Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - Loxo Oncology, Inc. | a16-23351_1ex99d3.htm |
| EX-99.2 - EX-99.2 - Loxo Oncology, Inc. | a16-23351_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Loxo Oncology, Inc. | a16-23351_1ex99d1.htm |
| 8-K - 8-K - Loxo Oncology, Inc. | a16-23351_18k.htm |
Exhibit 99.4
Corporate Update December 19, 2016 1

Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: "anticipate," "intend," "plan," "goal," "seek," "believe," "project," "estimate," "expect," "strategy," "future, "likely," "may," "should," "will" and similar references to future periods. Examples of forward-looking statements include, among others, statements we make regarding our future financial performance, business plans and objectives, timing and success of our clinical trials, our ability to obtain regulatory approval or the timing of regulatory filings, the potential therapeutic benefits and economic value of our lead product candidate and second generation product candidate, potential growth opportunities, financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: those related to our future financial performance, our ability to develop and maintain partnerships, our ability to identify and develop new products in a timely manner, the outcome, cost and timing of our product development activities and clinical trials, market size and acceptance of our targeted small molecule therapeutics and diagnostics, our ability to maintain, protect and enhance our brand and intellectual property, our ability to continue to stay in compliance with applicable laws and regulations, our ability to scale our business and make key hires and such other factors as discussed under the section titled “Risk Factors” and elsewhere in a registration statement (including a prospectus) that we filed with the Securities and Exchange Commission (“SEC”) as well as our other filings and the documents incorporated by reference therein, with the SEC. Any forward-looking statement made by us in this presentation and the accompanying oral presentation is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Certain information in this slide deck on larotrectinib is derived from the presentation made at the 2016 ESMO Asia Congress by Dr. Bauer, an independent third-party investigator. 2

Agenda for Today’s Call Introduction Review updated larotrectinib (LOXO-101) adult Phase 1 data presented at ESMO Asia Outline larotrectinib development timelines and commercial opportunity LOXO-195 overview and program rationale LOXO-292 overview and program rationale Guidance on corporate milestones 3

Loxo Oncology: Foundational Theses Targeted therapies remain an essential category for new drug development in oncology Address low mutational burden/ IO-refractory, oncogene addicted cancers Leverage clinical and pathology trends towards comprehensive tumor profiling Potency, specificity and well-behaved pharmacology define the best-in-class drugs Should carry through to response rate, duration of response, safety Acquired resistance (when responding tumors progress) may be drugged through second generation profiles and extend durable disease control for patients 4

