Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AVADEL PHARMACEUTICALS PLC | form8-kcover_presentation.htm |

Avadel Pharmaceuticals plc
January 2017
Corporate Presentation

2January 2017
Safe Harbor
This presentation may include "forward-looking statements" within the meaning of the Private Securities Litigation
Reform Act of 1995. All statements herein that are not clearly historical in nature are forward-looking, and the words
"anticipate," "assume," "believe," "expect," "estimate," "plan," "will," "may," and the negative of these and similar
expressions generally identify forward-looking statements. All forward-looking statements involve risks, uncertainties
and contingencies, many of which are beyond Flamel's control and could cause actual results to differ materially from
the results contemplated in such forward-looking statements. These risks, uncertainties and contingencies include the
risks relating to: our dependence on a small number of products and customers for the majority of our revenues; the
possibility that our Bloxiverz®, Vazculep® and Akovaz™ products, which are not patent protected, could face substantial
competition resulting in a loss of market share or forcing us to reduce the prices we charge for those products; the
possibility that we could fail to successfully complete the research and development for the pipeline product we are
evaluating for potential application to the FDA pursuant to our "unapproved-to-approved" strategy, or that competitors
could complete the development of such product and apply for FDA approval of such product before us; our
dependence on the performance of third parties in partnerships or strategic alliances for the commercialization of some
of our products; the possibility that our products may not reach the commercial market or gain market acceptance; our
need to invest substantial sums in research and development in order to remain competitive; our dependence on
certain single providers for development of several of our drug delivery platforms and products; our dependence on a
limited number of suppliers to manufacture our products and to deliver certain raw materials used in our products; the
possibility that our competitors may develop and market technologies or products that are more effective or safer than
ours, or obtain regulatory approval and market such technologies or products before we do; the challenges in
protecting the intellectual property underlying our drug delivery platforms and other products; our dependence on key
personnel to execute our business plan; the amount of additional costs we will incur to comply with U.S. securities laws
as a result of our ceasing to qualify as a foreign private issuer; and the other risks, uncertainties and contingencies
described in the Company's filings with the U.S. Securities and Exchange Commission, including our annual report on
Form 10-K for the year ended December 31, 2015, all of which filings are also available on the Company's website.
Flamel undertakes no obligation to update its forward-looking statements as a result of new information, future events
or otherwise, except as required by law.
3January 2017
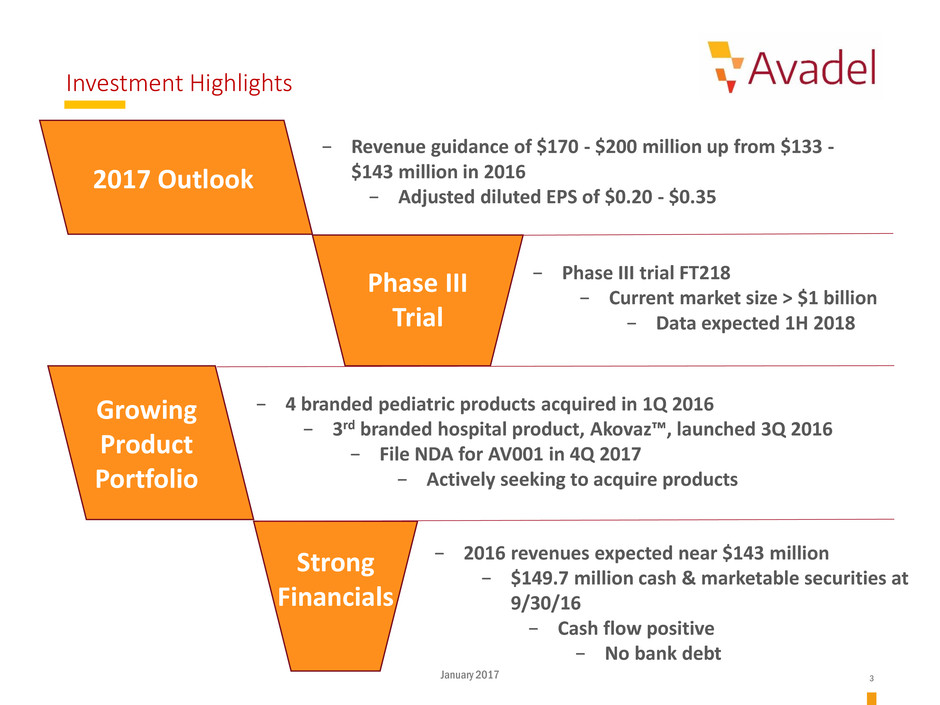
Investment Highlights
Phase III
Trial
Growing
Product
Portfolio
2017 Outlook
− 2016 revenues expected near $143 million
− $149.7 million cash & marketable securities at
9/30/16
− Cash flow positive
− No bank debt
− Phase III trial FT218
− Current market size > $1 billion
− Data expected 1H 2018
− 4 branded pediatric products acquired in 1Q 2016
− 3rd branded hospital product, Akovaz™, launched 3Q 2016
− File NDA for AV001 in 4Q 2017
− Actively seeking to acquire products
− Revenue guidance of $170 - $200 million up from $133 -
$143 million in 2016
− Adjusted diluted EPS of $0.20 - $0.35
Strong
Financials

