Attached files
| file | filename |
|---|---|
| EX-21 - EXHIBIT 21 - China Biologic Products Holdings, Inc. | exhibit21.htm |
| EX-32.2 - EXHIBIT 32.2 - China Biologic Products Holdings, Inc. | exhibit32-2.htm |
| EX-32.1 - EXHIBIT 32.1 - China Biologic Products Holdings, Inc. | exhibit32-1.htm |
| EX-31.1 - EXHIBIT 31.1 - China Biologic Products Holdings, Inc. | exhibit31-1.htm |
| EX-23.2 - EXHIBIT 23.2 - China Biologic Products Holdings, Inc. | exhibit23-2.htm |
| EX-31.2 - EXHIBIT 31.2 - China Biologic Products Holdings, Inc. | exhibit31-2.htm |
| EX-23.1 - EXHIBIT 23.1 - China Biologic Products Holdings, Inc. | exhibit23-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.
20549
FORM 10-K
(Mark One)
[x] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 2010
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ____________to _____________
Commission File No. 001-34566
CHINA BIOLOGIC PRODUCTS,
INC.
(Exact name of registrant as specified in its
charter)
| Delaware | 75-2308816 |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) |
| incorporation or organization) |
No. 14 East Hushan Road
Tai’an City, Shandong 271000
People’s Republic of China
(Address of principal executive offices)
(+86) 538-620-2306
(Registrant’s telephone
number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [x]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] No [x]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [x] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [ ] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [x]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer [ ] | Accelerated Filer [x] |
| Non-Accelerated Filer [ ] | Smaller reporting company [x] |
| (Do not check if a smaller reporting company) |
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ] No [x]
As of June 30, 2010 (the last business day of the registrant’s most recently completed second fiscal quarter), the aggregate market value of the shares of the registrant’s common stock held by non-affiliates (based upon the closing sale price of such shares as reported on the NASDAQ Global Select Market) was approximately $96.3 million. Shares of the registrant’s common stock held by each executive officer and director and each by each person who owns 10% or more of the outstanding common stock have been excluded from the calculation in that such persons may be deemed to be affiliates of the registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
There were a total of 24,351,125 shares of the registrant’s common stock outstanding as of March 25, 2011.
DOCUMENTS INCORPORATED BY REFERENCE
None.

| Annual Report on Form 10-K |
| For the Fiscal Year Ended December 31, 2010 |
TABLE OF CONTENTS
| Item 1. | Business. | 1 |
| Item 1A. | Risk Factors | 16 |
| Item 1B. | Unresolved Staff Comments. | 29 |
| Item 2. | Properties. | 29 |
| Item 3. | Legal Proceedings. | 29 |
| Item 4. | (Removed and Reserved). | 33 |
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 33 |
| Item 6. | Selected Financial Data | 34 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 34 |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. | 42 |
| Item 8. | Financial Statements and Supplementary Data. | 43 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 43 |
| Item 9A. | Controls and Procedures | 44 |
| Item 9B. | Other Information | 45 |
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance. | 45 |
| Item 11. | Executive Compensation | 52 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 56 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. | 56 |
| Item 14. | Principal Accounting Fees and Services. | 60 |
PART IV
| Item 15. | Exhibits, Financial Statement Schedules | 61 |
Special Note Regarding Forward Looking Statements
In addition to historical information, this report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. We use words such as “believe,” “expect,” “anticipate,” “project,” “target,” “plan,” “optimistic,” “intend,” “aim,” “will” or similar expressions which are intended to identify forward-looking statements. Such statements include, among others, those concerning market and industry segment growth and demand and acceptance of new and existing products; any projections of sales, earnings, revenue, margins or other financial items; any statements of the plans, strategies and objectives of management for future operations; any statements regarding future economic conditions or performance; as well as all assumptions, expectations, predictions, intentions or beliefs about future events. You are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, as well as assumptions, which, if they were to ever materialize or prove incorrect, could cause the results of the Company to differ materially from those expressed or implied by such forward-looking statements. Risks and uncertainties that could cause actual results to differ materially from those anticipated include risks related to, among others: our ability to overcome competition from local and overseas pharmaceutical enterprises; decrease in the availability, or increase in the cost, of plasma; failure to obtain PRC governmental approval to increase retail prices of certain of our biopharmaceutical products; difficulty in servicing our debt; loss of key members of our senior management; and unexpected changes in the PRC government’s regulation of the biopharmaceutical industry in China, or changes in China’s economic situation and legal environment. Additional disclosures regarding factors that could cause our results and performance to differ from results or performance anticipated by this report are discussed in Item 1A. “Risk Factors.”
Readers are urged to carefully review and consider the various disclosures made by us in this report and our other filings with the SEC. These reports attempt to advise interested parties of the risks and factors that may affect our business, financial condition and results of operations and prospects. The forward-looking statements made in this report speak only as of the date hereof and we disclaim any obligation, except as required by law, to provide updates, revisions or amendments to any forward-looking statements to reflect changes in our expectations or future events.
Use of Terms
Except as otherwise indicated by the context and for the purposes of this report only, references in this report to:
- “China Biologic,” the “Company,” “we,” “us,” or “our,” are to the combined business of China Biologic Products, Inc., a Delaware corporation, and its direct and indirect subsidiaries;
- “Logic Express” are to our wholly owned subsidiary Logic Express Limited, a BVI company;
- “Logic Holdings” are to our wholly-owned subsidiary Logic Holdings (Hong Kong) Limited, a Hong Kong company;
- “Logic China” are to our wholly owned subsidiary Logic Management and Consulting (China) Co., Ltd., a PRC company;
- “Logic Beijing” are to our wholly owned subsidiary Logic Taibang Bio-Tech Institute (Beijing), a PRC company;
- “Dalin” are to our majority owned subsidiary Guiyang Dalin Biologic Technologies Co., Ltd., a PRC limited company;
- “Shandong Taibang” are to our majority owned subsidiary Shandong Taibang Biological Products Co. Ltd., a sino-foreign joint venture incorporated in China;
- “Taibang Medical” are to our wholly owned subsidiary Shandong Taibang Medical Company, a PRC company;
- “Guizhou Taibang” are to our majority owned subsidiary Guizhou Taibang Biological Products Co., Ltd., a PRC company, formerly, Guiyang Qianfeng Biological Products Co., Ltd.;
- “Huitian” are to our minority owned subsidiary Xi'an Huitian Blood Products Co., Ltd., a PRC company;
- “BVI” are to the British Virgin Islands;
- “Hong Kong” are to the Hong Kong Special Administrative Region of the People’s Republic of China;
- “PRC” and “China” are to the People’s Republic of China;
- “SEC” are to the Securities and Exchange Commission;
- “Securities Act” are to the Securities Act of 1933, as amended;
- “Exchange Act” are to the Securities Exchange Act of 1934, as amended;
- “Renminbi” and “RMB” are to the legal currency of China; and
- “U.S. dollars,” “dollars” and “$” are to the legal currency of the United States.
1
PART I
ITEM 1. BUSINESS.
Overview of Our Business
We are a biopharmaceutical company and through our indirect PRC subsidiaries, Shandong Taibang and Guizhou Taibang, and our minority-owned PRC investee, Huitian, we are principally engaged in the research, development, manufacturing and sales of plasma-based pharmaceutical products in China. Shandong Taibang operates from our manufacturing facility located in Tai’an, Shandong Province, and Guizhou Taibang operates from our manufacturing facility located in Guiyang City, Guizhou Province. Our minority owned investee, Huitian, operates from its facility in Shaanxi Province. The plasma-based biopharmaceutical manufacturing industry in China is highly regulated by both the provincial and central governments. Accordingly, the manufacturing process of our products is strictly monitored from the initial collection of plasma from human donors to finished products. Our principal products include our approved human albumin and immunoglobulin products.
We are approved to sell human albumin 20%/10ml, 20%/25ml and 20%/50ml, 10%/10ml, 10%/25ml, 10%/50ml and 25%/50ml. Human albumin is our top-selling product. Sales of these human albumin products represented approximately 48.0%, 49.7% and 57.8% of our total revenues, respectively, for the each of the years ended December 31, 2010, 2009 and 2008. Human albumin is principally used to increase blood volume while immunoglobulin, one of our other major products, is used for certain disease preventions and cures. The Company’s approved human albumin and immunoglobulin products use human plasma as raw material. Albumin has been used for almost 50 years to treat critically ill patients by replacing lost fluid and maintaining adequate blood volume and pressure. All of our products are prescription medicines administered in the form of injections.
We sell our products to customers in the PRC, mainly hospitals and inoculation centers. Our sales have historically been made on the basis of short-term arrangements and our largest customers have changed over the years. For the years ended December 31, 2010, 2009 and 2008, our top 5 customers accounted for approximately 12.3%, 10.7% and 16.2%, respectively, of our total revenue. For the years ended December 31, 2010, 2009 and 2008, our largest customer accounted for approximately 2.8%, 4.0% and 6.4%, of our revenue, respectively. As we continue to diversify our geographic presence, customer base and product mix, we expect that our largest customers will continue to change from year to year. We have product liability insurance covering all of our products. However, since our establishment in 2002, there has not been any product liability claims nor has any legal action been filed against the Company brought by patients related to the use of our products.
Our principal executive offices are located at No. 14 East Hushan Road, Tai’an City, Shandong, the People’s Republic of China 271000. Our corporate telephone number is (86) 538-620-2306 and our fax number is (86)538-620-3895. We maintain a website at http://www.chinabiologic.com that contains information about our operating company, but that information is not part of this report.
Our History and Background
China Biologic Products, Inc. was originally incorporated on December 20, 1989 under the laws of the State of Texas, as Shepherd Food Equipment, Inc. On November 20, 2000, Shepherd Food Equipment, Inc. changed its corporate name to Shepherd Food Equipment, Inc. Acquisition Corp., which is the survivor of a May 28, 2003 merger with GRC Holdings, Inc. or GRC. In the merger, the Company adopted the Articles of Incorporation and By-Laws of GRC and changed its corporate name to GRC Holdings, Inc. On January 10, 2007, a Plan of Conversion became effective pursuant to which GRC was converted into a Delaware corporation and changed its name to China Biologic Products, Inc.
Acquisition of Logic Express
On July 19, 2006, we completed a reverse acquisition with Logic Express, whereby we issued to the shareholders of Logic Express 18,484,715 shares of our common stock in exchange for 100% of the issued and outstanding shares of capital stock of Logic Express and its majority-owned Chinese operating subsidiary, Shandong Taibang. As a result of the reverse acquisition, Logic Express became our 100% owned subsidiary, the former shareholders of Logic Express became our controlling stockholders with 96.1% of our common stock, and Shandong Taibang became our 82.76% majority-owned indirect subsidiary. Shandong Taibang is a sino-foreign joint venture company established on October 23, 2002 with a registered capital of RMB 80 million (then approximately $10.3 million).
Acquisition of Plasma Stations
In December 2006, our subsidiary, Shandong Taibang, acquired all the assets of five plasma stations in Shandong Province. We obtained the permit to operate the stations in January 2007. In April 2007, Shandong Taibang acquired certain assets of two plasma stations in Guangxi Province. The two plasma stations obtained their operating permits in February and April 2007, respectively.
2
We acquired the assets of these plasma stations through separate Shandong Taibang subsidiaries, specially formed for this purpose. The subsidiaries holding six of our new plasma stations are the Xia Jin Plasma Company, the Qi He Plasma Company, the He Ze Plasma Company, the Huan Jiang Plasma Company, the Liao Cheng Plasma Company, and the Zhang Qiu Plasma Company. The seventh plasma station is held by the Fang Cheng Plasma Company, which at the time was 80% owned by Shandong Taibang and 20% owned by Feng Lin, an unrelated third party. On January 13, 2010, Shandong Taibang acquired the 20% non-controlling interest in the Fang Cheng Plasma Company, and it is now wholly-owned by Shandong Taibang. In January 2007, Shandong Taibang also signed a letter of intent to acquire certain assets of a plasma station in Guangxi Province, however, we have not consummated this acquisition as the permit for this station is in dispute, as described in Item 3, “Legal Proceedings.”
In June 2008, we received approval from the Guangxi Province Bureau of Health to set up a new plasma collection station in Pu Bei County, Guangxi Province. The new plasma collection station will be located in the Centralized Industry Zone of Pu Bei County and when it becomes operational, it will replace our existing Fang Cheng Plasma Collection Station. We decided to relocate the Fang Cheng Plasma Collection Station to a more strategic location, also in Guangxi, to increase collection volumes.
On January 22, 2010, Shandong Taibang entered into an equity transfer agreement with Yuncheng Ziguang Biotechnology Co., Ltd. (“Yuncheng Ziguang”), located in Yuncheng, Shandong Province. Under the terms of the equity transfer agreement, Shandong Taibang agreed to purchase 100% of Yuncheng Ziguang’s equity interest at a purchase price of RMB 10,066,672 (approximately $1,476,781), which was paid on February 24, 2010. The purpose of this acquisition is for relocation of Shandong Taibang’s He Ze Plasma Company into the nearby Yuncheng Ziguang facility. In February 2011, the He Ze Plasma Company moved into the Yuncheng Ziguang facility and began collecting plasma. Currently Yuncheng Ziguang has no other operations.
On July 7, 2010, Shandong Taibang invested RMB 6,000,000 (approximately $910,200) to establish a wholly-owned subsidiary, Ning Yang Plasma Company, in Shandong Province. The Ning Yang Plasma Company was still under construction as of December 31, 2010.
On July 20, 2010, Shandong Taibang invested another RMB 6,000,000 (approximately $910,200) to establish a wholly-owned subsidiary, Yishui Plasma Company, in Shandong Province. The Yi Shui Plasma Company obtained its operating permits from relevant PRC authorities on December 6, 2010, and had commenced operation as of December 31, 2010.
Establishment of Taibang Medical
In September 2006, Shandong Taibang established a wholly owned subsidiary, Taibang Medical (known then as Shandong Missile Medical Co., Ltd.), with registered capital of $384,600, fully paid on March 1, 2007. On February 7, 2007, Taibang Medical obtained a distribution license for biological products, except for vaccine, from the Shandong Food and Drug Administration, for a license period of five years from the date of obtaining the license. The registration of Taibang Medical was ultimately approved by Shandong Provincial Department of Foreign Trade and Economic Cooperation on July 4, 2007 and Taibang Medical was registered on July 19, 2007. The scope of business is wholesale of biological products, except vaccines, with a license period of 25 years from the date of registration.
On August 14, 2009, we changed Taibang Medical’s name from Shandong Missile Medical Co., Ltd. to Shandong Taibang Medical Company. In addition, the registered capital of Taibang Medical was increased by RMB 2,000,000 (approximately $293,400) to $733,500.
On July 8, 2010, Logic China, our PRC operating subsidiary, entered into an equity transfer agreement to purchase 100% of the equity interest in Taibang Medical from Shandong Taibang with a cash purchase price of RMB 6,440,000 (approximately $947,327). The equity transfer was registered with the local Administration for Industry and Commerce, or the AIC, on September 10, 2010 and the purchase price was fully paid on September 23, 2010. With this equity transfer, Taibang Medical is now the Company’s indirect wholly-owned subsidiary and the Company will be able to consolidate its resources in the sale and marketing of Shandong Taibang and Guizhou Taibang’s products.
Formation of Hong Kong Subsidiary
On December 12, 2008, we established Logic Holdings, our wholly-owned Hong Kong subsidiary, for the purpose of being a holding company for our majority equity interest in Dalin.
Dalin Acquisition and Entrustment Agreement
We completed the acquisition of 90% interest in Dalin in April 2009 upon payment of 90% of the purchase price. We substantially paid the remaining 10% of the purchase price, RMB 19,440,000 (approximately $2,844,350), on April 9, 2010, the one-year anniversary of the approval of the equity transfer by the local Administration for Industry and Commerce, or AIC.
3
On January 4, 2011, we entered into an equity transfer agreement with Shaowen Fan to acquire the remaining 10% minority interest in Dalin for a purchase price of RMB 50,000,000 (approximately $7,585,000). The equity transfer was registered with the local AIC on January 26, 2011 and the purchase price was fully paid as of February 22, 2011 in accordance with the equity transfer agreement. With this equity transfer, Guiyang Dalin is now the Company’s indirect wholly-owned subsidiary.
On April 6, 2009, Logic Express entered into an agreement with Shandong Institute, the noncontrolling interest holders in Shandong Taibang, pursuant to which, Shandong Institute would provide an advance to assist Logic Express’s purchase of 90% Dalin's equity interests. Under the terms of the agreement, Shandong Institute agreed to provide advance of $3,792,500 (RMB25,000,000), representing 12.86% of the Company’s purchase consideration in Dalin to the Company for one year, bearing interest equal to the higher of a proportionate share of the net income of Dalin during the year ended December 31, 2009 or 6% per annum. On April 12, 2010, the Company fully paid the advance from Shandong Institute and the interest of approximately $1.3 million, which was less than the Company’s previous estimate by approximately $0.9 million. The Company recorded the difference between the previous estimate and actual payment in other income of the consolidated statement of income for the year ended December 31, 2010.
As part of our due diligence investigation into Dalin and Guizhou Taibang, we discovered that our indirect interest in Guizhou Taibang acquired under the equity transfer agreement may be diluted to as low as 41.3%. The local AIC records show Dalin as a 54% shareholder of Guizhou Taibang; however, the AIC records do not reflect a potential issuance of Guizhou Taibang’s equity interests to certain investors in May 2007, pursuant to a capital increase agreement. Guizhou Taibang has received the consideration for the potential issuance of equity interests, but the increase in registered capital and the related issuance of the equity interest has not yet been registered with the local AIC, pending the outcome of a minority shareholder suit against Guizhou Taibang and its then shareholders, alleging violation of the shareholder’s right of first refusal in connection with the May 2007 equity issuance. For details regarding the Guizhou Taibang shareholder suit and our position with respect to the May 2007 equity issuance of Guizhou Taibang’s equity interests, see our disclosure under Item 3, “Legal Proceedings” herein.
Guizhou Taibang is one of the largest plasma-based biopharmaceutical companies in China and is the only manufacturer currently operating in Guizhou Province. With a population of 39 million, Guizhou Province has historically produced the highest volumes of plasma collection in China, because a higher proportion of its population has been willing to engage in the collection process. Guizhou Province has a total of 19 plasma collection stations in operation, collecting approximately 1,200 tons of plasma supply every year. Guizhou Taibang owns seven of these plasma collection stations, of which five are currently in operation and collecting approximately 300 tons of plasma supply per year, with an annual capacity of 400 tons. We intend to employ more advanced collection techniques at these stations to improve yields and generate additional plasma supply. Guizhou Taibang is in compliance with Good Manufacturing Practices, or GMP, standards, and has been approved by the PRC’s State Food and Drug Administration, or SFDA, to produce six types of plasma-based products including Human Albumin, Human Immunoglobulin, Human Intravenous Immunoglobulin, Human Hepatitis B Immunoglobulin, Human Tetanus Immunoglobulin and Human Rabies Immune Globulin.
On December 30, 2010, the Guiyang AIC approved Guizhou Taibang’s application to change its name to Guizhou Taibang Biological Products Co., Ltd. We expect that the name change will facilitate the Company’s promotion of the “Taibang” brand name and further the Company’s integration of its marketing efforts.
On November 11, 2010, the Company established Guiyang Qianfeng Biological Technology Co., Ltd., a wholly-owned subsidiary of Guizhou Taibang, in Guiyang, Guizhou, for the purpose of research and development of placenta based products.
Huitian Acquisition
We purchased a 35% equity interest in Huitian at a purchase price of RMB 44,000,000 (approximately $6,454,800) in October 2008. Huitian is a manufacturer of plasma-based biopharmaceutical products in Shaanxi Province and is one of only 32 such manufacturers in China which are government approved. Shaanxi Province, which has a population of 37 million, has had a historically high collection volume with approximately ten plasma collection stations in operation, collecting approximately 300 tons of plasma supply each year. Only four of the collection stations in Shaanxi Province are government approved and three of these are owned by Huitian. Huitian produces about 80 tons of plasma-based products per year and has 200 tons of annual production capacity. We believe Huitian provides a strong long-term growth potential. Huitian is in compliance with GMP standards and it is also approved by the SFDA for the production of Human Albumin, Human Immunoglobulin, Human Immunoglobulin for Intravenous Injection, and Human Hepatitis B Immunoglobulin products.
4
Formation of PRC Subsidiaries
On December 21, 2009, our Hong Kong subsidiary, Logic Holdings, established Logic China for the purpose of holding our majority equity interest in Dalin and to facilitate our Chinese operations at the holding company level. On December 28, 2009, the Company transferred its 90% equity interest in Dalin from Logic Holdings to Logic China to complete this process.
On August 5, 2010, Logic China formed a wholly-owned subsidiary, Logic Taibang Bio-Tech Institute (Beijing) (“Logic Beijing”), with a registered capital of RMB 1 million (approximately, $149,700). Logic Beijing was established to operate all research and development activities of the Company and its subsidiaries.
Corporate Structure
The following chart reflects our current corporate organizational structure:
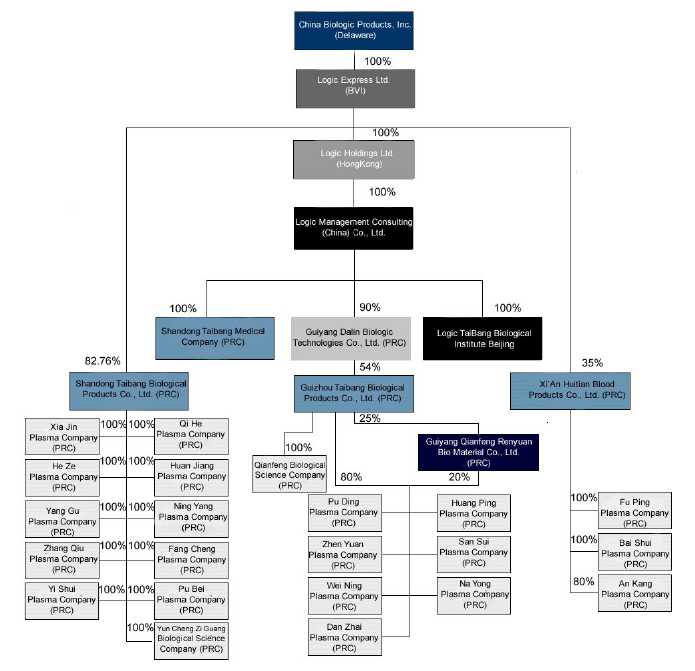
5
Our Industry
Plasma Collection in China
The collection of human plasma in China is generally influenced by a number of factors such as government regulations, geographical locations of collection stations, sanitary conditions of collection stations, living standards of the donors, and cultural and religious beliefs. Until 2006, only licensed Plasmapheresis stations owned and operated by the government could collect human plasma. Furthermore, each collection station was only allowed to supply plasma to the one manufacturer that had signed the “Quality Responsibility” statement with them. However, in March 2006, the Ministry of Health promulgated certain “Measures on Reforming Plasma Collection Stations,” or the Blood Collection Measures, whereby the ownership and management of PRC plasma stations are required to be transferred to plasma-based biopharmaceutical companies and the local government is charged with regulatory supervision and administrative control in accordance with the policies of the central government. Plasma stations that did not complete their reform by December 31, 2006 risked revocation of their license to collect plasma.
The supply of plasma for plasma-based products in the PRC has been on the decline since 2003 from the historical high of annual supply of approximately 7,000 tons to approximately 4,000 tons. We believe that this decline is a direct result of the government’s industry reforms of the country’s collection practices which led to the closure of many stations that did not meet the new industry standards. Based on reports promulgated by the PRC Ministry of Health, we estimate that the current annual supply of plasma in China amounts to approximately 4,000 tons, as compared to 30,000 tons in the global market, with the six largest manufacturers of plasma products accounting for approximately 50% of the annual plasma collection. In spite of the shortage of plasma supply, revenues from the sale of plasma products in China amounted to approximately $1.1 billion in 2009 per management’s estimate, and revenues from the sale of human albumin products accounted for about 70%. We expect that the plasma derivatives market to grow at a 15% rate per year through 2011.
We believe that these regulatory changes, including measures which limit illegal selling of blood, have improved the quality of blood and plasma by increasing cleanliness standards at blood collection stations. As the operation of the plasma stations become more regulated and the donor population expands, we believe that the overall quality of raw materials, such as human albumin will continue to improve, leading to a safer, more reliable finished product.
Plasma-Based Products Industry in China
We produce approved human albumin and immunoglobulin products, with human plasma as the main ingredient. In addition to the low usage ratio of such products in China as compared to other more developed countries, there is a significant difference in the make up and range of the plasma-based pharmaceutical products. Based on our analysis, in most developed countries like the United States, clotting factor products accounts for the majority of the plasma-based biopharmaceutical products, while in China, human albumin products accounts for the vast majority of such products. Specifically, total clotting factor products and human albumin products, account for approximately 40% and 25%, respectively, of United States’ total annual plasma-derived products, and account for approximately 3% and 59%, respectively, of China’s.
Our Growth Strategy
Our mission is to become a first-class biopharmaceutical enterprise in China. To achieve this objective, we have implemented the following strategies:
- Securing the supply of plasma. Due to the shortage of plasma and the reform of the ownership of plasma stations, our immediate strategy is to negotiate and acquire plasma stations in order to secure our plasma supply. In December 2006, we acquired five of the plasma stations in Shandong Province. Furthermore, in January 2007, we acquired two additional plasma stations in Guangxi Province. In June 2008, we received approval from the Guangxi Province Bureau of Health to set up a new plasma collection station in Pu Bei County, Guangxi Province, which, when operational, will replace our existing Fang Cheng Plasma Collection Station. We decided to relocate the Fang Cheng Plasma Collection Station to a more strategic location to increase collection volumes. During the construction period, the station will still continue with its normal operations. With the approval of the Centralized Industry Zone of Pu Bei County, once the Fang Cheng Plasma Collection Station becomes operational, we hope to expand its coverage area to secure higher collection volumes in the future. In January, 2010, Shandong Taibang purchased 100% of Yuncheng Ziguang Biotechnology Co., Ltd., or Yuncheng Ziguang, located in Yuncheng, Shandong Province, for the purpose of relocation of Shandong Taibang’s He Ze Plasma Company, or He Ze, into the nearby Yuncheng Ziguang facility. In February 2011, the He Ze Plasma Company moved into Yuncheng Ziguang and began collecting plasma. We hope to expand He Ze to secure higher collection volumes in the future. In 2010, we additionally established two new plasma companies in Shandong Province, one of which began operation in December 2010 and the other of which is still under construction. We also expect that our acquisition of Dalin and its PRC operating subsidiary, Guizhou Taibang, and our acquisition of a minority equity interest in Huitian, will help secure our plasma supply as well as expand production capacity and market coverage.
6
-
Acquisition of competitors and/or other biologic related companies. In addition to organic growth, acquisition is an important part of our expansion strategy. Although there are about 32 approved plasma-based biopharmaceutical manufacturers in the market, we believe that there are only 26 manufacturers in operation, only about half of whom will be competitive. The top six manufacturers in China account for more than 50% market share. Furthermore, we believe that the regulatory authorities are considering further reforming the industry and those smaller, less competitive manufacturers will face the possibility of having their manufacturing permits revoked by the regulators, making them potential targets for acquisition. Also, if we are presented with appropriate opportunities, we may acquire additional companies, products or technologies in the biologic related sectors (including but not limited to medical, pharmaceutical and biopharmaceutical).
-
Further strengthening of research and development capability. We believe that, unlike other more developed countries such as the U.S., China’s plasma-based biopharmaceutical products are at the initial stage of development. There are many other plasma-based products that are being used in the U.S. which are not currently being manufactured in China. We intend to strengthen our research and development capability so as to expand our product line to include higher-margin, technologically more advanced plasma-based biopharmaceutical products. We believe that our increased focus on research and development will give us a competitive advantage over our competitors.
-
Market development and network expansion. Leveraging on the high quality and excellent safety record of our products, we intend to (i) enhance our product penetration with our existing customers by introducing new products and (ii) extend the reach of our products from our current market to include other provinces where we envision significant market potential.
Our Products
Our principal products are our approved human albumin and immunoglobulin products. We are currently approved to produce 21 biopharmaceutical products in eight major categories as follows:
| Approved Products (1)(2) | Cure/Use |
| Human Albumin: - 20%/10ml, 20%/25ml, 20%/50ml, 10%/10ml, 10%/25ml, 10%/50ml and 25%/50ml | Shock caused by blood loss trauma or burn; raised intracranial pressure caused by hydrocephalus or trauma; Oedema or ascites caused by hepatocirrhosis and nephropathy; prevention and cure of low-density- lipoproteinemia; and Neonatal hyperbilirubinemia. |
| Human Hepatitis B Immunoglobulin – 100 International Units, or IU, 200IU, 400IU | Prevention of measles and contagious hepatitis. When applied together with antibiotics, its curative effect on certain severe bacteria or virus infection may be improved. |
| Human Immunoglobulin – 10%/3ml and 10%/1.5ml | Original immunoglobulin deficiency, such as X chain low immunoglobulin, familiar variable immune deficiency, immunoglobulin G secondary deficiency; Secondary immunoglobulin deficiency: such as severe infection, newborn sepsis; and Auto-immune deficiency diseases, such as original thrombocytopenia purpura or kawasaki disease. |
| Human Immunoglobulin for Intravenous Injection – 5%/25ml and 5%/50ml | Same as above |
| Human Immunoglobulin-5g/vial | Same as above |
| Thymopolypeptides Injection – 20mg/2ml,5mg/2ml | Cure for various original and secondary T-cell deficiency syndromes, some auto-immune deficiency diseases and various cell immunity deficiency diseases, and assists in the treatment for tumors. |
| Human Rabies Immunoglobulin – 100IU, 200IU and 500IU | Mainly for passive immunity from bites or claws by rabies or other infected animals. All patients suspected of being exposed to rabies will be treated with a combined dose of rabies vaccine and human rabies immunoglobulin. |
| Human Tetanus Immunoglobulin – 250IU | Mainly used for the prevention and therapy of tetanus. Particularly applied to patients who have allergic reactions to Tetanus Antitoxin. (3) |
| (1) |
“%” represents the degree of dosage concentration for the product and each product has its own dosage requirement. For example, Human Albumin 20%/10ml means 2g of Human Albumin is contained in each 10ml packaging and Human Immunoglobulin 10%/3ml means 300mg of Human Immunoglobulin is contained in each 3ml packaging. Under PRC law, each variation in the packaging, dosage and concentration of medical products requires registration and approval by the SFDA. During this process the altered product is not commercially available for sale. For example, among our Human Albumin products only Human Albumin 20%/10ml, 20%/25ml, 20%/50ml, 10%/10ml, 10%/25ml, 10%/50ml, and 25%/50ml products are currently approved and are commercially available. |
7
| (2) | “IU” means International Units, or IU. IU is a unit used to measure the activity of many vitamins, hormones, enzymes, and drugs. An IU is the amount of a substance that has a certain biological effect. For each substance there is an international agreement on the biological effect that is expected for 1 IU. In the case of Immunoglobulin, it means the number of effective units of antibodies in each package. When exposed to an antigen, the body views it as foreign material, and takes steps to neutralize the antigen. Typically, the body accomplishes this by making antibodies, which are intended to defend the body from invasion by potentially dangerous substances. These antibodies can be beneficial, as is the case when the body learns to fight a virus, or they can be harmful, in the instance of allergies. In a situation when the body cannot effectively react with these antigens, injection of our product will provide sufficient antibodies to neutralize the antigens. |
| (3) | “Tetanus Antitoxin” is a cheaper injection treatment for tetanus. However it is not widely used because most people are allergic to it. |
______________
Human albumin is principally used to increase blood volume while immunoglobulin is used for certain disease preventions and cures. Albumin is also used to treat critically ill patients by replacing lost fluid and maintaining adequate blood volume and pressure. Our approved human albumin and immunoglobulin products use human plasma as the basic raw material. All of our approved products are prescription medicines administered in the form of injections.
Under PRC law, each variation in the packaging, dosage and concentration of medical products requires registration and approval by the SFDA. During this process the altered product is not commercially available for sale. For example, among our human albumin products only Human Albumin 20%/10ml, 20%/25ml, 20%/50ml, 10%/10ml, 10%/25ml, 10%/50ml, and 25%/50ml products are currently approved and are commercially available. Accordingly, all references, in this report, to our manufacture and sale of human albumin relate to our approved human albumin products.
We have two product liability insurances covering Shandong Taibang and Guizhou Taibang’s products in the amount of approximately $3,034,000 (RMB 20,000,000) each. Since our establishment in 2002, there has not been any product liability claims nor has any legal action been filed against us by patients related to the use of our products.
Raw Materials
Plasma
Plasma is the principal raw material for our biopharmaceutical products. Until 2006, all plasma collection stations were owned by the PRC government. Following the mandated privatization of plasma stations resulted from the Ministry of Health’s Blood Collection Measures, we acquired our stable of plasma collection stations. We believe that the acquisitions of plasma stations will give us a controlled source of plasma and better control over the quality and quantity produced. We will also be able to have increased control over the cost of plasma. Finally, we believe that we will enjoy benefits of economies of scale with respect to the administration and management expenses of our several plasma stations.
We spent $51.0 million, $35.6 million and $14.0 million on the collection of plasma in 2010, 2009 and 2008, respectively. Currently, we own six operating plasma collection stations in Shandong province, two in Guangxi province and five in Guizhou province, and two under construction in each of Shandong and Guangxi provinces. We currently maintain sufficient plasma supply for approximately 6 months of production. In March 2007, the SFDA implemented new measures on biopharmaceutical industry effective as of July 1, 2008, requiring plasma raw material to be kept for at least 90 days before being put into production. In view of the new measures, in due course we will extend our plasma supply for approximately four months. We have not experienced any interruptions to our production due to shortage of plasma.
Other Raw Materials and Packaging Materials
Other raw materials used in the production of our biopharmaceutical products include: reagents, consumables and packaging materials. The principal packaging materials we use include glass bottles for our injection products, external packaging and printed instructions for our biopharmaceutical products. We acquire our raw materials and packaging materials from our approved suppliers in China and overseas. We select our suppliers based on quality, consistency, price and delivery of the raw materials which they supply.
We have not experienced any shortage of supply on these raw materials and packaging materials and there has not been any significant problem with the quality of materials supplied by these suppliers.
Our Major Suppliers
The table below lists our major suppliers as of December 31, 2010, showing the cumulative dollar amount of raw materials and supplies purchased from them during the fiscal year ended December 31, 2010, and the percentage of purchases from each supplier as compared to procurement of all raw materials.
8
Rank |
Supplier's Name |
Cumulative Amount Purchased During Fiscal Year 2010 (US$) |
Percentage of Total Purchases During Fiscal Year 2010 |
| 1 | Sansui Plasma Station | 3,086,695 | 20.6% |
| 2 | Chongqing Sanda Weiye Pharmaceutical Products Company | 1,575,679 | 10.5% |
| 3 | Sichuan Nangeer Biological Medical Company | 940,706 | 6.3% |
| 4 | Chengdu Yingde Industrial Equipment Installation Company | 888,406 | 5.9% |
| 5 | Tai'an City Ruifeng Company | 603,326 | 4.0% |
| 6 | Guizhou Nengji Industrial Company | 483,010 | 3.2% |
| 7 | Beijing Wantai Biological Pharmacy Enterprise | 397,938 | 2.7% |
| 8 | Shijiangzhuang Dongsai Trading Company | 389,230 | 2.6% |
| 9 | Beijing Zhongtianbaiyi Technology Development Company | 360,451 | 2.4% |
| 10 | Guangzhou Maige Biologic Technology Company | 249,934 | 1.7% |
| TOTAL | 8,975,375 | 60.0% |
Except for the Sansui Plasma Station, none of the above suppliers are plasma suppliers. The majority of our plasma were collected through our majority-owned plasma stations. These stations purchase, collect, examine and deepfreeze plasma on behalf of Shandong Taibang and Guizhou Taibang, subject to rules and specifications that meet the Provincial SFDA’s requirements for quality, packaging and storage. The stations must only collect plasma from healthy donors within their respective districts and in accordance with a time table set by Shandong Taibang or Guizhou Taibang. The plasma must: be negative HbsAg, anti-HCV, anti-HIV and reaction of serum to RPR; contain an ALT ≤25 units (ALT), plasma protein ≥55g/l; contain no virus pollution or visible erythrolysis, lipemia, macroscopic red blood cell or any other irregular finding. In addition, the plasma must be packaged in 25 separate 600g bags, boxed with a packing list and labeled to be consistent with computer records and must be stored at -20°C within limited time after collection to ensure that it will congeal within 6 hours. Shandong Taibang and Guizhou Taibang are fully responsible for the overall technical guidance and quality supervision.
Our Major Customers
Due to the nature of our products and the current regulations, all of our customers are located in China. We have established relationships with most of our key customers since our establishment in 2002. For the fiscal year ended December 31, 2010, our top five customers, based on sales revenue and the percentage of their contribution to our revenues, were as follows:
Rank |
Customer’s Name |
Revenues During Fiscal Year 2010 (US$) |
Percentage of Total Sales
During Fiscal Year 2010 |
| 1 | Yunnan JianRong Biologic Product Company | 3,895,986 | 2.8% |
| 2 | Handan Zhiying Medical Company | 3,675,226 | 2.6% |
| 3 | Guangdong Meheco Medicine Company | 3,278,723 | 2.3% |
| 4 | Guangdong Minshengtang Medicine Company | 3,272,824 | 2.3% |
| 5 | Shanghai Pharmaceutical Co., Ltd. | 3,170,650 | 2.3% |
| TOTAL | 17,293,409 | 12.3% |
Sales, Marketing and Distribution
Because all of our products are prescription drugs, we can only sell to hospitals and inoculation centers directly or through approved distributors. For the years ended December 31, 2010, 2009 and 2008, direct sales to distributors represented approximately 48.9%, 67.3% and 65.6%, respectively, of our revenues. Our five largest customers in the aggregate accounted for approximately 12.3%, 10.7% and 16.2% of our total revenues for the years ended December 31, 2010, 2009 and 2008, respectively. Our largest customer accounted for approximately 2.8%, 4.0% and 6.2% of our total revenues for the years ended December 31, 2010, 2009 and 2008, respectively.
As part of our effort to ensure the quality of our distributors, we conduct due diligence to verify whether potential distributors have obtained necessary permits and licenses and facilities (such as cold storage) for the distribution of our biopharmaceutical products. We also assess the distributors’ financial condition before appointing them as distributors. Certain of our regional distributors are appointed on an exclusive basis within a specified area. The supply contracts normally set out the quantity and price of products. For distributors, they also contain guidelines for the sale and distribution of our products, including restrictions on the geographical area to which the products could be sold. We provide our distributors with training in relation to our products and on sales techniques. We have implemented a coding system for our products for easy tracking. Depending on the relationship and the creditability of the distributors, we generally grant a credit period of no longer than 30 days to distributors with some exceptions. For hospitals and clinics, we generally grant a credit period of no longer than 90 days. We had a bad debt credit of $0.1 million for 2010, a bad debt expense of $0.3 million for 2009 and a bad debt credit of $0.1 million for 2008 related to the sales of our products. The $0.1 million bad debt credit for both 2010 and 2008 is due to recovery of bad debt previously reserved.
9
Our current key market is in Shandong province, representing approximately 22.0%, 25.5% and 48.1% of our total revenues for the years ended December 31, 2010, 2009 and 2008, respectively. Prior to the acquisition of Dalin and Huitian, our strategy has been to focus our marketing efforts in Jiangsu, Zhejiang, Henan and the northeastern part of China. With the advantage of the scale of economy, the Company has been expanding its sales efforts into 30 provinces and municipal cities, especially those provinces that were untapped by Shandong Taibang previously, with Shandong and Guangdong provinces accounting for more than 30.8% of the total sales during 2010.
Our marketing and after-sales services department currently employs approximately 93 employees.
We believe that due to the unique nature of our products, the key emphasis on our marketing efforts centers on product safety, brand recognition, timely availability and pricing. As all of our products are prescription medicines, we are not allowed to advertise our products in the mass media. For the years ended December 31, 2010, 2009 and 2008, total sales and marketing expenses amounted to approximately $7.4 million, $3.5 million and $2.2 million, respectively, representing approximately 3.0%, 4.4% and 4.7%, respectively, of our revenues.
Our Research and Development Efforts
The Shandong Institute was established in 1971. The Shandong Institute is the research arm established by and directly administrated by the Shandong Provincial health department. It was the only entity approved for the research, development and production of biological and plasma-based biopharmaceutical products in Shandong Province, the second largest province in China. Since 1998, it promoted GMP management in the production process of blood products and became one of the first blood products manufacturing enterprises to obtain GMP Certification in China. In 2002, the Shandong Institute transferred all of its business and the licenses necessary to carry on its business and seconded certain of its employees to our subsidiary, Shandong Taibang. We were awarded the advanced high-tech enterprise certification by the Department of Science and Technology of Shandong Province in 2005 and 2008 and by the Ministry of Science and Technology of China in 2006. In 2007, we were admitted as a member of the Shandong Institute of Medicine and awarded the “Advanced Enterprise” accolade by the Shandong Blood Center. We were also awarded the “Advanced Technology Certification for Foreign Funded Enterprises” by the Department of Foreign Trade and Economic Cooperation of Shandong Province in 2008.
We employ a market driven approach to initiate research and development projects including both product and production technique development. We believe that the key to the industry revolves around (i) safety of products and (ii) maximizing the yield per unit volume of plasma. Our research and development efforts are focused around the following areas:
- broaden the breadth and depth of our portfolio of plasma-based biopharmaceutical products;
- enhance the yield per unit volume of plasma through new collection techniques;
- maximize manufacturing efficiency and safety;
- promote product safety through implementation of new technologies; and
- refine production technology for existing products.
Our research center is located on the same premises as the factory, which is located in Tai’an City, Shandong Province. The research center is equipped with specialized equipment including advanced testing and analytical equipment, such as atomic absorptimeter, fully automated blood coagulation analyzer, high performance liquid chromatograph, gas chromatograph, radioimmunoassay analyzer, ultraviolet-visible spectrophotometer, and protein chromatograph, most of which have been imported from the US, Japan, Italy, Germany and Australia. Our research and development department is comprised of about 30 researchers. All of them hold degrees in areas such as medicine, pharmacy, biology, and biochemistry. Our research center carries out development and registration of our products.
All the products we currently manufacture have been developed in-house. The following table outlines our research and development work in progress:
| Products Currently in Development |
Cure/Use |
Status of Product Development |
Stage* |
| Human Prothrombin Complex Concentrate | Used for the prophylaxis and treatment of bleeding in patients with single or multiple congenital deficiencies of factor II or X and in patients with single or multiple acquired prothrombin complex factor deficiency requiring partial or complete reversal. | Application made to the SFDA for official production permit and product certification. Commercial production expected in second half of 2011. | 9 |
| Human Coagulation Factor VIII | Use for coagulopathie such as Hemophilia A and increase concentration of coagulation factor VIII. | Application made to the SFDA for official production permit and product certification Commercial production expected in first half of 2011. | 9 |
10
| Human Hepatitis B Immunoglobulin (PH4) for Intravenous Injection | Prevention of measles and contagious hepatitis. When applied together with antibiotics, its curative effect on certain severe bacteria or virus infection may be improved. | Clinical trial just commenced Commercial production expected in 2014. | 8 |
| Human Fibrinogen | Cure for lack of fibrinogen and increase human fibrinogen concentration. | Clinical trial program under SFDA review. Commercial production in 2014. | 7 |
| Varicella Hyperimmune Globulins | Used for treatment of eczema vaccinatum, vaccinia necrosum, and ocular vaccinia | Develop scope and technique for testing the new medicine. | 3 |
| Human Immunoglobulin for Intravenous Injection – 10% | Cure for original immunoglobulin deficiency; secondary immunoglobulin deficiency and Auto-immune deficiency diseases | Develop laboratory-scale manufacturing process. | 3 |
* These stages refer to the stages in the regulatory approval process for our products disclosed under the heading “Regulation” in this report.
For the fiscal years ended December 31, 2001, 2009 and 2008, total research and development expenses amounted to approximately $2.3 million, $1.7 million and $1.2 million, respectively, representing approximately 1.7%, 1.4% and 2.5%, respectively, of our revenues.
Our Competition
We are subject to intense competition. There are both local and overseas pharmaceutical enterprises that are engaged in the manufacture and sale of potential substitute or similar biopharmaceutical products as our products in the PRC. These competitors may have more capital, better research and development resources, manufacturing and marketing capability and experience than we do. In our industry, we compete based upon product quality, product cost, ability to produce a diverse range of products and logistical capabilities.
We believe that we have strengthened our position in the marketplace with our acquisition of Dalin and its 54% majority-owned operating subsidiary, Guizhou Taibang and a 35% equity interest in Huitian, Xi’an-based biopharmaceutical company.
Our profitability may be adversely affected if (i) competition intensifies; (ii) competitors drastically reduce prices; (iii) PRC government’s interference on prices; or (iv) competitors develop new products or product substitutes having comparable medicinal applications or therapeutic effects which are more effective and /or less costly than those produced by us.
Other approved biopharmaceutical manufacturers in the PRC are entitled to produce many of the products produced by us. There are currently about 32 approved manufacturers of plasma-based pharmaceutical products in China. Many of these manufacturers are essentially producing the same type of products that we produce: human albumin and various types of immunoglobulin. However, due to recent Ministry of Health regulations, we believe that it is difficult for new manufacturers to enter into the industry. We believe that our major competitors in the albumin and immunoglobulin market in China are Hua Lan Biological Engineering, Shanghai Institute of Biological Products, Shanghai RAAS Blood Products Co. Ltd., Beijing Tiantan Biological Products, and Sichuan Yuanda Shuyang Pharmaceutical Co.
In addition, competition from imported products and China’s admission as a member of the WTO creates increased competition. The PRC became a member of the WTO in December 2001. Competition in the biopharmaceutical industry in the PRC will intensify generally in two respects. With lower import tariffs, we anticipate that imported biopharmaceutical products manufactured overseas may become increasingly competitive with domestically produced products in terms of pricing. We also believe that well-established foreign biopharmaceutical manufacturers may set up production facilities in the PRC and compete with domestic manufacturers directly. With the expected increased supply of competitively priced biopharmaceutical products in the PRC, we may face with increased competition from foreign biopharmaceutical products, including the types of products manufactured by US manufacturers and other manufacturers. In 2009 and 2010, we have seen a substantial increase in the volume of imported human albumin in China. If the trend of importation of human albumin continues, we may face more fierce competition in domestic human albumin market.