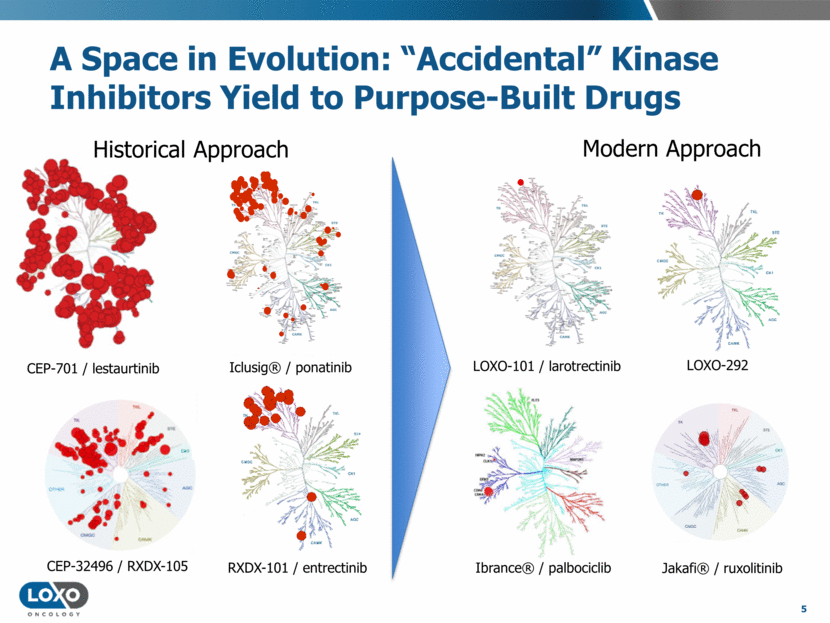
A Space in Evolution: “Accidental” Kinase Inhibitors Yield to Purpose-Built Drugs 5 Historical Approach Modern Approach CEP-701 / lestaurtinib TRK LOXO-101 / larotrectinib LOXO-292 CEP-32496 / RXDX-105 RXDX-101 / entrectinib Ibrance® / palbociclib Jakafi® / ruxolitinib Iclusig® / ponatinib
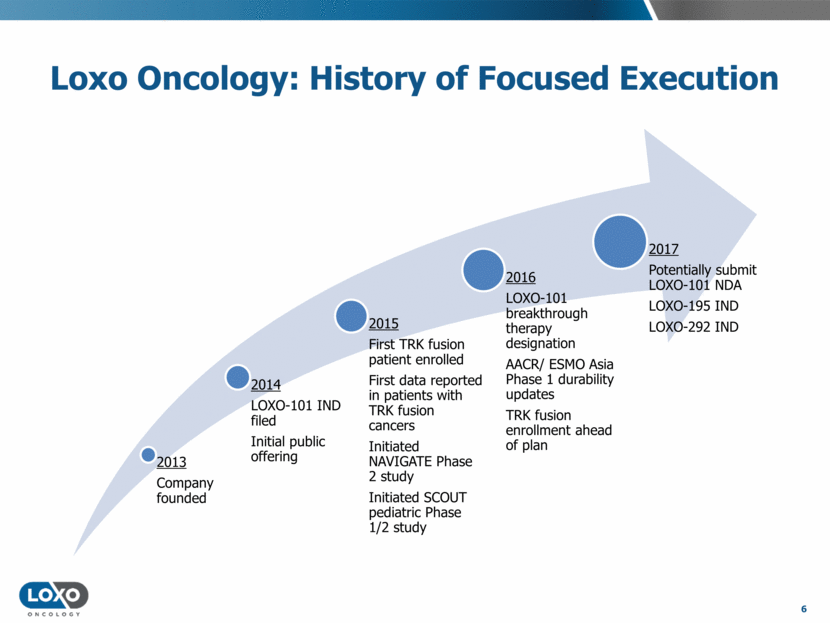
Loxo Oncology: History of Focused Execution 6 2013 Company founded 2014 LOXO-101 IND filed Initial public offering 2015 First TRK fusion patient enrolled First data reported in patients with TRK fusion cancers Initiated NAVIGATE Phase 2 study Initiated SCOUT pediatric Phase 1/2 study 2016 LOXO-101 breakthrough therapy designation AACR/ ESMO Asia Phase 1 durability updates TRK fusion enrollment ahead of plan 2017 Potentially submit LOXO-101 NDA LOXO-195 IND LOXO-292 IND
larotrectinib 7

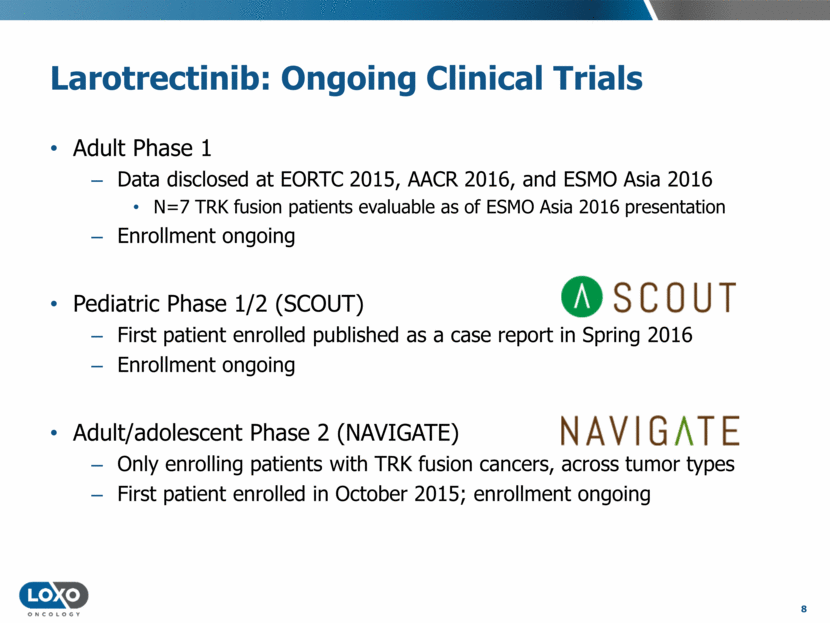
Larotrectinib: Ongoing Clinical Trials Adult Phase 1 Data disclosed at EORTC 2015, AACR 2016, and ESMO Asia 2016 N=7 TRK fusion patients evaluable as of ESMO Asia 2016 presentation Enrollment ongoing Pediatric Phase 1/2 (SCOUT) First patient enrolled published as a case report in Spring 2016 Enrollment ongoing Adult/adolescent Phase 2 (NAVIGATE) Only enrolling patients with TRK fusion cancers, across tumor types First patient enrolled in October 2015; enrollment ongoing 8
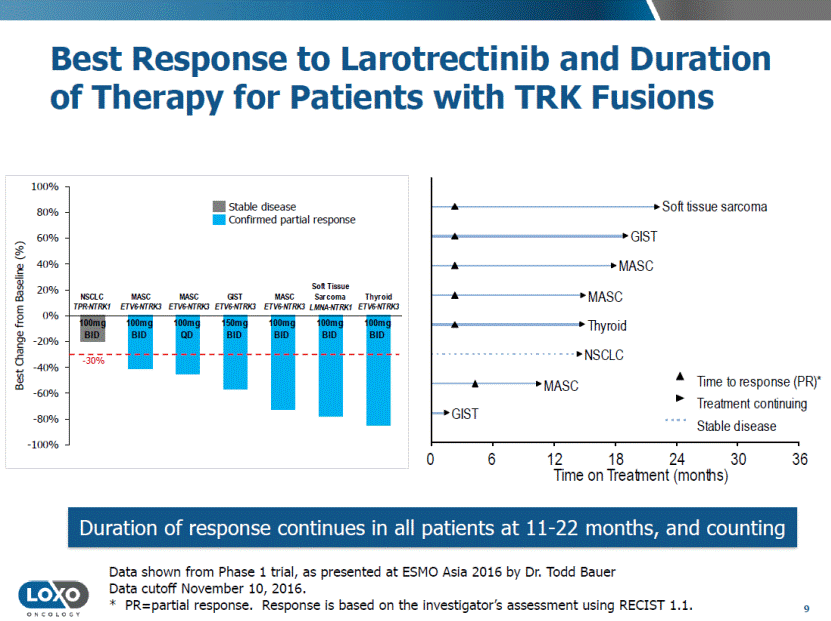
Best Response to Larotrectinib and Duration of Therapy for Patients with TRK Fusions 9 Data shown from Phase 1 trial, as presented at ESMO Asia 2016 by Dr. Todd Bauer Data cutoff November 10, 2016. * PR=partial response. Response is based on the investigator’s assessment using RECIST 1.1. Duration of response continues in all patients at 11-22 months, and counting -100% -80% -60% -40% -20% 0% 20% 40% 60% 80% 100% Best Change from Baseline (%) Stable disease Confirmed partial response - 30% Time to response (PR)* Treatment continuing Stable disease GIST MASC NSCLC Thyroid MASC MASC GIST Soft tissue sarcoma Time on Treatment (months) 0 6 12 18 24 30 36 100mg BID 150mg BID 100mg BID 100mg BID 100mg BID 100mg QD 100mg BID NSCLC TPR - NTRK1 MASC ETV6 - NTRK3 MASC ETV6 - NTRK3 MASC ETV6 - NTRK3 GIST ETV6 - NTRK3 Soft Tissue Sarcoma LMNA - NTRK1 Thyroid ETV6 - NTRK3
Larotrectinib Well-Positioned for Infantile Fibrosarcoma (IFS) IFS presents unique opportunity for LOXO-101 Different commercially-facing product (i.e. liquid) Clinical impact may be very high (i.e. limb salvage) Market opportunity Estimated ~100 cases/year in the US1 Tumor type identified by histology instead of fusion status Currently enrolling in pediatric Phase 1/2 SCOUT trial; clinical data expected mid-2017 Loxo Oncology granted rare pediatric disease designation for larotrectinib in infantile fibrosarcoma 10 1 Loxo Oncology proprietary market research, conducted by Decision Resources. Image from Bhatnagar, J. Neonatal Surg 2012; 1: 5. Larotrectinib liquid formulation
