Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Impax Laboratories, LLC | ipxl-3x1x2017xex991.htm |
| 8-K - 8-K - Impax Laboratories, LLC | ipxl-3x1x2017x8k.htm |

1
Fourth Quarter and
Full Year 2016 Results
March 1, 2017

2
Impax Cautionary Statement Regarding
Forward Looking Statements
"Safe Harbor" statement under the Private Securities Litigation Reform Act of 1995:
To the extent any statements made in this presentation contain information that is not historical; these statements are forward-looking in nature
and express the beliefs and expectations of management. Such statements are based on current expectations and involve a number of known
and unknown risks and uncertainties that could cause the Company’s future results, performance, or achievements to differ significantly from
the results, performance, or achievements expressed or implied by such forward-looking statements. Such risks and uncertainties include, but
are not limited to, fluctuations in the Company’s operating results and financial condition, the volatility of the market price of the Company’s
common stock, the Company’s ability to successfully develop and commercialize pharmaceutical products in a timely manner, the impact of
competition, the effect of any manufacturing or quality control problems, the Company’s ability to manage its growth, risks related to
acquisitions of or investments in technologies, products or businesses, risks relating to goodwill and intangibles, the reduction or loss of
business with any significant customer, the substantial portion of the Company’s total revenues derived from sales of a limited number of
products, the impact of consolidation of the Company’s customer base, the Company’s ability to sustain profitability and positive cash flows, the
impact of any valuation allowance on the Company’s deferred tax assets, the restrictions imposed by the Company’s credit facility and
indenture, the Company’s level of indebtedness and liabilities and the potential impact on cash flow available for operations, the availability of
additional funds in the future, any delays or unanticipated expenses in connection with the operation of the Company’s manufacturing facilities,
the effect of foreign economic, political, legal and other risks on the Company’s operations abroad, the uncertainty of patent litigation and other
legal proceedings, the increased government scrutiny on the Company’s agreements to settle patent litigations, product development risks and
the difficulty of predicting FDA filings and approvals, consumer acceptance and demand for new pharmaceutical products, the impact of market
perceptions of the Company and the safety and quality of the Company’s products, the Company’s determinations to discontinue the
manufacture and distribution of certain products, the Company’s ability to achieve returns on its investments in research and development
activities, changes to FDA approval requirements, the Company’s ability to successfully conduct clinical trials, the Company’s reliance on third
parties to conduct clinical trials and testing, the Company’s lack of a license partner for commercialization of Numient® (IPX066) outside of the
United States, impact of illegal distribution and sale by third parties of counterfeits or stolen products, the availability of raw materials and
impact of interruptions in the Company’s supply chain, the Company’s policies regarding returns, rebates, allowances and chargebacks, the use
of controlled substances in the Company’s products, the effect of current economic conditions on the Company’s industry, business, results of
operations and financial condition, disruptions or failures in the Company’s information technology systems and network infrastructure caused
by third party breaches or other events, the Company’s reliance on alliance and collaboration agreements, the Company’s reliance on licenses
to proprietary technologies, the Company’s dependence on certain employees, the Company’s ability to comply with legal and regulatory
requirements governing the healthcare industry, the regulatory environment, the effect of certain provisions in the Company’s government
contracts, the Company’s ability to protect its intellectual property, exposure to product liability claims, changes in tax regulations, uncertainties
involved in the preparation of the Company’s financial statements, the Company’s ability to maintain an effective system of internal control over
financial reporting, the effect of terrorist attacks on the Company’s business, the location of the Company’s manufacturing and research and
development facilities near earthquake fault lines, expansion of social media platforms and other risks described in the Company’s periodic
reports filed with the Securities and Exchange Commission. Forward-looking statements speak only as to the date on which they are made,
and the Company undertakes no obligation to update publicly or revise any forward-looking statement, regardless of whether new information
becomes available, future developments occur or otherwise.
Trademarks referenced herein are the property of their respective owners.
©2017 Impax Laboratories, Inc. All Rights Reserved.

