Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - China Biologic Products Holdings, Inc. | v456056_ex99-1.htm |
| 8-K - FORM 8-K - China Biologic Products Holdings, Inc. | v456056_8k.htm |
Exhibit 99.2

China Biologic Products, Inc. (NASDAQ: CBPO) 35 th Annual J.P . Morgan Healthcare Conference January 2017 Creating Miracles in Life

Safe Harbor Statement This presentation contains forward - looking statements, including statements about the business outlook, strategy and market opportunity of China Biologic Products, Inc . (the “Company” or “we”), and statements that may suggest trends for its business . Such forward - looking statements can be identified by the use of forward - looking terminology such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “should,” “will,” “would,” and similar expressions, or the negatives thereof . These statements are individually and collectively forward - looking statements within the meaning of Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act of 1934 . These forward - looking statements are made only as of the date of this presentation and are based on estimates and information available to the Company at the time of this presentation . These statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict and may be beyond the Company’s control . Therefore, the Company cautions that actual results may differ materially from those set forth in any forward - looking statements herein, and are subject to numerous assumptions, risks, uncertainties and other factors, including those discussed under “Risk Factors” in the Company’s annual report on Form 10 - K filed with the SEC for the year ended December 31 , 2015 . In light of these risks and uncertainties, there can be no assurance that the forward - looking statements made during this presentation will in fact be realized . Any forward - looking statements and projections made by others in this presentation are not adopted by the Company and the Company is not responsible for the forward - looking statements and projections of others . Except as otherwise required by applicable securities laws, the Company disclaims any intention or obligation to publicly update or revise forward - looking statements, whether as a result of new information, future events or otherwise . In evaluating our business, we use certain non - GAAP measures as supplemental measures to review and assess our operating performance . These non - GAAP financial measures have limitations as analytical tools and investors should not consider them in isolation, or as a substitute for net income attributable to Company or other consolidated statement of comprehensive income data prepared in accordance with U . S . GAAP . China Biologic Products, Inc. 01
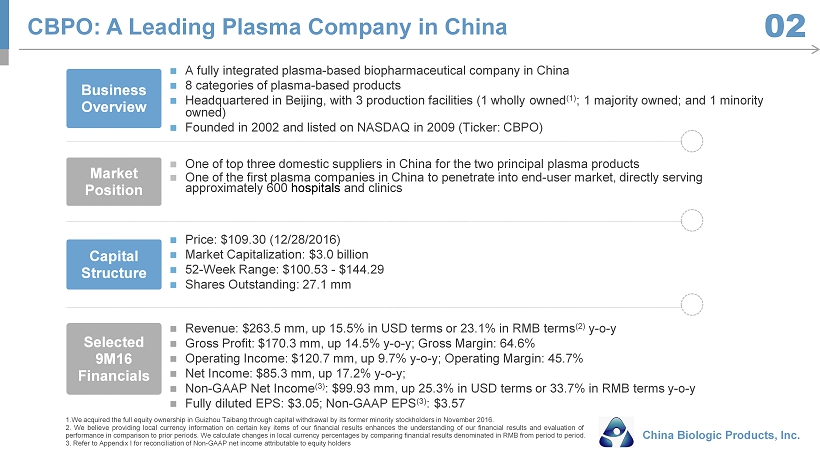