We believe that we have secured better ranking in 2010 based on our analysis of data regarding the approval for sales of plasma-derived products published by China National Institute for the Control of Pharmaceutical and Biological Products throughout of the year. Our past financial performance is attributable to our market position in the industry. Furthermore, while each of the plasma products related companies have their own product composition which include 3 main categories namely human albumin, human immunoglobulin and lyophilized human factor, we are currently developing lyophilized human factor products which we expect to launch in 2011. We will continue to meet challenges and secure our market position by enhancing our existing products, introducing new products to meet customer demand, delivering quality products to our customers in a timely manner and maintaining our established industry reputation.
11
Our Intellectual Property
Pursuant to a Trademark License Agreement with the Shandong Institute, we hold the exclusive license to a Trademark Registration Certificate (No.3375484) issued by the PRC Industry and Commerce Administration Trademark Bureau. The class of goods on which the trademark has been approved to use include: drug for human beings, serum, microorganism products for medicine and veterinary medicine, plasma, medical blood, and medical biological product. The registration will expire in June 2014. The Shandong Institute has allowed us to use the trademark for free until May 2011. We expect to develop and register our own trademark before the termination of this license.
In addition, we have registered the following domain names: www.chinabiologic.com and www.ctbb.com.cn.
Regulation
This section summarizes the major PRC regulations relating to our business.
Due to the nature of our products, we are supervised by various levels of the PRC Ministry of Health and/or SFDA. Such supervision includes the safety standards regulating our source supplies (mainly plasma), our manufacturing process through the issuance of our GMP Certification and the inspection of our finished products.
We are also subject other PRC regulations, including those relating to taxation, foreign currency exchange and dividend distributions.
Plasma Collection
Substantially all plasma donations for commercialized plasma-based biopharmaceutical products are done through plasmapheresis donation stations. Plasmapheresis donation means donors give only selected blood components — platelets, plasma, red cells, infection-fighting white cells called granulocytes, or a combination of these, depending on donors blood type and the needs of the community. Plasmapheresis stations in China are commonly used to collect plasma. In China, current regulations only allow an individual donor to donate blood in 14-day intervals, with a maximum quantity of 580ml (or about 600 gram) per donation.
The following are the regulatory requirements to establish a plasmapheresis station in China:
- meet the overall plan in terms of the total number, distribution, and operational scale of plasmapheresis stations;
- have the required professional health care technicians to operate a station;
- have the facility and a hygienic environment to operate a station;
- have an identification system to identify donors;
- have the equipment to operate a station; and
- have the equipment and quality control technicians to ensure the quality of the plasma collected.
As a result of the overhaul by the four ministries of the State Council in May 2004, we estimate that the number of collection stations (including plasma stations) that meet the standards imposed by the PRC has been reduced from approximately 156 to approximately 120. Plasma stations were customarily owned and managed by the PRC health authorities. In March 2006, the Ministry of Health promulgated the Blood Collection Measures whereby the ownership and management of the plasma stations must be transferred to plasma-based biopharmaceutical companies while the regulatory supervision and administrative control remain with the government. For those plasma stations which did not complete their reform by December 31, 2006, their license to collect plasma will be revoked. As a result, all plasma stations are now having direct supply relationship with their parent fractionation facilities.
Set out below are some of the safety features at China’s collection stations:
-
Collection stations can only source plasma from donors within the assigned district approved by the provincial health authorities.
-
Collection stations must perform a health check on the donor. Once the donor passes the health check, a “donor permit” is issued to the donor. The standards of the health check are established by the health authorities at the State Council level.
-
The design and printing of the “donor permit” is administrated by the provincial health authorities, autonomous region or municipality government, as the case maybe. The “donor permit” cannot be altered, copied or assigned.
-
Before donors can donate plasma, the station must verify their identities and the validity of their “donor permits.” The donors must pass the verification procedures before they are given a health check and blood test. For those donors who have passed the verification, health check and blood test and whose plasma were donated according to prescribed procedures, the station will setup a record.
12
- All collection stations are subject to the regulations on transmittable diseases prevention. They must strictly adhere to the sanitary requirements and reporting procedures in the event of an epidemic situation.
The operation of plasma collection stations is strictly regulated by the PRC government. With the restarts of previous stations and newly built stations, the Company estimated that there are approximately 140 plasma stations in operation in China.
Importation of Blood Products
According to current Chinese regulations, the following blood products are banned from importation to China:
-
Plasma – frozen, liquid and freeze-dried Human Plasma;
-
Immunoglobulin – Human Normal Immunoglobulin, Specific Immunoglobulin, Human Anti-Tetanus Immunoglobulin, Human Anti-hemophilia Globulin, Human Anti-HBs Immunoglobulin, Human Anti- D(Rho) Immunoglobulin and Immunoglobulin For Intravenous Administration;
-
Factor VIII – Cryoprecipitated Factor VIII and Factor VIII Concentrate (only Bayer is allowed, under a special arrangement with PRC government, to import this product into PRC, commencing November 2007);
-
Factor IX Concentrate;
-
Human Fibrinogen;
-
Platelet Concentrate;
-
Human Prothrombin Complex;
-
Whole blood or blood components.
Production of Plasma-based Products
The manufacture and sale of plasma-based biopharmaceutical products is strictly regulated by the PRC government. For example, under PRC law, each variation in the packaging, dosage and concentration of medical products requires registration and approval by the SFDA. During this process the altered product is not commercially available for sale. For example, among our human albumin products only Human Albumin 20%/10ml, 20%/25ml, 20%/50ml, 10%/10ml, 10%/25ml, 10%/50ml, and 25%/50ml products are currently approved and are commercially available. Accordingly, all references, in this report, to our manufacture and sale of human albumin relate to our approved human albumin products.
The table below shows the PRC approval process for the manufacture and sale of new medicines:
| Stage (Estimated Time Period) | Activities | |
| 1 | Planning Stage (1 month) | Prior to the development of potential new products, our Research & Development department will engage in a comprehensive review of existing medical literature, patent status and market information, including expected product demand and other competition, in order to determine the feasibility of development and production of a new product offering. Although this typically takes about 1 month to complete, this stage precedes development efforts for a new product, which could take several months or even years to complete. For products with lengthy development periods, we may be required to periodically revisit this stage to confirm the feasibility of continued development efforts. |
| 2 | Feasibility study and assumption clarification (2 months) | If we determine that development, ownership and marketing of a potential new product is possible and potentially advantageous, we proceed with development efforts. However, potential new products are typically developed in a laboratory or small batch setting, and in order to obtain approval for potential new products and to market new products, we must develop a plan for testing and producing the new product. The first step in developing such plan is a feasibility study and assumption clarification. This study is conducted following or during development of a new product, and involves a review and study of the feasibility of our technical, production and financial capabilities, production conditions and financial forecasts. We also review the feasibility of preparing and conducting a clinical study, or a Clinical Trial program, during this stage. |
| 3 | Develop scope and technique for testing the new medicine (6 months) | If following completion of a Stage 2 study we make a determination that producing and testing a potential new product is feasible and potentially advantageous, we will develop the scope and techniques for testing the potential new product. This involves confirming the sourcing of materials needed for production and marketing of the potential new product and development of the method of production, dosage design and prescription selections. During this stage, we will also develop a clinical research sample. |
13
| 4 | Preparation of a virus inactivation report and submission to the National Institute for the Control of Pharmaceutical and Biological Products, or NICPBP, for preliminary review (4-6 months) | If following development of testing methods for the potential new product we determine that testing can be successfully completed, we will prepare and finalize the virus inactivation method for the potential new product. We are then required to prepare a report with details on the production method and procedures and basis of quality evaluation for preliminary review by the NICPBP. NICPBP staff usually makes an onsite visit during this stage to supervise testing and re-testing of the virus inactivation process. Tested samples will be sent back to the NICPBP central office in Beijing for evaluation. |
| 5 | R&D test product information submitted to the SFDA for preliminary assessment (4-6 months) | Before the NICPBP can determine that our clinical research sampling and virus inactivation method and procedures are successful, we are required to submit our clinical research sampling and virus inactivation method and procedures to the SFDA via the provincial FDA for preliminary assessment. We also develop the parameters for a Clinical Trial program at this stage. Our program usually requires the establishment of a committee comprised of our Research and Development staff whose responsibility is to communicate with the hospitals and doctors who are invited to participate in the trial. After our submission of information to the SFDA we will become subject to random onsite sampling by the SFDA as they review our reports and procedures regarding testing of the potential product. The SFDA will usually inform us of the exact sampling date and SFDA staff will randomly select certain samples during their visit for additional testing. The SFDA will then provide us with their preliminary assessment of our new product and our related procedures. Depending on the results of its preliminary assessment the SFDA may recommend that we alter certain aspects of our reports and proposed Clinical Trial programs, or even repeat our Stage 3 and Stage 4 trials and resubmit related reports. The SFDA review process typically takes 4-6 months, but this process could take longer if we are required to amend or repeat our trials or if we amend our reports in order to obtain more a favorable preliminary assessment. |
| 6 | Formal application to the NICPBP for test of virus inactivation and for CDE certification of Clinical Trial (6-7 months) | Once we receive a favorable or satisfactory preliminary assessment from the SFDA, the NICPBP will continue the process begun at Stage 4. The NICPBP will conduct tests of virus inactivation based on defined medical literature and on our prescribed procedures and method of production. If the tests are successful, the NICPBP will transfer the application to the CDE for review of our prescribed procedures and method of production and the CDE may request additional information before making a determination. If the CDE is satisfied with our procedures and method of production it will certify the new product for production for Clinical Trial. |
| 7 | SFDA review of Clinical Trial program for approval (1 month) | Following provision of the CDE product certification, we must submit our Clinical Trial program (developed at Stage 5 and 6) to the SFDA for formal approval. The SFDA may request additional information regarding our proposed Clinical Trial program. If the SFDA rejects our Clinical Trial program or requires changes to any of our procedures and methods, we may be required to amend our Clinical Trial program, which may require repeating several of the processes previously conducted. The criteria for SFDA approval for Clinical Trial programs are based on Good Clinical Practice which is publicly available in the PRC. |
| 8 |
Clinical Trial: Phases 1 to 4 (3 years for a
new drug and 2 years for a generic drug) |
Following approval of our Clinical Trial
program by the SFDA, we will begin Clinical Trials of the potential new
product. There are four phases to the clinical trial process and any
failure of the potential new product at any of the Clinical Trial phases,
could cause a significant delay in approval of the new product, or
termination of the new product launch: Phase 1: Basic clinical pharmacology and human safety evaluation studies are conducted by the Company. Prior to determining the effectiveness of our potential new product, we must determine that certain pharmacological and safety standards are met by our potential new product. These standards are set in stage 4 or according to medical literature. If the clinical trial indicates that such standards are met, we then move on to Phase 2 of the trials. If the Phase 1 standards are not met, we may be required to conduct further R&D on the potential new product, alter the new product formulation and amend the Clinical Trial program, which could require that we repeat several of the stages referenced above. Phase 2: A preliminary exploration of the product's therapeutic efficacy is conducted by the Company. If we determine at this stage that the potential new product is not effective, we may conduct further R&D on the potential new product, alter the new product formulation and amend the Clinical Trial program, which would require that we repeat several of the stages referenced above. Phase 3: If we determine that the potential new product meets the required standards of Phases 1 and 2 above, we must then submit a report of the Clinical Trial results to the SFDA together with an application for trial production of the product. If the SFDA rejects application for trial production or otherwise requires a repeat of our Clinical Trials, we may be required to repeat all or a portion of our Clinical Trial program, which may require repeating several of the processes previously conducted. Phase 4: If we receive SFDA approval to conduct a trial production of the new product, we will then conduct a larger test of approximately 2,000 samples. We will conduct this test while also conducting a new drug post- marketing study. |
14
| 9 | Application to the SFDA for official production permit and product certification (8- 9 months) | The trial production of the potential new product will be monitored by an SFDA inspector who will also make onsite visits and assess the results of the trial production. We will also be required to prepare and submit to the SFDA a report of the trial production results by gathering statistical information obtained during the trial period. The CDE will also conduct a final review of the trial production for the potential new product. Upon satisfactory completion of the trial production, the CDE will inform the SFDA. The SFDA will then issue a permit to us for official production, the issuance of which is announced on the SFDA's website, and copied to the NICPBP and the provincial FDA. The SFDA will also issue the new product a Good Manufacturing Practice, or GMP, certification. The provincial FDA will follow with the issuance of a provincial production permit for the new product. Although the SFDA's criteria for final approval of new products are not publicly available in the PRC, if a manufacturer makes the adjustments to its methods and procedures recommended by the SFDA earlier on in the product approval process, it is likely that the SFDA will approve the new product for production. |
| 10 | Commercial Production | Following issuance of state and provincial production permits and certifications, we may begin production of the new product. |
Pricing
In addition, there are regulations regarding the retail price, rather than regulations of wholesale prices, of our products. According to the “Regulations on controlling blood products” promulgated by the State Council in 1996, the price (retail) setting standard and regulatory functions reside with regional offices of the Pricing Bureau and the Ministry of Health. Presently, there are retail pricing guidelines for hospitals which sell our human albumin and immunoglobulin products to patients as prescribed by the relevant regulators in each region. The retail pricing guidelines are established based on, amongst other things, the regional living standards and the cost of production of the manufacturers. The hospitals cannot sell the products to patients at prices exceeding the highest retail price prescribed by the relevant regulators. There is no pricing guideline on the ex-factory price to the hospital and the distributors. The highest retail price guideline is revised occasionally.
Taxation
On March 16, 2007, the National People's Congress of China passed a new Enterprise Income Tax Law, or EIT Law, and on November 28, 2007, the State Council of China passed its implementing rules, which took effect on January 1, 2008. Before the implementation of the EIT Law, foreign invested enterprises, or FIEs, established in the PRC, unless granted preferential tax treatments by the PRC government, were generally subject to an earned income tax, or EIT, rate of 33.0%, which included a 30.0% state income tax and a 3.0% local income tax. The EIT Law and its implementing rules impose a unified EIT of 25.0% on all domestic-invested enterprises and FIEs, unless they qualify under certain limited exceptions. However, the EIT Law gives FIEs established before March 16, 2007, or Old FIEs, a five-year grandfather period during which they can continue to enjoy their existing preferential tax treatments. During this five-year grandfather period, Old FIEs that enjoyed tax rates lower than 25% under the original EIT Law can gradually increase their EIT rate by 2% per year until their tax rate reaches 25%. In addition, the Old FIEs that are eligible for the “two-year exemption and three-year half reduction” or “five-year exemption and five-year half-reduction” under the original EIT law, are allowed to continue enjoying their preference until these holidays expire.
In addition to the changes to the current tax structure, under the EIT Law, an enterprise established outside of China with “de facto management bodies” within China is considered a resident enterprise and will normally be subject to an EIT of 25% on its global income. The implementing rules define the term “de facto management bodies” as “an establishment that exercises, in substance, overall management and control over the production, business, personnel, accounting, etc., of a Chinese enterprise.” If the PRC tax authorities subsequently determine that we should be classified as a resident enterprise, then our organization’s global income will be subject to PRC income tax of 25%. For detailed discussion of PRC tax issues related to resident enterprise status, see Item 1A, “Risk Factors – Risks Related to Doing Business in China – Under the New Enterprise Income Tax Law, we may be classified as a ‘resident enterprise’ of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC stockholders.”
15
Foreign Currency Exchange
The principal regulation governing foreign currency exchange in China is the Foreign Currency Administration Rules (1996), as amended (2008). Under these Rules, RMB is freely convertible for current account items, such as trade and service-related foreign exchange transactions, but not for capital account items, such as direct investment, loan or investment in securities outside China unless the prior approval of, and/or registration with, the State Administration of Foreign Exchange of the People’s Republic of China, or SAFE, or its local counterparts (as the case may be) is obtained.
Pursuant to the Foreign Currency Administration Rules, FIEs in China may purchase foreign currency without the approval of SAFE for trade and service-related foreign exchange transactions by providing commercial documents evidencing these transactions. They may also retain foreign exchange (subject to a cap approved by SAFE) to satisfy foreign exchange liabilities or to pay dividends. In addition, if a foreign company acquires a company in China, the acquired company will also become an FIE. However, the relevant PRC government authorities may limit or eliminate the ability of FIEs to purchase and retain foreign currencies in the future. In addition, foreign exchange transactions for direct investment, loan and investment in securities outside China are still subject to limitations and require approvals from, and/or registration with, SAFE.
Dividend Distributions
Under applicable PRC regulations, FIEs in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a FIE in China is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its general reserves until the accumulative amount of such reserves reach 50% of its registered capital. These reserves are not distributable as cash dividends. The board of directors of a FIE also has the discretion to allocate a portion of its after-tax profits to staff welfare and bonus funds, which may not be distributed to equity owners except in the event of liquidation.
In addition, under the EIT law, the Notice of the State Administration of Taxation on Negotiated Reduction of Dividends and Interest Rates, which was issued on January 29, 2008, the Arrangement between the PRC and the Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion, or the Double Taxation Treaty, which became effective on December 8, 2006, and the Notice of the State Administration of Taxation Regarding Interpretation and Recognition of Beneficial Owners under Tax Treaties, which became effective on October 27, 2009, dividends from our PRC operating subsidiary, Logic China, paid to us through our Hong Kong subsidiary, Logic Holdings, may be subject to a withholding tax at a rate of 10%, or at a rate of 5% if Logic Holdings is considered a “beneficial owner” that is generally engaged in substantial business activities and entitled to treaty benefits under the Double Taxation Treaty.
Our Employees
As of December 31, 2010, we employed approximately 1,446 full-time employees, including Shandong Taibang and Dalin and all of their subsidiaries and Taibang Medical, of which approximately 104 were seconded to us by the Shandong Institute.
We believe that we maintain a satisfactory working relationship with our employees and we have not experienced any significant labor disputes or any difficulties in recruiting staff for our operations. As required by applicable Chinese law, we have entered into employment contracts with most of our officers, managers and employees. The PRC enacted a new Labor Contract Law, which became effective on January 1, 2008. We have updated our employment contracts and employee handbook and are in compliance with the new law. We will work with the employees and the labor union to insure that our employees obtain the full benefit of the law. We do not anticipate that changes in the law will materially impact our balance sheet and cash flows.
ITEM 1A. RISK FACTORS.
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this report, before making an investment decision. If any of the following risks actually occurs, our business, financial condition or results of operations could suffer. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment. You should read the section entitled “Special Note Regarding Forward Looking Statements” above for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this report.
16
RISKS RELATED TO OUR BUSINESS
We identified material weaknesses in our internal controls over financial reporting that require us to restate our financial statements.
Our internal control over financial reporting is a process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer and effected by our board of directors to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external reporting purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes policies and procedures that pertain to the maintenance of records that in reasonable detail accurately reflect the transactions and dispositions of our assets; provide reasonable assurance that transactions are recorded as necessary to permit preparation of our financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with the authorization of our board of directors and management; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation under the criteria established in Internal Control – Integrated Framework, our management concluded that our internal control over financial reporting was not effective as of December 31, 2010 because of the material weakness in our internal control over financial reporting described below. A material weakness (within the meaning of PCAOB Auditing Standard No. 5) is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
Management believes that, as of December 31, 2010, our corporate accounting lacked the expertise in nonrecurring transactions to effectively recognize deferred tax liabilities and derivative instrument valuation review controls, which resulted in an inadvertent omission of the fair value of embedded options in warrants and a misinterpretation of US GAAP regarding the recognition of deferred tax liabilities upon a business combination. We have taken measures to remediate these material weaknesses by adding two additional qualified accountants in late 2010, and we have engaged outside consultants specializing in deferred tax accounting and derivative instrument valuation. We have also implemented more formal review procedures and documentation standards for the accounting and monitoring of non-routine and complex transactions and will continue to evaluate other measures to enhance the effectiveness of our internal control over financial reporting.
We face risks related to general domestic and global economic conditions and to the credit crisis. Disruptions in the capital and credit markets related to the current national and worldwide financial crisis, which may continue indefinitely or intensify, could adversely affect our results of operations, cash flows and financial condition, or those of our customers, suppliers and creditors.
We currently generate sufficient operating cash flows, which combined with access to the credit markets, provide us with significant discretionary funding capacity. However, the current uncertainty arising out of domestic and global economic conditions, including the disruption in credit markets, may impact our ability to manage normal relationships with our customers, suppliers and creditors. The disruptions in the capital and credit markets may continue indefinitely or intensify, and adversely impact our results of operations, cash flows and financial condition, or those of our customers, suppliers and creditors. Disruptions in the capital and credit markets as a result of uncertainty, changing or increased regulation, reduced alternatives or failures of significant financial institutions could adversely affect our access to liquidity needed to conduct or expand our businesses or conduct acquisitions or make other discretionary investments. Such disruptions may also adversely impact the capital needs of our customers and suppliers, which, in turn, could adversely affect our results of operations, cash flows and financial condition.
In addition, the demand for our products is largely affected by the general economic conditions in China as our products are still not affordable to many patients. As China's economy grows, we expect more Chinese people will become consumers of medical treatments and procedures, including procedures requiring human plasma. However, we expect that continuation of the global economic slowdown may result in slower economic growth in China and an unfavorable economic environment which in turn may make our products less affordable to more patients and result in an overall decreased demand for our products. Such reductions and disruptions could have a material adverse effect on our business operations.
In order to grow at the pace expected by management, we will require additional capital to support our long-term business plan. If we are unable to obtain additional capital in future years, we may be unable to proceed with our long-term business plan and we may be forced to curtail or cease our operations or further business expansion.
We will require additional working capital to support our long-term business plan, which includes identifying suitable targets for horizontal or vertical mergers or acquisitions, so as to enhance the overall productivity and benefit from economies of scale. Our working capital requirements and the cash flow provided by future operating activities, if any, will vary greatly from quarter to quarter, depending on the volume of business during the period and payment terms with our customers. We may not be able to obtain adequate levels of additional financing, whether through equity financing, debt financing or other sources, especially in light of the global financial crisis and the market downturn. To raise funds, we may need to issue new equities or bonds which could result in additional dilution to our shareholders and investors. Additional financings could result in significant dilution to our earnings per share or the issuance of securities with rights superior to our current outstanding securities or contain covenants that would restrict our operations and strategy. In addition, we may grant registration rights to investors purchasing our equity or debt securities in the future. If we are unable to raise additional financing, we may be unable to implement our long-term business plan, develop or enhance our products and services, take advantage of future opportunities or respond to competitive pressures on a timely basis. In addition, a lack of additional financing could force us to substantially curtail or cease operations.
Our limited operating history may not serve as an adequate basis to judge our future prospects and results of operations.
We have a limited operating history. Shandong Taibang as began its operation in October 2002. With the rapid growth of the industry, it has experienced a high growth rate since 2002. Furthermore, we did not acquire a controlling interest in Shandong Taibang until September 2005 and Guizhou Taibang until January 2009. As such, our historical operating results may not provide a meaningful basis for evaluating our business, financial performance and prospects. We may not be able to achieve a similar growth rate in future periods. Accordingly, you should not rely on our results of operations for any prior periods as an indication of our future performance.
We have a significant amount of debt, which could have negative consequences to us.
We have certain amount of debt. As of December 31, 2010, we had, on a consolidated basis, approximately $3.0 million principal amount of indebtedness outstanding. Our substantial indebtedness could have important consequences, including:
-
increasing our vulnerability to adverse general economic and industry conditions and adverse changes in governmental regulations;
-
limiting our ability to obtain additional financing to fund capital expenditures and other general corporate requirements;
-
requiring us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund capital expenditures or other general corporate purposes;
-
limiting our flexibility in planning for or reacting to changes in our business and the industry in which we operate; and
-
placing us at a competitive disadvantage compared to our less leveraged competitors.
Our ability to pay interest on our indebtedness and to satisfy our other debt obligations will depend upon, among other things, our future operating performance and cash flow and our ability to refinance indebtedness when necessary. Each of these factors is, to a large extent, dependent on general economic, financial, competitive, legislative, regulatory and other factors beyond our control. If in the future we cannot generate sufficient cash from operations to make scheduled payments on our indebtedness or to meet our liquidity needs or other obligations, we will need to refinance our existing debt, obtain additional financing or sell assets. We cannot assure you that we will be able to renegotiate or refinance any of our debt on commercially reasonable terms or at all. In addition, our interest expense may increase if general economic conditions result in an increasing interest rate environment. We cannot assure you that our business will generate cash flow, or that we will be able to obtain funding sufficient to satisfy our debt service requirements.
17
Our cash flow could be negatively affected as a result of our extension of relatively long payment terms to customers that we believe are credit worthy.
As is customary in our industry, we extend relatively long payment terms (up to six months) to customers that we believe are credit worthy. The dollar amount of our accounts receivable, net of our allowance for doubtful accounts as of December 31, 2010, 2009 and 2008 was $9,922,111, $1,767,076 and $313,087, respectively. The bad debt (credit) expenses for the years ended December 31, 2010, 2009 and 2008 were $(57,623), $(13,089) and $(56,462), respectively. Although we attempt to establish appropriate reserves for our receivables, those reserves may not prove to be adequate in view of actual levels of bad debts. The failure of our customers to pay us timely would negatively affect our working capital, which could in turn adversely affect our cash flow.
If the PRC government bans or limits plasma-based biopharmaceutical products, our operations, revenues and profitability would be adversely affected.
The principal raw materials of our existing and planned biopharmaceutical products is human source plasma, which, due to its unique nature, is subject to various quality and safety control issues which include, but are not limited to, contaminations and blood-borne diseases. In addition, limitations of current technology pose biological hazards inherent in plasma that have yet to be discovered which could result in a wide spread epidemic due to blood infusion. The primary law that regulates plasma products in China is the PRC Pharmaceutical Law, the Implementation Rules on the PRC Pharmaceutical Law and the Regulations on the Administration of Blood Products. These rules and regulations require entities producing blood products to strictly comply with certain hygienic standards and specifications promulgated by the government. In the event that human plasma is discovered to be noncompliant with the government's hygienic standards and specifications, the health department may revoke the registration and/or the approval of the blood product, or otherwise limit the use of such blood product. If the PRC government bans or limits plasma-based biopharmaceutical products, our operations, revenues and profitability would be adversely affected.
If the plasma we source is found to be contaminated, our operation, revenues and profitability would be adversely affected.
We currently source plasma mainly from human donations to our plasma stations in Shandong, Guangxi and Guizhou Provinces. If any of our human donors is infected with certain diseases, then the plasma from such donor may be infected. If such contaminated plasma is not appropriately screened out, our entire plasma source for the relevant collection station may become contaminated. If the plasma from our collection stations is found to be contaminated, our operation, revenues and profitability would be adversely affected.
If our supply of quality plasma is interrupted, our results of operations and profitability will be adversely affected.
The production of plasma-based biopharmaceutical products relies on the supply of plasma of suitable quality. For the years ended December 31, 2010, 2009 and 2008, the cost of plasma used by us for production accounted for approximately 73%, 83% and 76%, respectively, of total production cost. The supply and market prices of plasma may be adversely affected by factors such as regulatory restrictions, weather conditions or outbreak of diseases which would impact our costs of production. We may not be able to pass on any resulting increase in costs to our customers and therefore any substantial fluctuation in supply or market prices of plasma may adversely affect our results of operations and profitability.
The biopharmaceutical industry in the PRC is strictly regulated and changes in such regulations may have an adverse effect on our business.
The biopharmaceutical industry in the PRC is strictly regulated by the government. The regulatory regime, such as administrative approval of medicines and production approvals, comprises of series of regulations and administrative rules. The PRC regulatory authorities may amend such regulations and administrative rules and promulgate new regulations and administrative rules from time to time. Changes in these regulations and administrative rules could have a significant impact on our business. Such changes may have any adverse impact on our business.
18
We may not be able to carry on our business if we lose any of the permits and licenses required by the PRC Government in order to carry on our business.
All pharmaceutical manufacturing and distribution enterprises in the PRC are required to obtain from various PRC governmental authorities certain permits and licenses, including, in the case of manufacturing enterprises, a Pharmaceutical Manufacturing Permit and, in the case of distribution enterprises, a Pharmaceutical Distribution Permit.
We have obtained permits and licenses and the GMP certificates, required for the manufacturing and sales of our pharmaceutical products. These permits and licenses held by us are subject to periodic renewal and/or reassessment by the relevant PRC Government authorities and the standards of compliance required in relation thereto may from time to time be subject to changes. We intend to apply for the renewal of such permits and licenses when required by applicable laws and regulations. Any changes in compliance standards, or any new laws or regulations that may prohibit or render it more restrictive for us to conduct our business or increase our compliance costs may adversely affect our operations or profitability. Any failure by us to obtain such renewals may have a material adverse effect on the operation of our business. In addition, we may not be able to carry on business without such permits and business licenses being renewed.
If we do not receive PRC governmental approval to increase the retail prices of certain of our biopharmaceutical products our revenues may be adversely affected.
Retail prices of certain of our biopharmaceutical products in the PRC are subject to the control of the relevant central and provincial price administration authorities. The actual price for any given price-controlled product set by manufacturers, wholesalers and retailers cannot exceed the price ceiling imposed in accordance with the applicable government price control rules. Pharmaceutical products which are included in China’s National (Medical) Insurance Catalogue, or the NIC, administered at the central or provincial level are subject to price control by the National Reform and Development Commission of China, or the NDRC, China’s pricing authority.
The 2009 NIC was published by China's Ministry of Human Resources and Social Security in November, 2009 as part of China's healthcare reform, to make more drugs affordable to PRC consumers. The NIC is China’s official drug reimbursement list for its universal healthcare system, which the PRC government expected to cover 90% of PRC citizens as at the 2010 year end. The 2009 NIC features 2,151 drugs, categorized as Class A (fully covered) or Class B (partially covered). Five of our principal products, Human Albumin Human Immunoglobulin for intravenous injection, Human Rabies Immunoglobulin, Human Tetanus Immunoglobulin, Human Immunoglobulin have been included in the 2009 NIC as Class B drugs and are subject to national price control by the NDRC.
The 2009 NIC was expected to be fully implemented in 2010, but has been postponed. Once the 2009 NIC is fully implemented, the price of our included products may not be increased at our discretion above the relevant controlled retail price ceiling without prior governmental approval. This, in turn, may affect the ex-factory prices set by us for our products and we therefore do not have unfettered freedom to maximize our profits. It is uncertain whether we will be able to obtain necessary approvals to increase the price of any of our products.
If we are unable to adequately monitor our plasma stations our plasma supply may be tainted and we will be subject to sanctions by the government which would have a material adverse effect on our business.
As part of the industry reform initiative by the Chinese government, in 2006 we acquired the assets of five of the six then existing plasma stations in Shandong Province through our wholly owned subsidiaries, Xia Jin Plasma Company, the Qi He Plasma Company, the He Ze Plasma Company, the Zhang Qiu Plasma Company and the Liao Cheng Plasma Company. We received permits to operate these subsidiaries in January 2007. In April 2007, we acquired the assets of two additional plasma stations, one through Huan Jiang Plasma Company and the other through Fang Cheng Plasma Company, which is then 80% owned by Shandong Taibang and 20% owned by Feng Lin, an unrelated third party, which we subsequently acquired in January 2010. We obtained necessary permits and commenced their operation in July and August 2007, respectively. In 2010, we established two plasma stations, Yi Shui Plasma Company, which has begun operation in December 2010, and Ning Yang Plasma Company, which is still under construction. Guizhou Taibang, the main operating subsidiary of Dalin, is the 85% owner of the seven plasma stations in Guizhou province. Huitian, the 35% minority owned affiliated company by the Company, has three plasma stations operating in Shaanxi province. While we monitor our blood plasma intake procedures through frequent unscheduled inspections of our stations, there remains a risk that our blood supply may become tainted during the collection process. Our blood supply may become tainted if we accept blood from donors whose blood shows any irregular findings including HIV, Hepatitis C and liver disease. We pre-screen all donors in order to ensure that these diseases are not present. If our blood supply becomes tainted, the consequences for our business could be severe. We could be subject to civil liability from suits brought by consumers and to criminal liability and loss of our registration if we are found by the government to have been criminally negligent.
Our operations, sales, profit and cash flow will be adversely affected if our albumin products fail inspection or are delayed by regulators.
Each batch of our albumin products requires inspection by Chinese government regulators before we can ship it to our customers. The SFDA has a quality standard which considers, among other things, the appearance, packing capacity, thermal stability, pH value, protein content and percentage of purity of the product. In order to pass inspection, our plasma must test negative for any blood irregularities, including Hepatitis C, HIV and liver disease. The plasma must be packaged in 25 to 30 separate 600g bags and boxed with a packing list and labeled to be consistent with computer records. The plasma must then be stored at -20°C as soon as possible after collection to ensure that it will congeal within 6 hours. Government regulators usually take one month to inspect a batch of albumin products. The process begins when the regulator randomly selects samples of our albumin products and delivers them to the National Institute for the Control of Pharmaceutical and Biological Products, or the NICBPB, in Beijing for testing, and the process ends when the products are given final approval by the NICBPB. In the event that the regulators delay the approval of our products, change the requirements in such a way that we are unable to comply with those requirements, or require our other products to be inspected by regulators before we can ship them to our customers, our operations, sales, profit and cash flow will be adversely affected.
19
We rely on a Secondment Agreement with the Shandong Institute, which is expected to terminate upon the future privatization of the Shandong Institute, for over 39% of our Shandong Taibang employees. If the Secondment Agreement is breached or terminated, it could have an adverse effect on our operations and on our financial results.
The Shandong Institute has provided us with approximately 104 of our employees out of a total of approximately 1,446 employees, pursuant to a secondment agreement, or Secondment Agreement, dated October 28, 2002, between Shandong Taibang and the Shandong Institute. Pursuant to the Secondment Agreement, we are responsible for the salaries of these employees, as well as for their social benefits such as insurance. Our Secondment Agreement with the Shandong Institute will expire on the sooner to occur of October 2032 or upon the privatization of the Shandong Institute, which was originally expected to occur before the end of 2008. However, the completion of privatization of Shandong Institute has been further delayed indefinitely due to slower action taken by the Shandong Ministry of Health in implementing the privatization plan. Upon expiration or termination of the Secondment Agreement, we plan to hire the seconded employees directly. However, we cannot be sure that all of the employees will accept our employment offers at that time. Guang Li Pang, Shandong Taibang’s Deputy Chief Executive Officer, Yun Hua Gao and Dian Cong Liu, our Senior Technical Advisors are employed through the Secondment Agreement. Although none of our seconded employees have indicated that they do not plan to continue working for our Company after the privatization, if the Secondment Agreement is terminated or expires and we are unable to hire those employees or replacement employees on time, our operations, as well as our financial results, may suffer.
If the distributors who we rely on do not purchase our products, our business and results of operations will be adversely affected.
We sell almost a half of our products in China through our network of about 279 distributors located in about 30 provinces and municipal cities throughout China. While we have established working relationships with many of our distributors and strictly regulate their sales and marketing activities by annual distribution agreements, there are no restrictions in these distribution agreements preventing our distributors from also supplying products produced by our competitors. Our own marketing and sales staff work to develop and maintain relationships with our distributors, but there can be no assurance that we will be able to maintain such relationships. For the years ended December 31, 2010, 2009 and 2008, direct sales to distributors represented approximately 48.9%, 67.3% and 65.6%, respectively, of our total revenues. If a number of our distributors cease to purchase our products and we are unable to find suitable replacements, our business and results of operations will be adversely affected.
Our inability to successfully research and develop new biological pharmaceutical products could have an adverse effect on our future growth.
We believe that the successful development of biological pharmaceutical products can be affected by many factors. Products that appear to be promising in the early phases of research and development may fail to be commercialized for various reasons, including the failure to obtain the necessary regulatory approvals. In addition, the research and development cycles for new medicine for which we must obtain a Certificate of New Medicine from the PRC Ministry of Health, is a relatively lengthy process. In our experience, the process of conducting research and various tests on new products before obtaining a Certificate of New Medicine and subsequent procedures may take approximately three to five years. There is no assurance that our future research and development projects will be successful or that they will be completed within the anticipated time frame or budget. Also, there is no guarantee that we will receive the necessary approvals from relevant authorities for the production of our newly developed products. Even if such products could be successfully commercialized, there is no assurance that they will be accepted by the market as anticipated.
Our financial position and operations may be materially and adversely affected if our product liability insurance does not sufficiently cover our liabilities.
Under current PRC laws, manufacturers and vendors of defective products in the PRC may incur liability for loss and injury caused by such products. Pursuant to the General Principles of the Civil Law of the PRC, or the PRC Civil Law, which became effective in 1987, a defective product which causes property damage or physical injury to any person may subject the manufacturer or vendor of such product to civil liability.
In 1993, the PRC promulgated the Product Quality Law of the PRC, or the Product Quality Law, which was revised in 2000. The Product Quality Law was enacted to protect the rights and interests of end-users and consumers and to strengthen the supervision and control of the quality of products. Under the Product Quality Law, manufacturers who produce defective products may be subject to fines and required to cease production, and in severe cases, be subject to criminal liability and may have their business licenses revoked.
In 1993, the Law of the PRC on the Protection of the Rights and Interests of Consumers, or the Consumers' Rights Law, was promulgated to further protect the legal rights and interests of consumers in connection with the purchase or use of goods and services. All businesses, including our business, must observe and comply with the Consumers' Rights Law.
20
We maintain two product liability insurances for sales in the PRC for Shandong Taibang and Guizhou Taibang’s products in the amount of approximately $3.0 million (RMB 20 million) each. Although no one has filed any claims in relation to the use of our pharmaceutical products, our financial position and operations may be materially and adversely affected, if our insurance coverage is insufficient to cover a successful claim.
We may encounter increased competition from both local and overseas pharmaceutical enterprises as a result of a relaxation of the PRC regulatory approval process for plasma-based biopharmaceutical products or a relaxation of international trade restrictions. A change in our competitive environment could adversely affect our profitability and prospects.
Our continued ability to compete depends on the development of the plasma-based biopharmaceutical manufacturing industry in China. The plasma-based biopharmaceutical manufacturing industry in China is highly regulated by both provincial and central governments. Prior to engaging in the collection and production of plasma products, companies such as ours are required to obtain collection permits from the central health department and production permits and certificates for each new product formulation from the various provincial food and drug authorities. We have the advantage of being already approved by the state to collect plasma from human donors and manufacture and sell plasma-based biopharmaceutical products in Shandong Province, as well as in all other provinces in China, and our research and development department has become familiar with the provincial product approval process. However, although we believe that the regulatory requirements pose a competitive barrier to entry into the biopharmaceutical industry, over time there may be new entrants. If the government relaxes these restrictions and allow more competitors to enter into the market, these competitors may have more capital, better research and development resources, manufacturing and marketing capability and experience than us. Our profitability may be adversely affected if (i) competition intensifies; (ii) competitors drastically reduce prices; or (iii) competitors develop new products having comparable medicinal applications or therapeutic effects which are more effective or less costly than those produced by us.
In addition we expect that competition from imported products will increase as a result of a trend towards lower import tariffs and China's admission as a member of the WTO in December 2001. We believe that lower import tariffs will result in more affordable pricing for imported biopharmaceutical products manufactured overseas as compared to domestically manufactured products such as ours. In addition, China's membership in the WTO makes it more accessible to foreign biopharmaceutical manufacturers who may wish to set up production facilities in the PRC and compete directly with domestic manufacturers. The expected increased supply of both domestic and foreign competitively priced biopharmaceutical products in the PRC will result in increased competition. There is no assurance that our strategies to remain competitive can be implemented successfully as scheduled or at all. Our inability to remain competitive may have an adverse effect on our profitability and prospects.
We depend heavily on key personnel, and turnover of key employees and senior management could harm our business.
Our success, to a certain extent, is attributable to the expertise and experience of our senior management and key research and technical personnel, including Chao-Ming Zhao, our Chief Executive Officer, Y. Tristan Kuo, our President, Stanley Lau, our Chief Financial Officerand Yiwu Xie, the Chief Technical Officer of the Company, who carry out key functions in our operation. If we lose the service of any of our senior management or key research or technical personnel or fail to attract additional personnel with suitable experience and qualification, our business operations and research capability may be adversely affected.
Our senior management and employees have worked together for a short period of time, which may make it difficult for you to evaluate their effectiveness and ability to address challenges.
Due to our limited operating history and recent additions to our management team, certain of our senior management and employees have worked together at our company for only a relatively short period of time. Specifically, Chao Ming Zhao became our Chief Executive Officer in June 2008 after serving as our Chief Financial Officer since November 2006 and Y. Tristan Kuo became our Chief Financial Officer in June 2008 and had served as our Vice President-Finance since September 2007. Stanley Lau became our President in December 2010. Siu Ling Chan became our directors in July 2006. While Mr. Zhao and Ms. Chen were employed in various capacities by Logic Express and Shandong Taibang, Mr. Lau is a relative newcomer to our Company. As a result of these circumstances, it may be difficult for you to evaluate the effectiveness of our senior management and other key employees and their ability to address future challenges to our business.
Future acquisitions may have an adverse effect on our ability to manage our business.
Selective acquisitions form part of our strategy to further expand our business. If we are presented with appropriate opportunities, we may acquire additional companies, products or technologies. Future acquisitions and the subsequent integration of new companies into ours would require significant attention from our management. Our company has little experience with integrating newly acquired businesses. Potential problems encountered by each organization during mergers and acquisitions would be unique, posing additional risks to the company. The diversion of our management's attention and any difficulties encountered in any integration process could have an adverse effect on our ability to manage our business. Future acquisitions would expose us to potential risks, including risks associated with the assimilation of new operations, technologies and personnel, unforeseen or hidden liabilities, the diversion of resources from our existing businesses and technologies, the inability to generate sufficient revenue to offset the costs and expenses of acquisitions, and potential loss of, or harm to, relationships with employees, customers and suppliers as a result of integration of new businesses.
21
We may lose our competitive advantage and our operations may suffer if we fail to prevent the loss or misappropriation of, or disputes over, our intellectual property.
None of our products are currently covered by patents. The trademark “Lu Yue” is licensed to us by the Shandong Institute for our use as in the labeling of human-use medicine, biopreparate and blood products, pursuant to a trademark license agreement, dated February 27, 2007. We plan to apply for patents for our manufacturing processes. The patent application will be subject to approval from the relevant PRC authorities. We may not be able to successfully obtain the approval of the PRC authorities for our patent applications. Furthermore, third parties may assert claims to our proprietary procedures, technologies and systems. These proprietary procedures, technologies and systems are important to our business as they allow us to maintain our competitive edge over our competitors.
While we are not aware of any infringement on our intellectual property and we have not been notified by any third party that we are infringing on their intellectual property, our ability to compete successfully and to achieve future revenue growth will depend, in significant part, on our ability to protect our proprietary technology and operate without infringing upon the intellectual property rights of others. The legal regime in China for the protection of intellectual property rights is still at its early stage of development. Intellectual property protection became a national effort in China in 1979 when China adopted its first statute on the protection of trademarks. Since then, China has adopted its Patent Law, Trademark Law and Copyright Law and promulgated related regulations such as Regulation on Computer Software Protection, Regulation on the Protection of Layout Designs of Integrated Circuits and Regulation on Internet Domain Names. China has also acceded to various international treaties and conventions in this area, such as the Paris Convention for the Protection of Industrial Property, Patent Cooperation Treaty, Madrid Agreement and its Protocol Concerning the International Registration of Marks. In addition, when China became a party to the World Trade Organization in 2001, China amended many of its laws and regulations to comply with the Agreement on Trade-Related Aspects of Intellectual Property Rights. Despite many laws and regulations promulgated and other efforts made by China over the years with a view to tightening up its regulation and protection of intellectual property rights, private parties may not enjoy intellectual property rights in China to the same extent as they would in many Western countries, including the United States, and enforcement of such laws and regulations in China have not achieved the levels reached in those countries. Both the administrative agencies and the court system in China are not well-equipped to deal with violations or handle the nuances and complexities between compliant technological innovation and noncompliant infringement.
We rely on confidentiality agreements with our management and employees to protect our confidential proprietary information. However, the protection of our intellectual properties may be compromised as a result of:
- departure of any of our management members or employees in possession of our confidential proprietary information;
- breach by such departing management member or employee of his or her confidentiality and non- disclosure undertaking to us;
- infringement by others of our proprietary information and intellectual property rights; or
- refusal by relevant regulatory authorities to approve our patent or trademark applications.
Any of these events or occurrences may have a material adverse effect on our operations and the measures that we have put into place to protect our intellectual property rights may not be sufficient. Litigation to enforce our intellectual property rights could result in substantial costs and may not be successful. If we are not able to successfully defend our intellectual property rights, we might lose rights to technology that we need to conduct and develop our business. This may seriously harm our business, operating results and financial condition, and enable our competitors to use our intellectual property to compete against us.
Furthermore, if third parties claim that our products infringe their patents or other intellectual property rights, we may be required to devote substantial resources to defend against such claims. If we are unsuccessful in defending against such infringement claims, we may be required to pay damages, modify our products or suspend the production and sale of such products. We cannot guarantee that we will be able to modify our products on commercially reasonable terms.
A disruption in the supply of utilities, fire or other calamity at our manufacturing plant would disrupt production of our products and adversely affect our sales.
Our products are manufactured at our production facilities located in Tai’an, Shandong Province and Guiyang, Guizhou Province in the PRC. While we have not in the past experienced any calamities which disrupted production, any disruption in the supply of utilities, in particular, electricity or power supply, or any outbreak of fire, flood or other calamity resulting in significant damage at our facilities would severely affect our production and have a material adverse effect on our business, financial condition and results of operations.
We maintain insurance policies covering losses with respect to damages to our properties and products. We do not have insurance coverage for inventories of raw materials or business interruption. There is no assurance that our insurance would be sufficient to cover all of our potential losses.
22
There are allegations of past criminal conduct against certain members of our Board of Directors and a significant employee. Our business and results of operations could be adversely affected if any of these allegations are proven true.
