Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - GI DYNAMICS, INC. | d354504d8k.htm |

GI Dynamics, Inc. (ASX.GID) Pioneering treatment for type 2 diabetes /obesity without invasive surgery SHAREHOLDER UPDATE: 2016 Results and 2017 Strategy Exhibit 99.1

3/30/2017 GI Dynamics, Inc. Important Notice Currency References Financial amounts in this presentation are expressed in US Dollars, except where specifically noted. Forward-Looking Statements This presentation contains forward-looking statements concerning: our development and commercialization plans; our potential revenues and revenue growth, costs, excess inventory, profitability and financial performance; our ability to obtain reimbursement for our products; our clinical trials, and associated regulatory submissions and approvals; the number and location of commercial centers offering the EndoBarrier®; and our intellectual property position. These forward-looking statements are based on the current estimates and expectations of future events by the management of GI Dynamics, Inc. as of the date of this presentation and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those indicated in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks associated with the possibility that clinical trials will not be successful or confirm earlier results; risks associated with obtaining funding from third parties; risks relating to the timing and costs of clinical trials, the timing of regulatory submissions, the timing, receipt and maintenance of regulatory approvals, the timing and amount of other expenses, and the timing and extent of third-party reimbursement; risks associated with commercial product sales, including product performance; competition; risks related to market acceptance of products; intellectual property risks; risks related to excess inventory; risks related to assumptions regarding the size of the available market, benefits of our products, product pricing, timing of product launches, future financial results and other factors including those described in our filings with the U.S. Securities and Exchange Commission. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law. Disclaimer This presentation and any supplemental materials have been prepared by GI Dynamics, Inc. based on available information. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness, or correctness of such information and opinions and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of GI Dynamics, Inc., or any of its members, directors, officers, employees, or agents or advisors, nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of GI Dynamics, Inc. or any of its directors, officers, employees or agents. EndoBarrier® is not available for sale in the United States (OUS patients in data disclosed).