Larotrectinib: Emerging Regulatory Clarity Breakthrough therapy designation (BTD) granted in July 2016 BTD indication: “For the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments” Company has engaged in longitudinal conversations with U.S. and ex-U.S. regulators; today’s update based on written meeting minutes 11
Larotrectinib: Emerging Regulatory Clarity Breakthrough therapy designation (BTD) granted in July 2016 BTD indication: “For the treatment of unresectable or metastatic solid tumors with NTRK-fusion proteins in adult and pediatric patients who require systemic therapy and who have either progressed following prior treatment or who have no acceptable alternative treatments” Company has engaged in longitudinal conversations with U.S. and ex-U.S. regulators; today’s update based on written meeting minutes 12 Potential for larotrectinib to be the first cancer drug approved with a genetic, tumor-agnostic indication
Larotrectinib Regulatory Feedback Trial design and endpoints Ongoing Phase 2 NAVIGATE trial will form the basis of an NDA Primary endpoint: RECIST v1.1 overall response rate (ORR) based on independent radiology review Durability and safety will be key components of the risk-benefit determination upon NDA review Size of approval database Efficacy and safety database sizes for larotrectinib will be within precedents set by prior targeted cancer drug approvals 13
Larotrectinib Enrollment Update Currently ~85% enrolled to goal for primary efficacy analysis population of NDA submission Expect full enrollment early 2017 Enrolling worldwide US, EU, S Korea, Singapore, Japan Streamlined site selection Dozens of patients with TRK fusions enrolled across >10 different tumor types Clinical trials will remain open and continue to enroll beyond target goal Patients require long-term follow-up Data will support submissions Mechanism for continued drug access to newly identified patients through trial enrollment during regulatory interactions 14
Standard NDA/MAA Elements Clinical Last patient in Patient follow-up Independent radiology review Clinical study report CMC scale-up and stability testing Dictates the expiration date of the commercial product Long-term toxicology Clinical pharmacology studies Drug-drug interactions Food effect ADME Companion diagnostics development 15 – NDA submission expected in late 2017 or early 2018 – – MAA submission expected in 2018 –
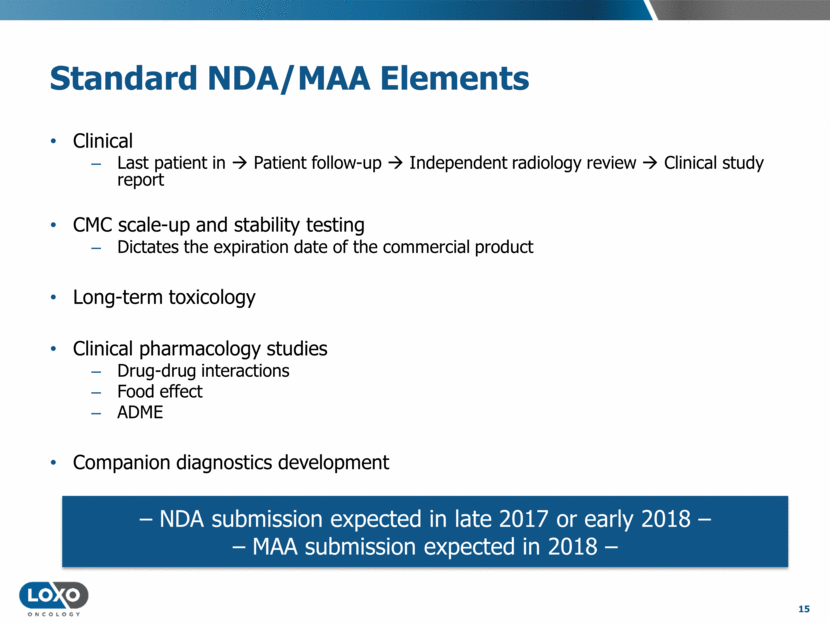
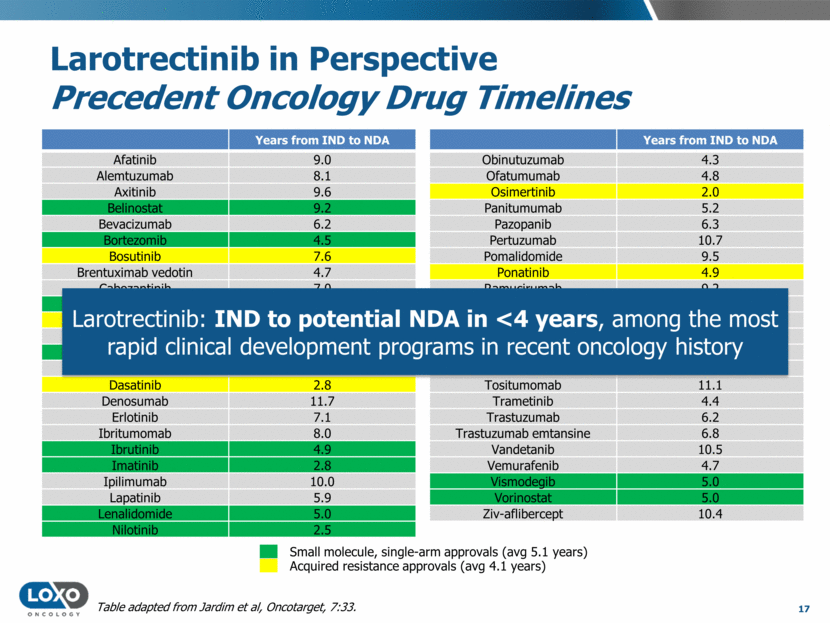