3
Agenda
Business Update
Kevin Buchi – Interim President & Chief Executive Officer
Financial Review
Bryan Reasons – Senior Vice President, Chief Financial Officer
Additional Participants for Q&A Session
Doug Boothe – President Impax Generics
Michael Nestor – President Impax Specialty Pharma

4
Business Update
Kevin Buchi
Interim President & Chief Executive Officer

5
Increased rate of ANDA approvals by FDA
Consolidation of payers and customers
Aggressive competition and pricing activities; impacted our 2016 results
Generic division pricing declined ~22%
Excluding diclofenac sodium gel and metaxalone; pricing declined ~12%
Generic Industry Challenges…
Summary of 2016
$0
$50
$100
$150
$200
$250
1Q 2Q 3Q 4Q
2016 Quarterly Revenue
Generic Specialty Pharma
…Resulted in Significant Sales Volatility
$226
$173
$228
$198
$711
$606
$150
$218
$0
$200
$400
$600
$800
$1,000
2015 2016
Annual Revenue
Generic Specialty Pharma
$861 $824
($ millions)
Note: The sum of the individual amounts may not equal due to rounding.

6
Expanded production/supply
Enhanced greater awareness
The “Go To Choice”
Significantly expanded preferred drug list status
CVS agreement
2016 revenue of $92MM; up 139% over 2015
Generics Business Highlights
IMS NPA Weekly Feb. 17, 2017
0%
5%
10%
15%
20%
25%
30%
Epinephrine Auto-Injector
Share Growth
Jan. 1, 2016 – Feb. 17, 2017
4
26%
0%
10%
20%
30%
40%
50%
60%
70%
Oxymorphone ER
Share Growth
Jan. 1, 2016 – Feb. 17, 2017
7.5 & 15 mg 5, 10, 20, 30 & 40 mg
34%
46%
Sole generic player
Captured share of exiting generic player
2016 revenue of $73MM; up 23% over 2015
48%
58%
Despite Industry challenges, several products in the generic
portfolio delivered strong growth

7
Redefined clinical messaging related to formulation
Simplified dose conversion guidance for general neurologists
Launched myRytary Patient Support Program www.myrytary.com
74% revenue growth and 114% script growth in 2016 (a)
Delivering Solid Growth Across Specialty Portfolio
Emphasis on second position promotion
Increased non-personal promotion
Solid growth from general neurologists, headache
specialists and pediatricians
9% revenue growth and 8% script growth in 2016
0%
1%
2%
3%
0
2,000
4,000
6,000
8,000
10,000
12,000
14,000
Jan-16 Feb-16 Mar-16 Apr-16 May-16 Jun-16 Jul-16 Aug-16 Sep-16 Oct-16 Nov-16 Dec-16 Jan-17
CD
L
D
S
h
a
re
T
R
x
Monthly TRx and Share of National CD-LD TRx
Rytary TRx CD-LD Share
(a) Rytary was launched in Feb. 2015.
(b) Acquired in the Tower Holdings Acquisition in March 2015.
Source: IMS NPA Monthly Jan. 2017
Emverm launched late 1Q 2016
Focusing on Albenza to Emverm conversion
Increased non-personal promotion of Emverm
Albenza: 43% revenue growth in 2016 (b)