4January 2017
Corporate Transformation
− Established in 1990 to
provide life cycle solutions
to large pharma using its
polymer-based drug
delivery technology
− Coreg CR® using
Micropump® received FDA
approval (GSK partnered
product)
− Acquired Éclat
Pharmaceuticals and
transitions to specialty
pharma model
− FDA approval for
Boxiverz®
− FDA approval for
Vazculep®
− Moved IP to Irish
subsidiary
− FDA approval for
Akovaz™
− Acquired FSC Pediatrics
− Phase III Trial for FT218
initiated
− Reincorporated in
Ireland
− Avadel established

5January 2017
Management Team
Michael Anderson
Chief Executive Officer
• Appointed CEO in 2012
• Former CEO of Éclat
Pharmaceuticals
• Former President & CEO of
generics business at KV
Pharmaceutical Company
• Former President & CEO of
Ther-Rx
Michael Kanan
SVP, Chief Financial Officer
• Appointed in 2015
• Former VP, Finance,
Corporate Controller & Chief
Accounting Officer at Sigma
Aldrich
• Various finance leadership
roles Meritor
Gregory Divis
EVP, Chief Commercial Officer
• Appointed CCO in January
2017
• Former President & CEO of
Lumara Health
• VP, Business Development
& Lifecycle Management at
Sanofi-Aventis
• VP & General Manager, UK
and Ireland, for Schering-
Plough
Phil Thompson
SVP, General Counsel
• Appointed in 2013
• VP, Legal Affairs at West-
Ward Pharmaceutical Corp
• VP, General Counsel for
Paddock Laboratories
• VP, Strategic Business
Transactions & Assistant
General Counsel at KV
Pharmaceutical Co.
Sandy Hatten
SVP, Quality & Regulatory
• Appointed in 2015
• Former SVP, Quality &
Regulatory Compliance at
Mallinckrodt plc
• VP, Quality Assurance at KV
Pharmaceutical Co
• Director, Quality Assurance
at Perrigo
Dhiren D’Silva
SVP, Irish & European Operations
• Appointed in 2015
• Former Sr. Director of
International Business Operations
at NPS Pharmaceuticals, Inc.
• Served as Director of Business
Development for Product
Ventures Group at Catalent
Pharma
David Monteith
VP, Research & Development
• Appointed in 2014
• Former AVP, Pharmaceutical
Development for Emerging
Markets at Merck & Co
• Worked at Schering-Plough
in various positions from Ass.
Director, Pharmaceutical
Development to Sr. Director,
Product Value Enhancement
Gregg Davis
VP, Business Development
• Appointed in 2015
• Previously co-founder & CBO
of Flag Therapeutics, Inc.
• Former VP, Corporate
Development of Patheon
• Former Director, Worldwide
Business Development at
GlaxoSmithKline.

6January 2017
Performance Highlights
Generated total revenue at the top end of guidance: $133 - $143 million
Completed cross-border merger from France to Ireland
Launched Akovaz™ (approved 4/29/16)
Integrated pediatric products (acquired 2/8/16)
Commenced dosing for REST-ON Phase III trial of Micropump® sodium
oxybate (FT218)
Began licensing discussion for Trigger Lock™ & Medusa™ technologies
Initiated development of 4th unapproved marketed product (UMD),
AV001
2016