3/30/2017 GI Dynamics, Inc. Overview EndoBarrier is the only implantable device cleared for treating patients with type 2 diabetes + obesity ~3,700 implants worldwide since inception Clinical data shows EndoBarrier therapy reduces weight, HbA1c, the need for insulin, and lowers dosages of other prescribed medications Multiple post market and investigator studies underway, aimed at bolstering our efficacy and safety profile Incidence and prevalence rates of patients with combined obesity and type 2 diabetes are high and rising quickly Recent review of EndoBarrier safety profile à EndoBarrier is a safe and effective device Treatment gap in type 2 diabetes widening at alarming rate
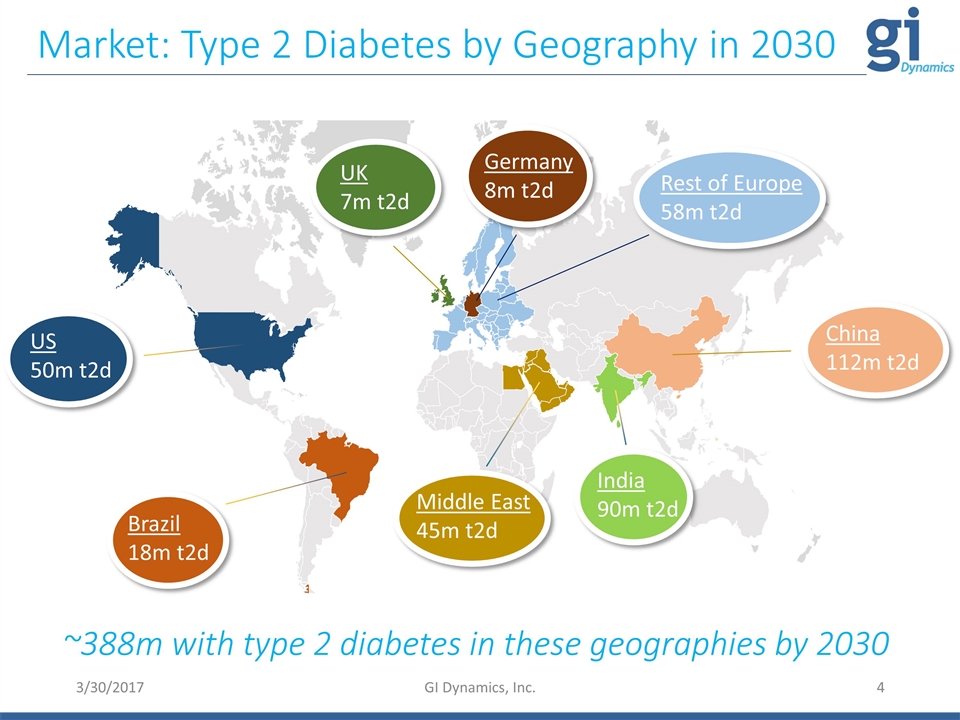
3/30/2017 GI Dynamics, Inc. Market: Type 2 Diabetes by Geography in 2030 US 50m t2d UK 7m t2d Brazil 18m t2d China 112m t2d India 90m t2d Germany 8m t2d Rest of Europe 58m t2d Middle East 45m t2d ~388m with type 2 diabetes in these geographies by 2030
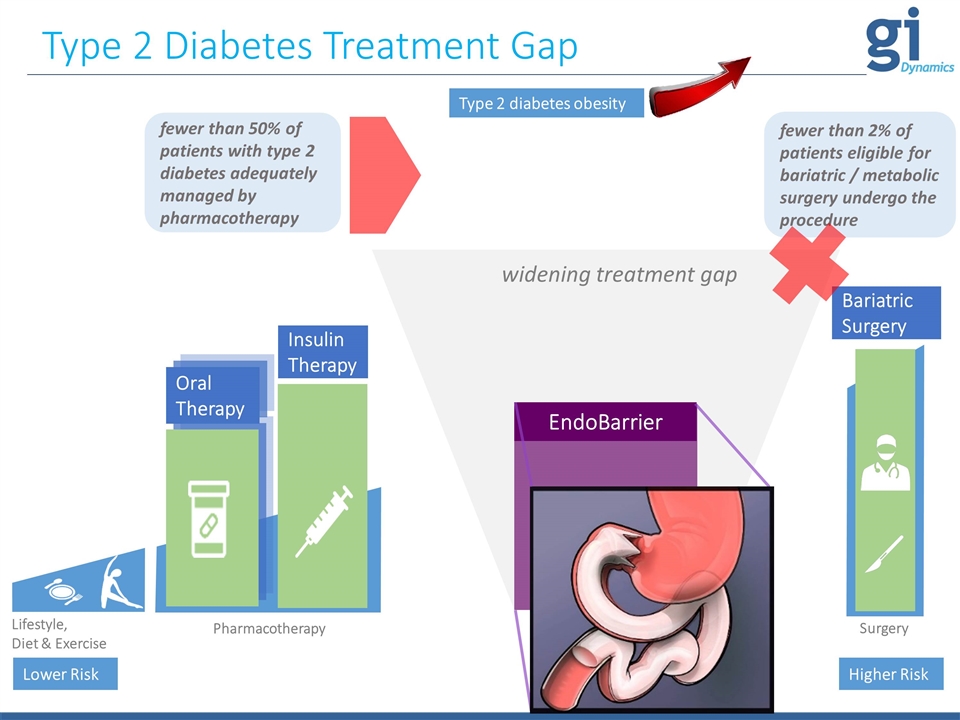
Type 2 Diabetes Treatment Gap Lifestyle, Diet & Exercise Pharmacotherapy Lower Risk Higher Risk Bariatric Surgery EndoBarrier Surgery fewer than 50% of patients with type 2 diabetes adequately managed by pharmacotherapy Oral Therapy Insulin Therapy widening treatment gap Type 2 diabetes obesity fewer than 2% of patients eligible for bariatric / metabolic surgery undergo the procedure
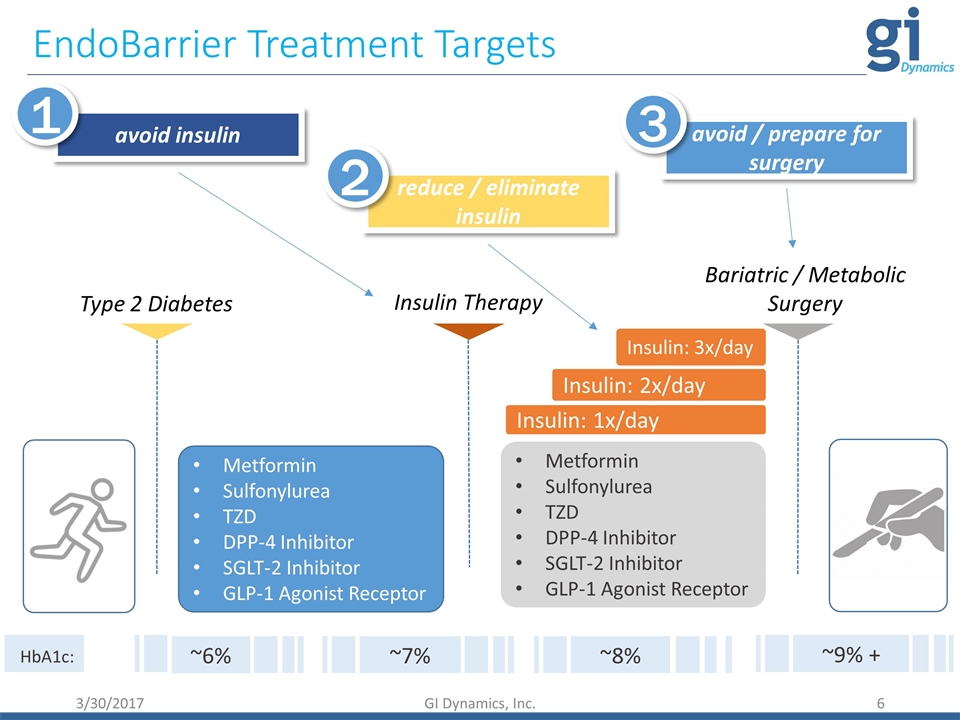
Metformin Sulfonylurea TZD DPP-4 Inhibitor SGLT-2 Inhibitor GLP-1 Agonist Receptor Insulin: 1x/day Insulin: 2x/day Insulin: 3x/day Type 2 Diabetes Insulin Therapy Metformin Sulfonylurea TZD DPP-4 Inhibitor SGLT-2 Inhibitor GLP-1 Agonist Receptor Bariatric / Metabolic Surgery EndoBarrier Treatment Targets avoid insulin 1 reduce / eliminate insulin 2 avoid / prepare for surgery 3 ~7% ~8% ~9% + HbA1c: ~6% 3/30/2017 GI Dynamics, Inc.

3/30/2017 GI Dynamics, Inc. Value Proposition There remains a significant need in the market for a device to aid HbA1c and weight reduction in patients for whom prior treatments have failed to halt disease. New management team is focused on executing clinical and commercial strategies aimed at achieving regulatory and sales goals, increasing company value and regaining investor credibility. Pricing favorable with significant investment upside potential unique diabetes / obesity implant with extensive clinical data