Capital Structure Price: $109.30 (12/28/2016) Market Capitalization: $3.0 billion 52 - Week Range: $100.53 - $144.29 Shares Outstanding : 27.1 mm Selected 9M16 Financials Revenue : $263.5 mm, up 15.5% in USD terms or 23.1% in RMB terms (2) y - o - y Gross Profit: $170.3 mm, up 14.5% y - o - y; Gross Margin: 64.6% Operating Income: $120.7 mm, up 9.7% y - o - y; Operating Margin: 45.7% Net Income: $85.3 mm, up 17.2% y - o - y; Non - GAAP Net Income (3) : $99.93 mm, up 25.3% in USD terms or 33.7% in RMB terms y - o - y Fully diluted EPS: $3.05; Non - GAAP EPS (3) : $3.57 Business Overview A fully integrated plasma - based biopharmaceutical company in China 8 categories of plasma - based products Headquartered in Beijing, with 3 production facilities (1 wholly owned (1) ; 1 majority owned; and 1 minority owned ) Founded in 2002 and listed on NASDAQ in 2009 (Ticker: CBPO) Market Position One of top three domestic suppliers in China for the two principal plasma products One of the first plasma companies in China to penetrate into end - user market, directly serving approximately 600 hospitals and clinics CBPO: A Leading Plasma Company in China China Biologic Products, Inc. 02 1 . We acquired the full equity ownership in Guizhou Taibang through capital withdrawal by its former minority stockholders in November 2016 . 2 . We believe providing local currency information on certain key items of our financial results enhances the understanding of our financial results and evaluation of performance in comparison to prior periods . We calculate changes in local currency percentages by comparing financial results denominated in RMB from period to period . 3 . Refer to Appendix I for reconciliation of Non - GAAP net income attributable to equity holders
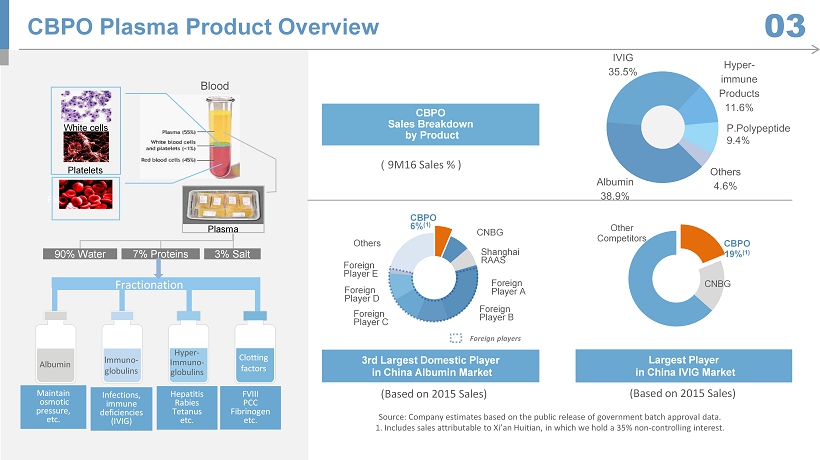
CBPO 19% (1) CNBG Other Competitors Hepatitis Rabies Tetanus etc. FVIII PCC Fibrinogen etc. Fractionation lmmuno - globulins Hyper - Immuno - g lobulins Clotting f actors A lbumin Maintain osmotic pressure, etc. Infections , immune deficiencies (IVIG) CBPO Plasma Product Overview 03 Red cells ( 9M16 Sales % ) CBPO Sales Breakdown by Product Albumin 38.9% IVIG 35.5% Hyper - immune Products 11.6% P. Polypepti de 9.4% Others 4.6% CBPO 6% (1) CNBG Shanghai RAAS Foreign Player A Foreign Player B Foreign Player C Foreign Player D Foreign Player E Others (Based on 2015 Sales) 3rd Largest Domestic Player in China Albumin Market Largest Player in China IVIG M arket ( Based on 2015 Sales) Source: Company estimates based on the public release of government batch approval data. 1. Includes sales attributable to Xi’an Huitian , in which we hold a 35% non - controlling interest. F oreign players 90% Water 7% Proteins 3% Salt Blood White cells Platelets Plasma P.Polypeptide 9.4%