On January 26, 2010, certain allegations of fraud and criminal activity involving smuggling and related activities allegedly engaged in prior to 2005 by the CEO of the Company's primary operating subsidiary, Shandong Taibang, and by a relative of one of our directors surfaced on certain financial websites. On January 27, 2010, in response to these allegations, the Company's board of directors established a special independent subcommittee comprised of the Company's independent directors, Mr. Sean Shao and Dr. Tong Jun Lin (who were later joined by new director Dr. Xiangmin Cui), or the Special Committee, to investigate the allegations with the assistance of a reputable international firm, and report its findings to the board of directors as soon as practicable. On March 1, 2010, the Special Committee retained O'Melveny & Myers LLP, an international law firm, to advise the Special Committee and to assist in the investigation of the allegations. On November 26, 2010, the Special Committee reported its findings to the Company’s board of directors, a summary of which the Company disclosed in a Current Report on Form 8-K filed with the Commission on December 3, 2010. The Special Committee could not find support for a majority of the allegations, however, the Special Committee found support that Mr. Ze Qin Lin, the husband of our former director Ms. Lin Ling Li, was imprisoned in China in connection with smuggling offenses, and with respect to the allegation that Mr. Tung Lam, the Chief Executive Officer of one of our primary operating subsidiaries, Shandong Taibang, and spouse of Mrs. Siu Ling Chan, our board chair, was previously known as Mr. Lin Ziping and was imprisoned for smuggling offenses in China, the Special Committee found evidence supporting Mr. Lam's denial of the allegation, as well as conflicting evidence with respect to this claim. As a result, the Special Committee concluded that it could neither confirm nor exclude the allegation against Mr. Lam. The findings of the Special Committee regarding Mr. Lin and its inability to reach a conclusion regarding the allegations against Mr. Lam may make investing in our Company unattractive to certain investors and may cause existing investors to end their investment in the Company, which may cause our stock price to decline.
RISKS RELATED TO DOING BUSINESS IN CHINA
Changes in China's political or economic situation could harm us and our operating results.
Economic reforms adopted by the Chinese government have had a positive effect on the economic development of the country, but the government could change these economic reforms or any of the legal systems at any time. This could either benefit or damage our operations and profitability. Some of the things that could have this effect are:
- Level of government involvement in the economy;
- Control of foreign exchange;
- Methods of allocating resources;
- Balance of payments position;
- International trade restrictions; and
- International conflict.
The Chinese economy differs from the economies of most countries belonging to the Organization for Economic Cooperation and Development, or OECD, in many ways. For example, state-owned enterprises still constitute a large portion of the Chinese economy, and weak corporate governance and the lack of a flexible currency exchange policy still prevail in China. As a result of these differences, we may not develop in the same way or at the same rate as might be expected if the Chinese economy was similar to those of the OECD member countries.
Uncertainties with respect to the PRC legal system could limit the legal protections available to you and us.
We conduct substantially all of our business through our operating subsidiaries in the PRC. Our operating subsidiaries are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to FIEs. The PRC legal system is based on written statutes, and prior court decisions may be cited for reference but have limited precedential value. Since 1979, a series of new PRC laws and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, since the PRC legal system continues to evolve rapidly, the interpretations of many laws, regulations, and rules are not always uniform, and enforcement of these laws, regulations, and rules involve uncertainties, which may limit legal protections available to you and us. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention. In addition, most of our executive officers and directors are residents of China and not of the United States, and substantially all the assets of these persons are located outside the United States. As a result, it could be difficult for investors to affect service of process in the United States or to enforce a judgment obtained in the United States against our Chinese operations and subsidiary.
23
You may have difficulty enforcing judgments against us.
Most of our assets are located outside of the United States and most of our current operations are conducted in the PRC. In addition, most of our directors and officers are nationals and residents of countries other than the United States. A substantial portion of the assets of these persons is located outside the United States. As a result, it may be difficult for you to effect service of process within the United States upon these persons. It may also be difficult for you to enforce in U.S. courts judgments on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors, most of whom are not residents in the United States and the substantial majority of whose assets are located outside of the United States. In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of U.S. courts. Our counsel as to PRC law has advised us that the recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. Courts in China may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based on treaties between China and the country where the judgment is made or on reciprocity between jurisdictions. China does not have any treaties or other arrangements that provide for the reciprocal recognition and enforcement of foreign judgments with the United States. In addition, according to the PRC Civil Procedures Law, courts in the PRC will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates basic principles of PRC law or national sovereignty, security, or the public interest. So it is uncertain whether a PRC court would enforce a judgment rendered by a court in the United States.
The PRC government exerts substantial influence over the manner in which we must conduct our business activities.
The PRC government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those relating to taxation, import and export tariffs, environmental regulations, land use rights, property, and other matters. We believe that our operations in China are in material compliance with all applicable legal and regulatory requirements. However, the central or local governments of the jurisdictions in which we operate may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations.
Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof and could require us to divest ourselves of any interest we then hold in Chinese properties or joint ventures.
Future inflation in China may inhibit our ability to conduct business in China.
In recent years, the Chinese economy has experienced periods of rapid expansion and highly fluctuating rates of inflation. During the past ten years, the rate of inflation in China has been as high as 5.9% and as low as -0.8%. These factors have led to the adoption by the Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. High inflation may in the future cause the Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products and our company.
Restrictions on currency exchange may limit our ability to receive and use our sales effectively.
The majority of our sales will be settled in RMB, and any future restrictions on currency exchanges may limit our ability to use revenue generated in RMB to fund any future business activities outside China or to make dividend or other payments in U.S. dollars. Although the Chinese government introduced regulations in 1996 to allow greater convertibility of the RMB for current account transactions, significant restrictions still remain, including primarily the restriction that FIEs may only buy, sell or remit foreign currencies after providing valid commercial documents, at those banks in China authorized to conduct foreign exchange business. In addition, conversion of RMB for capital account items, including direct investment and loans, is subject to governmental approval in China, and companies are required to open and maintain separate foreign exchange accounts for capital account items. We cannot be certain that the Chinese regulatory authorities will not impose more stringent restrictions on the convertibility of the RMB.
Fluctuations in exchange rates could adversely affect our business and the value of our securities.
The value of our common stock will be indirectly affected by the foreign exchange rate between the U.S. dollar and RMB and between those currencies and other currencies in which our sales may be denominated. Appreciation or depreciation in the value of the RMB relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. Fluctuations in the exchange rate will also affect the relative value of any dividend we issue that will be exchanged into U.S. dollars, as well as earnings from, and the value of, any U.S. dollar-denominated investments we make in the future.
24
Since July 2005, the RMB has no longer been pegged to the U.S. dollar. Although the People’s Bank of China regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the RMB may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is possible that in the future PRC authorities may lift restrictions on fluctuations in the RMB exchange rate and lessen intervention in the foreign exchange market.
Very limited hedging transactions are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions. While we may enter into hedging transactions in the future, the availability and effectiveness of these transactions may be limited, and we may not be able to successfully hedge our exposure at all. In addition, our foreign currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert RMB into foreign currencies. Currently, some of our raw materials and major equipment are imported. In the event that the U.S. dollars appreciate against RMB, our costs will increase. If we cannot pass the resulting cost increases on to our customers, our profitability and operating results will suffer. In addition, if our sales to international customers grow, we will be increasingly subject to the risk of foreign currency depreciation.
Restrictions under PRC law on our PRC subsidiaries’ ability to make dividends and other distributions could materially and adversely affect our ability to grow, make investments or acquisitions that could benefit our business, pay dividends to you, and otherwise fund and conduct our business.
Substantially all of our sales are earned by our PRC subsidiaries. However, PRC regulations restrict the ability of our PRC subsidiaries to make dividends and other payments to their offshore parent companies. PRC legal restrictions permit payments of dividends by our PRC subsidiaries only out of their accumulated after-tax profits, if any, determined in accordance with PRC accounting standards and regulations. Our PRC subsidiaries are also required under PRC laws and regulations to allocate at least 10% of their annual after-tax profits determined in accordance with PRC GAAP to a statutory general reserve fund until the amounts in said fund reaches 50% of their registered capital. Allocations to these statutory reserve funds can only be used for specific purposes and are not transferable to us in the form of loans, advances, or cash dividends. Any limitations on the ability of our PRC subsidiaries to transfer funds to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends and otherwise fund and conduct our business.
Failure to comply with PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident stockholders to personal liability, limit our ability to acquire PRC companies or to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute profits to us or otherwise materially adversely affect us.
In October 2005, SAFE issued the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, generally referred to as Circular 75, which required PRC residents to register with the competent local SAFE branch before establishing or acquiring control over an offshore special purpose company, or SPV, for the purpose of engaging in an equity financing outside of China on the strength of domestic PRC assets originally held by those residents. Internal implementing guidelines issued by SAFE, which became public in June 2007 (known as Notice 106), expanded the reach of Circular 75 by (1) purporting to cover the establishment or acquisition of control by PRC residents of offshore entities which merely acquire “control” over domestic companies or assets, even in the absence of legal ownership; (2) adding requirements relating to the source of the PRC resident's funds used to establish or acquire the offshore entity; (3) covering the use of existing offshore entities for offshore financings; (4) purporting to cover situations in which an offshore SPV establishes a new subsidiary in China or acquires an unrelated company or unrelated assets in China; and (5) making the domestic affiliate of the SPV responsible for the accuracy of certain documents which must be filed in connection with any such registration, notably, the business plan which describes the overseas financing and the use of proceeds. Amendments to registrations made under Circular 75 are required in connection with any increase or decrease of capital, transfer of shares, mergers and acquisitions, equity investment or creation of any security interest in any assets located in China to guarantee offshore obligations, and Notice 106 makes the offshore SPV jointly responsible for these filings. In the case of an SPV which was established, and which acquired a related domestic company or assets, before the implementation date of Circular 75, a retroactive SAFE registration was required to have been completed before March 31, 2006. This date was subsequently extended indefinitely by Notice 106, which also required that the registrant establish that all foreign exchange transactions undertaken by the SPV and its affiliates were in compliance with applicable laws and regulations. Failure to comply with the requirements of Circular 75, as applied by SAFE in accordance with Notice 106, may result in fines and other penalties under PRC laws for evasion of applicable foreign exchange restrictions. Any such failure could also result in the SPV’s affiliates being impeded or prevented from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the SPV, or from engaging in other transfers of funds into or out of China.
We have asked our stockholders who are PRC residents as defined in Circular 75 to register with the relevant branch of SAFE, as currently required, in connection with their equity interests in us and our acquisitions of equity interests in our PRC subsidiaries. However, we cannot provide any assurances that they can obtain the above SAFE registrations required by Circular 75 and Notice 106. Moreover, because of uncertainty over how Circular 75 will be interpreted and implemented, and how or whether SAFE will apply it to us, we cannot predict how it will affect our business operations or future strategies. For example, our present and prospective PRC subsidiaries’ ability to conduct foreign exchange activities, such as the remittance of dividends and foreign currency-denominated borrowings, may be subject to compliance with Circular 75 and Notice 106 by our PRC resident beneficial holders.
25
In addition, such PRC residents may not always be able to complete the necessary registration procedures required by Circular 75 and Notice 106. We also have little control over either our present or prospective direct or indirect stockholders or the outcome of such registration procedures. A failure by our PRC resident beneficial holders or future PRC resident stockholders to comply with Circular 75 and Notice 106, if SAFE requires it, could subject these PRC resident beneficial holders to fines or legal sanctions, restrict our overseas or cross-border investment activities, limit our subsidiaries’ ability to make distributions or pay dividends or affect our ownership structure, which could adversely affect our business and prospects.
We may be unable to complete a business combination transaction efficiently or on favorable terms due to complicated merger and acquisition regulations which became effective on September 8, 2006.
On August 9, 2006, six PRC regulatory agencies, including the China Securities Regulatory Commission, or CSRC, promulgated the Regulation on Mergers and Acquisitions of Domestic Companies by Foreign Investors, which became effective on September 8, 2006. This new regulation, among other things, governs the approval process by which a PRC company may participate in an acquisition of assets or equity interests. Depending on the structure of the transaction, the new regulation will require the PRC parties to make a series of applications and supplemental applications to the government agencies. In some instances, the application process may require the presentation of economic data concerning a transaction, including appraisals of the target business and evaluations of the acquirer, which are designed to allow the government to assess the transaction. Government approvals will have expiration dates by which a transaction must be completed and reported to the government agencies. Compliance with the new regulations is likely to be more time consuming and expensive than in the past and the government can now exert more control over the combination of two businesses. Accordingly, due to the new regulation, our ability to engage in business combination transactions has become significantly more complicated, time consuming and expensive, and we may not be able to negotiate a transaction that is acceptable to our stockholders or sufficiently protect their interests in a transaction.
The new regulation allows PRC government agencies to assess the economic terms of a business combination transaction. Parties to a business combination transaction may have to submit to the PRC Ministry of Commerce, or MOFCOM, and other relevant government agencies an appraisal report, an evaluation report and the acquisition agreement, all of which form part of the application for approval, depending on the structure of the transaction. The regulations also prohibit a transaction at an acquisition price obviously lower than the appraised value of the PRC business or assets and in certain transaction structures, require that consideration must be paid within defined periods, generally not in excess of a year. The regulation also limits our ability to negotiate various terms of the acquisition, including aspects of the initial consideration, contingent consideration, holdback provisions, indemnification provisions and provisions relating to the assumption and allocation of assets and liabilities. Transaction structures involving trusts, nominees and similar entities are prohibited. Therefore, such regulation may impede our ability to negotiate and complete a business combination transaction on financial terms that satisfy our investors and protect our stockholders’ economic interests.
Under the New Enterprise Income Tax Law, we may be classified as a “resident enterprise” of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC stockholders.
The EIT Law and its implementing rules became effective on January 1, 2008. Under the EIT Law, an enterprise established outside of China with “de facto management bodies” within China is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. The implementing rules of the EIT Law define de facto management as “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise.
On April 22, 2009, the State Administration of Taxation issued the Notice Concerning Relevant Issues Regarding Cognizance of Chinese Investment Controlled Enterprises Incorporated Offshore as Resident Enterprises pursuant to Criteria of de facto Management Bodies, or the Notice, further interpreting the application of the EIT Law and its implementation non-Chinese enterprise or group controlled offshore entities. Pursuant to the Notice, an enterprise incorporated in an offshore jurisdiction and controlled by a Chinese enterprise or group will be classified as a “non-domestically incorporated resident enterprise” if (i) its senior management in charge of daily operations reside or perform their duties mainly in China; (ii) its financial or personnel decisions are made or approved by bodies or persons in China; (iii) its substantial assets and properties, accounting books, corporate chops, board and shareholder minutes are kept in China; and (iv) at least half of its directors with voting rights or senior management often resident in China. A resident enterprise would be subject to an enterprise income tax rate of 25% on its worldwide income and must pay a withholding tax at a rate of 10% when paying dividends to its non-PRC shareholders. However, it remains unclear as to whether the Notice is applicable to an offshore enterprise incorporated by a Chinese natural person. Nor are detailed measures on imposition of tax from non-domestically incorporated resident enterprises are available. Therefore, it is unclear how tax authorities will determine tax residency based on the facts of each case.
26
We may be deemed to be a resident enterprise by Chinese tax authorities. If the PRC tax authorities determine that we are a “resident enterprise” for PRC enterprise income tax purposes, a number of unfavorable PRC tax consequences could follow. First, we may be subject to the enterprise income tax at a rate of 25% on our worldwide taxable income as well as PRC enterprise income tax reporting obligations. In our case, this would mean that income such as interest on financing proceeds and non-China source income would be subject to PRC enterprise income tax at a rate of 25%. Second, although under the EIT Law and its implementing rules dividends paid to us from our PRC subsidiaries would qualify as “tax-exempt income,” we cannot guarantee that such dividends will not be subject to a 10% withholding tax, as the PRC foreign exchange control authorities, which enforce the withholding tax, have not yet issued guidance with respect to the processing of outbound remittances to entities that are treated as resident enterprises for PRC enterprise income tax purposes. Finally, it is possible that future guidance issued with respect to the new “resident enterprise” classification could result in a situation in which a 10% withholding tax is imposed on dividends we pay to our non-PRC stockholders and with respect to gains derived by our non-PRC stockholders from transferring our shares. We are actively monitoring the possibility of “resident enterprise” treatment for the 2011 tax year and are evaluating appropriate organizational changes to avoid this treatment, to the extent possible.
If we were treated as a “resident enterprise” by PRC tax authorities, we would be subject to taxation in both the U.S. and China, and our PRC tax may not be creditable against our U.S. tax.
We face uncertainty from China’s Circular on Strengthening the Administration of Enterprise Income Tax on Non-Resident Enterprises' Share Transfer that was released in December 2009 with retroactive effect from January 1, 2008.
The Chinese State Administration of Taxation, or SAT, released a circular on December 15, 2009 that addresses the transfer of shares by nonresident companies, generally referred to as Circular 698. Circular 698, which is effective retroactively to January 1, 2008, may have a significant impact on many companies that use offshore holding companies to invest in China. Circular 698, which provides parties with a short period of time to comply with its requirements, indirectly taxes foreign companies on gains derived from the indirect sale of a Chinese company. Where a foreign investor indirectly transfers equity interests in a Chinese resident enterprise by selling the shares in an offshore holding company, and the latter is located in a country or jurisdiction where the effective tax burden is less than 12.5% or where the offshore income of his, her, or its residents is not taxable, the foreign investor is required to provide the tax authority in charge of that Chinese resident enterprise with the relevant information within 30 days of the transfers. Moreover, where a foreign investor indirectly transfers equity interests in a Chinese resident enterprise through an abuse of form of organization and there are no reasonable commercial purposes such that the corporate income tax liability is avoided, the PRC tax authority will have the power to re-assess the nature of the equity transfer in accordance with PRC’s “substance-over-form” principle and deny the existence of the offshore holding company that is used for tax planning purposes. There is uncertainty as to the application of Circular 698. For example, while the term “indirectly transfer” is not defined, it is understood that the relevant PRC tax authorities have jurisdiction regarding requests for information over a wide range of foreign entities having no direct contact with China. Moreover, the relevant authority has not yet promulgated any formal provisions or formally declared or stated how to calculate the effective tax in the country or jurisdiction and to what extent and the process of the disclosure to the tax authority in charge of that Chinese resident enterprise. In addition, there are not any formal declarations with regard to how to decide “abuse of form of organization” and “reasonable commercial purpose,” which can be utilized by us to balance if our Company complies with the Circular 698. As a result, we may become at risk of being taxed under Circular 698 and we may be required to expend valuable resources to comply with Circular 698 or to establish that we should not be taxed under Circular 698, which could have a material adverse effect on our financial condition and results of operations.
We may be exposed to liabilities under the Foreign Corrupt Practices Act and Chinese anti-corruption laws, and any determination that we violated these laws could have a material adverse effect on our business.
We are subject to the Foreign Corrupt Practice Act, or FCPA, and other laws that prohibit improper payments or offers of payments to foreign governments and their officials and political parties by U.S. persons and issuers as defined by the statute, for the purpose of obtaining or retaining business. We have operations, agreements with third parties, and make most of our sales in China. The PRC also strictly prohibits bribery of government officials. Our activities in China create the risk of unauthorized payments or offers of payments by the employees, consultants, sales agents, or distributors of our Company, even though they may not always be subject to our control. It is our policy to implement safeguards to discourage these practices by our employees. However, our existing safeguards and any future improvements may prove to be less than effective, and the employees, consultants, sales agents, or distributors of our Company may engage in conduct for which we might be held responsible. Violations of the FCPA or Chinese anti-corruption laws may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could negatively affect our business, operating results and financial condition. In addition, the U.S. government may seek to hold our Company liable for successor liability FCPA violations committed by companies in which we invest or that we acquire.
If we become directly subject to the recent scrutiny, criticism and negative publicity involving U.S.-listed Chinese companies, we may have to expend significant resources to investigate and resolve the matter which could harm our business operations, stock price and reputation and could result in a loss of your investment in our stock, especially if such matter cannot be addressed and resolved favorably.
Recently, U.S. public companies that have substantially all of their operations in China, particularly companies like us which have completed so-called reverse merger transactions, have been the subject of intense scrutiny, criticism and negative publicity by investors, financial commentators and regulatory agencies, such as the SEC. Much of the scrutiny, criticism and negative publicity has centered around financial and accounting irregularities and mistakes, a lack of effective internal controls over financial accounting, inadequate corporate governance policies or a lack of adherence thereto and, in many cases, allegations of fraud. As a result of the scrutiny, criticism and negative publicity, the publicly traded stock of many U.S. listed Chinese companies has sharply decreased in value and, in some cases, has become virtually worthless. Many of these companies are now subject to shareholder lawsuits, SEC enforcement actions and are conducting internal and external investigations into the allegations. It is not clear what effect this sector-wide scrutiny, criticism and negative publicity will have on our company, our business and our stock price. If we become the subject of any unfavorable allegations, whether such allegations are proven to be true or untrue, we will have to expend significant resources to investigate such allegations and/or defend our company. This situation will be costly and time consuming and distract our management from growing our company. If such allegations are not proven to be groundless, our company and business operations will be severely and your investment in our stock could be rendered worthless.
27
RISKS RELATED TO THE MARKET FOR OUR STOCK
Although publicly traded, the trading market in our common stock has been substantially less liquid than the average trading market for a stock quoted on the NASDAQ Stock Market and this low trading volume may adversely affect the price of our common stock.
Our common stock started trading on the NASDAQ Global Select Market under the symbol “CBPO” on December 7, 2009. The trading market in our common stock has been substantially less liquid than the average trading market for companies trading on the NASDAQ Stock Market. Reported average daily trading volume in our common stock for the three months immediately prior to March 1, 2011, was approximately 0.1 million shares. Limited trading volume will subject our shares of common stock to greater price volatility and may make it difficult for you to sell your shares of common stock at a price that is attractive to you.
The market price of our common stock is volatile, leading to the possibility of its value being depressed at a time when you want to sell your holdings.
The market price of our common stock is volatile, and this volatility may continue. Numerous factors, many of which are beyond our control, may cause the market price of our common stock to fluctuate significantly. These factors include:
- our earnings releases, actual or anticipated changes in our earnings, fluctuations in our operating results or our failure to meet the expectations of financial market analysts and investors;
- changes in financial estimates by us or by any securities analysts who might cover our stock;
- speculation about our business in the press or the investment community;
- significant developments relating to our relationships with our customers or suppliers;
- stock market price and volume fluctuations of other publicly traded companies and, in particular, those that are in the our industry;
- customer demand for our products;
- investor perceptions of the our industry in general and our company in particular;
- the operating and stock performance of comparable companies;
- general economic conditions and trends;
- major catastrophic events;
- announcements by us or our competitors of new products, significant acquisitions, strategic partnerships or divestitures;
- changes in accounting standards, policies, guidance, interpretation or principles;
- loss of external funding sources;
- sales of our common stock, including sales by our directors, officers or significant stockholders; and
- additions or departures of key personnel.
Securities class action litigation is often instituted against companies following periods of volatility in their stock price. This type of litigation could result in substantial costs to us and divert our management’s attention and resources. Moreover, securities markets may from time to time experience significant price and volume fluctuations for reasons unrelated to operating performance of particular companies. For example, in July 2008, the securities markets in the United States, China and other jurisdictions experienced the largest decline in share prices since September 2001. These market fluctuations may adversely affect the price of our common stock and other interests in our company at a time when you want to sell your interest in us.
Provisions in our certificate of incorporation and bylaws or Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore depress the trading price of the common stock.
Delaware corporate law and our certificate of incorporation and bylaws contain provisions that could discourage, delay or prevent a change in control of our Company or changes in its management that our stockholders may deem advantageous. These provisions:
-
deny holders of our common stock cumulative voting rights in the election of directors, meaning that stockholders owning a majority of our outstanding shares of common stock will be able to elect all of our directors; and
-
allow any vacancy on the board of directors, however the vacancy occurs, to be filled by the directors.
In addition, Section 203 of the Delaware General Corporation Law generally limits our ability to engage in any business combination with certain persons who own 15% or more of our outstanding voting stock or any of our associates or affiliates who at any time in the past three years have owned 15% or more of our outstanding voting stock. These provisions may have the effect of entrenching our management team and may deprive you of the opportunity to sell your shares to potential acquirers at a premium over prevailing prices. This potential inability to obtain a control premium could reduce the price of our common stock.
We do not intend to pay dividends for the foreseeable future.
For the foreseeable future, we intend to retain any earnings to finance the development and expansion of our business, and we do not anticipate paying any cash dividends on our common stock. Accordingly, investors must be prepared to rely on sales of their common stock after price appreciation to earn an investment return, which may never occur. Investors seeking cash dividends should not purchase our common stock. Any determination to pay dividends in the future will be made at the discretion of our board of directors and will depend on our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board deems relevant.
28
ITEM 1B. UNRESOLVED STAFF COMMENTS.
We have no outstanding or unresolved comments from the SEC staff.
ITEM 2. PROPERTIES.
All land in China is owned by the government. Individuals and companies are permitted to acquire land use rights for specific purposes. Industrial land use rights are granted for a period of 50 years. This period may be renewed at the expiration of the initial and any subsequent terms. Granted land use rights are transferable and may be used as security for borrowings and other obligations.
In July 2003, Shandong Taibang obtained certain land use rights from the PRC municipal government to 43,663 square meters consisting of manufacturing facilities, warehouses and office buildings in Tai'an City, Shandong Province. Shandong Taibang is required to make payments totaling approximately $21,063 (RMB138,848) per year to the local state-owned entity, for the 50 year life of the rights or until the Shandong Institute completes its privatization process. We recorded “land use rights” equal to “other payable – land use rights” totaling $333,008, $323,687 and $325,390 as of December 31, 2010, 2009 and 2008, respectively, determined using present value of annual payments over 50 years.
Guizhou Taibang entered into a lease agreement on June 1, 2006 with a group of individuals in an area located next to its production facility to lease and use the space for processing industrial wastes for 10 years. The annual lease amount is approximately $1,583 (RMB 10,438).
We believe that all of our properties have been adequately maintained, are generally in good condition, and are suitable and adequate for our business.
Some of our properties are leased from third parties. We have entered into formal lease agreements with two of them. The remaining leases are on a verbal basis. In all cases, the lessors have not been able to provide copies of documentation evidencing their rights to use the leased property. In most cases, the leased properties are small operating spaces we leased for our sales offices in different parts of China. In the event of any future dispute over the ownership of the leased properties, we believe we could easily and quickly find replacement premises so that the operations would not be affected.
ITEM 3. LEGAL PROCEEDINGS.
From time to time, we may become involved in various lawsuits and legal proceedings, which arise, in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these, or other matters, may arise from time to time that may harm our business. Other than the legal proceedings set forth below, we are currently not aware of any such legal proceedings or claims that we believe will have a material adverse affect on our business, financial condition or operating results.
Bobai County Collection Station
In January 2007, the Company's PRC subsidiary, Shandong Taibang, advanced approximately $455,100 (RMB3.0 million) to Feng Lin, the 20% minority shareholder in Fang Cheng Plasma Company, the Company's majority owned subsidiary, for the purpose of establishing or acquiring a plasma collection station. Mr. Lin and Shandong Taibang intended to establish the Bobai Kangan Plasma Collection Co., Ltd. (“Bobai”) in Bobai County, Guangxi and on January 18, 2007, Shandong Taibang signed a letter of intent to acquire the assets of the Bobai Plasma Collection Station, which was co-owned by Mr. Lin and Mr. Keliang Huang. However, in January 2007, Hua Lan Biological Engineering Co., Ltd., or Hua Lan, filed suit in the District Court of Hong Qi District, Xin Xiang City, Henan Province, alleging that Feng Lin, Keliang Huang and Shandong Taibang established and/or sought to operate the Bobai Plasma Collection Station using a permit for collecting and supplying human plasma in Bobai County, that was originally granted to Hua Lan by the government of the Guangxi region, without Hua Lan's permission. The establishment and registration of Bobai was never realized as a result of this law suit. On January 29, 2007, on Hua Lan's motion, the District Court entered an order to freeze funds in the amount of approximately $455,100 (RMB3,000,000) held by the defendants in the case, including approximately $75,850 (RMB500,000) in funds held in Shandong Taibang's bank account in Tai'an City. A hearing was held on June 25, 2007 and judgment was entered against the defendants along with a $257,890 (RMB1,700,000) joint financial judgment. The Company appealed the District Court judgment to the Xinxiang City Intermediate Court. In November 2007, the Intermediate Court affirmed the judgment against the three defendants and increased the amount of the joint financial judgment to approximately $455,100 (RMB3,000,000).
29
In January 2008, Hua Lan enforced the judgment granted by the Intermediate Court to freeze the Company's bank accounts. Shandong Taibang has filed a separate action against Hua Lan before the Tai'an City District Court to seek recovery of any losses in connection with Hua Lan's claim and to request that the Tai'an City District Court preserve Hua Lan's property or freeze up to approximately $455,100 (RMB 3 million) of Hua Lan's assets to secure the return of such funds to the Company. The intermediate court in Tai'an City accepted the application on February 14, 2008 but the matter is still pending. Pending the outcome of the proceedings, Shandong Taibang increased its loss contingency reserve during its fourth quarter of 2007 from approximately $85,963 (RMB566,667) to $151,700 (RMB1,000,000) to cover its share of the enforcement of this judgment. During the fourth quarter of 2008, full amount of the judgment, including Feng Lin and Keliang Huang's portions of the judgment and the related fees, approximately $471,772 (RMB 3,109,900) has been withdrawn from Shandong Taibang's account. The Company recorded Feng Lin and Keliang Huang's portion of the judgment, approximately $314,510 (RMB 2,073,234), as receivable as a result of the withdrawal. As of December 31, 2008, the Company determined that it is unlikely that the Company will be able to recover such receivable from those two individuals and wrote off the receivable as bad debt expense. In January 2010, Feng Lin transferred his 20% equity in Fang Cheng Plasma Company as a repayment to such receivable. As a result, the Company is now the 100% owner of the Fang Cheng Plasma Company.
In October 2009, Shandong Taibang appealed to the High Court of Henan Province requesting the court to reverse judgments from the Hong Qi District Court based on Shandong Taibang's belief that Hua Lan’s involvement in Bobai was in violation of PRC Blood Products Regulations as Hua Lan did not invest, as Shandong Taibang did, in Bobai as required by the Regulation. The Company was awaiting the judgment of the Henan High Court as of the date of this report. In light of the foregoing, it is unlikely that the Company's planned acquisition of the assets of Bobai will go forward.
Dispute among Guizhou Taibang Shareholders over Raising Additional Capital
On May 28, 2007, a 91% majority of Guizhou Taibang's shareholders approved a plan to raise additional capital from private strategic investors through the issuance of an additional 20,000,000 shares of Guizhou Taibang equity interests at RMB 2.80 per share. The plan required all existing Guizhou Taibang shareholders to waive their rights of first refusal to subscribe for the additional shares. The remaining 9% minority holder of Guizhou Taibang's shares, the Guizhou Jie'an Company, or Jie'an, did not support the plan and did not agree to waive its right of first refusal. On May 29, 2007, the majority shareholders caused Guizhou Taibang to sign an Equity Purchase Agreement with certain investors, pursuant to which the investors agreed to invest an aggregate of RMB 50,960,000 (approximately $7,730,632) in exchange for 18,200,000 shares, or 21.4%, of Guizhou Taibang's equity interests. At the same time, Jie'an also subscribed for 1,800,000 shares, representing its 9% pro rata share of the 20,000,000 shares being offered. The proceeds from all parties were received by Guizhou Taibang in accordance with the agreement.
In June 2007, Jie'an brought suit in the High Court of Guizhou province, China, against Guizhou Taibang and the three other original Guizhou Taibang shareholders, alleging the illegality of the Equity Purchase Agreement. In its complaint, Jie'an alleged that it had a right to acquire the shares waived by the original Guizhou Taibang shareholders and offered to the investors in connection with the Equity Purchase Agreement. On September 12, 2008, the Guizhou High Court ruled against Jie'an and sustained the Equity Purchase Agreement, but on November 2008, Jie'an appealed the Guizhou High Court judgment to the People's Supreme Court in Beijing. On May 13, 2009, the People's Supreme Court sustained the original ruling and denied the rights of first refusal of Jie'an over the additional shares waived by the original Guizhou Taibang's shareholders. The registration of the new investors as Guizhou Taibang's shareholders and the related increase in registered capital of Guizhou Taibang with the Administration for Industry and Commerce are still pending. On January 27, 2010, the strategic investors brought suit in the High Court of Guizhou Province against Guizhou Taibang alleging Guizhou Taibang’s failure to register their equity interest in Guizhou Taibang with the local AIC and requesting the distribution of their share of Guizhou Taibang’s dividends. Dalin was also joined as a co-defendant as it is the majority shareholder and exercises control over Guizhou Taibang’s day-to-day operations. The Company does not expect the strategic investors to prevail because, upon evaluation of the Equity Purchase Agreement, the Company believes that the Equity Purchase Agreement is void due to certain invalid pre-conditions and the absence of shareholder authorization of the initial investment. In the event that Guizhou Taibang is required to return their original investment amount to the strategic investors, as of December 31, 2010, Guizhou Taibang has set aside the strategic investors’ fund along with RMB 10,056,242 (approximately $1,525,532) in accrued interests, and RMB 509,600 (approximately $77,306) for the 1% penalty imposed by the agreement for any breach. If strategic investors prevail in their suit, Dalin's interests in Guizhou Taibang may be reduced to approximately 41.3%. The High Court of Guizhou heard the case on April 8, 2010 and encouraged, and accepted by both parties, to settle the dispute outside the court but both parties failed to reach a mutual agreeable term.
On October 14, 2010, the High Court of Guizhou ruled in favor of the Company and denied the strategic investors’ right as shareholders of Guizhou Taibang, as well as their entitlement to the dividends. On October 26, 2010, the strategic investors appealed to, and subsequently accepted by, the PRC Superior Court in Beijing on the ruling. As of the date of this report, the Company is waiting to hear the ruling from the Court. In light of the Guizhou ruling, in November 2010 the Company returned the proceeds in the amount of RMB 11,200,000 (approximately $1,699,040) to one of the strategic investors.
30
During the second quarter of 2010, Jie’an requested that Guizhou Taibang register its 1.8 million shares of additional capital infusion with the local AIC, pursuant to the Equity Purchase Agreement, and such request was approved by the majority shareholders of Guizhou Taibang in a shareholders meeting held in the second quarter of 2010. However, the Board of Directors of the Company is withholding its required ratification of the shareholders’ approval of Jie’an’s request, pending the outcome of the ongoing litigation. If the Company decides to ratify the approval, Dalin’s ownership in Guizhou Taibang will be diluted from 54% to 52.54% and Jie’an may be entitled to receive its pro rata share of Guizhou Taibang’s profits from the prior 2 years.
Dispute over Guizhou Taibang Technical Consulting Agreement
In 1997, Guizhou Taibang entered into a Technical Cooperation Agreement with Sin Kyung Ye, or Sin, a Korean individual, to provide certain fractionation equipment and transfer processing know-how to Guizhou Taibang. In August 2004, Sin filed a law suit against Guizhou Taibang with the Intermediate Court in Guiyang City, China, alleging non-payment of RMB 100,000 (approximately, $15,170) for his fractionation equipment and RMB 5,000,000 (approximately, $758,500) for the transfer of his technological know-how. The Intermediate Court ruled in favor of Sin and found that Guizhou Taibang owed Sin RMB 10,376,160 (approximately, $ 1,574,063), but Guizhou Taibang appealed the Intermediate Court ruling to the Guizhou High Court. The Guizhou High Court agreed in part with Guizhou Taibang's grounds for appeal and reduced the amount of know-how transfer fee to RMB 1,970,413 (approximately, $298,912). In May 2007, Sin appealed the Guizhou High Court's decision to the People's Supreme Court in Beijing. The People's Supreme Court heard in April 2008 and ruled on December 29, 2009 for Guizhou Taibang pay RMB 4,700,000 (approximately, $712,990) as compensation to Sin for technology transfer and RMB 100,000 (approximately, $15,170) for unpaid equipment purchase. In December 2010, Sin filed another law suit against Guizhou Taibang with the Guizhou High Court for additional compensation of RMB5,376,160 (approximately $815,563). The Guizhou High Court ruled in favor of Sin on December 7, 2010 for Guizhou Taibang to pay the additional compensation. Guizhou Taibang has accrued and accounted for all these expenses as of December 31, 2010 and subsequently paid the judgment in full as of the date of this report. In January 2011, Sin appealed to the Guizhou High Court again for interest due to the late payment of the technology transfer fee since 2002. On March 16, the Company settled with Sin in court to pay RMB 752,834 (approximately $114,205) as interest for the delay in payment since 2002 and both parties also agreed not to appeal further.
Guizhou Taibang's Guarantee to a Third Party
In 2007, as a condition to purchase Huang Ping Plasma Station, Guizhou Taibang entered into an agreement with the Guizhou Zhongxin Investment Company, or Zhongxin, in which Guizhou Taibang agreed to repay Zhongxin's debt out of Guizhou Taibang's payables to Zhongxin arising from plasma purchased from Zhongxin. In the same agreement, Guizhou Taibang also delivered a guarantee to the Huang Ping County Hospital, the former co-owner of the Huang Ping Plasma Station, that it would pay RMB3,074,342 (approximately, $466,378) in debt that Zhongxin owed to the hospital. On June 1, 2009, the Huang Ping Hospital brought suit, in the Huang Ping County People's Court of Guizhou Province, against Zhongxin for non-payment of its payables and debt due to Huang Ping Hospital and against Guizhou Taibang as the guarantor. On November 2, 2009, the court ruled in favor of the plaintiff and Guizhou Taibang as the guarantor became obligated to repay the Zhongxin’s debt to the Huang Ping Hospital on behalf of Zhongxin. In October 2009, Guizhou Taibang appealed to the Middle Court of Kaili District in Guizhou Province which sustained the original judgment on April 8, 2010. Under the Equity Transfer Agreement pursuant to which we acquired a 90% interest in Dalin, Guizhou Taibang's majority shareholder, provides that the sellers will be responsible, based on their pro rata equity interest in Guizhou Taibang, for damages incurred by Guizhou Taibang from Zhongxin's debt and that they will repay Dalin their pro rata share of payments made by Guizhou Taibang to creditors in connection with Zhongxin's debt within 10 days after payment by Guizhou Taibang. The RMB 3,074,342 contingent liability and proportionate share of the liability to be recovered from the sellers were properly reflected in the financials as of December 31, 2009. On December 31, 2010, Guizhou Taibang brought suit against Zhongxin in the Middle Court of Guiyang City, to recover the full judgment amount of RMB 3,074,342 plus court fee of RMB 32,340 that Guizhou Taibang has already paid on behalf of Zhongxin.
On September 13, 2010, Zhongxin countersued the Company for a consideration of RMB 500,000 (approximately $75,850) for the alleged loss of its share of income from the Huang Ping Plasma Station since the Company acquired the station in April 2007. The Company believes Zhongxin’s claim is unwarranted since the Company acquired the station from its rightful owner, the Treasury Department of Huangpin County, Guizhou Province.
Shandong Taibang Equity Interests
Mr. Zu Ying Du was one of the original equity holders in the Company’s operating subsidiary, Shandong Taibang. Pursuant to a joint venture agreement, among the original equity holders, Mr. Du was obligated to make a capital contribution of RMB 20 million (or approximately $3.0 million) for a 25% interest in Shandong Taibang. Mr. Du made this contribution using funds borrowed from the Beijing Chen Da Technology Investment Company, or Beijing Chen Da. Mr. Du failed to repay Beijing Chen Da for his loan of the capital contribution amount. Mr. Du disputes that the money was due and owing. A Beijing court found that Beijing Chen Da had given money to Mr. Du but found that the loan agreement failed to comply with Chinese law. A notice was issued on July 5, 2004 by the Shenzhen Public Security Bureau Economic Crime Investigation Unit requesting a stay of the Beijing action pending their investigation into money laundering relating to the 20 million RMB loan to Zu Ying Du.
31
Subsequently, Beijing Chen Da entered into an equity transfer agreement with Mr. Du, pursuant to which Mr. Du's 25% equity interest in Shandong Taibang was transferred to Beijing Chen Da as repayment of the RMB 20 million debt. This agreement was signed by Mr. Du's brother who held a power of attorney from Mr. Du. Mr. Du disputes the legitimacy of this transfer and has argued that his brother, Du Hai Shan, exceeded the scope of the power of attorney. Mr. Du sued his brother in the court of Jianli County, Hubei province, relating to the propriety of the brother's actions under the power of attorney. Initially the county court found in its judgment that the act had exceeded the scope of the power of attorney. Subsequently the Intermediate Court of Jingzhou City, Hubei province, ruled on December 10, 2008 to suspend the judgment based on the grounds that the original court lacked jurisdiction to hear the case. The case is slated to be reviewed again by the Hubei Jingzhou Intermediate Court.
Missile Engineering, another original equity holder wholly controlled by Mr. Du, was obligated to contribute RMB 32.8 million (or $5.0 million) for a 41% interest in Shandong Taibang by means of cash, equipment and patent technology. It was obligated to obtain new drug certificate and production license of its patent technology from the government within a stipulated period in order to be recognized as a valid capital contribution, or in the alternative, make a cash payment. The patent technology was valued as RMB 26.4 million (or approximately $4.0 million). However, Missile Engineering failed to obtain the new drug certificate and production license within the stipulated period. Mr. Du also disputes whether the period for obtaining the certificate and license had expired. Pursuant to a stockholders resolution on September 26, 2004, Missile Engineering agreed to sell its 41% interest in Shandong Taibang to Up-Wing and Up-Wing agreed to take up the obligation of Missile Engineering to pay the RMB 26.4 million in cash. Missile Engineering disputes this transaction and sued the brother of Mr. Du in the court of Jianli County, Hubei province, relating to the propriety of the brother's actions under the power of attorney. Initially the county court found in its judgment that the act had exceeded the scope of the power of attorney. Subsequently the Intermediate Court of Jingzhou City, Hubei province, ruled on December 10, 2008 to suspend the judgment based on the grounds that the original court lacked jurisdiction to hear the case. The case is slated to be reviewed again by the Hubei Jingzhou Intermediate Court.
In June 10, 2005, Beijing Chen Da also sold its equity interest in Shandong Taibang to Up-Wing Investments Limited, or Up-Wing, pursuant to a share transfer agreement, which became effective on September 2, 2005, upon approval by the Shandong Provincial Department of Foreign Trade and Economic Cooperation, or the Shandong COFTEC. In March 2006, Up-Wing sold its equity interests in Shandong Taibang to Logic Express, the Company’s subsidiary.
In 2006, Missile Engineering applied for arbitration before the China International Economic and Trade Arbitration Commission, or CIETAC, to challenge the effectiveness of the transfer to Up-Wing Investments Limited, of the equity interests in Shandong Taibang formerly owned by Missile Engineering. The equity transfer had been approved by the Shandong Provincial Department of Foreign Trade and Economic Cooperation, or the Shandong COFTEC. Missile Engineering later voluntarily withdrew this application and instead applied for administrative reconsideration of the equity transfer, but this application was rejected by the Ministry of Commerce in 2007. Missile Engineering applied with the District Court of Lixia District, Jinan City, Shandong province requesting revocation of Shandong COFTEC's approval of the equity transfer to Up-wing by Missile Engineering. Missile Engineering later voluntarily withdrew the action. In April 2007, Logic Express initiated an arbitration proceeding before the Shandong Tai'an Arbitration Committee, to establish that Logic Express is the lawful shareholder of Shandong Taibang. The parties to that proceeding were Logic Express Ltd. and Shandong Taibang Biological Products Co., Ltd. The Arbitration Committee's decision on September 6, 2007 confirmed that Logic Express had legitimate ownership as a result of the transfer of Shandong Taibang. Up-Wing started an action in the Intermediate Court of Tai'an City, Shandong province requesting the court to establish that Up-Wing is the lawful shareholder of Shandong Taibang. The Intermediate Court of Tai'an City, Shandong province on December 20, 2007 rejected the application on the basis that the same matter had been tried by the arbitration panel.
Up-Wing filed a defamation case in the District Court of Hi-technology and Industry Development District, Tai'an City, Shandong province claiming defamation against Mr. Du and the 21st Century Economic Report Newspaper. Judgment in favor of Up-Wing was rendered on July 22, 2008 ordering the newspaper and Mr. Du to publish an apology to Up-Wing.
Mr. Du and Missile Engineering subsequently filed two actions in the Intermediate Court of Wuhan City, Hubei province, against Du Hai Shan, his brother, Beijing Chen Da and Logic Express, requesting that the court restore the equity interests originally held by the plaintiffs, 25% equity interest held by Mr. Du and 41% equity interest held by Missile Engineering and the court issued a preliminary order attaching 66% of the equity of Shandong Taibang pending the outcome of the case. On September 25, 2009, the Higher People's Court of Hubei overruled the Wuhan Intermediate Court's acceptance of jurisdiction over the case and ruled that the Tai'an Intermediate Court in Shandong Province, where the Company is located, had the proper jurisdiction over the parties' dispute. The court ruled that while the plaintiffs had the right to bring a lawsuit for the validity of the share transfer agreement because they did not attend the previous arbitration hearing and never reached an arbitration agreement regarding their dispute, the Tai'an Intermediate Court has the proper jurisdiction over the dispute pursuant to the prior agreement of the parties. As a result, the attached 66% of the equity of Shandong Taibang was released. On November 17, 2009, the Wuhan Intermediate Court permitted Mr. Du and Missile Engineering to withdraw their suits against Logic Express and the other defendants.
32
On September 28, 2010, the Company received a Notice from the PRC Supreme Court, advising the Company that the PRC Supreme Court has accepted an appeal for judicial review of the Hubei High Court ruling dismissing case. On November 2, 2010, the Company submitted its counter-argument and related materials to the PRC Supreme Court and the PRC Supreme Court ruled in favor of the Company on November 30, 2010 and denied Mr. Du and Missile Engineering’s appeal to the ruling of the Wuhan Intermediate Court.
Dispute over Raw Plasma Supply Agreement with Xintai
On March 10, 2009, Henan Xintai Medicine Company (previously known as Henan Zhongtai Medicine, “Xintai”) brought suit against Shandong Taibang and its two wholly-owned plasma collecting subsidiaries in Shandong for breach of a raw plasma supply agreement. The suit was subsequently withdrawn by Xintai on May 31, 2009. The agreement, signed by Shandong Taibang and Xintai on October 10, 2006, requires the two subsidiaries to provide to Xintai 45 metric tons of raw plasma per year from 2007 to 2009. The subsidiaries provided more than 34 metric tons of plasma to Xintai during 2007. However, the Company ceased its delivery of plasma to Xintai in late 2007 to avoid contravention of new PRC regulation. Clause 43 of an October 31, 2007, PRC State Department Regulation on Plasma Collection Stations, prohibits plasma collecting stations from providing raw plasma to any manufacturer other than their direct parent (the “Regulation”). On March 12, 2009, Shandong Taibang filed a suit in the Shandong Tai'an Middle Court against Xintai seeking damages of RMB50,000 (approximately, $7,585) for the plasma already supplied to Xintai during 2007. On June 29, 2009, Xintai re-filed its suit in the Shandong Tai'an Middle Court against Shandong Taibang and the two subsidiaries seeking compensation of RMB6,000,000 (approximately, $910,200) for alleged breach of contract and demanding that Shandong Taibang and the subsidiaries continue to honor the agreement. On October 20, 2009, the Tai'an Middle Court combined and heard the two suits and ruled on January 20, 2010 in favor of Shandong Taibang on all accounts.