3/30/2017 GI Dynamics, Inc. Corporate Priorities- Final Review 2016 1 2 3 4 5 Modify Cost Structure. We will implement a leaner, more efficient cost structure by cutting expenses to extend our cash runway. Rebuild Team. As CEO, I will appoint a new chief financial officer and chief compliance officer (responsible for clinical, regulatory, and quality), in addition to adding other experienced team members. Develop Clinical Data and Core Science. We will continue to support investigator-initiated studies around the world in addition to our internal analysis of the safety and efficacy of EndoBarrier therapy. Focus Revenue Efforts. We will focus on strategic commercial centers outside the United States. Improve Regulatory Relationships. We will collaborate with the FDA to review lessons learned as we design our next EndoBarrier therapy trial, engage with our European Notified Body and the TGA in Australia to refine our post-market surveillance. Ongoing, completed significant work Full review of safety profile Working towards reimbursement, supporting clinical studies, positioning for future revenue Multiple legacy issues addressed TGA cancellation Focused on new FDA study design Completed Continuing to attract experienced professionals Completed / ongoing Extended cash runway by ~12 months
2016 Summary Presented ENDO preliminary clinical data trial at ADA Appointed Dan Moore as nonexecutive chairman of the board Presented ABCD REVISE clinical study data at ADA Presented NAFLD data at ADA Presented “The Effect of 1-Year’s Endoscopic Proximal Intestinal Exclusion Using EndoBarrier on 10-Year Cardiovascular Risk in Type 2 Diabetes” at ADA German hospitals won reimbursement arbitration court cases supporting EndoBarrier reimbursement under NUB-1 Released German registry data on over 235 patients at EASD Closed financing of 15% shelf registration, raising ~USD $1.1 million Association of British Clinical Diabetologists initiated Worldwide EndoBarrier Registry FDA Accepted ENDO clinical study report U.S. Patent Office, European Patent Office granted 8 patent allowances Closed Lexington Massachusetts office, terminated lease, moved into Boston Innovation District 3/30/2017 GI Dynamics, Inc.