8
Investing to Expand Pipeline Opportunities
Source of sales data: IMS NSP Dec 2016; *U.S. Brand/Generic market sales; Pipeline data as of Feb 24, 2017
Generic R&D
16
14
7
6
Pending at FDA
$14B
Under Development
$10B
Solid Oral Dose Alternative Dose
23
20
# of Potential
Products
FTF or FTM
8 17
Specialty Pharma R&D
Portfolio of 43 Products
Current U.S. Brand/Generic Market of $24B
IPX-203 Carbidopa-Levodopa
Ongoing Phase 2b study
Multiple dose study in patients with
advanced Parkinson’s disease
Interim results by end of first half 2017
Held meeting with FDA in Feb. 2017 to
discuss Phase 3 development plan
Pipeline provides solid platform for growth
9
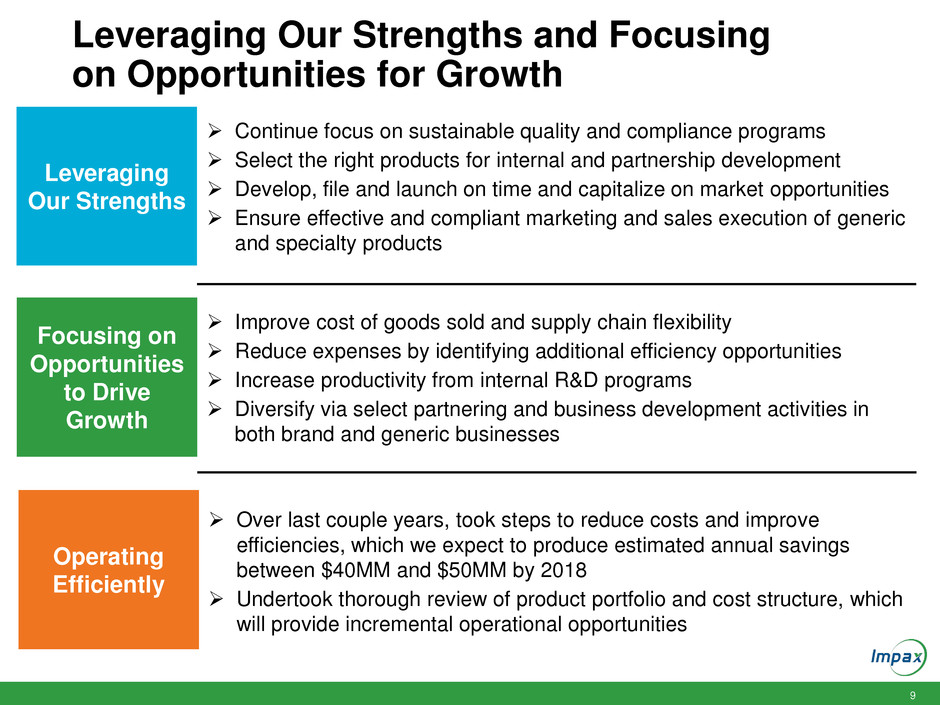
Leveraging Our Strengths and Focusing
on Opportunities for Growth
Continue focus on sustainable quality and compliance programs
Select the right products for internal and partnership development
Develop, file and launch on time and capitalize on market opportunities
Ensure effective and compliant marketing and sales execution of generic
and specialty products
Leveraging
Our Strengths
Improve cost of goods sold and supply chain flexibility
Reduce expenses by identifying additional efficiency opportunities
Increase productivity from internal R&D programs