7January 2017
Pipeline
Completed feasibility and PK studies for both Medusa™ exenatide and Trigger Lock™
hydromorphone, and actively seeking to out license or divest these platforms*
(AV001)
* Please see our appendix for more details on these technologies
OTC
8January 2017
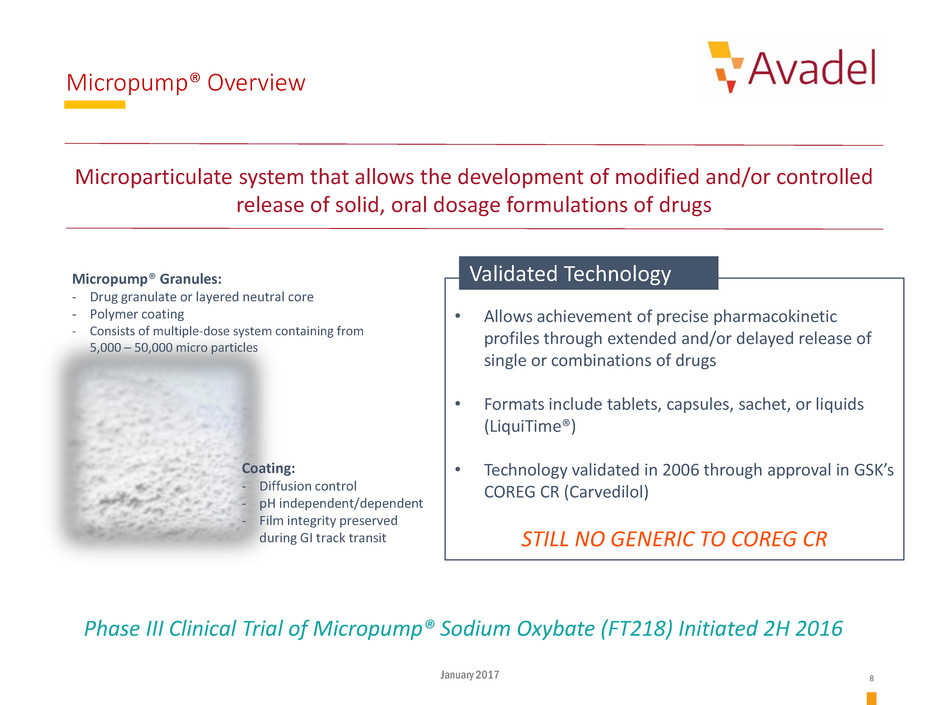
Micropump® Overview
Micropump® Granules:
- Drug granulate or layered neutral core
- Polymer coating
- Consists of multiple-dose system containing from
5,000 – 50,000 micro particles
Coating:
- Diffusion control
- pH independent/dependent
- Film integrity preserved --
during GI track transit
• Allows achievement of precise pharmacokinetic
profiles through extended and/or delayed release of
single or combinations of drugs
• Formats include tablets, capsules, sachet, or liquids
(LiquiTime®)
• Technology validated in 2006 through approval in GSK’s
COREG CR (Carvedilol)
STILL NO GENERIC TO COREG CR
Microparticulate system that allows the development of modified and/or controlled
release of solid, oral dosage formulations of drugs
Validated Technology
Phase III Clinical Trial of Micropump® Sodium Oxybate (FT218) Initiated 2H 2016

9January 2017
LiquiTime® Overview
• Intended for development of modified/controlled release liquid
formulations for patients having issues swallowing
tablets/capsules
• Not limited to working solely with ionic drugs as with resin-
complex based technologies
• Easy-to-swallow, good mouthfeel, taste-masked and dosing
flexibility
Potential Advantages
Out licensed rights to Perrigo for OTC cough / cold products, and internally conducting
feasibility assessment on a number of Rx products

10January 2017
Narcolepsy Overview
A sleep disorder, involving irregular patterns in Rapid Eye Movement (REM) sleep and
significant disruptions of the normal sleep/wake cycle
- Only 25% of people
with narcolepsy have
been diagnosed and are
receiving treatment*
- Sodium oxybate (Xyrem®)
dosed 2x / night – totaling 9g -
only drug indicated for BOTH
EDS and Cataplexy***
- Xyrem® expected to
generate between $1.1 -
$1.125 billion in revenue in
2016**
~
- ~12,800 of diagnosed
patients treated with sodium
oxybate**
*Narcolepsy Network foundation http://narcolepsynetwork.org/about-narcolepsy/
**Jazz Pharmaceuticals plc 3Q2016 Earnings Conference Call
*** Xyrem prescribing information
- Estimated ~ 200,000
Americans suffer from
Narcolepsy*
- Prevalent symptoms
include Excessive
Daytime Sleepiness
(EDS) and Cataplexy*
11January 2017
FT218: Potential for Improved Treatment
FT218: Once-nightly formulation of sodium oxybate utilizing Avadel’s proprietary
extended-release Micropump® micro/nano particle technology for oral suspension
Studied in 40 healthy volunteers:
Comparable AUC as Xyrem® on dose-
for-dose basis
Similar onset of action to Xyrem®
Similar blood levels at hrs 7-8
Slightly lower C-max
FT218 potential to provide:
• One single dose at bedtime
• Possible reduction of sleep
disruption
• Potential for additional benefits,
including improved safety
Goal: Provide 7-8 hours of restful sleep and effective relief of EDS and Cataplexy with a
single dose of medication