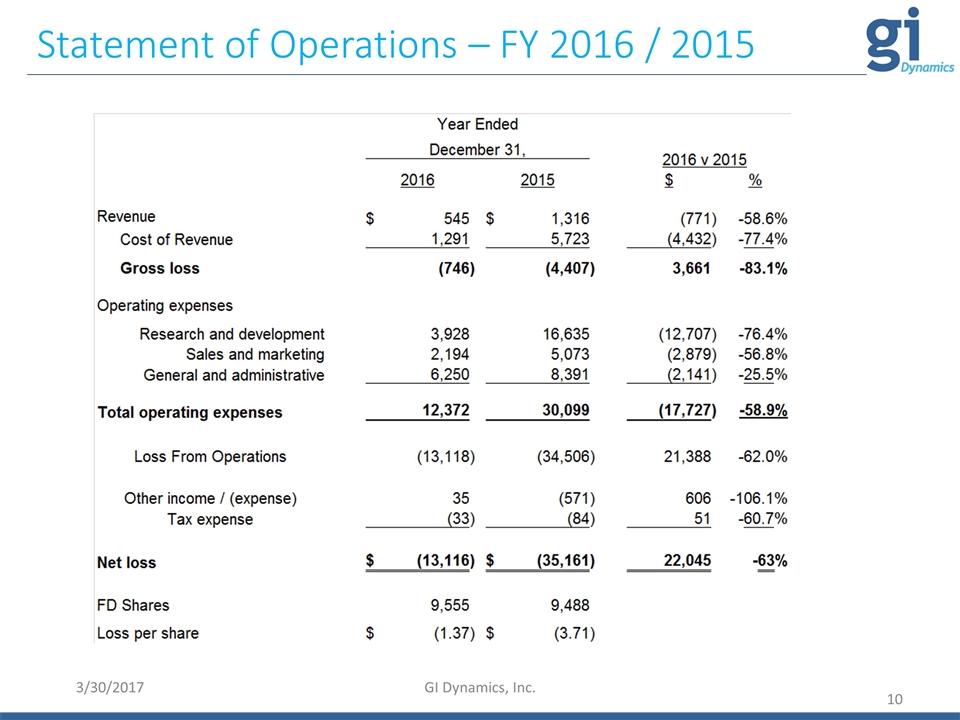
Statement of Operations – FY 2016 / 2015 3/30/2017 GI Dynamics, Inc.
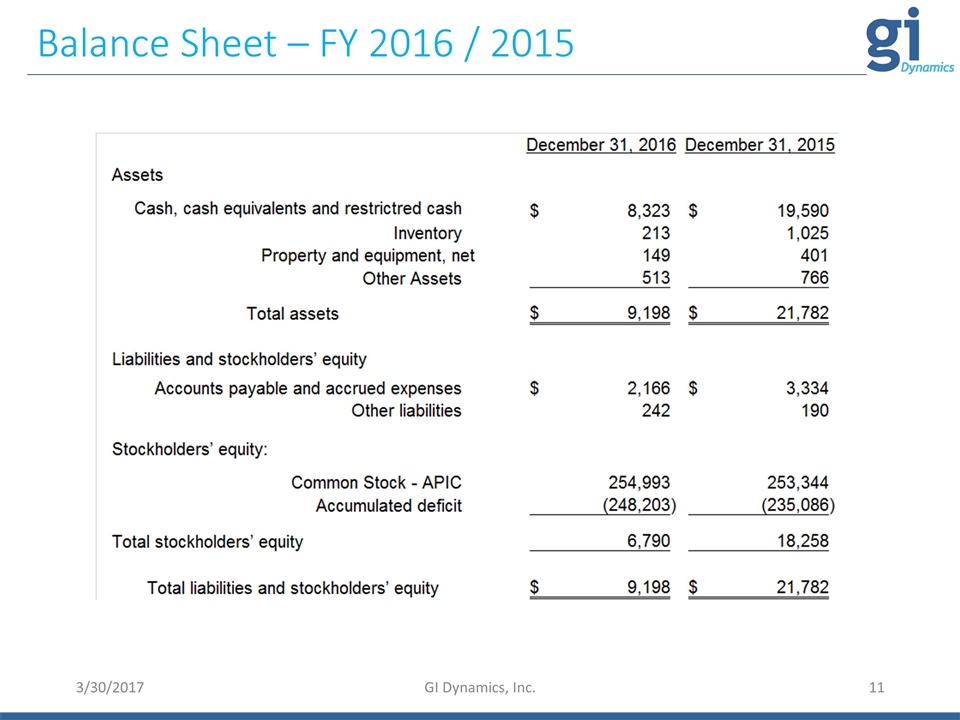
Balance Sheet – FY 2016 / 2015 3/30/2017 GI Dynamics, Inc.