On February 17, 2010, Xintai appealed to the High Court of Shandong Province and on August 10, 2010, the High Court remanded the suit to the Tai’an Middle Court for retrial on grounds of insufficient evidence.
On November 8, 2010, the Company and Xintai reached an out of court settlement whereby Shandong Taibang agreed to pay Xintai RMB 4,000,000 (approximately $606,800), payable on or before November 22, 2010, and RMB 43,950 (approximately $6,667) in court costs. The Company has paid the costs of its settlement agreement with Xintai under legal expenses as of December 31, 2010.
ITEM 4. (REMOVED AND RESERVED).
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common stock is traded on the Nasdaq Global Select Market under the symbol “CBPO.”
The following table sets forth, for the periods indicated, the high and low closing prices of our common stock. These prices reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions.
| Closing Prices(1) | ||||||
| High | Low | |||||
| Year Ended December 31, 2010 | ||||||
| 1st Quarter | $ | 13.48 | $ | 7.62 | ||
| 2nd Quarter | 13.95 | 10.28 | ||||
| 3rd Quarter | 13.95 | 9.61 | ||||
| 4th Quarter | 17.23 | 9.38 | ||||
| Year Ended December 31, 2009 | ||||||
| 1st Quarter | $ | 2.45 | $ | 1.69 | ||
| 2nd Quarter | 5.00 | 2.25 | ||||
| 3rd Quarter | 7.54 | 3.69 | ||||
| 4th Quarter | 12.08 | 7.10 | ||||
| (1) |
The above table sets forth the range of high and low closing prices per share of our common stock as reported by www.quotemedia.com for the periods indicated. |
33
Approximate Number of Holders of Our Common Stock
As of March 25, 2011, there were approximately 441 holders of record of our common stock. This number excludes the shares of our common stock owned by stockholders holding stock under nominee security position listings.
Dividend Policy
We have never declared dividends or paid cash dividends. Any future decisions regarding dividends will be made by our board of directors. We currently intend to retain and use any future earnings for the development and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
See Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters - Securities Authorized for Issuances under Equity Compensation Plans.”
Recent Sales of Unregistered Securities
We have not sold any equity securities during the fiscal year ended December 31, 2010 that were not previously disclosed in a quarterly report on Form 10-Q or a current report on Form 8-K that was filed during the 2010 fiscal year.
Purchases of Equity Securities
No repurchases of our common stock were made during the fourth quarter of 2010.
ITEM 6. SELECTED FINANCIAL DATA.
Not Applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following management’s discussion and analysis should be read in conjunction with our financial statements and the notes thereto and the other financial information appearing elsewhere in this report. In addition to historical information, the following discussion contains certain forward-looking information. See “Special Note Regarding Forward Looking Statements” above for certain information concerning those forward looking statements. Our financial statements are prepared in U.S. dollars and in accordance with U.S. GAAP.
Overview
We are a biopharmaceutical company and through our indirect majority-owned PRC subsidiaries, Shandong Taibang and Guizhou Taibang, and our minority-owned PRC investee, Huitian, we are principally engaged in the research, development, manufacturing and sales of plasma-based pharmaceutical products in China. Shandong Taibang operates from our manufacturing facility located in Tai’an, Shandong Province and Guizhou Taibang operates from our manufacturing facility located in Guiyang City, Guizhou Province. Our minority owned investee, Huitian, operates from its facility in Shaanxi Province. The plasma-based biopharmaceutical manufacturing industry in China is highly regulated by both the provincial and central governments. Accordingly, the manufacturing process of our products is strictly monitored from the initial collection of plasma from human donors to finished products. Our principal products include our approved human albumin and immunoglobulin products.
We are approved to sell human albumin 20%/10ml, 20%/25ml, 20%/50ml, 10%/10ml, 10%/25ml, 10%/50ml and 25%/50ml. Human albumin is our top-selling product. Sales of these human albumin products represented approximately 47.8%, 49.5% and 57.8% of our total revenues, respectively, for the each of the years ended December 31, 2010, 2009 and 2008. Human albumin is principally used to increase blood volume while immunoglobulin, one of our other major products, is used for certain disease preventions and cures. The Company’s approved human albumin and immunoglobulin products use human plasma as the basic raw material. Albumin has been used for almost 50 years to treat critically ill patients by replacing lost fluid and maintaining adequate blood volume and pressure. All of our products are prescription medicines administered in the form of injections.
We sell our products to customers in the PRC, mainly hospitals and inoculation centers directly or through approved distributors. Our sales have historically been made on the basis of short-term arrangements and our largest customers have changed over the years. For the years ended December 31, 2010, 2009 and 2008, our top 5 customers accounted for approximately 12.3%, 10.7% and 16.2%, respectively, of our total revenue. For the years ended December 31, 2010, 2009 and 2008, our largest customer accounted for approximately 2.8%, 4.0% and 6.4%, of our revenue, respectively. As we continue to diversify our geographic presence, customer base and product mix, we expect that our largest customers will continue to change from year to year.
34
We operate and manage our business as a single segment. We do not account for the results of our operations on a geographic or other basis.
2010 Financial Performance Highlights
We continued to experience strong demand for our products and services during the fiscal year ended December 31, 2010, which resulted in revenue and net income growth. The following are some financial highlights for the year:
-
Sales: Sales increased $20,697,262, or 17.4%, to $139,695,417 for the year ended December 31, 2010, from $118,998,155 for 2009.
-
Gross Profit: Gross profit increased $16,368,021, or 18.9%, to $102,744,268 for the year ended December 31, 2010, from $86,376,247 for 2009. As a percentage of revenues, gross profit increased 0.9% to73.5% for year 2010 from 72.6% for 2009.
-
Income from operations: Income from operations increased $8,148,036, or 13.3%, to $69,525,228 for the year ended December 31, 2010, from $61,377,192 for 2009.
-
Net income: Net income increased $33,168,520, or 176.2%, to $51,992,304 for the year ended December 31, 2010, from $18,823,784 for 2009.
-
Fully diluted net income per share: Fully diluted net income per share was $1.30 for the year ended December 31, 2010, as compared $0.10 for 2009.
Principal Factors Affecting our Financial Performance
The following are key factors that affect our financial condition and results of operations and we believe them to be important to the understanding of our business:
Raw Material Supply and Prices
The primary raw material used in the production of our albumin and immunoglobulin products is human plasma. Collection of human plasma in China is regulated and, until 2006, only licensed Plasmapheresis stations owned and operated by the government could collect human plasma. Each collection station was only allowed to supply plasma to the one manufacturer that has signed the “Quality Responsibility” statement with them. The price of human plasma is negotiated on an annual basis and is determined by a number of factors including, but not limited to, the cost of operating the collection stations, the nutritional supplement fee awarded to the donors for each donation, and the anticipated volume of total plasma donated. However, in March 2006, the Ministry of Health promulgated certain “Measures on Reforming Plasma Collection Stations,” or the Blood Collection Measures, whereby the ownership and management of PRC plasma stations are required to be transferred to plasma-based biopharmaceutical companies while the regulatory supervision and administrative control remain with the State. Plasma stations that did not complete their reform by December 31, 2006, risked revocation of their license to collect plasma.
In December 2006, we acquired five of the six then existing plasma stations in Shandong and on January 1, 2007 we obtained the permits to operate these stations. These acquisitions have allowed us to have a direct influence on the operation of these collection stations and secure a stable source of plasma supply for production. The foregoing acquisitions, as well as the acquisition of Dalin and its indirectly owned plasma stations, have led to an increase in our plasma supply for production and did not result in any material differences in our cost structure. Due to current market conditions, we have generally been able to pass substantially all cost increases in recent years on to our customers.
Prices of and Demand for Our Products
In recent years, due to increased regulatory restrictions and market demand, we have been able to increase the selling price of most of our key products. The demand for our products is largely affected by the general economic conditions in China because they are still not affordable to many patients. As China's economy grows, we expect more Chinese people will become consumers of medical treatments and procedures, including procedures requiring human plasma. A significant improvement in the economic environment in China will likely improve consumer income which in turn would make our products more affordable and consequently increase the demand for our products. We have been able to expand our product range and markets by introducing new products required by customers. We believe that our technical expertise is important in introducing products that are in demand.
35
Production Capacity
Our sales volume is limited by our annual production capacity. As we grow our business in the future, our ability to fulfill additional and larger orders will depend on our ability to increase our production capacity. Our plan to expand our production capacity will depend on, inter alia, the availability of capital to meet our needs of expansion or upgrading of production lines, and the availability of stable plasma supply.
As of December 31, 2010, the aggregate production capacity of Shandong Taibang and Guizhou Taibang was 1,100 metric tons per annum. We estimate that the production capacity of our major competitors ranges from 300 tons to 1,000 tons per annum. We believe that our current production capacity is sufficient to meet the current demand for our products for the next two years.
Competition
We are subject to intense competition. There are both local and overseas pharmaceutical enterprises that are engaged in the manufacture and sale of potential substitute or similar biopharmaceutical products as our products in the PRC. These competitors may have more capital, better research and development resources, manufacturing and marketing capability and experience than we do. In our industry, we compete based upon product quality, product cost, ability to produce a diverse range of products and logistical capabilities.
We believe that we have strengthened our position in the marketplace with our acquisition of Dalin and its 54% majority-owned operating subsidiary, Guizhou Taibang, and a 35% equity interest in Huitian, a Xi'an-based biopharmaceutical company.
Our profitability may be adversely affected if (i) competition intensifies; (ii) competitors drastically reduce prices; (iii) PRC government’s interference on prices; or (iv) competitors develop new products or product substitutes with comparable medicinal applications or therapeutic effects which are more effective or less costly than those produced by us. Please refer to Item 1, “Business - Competition” for more information regarding this factor.
Taxation
China Biologic is subject to United States tax at a tax rate of 34%. No provision for income taxes in the United States has been made as China Biologic has no income taxable in the United States. Logic Express was incorporated in the BVI, but is not subject to taxation in that jurisdiction. Logic Holdings was incorporated in Hong Kong and under the current laws of Hong Kong, are subject to a Profits Tax of 16.5%. However, no provision for Hong Kong Profits Tax has been made as Logic Holdings has no taxable income.
According to the PRC's central government policy, new or high technology companies will enjoy preferential tax treatment of 15%, instead of 25% under the EIT Law. On December 5, 2008, Shandong Taibang received the new technology or high technology certification from Shandong provincial government. The Certification allows Shandong Taibang to receive the 15% preferential income tax rate, for a period of three years starting from January 1, 2008. Guizhou Taibang enjoyed the preferential income tax rate of 15% also under the 10-year Western Development Tax Concession, which started on January 2001 and ended on December 2010. The PRC tax authority is studying the possibility of extending the concession, especially for those industries that are encouraged by the PRC government, such as ours. In the event that PRC tax authorities discontinue the concession, Guizhou Taibang will apply for the new or high technology preferential tax treatment of 15% like Shandong Taibang. See Item 1, “Business – Regulation – Taxation” for a detailed description of the EIT Law and tax regulations applicable to our PRC subsidiaries.
36
Results of Operations
Comparison of Fiscal Years Ended December 31, 2010 and 2009
The following table sets forth key components of our results of operations for fiscal years ended December 31, 2010 and 2009.
(All amounts, other than percentages, in U.S. dollars)
| Year Ended | $ | Increase | % Increase | |||||||||
| December 31, | (Decrease) | (Decrease) | ||||||||||
| 2010 | 2009 | |||||||||||
| SALES | ||||||||||||
| External customers | $ | 138,674,983 | 118,293,137 | $ | 20,381,846 | 17.2% | ||||||
| Related party | 1,020,434 | 705,018 | 315,416 | 44.7% | ||||||||
| Total sales | 139,695,417 | 118,998,155 | 20,697,262 | 17.4% | ||||||||
| COST OF SALES | ||||||||||||
| External customers | 36,793,775 | 32,544,743 | 4,249,032 | 13.1% | ||||||||
| Related party | 157,374 | 77,165 | 80,209 | 103.9% | ||||||||
| Total cost of sales | 36,951,149 | 32,621,908 | 4,329,241 | 13.3% | ||||||||
| GROSS PROFIT | 102,744,268 | 86,376,247 | 16,368,021 | 18.9% | ||||||||
| OPERATING EXPENSES: | ||||||||||||
| Selling expenses | 7,372,348 | 3,529,242 | 3,843,106 | 108.9% | ||||||||
| General and administrative expenses | 23,510,566 | 19,807,123 | 3,703,443 | 18.7% | ||||||||
| Research and development expenses | 2,336,126 | 1,662,690 | 673,436 | 40.5% | ||||||||
| Total operating expenses | 33,219,040 | 24,999,055 | 8,219,985 | 32.9% | ||||||||
| INCOME FROM OPERATIONS | 69,525,228 | 61,377,192 | 8,148,036 | 13.3% | ||||||||
| OTHER EXPENSES (INCOME): | ||||||||||||
| Equity in income of equity method investee | (1,070,241 | ) | (566,984 | ) | (503,257 | ) | 88.8% | |||||
| Change in fair value of derivative liabilities | 3,233,288 | 28,915,328 | (25,682,040 | ) | (88.8% | ) | ||||||
| Interest expense, net | 1,930,165 | 3,930,249 | (2,000,084 | ) | (50.9% | ) | ||||||
| Other (income)/expense, net | (169,043 | ) | 261,252 | (430,295 | ) | (164.7% | ) | |||||
| Total other expenses, net | 3,924,169 | 32,539,845 | (28,615,676 | ) | (87.9% | ) | ||||||
| EARNINGS BEFORE INCOME TAX EXPENSE | 65,601,059 | 28,837,347 | 36,763,712 | 127.5% | ||||||||
| INCOME TAX EXPENSES | 13,608,755 | 10,013,563 | 3,595,192 | 35.9% | ||||||||
| NET INCOME | $ | 51,992,304 | $ | 18,823,784 | $ | 33,168,520 | 176.2% | |||||
| Less: Net income attributable to noncontrolling interest | 20,449,421 | 16,615,658 | 3,833,763 | 23.1% | ||||||||
| NET INCOME ATTRIBUTABLE TO CHINA BIOLOGIC PRODUCTS, INC. | $ | 31,542,883 | $ | 2,208,126 | $ | 29,334,757 | 1328.5% | |||||
Sales. Our sales are derived primarily from the sales of human albumin and various types of immunoglobulin. Our sales increased 17.4%, or $20,697,262, to $139,695,417 for the fiscal year ended December 31, 2010, compared to sales of $118,998,155 for the fiscal year ended December 31, 2009. The increase in sales during fiscal year 2010 is primarily attributable to a general increase in the price and volume of plasma based products. Among the factors that contributed to the growth in revenue, foreign exchange translation accounted for 1.1% of the increase.
Most of our approved products recorded price increases ranging from 12.6% to 186.4%, except for human albumin products, which decreased by 1.8%. The decrease in the price of human albumin in 2010 is primarily due to the increased volume of imported human albumin products in the PRC market during the period. We expect that this trend may continue as long as the volume of the imported human albumin products continue to grow. For the fiscal year ended December 31, 2010, the average price for our approved human albumin products, which contributed 48.0% to our total revenue, decreased 1.8%, the average price for our approved human immunoglobulin for intravenous injection, which contributed 34.4% to our revenue, increased 26.9%, the average price for our approved human tetanus immunoglobulin, which contributed 2.9% to our revenue, increased 12.6%, the average price for our approved human rabies immunoglobulin, which contributed 5.3% to our revenue, increased 24.6%, and the average price for our approved human hepatitis B immunoglobulin, which contributed 7.6% to our revenue, increased 186.4%, as compared to the same period in 2009. The price increase of our products was primarily attributable to the continuing shortage in supply of the plasma-based products. We were able to adjust our production plan to take advantage of the limited market supply of plasma resources to realize higher profit margins.
Volume in sales for our human albumin, human hepatitis B immunoglobulin, human rabies immunoglobulin products and human tetanus immunoglobulin products increased by 15.7%, 7.4%, 26.5% and 24.6%, respectively, for the fiscal year ended December 31, 2010, as compared to the same period in 2009. Volume in sales for our human immunoglobulin for intravenous injection decreased by 15.8%, for the fiscal year ended December 31, 2010, as compared to the same period in 2009, because in 2009 the market demand for human immunoglobulin for intravenous injection increased due to the outbreak of hand-foot-mouth disease and the price of human immunoglobulin for intravenous injection was also much enhanced. As a result we used the large amount of Factor II+III, the material segregated from plasma and restored separately when making centralized production of human albumin in 2008, to produce and sell high volume of human immunoglobulin for intravenous injection in 2009. The volume in sales for human immunoglobulin for intravenous injection decreased to a comparatively normal level in 2010.
37
Cost of Sales. Our cost of sales increased $4,329,241, or 13.3%, to $36,951,149 for the year ended December 31, 2010, from $32,621,908 during the same period in 2009. Cost of sales as a percentage of sales was 26.5% for the fiscal year ended December 31, 2010, as compared to 27.4% during 2009. The increase in cost of sales is due to the increase in sales, while the decrease in cost of sales as a percentage of sales is due to a change in the mix of products, as well as the price increase in most of the products, that were sold during 2010.
Gross profit and gross margin. The gross profit increased by $16,368,021, or 18.9%, to $102,744,268 for the fiscal year ended December 31, 2010 from $86,376,247 for the same period in 2009. As a percentage of sales revenue, our gross profit increased by 0.9% to 73.5% for the fiscal year ended December 31, 2010, from 72.6% for 2009. The increase in gross profit is due mainly to increases in the selling price and sales volume of our products during 2010, as compared to 2009.
Operating expenses. Our total operating expenses increased by $8,219,985, or 32.9%, to $33,219,040 for the fiscal year ended December 31, 2010, from $24,999,055 for 2009. The increase was primarily attributable to a 40.5% increase in our research and development expenses, a 108.9% increase in our selling expense and an 18.7% increase in our general and administrative expenses during 2010. As a percentage of sales revenue, total expenses increased by 2.8% to 23.8% for the fiscal year ended December 31, 2010 from 21.0% for 2009.
Selling expenses. For the fiscal year ended December 31, 2010, our selling expenses increased to $7,372,348, from $3,529,242 for the fiscal year ended December 31, 2009, an increase of $3,843,106, or 108.9%. As a percentage of sales, our selling expenses for the fiscal year ended December 31, 2010 increased by 2.3%, to 5.3%, from 3.0% for the fiscal year ended December 31, 2009. The increase in selling expenses is primarily due to an increase in our promotional and conference activities as we continue our efforts in expanding our customer base into hospital and inoculation centers throughout the PRC.
General and administrative expenses. For the fiscal year ended December 31, 2010, our general and administrative expenses increased to $23,510,566, from $19,807,123 for the fiscal year ended December 31, 2009, a $3,703,443, or 18.7% increase. General and administrative expenses as a percentage of sales increased by 0.2% to 16.8% for fiscal year 2010 from 16.6% for the fiscal year 2009. The increase in general and administrative expenses is primarily due to the increases in legal expense, non-cash employee compensation, travel and general office expenses as we continue to our efforts to integrate our two main operating entities, as well as and inventory allowance, which were offset by the decrease in payroll. The increase in legal expense is due to the $598,114 settlement of a law suit with Henan Xintai and the $1,177,836 settlement of a law suit with Sin Kyung Ye as described under Item 3, Legal Proceedings. Non-cash employee compensation for the fiscal year ended December 31, 2010 increased by $2,279,502 to $2,341,783, from $62,281 for 2009, as a result of the amortization of the grant of stock options to the Company’s senior management staff in the January, February and July of 2010.
Research and development expenses. For the fiscal years ended December 31, 2010 and 2009, our research and development expenses were $2,336,126 and $1,662,690, respectively, an increase of $673,436, or 40.5%. As a percentage of sales, our research and development expenses for the fiscal year ended December 31, 2010 and 2009 were 1.7% and 1.4%, respectively. The increase in research and development expenses is primarily due to the cost associated with the development of two new products that are at the end of their respective development stage. We expect to receive SFDA approval for these two new products in 2011.
Change in fair value of derivative liabilities. The embedded derivatives (including the conversion option) in our senior secured convertible notes and warrants that were issued in June 2009 are classified as derivative liabilities carried at fair value. For the year ended December 31, 2010 and 2009, the Company recognized a loss on the change in the fair value of derivative liabilities of $3,233,288 and $28,915,328, respectively. The recognized loss on the change in the fair value for the fiscal year ended December 31, 2010 is mainly due to the Company’s stock price increase from $12.08 to $16.39, which increased the fair value of derivative instruments, as of December 31, 2009 and December 31, 2010, respectively. Future changes in the market price of our common stock could cause the fair value of these derivative financial instruments to change significantly in future periods.
Interest expense (income), net. Our net interest expense decreased $2,000,084 to $1,930,165 for the year ended December 31, 2010, from interest expense of $3,930,249 for the same period in 2009. The decrease in net interest expense is primarily due to our payment of a related party loan related to the acquisition of Dalin in the second quarter of 2010, conversion of $4.9 million of our outstanding convertible notes in 2009 and 2010 and an increase in interest income from our short term deposits with financial institutions.
Income tax expense. Our provision for income taxes increased $3,595,192, or 35.9%, to $13,608,755 for the year ended December 31, 2010, from $10,013,563 for the same period in 2009. Our effective tax rate for the year ended December 31, 2010 was 20.7%, and our 2009 effective tax rate was 34.7%. The decrease in effective tax rate is mainly due to the decrease of $25.7 million in change in fair value of derivative liabilities that is not tax deductible. Among the increase in income taxes, $1.3 million is due to the dividend tax imposed by PRC tax authorities on dividends distributed by our two main operating entities to Logic Express during the 2010 period.
Net income. Our net income increased $33,168,520, or 176.2%, to $51,992,304 for the year ended December 31, 2010, from $18,823,784 for the same period in 2009. Net income as a percentage of sales was 37.2% and 15.8% for the year ended December 31, 2010 and 2009, respectively, as a result of the foregoing factors.
38
Liquidity and Capital Resources
As of December 31, 2010, we had cash and cash equivalents of $64,941,368, primarily consisting of cash on hand and demand deposits. To date, we have financed our operations primarily through cash flows from operations, augmented by short-term bank borrowings and equity contributions by our stockholders. We expect that cash on hand, funds generated from our operations and funds generated from companies that we may acquire in the future will be sufficient to satisfy our current and future commitments for at least the next twelve months. We do not believe that we have any significant short term liquidity problems.
The following table sets forth a summary of our cash flows for the periods indicated:
Cash Flow
(all amounts in U.S. dollars)
|
Year Ended December 31, |
||||||
| 2010 | 2009 | |||||
| Net cash provided by operating activities | 38,787,226 | 50,300,987 | ||||
| Net cash used in investing activities | (15,851,475 | ) | (6,860,454 | ) | ||
| Net cash provided by (used in) financing activities | (14,278,870 | ) | 1,564,925 | |||
| Effects of exchange rate change in cash | 2,440,536 | 23,877 | ||||
| Net increase in cash and cash equivalents | 11,097,417 | 45,029,335 | ||||
| Cash and cash equivalents at beginning of the year | 53,843,951 | 8,814,616 | ||||
| Cash and cash equivalent at end of the year | 64,941,368 | 53,843,951 | ||||
Operating activities
Net cash provided by operating activities was $38.8 million for the fiscal year ended December 31, 2010, as compared to $50.3 million net cash provided by operating activities for 2009. The net cash provided by operating activities in the year ended December 31, 2010 was mainly due to the net income of $65.4 million and offset by cash outflow for inventory and accounts receivable of $16.0 million and $7.8 million, respectively. The cash outflow for inventory is a direct result of the implementation of the 90-day quarantine period by the PRC government, which caused a longer staging period for its raw material plasma inventory. As the Company increased its sales directly to the end-users, hospitals and inoculation centers with extended credit term, we experienced a slower turn-over with our accounts receivable.
Investing activities
Our use of cash for investing activities is primarily for the acquisition of property, plant and equipment and intangibles, and advances on non-current assets. Net cash used for investing activities for the fiscal year ended December 31, 2010 was $15.9 million, as compared to $6.9 million in 2009. During the fiscal year ended December 31, 2010, we paid $1.5 million to acquire a new Company, Ziguang Bio-tech Company, paid the final $2.5 million payment for the acquisition of 90% equity in Dalin, $5.3 million for equipment for Shandong Taibang and $6.5 million for our plasma companies' buildings and construction in progress in Guizhou Taibang.
39
Financing activities
Net cash used in financing activities for the year ended December 31, 2010 totaled $14.3 million as compared to $1.6 million net cash provided by financing activities in 2009. The increase of the cash used in financing activities was mainly attributable to the $10.4 million dividend paid by our subsidiaries to the noncontrolling interest holder, repayment of a non-controlling shareholder loan of $3.7 million, repayment of short term bank loan of $7.4 million and offset by short-term bank loans and proceeds from warrants conversion of $5.9 million and $1.2 million, respectively.
Management believes that the Company has sufficient cash on hand and continuing positive cash inflow, from the sale of its plasma-based products in the PRC market. Our management expects continued growth in revenues throughout the term of the convertible notes, largely due to the ongoing limited supply of plasma-based products in the PRC market due to the introduction of more stringent health and safety measures which we already meet. In light of the foregoing, we believe that the Company will have the financial ability to fulfill its payment obligations under the convertible notes when they come due.
Obligations under Material Contracts
The following table sets forth our material contractual obligations as of December 31, 2010:
(All amounts in thousands of U.S. dollars)
| Payments Due by Period | ||||||||||||||||
| Less than | More than | |||||||||||||||
| Contractual Obligations | Total | 1 year | 1-3 years | 3-5 years | 5 years | |||||||||||
| Convertible notes | $ | 1,196,233 | $ | 1,196,233 | $ | - | $ | - | $ | - | ||||||
| Due to related parties | 2,195,123 | 2,195,123 | - | - | - | |||||||||||
| Operating lease obligations | 1,076,168 | 328,231 | 400,569 | 108,550 | 238,818 | |||||||||||
| Total | $ | 4,467,524 | $ | 3,719,587 | $ | 400,569 | $ | 108,550 | $ | 238,818 | ||||||
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires our management to make assumptions, estimates and judgments that affect the amounts reported in the financial statements, including the notes thereto, and related disclosures of commitments and contingencies, if any. We consider our critical accounting policies to be those that require the more significant judgments and estimates in the preparation of financial statements, including the following:
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. Significant items subject to such estimates and assumptions include the useful lives of fixed assets; the allowance for doubtful accounts; the fair value determinations of financial and equity instruments and stock compensation awards, assets acquired and liabilities assumed in a business combination; the realizability of deferred tax assets and inventories; the recoverability of goodwill, intangible asset, land use right and property, plant and equipment; and accruals for income tax uncertainties and other contingencies. The current economic environment has increased the degree of uncertainty inherent in those estimates and assumptions.
40
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, delivery of the product has occurred, the sales price is fixed or determinable and collectability is reasonably assured. The Company mainly sells human albumin and human immunoglobulin to hospitals, inoculation centers and pharmaceutical distributors. The Company requires a contract or purchase order which specify pricing, quantity and product specifications for all sales. Delivery of the product occurs at the point in time the product is received by the customer, which is when the risks and rewards of ownership have been transferred. Delivery is evidenced by a signed customer acceptance form. Sales are presented net of any discounts given to customers. For the year ended December 31, 2009 and 2010, there were no significant sales return from the customers of the Company.
Fair Value Measurements
On January 1, 2008, the Company adopted FASB's accounting standard related to fair value measurements and began recording financial assets and liabilities subject to recurring fair value measurement at the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. These fair value principles prioritize valuation inputs across three broad levels. The Company considers the carrying amount of cash, receivables, payables including accrued liabilities and short term loans to approximate their fair values because of the short period of time between the origination of such instruments and their expected realization and if applicable, their stated rates of interest are equivalent to interest rates currently available. The fair values are measured pursuant to the three levels defined by the FASB's accounting standard as follow:
-
Level 1: inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
-
Level 2: inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
-
Level 3: inputs to the valuation methodology are unobservable and significant to the fair value.
The fair value of the embedded conversion option in the Notes of December 31, 2010 and 2009 was determined based on the Binominal option pricing model, using the following key assumptions:
| December 31, 2010 | December 31, 2009 | |||||
| Expected dividend yield | 0% | 0% | ||||
| Risk-free interest rate | 0.17% | 0.75% | ||||
| Time to maturity (in years) | 0.43 | 1.43 | ||||
| Expected volatility | 50.0% | 130.0% | ||||
| Fair value of underlying common shares (per share) | $ | 16.39 | $ | 12.08 |
The fair values of the warrants outstanding as of December 31, 2010 and 2009 were determined based on the Binominal option pricing model, using the following key assumptions:
| December 31, 2010 | December 31, 2009 | |||||
| Expected dividend yield | 0% | 0% | ||||
| Risk-free interest rate | 0.43% | 1.38% | ||||
| Time to maturity (in years) | 1.43 | 2.43 | ||||
| Expected volatility | 70.0% | 130.0% | ||||
| Fair value of underlying common shares (per share) | $ | 16.39 | $ | 12.08 |
Accounts Receivable and Allowance For Doubtful Accounts
Accounts receivable are recorded at the invoiced amount and do not bear interest. The Company maintains an allowance for doubtful accounts for estimated losses inherent in its accounts receivable portfolio. In establishing the required allowance, management considers historical losses adjusted to take into account current market conditions and the customers’ financial condition, the amount of receivables in dispute, and the current receivables aging and current payment patterns. The Company reviews its allowance for doubtful accounts monthly. Past due balances are reviewed individually for collectability. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers.
Depending on the relationship and the creditability of the distributors, we generally grant a credit period of no longer than 30 days to distributors with some exceptions. For hospitals and clinics, we generally grant a credit period of no longer than 90 days. We had a bad debt credit of $0.1 million for 2010, a bad debt expense of $0.3 million for 2009 and a bad debt credit of $0.1 million for 2008 related to the sales of our products. The $0.1 million bad debt credit for both 2010 and 2008 is due to recovery of bad debt previously reserved.
Inventories
Inventories are stated at the lower of cost or market. Cost is determined using the weighted average method. Cost of work in progress and finished goods comprise direct materials, direct production costs and an allocation of production overheads based on normal operating capacity.
The Company reviews its inventory periodically for possible obsolete goods and cost in excess of net realizable value to determine if any reserves are necessary. As of December 31, 2010 and 2009, the Company wrote off $1,000,277 and $519,333 relating to obsolete raw material plasma that may not qualify for production due to the 90-day quarantine period rules implemented by State Food and Drug Administration on July 1, 2008.
Stock-based Compensation
The Company measures the cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award and recognizes the cost over the period during which an employee is required to provide service in exchange for the award, which generally is the vesting period.
The fair value of each option granted on May 9, 2008, July 24, 2008, January 7, 2010, February 4, 2010 and July 11, 2010 are estimated on the respective dates of grant using the Black-Scholes option pricing model with the following major assumptions:
| Granted on | May 9, 2008 | July 24, 2008 | January 7, 2010 | February 4, 2010 | July 11, 2010 | ||||||||||
| Expected dividend yield | 0% | 0% | 0% | 0% | 0% | ||||||||||
| Risk-free interest rate | 3.56% | 3.56% | 2.62% | 2.29% | 1.85% | ||||||||||
| Expected term (in years) | 5 | 5 | 5 | 5 | 6.5 | ||||||||||
| Expected volatility | 59.4% | 81.2% | 130.0% | 130.0% | 135.0% |
The volatility of the Company’s common stock was estimated by management based on the historical volatility of the Company’s common stock. The risk free interest rate was based on Treasury Constant Maturity Rates published by the U.S. Federal Reserve for periods applicable to the estimated term of the options. The expected dividend yield was based on the Company’s current and expected dividend policy. The weighted average grant date fair value of options granted during the year of 2010 was $10.70.
41
Impairment of Long-Lived Assets
In accordance with Impairment or Disposal of Long-Lived Assets Subsections of FASB ASC Subtopic 360-10, Property, Plant, and Equipment. Overall, long-lived assets, such as property, plant and equipment, and purchased intangible asset subject to amortization, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If circumstances require a long-lived asset or asset group be tested for possible impairment, the Company first compares undiscounted cash flows expected to be generated by that asset or asset group to its carrying value. If the carrying value of the long-lived asset or asset group is not recoverable on an undiscounted cash flow basis, an impairment is recognized to the extent that the carrying value exceeds its fair value. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary. No impairment of long-lived assets was recognized for the years ended December 31, 2010 and 2009.
Recent Accounting Pronouncements
In October 2009, the FASB issued Accounting Standards Update (“ASU”) 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements (EITF Issue No. 08-1, “Revenue Arrangements with Multiple Deliverables”). ASU 2009-13 amends FASB ASC Subtopic 605-25 to eliminate the requirement that all undelivered elements have vendor specific objective evidence of selling price (“VSOE”) or third party evidence of selling price (“TPE”) before an entity can recognize the portion of an overall arrangement fee that is attributable to items that already have been delivered. In the absence of VSOE or TPE for one or more delivered or undelivered elements in a multiple-element arrangement, entities will be required to estimate the selling prices of those elements. The overall arrangement fee will be allocated to each element (both delivered and undelivered items) based on their relative selling prices, regardless of whether those selling prices are evidenced by VSOE or TPE or are based on the entity’s estimated selling price. Application of the “residual method” of allocating an overall arrangement fee between delivered and undelivered elements will no longer be permitted upon adoption of ASU 2009-13. Additionally, the new guidance will require entities to disclose more information about their multiple-element revenue arrangements. ASU 2009-13 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. Management is currently evaluating the potential impact, if any, of adopting ASU 2009-13 on the Company’s financial position and results of operations.
Seasonality of our Sales
Our operating results and operating cash flows historically have not been subject to seasonal variations. This pattern may change, however, as a result of new market opportunities or new product introductions.
Inflation
Inflation does not materially affect our business or the results of our operations.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our investors.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Our operations are carried out in the PRC and we are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. Accordingly, our business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC economy. Our results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Interest Rate Risk
We are exposed to interest rate risk primarily with respect to our short-term and long-term bank loans. Although the interest rates, which are based on the banks’ prime rates with respect to our short-term loans are fixed for the terms of the loans, the terms are typically three to twelve months for short-term bank loans and interest rates are subject to change upon renewal. There were no material changes in interest rates for short-term bank loans renewed during the fiscal year ended December 31, 2010.
A hypothetical 1.0% increase in the annual interest rates for all of our credit facilities under which we had outstanding borrowings as of December 31, 2010, would decrease net income before provision for income taxes by approximately $30,340 for the fiscal year ended December 31, 2010. Management monitors the banks’ prime rates in conjunction with our cash requirements to determine the appropriate level of debt balances relative to other sources of funds. We have not entered into any hedging transactions in an effort to reduce our exposure to interest rate risk.
42
Foreign Exchange Risk
While our reporting currency is the U.S. Dollar, all of our consolidated revenues and consolidated costs and majority of expenses are denominated in RMB. All of our assets are denominated in RMB, except certain cash balances. As a result, we are exposed to foreign exchange risk as our revenues and results of operations may be affected by fluctuations in the exchange rate between U.S. Dollars and RMB. If RMB depreciates against the U.S. Dollar, the value of our RMB revenues, earnings and assets as expressed in our U.S. Dollar financial statements will decline. Assets and liabilities are translated at exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates and shareholders’ equity is translated at historical exchange rates. Any resulting translation adjustments are not included in determining net income but are included in determining other comprehensive income, a component of stockholder’s equity. We have not entered into any hedging transactions in an effort to reduce our exposure to foreign exchange risk.
The value of the RMB against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions. Since July 2005, the RMB has not been pegged to the U.S. dollar. Although the People’s Bank of China regularly involved in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the RMB may appreciate or depreciate significantly in value against the U.S. dollar or Euro in the medium to long term. Moreover, it is possible that in the future, PRC authorities may lift restrictions on fluctuations in RMB exchange rate and lessen involvement in the foreign exchange market.
Account Balances
We maintain balances at financial institutions which, from time to time, may exceed Federal Deposit Insurance Corporation insured limits for the banks located in the United States or may exceed Hong Kong Deposit Protection Board insured limits for the banks located in Hong Kong. Balances at financial institutions or state-owned banks within the PRC are not covered by insurance. Total cash in banks as of December 31, 2010 and 2009 amounted to $64,443,315 and $53,576,495, respectively, $1,473,917 and $1,009,053 of which are covered by insurance, respectively. We have not experienced any losses in such accounts and we do not believe that we are exposed to any significant risks on our cash in bank accounts.
Inflation
Inflationary factors such as increases in the cost of our sales and overhead costs may adversely affect our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of gross margin and selling, general and administrative expenses as a percentage of net sales if the selling prices of our products do not increase with these increased costs.
Market for Human Albumin
The Company’s major product, human albumin: 20%/10ml, 20%/25ml, 20%/50ml, 10%/10ml, 10%/25ml and 10%/50ml, accounted for 48.0% and 49.7% of the total revenues for the years ended December 31, 2010 and 2009, respectively. If the market demands for human albumin cannot be sustained in the future or if the price of human albumin decreases, it would adversely affect our operating results.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
Consolidated Financial Statements
The full text of our audited consolidated financial statements as of December 31, 2010 and 2009 begins on page F-1 of this report.
Quarterly Financial Results
The following table sets forth certain unaudited financial information for each of the eight quarters ended December 31, 2010. The consolidated financial statements for each of these quarters have been prepared on the same basis as the audited consolidated financial statements included in this annual report and, in the opinion of management, include all adjustments necessary for the fair presentation of the results of operations for these periods. This information should be read together with our audited consolidated financial statements and the related notes included elsewhere in this annual report.
43
(All amounts in thousands of U.S. dollars)
| Dec 31, | Sep 30, | Jun 30, | Mar 31, | Dec 31, | Sep 30, | Jun 30, | Mar 31, | |||||||||||||||||
| 2010 | 2010 | 2010 | 2010 | 2009 | 2009 | 2009 | 2009 | |||||||||||||||||
| Sales | $ | 35,684 | $ | 36,004 | $ | 40,908 | $ | 27,099 | $ | 37,627 | $ | 27,040 | $ | 33,182 | $ | 21,149 | ||||||||
| Gross profit | 24,860 | 25,735 | 31,849 | 20,300 | 27,343 | 20,079 | 24,020 | 14,934 | ||||||||||||||||
| Earnings before income tax expenses | 768 | 22,290 | 24,656 | 17,887 | 4,805 | (382 | ) | 15,142 | 9,272 | |||||||||||||||
| Net income attributable to China Biologic Products, Inc. | (5,992 | ) | 13,801 | 13,003 | 10,731 | (3,713 | ) | (6,271 | ) | 7,883 | 4,309 | |||||||||||||
| Basic earnings per share | (0.25 | ) | 0.59 | 0.55 | 0.46 | (0.17 | ) | (0.29 | ) | 0.37 | 0.20 | |||||||||||||
| Diluted earnings per share | (0.19 | ) | 0.54 | 0.50 | 0.41 | (0.16 | ) | (0.29 | ) | 0.36 | 0.20 |
Earnings per share are computed independently for each of the quarters presented. Therefore, the sum of the quarterly net earnings per share will not necessarily equal the total for the year.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a] 15(e) and 15d] 15(e) under the Securities Exchange Act of 1934 (the Exchange Act)). Disclosure controls and procedures are controls and procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is accumulated and communicated to management, including our principal executive officer and our principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. Based on our assessment, Mr. Zhao and Mr. Kuo determined that, as of December 31, 2010, and as of the date that the evaluation of the effectiveness of our disclosure controls and procedures was completed, because of the material weaknesses in our internal control over financial reporting described below, our disclosure controls and procedures were not effective to satisfy the objectives for which they are intended.
Notwithstanding management’s assessment that our internal control over financial reporting was ineffective as of December 31, 2010 due to the material weakness described below under Management’s Report on Internal Control Over Financial Reporting, we believe that the consolidated financial statements included in this Annual Report on Form 10-K correctly present our financial condition, results of operations and cash flows for the fiscal years covered thereby in all material respects.
Internal Control over Financial Reporting
(a) Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a] 15(f) and 15d] 15(f) under the Securities Exchange Act. The Company’s internal control over financial reporting is a process that is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States and includes those policies and procedures that:
(1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets;
(2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States and that our receipts and expenditures are being made only in accordance with the authorization of our management and directors; and
(3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as based on framework established in Internal Control Integrated Framework issued by the committee of Sponsoring Organizations of the Treadway Commission (COSO) as of December 31, 2010. Based on such evaluation, our management, including the CEO and CFO, has concluded that the Company’s internal control over financial reporting (as defined in Rules 13a] 15(f) and 15d] 15(f) of the Securities Exchange Act of 1934, as amended) as of December 31, 2010 is ineffective.
This assessment identified ineffective review controls on the recognition of deferred tax liabilities and derivative instrument valuation, because of lack of resources with expertise in non] recurring transactions, which resulted in inadvertently omission of the fair value of embedded option in warrants and misinterpretation of US GAAP regarding the recognition of deferred tax liabilities upon business combination. The Company has already taken measures to remediate these material weaknesses by adding two additional qualified accountants in late 2010 and enhancing the supervision, monitoring and reviewing of financial statement preparation processes. Furthermore, the Company has already engaged outside consultants specializing in deferred tax accounting and derivative instrument valuation, as well as in reinforcing the rigorous process for collecting and reviewing information required for the preparation of the financial statements including footnotes.
The Company’s internal control over financial reporting as of December 31, 2010 has been audited by our registered public accounting firm as stated in their report appearing herein under Item 9(b) of this Annual Report on Form 10] K . Our independent registered public accounting firm has issued an adverse opinion on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2010.
44
(b) Attestation Report of the Registered Public Accounting Firm
The Board of Directors and Stockholders
China Biologic Products, Inc.:
We have audited China Biologic Products, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). China Biologic Products, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. Material weaknesses related to review controls on the determination of fair values of derivative instruments and the recognition of deferred tax liabilities upon business combination have been identified and included in management’s assessment. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of China Biologic Products, Inc. and subsidiaries as of December 31, 2010, and the related consolidated statements of income, stockholders’ equity and comprehensive income, and cash flows for the year then ended. These two material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of those consolidated financial statements, and this report does not affect our report dated March 31, 2011, which expressed an unqualified opinion on those consolidated financial statements.
In our opinion, because of the effects of the aforementioned material weaknesses on the achievement of the objectives of the control criteria, China Biologic Products, Inc. has not maintained effective internal control over financial reporting as of December 31, 2010, based on criteria established in
Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.We do not express an opinion or any other form of assurance on management’s statements referring to corrective actions taken after December 31, 2010, relative to the aforementioned material weaknesses in internal control over financial reporting.
KPMG
Hong Kong, China
March 31, 2011
Changes in Internal Controls over Financial Reporting
Except for the addition of qualified accountants and consultants and our enhancement of internal control procedures, during the year ended December 31, 2010, there was no major change in our internal control over financial reporting (as such term is defined in Rule 13a-15(f) under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
We have no information to disclose that was required to be disclosed in a report on Form 8-K during fourth quarter of fiscal year 2010, but was not reported.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors and Executive Officers
The following sets forth information about our directors and executive officers:
| NAME | AGE | POSITION | ||||
| Siu Ling Chan | 48 | Chairwoman of the Board | ||||
| Chao Ming Zhao | 39 | Chief Executive Officer | ||||
| Stanley Lau | 56 | President | ||||
| Yu-Yun Tristan Kuo | 56 | Chief Financial Officer | ||||
| Sean Shao | 53 | Director | ||||
| Xiangmin Cui | 42 | Director | ||||
| Tong Jun Lin | 49 | Director | ||||
| Chong Yang Li | 37 | Director | ||||
| Bing Li | 42 | Director | ||||
| Wenfang Liu | 72 | Director |
Siu Ling Chan. Ms. Chan has been a member of our board of directors since July 19, 2006. She has been our chairwoman since January 1, 2007 and served as our CEO from January 2007 to March 2007. Ms. Chan is also currently a director of our subsidiary Logic Express. She was also appointed as the director of Shandong Taibang in April 2006. Prior to joining us, Ms. Chan worked from 1991 to 2005, as an administrator at the Fujian Academy of Social Sciences, and from 1989 to 1991 as a statistician at the Fujian Pingtan Economy Committee. She received her diploma in Statistics from Xiamen University in 1989 and a diploma in management from the Fujian Party Committee School in 2004.
Chao Ming Zhao. Mr. Zhao has been our Chief Executive Officer since June 1, 2008. Mr. Zhao was our Chief Financial Officer from November 2006 to until his appointment as our Chief Executive Officer, and has been the Chief Financial Officer of our operating subsidiary, Shandong Taibang since September 2003. From February 2002 to June 2003, Mr. Zhao was the financial manager at EF English First (Fuzhou) School, where he was responsible for managing the school's accounting and its internal control. He was a manager and auditor at Fujian (CFC) Group from July 1996 to January 2002, and was in charge of internal audit. Mr. Zhao is a certified accountant in the PRC and is an international registered internal auditor. Mr. Zhao obtained his Bachelor's degree in Investment Economy Management from Fuzhou University in 1996 and received his MBA from the Chinese University of Hong Kong in 2006.
45
Stanley Lau. Mr. Lau has been our President since December 1, 2010. Mr. Lau offers over 30 years of experience in the healthcare, medical device and pharmaceutical arena, in both international and domestic markets. He brings expertise in profit and loss management, operations, sales and marketing, product development, start-ups, turnaround initiatives, profitable growth and change management. Prior to joining us, Mr. Lau served from June, 2002 to March 2009 in various executive positions with Baxter Healthcare International, an international pharmaceutical company, including as General Manager of Baxter Healthcare Ltd. (Hong Kong), Baxter’s wholly-owned subsidiary, where he oversaw four joint ventures and a 500% increase in revenue in less than four years. Mr. Lau also served in various capacities with Pfizer, Inc. and with Pharmacia, Merck & Co., where he established its first wholly-owned subsidiary in Taiwan. Mr. Lau holds a Bachelor of Pharmacy degree from the University of London.