Diversify via select partnering and business development activities in
both brand and generic businesses
Focusing on
Opportunities
to Drive
Growth
Over last couple years, took steps to reduce costs and improve
efficiencies, which we expect to produce estimated annual savings
between $40MM and $50MM by 2018
Undertook thorough review of product portfolio and cost structure, which
will provide incremental operational opportunities
Operating
Efficiently

10
Financial Review
Bryan Reasons
Senior Vice President, Chief Financial Officer
11
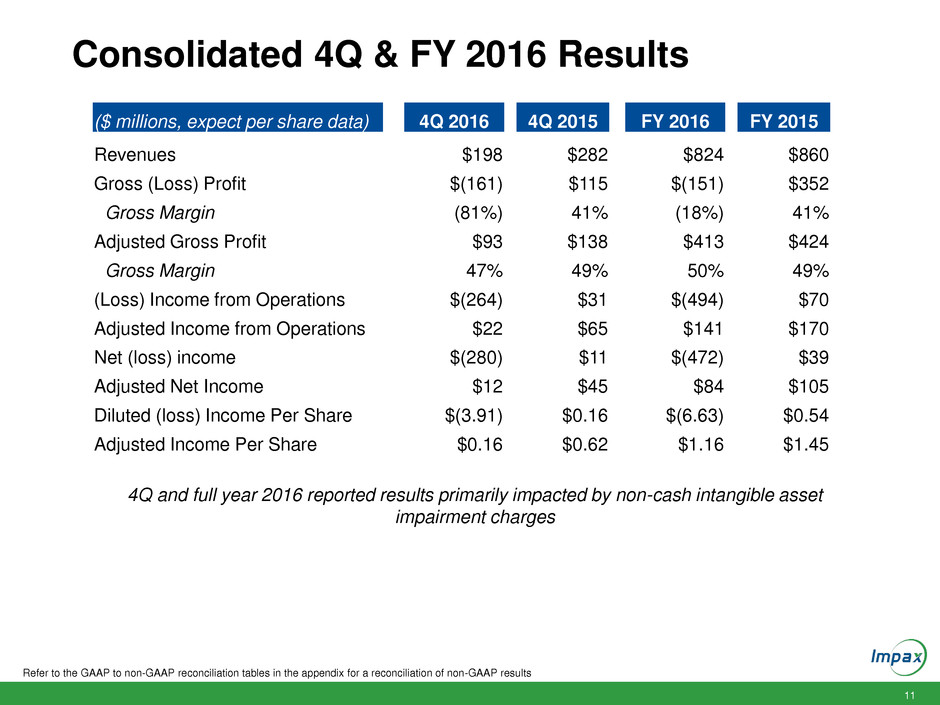
Consolidated 4Q & FY 2016 Results
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results
($ millions, expect per share data) 4Q 2016 4Q 2015 FY 2016 FY 2015
Revenues $198 $282 $824 $860
Gross (Loss) Profit $(161) $115 $(151) $352
Gross Margin (81%) 41% (18%) 41%
Adjusted Gross Profit $93 $138 $413 $424
Gross Margin 47% 49% 50% 49%
(Loss) Income from Operations $(264) $31 $(494) $70
Adjusted Income from Operations $22 $65 $141 $170
Net (loss) income $(280) $11 $(472) $39
Adjusted Net Income $12 $45 $84 $105
Diluted (loss) Income Per Share $(3.91) $0.16 $(6.63) $0.54
Adjusted Income Per Share $0.16 $0.62 $1.16 $1.45
4Q and full year 2016 reported results primarily impacted by non-cash intangible asset
impairment charges