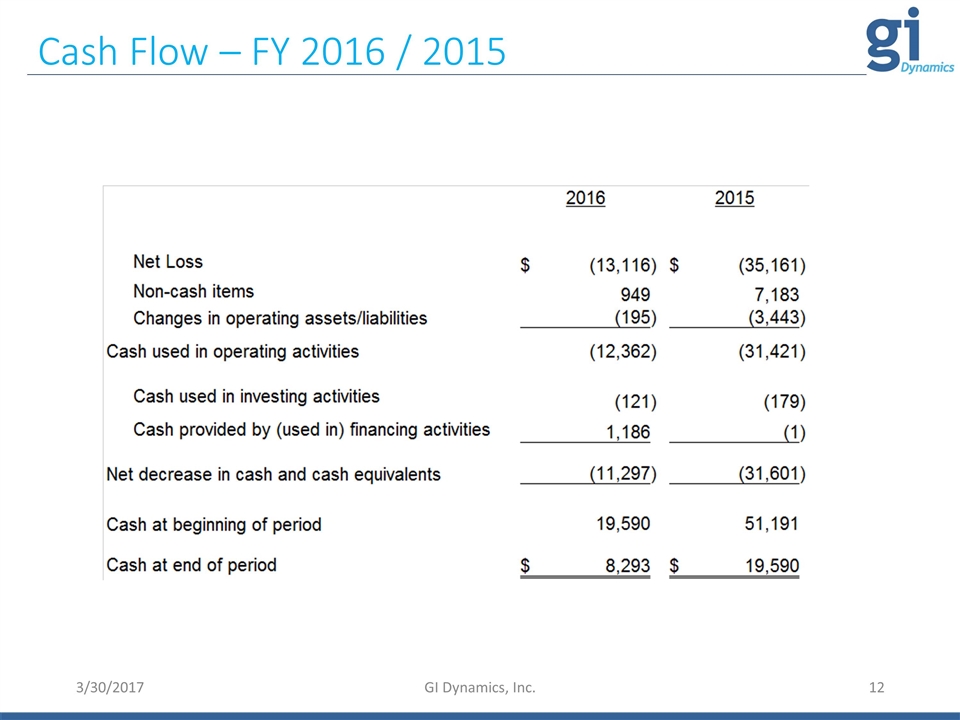
Cash Flow – FY 2016 / 2015 3/30/2017 GI Dynamics, Inc.

3/30/2017 GI Dynamics, Inc. Corporate Priorities- 2017 1 2 3 4 5 Process improvement across all internal systems and Quality Management System, Updated Risk Management à Continuous Compliance and Continuous Improvement Maintain lean operations Evolve culture of accountability and teamwork, retain / motivate / add talented team members Primary focus on UK & Germany Secondary focus: Middle East, Rest of Europe Continue to drive ongoing reimbursement efforts Focused advocacy and targeted marketing support Revitalize and revamp corporate branding Optimize commercial model and sales force effectiveness Seek agreement with FDA to initiate US clinical trial Evaluate financing options and choose best options to support operations and maximize shareholder value GI Dynamics must raise capital by Q3 2017 Support ongoing clinical trials and registries Attract and leverage world-class scientific advisory board (SAB) Drive for Continuous Improvement / Build from Ground Up Focus on Commercial Outcomes in Target Geographies Continue to develop EndoBarrier safety and efficacy data Move towards new IDE study with FDA Appropriately Capitalize

2017 Update Oern Stuge, MD added to Board of Directors Jack Meyer resigned from Board of Directors EndoBarrier receives NUB-1 designation in Germany First adolescent obesity study featuring EndoBarrier releases results GI Dynamics Scientific Advisory Board created: Professor David Cummings, MD Manoel Galvao, MD Professor Jan-Willem Greve, MD Professor Carel Le Roux, MD Continued dialogue with FDA Expecting numerous investigator-initiated clinical data releases at Digestive Disease Week (May), American Diabetes Association (June), IFSO (June) meetings 3/30/2017 GI Dynamics, Inc.