Y. Tristan Kuo. Mr. Kuo has been our Chief Financial Officer since June 1, 2008 and has served as the Vice President-Finance of the Company since September 2007. Mr. Kuo has more than 28 years of experience in accounting, financing and information system for companies in the manufacturing, commodity trading and banking industries and has served in the capacity of CFO, CIO and Controller. Of these years, Mr. Kuo has worked in the United States for 24 years and in Asia for 4 years. Prior to joining our company, Mr. Kuo worked for the Noble Group in Hong Kong as the Senior Business Analysis Manager from February through August 2007. Prior to that, Mr. Kuo served as the CFO of Cuisine Solution, Inc., a publicly traded company in Alexandria, Virginia, from December 2002 to January 2007. Mr. Kuo also served as the Vice President of Information System for Zinc Corporation of America in Monaca, Pennsylvania from 2001 and 2002 and as Chief Information Officer and Controller of Wise Metals Group in Baltimore, Maryland, the largest independent aluminum sheet producer in the U.S., from 1991 to 2001. Mr. Kuo obtained his Master's degree in Accounting from the Ohio State University and Bachelors degree in Economics from Soochow University in Taipei.
Sean Shao. Mr. Shao has been a member of our board of directors since July 24, 2008. Mr. Sean Shao currently also serves as (i) independent director and chairman of the audit committee of: American Dairy, Inc., a Chinese dairy products company listed on NYSE, Renhuang Pharmaceuticals, Inc., a Chinese pharmaceutical company listed on AMEX, China Recycling Energy Corporation, an energy recycling system design company listed on NASDAQ and Yongye International, Inc., a Chinese agricultural company listed on NASDAQ; (ii) independent director of AsiaInfo-Linkage, Inc., a Chinese telecom software solutions provider listed on NASDAQ and China Medicine Corporation, a distributor and developer of medicines listed on bulletin board; (iii) independent director and chairman of the nominating committee of Agria Corporation, a Chinese agricultural company listed on NYSE; and (iv) independent director and chairman of the audit committee and compensation committee of China Nuokang Bio-Pharmaceutical, Inc., a biopharmaceutical company listed on NASDAQ. He served as the chief financial officer of Trina Solar Limited from 2006 to 2008. In addition, Mr. Shao served from 2004 to 2006 as the chief financial officer of ChinaEdu Corporation, an educational service provider, and of Watchdata Technologies Ltd., a Chinese security software company. Prior to that, Mr. Shao worked at Deloitte Touche Tohmatsu CPA Ltd. for approximately a decade. Mr. Shao received his master’s degree in health care administration from the University of California at Los Angeles in 1988 and his bachelor’s degree in art from East China Normal University in 1982. Mr. Shao is a member of the American Institute of Certified Public Accountants.
Dr. Xiangmin Cui. Dr. Cui has been a member of our board of directors since February 2010. Dr. Cui is a Principal at Bay City Capital LLC, a venture capital firm managing approximately $1.6 billion of capital invested across various healthcare sectors. Prior to joining it in 2006, Dr. Cui was Director of Strategic Investment Planning for Southern Research Institute, an organization that discovered and developed six anti-cancer drugs that have been approved by the U.S. Food and Drug Administration. Prior to that, Dr. Cui co-founded Pan Pacific Pharmaceuticals, a U.S. biotech company, and Hucon Biopharmaceuticals, a PRC pharmaceutical company. He served as the Chief Scientific Officer and Executive Vice President of Pan Pacific Pharmaceuticals from 1998 to 2002 and Chief Executive Officer and President of Hucon Biopharmaceuticals 2003 to 2005, respectively. In these positions, he led the efforts to evaluate and acquire several key technologies in the fields of oncology, infectious and inflammatory diseases. Dr. Cui was also a co-founder of CNetwork, a San Francisco based non-profit organization dedicated to serving Chinese communities in North America. He received his Ph.D. in Cancer Biology from Stanford University, and his B.S. in Molecular Biology from Peking University.
Dr. Tong Jun Lin. Dr. Lin has been a member of our board of directors since July 24, 2008. He is a Professor in the Departments of Microbiology and Immunology and Pediatrics, Dalhousie University and has focused his research in immune response to microbial pathogens. Dr. Lin received his PhD degrees (1990) from the Chinese Academy of Medical Sciences, and his post-doctoral training at the University of Alberta (1993-1997), Duke University (1997-1998) and Dalhousie University (1998-2000). He has published extensively in leading scientific journals and has served on the Editorial Advisory Board of the journal of Inflammation and Allergy –Drug Targets. He has received continuous funding from Canadian Institutes of Health Research and other national granting agencies. Dr. Lin is a Scholar of Canadian Institutes of Health Research, a recipient of the Award of Excellence in Medical Research from Dalhousie University (2004), and a recipient of an Investigator Award from Canadian Society for Immunology (2007).
Chong Yang Li. Mr Li has been a member of our board of directors since December 17, 2010. Mr. Li has been a certified appraiser since 1998 and has over 15 years’ experience in providing asset valuation services, in connection with IPOs and reorganizations, primarily to PRC listed companies across varied industries. Since 2006, Mr. Li has served in various managerial positions for Fujian Zhongxing Assets Real Estate and Land Appraisal Co., Ltd., an assets and real estate appraisal company where he currently serves as Vice Chairman. Prior to that time, Mr. Li served from 2000 to 2005 in various managerial positions, including as Executive Assistant to the General Manager, for the Fujian Assets Appraisal Center, an assets valuation and appraisal company. Mr. Li holds a Diploma in Accounting from the China Farmer University.
46
Bing Li. Dr. Bing Li was appointed as a director on February 27, 2011, and has served as an advisor of Warburg Pincus Asia LLC since June 2010, in which capacity Dr. Li advises on potential investment evaluation and portfolio management in the healthcare space. Prior to joining Warburg Pincus Asia LLC, Dr. Li served from November 2007 to June 2010 as the General Manager of Enterprise Business and Business Development at GlaxoSmithKline China/Hong Kong, and from August 2006 to October 2007, as the Commercial Development Director of GlaxoSmithKline China/Hong Kong. Prior to that, Dr. Li served from April 1999 to August 2006 in various positions with Eli Lilly and Company in the United States, including Manager of China/India strategy, Manager of Global New Product Planning for Drug Delivery System, and Consultant to Biotechnology Strategy Group. While at GlaxoSmithKline, Dr. Li led efforts in creating the brand generics business and other new commercial models. Dr. Li also served as a Director of Shenzhen GSK NB, a joint venture for vaccine development and production between GlaxoSmithKline and Neptunus Group. Dr. Li holds a Master of Business Administration and Master of Engineering Management from the Kellogg Graduate School of Management, a Ph.D. in Cell and Molecular Biology from the University of Rochester, and a Bachelor of Science in Biophysics from Fudan University.
Wenfang Liu. Prof. Wenfang Liu was appointed as a director on February 27, 2011, and has served since February 2007 as the Chief Consultant for Sichuan Yuanda Shuyang Pharmaceuticals. Prior to that, Prof. Liu served from 2000-2007, in various managerial positions including as Chief Engineer and Director of Hualan Biological Engineering, and as Director of Blood Separating, from 2005 to 2006, at Chengdu Jiaying Medical Product Co Ltd.. Prior to that, Prof. Liu served, from 1998 to 1999, as Chief Engineer of Guiyang Qianfeng Biological Products Co. Ltd., and from 1988 to 1998 as Vice Chairman of the Institute of Blood Transfusion of Chinese Academy of Medical Sciences. Prof. Liu is currently a Member of the Sichuan CPPCC Standing Committee, and previously served as a member of the Chinese Society of Blood Transfusion and the China Medical Biotech Association. Prof. Liu holds a Bachelors Degree in Bio-Chemistry from the Chinese Academy of Sciences, Forest and Soil College and was a Ph.D advisor from 1997 to 1998.
There are no agreements or understandings for any of our executive officers or directors to resign at the request of another person and no officer or director is acting on behalf of nor will any of them act at the direction of any other person. To the best of our knowledge and belief, there are no arrangements or understandings with any of our principal stockholders, customers, suppliers, or any other person, pursuant to which any of our directors or executive officers were appointed.
Directors are elected until their successors are duly elected and qualified.
Director Qualifications
Our board of directors has identified particular qualifications, attributes, skills and experience that are important to be represented on the board as a whole, in light of our current needs and business priorities. The Company is a NASDAQ listed biopharmaceutical company that is principally engaged in the research, development and manufacturing of plasma-based pharmaceutical products in China. Therefore, the board believes that a diversity of professional experiences in the biopharmaceutical industry, specific knowledge of key geographic growth areas, and knowledge of U.S. capital markets and of U.S. accounting and financial reporting standards should be represented on the board. In addition, the market in which we compete is characterized by introductions of new products and changes in customer demands and our future success depends upon our ability to keep pace through strong research and development. Therefore, the board believes that academic and professional experience in research and development in the biopharmaceutical industry should also be represented on the board.
Set forth below is a summary of some of the specific qualifications, attributes, skills and experiences of our directors that led us to the conclusion that they should serve as members of our board of directors.
Siu Ling Chan
- co-founder of the Company and Chairwoman of the Company’s subsidiaries, Logic Express and Shandong Taibang, since its 2006
- served as a statistician and administrator at Fujian Academy of Social Sciences
- Ms. Chan’s long-term knowledge of the history and operations of the Company and background in administration has provided strategic guidance to the board and management over the years
Sean Shao
- as a U.S. certified accountant, with over 10 years experience as an auditor at Deloitte Touche Tohmatsu and Deloitte Touche Toronto, Mr. Shao led many independent audits of PRC-based companies
- served as CFO and assisted in the initial public offering and initial listing of companies on the NYSE and NASDAQ and led the implementation of related corporate governance requirements
47
- serves as an independent director of several NASDAQ-listed companies and one NYSE-listed company
- holds a master’s degree in health care administration from the University of California, Los Angeles
- Mr. Shao’s experience with U.S. public companies and his knowledge of the U.S. capital markets and of U.S. financial reporting requirements and U.S. GAAP is invaluable to the Company
Dr. Xiangmin Cui
- holds a Doctorate in Cancer Biology from Stanford University School of Medicine
- Principal of Bay City Capital, a healthcare venture capital fund, managing $1.6 billion in capital invested in over 85 companies
- led or actively participated in several investments, including GenturaDx, Ion Torrent Systems, Epizyme, Sunesis Pharmaceuticals, and Presidio Pharmaceuticals
- serves as a Director of Strategic Investment Planning at Southern Research Institute, a premier institution known for the discovery and development of six anti-cancer drugs; and as a director of Progentech and a board observer of Ion Torrent Systems
- co-founder and former executive of Hucon Biopharmaceuticals, Pan Pacific Pharmaceuticals, and CNetwork, a non-profit organization of over 5000 Chinese professionals in Silicon Valley
- Dr. Cui’s knowledge of the U.S. capital markets and of the healthcare industry in which the Company operates provides invaluable guidance and perspective to the board
Dr. Tong Jun Lin
- served as a Professor in the Dalhousie University’s Departments of Microbiology and Immunology, and Pediatrics since July 2000
- engaged in significant research and collaborative research with biotech companies in the field of immunology and the recipient of multiple grants as a principal investigator from competitive national funding agencies and currently focuses his research in the fields of innate and adaptive immunity, immune response to pathogens and allergens, vaccine and drug development
- serves as editor or reviewer for many academic journals, such as the Journal of Immunology, and national granting agencies, such as the Canadian Institutes of Health Research, and has published many high-impact research papers in the field of immunology and cell and molecular biology
- is a recipient of many academic accolades including an Award of Excellence in Medical Research from Dalhousie University and the recipient of the Canadian Society of Immunology’s prestigious annual Investigator Award for excellence in early stage of research career
- Dr. Lin’s academic excellence and his cutting edge industry research provides invaluable guidance and perspective to the board, especially in relation to the Company’s research and development efforts
Chong Yang Li
- has been a certified appraiser since 2006
- has over 15 years of experience in providing asset valuation services, in connection with IPOs and reorganizations, primarily to PRC listed companies across varied industries
- Mr. Li’s rich experiences in the valuation of listed companies, and his background and knowledge in accounting is beneficial to the Company
Dr. Bing Li
- serves as an advisor of Warburg Pincus Asia LLC on potential investment evaluation and portfolio management in healthcare space since June 2010
- served as General Manager of Enterprise Business and Business Development and Commercial Development Director of GlaxoSmithKline China/Hong Kong between 2006 to 2010
- served in various positions with Eli Lilly and Company in the United States, including Manager of China/India strategy, Manager of Global New Product Planning for Drug Delivery System, and Consultant to Biotechnology Strategy Group
- holds a Master of Business Administration and Master of Engineering Management from the Kellogg Graduate School of Management, a Ph.D. in Cell and Molecular Biology from the University of Rochester, and a Bachelor of Science in Biophysics from Fudan University
Prof. Wenfang Liu
- served as the Chief Consultant for Sichuan Yuanda Shuyang Pharmaceuticals, a plasma-based manufacture in China, from February 2007 to February 2011
48
- served in various managerial positions including as Chief Engineer and Director of Hualan Biological Engineering, a major player in PRC’s plasma-based manufacturer between 2000 to 2007
- served as Director of Blood Separating at Chengdu Jiaying Medical Product Co., Ltd. from 2005 to 2006
- served as Chief Engineer of Guiyang Qianfeng Biological Products Co., Ltd. between 1998 to 1999
- served as Vice Chairman of Institute of Blood Transfusion of Chinese Academy of Medical Sciences
- serves as a member of the Sichuan CPPC Standing Committee and previously served as a member of the Chinese Society of Blood Transfusion and the China Medical Biotech Association
- holds a Bachelors degree in Bio-Chemistry from the Chinese Academy of Sciences, Forest and Soil College and has been a Ph.D. advisor from 1007 to 1998
Significant Employees
In addition to the foregoing named officers and directors, the following employees are also key to our business and operations:
| NAME | AGE | POSITION | ||||
| Tung Lam | 51 | Chief Executive Officer of Shandong Taibang | ||||
| Yiwu Vincent Xie | 39 | Chief Technology Officer |
Tung Lam. Mr. Lam has been the Chief Executive Officer of our operating subsidiary, Shandong Taibang, since October 2003, and is responsible for the entire operation. He is the husband of Ms. Siu Ling Chan, the Chairwoman of our Board.
Dr. Yiwu Xie. Dr Xie has been our Chief Technology Officer since December 2009. He served from 2007 to 2009 as the general manager of R&D at New a-Ikor, a Hong Kong-based biopharmaceutical company, and from 2002 to 2007, as the director of R&D at Advantek Serum Laboratories.
Family Relationships
Ms. Siu Ling Chan is the wife of Mr. Tung Lam. There are no other family relationships among any of our officers and directors.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past ten years:
-
been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offences);
-
had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time;
-
been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity;
-
been found by a court of competent jurisdiction in a civil action or by the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated;
-
been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
-
been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
49
Except as set forth in our discussion below in Item 13, “Certain Relationships and Related Transactions, and Director Independence –Transactions with Related Persons,” none of our directors, director nominees or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Section 16(A) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our executive officers, directors and beneficial owner of more than 10% of a registered class of our equity securities to file with the SEC statements of ownership and changes in ownership. The same persons are required to furnish us with copies of all Section 16(a) forms they file. We believe that, during fiscal 2010, all of our executive officers, directors and beneficial owner of more than 10% of a registered class of our equity securities complied with the applicable filing requirements.
In making these statements, we have relied upon examination of the copies of all Section 16(a) forms provided to us and the written representations of our executive officers, directors and beneficial owner of more than 10% of a registered class of our equity securities.
Code of Ethics
On March 25, 2008, our board of directors adopted a code of ethics, which applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer, and principal accounting officer. The code of ethics is designed to deter wrongdoing and to promote: honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; full, fair, accurate, timely and understandable disclosure in reports and documents that we file with, or submit to, the SEC, and in other public communications that we made; compliance with applicable government laws, rules and regulations; the prompt internal reporting of violations of the code to the appropriate person or persons; and accountability for adherence to the code.
The code requires the highest standard of ethical conduct and fair dealing of its senior financial officers, defined as the Chief Executive Officer and Chief Financial Officer. While this policy is intended to only cover the actions of such officers, we expect our other officers, directors and employees will also review our code and abide by its provisions. We believe that our reputation is a valuable asset and must continually be guarded by all associated with us so as to cam the trust, confidence and respect of our suppliers, customers and stockholders.
Board Composition and Committees
Our Board is currently composed of five members, three of whom, Mr. Sean Shao, Dr. Xiangmin Cui, and Dr. Tong Jun Lin, are “independent” directors, within the meaning of the listing rules of The NASDAQ Stock Market, LLC, or the NASDAQ Listing Rules. All actions of the board of directors require the approval of a majority of the directors in attendance at a meeting at which a quorum is present.
Our board of directors currently has three standing committees: the Audit Committee, Compensation Committee and Governance and Nominating Committee, which, pursuant to delegated authority, perform various duties on behalf of and report to the board. Each of these Committees is comprised entirely of independent directors. From time to time, the board may establish other committees. Each of the Compensation Committee and Governance and Nominating Committee were formed on August 7, 2008 and the Audit Committee was formed on July 24, 2008. Copies of the charters for each of our standing committees may be obtained from our website at http://www.chinabiologic.com.
Audit Committee and Audit Committee Financial Expert
Our Audit Committee is currently composed of three members: Mr. Sean Shao, Dr. Xiangmin Cui, and Dr. Tong Jun Lin. Our board of directors determined that each member of the Audit Committee meets the independence criteria prescribed by applicable regulation and the rules of the SEC for audit committee membership and is an “independent” director within the meaning of the NASDAQ Listing Rules. Each Audit Committee member also meets NASDAQ’s financial literacy requirements. Mr. Shao serves as Chair of the Audit Committee.
Our Audit Committee oversees our accounting and financial reporting processes and the audits of our financial statements. Our Audit Committee is responsible for, among other things:
-
selecting our independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by our independent auditors;
-
reviewing with our independent auditors any audit problems or difficulties and management’s response;
-
reviewing and approving all proposed related-party transactions;
-
discussing the annual audited financial statements with management and our independent auditors;
50
-
reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of significant internal control deficiencies;
-
annually reviewing and reassessing the adequacy of our audit committee charter;
-
such other matters that are specifically delegated to our Audit Committee by our board of directors from time to time;
-
meeting separately and periodically with management and our internal and independent auditors; and
-
reporting regularly to the full board of directors.
Our board of directors has determined that Mr. Shao (i) is the “audit committee financial expert” as such term is defined in Item 407(d) of Regulation S-K promulgated by the SEC, and (ii) also meets NASDAQ’s financial sophistication requirements.
Compensation Committee
Our Compensation Committee is currently composed of three members: Mr. Sean Shao, Dr. Xiangmin Cui, and Dr. Tong Jun Lin, each of whom is “independent” within the meaning of the NASDAQ Listing Rules. Dr. Cui serves as Chair of the Compensation Committee.
Our Compensation Committee assists the board in reviewing and approving the compensation structure of our directors and executive officers, including all forms of compensation to be provided to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated.
The Compensation Committee is responsible for, among other things:
-
approving and overseeing the compensation package for our executive officers;
-
reviewing and making recommendations to the board with respect to the compensation of our directors;
-
reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer, evaluating the performance of our chief executive officer in light of those goals and objectives, and setting the compensation level of our chief executive officer based on this evaluation; and
-
reviewing periodically and making recommendations to the board regarding any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans.
Governance and Nominating Committee
Our Governance and Nominating Committee is currently composed of three members: Mr. Sean Shao, Dr. Xiangmin Cui, and Dr. Tong Jun Lin, each of whom is “independent” within the meaning of the NASDAQ Listing Rules. Dr. Lin serves as Chair of the Governance and Nominating Committee.
The Governance and Nominating Committee assists the board in identifying individuals qualified to become our directors and in determining the composition of the board and its committees.
The Governance and Nominating Committee is responsible for, among other things:
-
identifying and recommending to the board nominees for election or re-election to the board, or for appointment to fill any vacancy;
-
reviewing annually with the board the current composition of the board in light of the characteristics of independence, age, skills, experience and availability of service to us;
-
identifying and recommending to the board the directors to serve as members of the board's committees; and
-
monitoring compliance with our code of business conduct and ethics.
Material Changes to Director Nomination Procedures
There have been no material changes to the procedures by which shareholders may recommend nominees to our Board of Directors since such procedures were last disclosed.
51
ITEM 11. EXECUTIVE COMPENSATION.
Compensation Discussion and Analysis
Compensation Philosophy
Our executive compensation philosophy is to align the interests of executive management with stockholder interests and with our business strategy and success through an integrated executive compensation program that considers short-term performance, the achievement of long-term strategic goals and growth in total stockholder value. The key elements of our executive compensation philosophy are competitive base salary, annual incentive opportunities and equity participation. Our aggregate compensation package is designed to attract and retain individuals critical to the long-term success of the Company, to motivate these persons to perform at their highest levels, and to reward exceptional performance.
The compensation of our executive officers is determined by the Compensation Committee, except that the entire Board makes the final determination as to the compensation of our Chief Executive Officer. The Chief Executive Officer reviews and revises compensation proposals prepared by Human Resources and presents his recommendations to the Compensation Committee for the Committee’s ultimate review and approval. The Chief Executive Officer does not participate in Compensation Committee meetings and is not involved in decisions relating to his own compensation.
Elements of Compensation
We pay each of our named executive officers a base annual salary. Bonuses and equity incentives may also be provided to supplement base salary. The criteria for determining each of these components of compensation is described below. We do not provide retirement benefits and only minimal perquisites except as described below.
Base Salary
Base salary levels for executive officers are set forth in their individual employment agreements, and are reflected in the Summary Compensation Table below. The Compensation Committee considered the total compensation paid by other biopharmaceutical companies in Shandong, China to persons holding equivalent positions in setting base salary levels. However, the Compensation Committee did not conduct a peer group compensation analysis or target any particular compensation level in establishing the base salaries for named executive officers.
The Compensation Committee believes that any increases in base salary should be based upon a favorable evaluation of individual performance relative to individual goals, the functioning of the executive’s team within the corporate structure, success in furthering corporate strategy and goals, individual management skills and responsibilities, demonstrated loyalty, and the Company’s commitment to attract and retain executives. We expect that our Compensation Committee will reward superior individual and company performance with commensurate cash and other compensation. The Compensation Committee did not increase base salary during fiscal year 2010. Salary will next be reviewed when the Compensation Committee deems appropriate, but the Compensation Committee will not review salary more frequently than on an annual basis.
Bonuses
Executives are eligible to receive a discretionary bonus pursuant to the terms of their respective employment agreements. However, in fiscal 2010 we did not set any performance targets and no discretionary bonuses were paid. If our Compensation Committee determines to do so in the future, bonuses may be paid on an ad hoc basis to recognize superior performance. If the Compensation Committee determines to provide bonus compensation as a regular part of our executive compensation package, it will establish performance goals for each of the executive officers and maximum bonuses that may be earned upon attainment of such performance goals. Some of the components of our key performance appraisal index that we may use to consider in establishing these performance goals include the following: cash flow (accounts receivable, inventory, accounts payable); sales (revenue, accounts receivables collection, gross profit, customer credit management and goods delivery management); costs and expenses (financial costs, manufacturing costs, general and administrative expenses, sales and marketing expenses, research and development costs); and capital expenditures.
Equity Incentives
Named executive officers are eligible for equity awards in the form of stock options, stock appreciation rights, performance units, restricted stock, restricted stock units and performance shares under the China Biologic Products, Inc. 2008 Equity Incentive Plan, or the 2008 Plan. Equity awards are granted at the discretion of the Compensation Committee. The size of an award to any individual, including named executive officers, depends in part on individual performance, including the components of our key performance appraisal index described above and any other indicators of the impact that such employee’s productivity may have on stockholder value over time. Other factors include salary level and competitive data. In addition, in determining the awards granted to each named executive officer, the Compensation Committee considers the future benefits potentially available to the named executive officers from existing awards.
52
We have no program, plan or practice of granting equity awards that coincide with the release by the Company of material non-public information.
Retirement Benefits
Currently, we do not provide any company-sponsored retirement benefits or deferred compensation programs to any employee, including the named executive officers (other than a mandatory state pension scheme in which all of our employees in China participate) because it is not customary to provide such benefits and programs in China.
Perquisites
Historically, we have provided our named executive officers with minimal perquisites and other personal benefits that we believe are reasonable, such as company car-related benefits. We do not view perquisites as a significant element of compensation, but do believe they can be useful in attracting, motivating and retaining the executive talent for which we compete.
Summary Compensation Table — Fiscal Years Ended December 31, 2010, 2009 and 2008
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named persons for services rendered in all capacities during the noted periods. No other executive officer received total annual salary and bonus compensation in excess of $100,000.
Name and Principal Position |
Year |
Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($) |
All Other Compensation ($) |
Total ($) |
| Chao Ming Zhao, Chief Executive Officer (1) |
2010 2009 2008 |
177,630 184,046 148,208 |
133,146 43,803 34,377 |
- - - |
428,968 - 154,954 |
- - - |
739,744 227,849 337,539 |
| Y. Tristan Kuo, Chief Financial Officer (2) |
2010 2009 2008 |
223,168 227,095 179,805 |
56,217 37,996 20,582 |
- - - |
919,157 - 101,055 |
13,268 9,229 - |
1,211,810 274,320 301,442 |
| Stanley Lau, President (3) | 2010 | 17,522 | - | - | - | - | 17,522 |
| (1) |
Chao Ming Zhao has served as our Chief Executive Officer since June 1, 2008 and has also served as the Chief Financial Officer of our subsidiary Shandong Taibang since September 2003. He served as our Chief Financial Officer from November 2006 until June 1, 2008. The option awards in 2008 represents options granted to Mr. Zhao, in accordance with his employment agreement with the Company, to purchase 115,000 shares in May, 2008. The option awards in 2010 represents options granted to Mr. Zhao, along with the Company’s Board of Directors, other executives and certain other employees, to purchase 40,000 shares in July, 2010 as described in the section “Option” of footnote 15 in the accompanying financial statements. |
| (2) |
Y. Tristan Kuo has served as our Chief Financial Officer since June 1, 2008 and has served as the Vice President - Finance since September 2007. The option awards in 2008 represents options granted to Mr. Kuo, in accordance with his employment agreement with the Company, to purchase 75,000 shares in May, 2008 as described in the section “Option” of footnote 15 in the accompanying financial statements. On January 7, 2010, our board of directors granted Mr. Kuo options to purchase 50,000 shares of our common stock under the 2008 Plan, in accordance with his employment agreement with the Company. The option awards in 2010 represents options granted to Mr. Kuo, along with the Company’s Board of Directors, other executives and certain other employees, to purchase 35,000 shares in July, 2010 as described in the section “Option” of footnote 15 in the accompanying financial statements. |
| (3) |
Stanley Lau has served as our President since December 1, 2010. |
Summary of Employment Agreements and Material Terms
Pursuant to an employment agreement, as consideration for his services as our Chief Financial Officer and as a director, Chao Ming Zhao received a monthly salary of HK$50,000 (approximately $6,430), plus a guaranteed bonus of HK$50,000 (approximately $6,430), payable on December 31 of each year. On May 9, 2008, we entered into a new employment agreement with Mr. Zhao, pursuant to which we agreed to pay him an annual salary of RMB1,060,000 (approximately $160,802) per annum, as consideration for performance of his duties as Chief Executive Officer. We also agreed to pay Mr. Zhao an annual bonus equal to one month’s salary and Mr. Zhao may be eligible to receive additional bonus compensation as may be awarded by our board of directors at their sole discretion. We also agreed to grant to Mr. Zhao a ten-year nonstatutory stock option under the 2008 Plan, for the purchase of 115,000 shares of our common stock, at an exercise price of $4.00 per share. The stock option immediately vested.
53
Pursuant to the terms of Mr. Y. Tristan Kuo’s employment agreement, dated May 9, 2008, we agreed to pay Mr. Kuo an annual salary of RMB1,320,000 (approximately $200,244), as consideration for performance of his duties as Chief Financial Officer. We also agreed to pay Mr. Kuo an annual bonus equal to one month’s salary and Mr. Kuo may be eligible to receive additional bonus compensation as may be awarded by our board of directors at their sole discretion. We also agreed to grant to Mr. Kuo a ten-year nonstatutory stock option under the 2008 Plan, for the purchase of 75,000 shares of our common stock, at an exercise price of $4.00 per share. The stock option immediately vested. In addition, we were obligated to grant Mr. Kuo, within a month of our listing on NASDAQ, NYSE or AMEX, an option to purchase 50,000 shares of our common stock pursuant to the 2008 Plan immediately vesting and exercisable at the fair market value of the shares on the grant date. On January 7, 2010, our board of directors granted Mr. Kuo options to purchase 50,000 shares of our common stock under the 2008 Plan. The options have a ten-year term and are exercisable at an exercise price of $12.60, which was the fair market value of our common stock on the date of the grant.
Pursuant to the terms of Mr. Stanley Lau’s employment agreement, dated December 1, 2010, we agreed to pay Mr. Lau an annual salary of RMB 1,300,000 (approximately $197,210), as consideration for performance of his duties as President. We also agreed to grant to Mr. Lau a ten-year nonstatutory stock option under the 2008 Plan, for the purchase of 25,000 shares of our common stock, at an exercise price equal to the fair market value of the Company’s common stock on the date of the grant and will vest on a quarterly basis over twelve months, with the first portion vesting on May 1, 2011.of $4.00 per share. The Company is obligated to review Mr. Lau at the end of each fiscal year for the next five years, and to award him an annual discretionary bonus in the form of an additional option to purchase 50,000 shares of the Company's common stock under the Plan, which option will have an exercise price equal to the fair market value of the Company’s common stock on the date, and will vest quarterly in equal portions over 12 months, with the first portion vesting on March 31, 2011. The Company is also obligated to review Mr. Lau at the end of each fiscal year for the next five years, and to award him an annual discretionary bonus in the form of 10,000 restricted shares of the Company’s common stock under the Plan, which if granted, will vest in equal portions on a quarterly basis, with the first portion vesting on March 31st of the year immediately following the year that such bonus is granted.
Other than noted above and necessary social benefits required by the PRC government, which are defined in the employment agreements, we currently do not provide other benefits to our named executive officers at this time.
Grants of Plan-Based Awards
The following table sets forth information regarding equity grants to named executive officers during the fiscal year ended December 31, 2010.
Name |
Grant Date |
All other option awards: Number of securities underlying options (#) |
Exercise or base price of option awards ($/Sh) |
Grant date fair value of stock and option awards |
| Chao Ming Zhao | July 11, 2010 | 40,000 | $12.26 | 428,968 |
| Y. Tristan Kuo | July 11, 2010 | 35,000 | $12.26 | 375,347 |
Outstanding Equity Awards at Fiscal Year End
The following table sets forth the equity awards outstanding at December 31, 2010 for each of our named executive officers.
Name |
OPTION AWARDS | ||||
| Number of securities underlying unexercised options (#) exercisable |
Number of securities underlying unexercised options (#) unexercisable |
Equity incentive plan awards: number of securities underlying unexercised unearned options (#) |
Option exercise price ($) |
Option expiration date | |
| Chao Ming Zhao | 115,000 | - | - | 4.00 | June 1, 2018 |
| Y. Tristan Kuo | 75,000 | - | - | 4.00 | June 1, 2018 |
| Y. Tristan Kuo | 50,000 | - | - | 12.60 | January 1, 2020 |
| Chao Ming Zhao | 3,333 | 36,667 | - | 12.26 | July 11, 2020 |
| Y. Tristan Kuo | 2,917 | 32,083 | - | 12.26 | July 11, 2020 |
54
We use the Black-Scholes option pricing model to measure the fair value of stock options. The determination of the fair value of stock-based compensation awards on the date of grant using an option-pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables, including the expected volatility of our stock price over the term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rate and expected dividends.
Option Exercises and Stock Vested
No options to purchase our capital stock were exercised by any of our named executive officers, nor was any restricted stock held by such executive officers vested during the fiscal year ended December 31, 2010.
Pension Benefits
No named executive officers received or held pension benefits during the fiscal year ended December 31, 2010.
Nonqualified Deferred Compensation
No nonqualified deferred compensation was offered or issued to any named executive officer during the fiscal year ended December 31, 2010.
Potential Payments upon Termination or Change in Control
Our named executive officers are not entitled to severance payments upon the termination of their employment agreements or following a change in control.
Compensation of Directors
The following table sets forth the total compensation earned by our directors during fiscal year ended December 31, 2010:
Name |
Fees earned or paid in cash ($) |
Stock awards ($) |
Option awards ($) |
All other compensation ($) |
Total ($) |
| Siu Ling Chan(1) | 101,428 | - | - | 101,428 | |
| Sean Shao(2) | 24,000 | - | 428,968 | - | 452,968 |
| Tong Jun Lin(3) | 18,000 | - | 428,968 | 446,968 | |
| Xiangmin Cui(4) | 16,500 | - | 612,758 | 629,258 | |
| Lin Ling Li(5) | 98,312 | - | - | - | 98,312 |
| Chong Yang Li | 3,116 | - | - | - | 3,116 |
| (1) |
On July 19, 2006, we entered into a director employment agreement with Ms. Siu Ling Chan, pursuant to which she receives a monthly salary of HK$50,000 (approximately $6,430), plus a guaranteed bonus of HK$50,000 (approximately $6,430) payable on December 31 of each year, as consideration for her services as a director. |
| (2) |
On July 24, 2008, we entered into an independent director agreement with Mr. Sean Shao, pursuant to which we agreed to pay Mr. Shao an annual salary of $24,000 as compensation for the services to be provided by him as an independent director and head of our Audit Committee. In addition, we granted to Mr. Shao an option to purchase 20,000 shares of our common stock, with an exercise price of $4.00 per share. The option awards in 2010 represents options granted to Mr. Shao, along with the Company’s other directors, executives and certain other employees, to purchase 40,000 shares in July, 2010 as described in the section “Option” of footnote 15 in the accompanying financial statements. |
| (3) |
On July 24, 2008, we entered into an independent director agreement with Dr. Tong Jun Lin, pursuant to which we agreed to pay Dr. Lin an annual salary of $18,000 as compensation for the services to be provided by his as an independent director. In addition, we granted to Dr. Lin an option to purchase 20,000 shares of our common stock, with an exercise price of $4.00 per share. The option awards in 2010 represents options granted to Mr. Lin, along with the Company’s other directors, executives and certain other employees, to purchase 40,000 shares in July, 2010 as described in the section “Option” of footnote 15 in the accompanying financial statements. |
| (4) |
On February 4, 2010, we entered into an independent director agreement with Dr. Xiangmin Cui, pursuant to which we agreed to pay Dr. Cui an annual salary of $18,000 as compensation for the services to be provided by him as an independent director. In addition, we granted to Dr. Cui an option to purchase 20,000 shares of our common stock, with an exercise price of $10.66. 10,000 shares vested on August 4, 2010 and the remaining 10,000 shares vested on February 4, 2011. The option awards in 2010 represents options granted to Mr. Cui, along with the Company’s other directors, executives and certain other employees, to purchase 40,000 shares in July, 2010 as described in the section “Option” of footnote 15 in the accompanying financial statements. |
55
| (5) |
On July 19, 2006, we entered into a director employment agreement with Ms. Lin Ling Li, pursuant to which she received a monthly salary of HK$50,000 (approximately $6,430), plus a guaranteed bonus of HK$50,000 (approximately $6,430) payable on December 31 of each year, as consideration for her services as a director. Ms. Lin elected not to stand for re-election at our 2010 Annual Shareholders’ Meeting held on December 17, 2010 and was replaced by Mr. Chong Yang Li at such time. |
All directors receive reimbursements from us for expenses which are necessarily and reasonably incurred by them for providing services to us or in the performance of their duties. Our directors who are also our employees receive compensation in the form of salaries, housing allowances, employee insurance and benefits in kind. Our executive directors do not receive any compensation in addition to their salaries in their capacity as directors or other remunerations as members of our management team. However, we do pay their expenses related to attending board meetings and participating in board functions.
Compensation Committee Interlocks and Insider Participation
Mr. Sean Shao, Dr. Xiangmin Cui, and Dr. Tong Jun Lin served on the Compensation Committee during the fiscal year ended December 31, 2010. None of them was an employee, an officer, or former officer of the Company. No member of the Compensation Committee had any relationship with us requiring disclosure under Item 404 of SEC Regulation S-K during the fiscal year ended December 31, 2010. None of our executive officers has served on the board of directors or compensation committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served on our Board or Compensation Committee.
Compensation Committee Report
The Compensation Committee of the Company has reviewed and discussed the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K with management. Based on such review and discussions, the Compensation Committee recommended to the Board that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K.
/s/ Compensation Committee Dr. Xiangmin Cui, Chair Sean Shao Dr. Tong Jun Lin
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth information regarding beneficial ownership of our common stock as of March 14, 2011 (i) by each person who is known by us to beneficially own more than 5% of our common stock; (ii) by each of our officers and directors; and (iii) by all of our officers and directors as a group. Unless otherwise specified, the address of each of the persons set forth below is in care of the Company, No. 14 East Hushan Road, Tai'an City, Shandong 271000, People’s Republic of China.
Name and Address of Beneficial Owner |
Office, If Any |
Title of Class |
Amount and Nature of Beneficial Ownership(1) |
Percent of Class(2) |
| Officers and Directors | ||||
| Siu Ling Chan | Chairwoman of the Board | Common Stock | 5,515,957(3) | 22.5% |
| Chao Ming Zhao | Chief Executive Officer | Common Stock | 940,120(4) | 3.8% |
| Stanley Lau | President | Common Stock | - | - |
| Y. Tristan Kuo | Chief Financial Officer | Common Stock | 127,917(5) | 0.5% |
| Sean Shao | Director | Common Stock | 23,333(6) | 0.1% |
| Xiangmin Cui | Director | Common Stock | 23,333(6) | 0.1% |
| Tong Jun Lin | Director | Common Stock | 23,333(6) | 0.1% |
| Chong Yang Li | Director | Common Stock | - | |
| All officers and directors as a group
(8 persons named above) |
Common Stock |
6,653,993 |
27.2%
| |
56
| 5% Security Holders | ||||
| Siu Ling Chan | Chairwoman of the Board | Common Stock | 5,515,957(3) | 22.5% |
| Lin Ling Li | Common Stock | 4,592,624(7) | 19.0% | |
| IDG-Accel China Growth Fund II LP. | Common Stock | 1,700,132 | 7.0% | |
| Patrick J. McGoven | Common Stock | 1,839,174(8) | 7.6% | |
| Quan Zhou | Common Stock | 1,839,174(8) | 7.6% | |
| Essence International Investment LTD | Common Stock | 2,112,500 | 8.0% | |
| Lixin Tian | Common Stock | 2,112,500(9) | 8.0% | |
| Warburg Pincus Private Equity X, L.P. | Common Stock | 4,820,000(10) | 19.8% | |
| Charles R. Keye | Common Stock | 4,820,000(10) | 19.8% | |
| Joseph P. Landy | Common Stock | 4,820,000(10) | 19.8% | |
* Less than 1%
| (1) |
Beneficial Ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has direct ownership of and sole voting power and investment power with respect to our common stock. |
| (2) |
As of March 14, 2011, a total of 24,351,125 shares of our common stock are considered to be outstanding pursuant to SEC Rule 13d- 3(d)(1). For each Beneficial Owner above, any securities that are exercisable or convertible within 60 days have been included in the denominator. |
| (3) |
Includes 150,000 shares underlying a ten-year nonstatutory stock option granted under the 2008 Plan, exercisable at $4.00 per share, and 3,333 shares out of the 40,000 shares underlying a ten-year nonstatutory stock option granted under the 2008 Plan, exercisable at $12.26 per share, which will vest in equal portions on a quarterly basis over a three-year period, with the first portion vested and exercisable on October 11, 2010. |
| (4) |
Includes 115,000 shares underlying a ten-year nonstatutory stock option granted under the 2008 Plan, exercisable at $4.00 per share, and 3,333 shares out of the 40,000 shares underlying a ten-year nonstatutory stock option granted under the 2008 Plan, exercisable at $12.26 per share, which will vest in equal portions on a quarterly basis over a three-year period, with the first portion vested and exercisable on October 11, 2010. |
| (5) |
Includes 75,000 shares, exercisable at $4.00 per share, and 50,000 shares, exercisable at $12.60 per share, underlying a ten-year nonstatutory stock option granted under the 2008 Plan, and 2,917 shares out of the 35,000 shares underlying a ten-year nonstatutory stock option granted under the 2008 Plan, exercisable at $12.26 per share, which will vest in equal portions on a quarterly basis over a three-year period, with the first portion vested and exercisable on October 11, 2010. |
| (6) |
Represents shares underlying an option to purchase 20,000 shares of our common stock, with an exercise price of $4.00 per share, and 3,333 shares out of the 40,000 shares underlying a ten-year nonstatutory stock option granted under the 2008 Plan, exercisable at $12.26 per share, which will vest in equal portions on a quarterly basis over a three-year period, with the first portion vested and exercisable on October 11, 2010. |
| (7) |
Represents shares underlying an option to purchase 50,000 shares of our common stock, with an exercise price of $4.00 per share underlying a ten-year nonstatutory stock option granted under the 2008 Plan. |
| (8) |
Represents 1,700,132 shares held by IDG-Accel China Growth Fund II LP., or IDG Fund, and 139,042 shares held by IDG-Accel China Investors II L.P., or IDG Investors. Patrick J. McGoven and Quan Zhou are directors and executive officers of IDG-Accel China Growth Fund GP II Associates Ltd., which is the ultimate general partner of IDG Fund and IDG Investors. Patrick J. McGoven and Quan Zhou may be deemed to have shared voting and dispositive power with respect to the securities of the Company held by IDG Fund and IDG Investors. Each of Patrick J. McGoven and Quan Zhou disclaims beneficial ownership of the securities of the Company held by IDG Fund and IDG Investors. |
| (9) |
Represents (i) 1,875,000 shares issuable upon conversion of 3.8% convertible notes issued in our 2009 financing and (ii) 937,500 shares issuable upon the exercise of three-year warrants to purchase common stock at an exercise price of $4.80 per share held by Essence International Investment LTD. The general partner of Essence International Investment LTD is DT Capital Management Limited, which is controlled by Lixin Tian. |
| (10) |
Represents 4,670,580 shares held by Warburg Pincus Private Equity X, L.P., or WP X, and 149,420 shares held by Warburg Pincus X Partners, L.P., or WPP X. Messrs. Charles R. Kaye and Joseph P. Landy, each a Managing General Partner of Warburg Pincus & Co. and Co-President and Managing Member of Warburg Pincus LLC. Messrs Charles R. Kaye and Joseph P. Landy may be deemed to have shared voting and dispositive power with respect to the securities of the Company held by WP X and WPP X. |
Changes in Control
We are aware of no arrangements which if consummated may result in a change of control of our Company
Securities Authorized for Issuance under Equity Compensation Plans
The following table includes the information as of December 31, 2010 for each category of our equity compensation plan:
57
Plan category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) |
Weighted-average exercise price of outstanding options, warrants and rights (b) |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) |
| Equity compensation plans approved by security holders | - | - | - |
| Equity compensation plans not approved by security holders(1) | 1,975,350 | $8.21 | 2,961,500 |
| Total | 1,975,350 | $8.21 | 2,961,500 |
| (1) |
Effective May 9, 2008, our board of directors adopted the 2008 Plan. The 2008 Plan provides for grants of stock options, stock appreciation rights, performance units, restricted stock, restricted stock units and performance shares. A total of five million (5,000,000) shares of our common stock may be issued pursuant to the 2008 Plan. The exercise price per share for the shares to be issued pursuant to an exercise of a stock option will be no less than the fair market value per share on the grant date, except that, in the case of an incentive stock option granted to a person who holds more than 10% of the total combined voting power of all classes of our stock or any of our subsidiaries, the exercise price will be no less than 110% of the fair market value per share on the grant date. No more than an aggregate of 500,000 shares (or for awards denominated in cash, the fair market value of 5,000,000 shares on the grant date) may be subject to awards under the 2008 Plan to any individual participant in any one fiscal year. No awards may be granted under the 2008 Plan after May 9, 2018, except that any award granted before then may extend beyond that date. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Transactions with Related Persons
The following includes a summary of transactions since the beginning of the 2009 fiscal year, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds $120,000 and in which any related person had or will have a direct or indirect material interest (other than compensation described under Item 11, “Executive Compensation”). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
-
Guizhou Taibang provides processing services for Guizhou Eakan Co., Ltd. (“Guizhou Eakan”), the affiliate of one of the Guizhou Taibang’s noncontrolling interests holders. The Company’s total processing services income from Guizhou Eakan amounted to $499,128 and $705,018 for the years ended December 31, 2010 and 2009, respectively. In addition, Guizhou Taibang made sales to Guizhou Eakan, amounting to $521,306, for the year ended December 31, 2010. As of December 31, 2010 and 2009, accounts receivable due from Guizhou Eakan amounted to $212,611 and $222,617, respectively. The outstanding balance as of December 31, 2010 was settled in cash in January 2011.
-
On April 6, 2009, Logic Express entered into an agreement with Shandong Institute, the noncontrolling interest holders in Shandong Taibang, pursuant to which, Shandong Institute would provide an advance to assist Logic Express’s purchase of 90% Dalin's equity interests. Under the terms of the agreement, Shandong Institute agreed to provide advance of approximately $3,652,500 then (RMB25,000,000), representing 12.86% of the Company’s purchase consideration in Dalin to the Company for one year, bearing interest equal to the higher of a proportionate share of the net income of Dalin during the year ended December 31, 2009 or 6% per annum. On April 12, 2010, the Company fully paid the advance from Shandong Institute and the interest of approximately $1.3 million, which was less than the Company’s previous estimate by approximately $0.9 million. The Company recorded the difference between the previous estimate and actual payment in other income of the consolidated statement of income for the year ended December 31, 2010.
-
Guizhou Taibang has payables to Guizhou Eakan Investing Corp., amounting to approximately $2,195,123 (RMB14,470,160). Guizhou Eakan Investing Corp. is one of the noncontrolling interest holders of the Guizhou Taibang. The Company borrowed this interest free advance for working capital purpose for Guizhou Taibang. The balance is due on demand.
-
Guizhou Taibang has payables to Guizhou Jie’an, a noncontrolling interest holder of Guizhou Taibang, amounting to approximately $997,017 (RMB 6,569,840). In 2007, Guizhou Taibang received additional contributions from Guizhou Jie’an of approximately $962,853 then (RMB 6,569,840) to maintain Jie’an equity interest in Guizhou Taibang at 9%. However, due to a legal dispute among shareholders over raising additional capital as discussed in the legal proceeding section (see note 20), the money may be returned to Jie’an. During the second quarter of 2010, Jie’an requested that Guizhou Taibang register its 1.8 million shares of additional capital infusion with the local Administration for Industry and Commerce, pursuant to the equity purchase agreement, and such registration was approved by the majority shareholders of Guizhou Taibang in a shareholders meeting held in the second quarter of 2010. However, the Board of Directors of the Company is withholding its required ratification of the shareholders’ approval of Jie’an’s request until the outcome of the ongoing litigations. If the Company decided to ratify the approval, Dalin’s ownership in Guizhou Taibang will be diluted from 54% to 52.54% and Guizhou Jie’an will be entitled to receive its pro rata share of Guizhou Taibang’s profits from the prior 2 years.