Industry Overview China Biologic Products, Inc. China Biologic Products, Inc.

Per Capita Usage in U.S. / Per Capita Usage in China (x ) 2014 Market Size in China ($ MM) ( By 2015 Product Sales ) 2014 Plasma Products Per Capita Usage Plasma Products (1) Structure Comparison In China, albumin is the largest product in plasma products market. In U.S., IVIG is the largest product, and various coagulation factor products are growing fast. China’s 2014 market size is $2.5 billion, but its per capita penetration is still a fraction of that of the U.S. 1,560 557 45 2.5x 15.8x 15.9x 0.0 5.0 10.0 15.0 20.0 Albumin IVIG Factor VIII (2) (2) (3) China’s Plasma Protein Market – Early Stage of Growth, Low Penetration, Significant Potential 05 0.8 2.5 6.2 0.0 2.0 4.0 6.0 8.0 2009 2014 2019E China Plasma Products (1) Market Size ($ Billion) CAGR 20% CAGR 26% Source: The Marketing Research Bureau, Inc. and Company estimates 1. Excluding recombinant products 2. Based on 2014 per capita usage (kilogram per MM inhabitant) in U.S. divided by 2014 per capita usage in China 3. Based on 2014 per capita usage (international units per capita) in U.S. divided by 2014 per capita usage in China , excluding recombinant products China is the second largest plasma products market in the world Significant unmet clinical demand provides sustainable growth Growth potential driven by aging population and various diseases Seller’s m arket with sustainable pricing Albumin 66.5% IVIG 24.4% Hyper - immune 6.1% FVIII 1.5% Others 1.5% Albumin and plasma protein fraction 7.0% IVIG&SCIG 57.2% FVIII 2.5% Alpha - 1 Antitrypsin 8.1% C - 1 Esterase Inhibitor 8.2% Others 17.0% China U.S.

Regulatory framework in China has evolved into one of the most stringent globally due to crises of tainted plasma products over the past decade Fractionators permitted to process self - collected plasma only as required by law New plasma collection licenses are difficult to obtain Plasma fractionation licenses ceased to be granted since 2001 ; 28 active plasma product manufacturers in China currently Since 1986 , all imported blood products have been banned except for human albumin and recombinant factor VIII products China will continue to maintain stringent regulations for the plasma product industry as it is critical to China's public health security High Barriers to Entry Benefit Established Players 06 China Biologic Products, Inc.

United States China Population (million) 319 1,375 Donation Frequency Twice per 7 days, not within 48 hours Twice per month, not within 14 days Collection Volume Volumes by weight 50 - 68kg / 69 - 79kg / >79kg, donate 690 / 825 / 880 ml 580 ml Plasma Collection Centers Over 480 Approximately 200 Preapproval Requirement No Yes Plasma Donation Compensation (for one time donation) $25 - 35 $42 - 50 Regulation Agencies Food and Drug Administration (FDA) and Plasma Protein Therapeutics Association (PPTA) County - , Municipal - and Provincial - Level Governments Plasma Collected in 2015 (million liters) 31.0 5.8 Source: Broker Report, The Marketing Research Bureau, Inc., Plasma Protein Therapeutics Association (PPTA) and Company estima tes Plasma Collection Regulation Environment – U.S. vs. China China Biologic Products, Inc. 07

Plasma Companies Other Pharma Companies Commentary Regulatory Control China’s plasma - derived products market is highly regulated by the government Supply / Demand Significant supply shortage favors leading plasma players Historical Pricing Prices for most plasma products have remained steady whereas the average price of many pharma products has been in decline Domestic Competition 28 active plasma product manufacturers in China vs. thousands of pharmaceutical companies Foreign Competition Currently only albumin and recombinant factor VIII are open to imports Sales and Distribution Compared with pharmaceutical companies, the plasma industry in China is a seller’s market Growth Visibility Growth among plasma manufacturers is primarily driven by the supply/demand imbalance Plasma Is An Attractive Sector For Investment In China Pharmaceutical Industry China Biologic Products, Inc. 08
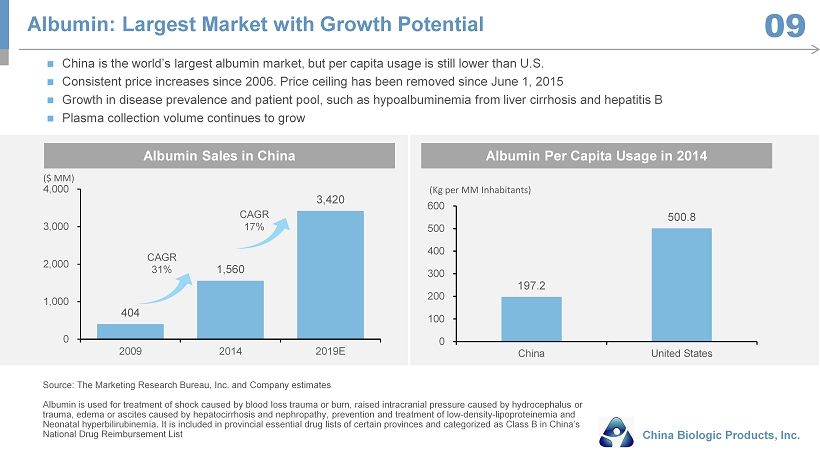
404 1,560 3,420 0 1,000 2,000 3,000 4,000 2009 2014 2019E 197.2 500.8 0 100 200 300 400 500 600 China United States China is the world’s largest albumin market, but per capita usage is still lower than U.S. Consistent price increases since 2006. Price ceiling has been removed since June 1, 2015 Growth in disease prevalence and patient pool, such as hypoalbuminemia from liver cirrhosis and hepatitis B Plasma collection volume continues to grow ($ MM) (Kg per MM Inhabitants) Albumin Sales in China Albumin Per Capita Usage in 2014 CAGR 17% CAGR 31% Source: The Marketing Research Bureau, Inc. and Company estimates Albumin is used for treatment of shock caused by blood loss trauma or burn, raised intracranial pressure caused by hydrocepha lus or trauma, edema or ascites caused by hepatocirrhosis and nephropathy, prevention and treatment of low - density - lipoproteinemia and Neonatal hyperbilirubinemia. It is included in provincial essential drug lists of certain provinces and categorized as Class B i n China’s National Drug Reimbursement List Albumin: Largest Market with Growth Potential China Biologic Products, Inc. 09