12
$ millions 4Q 2016 FY 2016
Tower Holdings Acquisition
Currently marketed portfolio $175 $177
In-process R&D $20 $46
Other --- $8
Teva Transaction
Currently marketed portfolio $54 $302
In-process R&D $4 $7
Other
Currently marketed portfolio $1 $1
Total non-cash impairment $254 $542
Note: The sum of the individual amounts may not equal due to rounding.
Non-Cash Intangible Asset Impairment Charges

13
Generic Division 4Q & FY 2016 Results
($ millions) 4Q 2016 4Q 2015 FY 2016 FY 2015
Revenues $139 $227 $606 $711
Gross (Loss) Profit $(176) $76 $(275) $261
Gross Margin (127%) 34% (45%) 37%
Adjusted Gross Profit $46 $92 $238 $303
Gross Margin 33% 41% 39% 43%
(Loss) Income from Operations $(212) $42 $(386) $169
Adjusted Income from Operations $22 $58 $157 $211
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results
4Q and full year 2016 reported results primarily impacted by non-cash intangible asset
impairment charges

14
Specialty Pharma Division 4Q & FY 2016 Results
($ millions) 4Q 2016 4Q 2015 FY 2016 FY 2015
Revenues $59 $55 $218 $150
Gross Profit $15 $38 $124 $92
Gross Margin 26% 70% 57% 61%
Adjusted Gross Profit $47 $46 $174 $121
Gross Margin 79% 84% 80% 81%
(Loss) Income from Operations $(17) $19 $12 $19
Adjusted Income from Operations $26 $27 $87 $49
Refer to the GAAP to non-GAAP reconciliation tables in the appendix for a reconciliation of non-GAAP results
4Q and full year 2016 reported results primarily impacted by non-cash intangible asset
impairment charges
15
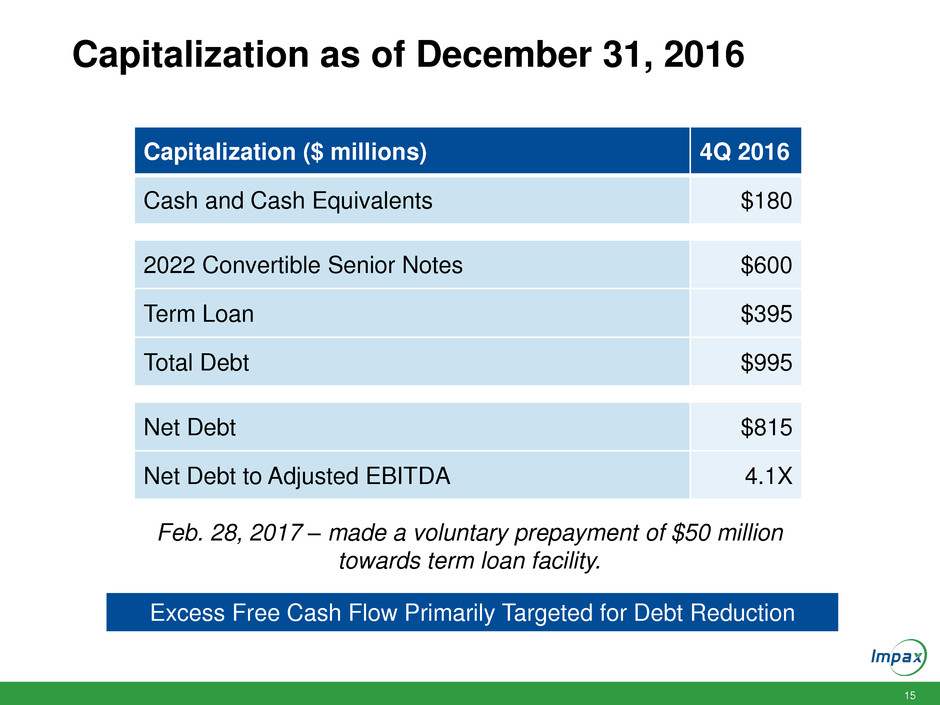
Capitalization as of December 31, 2016
Capitalization ($ millions) 4Q 2016
Cash and Cash Equivalents $180
2022 Convertible Senior Notes $600
Term Loan $395
Total Debt $995
Net Debt $815
Net Debt to Adjusted EBITDA 4.1X
Excess Free Cash Flow Primarily Targeted for Debt Reduction
Feb. 28, 2017 – made a voluntary prepayment of $50 million
towards term loan facility.

16
The Company’s full year 2017 estimates are based on management’s current expectations,
including with respect to prescription trends, pricing levels, inventory levels, and the anticipated
timing of future product launches and events. The following statements are forward-looking, and
actual results could differ materially depending on market conditions and the factors set forth
above under our “Safe Harbor” statement.
2017 Outlook

17
2017 Assumptions
Generic Division
Aggressive competition and pricing activities expected to continue
Pricing erosion in the high single to low double digit range
Minimal contribution from approved/tentatively approved products expected to launch throughout 2017
Generic Vytorin® (tentatively approved), patent expires late April
Not expecting approval of generics Renvela® and/or Welchol® until late 2017 – at the earliest
May be delayed until 2018
Total revenues, excluding products not yet approved, expected to decline year over year
Specialty Pharma Division
Continued Rytary® and Zomig® nasal spray growth
Possible generic competition on Albenza®
Partially offset by launch of our own authorized generic
Focusing on Albenza® to Emverm® conversion
The Company’s full year 2017 estimates are based on management’s current expectations, including with respect to prescription trends, pricing levels, inventory levels, and
the anticipated timing of future product launches and events. These statements are forward-looking, and actual results could differ materially depending on market conditions
and the factors set forth under our “Safe Harbor” statement above.

18
2017 Financial Guidance Summary
Guidance
Adjusted Gross Margin as a % of Revenues 47% to 49%
R&D & Patent Litigation Expense $90MM to $95MM
Selling, General & Administrative Expense $190MM to $195MM
Adjusted Interest Expense $30MM
Tax Rate 34% to 35%
Capital Expenditures $25MM to $30MM
The Company’s full year 2017 estimates are based on management’s current expectations, including with respect to prescription trends, pricing levels, inventory levels, and
the anticipated timing of future product launches and events. These statements are forward-looking, and actual results could differ materially depending on market conditions
and the factors set forth under our “Safe Harbor” statement above.
Aggressively pursuing additional efficiency opportunities to
free up resources to invest in growth initiatives and reduce
long-term debt