Larotrectinib in Perspective Precedent Oncology Drug Timelines Years from IND to NDA Afatinib 9.0 Alemtuzumab 8.1 Axitinib 9.6 Belinostat 9.2 Bevacizumab 6.2 Bortezomib 4.5 Bosutinib 7.6 Brentuximab vedotin 4.7 Cabozantinib 7.0 Carfilzomib 6.4 Ceritinib 3.3 Cetuximab 9.0 Crizotinib 5.3 Dabrafenib 3.1 Dasatinib 2.8 Denosumab 11.7 Erlotinib 7.1 Ibritumomab 8.0 Ibrutinib 4.9 Imatinib 2.8 Ipilimumab 10.0 Lapatinib 5.9 Lenalidomide 5.0 Nilotinib 2.5 16 Years from IND to NDA Obinutuzumab 4.3 Ofatumumab 4.8 Osimertinib 2.0 Panitumumab 5.2 Pazopanib 6.3 Pertuzumab 10.7 Pomalidomide 9.5 Ponatinib 4.9 Ramucirumab 9.2 Regorafenib 5.9 Ruxolitinib 4.2 Siltuximab 9.8 Sorafenib 5.2 Sunitinib 4.4 Tositumomab 11.1 Trametinib 4.4 Trastuzumab 6.2 Trastuzumab emtansine 6.8 Vandetanib 10.5 Vemurafenib 4.7 Vismodegib 5.0 Vorinostat 5.0 Ziv-aflibercept 10.4 Acquired resistance approvals (avg 4.1 years) Small molecule, single-arm approvals (avg 5.1 years) Table adapted from Jardim et al, Oncotarget, 7:33.
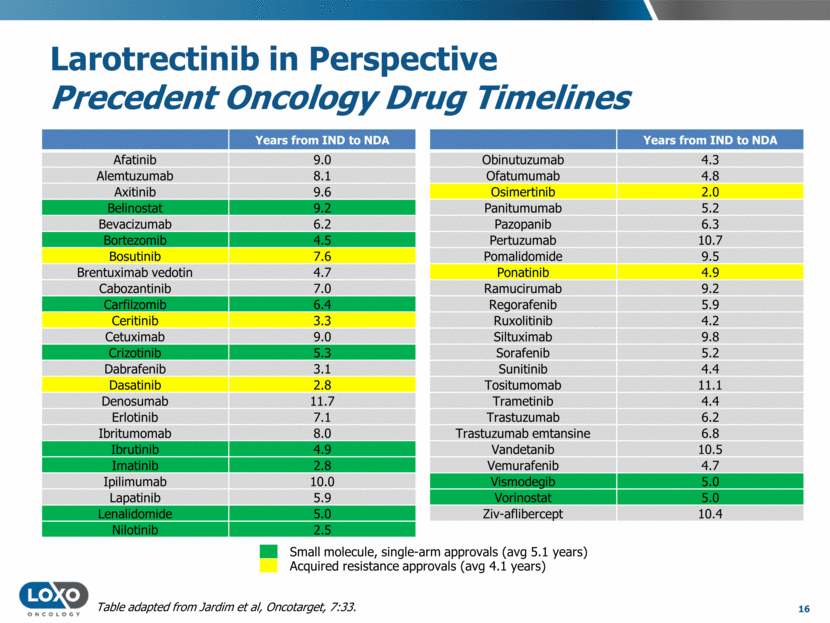
Larotrectinib in Perspective Precedent Oncology Drug Timelines Years from IND to NDA Afatinib 9.0 Alemtuzumab 8.1 Axitinib 9.6 Belinostat 9.2 Bevacizumab 6.2 Bortezomib 4.5 Bosutinib 7.6 Brentuximab vedotin 4.7 Cabozantinib 7.0 Carfilzomib 6.4 Ceritinib 3.3 Cetuximab 9.0 Crizotinib 5.3 Dabrafenib 3.1 Dasatinib 2.8 Denosumab 11.7 Erlotinib 7.1 Ibritumomab 8.0 Ibrutinib 4.9 Imatinib 2.8 Ipilimumab 10.0 Lapatinib 5.9 Lenalidomide 5.0 Nilotinib 2.5 17 Years from IND to NDA Obinutuzumab 4.3 Ofatumumab 4.8 Osimertinib 2.0 Panitumumab 5.2 Pazopanib 6.3 Pertuzumab 10.7 Pomalidomide 9.5 Ponatinib 4.9 Ramucirumab 9.2 Regorafenib 5.9 Ruxolitinib 4.2 Siltuximab 9.8 Sorafenib 5.2 Sunitinib 4.4 Tositumomab 11.1 Trametinib 4.4 Trastuzumab 6.2 Trastuzumab emtansine 6.8 Vandetanib 10.5 Vemurafenib 4.7 Vismodegib 5.0 Vorinostat 5.0 Ziv-aflibercept 10.4 Acquired resistance approvals (avg 4.1 years) Small molecule, single-arm approvals (avg 5.1 years) Table adapted from Jardim et al, Oncotarget, 7:33. Larotrectinib: IND to potential NDA in <4 years, among the most rapid clinical development programs in recent oncology history
Larotrectinib: Commercial Opportunity Loxo Oncology has commissioned proprietary work to better understand the frequency of TRK+ cancers i.e. tumor banks and third party lab relationships Proprietary datasets suggest 1,500-5,000 late-line eligible patients per year in US Similar estimates for EU5 and Japan, proportional to cancer incidence/mortality in those regions Potential to move into earlier lines of therapy over time Widespread adoption of sensitive diagnostic testing is key to patient identification TRK market opportunity compares favorably with other actionable US oncology markets1: Advanced basal cell carcinoma: ~3,000 patients Advanced systemic mastocytosis: 600-1,600 patients2 ALK+ NSCLC: ~5,500 patients Hodgkin’s lymphoma: ~1,000 patients IDH1/2-mutant AML: ~2,000 patients Unresectable/metastatic PDGFR-mutant GIST: <500 patients 18 1 Figures reflect estimated late-line patients per year or estimated deaths per year. 2 New diagnoses.