IVIG Sales in China IVIG Per Capita Usage in 2014 12.7 200.4 0 50 100 150 200 250 China United States 254 557 1,569 0 300 600 900 1,200 1,500 1,800 2009 2014 2019E ($ MM) (Kg per MM Inhabitants) CAGR 23% CAGR 13% Source: The Marketing Research Bureau, Inc. and Company estimates IVIG is used for treatment of hereditary immunoglobulin deficiency, such as X chain low immunoglobulin, familial variable imm une deficiency, immunoglobulin G secondary deficiency; secondary immunoglobulin deficiency, such as severe infection, newborn sepsis; and auto - immune deficiency diseases, such as original thrombocytopenia purpura or Kawasaki disease. It is included in provincial essential drug lists and categorized as Class B in China’s National Drug Reimbursement List IVIG: Early - stage Market with Strong Growth Drivers China Biologic Products, Inc. 10 Largest product category globally yet still at an early - stage in China Growth of IVIG market will be driven by expanded indications and physician education Per Capita usage in China is less than 10% of that in U.S. IVIG’s pricing in China is currently less than half of that of the US, providing strong upside potential

Source: The Marketing Research Bureau, Inc. and Company estimates 1 . As of December 31, 2014, according to China Hemophilia Association 2. Excluding recombinant products Factor VIII is used for treatment of coagulopathie such as hemophilia A and increase concentration of coagulation factor VIII . I t is included in national essential drug lists and categorized as Class A in China’s National Drug Reimbursement List Factor VIII: Nascent Market, Strong Growth Potential and Highly Profitable China Biologic Products, Inc. 11 Factor VIII is a life saving and a lifelong treatment Room for significant growth for Factor VIII products in China ‒ Only 8 domestic manufacturers in the plasma - derived Factor VIII segment ‒ Over 10,000 (1) registered hemophilia patients in China, many more unregistered ‒ Primarily acute usage vs. little preventive usage, providing huge room for growth Recombinant Factor VIII is not competitive due to higher cost and lack of insurance coverage 0.12 1.91 0.0 0.4 0.8 1.2 1.6 2.0 2.4 China United States 11 45 85 0 20 40 60 80 100 2009 2014 2019E ($ MM) (International Units per Inhabitant) Factor VIII (2) Sales in China Factor VIII (2) Per Capita Usage in 2014 CAGR 14% CAGR 33%

Company Overview China Biologic Products, Inc.
Strong Product Pipeline Enhancing Margins and Leadership 4 Stable and Growing Plasma Supply 2 Robust Cash Flow and Sustainable Profitability 5 Leading Plasma Player in China 1 Leader in China’s Fast Growing IVIG Market 3 Professional and Experienced Management Team 6 The Only Offshore Investment Opportunity in the Attractive Chinese Plasma Industry 13 China Biologic Products, Inc.