19
Q&A
20
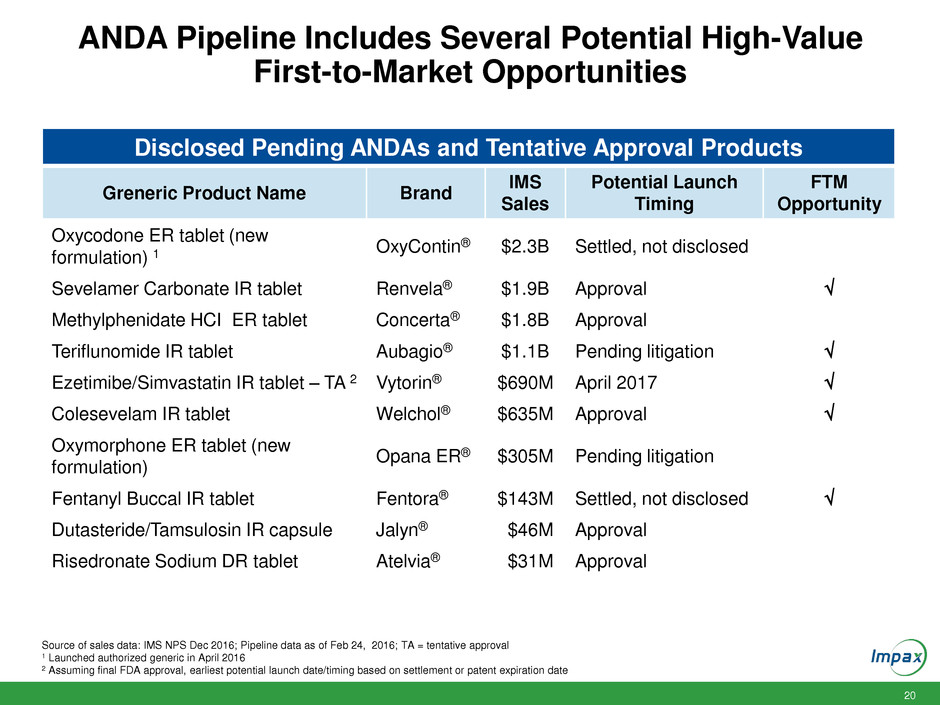
ANDA Pipeline Includes Several Potential High-Value
First-to-Market Opportunities
Source of sales data: IMS NPS Dec 2016; Pipeline data as of Feb 24, 2016; TA = tentative approval
1 Launched authorized generic in April 2016
2 Assuming final FDA approval, earliest potential launch date/timing based on settlement or patent expiration date
Disclosed Pending ANDAs and Tentative Approval Products
Greneric Product Name Brand
IMS
Sales
Potential Launch
Timing
FTM
Opportunity
Oxycodone ER tablet (new
formulation) 1
OxyContin® $2.3B Settled, not disclosed
Sevelamer Carbonate IR tablet Renvela® $1.9B Approval
Methylphenidate HCI ER tablet Concerta® $1.8B Approval
Teriflunomide IR tablet Aubagio® $1.1B Pending litigation
Ezetimibe/Simvastatin IR tablet – TA 2 Vytorin® $690M April 2017
Colesevelam IR tablet Welchol® $635M Approval
Oxymorphone ER tablet (new
formulation)
Opana ER® $305M Pending litigation
Fentanyl Buccal IR tablet Fentora® $143M Settled, not disclosed
Dutasteride/Tamsulosin IR capsule Jalyn® $46M Approval
Risedronate Sodium DR tablet Atelvia® $31M Approval

21
GAAP to Adjusted Results Reconciliation
The following table reconciles total Company reported cost of revenues and (Loss) income from operations to adjusted cost of
revenues, adjusted gross profit, adjusted gross margin and adjusted income from operations.
(Unaudited, amounts in thousands)
Refer to the Fourth Quarter 2016 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
(a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by
total revenues.
Three months ended Year Ended
December 31, December 31,
2016 2015 2016 2015
Cost of revenues $ 129,047 $ 160,019 $ 486,899 $ 500,762
Cost of revenues impairment charges 230,625 7,303 488,632 7,303
Adjusted to deduct:
Amortization 16,886 12,970 56,490 40,186
Intangible asset impairment charges 230,625 7,303 488,632 7,303
Restructuring and severance charges 6,414 1,136 18,708 6,387
Lease termination for office consolidation - - 53 -
Hayward facility remediation costs - 1,973 - 11,364
Fair value of inventory step-up - (9) - 6,458
Adjusted cost of revenues $ 105,747 $ 143,949 $ 411,648 $ 436,367
Adjusted gross profit (a) $ 92,675 $ 138,143 $ 412,781 $ 424,102
Adjusted gross margin (a) 46.7% 49.0% 50.1% 49.3%
GAAP (loss) income from operations $ (263,906) $ 30,804 $ (494,182) $ 69,568
Adjusted to deduct:
Amortization 16,886 12,970 56,490 40,186
Intangible asset impairment charges 230,625 7,303 488,632 7,303
Restructuring and severance charges 11,705 2,946 24,071 10,768
In-process research and development impairment charges 23,248 6,360 52,965 6,360
Business development expenses 251 2,363 4,540 17,334
Turing legal expenses 2,111 - 7,554 -
Lease termination for office consolidation - - 144 -
Hayward facility remediation costs - 1,973 - 11,364
Fair value of inventory step-up - (9) - 6,458
Other 600 - 1,522 750
Adjusted income from operations $ 21,520 $ 64,710 $ 141,736 $ 170,091