Crizotinib for ALK: A Diagnostics Case Study August 2011: Initial FDA approval with Abbott FISH diagnostic June 2015: FDA approval of Ventana IHC diagnostic November 2016: Submission of PMA for Thermo Fisher Oncomine Universal Dx Test (NGS) 19 FISH = Fluorescent in situ hybridization IHC = Immunohistochemistry NGS = Next-generation sequencing Enable diverse pathologists around the world to identify patients with as many suitable diagnostic tools as possible
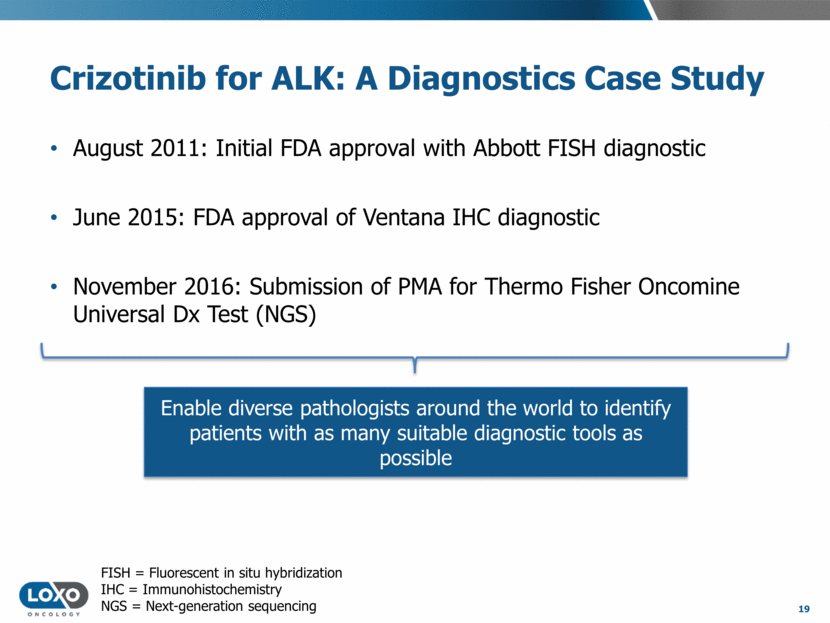
ALK+ NSCLC: A Commercial Case Study 20 Source: Pfizer, Novartis, and Roche financial reports. Xalkori, Zykadia, and Alecensa package inserts. Note: Market potential for larotrectinib may differ from that indicated by historical revenues for ALK+ NSCLC. * Xalkori was also FDA-approved for ROS1+ NSCLC in March 2016. 7.4-11.3 Median duration of response (mo.) ALK+ NSCLC: ~5,500 late-line patients/yr in US vs. TRK+ cancers: 1,500-5,000 late-line patients/yr in US 7.1-7.4 7.5-11.2 $0 $100 $200 $300 $400 $500 $600 $700 2011 2012 2013 2014 2015 2016 YTD, 9m* Worldwide Revenues ($MM) Crizotinib Zykadia Alecensa
LOXO-195 21

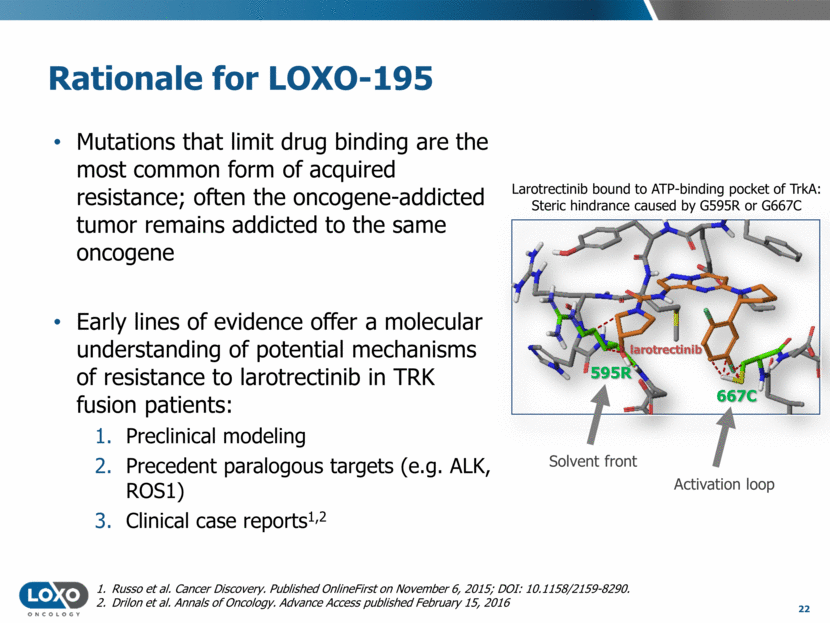
Mutations that limit drug binding are the most common form of acquired resistance; often the oncogene-addicted tumor remains addicted to the same oncogene Early lines of evidence offer a molecular understanding of potential mechanisms of resistance to larotrectinib in TRK fusion patients: Preclinical modeling Precedent paralogous targets (e.g. ALK, ROS1) Clinical case reports1,2 Russo et al. Cancer Discovery. Published OnlineFirst on November 6, 2015; DOI: 10.1158/2159-8290. Drilon et al. Annals of Oncology. Advance Access published February 15, 2016 667C 595R larotrectinib Rationale for LOXO-195 Solvent front Activation loop 22 Larotrectinib bound to ATP-binding pocket of TrkA: Steric hindrance caused by G595R or G667C