38.5 71.1 74.3 93.5 109.4 72.3 87.3 57.0 74.5 86.9 116.2 132.6 110.0 120.7 37.2% 40.3% 42.7% 47.8% 44.7% 48.2% 45.7% -8.0% 2.0% 12.0% 22.0% 32.0% 42.0% 52.0% 0 50 100 150 200 2011 2012 2013 2014 2015 9M'15 9M'16 Operating Cash Flow Operating income Operating Margin Unique distribution model, products sold directly to ~600 hospitals and clinics Non - GAAP Net Income Attributable to Equity Holders ( 1) ($ MM) 153.1 184.8 203.4 243.3 296.5 228.2 263.5 0 60 120 180 240 300 360 420 2011 2012 2013 2014 2015 2016E 9M'15 9M'16 36.5 48 59 75.6 100.1 79.7 99.9 -20 10 40 70 100 130 2011 2012 2013 2014 2015 2016E 9M'15 9M'16 ($ MM) 1 Leading Plasma Company in China - Solid Track Record of Delivering Sustainable Profitability 14 Total Sales China Biologic Products, Inc. 1. Non - GAAP net income attributable to equity holders excludes non - cash employee stock compensation and some non - recurring items. Recurring operating income excludes non - recurring items. Refer to Appendix I for reconciliations. Operating Cash flow and Recurring Operating Income and Margin (1) ($ MM) The largest domestic plasma player in China in terms of 2015 total sales * * source : MRB report * Subject to foreign exchange translation impact

Product portfolio consisting of eight plasma products with strong economics High product quality and safety record Large scale and reliable product supply to hospitals Government support for leading players Advantages from Leading Market Position 1 Leading Plasma Player in China 15 China Biologic Products, Inc.

Extensive plasma collection network of 14 captive plasma stations (including 1 branch station) With recently opened Xinglong plasma station, plasma collection network now covers four provinces with a total combined population of 220 MM Continue to explore new regions to expand plasma donor coverage Daming collection station in Hebei Province is expected to commence operation in 2017 D rive plasma collection growth volume at our existing stations Secured additional 500 tonnes of source plasma from August 2015 to 2018 through strategic collaboration with a third party fractionator Cao County Plasma Collection Center, Shandong Puding Plasma Collection Center, Guizhou Strong plasma supply growth: annual plasma collection 2012 to 2015 CAGR CBPO: 15% V.S. China Average: 11 %* * Source: data published by National Health and Family Planning Commission of the PRC. 2 Growing and Sustainable Plasma Supply 16 China Biologic Products, Inc.

3 Leader in China’s Fast Growing IVIG Market 1 7 China Biologic Products, Inc. CBPO 19% (1) (1st) CNBG (2nd) Other Competitors 49 125 0 40 80 120 2011 2015 IVIG Sales 32.3% ($ MM) 42.2% % of Total Sales (based on 2015 Sales) CBPO’s Increasing S ales in IVIG Leading Player in China’s IVIG Market Source: : The Marketing Research Bureau, Inc., and Company estimates 1. Includes sales attributable to Xi’an Huitian , in which we hold a 35% non - controlling stak e 200.4 12.7 0 200 United States China 2014 (Kg per MM Inhabitants) China’s IVIG Market Has Substantial Growth Potential Enhancing Physician Education Expanding Therapeutic Indication Improving Insurance Reimbursement Low per capita Consumption of IVIG in China Sustainable Growth Drivers

Products Preclinical Research Clinical Trial Application Clinical Trial Commercial Launch Development Status Human Fibrinogen Commercial production expected in 2017 New Generation IVIG Obtained the approval for clinical trial by CFDA Human Antithrombin III Obtained the approval for clinical trial by CFDA Human Cytomegalovirus Immunoglobulin Obtained the approval for clinical trial by CFDA Human coagulation factor IX Obtained the approval for clinical trial by CFDA Human Fibrin Sealant Completed the official virus inactivation by the PRC National Institutes for Food and Drug Control 4 Robust and Low Risk Product Pipeline Enhancing Margins and Leadership 18 China Biologic Products, Inc. China Biologic Products, Inc.