12January 2017
Double-Blind, Randomized, Placebo-Controlled, Study to Assess
Safety and Efficacy of Once Nightly Sodium Oxybate (FT218) for
the Treatment of Excessive Daytime Sleepiness (EDS) and
Cataplexy in Patients with Narcolepsy
REST-ON Phase III
Clinical Trial
13January 2017
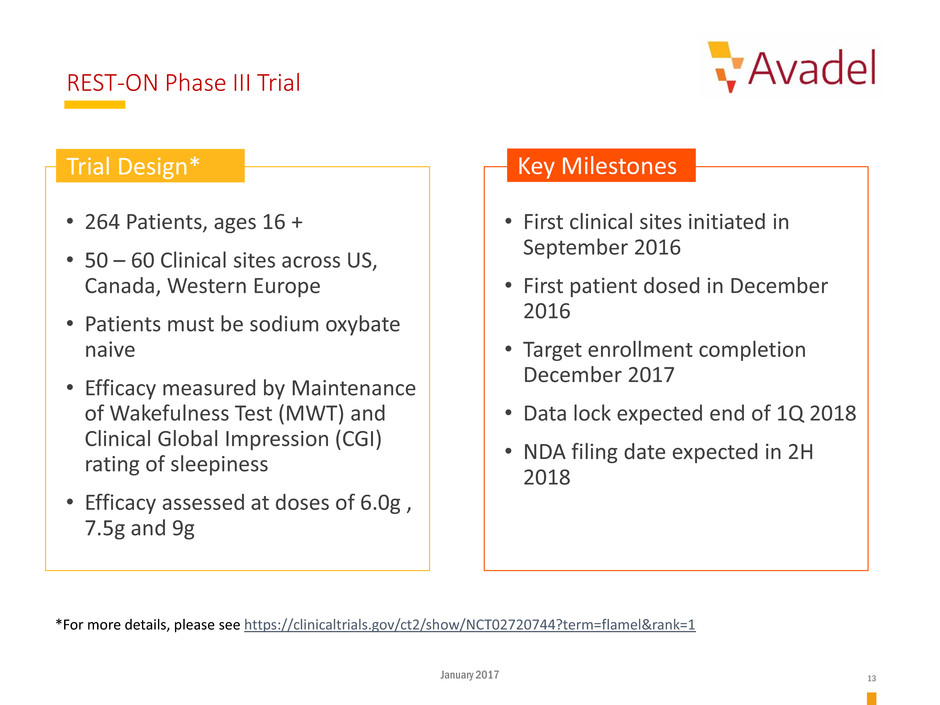
REST-ON Phase III Trial
• 264 Patients, ages 16 +
• 50 – 60 Clinical sites across US,
Canada, Western Europe
• Patients must be sodium oxybate
naive
• Efficacy measured by Maintenance
of Wakefulness Test (MWT) and
Clinical Global Impression (CGI)
rating of sleepiness
• Efficacy assessed at doses of 6.0g ,
7.5g and 9g
• First clinical sites initiated in
September 2016
• First patient dosed in December
2016
• Target enrollment completion
December 2017
• Data lock expected end of 1Q 2018
• NDA filing date expected in 2H
2018
Trial Design* Key Milestones
*For more details, please see https://clinicaltrials.gov/ct2/show/NCT02720744?term=flamel&rank=1

14January 2017
Current Product Portfolio
Hospital Products Pediatric Products

15January 2017
Hospital Products
Akovaz™ (ephedrine sulfate injection)
• Market volume ~ 7 million vials / year
Bloxiverz® (neostigmine methylsulfate injection)
• Market volume ~ 4 million vials / year
Vazculep® (phenylephrine hydrochloride)
• Market volume
1mL vial – 5.7 million 5mL vial – 1.2 M 10mL vial – 0.2 million
Hospital products generated $100.7 million in revenue through 9/30/16
First to gain FDA Approval for neostigmine, ephedrine and full-line phenylephrine
For full prescribing information on these products, please see the appendix.
16January 2017
Pediatrics
• Acquired 3 commercial stage pediatric-focused
products (February 2016)
• Flexichamber® launch planned for the end of 1Q
2017
• Actively seeking to acquire additional products to fold
into sales force
For full prescribing information on these products, please see the appendix.
17January 2017
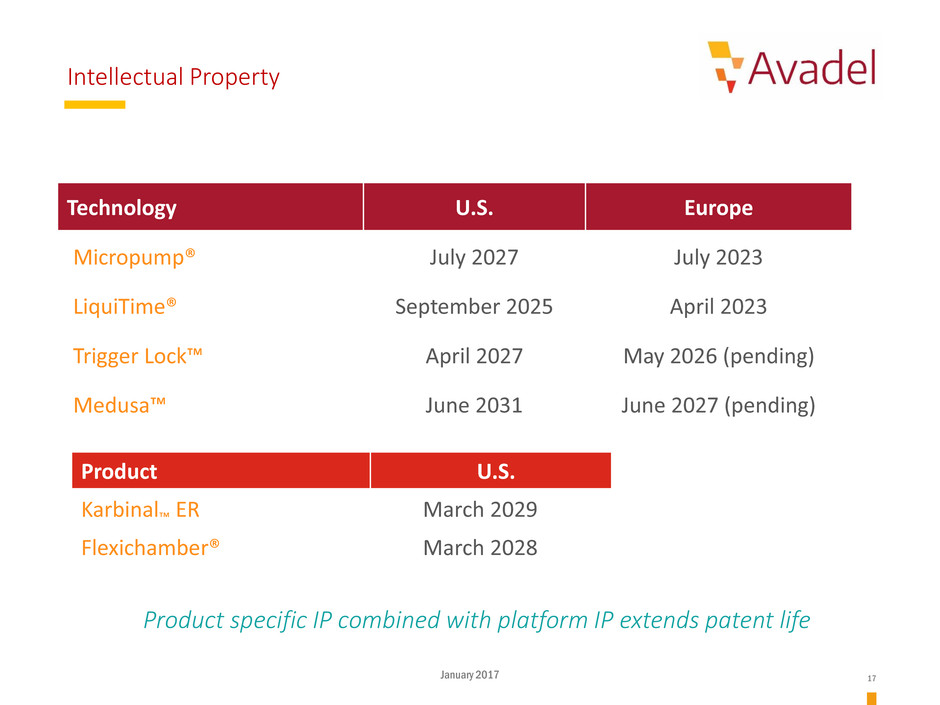
Intellectual Property
Technology U.S. Europe
Micropump® July 2027 July 2023
LiquiTime® September 2025 April 2023
Trigger Lock™ April 2027 May 2026 (pending)
Medusa™ June 2031 June 2027 (pending)
Product U.S.
Karbinal™ ER March 2029
Flexichamber® March 2028
Product specific IP combined with platform IP extends patent life