LOXO-195: Development Opportunity Foreshadowed by Other Classes Clinically, addressing acquired resistance allows clinicians and patients to maximize total duration of disease control over multiple lines of therapy ALK+ NSCLC Primary response on crizotinib 30-50% develop various mutations conferring resistance Potential for re-response to 2nd-line ceritinib/alectinib EGFR+ NSCLC Primary response on erlotinib/gefitinib 50-60% develop T790M mutation conferring resistance Potential for re-response to 2nd-line osimertinib TRK+ tumors Primary response on larotrectinib Patients may develop various mutations conferring resistance Potential for re-response to 2nd-line LOXO-195 Downstream signaling nodes (e.g. MEK) may be clinically useful as single agents post-progression 23

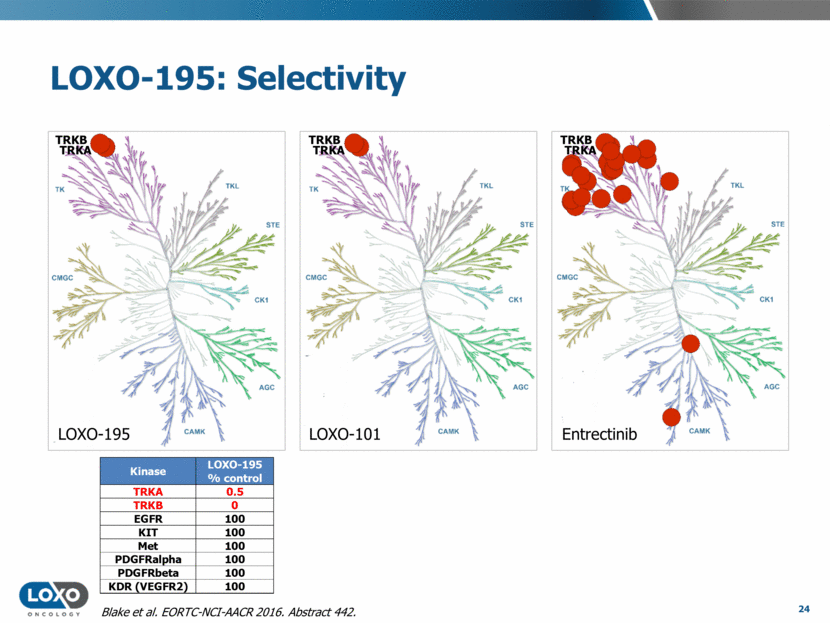
LOXO-195: Selectivity 24 LOXO-195 LOXO-101 Entrectinib TRKB TRKB TRKA TRKB TRKA TRKA Blake et al. EORTC-NCI-AACR 2016. Abstract 442. Kinase LOXO-195 % control TRKA 0.5 TRKB 0 EGFR 100 KIT 100 Met 100 PDGFRalpha 100 PDGFRbeta 100 KDR (VEGFR2) 100
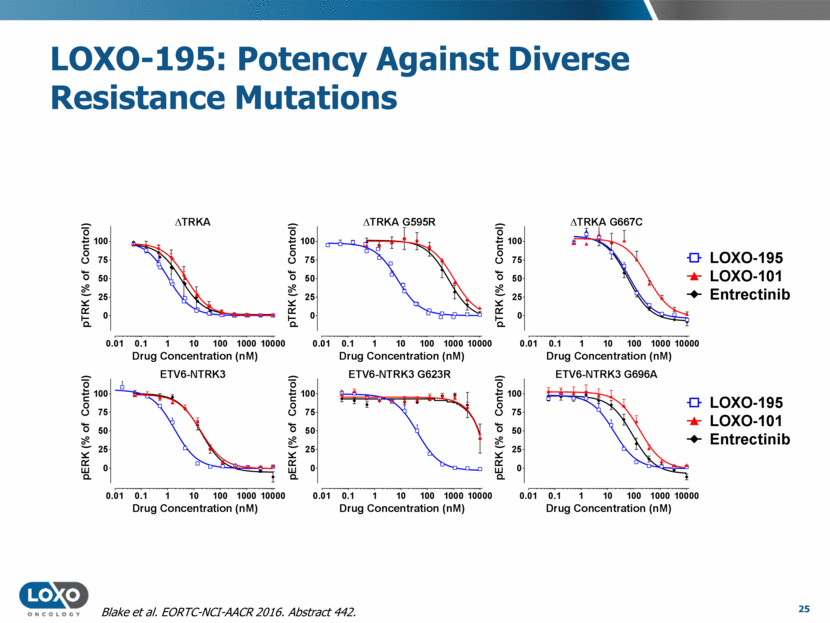
LOXO-195: Potency Against Diverse Resistance Mutations 25 Blake et al. EORTC-NCI-AACR 2016. Abstract 442. D TRKA 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p T R K ( % o f C o n t r o l ) ETV6-NTRK3 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p E R K ( % o f C o n t r o l ) D TRKA G595R 