22
GAAP to Adjusted Net Income Reconciliation
The following table reconciles reported net (loss) income to adjusted net income.
(Unaudited, amounts in thousands, except per share and per share data)
Refer to the Fourth Quarter 2016 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal
due to rounding.
Three months ended Year Ended
December 31, December 31,
2016 2015 2016 2015
Net (loss) income $(279,585) $ 11,427 $(472,031) $ 38,997
Adjusted to add (deduct):
Amortization 16,886 12,970 56,490 40,186
Non-cash interest expense 6,241 5,204 22,846 11,230
Business development expenses 251 2,363 4,540 17,334
Intangible asset impairment charges 253,873 13,664 541,597 13,664
Reserve for Turing receivable (7,731) - 40,312 -
Turing legal expenses 2,111 - 7,554 -
Restructuring and severance charges 11,705 2,947 23,896 10,768
Fixed asset impairment charges 1,644 - 1,644 -
Lease termination for office consolidation - - 144 -
Gain on sale of asset - - - (45,574)
Loss on extinguishment of debt - - - 16,903
Net change in fair value of derivatives - 9,000 - 13,000
Hayward facility remediation costs - 1,973 - 11,364
Fair value of inventory step-up - (9) - 6,458
Ticking Fees - - - 2,317
Other 1,118 520 2,040 1,270
Income tax effect 5,136 (15,540) (145,368) (33,399)
Adjusted net income $ 11,649 $ 44,519 $ 83,664 $ 104,518
Adjusted net income per diluted share $ 0.16 $ 0.62 $ 1.16 $ 1.45
Net (loss) income per diluted share $ (3.91) $ 0.16 $ (6.63) $ 0.54