18January 2017
Non GAAP Financial Results
*Reconciliations from GAAP to Non-GAAP can be found in the appendix
2016 2015
Total revenue 107,161 128,441 (21,280)
-
Cost of products and services sold (3rd Party) 9,132 8,473 659
Intercompany cost of products sold - - -
Cost of products and services sold 9,132 8,473 659
Research and development expenses 21,135 20,447 688
Selling, general and administrative expenses 33,491 14,904 18,587
Intangible asset amortization - - -
Changes in fair value of related party contingent consideration 19,321 23,923 (4,602)
Loss on early repayment of related party acquisition-related note - - -
Total operating expenses 83,079 67,747 15,332
-
Operating income (loss) 24,082 60,694 (36,612)
-
Interest & Other Expense (net) (2,240) (2,120) -
Income (loss) before income taxes 21,842 58,574 (36,732)
-
Income tax provision 24,485 27,604 (3,119)
-
Net Loss (2,643)$ 30,971$ (33,613)$
0
Net loss per share - Diluted (0.06)$ 0.77$ (0.83)$
Difference
Year/Year
Nine months ended September 30,
(in $000s)
19January 2017
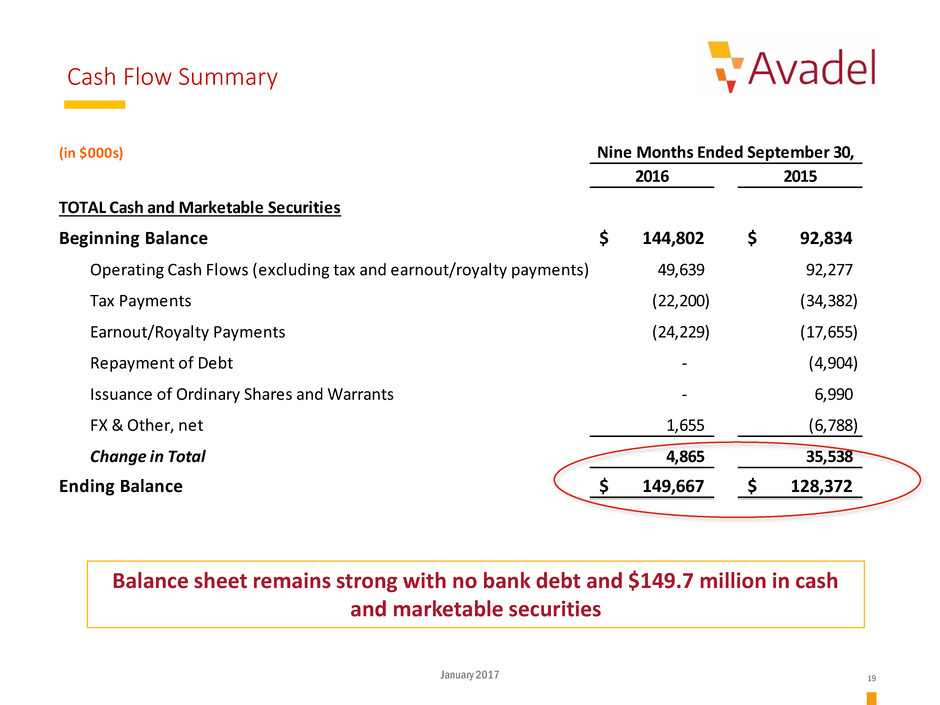
Cash Flow Summary
(in $000s)
2016 2015
TOTAL Cash and Marketable Securities
Beginning Balance 144,802$ 92,834$
Operating Cash Flows (excluding tax and earnout/royalty payments) 49,639 92,277
Tax Payments (22,200) (34,382)
Earnout/Royalty Payments (24,229) (17,655)
Repayment of Debt - (4,904)
Issuance of Ordinary Shares and Warrants - 6,990
FX & Other, net 1,655 (6,788)
Change in Total 4,865 35,538
Ending Balance 149,667$ 128,372$
Nine Months Ended September 30,
Balance sheet remains strong with no bank debt and $149.7 million in cash
and marketable securities
20January 2017
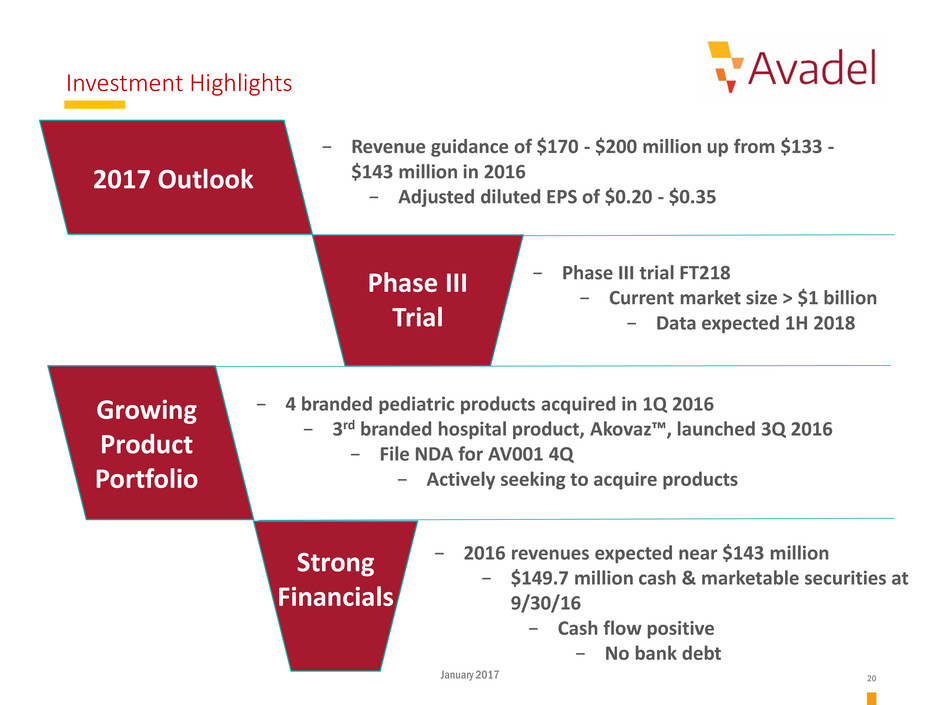
Investment Highlights
Phase III
Trial
Growing
Product
Portfolio
2017 Outlook
− 2016 revenues expected near $143 million
− $149.7 million cash & marketable securities at
9/30/16
− Cash flow positive
− No bank debt
− Phase III trial FT218
− Current market size > $1 billion
− Data expected 1H 2018
− 4 branded pediatric products acquired in 1Q 2016
− 3rd branded hospital product, Akovaz™, launched 3Q 2016
− File NDA for AV001 4Q
− Actively seeking to acquire products
− Revenue guidance of $170 - $200 million up from $133 -
$143 million in 2016
− Adjusted diluted EPS of $0.20 - $0.35
Strong
Financials