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p T R K ( % o f C o n t r o l ) ETV6-NTRK3 G623R 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p E R K ( % o f C o n t r o l ) D TRKA G667C 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p T R K ( % o f C o n t r o l ) ETV6-NTRK3 G696A 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p E R K ( % o f C o n t r o l ) LOXO-195 LOXO-101 Entrectinib LOXO-195 LOXO-101 Entrectinib
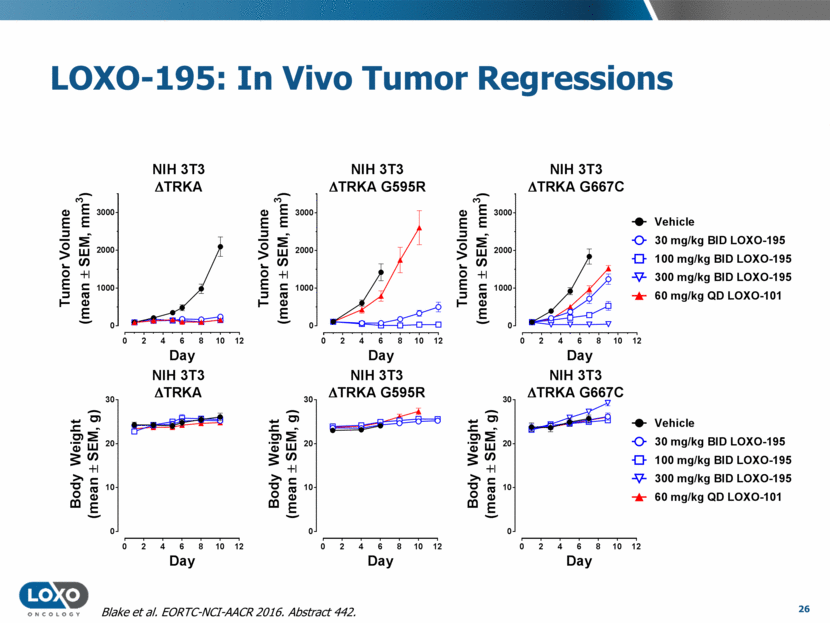
LOXO-195: In Vivo Tumor Regressions 26 Blake et al. EORTC-NCI-AACR 2016. Abstract 442. Vehicle 30 mg/kg BID LOXO-195 100 mg/kg BID LOXO-195 300 mg/kg BID LOXO-195 60 mg/kg QD LOXO-101 Vehicle 30 mg/kg BID LOXO-195 100 mg/kg BID LOXO-195 300 mg/kg BID LOXO-195 60 mg/kg QD LOXO-101 0 2 4 6 8 10 12 0 1000 2000 3000 NIH 3T3 D TRKA Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) NIH 3T3 D TRKA G595R 0 2 4 6 8 10 12 0 1000 2000 3000 Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 2 4 6 8 10 12 0 1000 2000 3000 NIH 3T3 D TRKA G667C Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 2 4 6 8 10 12 0 10 20 30 NIH 3T3 D TRKA Day B o d y W e i g h t ( m e a n ± S E M , g ) 0 2 4 6 8 10 12 0 10 20 30 NIH 3T3 D TRKA G595R Day B o d y W e i g h t ( m e a n ± S E M , g ) 0 2 4 6 8 10 12 0 10 20 30 NIH 3T3 D TRKA G667C Day B o d y W e i g h t ( m e a n ± S E M , g )
LOXO-195 LOXO-195 preclinically active against all acquired resistance mutations identified to date LOXO-195 Phase 1 study expected to start by mid-2017 Operational synergy with larotrectinib program Expect single agent activity at biologically relevant doses Assuming availability of appropriate patients, potential for initial clinical data by YE 2017 Loxo Oncology is well-positioned to be the first oncology company ever to simultaneously develop a first-generation inhibitor and a second-generation inhibitor to address acquired resistance 27