58
Except as set forth in our discussion above, none of our directors, director nominees or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Policies and Procedures for Review, Approval or Ratification of Transactions with Related Persons
On July 27, 2009, our board of directors adopted the China Biologic Products, Inc. Related Party Transactions Policy and Procedures, or the Policy. Under the Policy, all Interested Transactions with Related Parties are subject to approval or ratification in accordance with the procedures set forth below. There were no related party transactions since the beginning of the fiscal year ended December 31, 2010 for which our Policy did not require review, approval or ratification, or where our Policy was not followed.
The Policy defines an “Interested Transaction” is any transaction, arrangement or relationship or series of similar transactions, arrangements or relationships (including any indebtedness or guarantee of indebtedness) in which (1) the aggregate amount involved will or may be expected to exceed $100,000 in any calendar year, (2) the Company is a participant, and (3) any Related Party has or will have a direct or indirect interest (other than solely as a result of being a director or a less than 10 percent beneficial owner of another entity). A “Related Party” is defined as any (a) person who is or was (since the beginning of the last fiscal year for which the Company has filed a Form 10-K and proxy statement, even if they do not presently serve in that role) an executive officer, director or nominee for election as a director, (b) greater than 5 percent beneficial owner of our common stock, or (c) immediate family member of any of the foregoing.
Procedures
Under the Policy, the Governance and Nominating Committee shall review the material facts of all Interested Transactions that require the Committee’s approval and either approve or disapprove of the entry into the Interested Transaction, subject to the exceptions described below. If advance Committee approval of an Interested Transaction is not feasible, then the Interested Transaction shall be considered and, if the Committee determines it to be appropriate, ratified at the Committee’s next regularly scheduled meeting. In determining whether to approve or ratify an Interested Transaction, the Governance and Nominating Committee will take into account, among other factors it deems appropriate, whether the Interested Transaction is on terms no less favorable than terms generally available to an unaffiliated third-party under the same or similar circumstances and the extent of the Related Party’s interest in the transaction.
The Governance and Nominating Committee has reviewed the Interested Transactions described below in “Standing Pre-Approval for Certain Interested Transactions” and determined that each of the Interested Transactions described therein shall be deemed to be pre-approved or ratified (as applicable) by the Governance and Nominating Committee under the terms of the Policy. In addition, the board of directors has delegated to the Chair of the Governance and Nominating Committee the authority to pre-approve or ratify (as applicable) any Interested Transaction with a Related Party in which the aggregate amount involved is expected to be less than $250,000. In connection with each regularly scheduled meeting of the Governance and Nominating Committee, a summary of each new Interested Transaction deemed pre-approved pursuant to paragraph (3) or (4) under “Standing Pre- Approval for Certain Interested Transactions” below and each new Interested Transaction preapproved by the Chair in accordance shall be provided to the Committee for its review.
Under the Policy, no director shall participate in any discussion or approval of an Interested Transaction for which he or she is a Related Party, except that the director shall provide all material information concerning the Interested Transaction to the Governance and Nominating Committee.
If an Interested Transaction will be ongoing, the Governance and Nominating Committee may establish guidelines for our management to follow in its ongoing dealings with the Related Party. Thereafter, the Governance and Nominating Committee, on at least an annual basis, shall review and assess ongoing relationships with the Related Party to see that they are in compliance with the Committee’s guidelines and that the Interested Transaction remains appropriate.
Standing Pre-Approval for Certain Interested Transactions
The Governance and Nominating Committee has reviewed the types of Interested Transactions described below and determined that each of the following Interested Transactions shall be deemed to be pre-approved by the Committee, even if the aggregate amount involved will exceed $100,000.
| 1. |
Employment of Executive Officers. Any employment by the Company of an executive officer if (a) the related compensation is required to be reported in our proxy statement under Item 402 of the SEC’s compensation disclosure requirements; or (b) the executive officer is not an immediate family member of another executive officer or director of the Company, the related compensation would be reported in our proxy statement under Item 402 of the SEC’s compensation disclosure requirements if the executive officer was a “named executive officer,” and the Company’s Compensation Committee approved (or recommended that the Board approve) such compensation. |
59
| 2. |
Director Compensation. Any compensation paid to a director if the compensation is required to be reported in our proxy statement under Item 402 of the SEC’s compensation disclosure requirements; |
| 3. |
Certain Transactions with other Companies. Any transaction with another company at which a Related Party’s only relationship is as an employee (other than an executive officer), director or beneficial owner of less than 10% of that company’s shares, if the aggregate amount involved does not exceed the greater of $1,000,000, or 2 percent of that company’s total annual revenues; |
| 4. |
Certain Company Charitable Contributions. Any charitable contribution, grant or endowment by the Company to a charitable organization, foundation or university at which a Related Party’s only relationship is as an employee (other than an executive officer) or a director, if the aggregate amount involved does not exceed the lesser of $1,000,000, or 2 percent of the charitable organization’s total annual receipts; |
| 5. |
Transactions Where All Shareholders Receive Proportional Benefits. Any transaction where the Related Party’s interest arises solely from the ownership of our common stock and all holders of our common stock received the same benefit on a pro rata basis (e.g. dividends). |
| 6. |
Transactions Involving Competitive Bids. Any transaction involving a Related Party where the rates or charges involved are determined by competitive bids. |
| 7. |
Regulated Transactions. Any transaction with a Related Party involving the rendering of services as a common or contract carrier, or public utility, at rates or charges fixed in conformity with law or governmental authority. |
| 8. |
Certain Banking-Related Services. Any transaction with a Related Party involving services as a bank depositary of funds, transfer agent, registrar, trustee under a trust indenture, or similar services. |
Director Independence
Each of Mr. Sean Shao, Dr. Xiangmin Cui, Dr. Tong Jun Lin and Prof. Liu serves on our board as “independent directors as defined by the applicable rules of the SEC and NASDAQ.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Independent Auditors’ Fees
Our independent accountants for the audit of our annual financial statements and audit of internal control over financial reporting for our fiscal year ended December 31, 2010 was KPMG. Our independent accountants for the audit of our annual financial statements for our fiscal year ended December 31, 2009 was Frazer Frost, LLP ("Frazer Frost", Successor Entity of Moore Stephens Wurth Frazer and Torbet, LLP). The following table shows the fees paid and to be paid by us to KPMG and Frazer Frost.
| Year Ended December 31, | ||||||
| 2010 | 2009 | |||||
| Audit Fees | $ | 1,008,256 | $ | 420,000 | ||
| Audit-Related Fees | - | - | ||||
| Tax Fees | 41,550 | 17,000 | ||||
| All other Fees | 18,000 | 48,000 | ||||
Audit Fees were for professional services rendered for the audit of our company’s annual financial statements, the review of quarterly financial statements and the preparation of statutory and regulatory filings. We paid Frazer Frost $420,000 and $450,000 for the financial statements audit and quarterly financial statements reviews in 2009 and 2010, respectively. Tax fees paid by us to Frazer Frost of $17,000 and $41,500 were for tax services in 2009 and 2010, respectively. All Other Fees consisted of other fees billed for services provided by Frazer Frost.
For 2010, the audit fee payable by us to KPMG of $558,256 is for the audit of our 2010 annual financial statements.
60
Pre-Approval Policies and Procedures
Under the Sarbanes-Oxley Act of 2002, all audit and non-audit services performed by our auditors must be approved in advance by our board of directors to assure that such services do not impair the auditors’ independence from us. In accordance with its policies and procedures, our board of directors pre-approved the audit service performed by KPMG for our financial statements as of and for the year ended December 31, 2010.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
Financial Statements and Schedules
The financial statements are set forth under Item 8 of this annual report on Form 10-K. Financial statement schedules have been omitted since they are either not required, not applicable, or the information is otherwise included.
Exhibit List
The following exhibits are filed as part of this report or incorporated by reference:
| Exhibit No. | Description | |
| 2.1 | Share Exchange Agreement between the Company, Logic Express Limited and the selling stockholders signatory thereto, dated as of July 18, 2006 (incorporated by reference to Exhibit 2 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 3.1 | Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 3.2 | Amended and Restated By-Laws, adopted on March 31, 2009 | |
| 4.1 | Form of Registration Rights Agreement, dated June 5, 2009 (incorporated by reference to Exhibit 4.1 of the Current Report on Form 8-K filed by the Company on June 5, 2009). | |
| 4.2 | Form of 3.8% Convertible Senior Secured Note due 2011 (incorporated by reference to Exhibit 4.2 of the Current Report on Form 8-K filed by the Company on June 5, 2009). | |
| 4.3 | Form of Warrant (incorporated by reference to Exhibit 4.3 of the Current Report on Form 8-K filed by the Company on June 5, 2009) | |
| 10.1 | China Biologic Products, Inc. 2008 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.2 | Form of Stock Option Award Agreement of China Biologic Products, Inc. (incorporated by reference to Exhibit 10.5 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.3 | Group Secondment Agreement, dated October 28, 2002, between Shandong Taibang Biological Products Co., Ltd. and the Shandong Institute (English Translation) (incorporated by reference to Exhibit 10.1 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.4 | Amended and Restated Joint Venture Agreement, between Logic Express Limited and the Shandong Institute, dated as of March 12, 2006 (English Translation) (incorporated by reference to Exhibit 10.2 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.5 | Letter of Intent for Equity Transfer, between Logic Express Limited and the Shandong Institute, dated as of June 10, 2006 (English Translation) (incorporated by reference to Exhibit 10.3 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.6 | Joint Venture and Cooperation Agreement between Mr. Fan Qingchun, Shandong Taibang Biological Products Co., Ltd. and Shaanxi Power Construction Corporation, dated September 12, 2008 (incorporated by reference to Exhibit 10.2 of the current report on Form 8-K, filed by the Company on October 16, 2008) | |
| 10.7 | Agreement on Equity Transfer, Acquisition, Joint Venture and Cooperation, among Shandong Taibang Biological Products Co., Ltd., Shaanxi Power Construction Corporation and Mr. Fan Qingchun, dated September 12, 2008 (incorporated by reference to Exhibit 10.3 of the current report on Form 8-K, filed by the Company on October 16, 2008) |
61
| Exhibit No. | Description | |
| 10.8 | (Shareholder) Agreement among Shandong Taibang Biological Products Co., Ltd., Logic Express Limited and Biological Institute, dated September 12, 2008 (incorporated by reference to Exhibit 10.4 of the current report on Form 8-K, filed by the Company on October 16, 2008) | |
| 10.9 | Equity Transfer Agreement, dated September 26, 2008, among Logic Express Limited, Chongqing Dalin Biologic Technologies Co., Ltd. and certain shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on October 2, 2008) | |
| 10.10 | Equity Transfer Agreement, between Shandong Taibang Biological Products Co., Ltd. and Mr. Fan Qingchun, dated October 10, 2008 (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on October 16, 2008) | |
| 10.11 | Supplemental Agreement, dated November 3, 2008, among Logic Express Limited, Fan Shaowen, as representative of the shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. and Chongqing Dalin Biologic Technologies Co., Ltd. (English Translation) (incorporated by reference to Exhibit 10.2 of the current report on Form 8-K, filed by the Company on November 7, 2008) | |
| 10.12 | Second Supplemental Agreement, dated November 14, 2008, among Logic Express Limited, Fan Shaowen as representative of the shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. and Chongqing Dalin Biologic Technologies Co., Ltd. (English Translation) (incorporated by reference to exhibit 10.3 of the current report on Form 8-K, filed by the Company on November 20, 2008) | |
| 10.13 | Amended Equity Transfer Agreement, dated December 12, 2008, among Logic Express Limited, Chongqing Dalin Biologic Technologies Co., Ltd., and certain shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. (English Translation) (incorporated by reference to exhibit 10.4 of the current report on Form 8-K, filed by the Company on December 18, 2008) | |
| 10.14 | English Translation of the Equity Transfer and Entrustment Agreement, dated April 6, 2009, among Logic Express, Shandong Taibang Biological Products Co., Ltd. and the Shandong Institute of Biological Products (incorporated by reference to Exhibit 10.6 of the current report on Form 8-K filed by the Company on April 13, 2009) | |
| 10.15 | Raw Plasma Supply Agreement, between Shandong Taibang Biological Products Co., Ltd. and Qi He Plasma Collection Station, dated as of December 30, 2005 (English Translation) (incorporated by reference to Exhibit 10.4 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.16 | Raw Plasma Supply Agreement, between Shandong Taibang Biological Products Co., Ltd. and the Xia Jin Plasma Collection Station, dated as of December 30, 2005 (English Translation) (incorporated by reference to Exhibit 10.5 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.17 | Raw Plasma Supply Agreement, between Shandong Taibang and the Zhang Qiu Plasma Collection Station, dated as of December 30, 2005 (English Translation) (incorporated by reference to Exhibit 10.6 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.18 | Plasma Processing Agreement, between Shandong Taibang Biological Products Co., Ltd. and Qi He An Tai Plasma Collection Co., Ltd., dated as of January 2, 1007 (English Translation) (incorporated by reference to Exhibit 10.9 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.19 | Plasma Processing Agreement, between Shandong Taibang Biological Products Co., Ltd. and the Xia Jin An Tai Plasma Collection Co., Ltd., dated as of January 2, 2007 (English Translation) (incorporated by reference to Exhibit 10.10 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.20 | Plasma Processing Agreement, between Shandong Taibang Biological Products Co., Ltd. and the Zhang Qiu An Tai Plasma Collection Co., Ltd., dated as of January 2, 2007 (English Translation) (incorporated by reference to Exhibit 10.11 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.21 | Raw Plasma Supply Agreement, between Shandong Taibang Biological Products Co., Ltd. and Liao Cheng Tiantan Plasma Collection Co. Ltd., dated as of November 1, 2007 (English Translation) (incorporated by reference to Exhibit 10.23 of the registration statement on Form SB-2/A, filed by the Company on December 28, 2007) | |
| 10.22 | Asset Purchase Agreement, between Xia Jin An Tai Plasma Collection Co., Ltd. and Xia Jin County Plasma Collection Station, dated as of October 20, 2006 (English Translation) (incorporated by reference to Exhibit 10.15 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.23 | Asset Purchase Agreement, between Liao Cheng An Tai Plasma Collection Co., Ltd. and Yang Gu County Plasma Collection Station, dated as of November 3, 2006 (English Translation) (incorporated by reference to Exhibit 10.16 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) |
62
| Exhibit No. | Description | |
| 10.24 | Asset Purchase Agreement, between Qi He An Tai Plasma Collection Co., Ltd. and Qi He County Plasma Collection Station, dated as of November 9, 2006 (English Translation) (incorporated by reference to Exhibit 10.14 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.25 | Asset Purchase Agreement, between He Ze An Tai Plasma Collection Co., Ltd and Yun Cheng County Plasma Collection Station, dated as of December 15, 2006 (English Translation) (incorporated by reference to Exhibit 10.22 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.26 | Asset Purchase Agreement, between Zhang Qiu An Tai Plasma Collection Co., Ltd. and Zhang Qiu Plasma Collection Station, dated as of December 31, 2006 (English Translation) (incorporated by reference to Exhibit 10.12 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.27 | Asset Purchase Agreement, between Guang Xi Huan Jiang Missile Plasma Collection Co., Ltd. and Huan Jiang Maonan Autonomous County Plasma Collection Station, dated as of April 24, 2007 (English Translation) (incorporated by reference to Exhibit 10.13 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.28 | Asset Purchase Agreement, between Fang Cheng Plasma Collection Co., Ltd. and Fang Cheng Plasma Company, dated as of April 30, 2007 (English Translation) (incorporated by reference to Exhibit 10.21 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.29 | Asset Purchase Agreement, between Guang Xi Huan Jiang Missile Plasma Collection Co., Ltd. and Huan Jiang Maonan Autonomous County Plasma Collection Station, dated as of August 5, 2007 (English Translation) (incorporated by reference to Exhibit 10.13 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.30 | Trademark Licensing Agreement, dated as of February 27, 2007 (English Translation) (incorporated by reference to Exhibit 10.17 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.31 | Loan Agreement, dated as of November 30, 2006, among Shandong Taibang and the Shandong Institute and Logic Express (English Translation) (incorporated by reference to Exhibit 10.18 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.32 | Supplementary Agreement, dated as of September 1, 2007, among Shandong Taibang Biological Products Co., Ltd., the Shandong Institute and Logic Express Limited (English Translation) (incorporated by reference to Exhibit 10.19 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.33 | Form of Bank of Communications Loan Contract, among Shandong Taibang and the Tai’an Branch of the Bank of Communications (English Translation) (incorporated by reference to Exhibit 10.20 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.34 | China Bank of Communications Loan Contract, dated October 28, 2008, between Shandong Taibang Biological Products Co. Ltd. and Bank of Communications, Tai’an Branch (English Translation) (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on November 3, 2008) | |
| 10.35 | Loan Agreement between Shandong Taibang Biological Products Co., Ltd. and Bank Of China, dated January 8, 2009 (English Translation) (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on January 13, 2009) | |
| 10.36 | Employment Agreement, between Y. Tristan Kuo and China Biologic Products, Inc., dated May 9, 2008 (incorporated by reference to Exhibit 10.3 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.37 | Employment Agreement, between Chao Ming Zhao and China Biologic Products, Inc., dated May 9, 2008 (incorporated by reference to Exhibit 10.4 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.38 | Form of Director’s Employment Agreement of China Biologic (incorporated by reference to Exhibit 10.8 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.39 | Form of Independent Director Agreement of China Biologic Products, Inc. (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on July 30, 2008) | |
| 10.40 | Form of Indemnity Agreement of China Biologic Products, Inc. (incorporated by reference to Exhibit 10.2 of the current report on Form 8-K, filed by the Company on July 30, 2008) |
63
*Filed herewith.
64
SIGNATURES
In accordance with section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant caused this Report on Form 10-K to be signed on its behalf by the undersigned, thereto duly authorized individual.
Date: March 31, 2011
| CHINA BIOLOGIC PRODUCTS, INC. | ||
| By: | /s/ Chao Ming Zhao | |
| Chao Ming Zhao | ||
| Chief Executive Officer | ||
| By: | /s/ Yu-Yun Tristan Kuo | |
| Yu-Yun Tristan Kuo | ||
| Chief Financial Officer | ||
In accordance with the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Chao Ming Zhao | Chief Executive Officer | March 31, 2011 | ||
| Chao Ming Zhao | (Principal Executive Officer) | |||
| /s/ Yu-Yun Tristan Kuo | Chief Financial Officer | March 31, 2011 | ||
| Yu-Yun Tristan Kuo | (Principal Financial and Accounting Officer) | |||
| /s/ Siu Ling Chan | Chairwoman of the Board | March 31, 2011 | ||
| Siu Ling Chan | ||||
| /s/ Sean Shao | Director | March 31, 2011 | ||
| Sean Shao | ||||
| /s/ Xiangmin Cui | Director | March 31, 2011 | ||
| Xiangmin Cui | ||||
| /s/ Tong Jun Lin | Director | March 31, 2011 | ||
| Tong Jun Lin | ||||
| /s/ Chong Yang Li | Director | March 31, 2011 | ||
| Chong Yang Li | ||||
| /s/ Bing Li | Director | March 31, 2011 | ||
| Bing Li | ||||
| /s/ Wenfang Liu | Director | March 31, 2011 | ||
| Wenfang Liu |
65
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONTENTS
| Page | |
| Reports of Independent Registered Public Accounting Firms | F-1 - F-2 |
| Consolidated Balance Sheets | F-3 |
| Consolidated Statements of Income | F-4 |
| Consolidated Statements of Stockholders’ Equity and Comprehensive Income | F-5 |
| Consolidated Statements of Cash Flows | F-6 |
| Notes to Consolidated Financial Statements | F-8 - F-32 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
China Biologic Products, Inc.:
We have audited the accompanying consolidated balance sheet of China Biologic Products, Inc. and subsidiaries (the "Company") as of December 31, 2010, and the related consolidated statements of income, stockholders’ equity and comprehensive income, and cash flows for the year then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of China Biologic Products, Inc. and subsidiaries as of December 31, 2010, and the results of their operations and their cash flows for the year then ended, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), China Biologic Products, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 31, 2011 expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting.
As discussed in Note 20 to the consolidated financial statements, the Company’s subsidiary, Guizhou Taibang Biological Products Co., Ltd. ("Guizhou Taibang") is a defendant in a lawsuit brought by certain potential investors with respect to Guizhou Taibang’s failure to register their capital contributions in Guizhou Taibang with the local Administration for Industry and Commerce.
/s/KPMG
Hong Kong, China
March 31, 2011
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
China Biologic Products, Inc.
We have audited the accompanying consolidated balance sheets of China Biologic Products, Inc. and subsidiaries as of December 31, 2009 and 2008, and the related consolidated statements of income and other comprehensive income, changes in equity, and cash flows for each of the years in the two-year period ended December 31, 2009. China Biologic Products, Inc.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of China Biologic Products, Inc. and subsidiaries as of December 31, 2009 and 2008, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
/s/ Frazer Frost, LLP (Successor Entity of Moore Stephens
Wurth Frazer and Torbet, LLP, see Form 8-K filed on January 7, 2010)
Brea, California
March 23, 2010, except for the effects on the consolidated financial statements
of the restatement described in Note 2, as to which the date is March 31, 2011
F-2
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||
| 2010 | 2009 | |||||
| ASSETS | ||||||
| Current Assets | ||||||
|
Cash and cash equivalents |
$ | 64,941,368 | $ | 53,843,951 | ||
|
Accounts receivable, net of allowance for doubtful accounts |
9,922,111 | 1,767,076 | ||||
|
Accounts receivable - a related party |
212,611 | 222,617 | ||||
|
Inventories |
52,300,447 | 35,132,724 | ||||
|
Other receivables |
2,727,110 | 2,186,441 | ||||
|
Prepayments and prepaid expenses |
855,338 | 1,299,125 | ||||
|
Deferred tax assets |
1,860,753 | 1,053,771 | ||||
|
Total Current Assets |
132,819,738 | 95,505,705 | ||||
| Property, plant and equipment, net | 39,511,731 | 28,873,413 | ||||
| Intangible assets, net | 14,559,020 | 17,200,512 | ||||
| Land use rights, net | 4,701,450 | 3,979,810 | ||||
| Prepayments and deposits for property, plant and equipment | 4,254,423 | 3,223,960 | ||||
| Goodwill | 17,778,231 | 17,200,728 | ||||
| Equity method investment | 7,297,201 | 6,627,355 | ||||
| Total Assets | $ | 220,921,794 | $ | 172,611,483 | ||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||
| Current liabilities | ||||||
|
Short-term bank loans |
$ | 3,034,000 | $ | 4,401,000 | ||
|
Short term loans - noncontrolling interest |
- | 3,652,500 | ||||
|
Accounts payable |
4,392,772 | 3,750,441 | ||||
|
Due to related party |
3,192,140 | 3,086,940 | ||||
|
Other payables and accrued expenses |
21,606,730 | 21,516,116 | ||||
|
Accrued interest - noncontrolling interest |
- | 2,068,526 | ||||
|
Advance from customers |
3,560,018 | 3,868,577 | ||||
|
Income tax payable |
6,659,805 | 7,479,279 | ||||
|
Other taxes payable |
2,146,868 | 1,294,800 | ||||
|
Convertible notes |
1,196,233 | 89,760 | ||||
|
Derivative liabilities - embedded conversion option in convertible notes |
14,561,661 | 19,960,145 | ||||
|
Derivative liabilities - warrants |
11,095,592 | 12,701,262 | ||||
|
Total Current Liabilities |
71,445,819 | 83,869,346 | ||||
| Other payable | 333,008 | 323,687 | ||||
| Deferred tax liabilities | 4,098,834 | 4,275,295 | ||||
|
Total Liabilities |
75,877,661 | 88,468,328 | ||||
| Stockholder’s Equity | ||||||
|
Common stock: par value $.0001; 100,000,000 shares authorized; 24,351,126 and 23,056,442 shares issued and outstanding at December 31, 2010 and 2009, |
2,435 | 2,305 | ||||
|
Additional paid-in capital |
35,435,139 | 21,270,601 | ||||
|
Retained earnings |
55,739,101 | 24,196,218 | ||||
|
Accumulated other comprehensive income |
8,023,121 | 4,227,537 | ||||
|
Total stockholder’s equity attributable to China Biologic Products, Inc. |
99,199,796 | 49,696,661 | ||||
|
Noncontrolling interest |
45,844,337 | 34,446,494 | ||||
|
Total Stockholder’s Equity |
145,044,133 | 84,143,155 | ||||
|
Commitments and contingencies |
- | - | ||||
|
Total Liabilities and Stockholder’s Equity |
$ | 220,921,794 | $ | 172,611,483 |
F-3
See accompanying notes to Consolidated Financial Statements.
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
| For the Years Ended | ||||||
| December 31, | December 31, | |||||
| 2010 | 2009 | |||||
| Sales | ||||||
| External customers | $ | 138,674,983 | $ | 118,293,137 | ||
| Related party | 1,020,434 | 705,018 | ||||
| Total sales | 139,695,417 | 118,998,155 | ||||
| Cost of sales | ||||||
| External customers | 36,793,775 | 32,544,743 | ||||
| Related party | 157,374 | 77,165 | ||||
| Cost of sales | 36,951,149 | 32,621,908 | ||||
| Gross profit | 102,744,268 | 86,376,247 | ||||
| Operating expenses | ||||||
| Selling expenses | 7,372,348 | 3,529,242 | ||||
| General and administrative expenses | 23,510,566 | 19,807,123 | ||||
| Research and development expenses | 2,336,126 | 1,662,690 | ||||
| Income from operations | 69,525,228 | 61,377,192 | ||||
| Other expenses/(income) | ||||||
| Equity in income of equity method investee | (1,070,241 | ) | (566,984 | ) | ||
| Change in fair value of derivative liabilities | 3,233,288 | 28,915,328 | ||||
| Interest expense, net | 1,930,165 | 3,930,249 | ||||
| Other (income)/expense, net | (169,043 | ) | 261,252 | |||
| Total other expenses, net | 3,924,169 | 32,539,845 | ||||
| Earnings before income tax expense | 65,601,059 | 28,837,347 | ||||
| Income tax expense | 13,608,755 | 10,013,563 | ||||
| Net income | 51,992,304 | 18,823,784 | ||||
| Less: Net income attributable to the noncontrolling interest | 20,449,421 | 16,615,658 | ||||
| Net income attributable to China Biologic Products, Inc. | 31,542,883 | 2,208,126 | ||||
| Earnings per share: | ||||||
| Basic | $ | 1.34 | $ | 0.10 | ||
| Diluted | $ | 1.30 | $ | 0.10 | ||
| Weighted average shares used in computation: | ||||||
| Basic | 23,586,506 | 21,754,911 | ||||
| Diluted | 24,176,432 | 21,949,638 | ||||
See accompanying notes to Consolidated Financial Statements.
F-4
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE INCOME
| Accumulated other | |||||||||||||||||||||
| Common stock | Additional | Retained | comprehensive | Noncontrolling | |||||||||||||||||
| Shares | Par value | paid-in capital | earnings | income | interest | Total equity | |||||||||||||||
| Balance as of January 1, 2009 | 21,434,942 | $ | 2,143 | $ | 10,700,032 | $ | 22,382,054 | $ | 4,159,298 | $ | 4,805,381 | $ | 42,048,908 | ||||||||
| Cumulative effect of reclassification of 2006 Warrants (see Note 14) | - | - | (600,289 | ) | (393,962 | ) | - | - | (994,251 | ) | |||||||||||
| Balance as of January 1, 2009 (reclassified) | 21,434,942 | $ | 2,143 | $ | 10,099,743 | $ | 21,988,092 | $ | 4,159,298 | $ | 4,805,381 | $ | 41,054,657 | ||||||||
| Net income | - | - | - | 2,208,126 | - | 16,615,658 | 18,823,784 | ||||||||||||||
| Foreign currency translation adjustments, net of nil income taxes | - | - | - | - | 68,239 | 455,788 | 524,027 | ||||||||||||||
|
Comprehensive income |
19,347,811 | ||||||||||||||||||||
| Dividends declared by subsidiaries to noncontrolling interest | - | - | - | - | - | (8,955,392 | ) | (8,955,392 | ) | ||||||||||||
| Acquisition of Dalin | - | - | - | - | - | 21,525,059 | 21,525,059 | ||||||||||||||
| Stock compensation | - | - | 62,281 | - | - | - | 62,281 | ||||||||||||||
| Common stock issued in connection with: | |||||||||||||||||||||
| - Exercise of warrants | 1,284,000 | 128 | 8,571,281 | - | - | - | 8,571,409 | ||||||||||||||
| - Exercise of stock options | 87,500 | 9 | 349,991 | - | - | - | 350,000 | ||||||||||||||
| - Conversion of convertible notes | 250,000 | 25 | 2,187,305 | - | - | - | 2,187,330 | ||||||||||||||
| Balance as of December 31, 2009 | 23,056,442 | $ | 2,305 | $ | 21,270,601 | $ | 24,196,218 | $ | 4,227,537 | $ | 34,446,494 | $ | 84,143,155 | ||||||||
| Net income | - | - | - | 31,542,883 | - | 20,449,421 | 51,992,304 | ||||||||||||||
| Foreign currency translation adjustments, net of nil income taxes | - | - | - | - | 3,795,584 | 1,381,931 | 5,177,515 | ||||||||||||||
|
Comprehensive income |
57,169,819 | ||||||||||||||||||||
| Dividend declared by subsidiaries to noncontrolling interest | - | - | - | - | - | (10,446,179 | ) | (10,446,179 | ) | ||||||||||||
| Acquisition of noncontrolling interests | - | - | - | - | - | 12,670 | 12,670 | ||||||||||||||
| Stock compensation | - | - | 2,341,783 | - | - | - | 2,341,783 | ||||||||||||||
| Common stock issued in connection with: | |||||||||||||||||||||
| - Exercise of warrants | 294,019 | 30 | 4,278,160 | - | - | - | 4,278,190 | ||||||||||||||
| - Exercise of stock options | 37,130 | 4 | 97,596 | - | - | - | 97,600 | ||||||||||||||
| - Conversion of convertible notes | 963,535 | 96 | 7,446,999 | - | - | - | 7,447,095 | ||||||||||||||
| Balance as of December 31, 2010 | 24,351,126 | $ | 2,435 | $ | 35,435,139 | $ | 55,739,101 | $ | 8,023,121 | $ | 45,844,337 | $ | 145,044,133 | ||||||||
See accompanying notes to Consolidated Financial Statements.
F-5
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| 2010 | 2009 | |||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||
| Net income | $ | 51,992,304 | $ | 18,823,784 | ||
| Adjustments to reconcile net income to cash provided by operating activities: | ||||||
| Depreciation | 3,607,184 | 2,709,623 | ||||
| Amortization | 3,566,269 | 3,358,532 | ||||
| Loss on sale of property, plant and equipment | 120,224 | 224,548 | ||||
| Reversal of allowance for doubtful accounts, net | (57,624 | ) | (13,089 | ) | ||
| Provision for doubtful accounts - other receivables and prepayments | 475,346 | 280,796 | ||||
| Write-down of obsolete inventories | 451,761 | 519,333 | ||||
| Deferred tax benefit, net | (1,101,171 | ) | (1,552,661 | ) | ||
| Stock compensation | 2,341,783 | 62,281 | ||||
| Change in fair value of derivative liabilities | 3,233,288 | 28,915,328 | ||||
| Amortization of deferred note issuance cost | 258,753 | 247,199 | ||||
| Amortization of discount on convertible notes | 1,590,740 | 100,253 | ||||
| Equity in income of equity method investee | (1,070,241 | ) | (566,984 | ) | ||
| Change in operating assets and liabilities, net of acquisition in Dalin: | ||||||
| Accounts receivable – third parties | (7,837,681 | ) | (1,707,714 | ) | ||
| Accounts receivable - related party | 17,158 | 197,284 | ||||
| Other receivables | 182,686 | (1,744,794 | ) | |||
| Inventories | (16,026,215 | ) | (12,456,975 | ) | ||
| Prepayments and prepaid expenses | (91,307 | ) | (248,794 | ) | ||
| Accounts payable | 505,407 | (58,467 | ) | |||
| Other payables and accrued expenses | (596,938 | ) | 7,058,773 | |||
| Accrued interest - noncontrolling interest shareholders | (2,086,010 | ) | 2,068,526 | |||
| Advance from customers | (429,497 | ) | 274,768 | |||
| Income tax payable | (1,046,906 | ) | 2,943,767 | |||
| Other taxes payable | 787,913 | 865,670 | ||||
| Net cash provided by operating activities | 38,787,226 | 50,300,987 | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||
| Dividends received | - | 384,087 | ||||
| Acquisition of a subsidiary, net of cash acquired | (4,063,325 | ) | 1,573,079 | |||
| Acquisition of equity method investment | - | (3,225,420 | ) | |||
| Purchase of property, plant and equipment | (10,313,432 | ) | (3,522,768 | ) | ||
| Purchase of intangible assets and land use right | (1,474,718 | ) | (2,106,203 | ) | ||
| Proceeds from sale of property, plant and equipment | - | 36,771 | ||||
| Net cash used in investing activities | (15,851,475 | ) | (6,860,454 | ) |
See accompanying notes to Consolidated Financial Statements.
F-6
CHINA BIOLOGIC PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| 2010 | 2009 | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||
| Proceeds from warrants exercised | 1,232,482 | 3,649,770 | ||||
| Proceeds from option exercised | 97,600 | 350,000 | ||||
| Proceeds from issuance of convertible notes | - | 8,967,516 | ||||
| Repayments of former shareholders loan in a subsidiary | - | (2,841,302 | ) | |||
| Proceeds from short term bank loans | 5,917,600 | 13,536,688 | ||||
| Repayment of short term bank loans | (7,397,000 | ) | (18,355,572 | ) | ||
| Repayment of loan from noncontrolling interest holder | (3,683,373 | ) | (772,803 | ) | ||
| Dividends paid by subsidiaries to noncontrolling interest shareholders | (10,446,179 | ) | (2,969,372 | ) | ||
| Net cash (used)/provided by financing activities | (14,278,870 | ) | 1,564,925 | |||
| EFFECT OF FOREIGN EXCHANGE RATE CHANGES ON CASH | 2,440,536 | 23,877 | ||||
| NET INCREASE IN CASH | 11,097,417 | 45,029,335 | ||||
| Cash and cash equivalents at beginning of year | 53,843,951 | 8,814,616 | ||||
| Cash and cash equivalents at end of year | $ | 64,941,368 | $ | 53,843,951 | ||
| Supplemental cash flow information | ||||||
| Cash paid for income taxes | $ | 15,756,832 | $ | 8,021,981 | ||
| Cash paid for interest expense (net of capitalized interest) | $ | 810,643 | $ | 1,131,271 | ||
| Noncash investing and financing activities: | ||||||
| Reclassification of warrant liability to paid-in capital upon warrants conversion | $ | 3,045,611 | $ | 4,921,639 | ||
| Convertible notes conversion | $ | 7,191,763 | $ | 2,187,330 | ||
| Distribution paid by offsetting accounts receivable - related party | $ | - | $ | 944,036 | ||
| Distribution paid in exchange of noncontrolling interest shareholders loan | $ | - | $ | 3,665,250 | ||
| Distribution paid by offsetting loan and interest due from holder of noncontrolling interest | $ | - | $ | 4,647,924 | ||
| Net assets acquired with prepayments made in prior periods | $ | - | $ | 14,250,492 | ||
| Net assets acquired with unpaid investment | $ | - | $ | 2,850,098 | ||
| Transfer from prepayments and deposits to property, plant and equipment | $ | 1,078,348 | $ | 2,296,113 | ||
| Land use right acquired with prepayments made in prior periods | $ | - | $ | 146,610 |
See accompanying notes to Consolidated Financial Statements.
F-7
CHINA BIOLOGIC PRODUCTS INC. AND SUBSIDIARIES
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2010 AND 2009
NOTE 1 – ORGANIZATION BACKGROUND AND PRINCIPAL ACTIVITIES
A. Reorganization and Principal Activities
China Biologic Products, Inc. (the “Company” or “CBP”, formerly known as “GRC Holdings, Inc.”) was originally incorporated in the State of Texas in 1989. On July 19, 2006, the Company and its principle shareholders entered into a share exchange agreement (the “Exchange Agreement”) with Logic Express Ltd. (“Logic Express”), a privately held investment holding company incorporated on January 6, 2006 under the laws of the British Virgin Islands, and all the shareholders of Logic Express (the “Logic Express Shareholders”). Pursuant to the terms of the Exchange Agreement, the Logic Express Shareholders transferred to the Company all of their shares in exchange for 18,484,715 shares of the Company’s common shares (the “Share Exchange”). As a result of the Share Exchange, Logic Express became a wholly-owned subsidiary of the Company and the Logic Express Shareholders received approximately 96.1% of the Company’s issued and outstanding common shares. Immediately prior to the date of the Share Exchange, the Company was a publicly listed shell entity with no operations and, Logic Express, through its 82.76% owned subsidiary, Shandong Taibang Biological Products Co. Ltd. (“Shandong Taibang”), was engaged in the research, development, commercialization, manufacture and sale of human blood products primarily in the People’s Republic of China ( the “PRC” or China). The Share Exchange was accounted for as a reverse recapitalization, equivalent to the issuance of stock by Logic Express for the net monetary assets of the Company accompanied by a recapitalization. After consummation of the Share Exchange, the Company converted into a Delaware corporation and changed its name to China Biologic Products, Inc. on January 10, 2007.
The Company is a biopharmaceutical company and, through its PRC subsidiaries, is principally engaged in the research, development, manufacturing and sales of plasma-based pharmaceutical products in PRC. The PRC subsidiaries own plasma stations to purchase and collect plasma from individual donors for a fee. The plasma is processed into finished goods after passing through a series of fractionating processes. All of the Company’s products are prescription medicines which require government approval on the quality before the products are sold to customers. The Company primarily sells its products to hospitals and inoculation centers directly or through distributors in the PRC.
On September 26, 2008, the Company, through Logic Express, entered in an equity purchase agreement with Guiyang Dalin Biologic Technologies Co. Ltd. (“Dalin”, formerly known as “Chongqing Dalin Biologic Technologies Co. Ltd.”), an investment holding company, and certain equity owners of Dalin, to acquire 90% equity interest of Dalin. The purchase consideration for the 90% equity interest in Dalin was RMB 194,400,000 (or approximately $28,479,600) in cash (See Note 17).
Dalin holds 54% equity interest in Guiyang Qianfeng Biological Products Co., Ltd. (“Qianfeng”), which changed its name to Guizhou Taibang Biological Products Co., Ltd. (“Guizhou Taibang”) on December 30, 2010. Qianfeng is one of the largest plasma-based biopharmaceutical companies in China and is the only manufacturer currently operating in Guizhou Province. Qianfeng is in compliance with the Good Manufacturing Practices certified by State Food and Drug Administration (“SFDA”) for the manufacturing, sale and distribution of Human Albumin, Human Immunoglobulin, Human Intravenous Immunoglobulin, Human Hepatitis B Immunoglobulin, Human Tetanus Immunoglobulin and Human Rabies Immune Globulin.
The Company completed the acquisition of 90% equity interests in Dalin in Janurary, 2009. On December 28, 2009, the Company’s 90% equity interest in Dalin was transferred to Logic Management Consulting (China) Co., Ltd. (“Logic China”), a wholly owned subsidiary of the Company (see Note 17). The Company established Logic China in December 2009, for the purpose of being the holding company of the 90% equity interest in Dalin.
On August 5, 2010, Logic China established a wholly-owned subsidiary, Logic Taibang Biological Institute (Beijing) (“Logic Beijing”), with registered capital of $149,700 (RMB 1 million). Logic Beijing is principally engaged in the research and development of plasma-based pharmaceutical products and will coordinate the research and development activities of the Company and its subsidiaries.
F-8
On January 13, 2010, Shandong Taibang acquired the remaining 20% equity interest in Fangcheng Plasma Company from the noncontrolling interest holder (see Note 20). Since the additional purchase of 20% equity interest did not result in a change of the Company’s control over Fangcheng Plasma Company, this transaction was accounted for as an equity transaction. After the acquisition, Fangcheng Plasma Company became a wholly-owned subsidiary of Shandong Taibang.
On July 8, 2010, Logic China entered into an equity purchase agreement with Shandong Taibang, to acquire 100% equity interest in Shandong Taibang Medical Company (“Taibang Medical”), a wholly-owned subsidiary of Shandong Taibang. The cash consideration of the 100% equity interest in Taibang Medical was RMB 6,440,000 (approximately $947,327). The transaction was completed on September 23, 2010. The purpose of this transaction is to effectively acquire the 17.24% equity interest indirectly held by the noncontrolling interest in Shandong Taibang, which will enable the Company to consolidate its resources in the sales and marketing of Shandong Taibang and Guizhou Taibang’s products. This transaction was accounted for as an equity transaction.
On November 11, 2010, the Company established Qianfeng Biological Science Company (“Qianfeng Biologic”) for the purpose of research and development of placenta based products. As of December 31, 2010, Qianfeng Biologic, which is a wholly-owned subsidiary of Guizhou Taibang, had no operations.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation and Basis of Presentation
The accompanying consolidated financial statements of the Company have been prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”), and include the financial statements of the Company and its subsidiaries. All significant intercompany balances and transactions have been eliminated upon consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates. Significant items subject to such estimates and assumptions include the useful lives of fixed assets; the allowance for doubtful accounts; the fair value determinations of financial and equity instruments and stock compensation awards, assets acquired and liabilities assumed in a business combination; the realizability of deferred tax assets and inventories; the recoverability of goodwill, intangible asset, land use right and property, plant and equipment; and accruals for income tax uncertainties and other contingencies. The current economic environment has increased the degree of uncertainty inherent in those estimates and assumptions.
Foreign Currency Translation
The accompanying consolidated financial statements of the Company are reported in US dollar. The financial position and results of operations of the Company’s subsidiaries in the PRC are measured using the Renminbi, the local and functional currency of these entities. Assets and liabilities of the subsidiaries are translated at the prevailing exchange rate in effect at each period end. Income statement accounts are translated at the average rate of exchange during the period. Translation adjustments are included in accumulated other comprehensive income in the consolidated statements of stockholders’ equity and comprehensive income.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, delivery of the product has occurred, the sales price is fixed or determinable and collectability is reasonably assured. The Company mainly sells human albumin and human immunoglobulin to hospitals, inoculation centers and pharmaceutical distributors. The Company requires a contract or purchase order which specify pricing, quantity and product specifications for all sales. Delivery of the product occurs when the product is received by the customer, which is when the risks and rewards of ownership have been transferred. Delivery is evidenced by a signed customer acceptance form. Sales are presented net of any discounts given to customers. For the year ended December 31, 2009 and 2010, there were no significant sales return from the customers.
F-9
Fair Value Measurements
The Company applies the provisions of ASC Subtopic 820-10, Fair Value Measurements, for fair value measurements of financial assets and financial liabilities and for fair value measurements of nonfinancial items that are recognized or disclosed at fair value in the financial statements. ASC Subtopic 820-10 also establishes a framework for measuring fair value and expands disclosures about fair value measurements. The Company has included these disclosures in Note 18 of the consolidated financial statements.
ASC Subtopic 820-10 defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability.
ASC Subtopic 820-10 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC Subtopic 820-10 establishes three levels of inputs that may be used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to measurements involving significant unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are as follows:
-
Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
-
Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly.
- Level 3 inputs are unobservable inputs for the asset or liability.
The level in the fair value hierarchy within which a fair value measurement in its entirety falls is based on the lowest level input that is significant to the fair value measurement in its entirety.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and demand deposits. The Company considers all highly liquid investments with original maturities of three-month or less at the time of purchase to be cash equivalents. As of December 31, 2010, the Company had cash maintained at banks in the PRC, BVI, Hong Kong and the United States of $62,898,395, $1,376,822, $64,205 and $103,894, respectively.
Accounts Receivable and Allowance For Doubtful Accounts
Accounts receivable are recorded at the invoiced amount and do not bear interest. The Company maintains an allowance for doubtful accounts for estimated losses inherent in its accounts receivable portfolio. In establishing the required allowance, management considers historical losses adjusted to take into account current market conditions and the customers’ financial condition, the amount of receivables in dispute, and the current receivables aging and current payment patterns. The Company reviews its allowance for doubtful accounts monthly. Past due balances are reviewed individually for collectability. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers.
Inventories
Inventories are stated at the lower of cost or market. Cost is determined using the weighted average method. Cost of work in progress and finished goods comprise direct materials, direct production costs and an allocation of production overheads based on normal operating capacity.
Property, Plant and Equipment
Property, plant and equipment are stated at cost. Depreciation on property, plant and equipment is calculated on the straight-line method over the estimated useful lives of the assets: Estimated useful lives of the assets are as follows:
| Buildings | 30 years |
| Machinery and equipment | 10 years |
| Furniture, fixtures, office equipment and vehicles | 5-10 years |
F-10
Equity method investment
Investment in an investee in which the Company has the ability to exercise significant influence, but does not have a controlling interest is accounted for using the equity method. Significant influence is generally presumed to exist when the Company has an ownership interest in the voting stock between 20% and 50%, and other factors, such as representation on the board of directors and participation in policy-making processes, are considered in determining whether the equity method of accounting is appropriate. Under the equity method of accounting, the Company’s share of the investee’s results of operations is included in other income (expense) in the Company’s consolidated statements of income. The Company recognizes a loss if it is determined that other than temporary decline in the value of the investment exists.
Intangible Assets
Intangible assets are stated at cost less accumulated amortization. Amortization expense is recognized on the straight-line basis over the estimated useful lives, as the pattern in which the economic benefits of the intangible assets are used up cannot be reliably determined. The estimated useful live is the period over which the intangible asset is expected to contribute directly or indirectly to the future cash flows of the Company. The Company has no intangible assets with indefinite useful lives. Estimate useful lives of the assets are as follows:
| Permits and licenses | 10 years |
| GMP Certificate | 5.8 years |
| Long-term customer-relationship | 4 years |
Land use rights
Land use rights represent the exclusive right to occupy and use a piece of land in the PRC for a specified contractual term. Land use rights are carried at cost, less accumulated amortization. Amortization is calculated using the straight-line method over the lives of the rights ranging from 39 to 50 years.