January 2017 21
Appendix

22January 2017
GAAP to Non-GAAP Adjustments
Nine Months Ended September 30, 2016:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Purchase
accounting
adjustments -
FSC
Contingent
related party
payable
fair value
remeasurements
Contingent related
party payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 104,858$ -$ -$ -$ -$ -$ -$ 104,858$
License and research revenue 2,303 - - - - - - 2,303
Total revenue 107,161 - - - - - - 107,161
Cost of products and services sold (3rd Party) 10,657 - - (1,525) - - (1,525) 9,132
Intercompany cost of products sold - - - - - - - -
Cost of products and services sold 10,657 - - (1,525) - - (1,525) 9,132
Research and development expenses 21,135 - - - - - - 21,135
Selling, general and administrative expenses 33,491 - - - - - - 33,491
Intangible asset amortization 10,918 (10,918) - - - - (10,918) -
Changes in fair value of related party contingent consideration 52,989 - - - (52,989) 19,321 (33,668) 19,321
Total operating expenses 129,190 (10,918) - (1,525) (52,989) 19,321 (46,111) 83,079
Operating income (loss) (22,029) 10,918 - 1,525 52,989 (19,321) 46,111 24,082
Investment Income 1,080 - - - - - - 1,080
Interest Expense (702) - - - - - - (702)
Other Expense - changes in fair value of related party payable (6,135) - - - 6,135 (2,618) 3,517 (2,618)
Foreign exchange gain (loss) (12) - 12 - - - 12 -
Income (loss) before income taxes (27,798) 10,918 12 1,525 59,124 (21,939) 49,640 21,842
Income tax provision 18,212 3,920 - 533 2,986 (1,165) 6,273 24,485
Income Tax Rate (66%) 36% - 35% 5% 5% 13% 112%
Net Loss (46,010)$ 6,998$ 12$ 992$ 56,138$ (20,774)$ 43,367$ (2,643)$
Net loss per share - Diluted (1.12)$ 0.17$ -$ 0.02$ 1.36$ (0.50)$ 1.05$ (0.06)$
Adjustments
Exclude
Nine months ended September 30, 2016
23January 2017
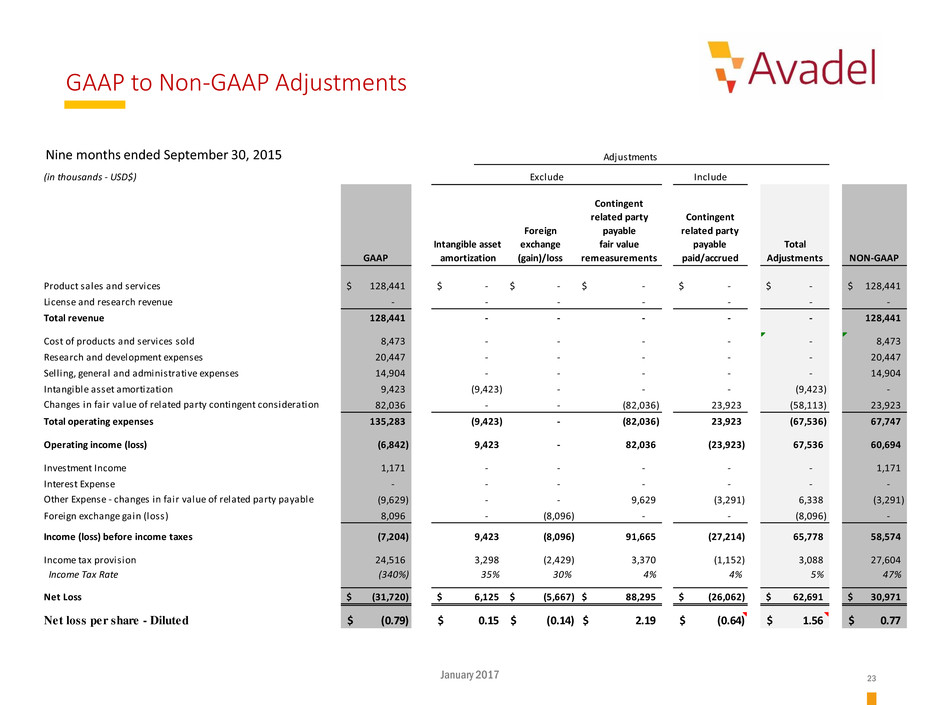