SAB We have announced 4 members and will announce more over the next months 3/30/2017 GI Dynamics, Inc. Professor Carel Le Roux, MD Head of Pathology University College Dublin Dublin, Ireland Professor David Cummings, MD Professor, Department of Medicine, Division of Metabolism, Endocrinology and Nutrition University Washington, Seattle Based at the Diabetes & Obesity Center of Excellence VA Puget Sound Health Care System Endocrinology Professor Jan Willem Greve, MD Medical Director Obesity Clinics South Chair Metabolic & Bariatric Surgery Zuyderland Medical Center Mastrich, Netherlands Manoel Galvao Neto, MD Advisory Board Member Association of Bariatric Endoscopy Associate Professor Herbert Wertheim College of Medicine at Florida International University in Miami, Florida Affiliate Professor of Surgery at ABC College of Medicine in Canto Andrew Sao Paulo, Brazil Gastroenterology Bariatric / Metabolic Surgery
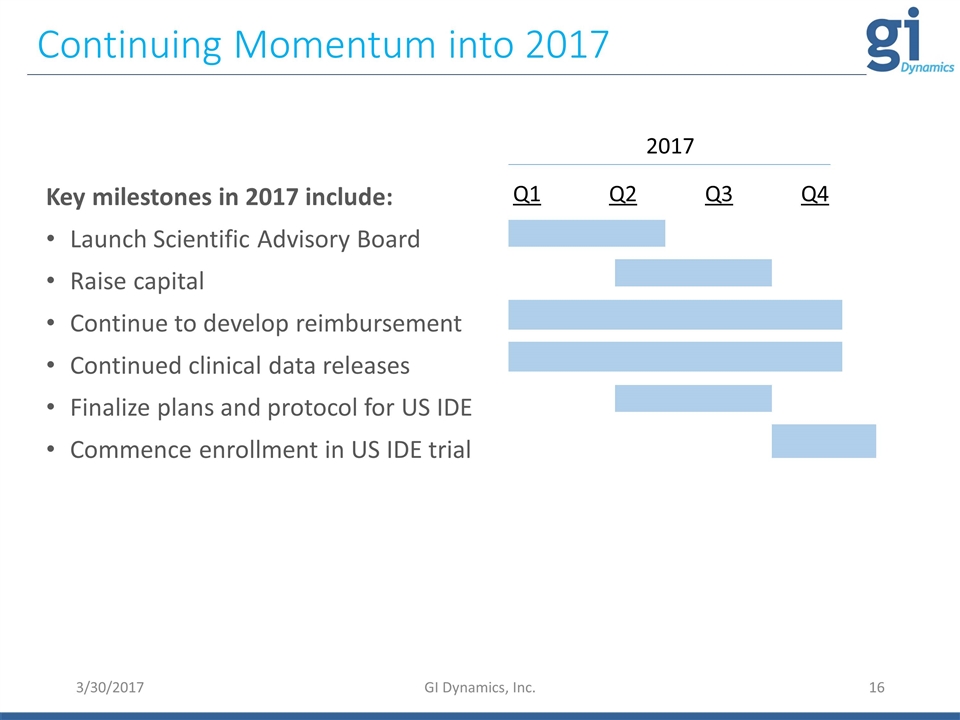
3/30/2017 GI Dynamics, Inc. Continuing Momentum into 2017 Key milestones in 2017 include: Launch Scientific Advisory Board Raise capital Continue to develop reimbursement Continued clinical data releases Finalize plans and protocol for US IDE Commence enrollment in US IDE trial Q1 Q2 Q3 Q4 2017

3/30/2017 GI Dynamics, Inc. Summary New team rebuilding confidence Applying lessons learned from thorough analysis of past Unique implant for treating the large unmet need of type 2 diabetes and obesity Significant efficacy shown in glucose control, weight loss and other risk factors Less invasive therapy with advantageous cost-benefit profile Substantial commercial & clinical experience > 3,500 shipped Significant upside potential