Goodwill
Goodwill represents the excess of the cost of an acquisition over the fair value of the net assets acquired. Goodwill is not amortized, but is instead tested for impairment. Goodwill is reviewed for impairment annually in accordance with the provisions of FASB ASC Topic 350, Intangibles - Goodwill and Other. The goodwill impairment test is a two-step test. Under the first step, the fair value of the reporting unit is compared with its carrying value (including goodwill). If the fair value of the reporting unit is less than its carrying value, an indication of goodwill impairment exists for the reporting unit and the enterprise must perform step two of the impairment test (measurement). Under step two, an impairment loss is recognized for any excess of the carrying amount of the reporting unit’s goodwill over the implied fair value of that goodwill. The implied fair value of goodwill is determined by allocating the fair value of the reporting unit in a manner similar to a purchase price allocation and the residual fair value after this allocation is the implied fair value of the reporting unit goodwill. Fair value of the reporting unit is determined using a discounted cash flow analysis. If the fair value of the reporting unit exceeds its carrying value, step two does not need to be performed.
The Company performs its annual impairment review of goodwill at each December 31, and when a triggering event occurs between annual impairment tests. No impairment of goodwill was recorded for the years ended December 31, 2010 and 2009.
Research and Development Expenses
Research and development costs are expensed as incurred. Research and development expenses for the years ended December 31, 2010 and 2009 were $2,336,126 and $1,662,690, respectively. These expenses include the costs of the Company’s internal research and development activities.
Product Liability
The Company’s products are covered by two separate product liability insurances each with coverages of approximately $2,934,000 (RMB 20,000,000) for the products sold by Shandong Taibang and Guizhou Taibang, respectively. There were no product liability claims as of December 31, 2010 and 2009.
F-11
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and tax loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the consolidated statements of income in the period that includes the enactment date. A valuation allowance is provided to reduce the amount of deferred tax assets if it is considered more likely than not that some portion or all of the deferred tax assets will not be realized.
The Company recognizes in the consolidated financial statements the impact of a tax position, if that position is more likely than not of being sustained upon examination, based on the technical merits of the position. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest related to unrecognized tax benefits in interest expense and penalties in selling, general, and administrative expenses.
Stock-based Compensation
The Company measures the cost of employee services received in exchange for an award of equity instruments based on the grant-date fair value of the award and recognizes the cost over the period during which an employee is required to provide service in exchange for the award, which generally is the vesting period.
Impairment of Long-Lived Assets
In accordance with Impairment or Disposal of Long-Lived Assets Subsections of FASB ASC Subtopic 360-10, Property, Plant, and Equipment - Overall, long-lived assets, such as property, plant and equipment, and purchased intangible asset subject to amortization, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If circumstances require a long-lived asset or asset group be tested for possible impairment, the Company first compares undiscounted cash flows expected to be generated by that asset or asset group to its carrying value. If the carrying value of the long-lived asset or asset group is not recoverable on an undiscounted cash flow basis, an impairment is recognized to the extent that the carrying value exceeds its fair value. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary. No impairment of long-lived assets was recognized for the years ended December 31, 2010 and 2009.
Earnings Per Share
Basic earnings per share is computed by dividing net income attributable to the Company by the weighted average number of common shares outstanding during the period. Diluted earnings per share reflects the potential dilution that would occur upon the exercise of outstanding warrants, options and the conversion of the convertible notes. Common share equivalents are excluded from the computation of the diluted earnings per share when their effect would be anti-dilutive.
Segment Reporting
The Company has one operating segment, which is the manufacture and sales of human blood products. Substantially all of the Company’s operations and customers are located in the PRC, and therefore, no geographic information is presented.
Contingencies
In the normal course of business, the Company is subject to loss contingencies, such as legal proceedings and claims arising out of its business, that cover a wide range of matters, including, among others, government investigations and tax matters. An accrual for a loss contingency is recognized when it is probable that a liability has been incurred and the amount of loss can be reasonably estimated.
Recently Issued Accounting Pronouncements
In October 2009, the FASB issued Accounting Standards Update (“ASU”) 2009-13, Revenue Recognition (Topic 605): Multiple-Deliverable Revenue Arrangements (EITF Issue No. 08-1, “Revenue Arrangements with Multiple Deliverables”). ASU 2009-13 amends FASB ASC Subtopic 605-25 to eliminate the requirement that all undelivered elements have vendor specific objective evidence of selling price (“VSOE”) or third party evidence of selling price (“TPE”) before an entity can recognize the portion of an overall arrangement fee that is attributable to items that already have been delivered. In the absence of VSOE or TPE for one or more delivered or undelivered elements in a multiple-element arrangement, entities will be required to estimate the selling prices of those elements. The overall arrangement fee will be allocated to each element (both delivered and undelivered items) based on their relative selling prices, regardless of whether those selling prices are evidenced by VSOE or TPE or are based on the entity’s estimated selling price. Application of the “residual method” of allocating an overall arrangement fee between delivered and undelivered elements will no longer be permitted upon adoption of ASU 2009-13. Additionally, the new guidance will require entities to disclose more information about their multiple-element revenue arrangements. ASU 2009-13 is effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. Management is currently evaluating the potential impact, if any, of adopting ASU 2009-13 on the Company’s financial position and results of operations.
F-12
NOTE 3 – ACCOUNTS RECEIVABLE
Accounts receivable at December 31, 2010 and 2009 consisted of the following:
| December 31, 2010 | December 31, 2009 | |||||
| Accounts receivable | $ | 11,160,751 | $ | 3,022,031 | ||
| Less: Allowance for doubtful accounts | (1,238,640 | ) | (1,254,955 | ) | ||
| Total | $ | 9,922,111 | $ | 1,767,076 |
The activities in the allowance for doubtful accounts for the years ended December 31, 2010 and 2009 are as follows:
| December 31, 2010 | December 31, 2009 | |||||
| Allowance for doubtful accounts at beginning of year | $ | 1,254,955 | $ | 1,268,052 | ||
| Charged to bad debt expense | 4,684 | 18,737 | ||||
| Recoveries of amounts previously reserved | (62,308 | ) | (31,826 | ) | ||
| Foreign currency translation adjustment | 41,309 | (8 | ) | |||
| Allowance for doubtful accounts at end of year | $ | 1,238,640 | $ | 1,254,955 |
NOTE 4 – INVENTORIES
Inventories at December 31, 2010 and 2009 consisted of the following:
| December 31, 2010 | December 31, 2009 | |||||
| Raw materials | $ | 24,933,485 | $ | 19,201,087 | ||
| Work-in-process | 15,262,139 | 8,407,319 | ||||
| Finished goods | 12,104,823 | 7,524,318 | ||||
| Total | $ | 52,300,447 | $ | 35,132,724 |
Raw materials mainly comprised the human blood plasma collected from the Company’s plasma stations. Work-in-process represented the intermediate products in the process of production. Finished goods mainly comprised human albumin, human immunoglobulin.
NOTE 5 – OTHER RECEIVABLES
Other receivables at December 31, 2010 and 2009 consisted of the following:
| December 31, 2010 | December 31, 2009 | |||||
| Advance to employees (1) | $ | 1,370,381 | $ | 1,512,583 | ||
| Dividends receivable (2) | 653,477 | - | ||||
| Deposits | 403,702 | 341,681 | ||||
| Others | 299,550 | 332,177 | ||||
| Total | $ | 2,727,110 | $ | 2,186,441 |
| (1) |
In 2009, 107 employees of the Company entered into agreements with developers in two housing projects. According to these agreements, the employees placed deposits equal to 80% of the purchase price of the residential units in these two housing projects with developers. To assist with their deposits, the Company entered into separate agreements with the employees by providing advances to them for an amount up to 50% of the purchase price of the residential units. The advances bear an annual interest rate of 3.89%. As of December 31, 2010, these two housing projects were still in construction stage. The Company expects the employees to settle these advances in 2011. |
|
| |
| (2) |
Dividends receivable represented the dividend declared by an equity method investment (see Note 9) during the year ended December 31, 2010. |
F-13
NOTE 6 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment at December 31, 2010 and 2009 consisted of the following:
| December 31, 2010 | December 31, 2009 | |||||
| Buildings | $ | 23,259,180 | $ | 12,901,205 | ||
| Machinery and equipment | 27,028,171 | 23,428,848 | ||||
| Furniture, fixtures, office equipment and vehicles | 5,441,115 | 3,862,385 | ||||
| Total property, plant and equipment, gross | 55,728,466 | 40,192,438 | ||||
| Accumulated depreciation | (17,434,512 | ) | (13,953,793 | ) | ||
| Total property, plant and equipment, net | 38,293,954 | 26,238,645 | ||||
| Construction in progress | 1,217,777 | 2,634,768 | ||||
| Property, plant and equipment, net | $ | 39,511,731 | $ | 28,873,413 |
Depreciation expense for the years ended December 31, 2010 and 2009 was $3,607,184 and $2,709,623, respectively. No interest was capitalized into construction in progress for the years ended December 31, 2010 and 2009.
NOTE 7 – INTANGIBLE ASSETS, NET
Intangible assets at December 31, 2010 and 2009 consisted of the following:
| December 31, 2010 | ||||||||||||
| Weighted | ||||||||||||
| average | Gross | Net | ||||||||||
| amortization | carrying | Accumulated | carrying | |||||||||
| period | amount | amortization | amount | |||||||||
| Amortizing intangible assets: | ||||||||||||
| Permits and licenses | 10 years | $ | 11,657,614 | (2,483,386 | ) | 9,174,228 | ||||||
| GMP certificate | 5.8 years | 2,414,275 | (821,015 | ) | 1,593,260 | |||||||
| Long-term customer-relationship | 4 years | 7,189,853 | (3,549,236 | ) | 3,640,617 | |||||||
| Others | 218,093 | (67,178 | ) | 150,915 | ||||||||
| Total | $ | 21,479,835 | (6,920,815 | ) | 14,559,020 | |||||||
| December 31, 2009 | ||||||||||||
| Weighted | ||||||||||||
| average | Gross | Net | ||||||||||
| amortization | carrying | Accumulated | carrying | |||||||||
| period | amount | Amortization | amount | |||||||||
| Amortizing intangible assets: | ||||||||||||
| Permits and licenses | 10 years | $ | 11,261,611 | (1,305,157 | ) | 9,956,454 | ||||||
| GMP certificate | 5.8 years | 2,327,885 | (401,661 | ) | 1,926,224 | |||||||
| Long-term customer-relationship | 4 years | 6,941,170 | (1,736,595 | ) | 5,204,575 | |||||||
| Others | 148,244 | (34,985 | ) | 113,259 | ||||||||
| Total | $ | 20,678,910 | (3,478,398 | ) | 17,200,512 | |||||||
Aggregate amortization expense for amortizing intangible assets was $3,422,418 and $3,218,274, for the years ended December 31, 2010 and 2009, respectively. Estimated amortization expenses for the next five years are $3,413,890 in 2011, $3,403,918 in 2012, $1,582,957 in 2013, $1,492,704 in 2014, and $1,160,168 in 2015.
F-14
NOTE 8 – LAND USE RIGHTS, NET
At December 31, 2010 and 2009, land use rights represented:
| December 31, 2010 | December 31, 2009 | |||||
| Land use rights | $ | 5,091,592 | $ | 4,163,140 | ||
| Accumulated amortization | (390,142 | ) | (183,330 | ) | ||
| Land use rights, net | $ | 4,701,450 | $ | 3,979,810 |
Aggregate amortization expense for amortizing land use right was $143,851 and $140,258, for the years ended December 31, 2010 and 2009, respectively.
NOTE 9 – EQUITY METHOD INVESTMENT
The Company’s equity method investment as of December 31, 2010 and 2009 represented 35% equity interest investment in Xi’an Huitian Blood Products Co., Ltd. (“Huitian”).
In October 2008, Shandong Taibang entered into an equity purchase agreement with one of the equity owners of Huitian (“Seller”) to acquire 35% equity interest in Huitian, for cash consideration of $6,454,800 (or RMB 44,000,000). In connection with this transaction, in October 2008, Logic Express entered into an entrust agreement (the “Entrust Agreement”) with Shandong Taibang and the noncontrolling interest holder of Shandong Taibang, pursuant to which, Logic Express would pay the cash consideration of $6,502,901 to the Seller, and would bear the risks and benefits as a 35% equity owner in Huitian. In addition, Logic Express would pay Shandong Taibang $18,204 (or RMB 120,000) per year as compensation for the administrative costs of its holding of the 35% equity interest in Huitian on behalf of Logic Express. Such amount paid and received is eliminated upon consolidation. Logic Express agreed to indemnify the noncontrolling interest holder of Shandong Taibang for any loss arising from the Entrust Agreement and has pledged the Company’s equity interest in Shandong Taibang as collateral against such loss.
The excess of cost over the Company's share of net assets of equity method investees is $1,145,966 at December 31, 2010.
The unaudited financial information for Huitian as of and for the years ended December 31, 2010 and 2009 is as follows:
| December 31, 2010 | December 31, 2009 | |||||
| Current assets | $ | 12,406,517 | $ | 9,912,775 | ||
| Non-current assets | 10,312,678 | 10,195,357 | ||||
| Total assets | 22,719,195 | 20,108,132 | ||||
| Current liabilities | 4,825,668 | 4,031,033 | ||||
| Non-current liabilities | 318,570 | 308,070 | ||||
| Total liabilities | 5,144,238 | 4,339,103 | ||||
| Owners' equity | 17,574,957 | 15,769,029 | ||||
| Total liabilities and owners' equity | $ | 22,719,195 | $ | 20,108,132 | ||
| For the Years Ended | ||||||
| December 31, 2010 | December 31, 2009 | |||||
| Net sales | $ | 10,729,345 | $ | 8,951,234 | ||
| Earnings before income tax expense | 3,694,582 | 2,100,164 | ||||
| Net income | 3,057,831 | 1,619,951 | ||||
| Company’s share of net income | $ | 1,070,241 | $ | 566,984 | ||
NOTE 10 – SHORT-TERM BANK LOANS
The Company’s bank loans at December 31, 2010 and 2009 consisted of the following:
| Maturity | Annual | December 31, | December 31, | |||||||||
| Loans | date | interest rate | 2010 | 2009 | ||||||||
| Short-term bank loan, secured | June 1, 2010 | 5.40% | $ | - | $ | 1,467,000 | ||||||
| Short-term bank loan, unsecured | January 28, 2010 | 5.31% | - | 2,934,000 | ||||||||
| Short-term bank loan, secured | March 21, 2011 | 5.84% | 3,034,000 | - | ||||||||
| Total | $ | 3,034,000 | $ | 4,401,000 |
F-15
Interest expense totaling $291,725 and $1,098,939 was incurred during the years ended December 31, 2010 and 2009, respectively.
As of December 31, 2009, the secured short-term bank loan was borrowed from the Bank of Communications, bore an annual interest rate of 5.40%, and was pledged by the Company’s buildings and land use right with a net carrying amount of $1,238,010 and $433,793, respectively. The pledges were released upon repayment of the loan. The unsecured short-term bank loan was borrowed from the Bank of China and bore an annual interest rate of 5.31% .
As of December 31, 2010, the secured short-term bank loan was borrowed from the Agricultural Bank of China and bears an annual interest rate of 5.84% . The loan is secured by the Company’s buildings with a net carrying amount of $1,584,927. The Company did not have any revolving line of credit as of December 31, 2010.
NOTE 11 – OTHER PAYABLES AND ACCRUED EXPENSES
Other payables and accrued expenses at December 31, 2010 and 2009 consisted of the following:
| December 31, 2010 | December 31, 2009 | |||||
| Payables to potential investors (1) | $ | 7,634,430 | $ | 8,613,272 | ||
| Salaries and bonuses payable | 4,588,641 | 4,725,374 | ||||
| Accruals for selling commission and promotion fee | 3,113,516 | 2,431,380 | ||||
| Payable for construction work | 2,605,583 | 373,397 | ||||
| Investment payable (2) | 71,496 | 2,195,365 | ||||
| Accruals for legal penalties (3) | 857,899 | 451,006 | ||||
| Others | 2,735,165 | 2,726,322 | ||||
| Total | $ | 21,606,730 | $ | 21,516,116 |
| (1) |
The payables to potential investors comprise deposits received from potential strategic investors of RMB 39,760,000 (or $6,031,592) and RMB 50,960,000 (or $7,465,640) as of December 31, 2010 and 2009, respectively, and related interest on these deposits of $1,602,838 and $1,147,632 as of December 31, 2010 and 2009, respectively. |
|
| |
|
In 2007, Guizhou Taibang received an aggregate amount of RMB 50,960,000 (or $7,506,408) from certain potential strategic investors in connection with their subscription to purchase shares in Guizhou Taibang. The registration of the new investors as Guizhou Taibang’s shareholders and the related increase in registered capital of Guizhou Taibang with the Administration for Industry and Commerce are pending due to shareholders dispute as described in the legal proceeding section (see Note 20). During the year ended December 31, 2010, the Company refunded RMB 11,200,000 (or $978,842) to one of the potential investors. | |
|
| |
| (2) |
As of December 31, 2009 and 2010, investment payable represented the payable for the acquisition of Dalin. |
|
| |
| (3) |
Accruals for legal penalties mainly comprise the compensation to Sin Kyung Ye in connection with the dispute over the Guizhou Taibang Technical Consulting Agreement (see Note 20). |
NOTE 12 – INCOME TAX
The Company and each of its subsidiaries file separate income tax returns.
The United States of America
The Company is incorporated in the State of Delaware in the U.S., and is subject to U.S. federal corporate income tax at gradual rates of up to 35%.
British Virgin Islands
Logic Express is incorporated in the British Virgin Islands. Under the current laws of the British Virgin Islands (BVI), Logic Express is not subject to tax on income or capital gains. In addition, upon payments of dividends by Logic Express, no British Virgin Islands withholding tax is imposed.
Hong Kong
Logic Holdings is incorporated in Hong Kong and is subject to Hong Kong’s profits tax rate of 16.5% for the years ended December 31, 2010 and 2009. Logic Holdings did not earn any income that was derived in Hong Kong for the years ended December 31, 2010 and 2009. The payments of dividends by Hong Kong companies are not subject to any Hong Kong withholding tax.
F-16
PRC
The PRC’s statutory income tax rate is 25%. The Company’s PRC subsidiaries are subject to income tax at 25% unless otherwise specified.
On February 12, 2009, Shandong Taibang received the High and New Technology Enterprise certificate from the Shandong provincial government. This certificate entitled Shandong Taibang to enjoy 15% preferential income tax rate for a period of three years from 2008 to 2010.
Guizhou Taibang was entitled to the preferential income tax rate of 15% under the 10-year Western Development Tax Concession, which ended in 2010.
The components of earnings (losses) before income taxes by jurisdictions are as follows:
| For the Years Ended | ||||||
| December 31, 2010 | December 31, 2009 | |||||
| PRC, excluding Hong Kong | $ | 78,868,026 | $ | 63,888,439 | ||
| U.S. | (11,948,208 | ) | (32,201,127 | ) | ||
| BVI | (474,777 | ) | (778,293 | ) | ||
| Hong Kong | (843,982 | ) | (2,071,672 | ) | ||
| Total | $ | 65,601,059 | $ | 28,837,347 | ||
Income tax expense for the years ended December 31, 2010 and 2009 represents PRC current income tax expense and deferred tax benefit:
| For the Years Ended | ||||||
| December 31, 2010 | December 31, 2009 | |||||
| Current income tax expense | $ | 14,709,926 | $ | 11,566,224 | ||
| Deferred tax benefit | (1,101,171 | ) | (1,552,661 | ) | ||
| $ | 13,608,755 | $ | 10,013,563 | |||
Reconciliation between income tax expense and the amounts computed by applying the PRC statutory tax rate of 25% to earnings before income tax expense is as follows:
| For the Years Ended | ||||||
| December 31, 2010 | December 31, 2009 | |||||
| Computed expected tax expense | $ | 16,400,263 | $ | 7,209,337 | ||
| Entities not subject to income tax | 329,690 | 712,491 | ||||
| Non-taxable income | (215,874 | ) | (19,035 | ) | ||
| Non-deductible expenses: | ||||||
| Salary and welfare | 429,134 | 434,323 | ||||
| Share-based compensation | 796,206 | 21,176 | ||||
| Fair value change of derivatives | - | 9,831,212 | ||||
| Convertible notes interest | - | 102,683 | ||||
| Bad debt expense | 887,320 | - | ||||
| Others | 752,722 | 497,394 | ||||
| Tax rate differential | (1,796,923 | ) | (2,898,101 | ) | ||
| Effect of PRC preferential tax rate | (8,126,918 | ) | (6,722,020 | ) | ||
| Bonus deduction on research and development expenses | (175,210 | ) | (124,702 | ) | ||
| Change in valuation allowance | 2,806,835 | 477,944 | ||||
| PRC dividend withholding tax | 1,331,631 | 490,861 | ||||
| Tax effect of equity method investment | 189,879 | - | ||||
| Income tax expense | $ | 13,608,755 | $ | 10,013,563 | ||
The PRC tax rate has been used because the majority of the Company’s consolidated pre-tax earnings arise in the PRC.
As of December 31, 2010 and 2009, significant temporary differences between the tax basis and financial statement basis of assets and liabilities that gave rise to deferred taxes were principally related to the following:
F-17
| For the Years Ended | ||||||
| December 31, 2010 | December 31, 2009 | |||||
| Deferred tax assets arising from: | ||||||
| -Accrued expenses | $ | 1,860,753 | $ | 1,053,771 | ||
| -Convertible notes and derivative liabilities | 5,881,778 | - | ||||
| -Tax loss carryforwards | 2,575,574 | 1,496,885 | ||||
| Gross deferred tax assets | 10,318,105 | 2,550,656 | ||||
| Less: valuation allowance | (8,457,352 | ) | (1,496,885 | ) | ||
| Net deferred tax assets | $ | 1,860,753 | $ | 1,053,771 | ||
| Deferred tax liabilities arising from: | ||||||
| - Intangible assets | 3,458,903 | 3,821,093 | ||||
| - Property, plant and equipment | 445,226 | 454,202 | ||||
| - Equity method investment | 194,705 | - | ||||
| Deferred tax liabilities | $ | 4,098,834 | $ | 4,275,295 | ||
| Classification on consolidated balance sheets: | ||||||
| Deferred tax assets - current | $ | 1,860,753 | $ | 1,053,771 | ||
| Deferred tax liabilities - non-current | $ | 4,098,834 | $ | 4,275,295 | ||
The deferred tax assets for tax loss carryforwards of $2,575,574 as of December 31, 2010 of which $279,578 and $2,295,996 relate to tax loss carryforwards of certain subsidiaries and the Company, respectively. For PRC income tax purposes, certain of the Company’s PRC subsidiaries had tax loss carryforwards of $1,118,311 which would expire in December 2015, if unused. For United States federal income tax purposes, the Company had tax loss carryforwards of approximately $6,752,930, of which $1,268,307, $614,982, $1,113,597, $1,405,718 and $2,350,326 would expire on December 31, 2026, 2027, 2028, 2029 and 2030, respectively, if unused. Management determined it is more likely than not that such deferred tax assets will not be realized, and therefore, full valuation allowances were provided as of December 31, 2010 and 2009. The increase in valuation allowance during the years ended December 31, 2010 and 2009 was $2,806,835 and $477,944, respectively.
In assessing the realizability of the Company’s deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets depends on the generation of future taxable income during the periods in which the deductible temporary differences and the tax loss carryforwards are utilized. The Company considers historical performance, projected future taxable income and tax planning strategies in making its assessment. Management believes it is more likely than not that the Company will realize the benefits of the deferred tax assets, net of the valuation allowances, as of December 31, 2010 and December 31, 2009.
According to the prevailing PRC income tax law and relevant regulations, dividends received by non-PRC-resident enterprises from PRC-resident enterprises are subject to withholding tax at 10%, unless reduced by tax treaties or similar arrangement, on earnings accumulated beginning on January 1, 2008. Undistributed earnings generated prior to January 1, 2008 are exempt from such withholding tax. Further, dividends received by the Company from its overseas subsidiaries are subject to the U.S. federal income tax at 34%, less any qualified foreign tax credits. Due to the Company’s policy of reinvesting its earnings in its overseas business, the Company has not provided for the related deferred tax liabilities on undistributed earnings of $90 million and $54 million as of December 31, 2010 and 2009, respectively. It is not practicable to estimate the amounts of unrecognized deferred tax liabilities thereof.
As of January 1, 2009 and for each of the years ended December 31, 2009 and 2010, the Company and its subsidiaries did not have any unrecognized tax benefits, and therefore no interest or penalties related to unrecognized tax benefits were accrued. The Company does not expect that the amount of unrecognized tax benefits will change significantly within the next 12 months.
The Company and each of its PRC subsidiaries file income tax returns in the United States and the PRC, respectively. The Company is subject to U.S. federal income tax examination by tax authorities for tax years beginning in 2007. According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of taxes is due to computational errors made by the taxpayer or the withholding agent. The statute of limitations is extended to five years under special circumstances where the underpayment of taxes is more than RMB100,000 ($15,000). In the case of transfer pricing issues, the statute of limitations is ten years. There is no statute of limitations in the case of tax evasion. The PRC tax returns for the Company’s PRC subsidiaries are open to examination by the PRC tax authorities for the tax years beginning in 2005.
F-18
NOTE 13 – CONVERTIBLE NOTES
| December 31, 2010 | December 31, 2009 | |||||
| $9,554,140, 3.8% Senior Secured Convertible Notes | $ | 9,554,140 | $ | 9,554,140 | ||
| Less: portion of notes converted | (4,854,140 | ) | (1,000,000 | ) | ||
| unamortized discount | (3,503,767 | ) | (8,464,380 | ) | ||
| Convertible notes | $ | 1,196,233 | $ | 89,760 |
On June 5, 2009, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with certain accredited investors (the “Investors”), pursuant to which the Company agreed to issue to the Investors 3.8% Senior Secured Convertible Notes in the aggregate principal amount of $9,554,140 (the “Notes”) and warrants (the “Warrants” and together with the Notes, the “Subscribed Securities”) to purchase up to 1,194,268 shares of common stock of the Company (the “Warrant Shares” and together with the shares to be converted in the Notes, the “Underlying Securities”). The transaction closed on June 10, 2009.
The coupon rate of the Notes is 3.8% per annum (the “Interest Rate”), payable from the closing until repayment, whether on maturity on June 5, 2011, by acceleration or otherwise. Interest on the Notes is due and payable in cash semi-annually on September 30 and March 31 of each year, commencing on September 30, 2009. The Company has the option to pay the interest due through the issuance of its common stock at a conversion price of $4.00 per share. If the Company defaults in the payment of the principal or interest on the Notes when due, subject to the Investors’ election, the Company is obligated to either (a) redeem all or a portion of the Notes pursuant to the redemption rights discussed below or (b) pay interest on such defaulted amount at a rate equal to the Interest Rate plus 2.0% . The Notes are convertible at any time before maturity into the Company’s common stock at a conversion price of $4.00 per share, subject to certain adjustments as specified in the Purchase Agreement.
The Warrants have a term of 3 years, with an exercise price of $4.80 per share, subject to adjustments as provided in the Warrants, from time to time pursuant to anti-dilution and other customary provisions, and are exercisable by the Investors at any time after the date on which their related Notes are converted, except that if any of the Notes is partially converted, the Investors could only exercise the corresponding portion of the Warrants.
The Company has granted the Investors demand and piggy-back registration rights with respect to the Underlying Securities, pursuant to a registration rights agreement among the Company and the Investors.
The Company paid its placement agent a cash fee, representing 6.1% of the proceeds received in connection with the issuance of the Notes, in addition to a 3-year warrant to purchase 93,750 shares of the Company’s common stock with an exercise price of $6.00 per share. The aggregate fee of $870,417 paid to the placement agent, including the fair value of the warrant issued, was deferred and amortized over the life of the Notes.
The Notes are secured by 3,000,000 shares of common stock of the Company held by Siu Ling Chan (“Ms. Chan”), the Company’s board of directors chairwoman and a principal shareholder of the Company, pursuant to the terms of a Guarantee and Pledge Agreement signed among the Company, the Investors and Ms. Chan. To induce Ms. Chan to enter into the Guarantee and Pledge Agreement with the Investors, the Company agreed to indemnify Ms. Chan for all damages, liabilities, losses and expenses of any kind (“Losses”), which may be sustained or suffered by Ms. Chan, arising out of or in connection with any enforcement action instituted by the Investors pursuant to the Guarantee and Pledge Agreement. The Company’s indemnification obligation is limited to Losses that arise as the result of any negligent or unlawful conduct of the Company that is caused unilaterally by the Company and is beyond Ms. Chan’s control in her capacity as a director of the Company, and will not exceed the market value of the pledged shares as of the closing of the transaction. On December 22, 2009, two of the Company’s Notes holders converted $1,000,000 of their Notes into an aggregate of 250,000 shares of the Company’s common stock. On January 13, 2010, these two Notes holders converted an additional $1,054,140 of their remaining Notes into an aggregate of 263,535 shares of the Company’s common stock. On November 10, 2010, another Notes holder converted $2,800,000 of Notes into an aggregate of 700,000 shares of the Company’s common stock. As of December 31, 2010, Notes in the principal amount of $4,700,000 was outstanding.
The terms of the Notes and Warrants include price adjustment provisions under which the conversion price for the Notes and the exercise price for the Warrants could be affected by future equity offerings undertaken by the Company. As a result, the embedded conversion option in the Notes and Warrants are not considered indexed to the Company’s own stock, and therefore accounted for as derivatives. The economic characteristics and risks of the embedded conversion option in the Notes are not considered clearly and closely related to the economic characteristics and risks of the host debt contract. The embedded conversion option in the Notes met all of the characteristics of a derivative instrument pursuant to ASC Subtopic 815-10. In accordance with ASC Subtopic 815-15, the embedded conversion option in the Notes was separated from the host debt contract and accounted for as a derivative.
F-19
Total principal of the Notes in the amount of $9,554,140 was first allocated to the embedded conversion option in the Notes and to the Warrants based on their fair value on the issuance date of $6,552,505 and $3,826,896, respectively. As a result, the Company recognized an initial charge to income of $825,261 (see Note 14) for the amount by which the fair value of these liabilities exceeded the face amount of the Notes for the year ended December 31, 2009. All changes in the fair value of the embedded conversion option in the Notes and Warrants are recognized in the statements of income until such time as the Notes are converted or redeemed and Warrants are exercised or expired.
The residual amount is allocated to the debt instrument in the amount of $0.01 and is accreted to the principal amount of the Notes using an effective annual interest rate of approximately 365% with the related interest expense recognized in the statements of income. Interest expense is expected to be $3,580,167 for the year ended December 31, 2011.
The fair value of the embedded conversion option in the Notes of December 31, 2010 and 2009 was determined based on the Binominal option pricing model, using the following key assumptions:
| December 31, | December 31, | |||||
| 2010 | 2009 | |||||
| Expected dividend yield | 0% | 0% | ||||
| Risk-free interest rate | 0.17% | 0.75% | ||||
| Time to maturity (in years) | 0.43 | 1.43 | ||||
| Expected volatility | 50.0% | 130.0% | ||||
| Fair value of underlying common shares (per share) | $ | 16.39 | $ | 12.08 |
NOTE 14 – WARRANTS AND OPTIONS
Warrants
On July 18, 2006, the Company entered into a securities purchase agreement with certain accredited investors and completed the sale of 2,200,000 shares of common stock of the Company and warrants to purchase 1,070,000 shares of the Company’s common stock at an exercise price of $2.8425 per share (the “2006 Warrants”). The warrants have a 5-year term and are callable by the Company if the shares trade at 160% of the exercise price for 15 consecutive trading days. On July 28, 2006, the Company issued additional warrants to purchase 214,000 shares of the Company’s common stock at an exercise price of $2.8425 to a placement agent (the “Placement Agent Warrants”). These warrants have a 5-year term and are non-callable. As a result of adopting ASC 815-40, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity's Own Stock” (“ASC 815-40”), effective January 1, 2009, both of the 2006 Warrants and Placement Agent Warrants which were previously equity classified were no longer equity classified because the exercise price of these warrants are denominated in US dollar, a currency other than the Company’s functional currency, Renminbi. In addition, the terms of the 2006 Warrants include a “down-round” provision under which the exercise price could be affected by future equity offerings undertaken by the Company. As a result, these warrants are not considered indexed to the Company’s own stock, and as such, are liability classified. All future changes in the fair value of these warrants are recognized in earnings until such time as the warrants are exercised or expired. As of January 1, 2009, the Company reclassified the original fair value of these warrants of $600,289 from additional paid-in capital to derivative liabilities, as if these warrants were treated as a derivative liability since their date of issuance in July 2006. In addition, the Company reclassified $393,962 from beginning retained earnings as of January 1, 2009 to derivative liabilities to recognize the change in fair value of 2006 Warrants and Placement Agent Warrants from the date of issuance to January 1, 2009. The cumulative-effect adjustment is the difference between the amounts recognized in the statement of financial position before initial application of ASC 815-40, and the amounts recognized in the statement of financial position at initial application of ASC 815-40. The amounts recognized in the statement of financial position as a result of the initial application of ASC 815-40 shall be determined based on the amounts that would have been recognized if the guidance in ASC 815-40 had been applied from the issuance date of the warrant. All of the 2006 Warrants and Placement Agent Warrants were exercised into the Company’s common stock during the year ended December 31, 2009.
In connection with the issuance of the Notes (see Note 13), the Company issued warrants to purchase up to 1,194,268 and 93,750 shares of common stock of the Company to the Investors and placement agent, respectively. During the year ended December 31, 2010, warrants to purchase 256,768 and 93,750 shares of common stock of the Company were exercised by the Investors and placement agent, respectively. At the time of the exercises, the fair value of the warrant derivative liabilities of $3,045,704 was transferred to additional paid-in capital.
F-20
The summary of warrant activities is as follows:
| Weighted | Average | ||||||||
| Warrants | Average | Remaining | |||||||
| Outstanding | Exercise Price | Contractual Life | |||||||
| January 1, 2009 | 1,284,000 $ | 2.84 | 2.55 | ||||||
| Granted | 1,288,018 | 4.89 | 2.44 | ||||||
| Exercised | (1,284,000 | ) | 2.84 | 1.80 | |||||
| December 31, 2009 | 1,288,018 | ||||||||
| Granted | - | - | - | ||||||
| Exercised - cash | (256,768 | ) | 4.80 | 1.44 | |||||
| Exercised – cashless | (93,750 | ) | 6.00 | 1.44 | |||||
| December 31, 2010 | 937,500 |
During the year ended December 31, 2010, the placement agents executed cashless exercise of all the 93,750 placement agent warrants and received 37,251 shares of the Company’s common stock.
The fair values of the warrants outstanding as of December 31, 2010 and 2009 were determined based on the Binominal option pricing model, using the following key assumptions:
| December 31, 2010 | December 31, 2009 | |||||
| Expected dividend yield | 0% | 0% | ||||
| Risk-free interest rate | 0.43% | 1.38% | ||||
| Time to maturity (in years) | 1.43 | 2.43 | ||||
| Expected volatility | 70.0% | 130.0% | ||||
| Fair value of underlying common shares (per share) | $ | 16.39 | $ | 12.08 |
Changes in the management’s estimates and assumptions regarding the expected volatility and valuation of Company’s common stock could significantly impact the estimated fair values of the warrants determined under the Binominal option pricing model and, as a result, the net income and the net income attributable to the Company’s stockholders.
Change in fair value of derivative liabilities for the years ended December 31, 2009 and 2010 is set forth below:
| Increase/ | |||||||||||||||
| Fair value at | (decrease) in fair | Fair value at | |||||||||||||
| January 1, | value for the year | date of | Fair value at | Fair value at | |||||||||||
| 2009 or | ended December | warrants | date of Notes | December 31, | |||||||||||
| issuance date | 31, 2009 | exercised | conversion | 2009 | |||||||||||
| Embedded conversion option in the Notes | $ | 6,552,505 | $ | 15,575,928 | $ | - | $ | (2,168,288 | ) | $ | 19,960,145 | ||||
| Warrants issued to Investors | 3,826,896 | 7,977,356 | - | - | 11,804,252 | ||||||||||
| Warrants issued to placement agent | 287,615 | 609,395 | - | - | 897,010 | ||||||||||
| 2006 Warrants | 994,251 | 3,927,388 | (4,921,639 | ) | - | - | |||||||||
| Loss upon issuance of the Notes (see Note 13) | - | 825,261 | - | - | - | ||||||||||
|
Total |
$ | 11,661,267 | $ | 28,915,328 | $ | (4,921,639 | ) | $ | (2,168,288 | ) | $ | 32,661,407 | |||
| Increase/ | |||||||||||||||
| (decrease) in fair | Fair value at | ||||||||||||||
| Fair value at | value for the year | date of | Fair value at | Fair value at | |||||||||||
| January 1, | ended December | warrants | date of Notes | December 31, | |||||||||||
| 2010 | 31, 2010 | exercised | conversion | 2010 | |||||||||||
| Embedded conversion option in the Notes | $ | 19,960,145 | $ | 1,793,254 | $ | - | $ | (7,191,738 | ) | $ | 14,561,661 | ||||
| Warrants issued to Investors | 11,804,252 | 1,668,067 | (2,376,727 | ) | - | 11,095,592 | |||||||||
| Warrants issued to placement agent | 897,010 | (228,033 | ) | (668,977 | ) | - | - | ||||||||
|
Total |
$ | 32,661,407 | $ | 3,233,288 | $ | (3,045,704 | ) | $ | (7,191,738 | ) | $ | 25,657,253 |
Options
On May 9, 2008, the Company was authorized under the 2008 Equity Incentive Plan to grant share options for the purchase up to 5,000,000 shares of Company’s common stock to employees and directors at various prices as determined by the Board of Directors of the Company.
On May 9, 2008, the Board of Directors granted options to certain directors and employees for the purchase of 937,500 shares of the Company’s common stock with an exercise price of $4.00 that immediate vest. These options expire on June 1, 2018.
F-21
On July 24, 2008, the Board of Directors granted options to three independent directors for the purchase of 60,000 shares of the Company’s common stock with an exercise price of $4.00, of which 30,000 shares vest on January 24, 2009 and the remaining 30,000 shares vest on July 24, 2009. These options expire on July 24, 2018.
On January 7, 2010, the Board of Directors granted options to one employee for the purchase of 50,000 shares of the Company’s common stock with an exercise price of $12.60 that immediate vest. These options expire on January 7, 2020.
On February 4, 2010, the Board of Directors granted options to a newly appointed director for the purchase of 20,000 shares of the Company’s common stock with an exercise price of $10.66, of which 10,000 shares vest on August 4, 2010 and the remaining 10,000 shares vest on February 4, 2011. These options expire on February 4, 2020.
On July 11, 2010, the Board of Directors granted options to four directors and certain employees for the purchase of 160,000 shares and 811,000 shares of the Company’s common stock with an exercise price of $12.26, respectively. These options vest in 12 equal quarters with an initial vesting date of October 11, 2010. These options expire on July 11, 2020.
The fair value of each option granted on May 9, 2008, July 24, 2008, January 7, 2010, February 4, 2010 and July 11, 2010 are estimated on the respective dates of grant using the Black-Scholes option pricing model with the following major assumptions:
| Granted on | May 9, 2008 | July 24, 2008 | January 7, 2010 | February 4, 2010 | July 11, 2010 | ||||||||||
| Expected dividend yield | 0% | 0% | 0% | 0% | 0% | ||||||||||
| Risk-free interest rate | 3.56% | 3.56% | 2.62% | 2.29% | 1.85% | ||||||||||
| Expected term (in years) | 5 | 5 | 5 | 5 | 6.5 | ||||||||||
| Expected volatility | 59.4% | 81.2% | 130.0% | 130.0% | 135.0% |
The volatility of the Company’s common stock was estimated by management based on the historical volatility of the Company’s common stock. The risk free interest rate was based on Treasury Constant Maturity Rates published by the U.S. Federal Reserve for periods applicable to the estimated term of the options. The expected dividend yield was based on the Company’s current and expected dividend policy. Changes in the management’s estimates and assumptions regarding the expected volatility and valuation of the Company’s common stock could significantly impact the estimated fair values of the share options determined under the Black-Scholes option pricing model and, as a result, the net income and the net income attributable to the Company’s stockholders. The weighted average grant date fair value of options granted during the year of 2010 was $10.70. The total intrinsic value of options exercised during the years ended December 31, 2010 and 2009 was $386,332 and $437,474, respectively. For the years ended December 31, 2010 and 2009, the Company recorded stock compensation expense of $2,341,783 and $62,281, respectively, in general and administrative expenses. As of December 31, 2010, approximately $8,799,015 of stock compensation expense with respect to non-vested stock-based awards is to be recognized over approximately 2.5 years. The options activity is as follows:
| Weighted | Average | |||||||||||
| Average | Remaining | Aggregate | ||||||||||
| Options | Exercise | Contractual | Intrinsic | |||||||||
| Outstanding | Price | Life | Value | |||||||||
| January 1, 2009 | 997,500 | $ | 4.00 | 9.43 | $ | - | ||||||
| Granted | - | - | - | |||||||||
| Vested | - | 4.00 | 9.06 | |||||||||
| Exercised | (87,500 | ) | 4.00 | 8.42 | ||||||||
| December 31, 2009 | 910,000 | $ | 4.00 | 8.43 | $ | 7,352,800 | ||||||
| Granted | 1,041,000 | 12.25 | 9.22 | |||||||||
| Vested | 12.26 | 9.22 | ||||||||||
| Exercised - cash | (24,400 | ) | 4.00 | 7.42 | ||||||||
| Exercised - cashless | (20,000 | ) | 4.00 | 7.42 | ||||||||
| December 31, 2010 | 1,906,600 | $ | 8.50 | 8.55 | $ | 15,039,114 |
During the year ended December 31, 2010, a holder of the share options executed cashless exercise of 20,000 share options and received 12,730 shares of common stock of the Company.
As of December 31, 2010, there were 1,006,530 options exercisable.
F-22
NOTE 15 – INTEREST EXPENSE (INCOME)
Interest expense (income), net for the years ended December 31, 2010 and 2009 comprised as following:
| Interest expense (income), net | 2010 | 2009 | ||||
| Interest expense – bank loans | $ | 291,725 | $ | 1,098,939 | ||
| Interest expense – noncontrolling interest holder | - | 2,068,897 | ||||
| Interest expense – potential investors | 405,778 | 1,072,216 | ||||
| Interest expense – Notes | 1,849,493 | 302,010 | ||||
| Interest expense – other | 135,486 | - | ||||
| Interest income | (752,317 | ) | (611,813 | ) | ||
| Total | $ | 1,930,165 | $ | 3,930,249 |
NOTE 16 – STATUTORY RESERVES
The Company’s PRC subsidiaries are required to allocate at least 10% of its after tax profits as determined under generally accepted accounting principal in the PRC to its statutory surplus reserve until the reserve balance reaches 50% of respective registered capital. The accumulated balance of the statutory reserve as of December 31, 2010 and 2009 was $28,820,686 and $17,414,769, respectively.
NOTE 17 – BUSINESS COMBINATION
On September 26, 2008, the Company entered into an equity purchase agreement with Dalin, an investment holding company, and certain equity owners of Dalin (“Equity Owners of Dalin”), to purchase 90% equity interest in Dalin, for cash consideration of RMB194,400,000 (approximately $28.9 million) (“Consideration”), payable in four installments. Dalin in turns held a 54% equity interest in Qianfeng and subsequently renamed as Guizhou Taibang on December 30, 2010. Pursuant to the equity purchase agreement (i) if the Company pays RMB 174,960,000 (approximately $25.6 million), representing 90% of the Consideration, on or before April 7, 2009, the Company will be entitled to share Dalin’s portion of the profit generated by Qianfeng starting from January 1, 2009, and (ii) if the Company fails to pay RMB174,960,000 on or before April 7, 2009, the profit generated by Qianfeng from January 1, 2009 until the day of payment of RMB174,960,000 will be shared by the Company and the Equity Owners of Dalin on a proportionate basis. In addition, the final installment, representing the 10% of the Consideration, should be paid on or before April 9, 2010 with interest accruing at 5.31% per annum.
The Company initiated payment of the third installment of the Consideration on April 7, 2009, in accordance with the instructions provided by Equity Owners of Dalin, which was subsequently paid on April 8 and April 14, 2009. The payment was deemed by Equity Owners of Dalin that the Company fulfilled its obligations under the equity purchase agreement. As a result, the Company was entitled to all the rights and privileges of a 90% shareholder in Dalin, including the right to receive its pro rata share of the profits generated by Dalin's 54% owned subsidiary, Qianfeng, since January 1, 2009, subject to a possible dilution to as low as 41.3%, if potential investors of Qianfeng prevail in a lawsuit to obtain additional equity interests in Qianfeng, or to 52.45%, if the Company decides to ratify a dissenting Qianfeng shareholder’s request to register its additional capital infusion (see Note 20). The Company paid a substantial portion of the final installment, representing 10% of the Consideration, on April 9, 2010. The remaining balance as of December 31, 2010 was $71,496.
According to the equity purchase agreement, as amended, the Company can exercise its shareholder's rights, as well as to take control over all the corporate seals and license of Dalin upon the payment of the second installment, which was paid by the Company on December 14, 2008. However, Dalin's related voting power over its subsidiary, Qianfeng, was not transferred to the Company until the Company's nominees gained control of the board of directors and the management positions of Qianfeng on January 16, 2009. The Company's four nominees were elected to Qianfeng's seven-member Board of Directors in a special meeting on January 16, 2009. In addition, on the same date, Qianfeng's Board of Directors elected a new management team consisting of all Company’s appointees, including Chief Executive Officer, Executive Senior Vice President, Chief Financial Officer and Director of Sales. Therefore, the Company believes that January 16, 2009, the date on which the Company legally obtained control, acquired the assets, assumed the liabilities and became entitled to Dalin's share of the profit generated by Qianfeng, as the acquisition date. The results of Dalin's and its subsidiaries' operations from January 1, 2009 through December 31, 2009 are included in the Company's consolidated statements of income and stockholders’ equity and comprehensive income.