Nine Months Ended September 30, 2015:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Contingent
related party
payable
fair value
remeasurements
Contingent
related party
payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 128,441$ -$ -$ -$ -$ -$ 128,441$
License and research revenue - - - - - - -
Total revenue 128,441 - - - - - 128,441
Cost of products and services sold 8,473 - - - - - 8,473
Research and development expenses 20,447 - - - - - 20,447
Selling, general and administrative expenses 14,904 - - - - - 14,904
Intangible asset amortization 9,423 (9,423) - - - (9,423) -
Changes in fair value of related party contingent consideration 82,036 - - (82,036) 23,923 (58,113) 23,923
Total operating expenses 135,283 (9,423) - (82,036) 23,923 (67,536) 67,747
Operating income (loss) (6,842) 9,423 - 82,036 (23,923) 67,536 60,694
Investment Income 1,171 - - - - - 1,171
Interest Expense - - - - - - -
Other Expense - changes in fair value of related party payable (9,629) - - 9,629 (3,291) 6,338 (3,291)
Foreign exchange gain (loss) 8,096 - (8,096) - - (8,096) -
Income (loss) before income taxes (7,204) 9,423 (8,096) 91,665 (27,214) 65,778 58,574
Income tax provision 24,516 3,298 (2,429) 3,370 (1,152) 3,088 27,604
Income Tax Rate (340%) 35% 30% 4% 4% 5% 47%
Net Loss (31,720)$ 6,125$ (5,667)$ 88,295$ (26,062)$ 62,691$ 30,971$
Net loss per share - Diluted (0.79)$ 0.15$ (0.14)$ 2.19$ (0.64)$ 1.56$ 0.77$
Adjustments
Exclude
Nine months ended September 30, 2015
GAAP to Non-GAAP Adjustments
24January 2017
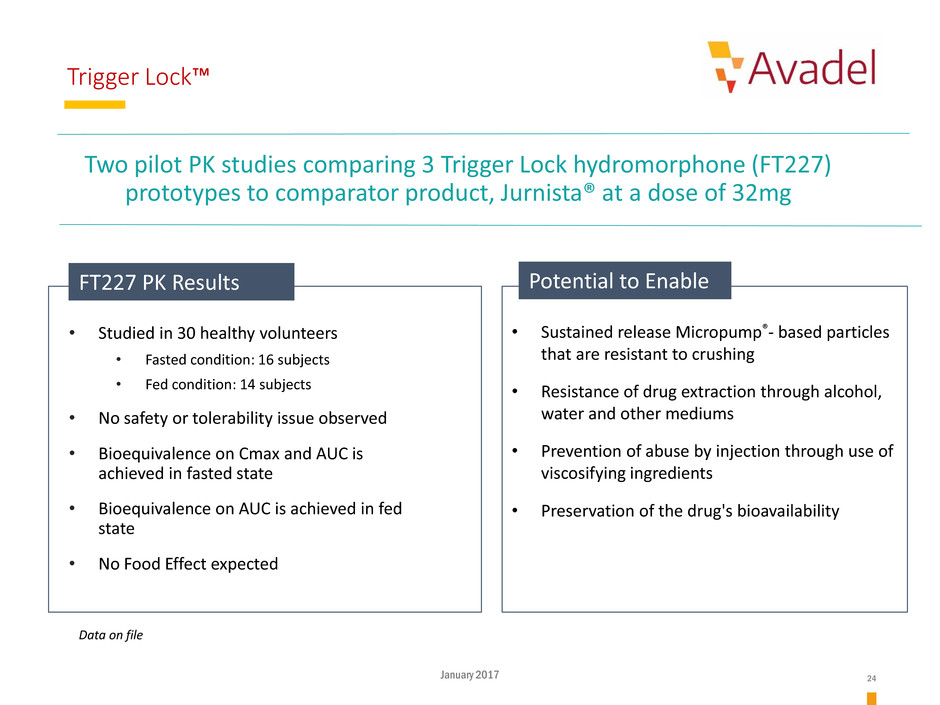
Trigger Lock™
• Sustained release Micropump®- based particles
that are resistant to crushing
• Resistance of drug extraction through alcohol,
water and other mediums
• Prevention of abuse by injection through use of
viscosifying ingredients
• Preservation of the drug's bioavailability
Potential to Enable
Data on file