23
Generic Division GAAP to Adjusted
Results Reconciliation
The following tables reconcile the Impax Generics Division reported cost of revenues and (Loss) income from operations to adjusted cost
of revenues, adjusted gross profit, adjusted gross margin and adjusted operating income.
(Unaudited, amounts in thousands)
Refer to the Fourth Quarter 2016 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
(a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by
total revenues.
Three Months Ended Year Ended
December 31, December 31,
2016 2015 2016 2015
Cost of revenues $ 109,380 $ 143,146 $ 417,316 $ 442,742
Cost of revenues impairment charges 206,312 7,303 464,319 7,303
Adjusted to deduct:
Amortization 9,470 5,155 30,599 15,692
Intangible asset impairment charges 206,312 7,303 464,319 7,303
Restructuring and severance charges 6,414 1,136 18,708 6,387
Lease termination for office consolidation - - 53 -
Hayward facility remediation costs - 1,973 - 11,364
Fair value of inventory step-up - (9) - 1,083
Adjusted cost of revenues $ 93,496 $ 134,891 $ 367,956 $ 408,216
Adjusted gross profit (a) $ 45,730 $ 91,955 $ 238,364 $ 302,716
Adjusted gross margin (a) 32.8% 40.5% 39.3% 42.6%
GAAP (loss) income from operations $ (212,088) $ 42,256 $ (386,397) $ 169,466
Adjusted to add (deduct):
Amortization 9,470 5,155 30,599 15,692
Intangible asset impairment charges 217,587 7,303 492,084 7,303
Restructuring and severance charges 6,414 472 18,708 5,723
Payments for licensing agreements 600 - 1,522 -
Lease termination for office consolidation - - 144 -
Hayward facility remediation costs - 1,973 - 11,364
Fair value of inventory step-up - (9) - 1,083
Hayward technical operations and R&D restructuring - 664 - 664
Tower Acquisition severance - - - -
Adjusted income from operations $ 21,983 $ 57,814 $ 156,660 $ 211,295
24
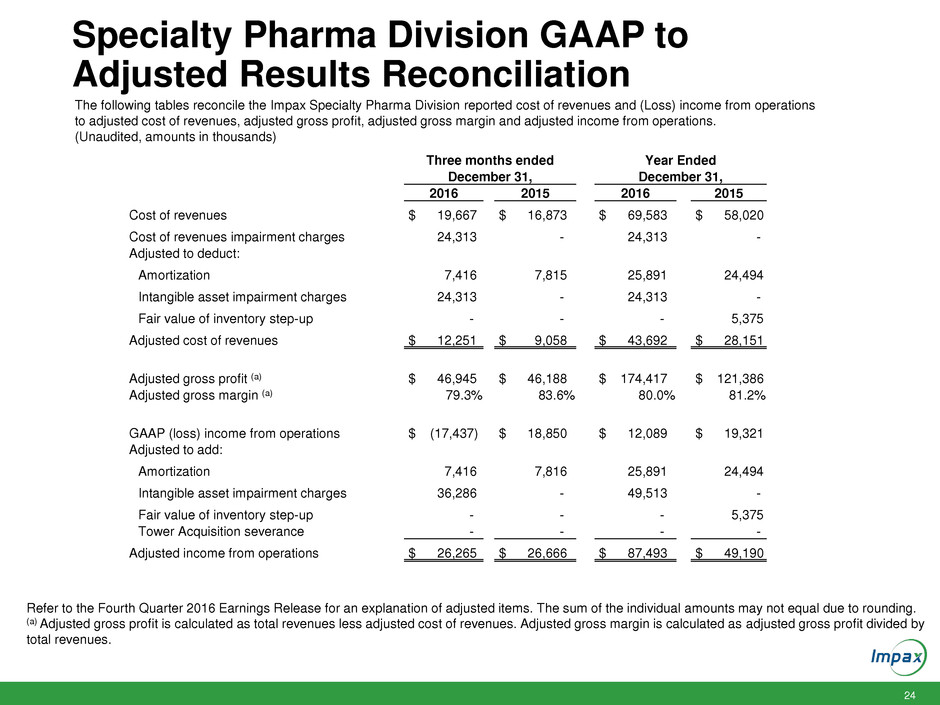
Specialty Pharma Division GAAP to
Adjusted Results Reconciliation
The following tables reconcile the Impax Specialty Pharma Division reported cost of revenues and (Loss) income from operations
to adjusted cost of revenues, adjusted gross profit, adjusted gross margin and adjusted income from operations.
(Unaudited, amounts in thousands)
Refer to the Fourth Quarter 2016 Earnings Release for an explanation of adjusted items. The sum of the individual amounts may not equal due to rounding.
(a) Adjusted gross profit is calculated as total revenues less adjusted cost of revenues. Adjusted gross margin is calculated as adjusted gross profit divided by
total revenues.
Three months ended Year Ended
December 31, December 31,
2016 2015 2016 2015
Cost of revenues $ 19,667 $ 16,873 $ 69,583 $ 58,020
Cost of revenues impairment charges 24,313 - 24,313 -
Adjusted to deduct:
Amortization 7,416 7,815 25,891 24,494
Intangible asset impairment charges 24,313 - 24,313 -
Fair value of inventory step-up - - - 5,375
Adjusted cost of revenues $ 12,251 $ 9,058 $ 43,692 $ 28,151
Adjusted gross profit (a) $ 46,945 $ 46,188 $ 174,417 $ 121,386
Adjusted gross margin (a) 79.3% 83.6% 80.0% 81.2%
GAAP (loss) income from operations $ (17,437) $ 18,850 $ 12,089 $ 19,321
Adjusted to add:
Amortization 7,416 7,816 25,891 24,494
Intangible asset impairment charges 36,286 - 49,513 -
Fair value of inventory step-up - - - 5,375
Tower Acquisition severance - - - -
Adjusted income from operations $ 26,265 $ 26,666 $ 87,493 $ 49,190