F-23
The following table summarizes the fair value of the assets acquired and liabilities assumed at the date of acquisition:
| Considerations: | |||
| Cash consideration payable by the Company | $ | 28,891,932 | |
| Fair value of noncontrolling interest | 21,525,059 | ||
| $ | 50,416,991 | ||
| Current assets | $ | 26,883,246 | |
| Property, plant and equipment, net | 8,098,959 | ||
| Intangibles | |||
| - Plasma collection permits | 10,891,092 | ||
| - Land use rights | 1,285,968 | ||
| - Long-term customer-relationship | 6,955,384 | ||
| - GMP Certificate | 2,332,652 | ||
| - Software | 6,312 | ||
| Other non-current assets | 3,449,162 | ||
| Total assets | 59,902,775 | ||
| Total liabilities | (21,911,373 | ) | |
| Deferred tax liabilities | (4,749,099 | ) | |
| Total identifiable net assets acquired | 33,242,303 | ||
| Goodwill | 17,174,688 | ||
| Total | $ | 50,416,991 |
The Company determined the fair values primarily based on the evaluation report prepared by an independent appraisal. The fair values of the assets acquired, liabilities assumed and noncontrolling interest were primarily estimated using a combination of income approach, cost approach and market approach valuation techniques. A goodwill of $17.2 million, representing the excess of the consideration and fair value of noncontrolling interest over the fair values assigned to assets acquired and liabilities assumed, was recognized at the acquisition date. The goodwill is mainly for the synergies and cost reduction expected to be achieved. The acquired goodwill is not deductible for tax purposes. The transaction costs of the acquisition were not material, and have been recorded in general and administrative expenses.
NOTE 18 – FAIR VALUE MEASUREMENTS
Management used the following methods and assumptions to estimate the fair value of financial instruments at the relevant balance sheet dates:
-
Short-term financial instruments (including cash and cash equivalents, accounts receivables, other receivables, short-term loans, accounts payable, other payables and accrued expenses, accrued interest, and amounts due to related parties) – The carrying amounts of the short-term financial instruments approximate their fair values because of the short maturity of these instruments.
-
Long-term other payable – The fair value of the Company’s long-term other payable is estimated by discounting future cash flows using current market interest rates offered to the Company and its subsidiaries for debts with substantially the same characteristics and maturities.
-
Derivative liabilities (the embedded conversion option in the Notes and Warrants) – The estimated fair values were determined by using Binominal Option Pricing Model with Level 2 inputs. The following table sets forth, by level within the fair value hierarchy, the Company’s financial instruments that were measured at fair value on a recurring basis as of December 31, 2010 and 2009.
F-24
| Fair Value Measurements Using: | ||||||||||||
| Quoted Prices | ||||||||||||
| in Active Markets | Significant | |||||||||||
| for Identical | Other | Significant | ||||||||||
| Financial Assets | Observable | Unobservable | ||||||||||
| and Liabilities | Inputs | Inputs | ||||||||||
| December 31, 2010 | Total | Level 1 | Level 2 | Level 3 | ||||||||
| Liabilities at fair value: | ||||||||||||
| Derivative liabilities—embedded conversion option in the Notes | $ | 14,561,661 | $ | - | $ | 14,561,661 | $ | - | ||||
| Derivative liabilities—Warrants | 11,095,592 | - | 11,095,592 | - | ||||||||
| December 31, 2009 | Total | Level 1 | Level 2 | Level 3 | ||||||||
| Liabilities at fair value: | ||||||||||||
| Derivative liabilities—embedded conversion option in the Notes | $ | 19,960,145 | - | $ | 19,960,145 | - | ||||||
| Derivative liabilities—Warrants | 12,701,262 | - | 12,701,262 | - | ||||||||
NOTE 19 – SALES
The Company’s sales are primarily derived from the manufacture and sale of Human Albumin and Immunoglobulin products. The Company’s sales by significant types of product for the years ended December 31, 2010 and 2009 are as follows:
| 2010 | 2009 | |||||
| Human Albumin | $ | 67,069,080 | $ | 59,160,664 | ||
| Immunoglobulin products: | ||||||
| Human Hepatitis B Immunoglobulin | 10,622,455 | 3,462,979 | ||||
| Human Immunoglobulin for Intravenous Injection | 47,952,716 | 43,748,854 | ||||
| Human Rabies Immunoglobulin | 7,458,151 | 4,745,205 | ||||
| Human Tetanus Immunoglobulin | 4,051,535 | 2,600,071 | ||||
| Human Immunoglobulin | 1,037,429 | 2,159,246 | ||||
| Others | 1,504,051 | 3,121,136 | ||||
| Totals | $ | 139,695,417 | $ | 118,998,155 |
The Company sells its human blood products to customers in China and India. The amount of human blood products sold to customers in India was less than 10% of total sales for the years ended December 31, 2010 and 2009, respectively.
NOTE 20 – COMMITMENTS AND CONTINGENCIES
Operating lease commitments
Total operating lease commitments for rental of offices and land use rights and buildings of the Company’s PRC subsidiaries as of December 31, 2010 is as follows:
| Year ending December 31, | |||
| 2011 | $ | 328,231 | |
| 2012 | 321,278 | ||
| 2013 | 79,291 | ||
| 2014 | 55,155 | ||
| 2015 | 53,395 | ||
| Years after | 238,818 | ||
| Total minimum payments required | $ | 1,076,168 |
For the years ended December 31, 2010 and 2009, total lease expense amounted to $216,943 and $172,922, respectively.
F-25
Legal proceedings
Bobai County Collection Station
In January 2007, the Company's PRC subsidiary, Shandong Taibang, advanced $413,697 (RMB3.0 million) to Feng Lin, the 20% minority shareholder of Fang Cheng Plasma Company, a Company's majority owned subsidiary, for the purpose of establishing or acquiring a plasma collection station. Mr. Lin and Shandong Taibang intended to establish the Bobai Kangan Plasma Collection Co., Ltd. (“Bobai”) in Bobai County, Guangxi. On January 18, 2007, Shandong Taibang signed a letter of intent to acquire the assets of the Bobai Plasma Collection Station, which was co-owned by Mr. Lin and Mr. Keliang Huang. However, in January 2007, Hua Lan Biological Engineering Co., Ltd. (“Hua Lan”) filed suit in the District Court of Hong Qi District, Xin Xiang City, Henan Province, alleging that Feng Lin, Keliang Huang and Shandong Taibang established and/or sought to operate the Bobai Plasma Collection Station using a permit for collecting and supplying human plasma in Bobai County, that was originally granted to Hua Lan by the government of the Guangxi region, without Hua Lan's permission. The establishment and registration of Bobai was never realized as a result of this law suit. On January 29, 2007, on Hua Lan's motion, the District Court entered an order to freeze funds in the amount of approximately $386,100 (RMB3,000,000) held by the defendants in the case, including approximately $65,750 (RMB500,000) in funds held in Shandong Taibang's bank account in Tai'an City. A hearing was held on June 25, 2007 and judgment was entered against the defendants along with a $226,780 (RMB1,700,000) joint financial judgment. The Company appealed the District Court judgment to the Xinxiang City Intermediate Court. In November 2007, the Intermediate Court affirmed the judgment against the three defendants and increased the amount of the joint financial judgment to approximately $405,954 (RMB3,000,000).
In January 2008, Hua Lan enforced the judgment granted by the Intermediate Court to freeze the Company's bank accounts. Shandong Taibang filed a separate action against Hua Lan before the Tai'an City District Court to seek recovery of any losses in connection with Hua Lan's claim and to request that the Tai'an City District Court preserve Hua Lan's property or freeze up to approximately $411,300 (RMB 3 million) of Hua Lan's assets to secure the return of such funds to the Company. The intermediate court in Tai'an City accepted the application on February 14, 2008 but the matter is still pending. Pending the outcome of the proceedings, Shandong Taibang increased its loss contingency reserve during its fourth quarter of 2007 from approximately $75,593 (RMB566,667) to $133,400 (RMB1,000,000) to cover its share of the enforcement of this judgment. During the fourth quarter of 2008, full amount of the judgment, including Feng Lin and Keliang Huang's portions of the judgment and the related fees, of approximately $456,222 (RMB3,109,900) was withdrawn from Shandong Taibang's account. The Company recorded Feng Lin and Keliang Huang's portion of the judgment, of approximately $304,143 (RMB2,073,234), as receivable as a result of the withdrawal. As of December 31, 2008, the Company determined that it is unlikely that the Company will be able to recover such receivable from those two individuals and wrote off the receivable as bad debt expense. In January 2010, Feng Lin transferred his 20% equity in Fang Cheng Plasma Company as a repayment for such receivable he owed to the Company. As a result, the Company is now the 100% owner of the Fang Cheng Plasma Company.
In October 2009, Shandong Taibang appealed to the High Court of Henan Province requesting the court to reverse judgments from the Hong Qi District Court based on Shandong Taibang's belief that Hua Lan’s involvement in Bobai was in violation of PRC Blood Products Regulations since Hua Lan did not invest, as Shandong Taibang did, in Bobai as required by the Regulation. The Company is awaiting the judgment of the Henan High Court as of the date of this report. In light of the foregoing, it is unlikely that the Company's plan acquisition of the assets of Bobai will go forward.
Dispute among Guizhou Taibang Shareholders over Raising Additional Capital
On May 28, 2007, a 91% majority of Guizhou Taibang's shareholders approved a plan to raise additional capital from private strategic investors through the issuance of an additional 20,000,000 shares of Guizhou Taibang equity interests at RMB2.80 per share. The plan required all existing Guizhou Taibang shareholders to waive their rights of first refusal to subscribe for the additional shares. The remaining 9% minority holder of Guizhou Taibang's shares, the Guizhou Jie'an Company, or Jie'an, did not support the plan and did not agree to waive its right of first refusal. On May 29, 2007, the majority shareholders caused Guizhou Taibang to sign an Equity Purchase Agreement with certain investors, pursuant to which the investors agreed to invest an aggregate of RMB50,960,000 (approximately $7,475,832) in exchange for 18,200,000 shares, or 21.4%, of Guizhou Taibang's equity interests. At the same time, Jie'an also subscribed for 1,800,000 shares, representing its 9% pro rata share of the 20,000,000 shares being offered. The proceeds from all parties were received by Guizhou Taibang in accordance with the agreement.
In June 2007, Jie'an brought suit in the High Court of Guizhou province, China, against Guizhou Taibang and the three other original Guizhou Taibang shareholders, alleging the illegality of the Equity Purchase Agreement. In its complaint, Jie'an alleged that it had a right to acquire the shares waived by the original Guizhou Taibang shareholders and offered to the investors in connection with the Equity Purchase Agreement. On September 12, 2008, the Guizhou High Court ruled against Jie'an and sustained the Equity Purchase Agreement. On November 2008, Jie'an appealed the Guizhou High Court judgment to the People's Supreme Court in Beijing. On May 13, 2009, the People's Supreme Court sustained the original ruling and denied the rights of first refusal of Jie'an over the additional shares waived by the original Guizhou Taibang's shareholders. The registration of the new investors as Guizhou Taibang's shareholders and the related increase in registered capital of Guizhou Taibang with the Administration for Industry and Commerce are still pending. On January 27, 2010, the strategic investors brought suit in the High Court of Guizhou Province against Guizhou Taibang alleging Guizhou Taibang’s failure to register their equity interest in Guizhou Taibang with the local Administration for Industry and Commerce (“AIC”) and requesting the distribution of their share of Guizhou Taibang’s dividends. Dalin was also joined as a co-defendant as it is the majority shareholder and exercises control over Guizhou Taibang’s day-to-day operations. The Company does not expect the strategic investors to prevail because, upon evaluation of the Equity Purchase Agreement, the Company believes that the Equity Purchase Agreement is void due to certain invalid pre-conditions and the absence of shareholder authorization of the initial investment. In the event that Guizhou Taibang is required to return their original investment amount to the strategic investors, Guizhou Taibang has set aside the strategic investors’ initial fund along with RMB10,056,242 (approximately $1,525,532) in accrued interest, and RMB509,600 (approximately $77,306) for the 1% penalty imposed by the agreement for any breach as of December 31, 2010. If strategic investors prevail in their suit, Dalin's interests in Guizhou Taibang could be reduced to approximately 41.3% . The High Court of Guizhou heard the case on April 8, 2010 and encouraged, and accepted by both parties, to settle the dispute outside the court but both parties failed to reach a mutual agreeable term.
F-26
On October 14, 2010, the High Court of Guizhou ruled in favor of the Company and denied the strategic investors’ right as shareholders of Guizhou Taibang, as well as their entitlement to the dividends. On October 26, 2010, the strategic investors appealed to, and subsequently accepted by, the PRC Superior Court in Beijing on the ruling. As of the date of this report, the Company is waiting to hear the ruling from the Court.
During the second quarter of 2010, Jie’an requested that Guizhou Taibang register its 1.8 million shares of additional capital infusion with the local AIC, pursuant to the Equity Purchase Agreement, and such request was approved by the majority shareholders of Guizhou Taibang in a shareholders meeting held in the second quarter of 2010. However, the Board of Directors of the Company is withholding its required ratification of the shareholders’ approval of Jie’an’s request until the outcome of the ongoing litigations. If the Company decides to ratify the approval, Dalin’s ownership in Guizhou Taibang will be diluted from 54% to 52.54% and Jie’an may be entitled to receive its pro rata share of Guizhou Taibang’s profits from the prior 2 years.
Dispute over Guizhou Taibang Technical Consulting Agreement
In 1997, Guizhou Taibang entered into a Technical Cooperation Agreement with Sin Kyung Ye, or Sin, a Korean individual, to provide certain fractionation equipment and transfer processing know-how to Guizhou Taibang. In August 2004, Sin filed a law suit against Guizhou Taibang with the Intermediate Court in Guiyang City, China, alleging non-payment of RMB100,000 (approximately $14,670) for his fractionation equipment and RMB5,000,000 (approximately $733,500) for the transfer of his technological know-how. The Intermediate Court ruled in favor of Sin and found that Guizhou Taibang owed Sin RMB10,376,160 (approximately $1,522,183). Guizhou Taibang appealed the Intermediate Court ruling to the Guizhou High Court. The Guizhou High Court agreed in part with Guizhou Taibang's grounds for appeal and reduced the amount of know-how transfer fee to RMB1,970,413 (approximately $289,060). In May 2007, Sin appealed the Guizhou High Court's decision to the People's Supreme Court in Beijing. The People's Supreme Court heard the case in April 2008 and ruled on December 29, 2009 for Guizhou Taibang to pay RMB4,700,000 (approximately $689,490) as compensation to Sin for technology transfer and RMB100,000 (approximately, $14,670) for unpaid equipment purchase. In December 2010, Sin filed another law suit against Guizhou Taibang with the Guizhou High Court for additional compensation of RMB5,376,160 (USD815,563). The Guizhou High Court ruled in favor of Sin and ruled on December 7, 2010 for Guizhou Taibang to pay the additional compensation. Guizhou Taibang has accrued and accounted for all these expenses as of December 31, 2010 and subsequently paid the judgment in full as of the date of this report.
Guizhou Taibang's Guarantee to a Third Party
In 2007, as a condition to purchase Huang Ping Plasma Station, Guizhou Taibang entered into an agreement with Guizhou Zhongxin Investment Company, or Zhongxin, in which Guizhou Taibang agreed to repay Zhongxin's debt out of Guizhou Taibang's payables to Zhongxin arising from plasma purchased from Zhongxin. In the same agreement, Guizhou Taibang also delivered a guarantee to the Huang Ping County Hospital, the former co-owner of the Huang Ping Plasma Station, that it would pay RMB3,074,342 (approximately, $451,006) in debt that Zhongxin owed to the hospital. On June 1, 2009, Huang Ping Hospital brought suit, in the Huang Ping County People's Court of Guizhou Province, against Zhongxin for non-payment of its payables and debt due to Huang Ping Hospital and against Guizhou Taibang as the guarantor. On November 2, 2009, the court ruled in favor of the plaintiff and Guizhou Taibang as the guarantor became obligated to repay the Zhongxin’s debt to the Huang Ping Hospital on behalf of Zhongxin. In October 2009, Guizhou Taibang appealed to the Middle Court of Kaili District in Guizhou Province which sustained the original judgment on April 8, 2010. Under the Equity Transfer Agreement pursuant to which we acquired a 90% interest in Dalin, Guizhou Taibang's majority shareholder, provides that the sellers will be responsible, based on their pro rata equity interest in Guizhou Taibang, for damages incurred by Guizhou Taibang from Zhongxin's debt and that they will repay Dalin their pro rata share of payments made by Guizhou Taibang to creditors in connection with Zhongxin's debt within 10 days after payment by Guizhou Taibang. The RMB3,074,342 contingent liability and proportionate share of the liability to be recovered from the sellers were properly reflected in the financials as of December 31, 2009. On December 31, 2010, Guizhou Taibang brought suit against Zhongxin in the Middle Court of Guiyang City, to recover the full judgment amount of RMB3,074,342 plus court fee of RMB32,340 that Guizhou Taibang has already paid on behalf of Zhongxin.
On September 13, 2010, Zhongxin countersued the Company for a consideration of RMB500,000 (approximately $74,850) for the alleged loss of its share of income from the Huang Ping Plasma Station since the Company acquired the station in April 2007. The Company believes Zhongxin’s claim is unwarranted since the Company acquired the station from its rightful owner, the Treasury Department of Huangpin County, Guizhou Province.
F-27
Shandong Taibang Equity Interests
Mr. Zu Ying Du was one of the original equity holders in the Company’s operating subsidiary, Shandong Taibang. Pursuant to a joint venture agreement, among the original equity holders, Mr. Du was obligated to make a capital contribution of RMB 20 million (or approximately $2.6 million) for a 25% interest in Shandong Taibang. Mr. Du made this contribution using funds borrowed from the Beijing Chen Da Technology Investment Company, or Beijing Chen Da. Mr. Du failed to repay Beijing Chen Da for his loan of the capital contribution amount. Mr. Du disputes that the money was due and owing. A Beijing court found that Beijing Chen Da had given money to Mr. Du but found that the loan agreement failed to comply with Chinese law. A notice was issued on July 5, 2004 by the Shenzhen Public Security Bureau Economic Crime Investigation Unit requesting a stay of the Beijing action pending their investigation into money laundering relating to the 20 million RMB loan to Zu Ying Du.
Subsequently, Beijing Chen Da entered into an equity transfer agreement with Mr. Du, pursuant to which Mr. Du's 25% equity interest in Shandong Taibang was transferred to Beijing Chen Da as repayment of the RMB 20 million debt. This agreement was signed by Mr. Du's brother who held a power of attorney from Mr. Du. Mr. Du disputes the legitimacy of this transfer and has argued that his brother, Du Hai Shan, exceeded the scope of the power of attorney. Mr. Du sued his brother in the court of Jianli County, Hubei province, relating to the propriety of the brother's actions under the power of attorney. Initially the county court found in its judgment that the act had exceeded the scope of the power of attorney. Subsequently the Intermediate Court of Jingzhou City, Hubei province, ruled on December 10, 2008 to suspend the judgment based on the grounds that the original court lacked jurisdiction to hear the case. The case is slated to be reviewed again by the Hubei Jingzhou Intermediate Court.
Missile Engineering, another original equity holder wholly controlled by Mr. Du, was obligated to contribute RMB 32.8 million (or $4.2 million) for a 41% interest in Shandong Taibang by means of cash, equipment and patent technology. It was obligated to obtain new drug certificate and production license of its patent technology from the government within a stipulated period in order to be recognized as a valid capital contribution, or in the alternative, make a cash payment. The patent technology was valued as RMB 26.4 million (or approximately $3.4 million). However, Missile Engineering failed to obtain the new drug certificate and production license within the stipulated period. Mr. Du also disputed whether the period for obtaining the certificate and license had expired. Pursuant to a stockholders resolution on September 26, 2004, Missile Engineering agreed to sell its 41% interest in Shandong Taibang to Up-Wing Investments Limited (“Up-Wing”) and Up-Wing agreed to take up the obligation of Missile Engineering to pay the RMB 26.4 million in cash. Missile Engineering disputed this transaction and sued the brother of Mr. Du in the court of Jianli County, Hubei province, relating to the propriety of the brother's actions under the power of attorney. Initially the county court found in its judgment that the act had exceeded the scope of the power of attorney. Subsequently the Intermediate Court of Jingzhou City, Hubei province, ruled on December 10, 2008 to suspend the judgment based on the grounds that the original court lacked jurisdiction to hear the case. The case is slated to be reviewed again by the Hubei Jingzhou Intermediate Court.
In June 10, 2005, Beijing Chen Da also sold its equity interest in Shandong Taibang to Up-Wing Investments Limited, or Up-Wing, pursuant to a share transfer agreement, which became effective on September 2, 2005, upon approval by the Shandong Provincial Department of Foreign Trade and Economic Cooperation, or the Shandong COFTEC. In March 2006, Up-Wing sold its equity interests in Shandong Taibang to Logic Express, the Company’s subsidiary.
In 2006, Missile Engineering applied for arbitration before the China International Economic and Trade Arbitration Commission, or CIETAC, to challenge the effectiveness of the transfer to Up-Wing Investments Limited, of the equity interests in Shandong Taibang formerly owned by Missile Engineering. The equity transfer had been approved by the Shandong Provincial Department of Foreign Trade and Economic Cooperation, or the Shandong COFTEC. Missile Engineering later voluntarily withdrew this application and instead applied for administrative reconsideration of the equity transfer, but this application was rejected by the Ministry of Commerce in 2007. Missile Engineering applied with the District Court of Lixia District, Jinan City, Shandong province requesting revocation of Shandong COFTEC's approval of the equity transfer to Up-wing by Missile Engineering. Missile Engineering later voluntarily withdrew the action. In April 2007, Logic Express initiated an arbitration proceeding before the Shandong Tai'an Arbitration Committee, to establish that Logic Express is the lawful shareholder of Shandong Taibang. The parties to that proceeding were Logic Express Ltd. and Shandong Taibang Biological Products Co., Ltd. The Arbitration Committee's decision on September 6, 2007 confirmed that Logic Express had legitimate ownership as a result of the transfer of Shandong Taibang. Up-Wing started an action in the Intermediate Court of Tai'an City, Shandong province requesting the court to establish that Up-Wing is the lawful shareholder of Shandong Taibang. The Intermediate Court of Tai'an City, Shandong province on December 20, 2007 rejected the application on the basis that the same matter had been tried by the arbitration panel.
Up-Wing filed a defamation case in the District Court of Hi-technology and Industry Development District, Tai'an City, Shandong province claiming defamation against Mr. Du and the 21st Century Economic Report Newspaper. Judgment in favor of Up-Wing was rendered on July 22, 2008 ordering the newspaper and Mr. Du to publish an apology to Up-Wing.
F-28
Mr. Du and Missile Engineering subsequently filed two actions in the Intermediate Court of Wuhan City, Hubei province, against Du Hai Shan, his brother, Beijing Chen Da and Logic Express, requesting that the court restore the equity interests originally held by the plaintiffs, 25% equity interest held by Mr. Du and 41% equity interest held by Missile Engineering and the court issued a preliminary order attaching 66% of the equity of Shandong Taibang pending the outcome of the case. On September 25, 2009, the Higher People's Court of Hubei overruled the Wuhan Intermediate Court's acceptance of jurisdiction over the case and ruled that the Tai'an Intermediate Court in Shandong Province, where the Company is located, had the proper jurisdiction over the parties' dispute. The court ruled that while the plaintiffs had the right to bring a lawsuit for the validity of the share transfer agreement because they did not attend the previous arbitration hearing and never reached an arbitration agreement regarding their dispute, the Tai'an Intermediate Court has the proper jurisdiction over the dispute pursuant to the prior agreement of the parties. As a result, the attached 66% of the equity of Shandong Taibang was released back to Logic Express. On November 17, 2009, the Wuhan Intermediate Court permitted Mr. Du and Missile Engineering to withdraw their suits against Logic Express and the other defendants.
On December 31, 2010, the Company received a Notice from the PRC Supreme Court, advising the Company that the PRC Supreme Court has accepted an appeal for judicial review of the Hubei High Court ruling dismissing case. The Company has submitted its counter-argument and related material to the PRC Supreme Court on November 2, 2010 and the PRC Supreme Court ruled in favor of the Company on November 30, 2010 and denied Mr. Du and Missile Engineering’s appeal to the ruling of Wuhan Intermediate Court.
Dispute over Raw Plasma Supply Agreement with Xintai
On March 10, 2009, Henan Xintai Medicine Company (previously known as Henan Zhongtai Medicine, “Xintai”) brought suit against Shandong Taibang and its two wholly-owned plasma collecting subsidiaries in Shandong for breach of a raw plasma supply agreement. The suit was subsequently withdrawn by Xintai on May 31, 2009. The agreement, signed by Shandong Taibang and Xintai on October 10, 2006, requires the two subsidiaries to provide to Xintai 45 metric tons of raw plasma per year from 2007 to 2009. The subsidiaries provided more than 34 metric tons of plasma to Xintai during 2007. However, the Company ceased its delivery of plasma to Xintai in late 2007 to avoid contravention of new PRC regulation. Clause 43 of an October 31, 2007, PRC State Department Regulation on Plasma Collection Stations, prohibits plasma collecting stations from providing raw plasma to any manufacturer other than their direct parent (the “Regulation”). On March 12, 2009, Shandong Taibang filed a suit in the Shandong Tai'an Middle Court against Xintai seeking damages of RMB50,000 (approximately, $7,335) for the plasma already supplied to Xintai during 2007. On June 29, 2009, Xintai re-filed its suit in the Shandong Tai'an Middle Court against Shandong Taibang and the two subsidiaries seeking compensation of RMB6,000,000 (approximately, $880,200) for alleged breach of contract and demanding that Shandong Taibang and the subsidiaries continue to honor the agreement. On October 20, 2009, the Tai'an Middle Court combined and heard the two suits and ruled on January 20, 2010 in favor of Shandong Taibang on all accounts.
On February 17, 2010, Xintai appealed to the High Court of Shandong Province and on August 10, 2010, the High Court remanded the suit to the Tai’an Middle Court for retrial on grounds of insufficient evidence.
On November 8, 2010, the Company and Xintai reached an out of court settlement whereby Shandong Taibang agreed to pay Xintai RMB 4,000,000 (approximately $598,800), payable on or before November 22, 2010, and RMB 43,950 (approximately $6,430) in court costs. The Company has paid the costs of its settlement agreement with Xintai under legal expenses as of December 31, 2010.
F-29
NOTE 21 – RELATED PARTY TRANSACTIONS
The material related party transactions undertaken by the Company with related parties for the years ended December 31, 2010 and 2009 are presented as follows:
| Assets | Purpose | December 31, 2010 | December 31, 2009 | ||||||
| Accounts receivable – related party(1) | Processing fees /sales | $ | 212,611 | $ | 222,617 | ||||
| Liabilities | Purpose | December 31, 2010 | December 31, 2009 | ||||||
| Short term loans – noncontrolling interest shareholders(2) | Loan | - | 3,652,500 | ||||||
| Accrued interest – noncontrolling interest shareholders(2) | Interest payable | $ | - | $ | 2,068,526 | ||||
| Other payable – related parties(3) | Loan | $ | 2,195,123 | $ | 2,122,772 | ||||
| Other payable – related parties(4) | Contribution | 997,017 | 964,168 | ||||||
| Total other payable – related parties | $ | 3,192,140 | $ | 3,086,940 |
(1) Guizhou Taibang provides processing services for Guizhou Eakan Co., Ltd. (“Guizhou Eakan”), the affiliate of one of the Guizhou Taibang’s noncontrolling interests holders. The Company’s total processing services income from Guizhou Eakan amounted to $499,128 and $705,018 for the years ended December 31, 2010 and 2009, respectively. In addition, Guizhou Taibang made sales to Guizhou Eakan, amounting to $521,306, for the year ended December 31, 2010. As of December 31, 2010 and 2009, accounts receivable due from Guizhou Eakan amounted to $212,611 and $222,617, respectively. The outstanding balance as of December 31, 2010 was settled in cash in January 2011.
(2) On April 6, 2009, Logic Express entered into an agreement with Shandong Institute, the noncontrolling interest holders in Shandong Taibang, pursuant to which, Shandong Institute would provide an advance to assist Logic Express’s purchase of 90% Dalin's equity interests (see Note 17). Under the terms of the agreement, Shandong Institute agreed to provide advance of $3,652,500 (RMB25,000,000), representing 12.86% of the Company’s purchase consideration in Dalin to the Company for one year, bearing interest equal to the higher of a proportionate share of the net income of Dalin during the year ended December 31, 2009 or 6% per annum. On April 12, 2010, the Company fully paid the advance from Shandong Institute and the interest of approximately $1.3 million, which was less than the Company’s previous estimate by approximately $0.9 million. The Company recorded the difference between the previous estimate and actual payment in other income of the consolidated statement of income for the year ended December 31, 2010.
(3) Guizhou Taibang has payables to Guizhou Eakan Investing Corp., amounting to approximately $2,195,123 (RMB14,470,160). Guizhou Eakan Investing Corp. is one of the noncontrolling interest holders of the Guizhou Taibang. The Company borrowed this interest free advance for working capital purpose for Guizhou Taibang. The balance is due on demand.
(4) Guizhou Taibang has payables to Guizhou Jie’an, a noncontrolling interest holder of Guizhou Taibang, amounting to approximately $997,017 (RMB 6,569,840). In 2007, Guizhou Taibang received additional contributions from Guizhou Jie’an of $962,853 to maintain Jie’an equity interest in Guizhou Taibang at 9%. However, due to a legal dispute among shareholders over raising additional capital as discussed in the legal proceeding section (see note 20), the money may be returned to Jie’an. During the second quarter of 2010, Jie’an requested that Guizhou Taibang register its 1.8 million shares of additional capital infusion with the local Administration for Industry and Commerce, pursuant to the equity purchase agreement, and such registration was approved by the majority shareholders of Guizhou Taibang in a shareholders meeting held in the second quarter of 2010. However, the Board of Directors of the Company is withholding its required ratification of the shareholders’ approval of Jie’an’s request until the outcome of the ongoing litigations. If the Company decided to ratify the approval, Dalin’s ownership in Guizhou Taibang will be diluted from 54% to 52.54% and Guizhou Jie’an will be entitled to receive its pro rata share of Guizhou Taibang’s profits from the prior 2 years.
F-30
NOTE 22 - EARNINGS PER SHARE
The following table sets forth the computation of basic and diluted income per share for the periods indicated:
| 2010 | 2009 | |||||
| Numerator used in basic net income per share: | ||||||
| Net income attributable to China Biologic Products, Inc. | $ | 31,542,883 | $ | 2,208,126 | ||
| Decrease in fair value of derivative liabilities | (228,033 | ) | - | |||
| Numerator used in diluted net income per share | $ | 31,314,850 | $ | 2,208,126 | ||
| Weighted average shares: | ||||||
| 2010 | 2009 | |||||
| Basic | 23,586,506 | 21,754,911 | ||||
| Effect of dilutive common share equivalents: | ||||||
| Diluted effect of placement agent warrants | 8,472 | - | ||||
| Diluted effect of stock option | 581,454 | 194,727 | ||||
| Diluted | 24,176,432 | 21,949,638 | ||||
| Net income per ordinary share-basic | $ | 1.34 | $ | 0.10 | ||
| Earnings per share - diluted | $ | 1.30 | $ | 0.10 |
During the year ended December 31, 2010, the Subscribed Securities and 1,021,000 options at an average exercise price of $12.43 are excluded from the calculation of diluted earnings per share since they did not have any dilutive effect.
During the year ended December 31, 2009, both the Subscribed Securities and all of the warrants are excluded from the calculation of diluted earnings per share since they did not have any dilutive effect.
NOTE 23 – CONCENTRATIONS AND CREDIT RISKS
The Company's operations are carried out in the PRC and are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. Accordingly, the Company's business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC economy. The Company's results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company maintains balances at financial institutions which, from time to time, may exceed Federal Deposit Insurance Corporation insured limits for its bank accounts located in the United States or may exceed Hong Kong Deposit Protection Board insured limits for its bank accounts located in Hong Kong. Cash balances maintained at financial institutions or state-owned banks in the PRC are not covered by insurance. Total cash in banks as of December 31, 2010 and 2009 amounted to $64,443,315 and $53,576,495, respectively, $1,473,917 and $1,009,053 of which are covered by insurance, respectively. The Company has not experienced any losses in uninsured bank deposits and does not believe that it is exposed to any significant risks on its cash in bank accounts.
The Company’s major product, human albumin: 20%/10ml, 20%/25ml, 20%/50ml, 10%/10ml, 10%/25ml and 10%/50ml, accounted for 48.0% and 49.7% of the total sales for the years ended December 31, 2010 and 2009, respectively. If the market demands for human albumin cannot be sustained in the future or if the price of human albumin decreases, it would adversely affect the Company’s operating results.
All of the Company’s customers are located in the PRC and India. As of December 31, 2010 and 2009, the Company had no significant concentration of credit risk. There were no customers that individually comprised 10% or more of the sales during the years ended December 31, 2010 and 2009. No individual customer represented 10% or more of trade receivables at December 31, 2010 and 2009. The Company performs ongoing credit evaluations of its customers’ financial condition and, generally, requires no collateral from its customers.
There was one vendor that individually comprised 10% or more of the purchase during the year ended December 31, 2010 and there were two vendors that individually comprised 10% or more of the purchase during the same period in 2009. There was one individual vendor that represented more than 10% of accounts payables at December 31, 2010 and 2009, respectively.
NOTE 24 – SUBSEQUENT EVENTS
On January 4, 2011, Logic China entered into an equity transfer agreement to purchase 10% of the equity interest in Dalin from its noncontrolling shareholder, Shaowen Fan, for cash consideration of $7,585,000 (RMB 50,000,000). The equity transfer was registered with the local Administration for Industry and Commerce on January 26, 2011 and the purchase price was fully paid as of February 22, 2011 in accordance with the equity transfer agreement. Upon completion of the transaction, Dalin became the Company’s indirect 100% owned subsidiary.
F-31
On January 19, 2011, the Board of Directors of Guizhou Taibang declared a $12,136,000 (RMB 80,000,000) distribution to its 54% shareholder, Dalin, its 19% shareholder, Guizhou Eakan, its 18% shareholder, Shenzhen Yigongshengda, and its 9% shareholder, Jie’an, in accordance with their respective equity interest in Guizhou Taibang. Guizhou Taibang paid $9,102,000 to the shareholders in March 2011.
NOTE 25 – CHINA BIOLOGIC PRODUCTS, INC. (PARENT COMPANY)
The following represents condensed unconsolidated financial information of the Parent Company only:
Condensed Balance Sheets
| December 31, 2010 | December 31, 2009 | |||||
| Cash and cash equivalents | $ | 103,894 | $ | 30,275 | ||
| Prepayments and prepaid expenses | 126,555 | 659,102 | ||||
| Property, plant and equipment, net | 11,644 | - | ||||
| Investment in and amounts due from subsidiaries | 130,004,635 | 85,299,954 | ||||
| Total Assets | 130,246,728 | 85,989,331 | ||||
| Other payables and accrued expenses | 4,193,446 | 3,541,503 | ||||
| Convertible notes | 1,196,233 | 89,760 | ||||
| Derivative liabilities-embedded conversion option in the Notes | 14,561,661 | 19,960,145 | ||||
| Derivative liabilities- Warrants | 11,095,592 | 12,701,262 | ||||
| Total Liabilities | 31,046,932 | 36,292,670 | ||||
| Total Equity | 99,199,796 | 49,696,661 | ||||
| Total Liabilities and Equity | $ | 130,246,728 | $ | 85,989,331 | ||
|
Condensed Statement of Income: |
||||||
| For the Years Ended | ||||||
| December 31, 2010 | December 31, 2009 | |||||
| Equity in income of subsidiaries | $ | 43,680,970 | $ | 34,409,253 | ||
| General and administrative expenses | (6,667,836 | ) | (2,975,114 | ) | ||
| Other expenses | (2,047,084 | ) | (310,685 | ) | ||
| Change in fair value of derivative liabilities | (3,233,288 | ) | (28,915,328 | ) | ||
| Profit before income tax expense | 31,732,762 | 2,208,126 | ||||
| Income tax expense | (189,879 | ) | - | |||
| Net Income | $ | 31,542,883 | $ | 2,208,126 | ||
|
Condensed Statement of Cash Flows: |
||||||
| For the Years Ended | ||||||
| December 31, 2010 | December 31, 2009 | |||||
| Net cash provided by operating activities | $ | 86,060 | $ | - | ||
| Net cash used in investing activities | - | - | ||||
| Net cash used in financing activities | (12,441 | ) | - | |||
| Net increase in cash | 73,619 | - | ||||
| Cash at beginning of year | 30,275 | 30,275 | ||||
| Cash at end of year | $ | 103,894 | $ | 30,275 | ||
F-32
EXHIBIT INDEX
| Exhibit No. | Description | |
| 2.1 | Share Exchange Agreement between the Company, Logic Express Limited and the selling stockholders signatory thereto, dated as of July 18, 2006 (incorporated by reference to Exhibit 2 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 3.1 | Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 3.2 | Amended and Restated By-Laws, adopted on March 31, 2009 | |
| 4.1 | Form of Registration Rights Agreement, dated June 5, 2009 (incorporated by reference to Exhibit 4.1 of the Current Report on Form 8-K filed by the Company on June 5, 2009). | |
| 4.2 | Form of 3.8% Convertible Senior Secured Note due 2011 (incorporated by reference to Exhibit 4.2 of the Current Report on Form 8-K filed by the Company on June 5, 2009). | |
| 4.3 | Form of Warrant (incorporated by reference to Exhibit 4.3 of the Current Report on Form 8-K filed by the Company on June 5, 2009) | |
| 10.1 | China Biologic Products, Inc. 2008 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.2 | Form of Stock Option Award Agreement of China Biologic Products, Inc. (incorporated by reference to Exhibit 10.5 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.3 | Group Secondment Agreement, dated October 28, 2002, between Shandong Taibang Biological Products Co., Ltd. and the Shandong Institute (English Translation) (incorporated by reference to Exhibit 10.1 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.4 | Amended and Restated Joint Venture Agreement, between Logic Express Limited and the Shandong Institute, dated as of March 12, 2006 (English Translation) (incorporated by reference to Exhibit 10.2 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.5 | Letter of Intent for Equity Transfer, between Logic Express Limited and the Shandong Institute, dated as of June 10, 2006 (English Translation) (incorporated by reference to Exhibit 10.3 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.6 | Joint Venture and Cooperation Agreement between Mr. Fan Qingchun, Shandong Taibang Biological Products Co., Ltd. and Shaanxi Power Construction Corporation, dated September 12, 2008 (incorporated by reference to Exhibit 10.2 of the current report on Form 8-K, filed by the Company on October 16, 2008) | |
| 10.7 | Agreement on Equity Transfer, Acquisition, Joint Venture and Cooperation, among Shandong Taibang Biological Products Co., Ltd., Shaanxi Power Construction Corporation and Mr. Fan Qingchun, dated September 12, 2008 (incorporated by reference to Exhibit 10.3 of the current report on Form 8-K, filed by the Company on October 16, 2008) |
66
| Exhibit No. | Description | |
| 10.8 | (Shareholder) Agreement among Shandong Taibang Biological Products Co., Ltd., Logic Express Limited and Biological Institute, dated September 12, 2008 (incorporated by reference to Exhibit 10.4 of the current report on Form 8-K, filed by the Company on October 16, 2008) | |
| 10.9 | Equity Transfer Agreement, dated September 26, 2008, among Logic Express Limited, Chongqing Dalin Biologic Technologies Co., Ltd. and certain shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on October 2, 2008) | |
| 10.10 | Equity Transfer Agreement, between Shandong Taibang Biological Products Co., Ltd. and Mr. Fan Qingchun, dated October 10, 2008 (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on October 16, 2008) | |
| 10.11 | Supplemental Agreement, dated November 3, 2008, among Logic Express Limited, Fan Shaowen, as representative of the shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. and Chongqing Dalin Biologic Technologies Co., Ltd. (English Translation) (incorporated by reference to Exhibit 10.2 of the current report on Form 8-K, filed by the Company on November 7, 2008) | |
| 10.12 | Second Supplemental Agreement, dated November 14, 2008, among Logic Express Limited, Fan Shaowen as representative of the shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. and Chongqing Dalin Biologic Technologies Co., Ltd. (English Translation) (incorporated by reference to exhibit 10.3 of the current report on Form 8-K, filed by the Company on November 20, 2008) | |
| 10.13 | Amended Equity Transfer Agreement, dated December 12, 2008, among Logic Express Limited, Chongqing Dalin Biologic Technologies Co., Ltd., and certain shareholders of Chongqing Dalin Biologic Technologies Co., Ltd. (English Translation) (incorporated by reference to exhibit 10.4 of the current report on Form 8-K, filed by the Company on December 18, 2008) | |
| 10.14 | English Translation of the Equity Transfer and Entrustment Agreement, dated April 6, 2009, among Logic Express, Shandong Taibang Biological Products Co., Ltd. and the Shandong Institute of Biological Products (incorporated by reference to Exhibit 10.6 of the current report on Form 8-K filed by the Company on April 13, 2009) | |
| 10.15 | Raw Plasma Supply Agreement, between Shandong Taibang Biological Products Co., Ltd. and Qi He Plasma Collection Station, dated as of December 30, 2005 (English Translation) (incorporated by reference to Exhibit 10.4 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.16 | Raw Plasma Supply Agreement, between Shandong Taibang Biological Products Co., Ltd. and the Xia Jin Plasma Collection Station, dated as of December 30, 2005 (English Translation) (incorporated by reference to Exhibit 10.5 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.17 | Raw Plasma Supply Agreement, between Shandong Taibang and the Zhang Qiu Plasma Collection Station, dated as of December 30, 2005 (English Translation) (incorporated by reference to Exhibit 10.6 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.18 | Plasma Processing Agreement, between Shandong Taibang Biological Products Co., Ltd. and Qi He An Tai Plasma Collection Co., Ltd., dated as of January 2, 1007 (English Translation) (incorporated by reference to Exhibit 10.9 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.19 | Plasma Processing Agreement, between Shandong Taibang Biological Products Co., Ltd. and the Xia Jin An Tai Plasma Collection Co., Ltd., dated as of January 2, 2007 (English Translation) (incorporated by reference to Exhibit 10.10 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.20 | Plasma Processing Agreement, between Shandong Taibang Biological Products Co., Ltd. and the Zhang Qiu An Tai Plasma Collection Co., Ltd., dated as of January 2, 2007 (English Translation) (incorporated by reference to Exhibit 10.11 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.21 | Raw Plasma Supply Agreement, between Shandong Taibang Biological Products Co., Ltd. and Liao Cheng Tiantan Plasma Collection Co. Ltd., dated as of November 1, 2007 (English Translation) (incorporated by reference to Exhibit 10.23 of the registration statement on Form SB-2/A, filed by the Company on December 28, 2007) | |
| 10.22 | Asset Purchase Agreement, between Xia Jin An Tai Plasma Collection Co., Ltd. and Xia Jin County Plasma Collection Station, dated as of October 20, 2006 (English Translation) (incorporated by reference to Exhibit 10.15 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.23 | Asset Purchase Agreement, between Liao Cheng An Tai Plasma Collection Co., Ltd. and Yang Gu County Plasma Collection Station, dated as of November 3, 2006 (English Translation) (incorporated by reference to Exhibit 10.16 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) |
67
| Exhibit No. | Description | |
| 10.24 | Asset Purchase Agreement, between Qi He An Tai Plasma Collection Co., Ltd. and Qi He County Plasma Collection Station, dated as of November 9, 2006 (English Translation) (incorporated by reference to Exhibit 10.14 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.25 | Asset Purchase Agreement, between He Ze An Tai Plasma Collection Co., Ltd and Yun Cheng County Plasma Collection Station, dated as of December 15, 2006 (English Translation) (incorporated by reference to Exhibit 10.22 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.26 | Asset Purchase Agreement, between Zhang Qiu An Tai Plasma Collection Co., Ltd. and Zhang Qiu Plasma Collection Station, dated as of December 31, 2006 (English Translation) (incorporated by reference to Exhibit 10.12 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.27 | Asset Purchase Agreement, between Guang Xi Huan Jiang Missile Plasma Collection Co., Ltd. and Huan Jiang Maonan Autonomous County Plasma Collection Station, dated as of April 24, 2007 (English Translation) (incorporated by reference to Exhibit 10.13 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.28 | Asset Purchase Agreement, between Fang Cheng Plasma Collection Co., Ltd. and Fang Cheng Plasma Company, dated as of April 30, 2007 (English Translation) (incorporated by reference to Exhibit 10.21 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.29 | Asset Purchase Agreement, between Guang Xi Huan Jiang Missile Plasma Collection Co., Ltd. and Huan Jiang Maonan Autonomous County Plasma Collection Station, dated as of August 5, 2007 (English Translation) (incorporated by reference to Exhibit 10.13 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.30 | Trademark Licensing Agreement, dated as of February 27, 2007 (English Translation) (incorporated by reference to Exhibit 10.17 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.31 | Loan Agreement, dated as of November 30, 2006, among Shandong Taibang and the Shandong Institute and Logic Express (English Translation) (incorporated by reference to Exhibit 10.18 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.32 | Supplementary Agreement, dated as of September 1, 2007, among Shandong Taibang Biological Products Co., Ltd., the Shandong Institute and Logic Express Limited (English Translation) (incorporated by reference to Exhibit 10.19 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.33 | Form of Bank of Communications Loan Contract, among Shandong Taibang and the Tai’an Branch of the Bank of Communications (English Translation) (incorporated by reference to Exhibit 10.20 of the registration statement on Form SB-2/A, filed by the Company on December 3, 2007) | |
| 10.34 | China Bank of Communications Loan Contract, dated October 28, 2008, between Shandong Taibang Biological Products Co. Ltd. and Bank of Communications, Tai’an Branch (English Translation) (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on November 3, 2008) | |
| 10.35 | Loan Agreement between Shandong Taibang Biological Products Co., Ltd. and Bank Of China, dated January 8, 2009 (English Translation) (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on January 13, 2009) | |
| 10.36 | Employment Agreement, between Y. Tristan Kuo and China Biologic Products, Inc., dated May 9, 2008 (incorporated by reference to Exhibit 10.3 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.37 | Employment Agreement, between Chao Ming Zhao and China Biologic Products, Inc., dated May 9, 2008 (incorporated by reference to Exhibit 10.4 of the current report on Form 8-K, filed by the Company on May 13, 2008) | |
| 10.38 | Form of Director’s Employment Agreement of China Biologic (incorporated by reference to Exhibit 10.8 of the registration statement on Form SB-2, filed by the Company on September 5, 2007) | |
| 10.39 | Form of Independent Director Agreement of China Biologic Products, Inc. (incorporated by reference to Exhibit 10.1 of the current report on Form 8-K, filed by the Company on July 30, 2008) | |
| 10.40 | Form of Indemnity Agreement of China Biologic Products, Inc. (incorporated by reference to Exhibit 10.2 of the current report on Form 8-K, filed by the Company on July 30, 2008) |
68
*Filed herewith.
69