FT227 PK Results
• Studied in 30 healthy volunteers
• Fasted condition: 16 subjects
• Fed condition: 14 subjects
• No safety or tolerability issue observed
• Bioequivalence on Cmax and AUC is
achieved in fasted state
• Bioequivalence on AUC is achieved in fed
state
• No Food Effect expected
Two pilot PK studies comparing 3 Trigger Lock hydromorphone (FT227)
prototypes to comparator product, Jurnista® at a dose of 32mg
25January 2017
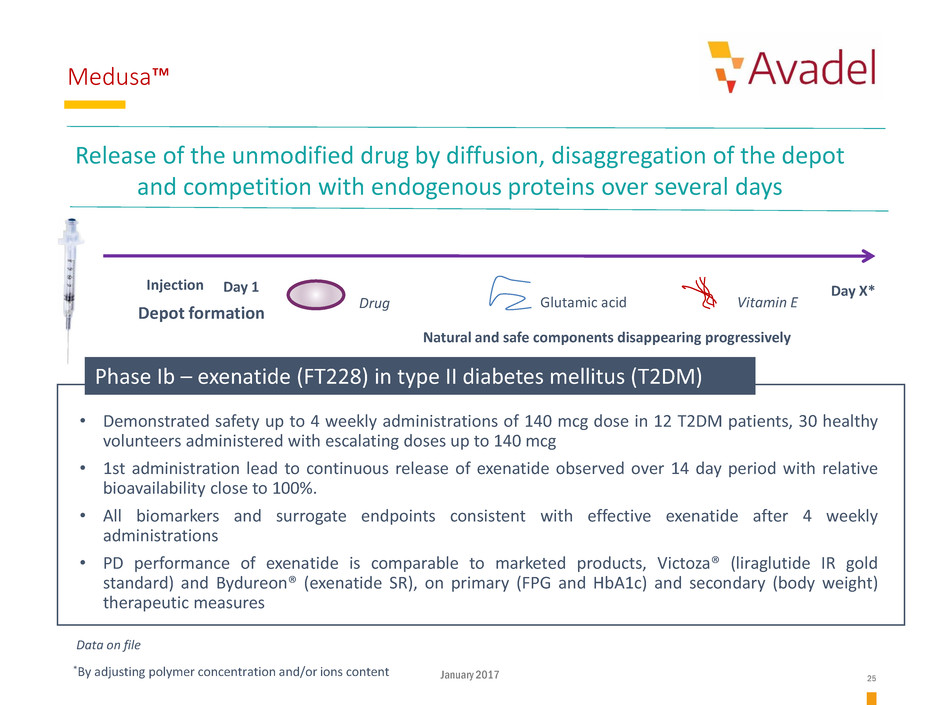
Medusa™
Release of the unmodified drug by diffusion, disaggregation of the depot
and competition with endogenous proteins over several days
Depot formation
Drug Vitamin EGlutamic acid
Day 1Injection Day X*
Natural and safe components disappearing progressively
*By adjusting polymer concentration and/or ions content
• Demonstrated safety up to 4 weekly administrations of 140 mcg dose in 12 T2DM patients, 30 healthy
volunteers administered with escalating doses up to 140 mcg
• 1st administration lead to continuous release of exenatide observed over 14 day period with relative
bioavailability close to 100%.
• All biomarkers and surrogate endpoints consistent with effective exenatide after 4 weekly
administrations
• PD performance of exenatide is comparable to marketed products, Victoza® (liraglutide IR gold
standard) and Bydureon® (exenatide SR), on primary (FPG and HbA1c) and secondary (body weight)
therapeutic measures
Phase Ib – exenatide (FT228) in type II diabetes mellitus (T2DM)
Data on file

26January 2017
Product & Safety Information
Please click below or visit our websites for full prescribing and safety
information for our marketed products
Bloxiverz®
www.bloxiverz.com
Vazculep®
www.vazculep.com
Akovaz™
www.akovaz.com
Karbinal™ ER
www.karbinaler.com
Aciphex® Sprinkle™
http://www.aciphexsprinkle.com
Cefaclor
http://cefaclororal.com
Flexichamber®
http://flexichamber.com