Cost Albumin Immunoglobulin Factor VIII PCC Other Products Total Fractionation Revenue Profit Hyper - Immune Products IVIG Indicative Revenue and Profit Breakdown per Liter of Plasma Each new product will incrementally improve profit 4 Robust and Low Risk Product Pipeline Enhancing Margins and Leadership (continued) 19 China Biologic Products, Inc.

38.5 71.1 74.3 93.5 109.4 72.3 87.3 0 25 50 75 100 125 2011 2012 2013 2014 2015 9M'15 9M'16 5 Robust Cash Flow Generation 20 China Biologic Products, Inc. ($ MM) Operating Cash Flow 8.0 13.9 19.7 20.2 52.3 42.5 0.0 10.0 20.0 30.0 40.0 50.0 60.0 2011 2012 2013 2014 2015 9M'16 ($ MM) Capital Expenditures m ainly for Shandong Taibang ‘s new fractionation facility

Planned New Facility in Shandong 21 China Biologic Products, Inc. Expected Capital Expenditure In 2016 and 2017(RMB mm) Scheduled open time: Second half of 2017 Estimated Investment : RMB 800 - 1,000 million Fractionation capacity: expanding to a minimum of 1,200 tonnes from 700 tonnes 2016 2017 400 200 Shandong Taibang New Fractionation Facility

Improve collection volume at existing centers Establish more collection centers and enlarge collection territories Increase our stake in existing subsidiaries Further consolidate subscale players in the i ndustry Explore new products by internal R&D and external cooperation Improve yield of existing products Partner with major distributors in tier - one cities to dominate high end market Source raw material through strategic collaboration Organic Growth is O ur Strategic Focus and Priority Further develop direct sales model Develop marketing initiatives and execution Expand into complementary business through possible acquisition or partnership Future Growth Drivers 22 China Biologic Products, Inc. Increased Plasma Collection Ongoing Innovation Expand Sales Model Potential M&A

Superior Capability of Generating H igh Growth with Sustainable Profitability and Robust Cash Flow China Plasma Market in Early - Stage of Development with Significant Growth Potential High Entry Barrier Creating A Seller’s Market A Leading Player with Substantial Competitive Advantages 3 4 1 2 Why Invest in CBPO and the Highly Attractive Plasma Sector? 23 China Biologic Products, Inc.

$ MM 2011 2012 2013 2014 2015 9M’15 9M’16 Total sales 153.1 184.8 203.4 243.3 296.5 228.2 263.5 Cost of sales 46.0 58.8 65.5 80.1 106.5 79.5 93.2 Gross profit 107.1 126.0 137.9 163.2 190.0 148.7 170.3 Operating expenses Selling expenses 14.6 14.5 10.6 10.7 10.0 7.2 7.3 G&A expenses 31.5 34.0 36.2 32.1 41.4 27.5 39.0 R&D expenses 4.0 3.0 4.2 4.2 6.0 4.0 3.4 Income from operations (1) 32.2 74.5 86.9 111.2 132.6 110.0 120.6 Income before income taxes 42.3 81.1 92.4 122.8 135.1 111.6 126.4 Net income 31.4 66.0 76.9 96.1 114.1 93.8 105.6 Net income attributable to CBPO 18.2 45.2 54.6 70.9 89.0 72.8 85.3 1. Excluding some non - recurring items, such as impairment loss of goodwill and loss on abandonment and write - off of long - lived assets in 2011, and provision for other receivables in respect of an employee housing development project in 2014, the recurring operating income for relevant periods is as below. Refer to Appendix I for reconciliation of income from operations to recurring operating income $ MM 2011 2012 2013 2014 2015 9M’15 9M’16 Recurring operating income 57.0 74.5 86.9 116.2 132.6 110.0 120.6 2011 2012 2013 2014 2015 9M’16 Total Cash (2) ($ MM) 89.4 132.5 181.2 144.5 182.9 203.2 Cash & Cash Equivalents ($ MM) 89.4 129.6 144.1 80.8 144.9 203.2 Restricted Cash and Deposit ($ MM) - 2.9 30.5 63.7 - - Time Deposits ($ MM) - - 6.6 - 38.0 - Short - term and Long - term Bank Loans ($ MM) 11.0 7.9 39.8 97.9 - - Accounts Receivable Days 32 28 26 28 27 33 Inventory Days 490 456 458 433 390 464 2. Calculated as the sum of cash & cash equivalents and restricted cash Income Statement and Balance Sheet Highlights 24 China Biologic Products, Inc.

