improvements made were not sufficiently embedded during the quarter ended December 31, 2020, to enable the Company to conclude that the material weakness noted above had been fully remediated.
| 17 |
PART II - OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
None.
ITEM 1A. RISK FACTORS
None.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
None.
ITEM 6. EXHIBITS
The exhibits listed on the Exhibit Index below are filed as part of this report.
| Exhibit No. | Document Description |
| 31.1 | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | Certification of the Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | Certification of the Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 101.INS | XBRL Instance Document |
| 101.SCH | XBRL Taxonomy Extension Schema |
| 101.CAL | XBRL Taxonomy Extension Calculation |
| 101.DEF | XBRL Taxonomy Extension Definition |
| 101.LAB | XBRL Taxonomy Extension Labels |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase |
| 18 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| NEMAURA MEDICAL INC. | ||
| February 12, 2021 | By: | /s/ Dewan F.H. Chowdhury |
Dewan F.H. Chowdhury (Principal Executive Officer) |
||
| February 12, 2021 | By: | /s/ Justin J. Mclarney |
Justin J. Mclarney (Principal Financial Officer and Principal Accounting Officer) |
||
| 19 |