Appendix China Biologic Products, Inc.

Reconciliation of Non - GAAP Net income Attributable to Equity Holders Appendix I 26 $ MM, except EPS in $ and number of shares in MM 2011 2012 2013 2014 2015 9M’16 Net Income Attributable to Equity holders 18.2 45.2 54.6 70.9 89.0 85.3 Non - cash employee stock compensation 4.9 4.6 4.4 4.7 11.1 14.6 Impairment loss of goodwill 18.2 - - - - - Loss on abandonment of long - lived assets attributable to controlling interest 3.6 - - - - - Written - off of raw material attributable to controlling interest due to closure of plasma stations 0.1 - - - - - Interest on the Notes 3.6 - - - - - Gain (loss) from change in fair value of embedded conversion option in the Notes - 6.4 - - - - - Gain (loss) from change in fair value of warrants - 5.7 - 1.8 - - - - Adjusted Net Income Attributable to Equity holders - Non GAAP 36.5 48.0 59.0 75.6 100.1 99.9 Diluted EPS - Non GAAP 1.37 1.79 2.12 2.89 3.68 3.57 Weighted average number of shares used in computation of Non GAAP diluted EPS 26.7 26.8 27.6 25.7 26.6 27.2 China Biologic Products, Inc. $ MM, except percentage 2011 2012 2013 2014 2015 9M’16 Income from operations 32.2 74.5 86.9 111.2 132.6 120.6 Operating margin 21.0% 40.3% 42.7% 45.7% 44.7% 45.7% Excludes non - recurring items: Impairment loss of goodwill 18.2 - - - - - Loss on abandonment and write - off of long - lived assets 6.6 - - - - - Provision for other receivables in respect of an employee housing development project - - - 5.0 - - Recurring operating income 57.0 74.5 86.9 116.2 132.6 120.6 Recurring operating margin 37.2% 40.3% 42.7% 47.8% 44.7% 45.7% Reconciliation of Income from Operations to Recurring Operating Income

Appendix II - Plasma Donor Management 27 China Biologic Products’ Fuping Plasma Collection Center in Shaanxi Five steps to assure safe plasma from healthy donors II. Five times fingerprint identification III. ID card identification system I. Facial identification V. Fully - automatic plasma collection machine; fully enclosed and disposable medical instruments IV . Physical examination before donating plasma to assure up to standards for HBsAg, HCV , HIV, ALT, and syphilis China Biologic Products, Inc.

Disqualification Qualification Appendix II - Plasma Donor Management 28 Safety measures in every production phase Plasma checking by collection station Plasma rechecking by fractionator Another plasma donation by the same donor after 90 days Checking of re - donated plasma by fractionator Plasma collected on first day goes to production Destruction Qualification Qualification Disqualification plasma waiting period for 110 days at least First day of plasma donation Disqualification Reducing safety risks of plasma collection with a TRIPLE virus detection defense system 2 3 1 China Biologic Products, Inc.

KPMG China Biologic Products, Inc. Ming Yin, Senior Vice President 18th Floor , 19 Chaoyang Park Road Chaoyang District, Beijing 100127, PRC. China: +86 10 6598 3099 ir@chinabiologic.com IR Agent ICR LLC Bill Zima, Partner China: +86 10 6583 7511 U.S.: +1 646 405 5191 William.Zima@icrinc.com Legal counsel Davis Polk & Wardwell LLP Independent auditor For more information please visit us at : www.chinabiologic.com Contacts 29 China Biologic Products, Inc.

THANK YOU