LOXO-292 (RET) 28

RET (REarranged during Transfection) Adapted from Mulligan, Nature Reviews Cancer (2014) 14: 173-185; Besset, JBC 2000 275: 39159-39166 Wild-type RET Extracellular mutation C634R Intracellular mutation M918T Oncogenic fusion KIF5B-RET CCDC6-RET ~60% medullary thyroid cancer ~2% NSCLC, 10-20% papillary thyroid cancer 29 ~5,000 late-line eligible patients per year in US
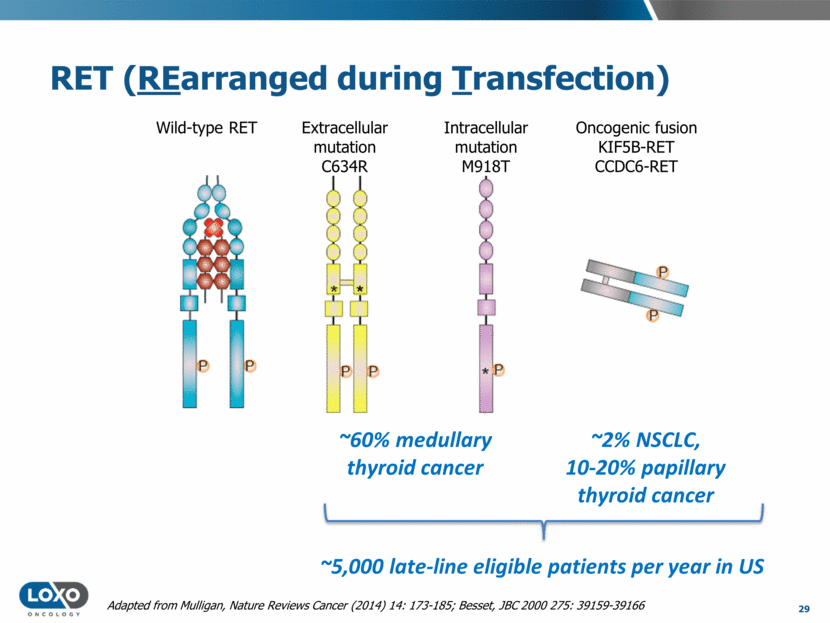
M918T Clinically Validated in MTC EXAM Phase 3 Trial, cabozantinib vs. pbo Source: Schlumberger, ASCO 2015 ITT Population HR 0.15 p<0.0001 HR 0.67 p=0.1875 30
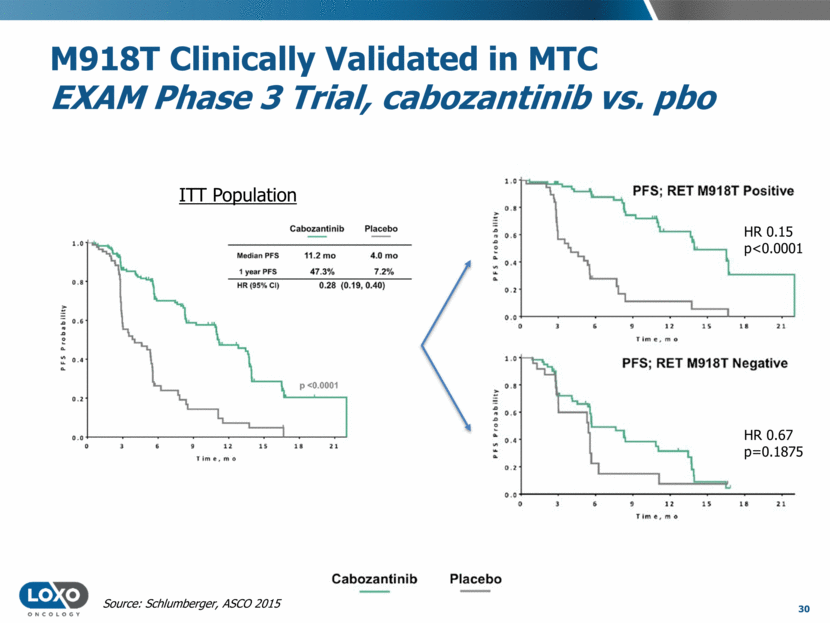
Multikinase Inhibitors Have Clinically Validated RET Fusions in NSCLC 31 Drug Publication N RECIST ORR1 Median PFS Issues Cabozantinib Initial data: ASCO 2015 Follow-up data: Lancet Oncol 2016 16 25 38% (6/16) 28% (7/25) 7m 5.5m Frequent AEs and dose reductions Lenvatinib ESMO 2016 25 16% (4/25) 7.3m Frequent dose reductions and D/Cs Vandetanib Lancet Respir 2016 Annals Oncology 2016 17 18 53% (9/17) 17% (3/18) 4.7m 4.5m Gr3/4 hypertension, diarrhea, rash, QTc prolongation CEP-32496/ RXDX-105 ASCO 2016, EORTC-NCI-AACR 2016 10 30% (3/10) NR Gr3 rash, diarrhea, continued dose-finding 1 Includes all RET fusion patients treated, regardless of prior therapy. Only includes confirmed responses.
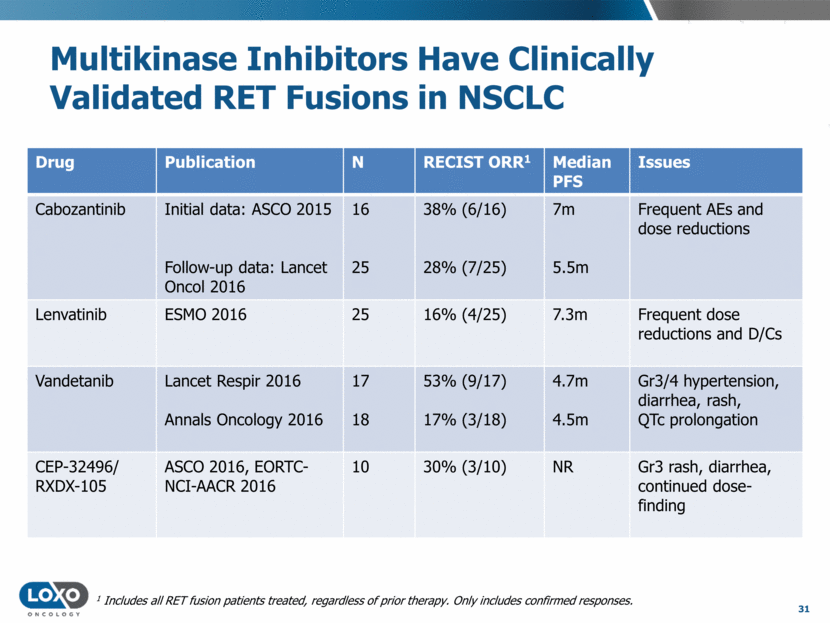
Multikinase Liabilities On Display 32 Excerpt from commentary “ ”
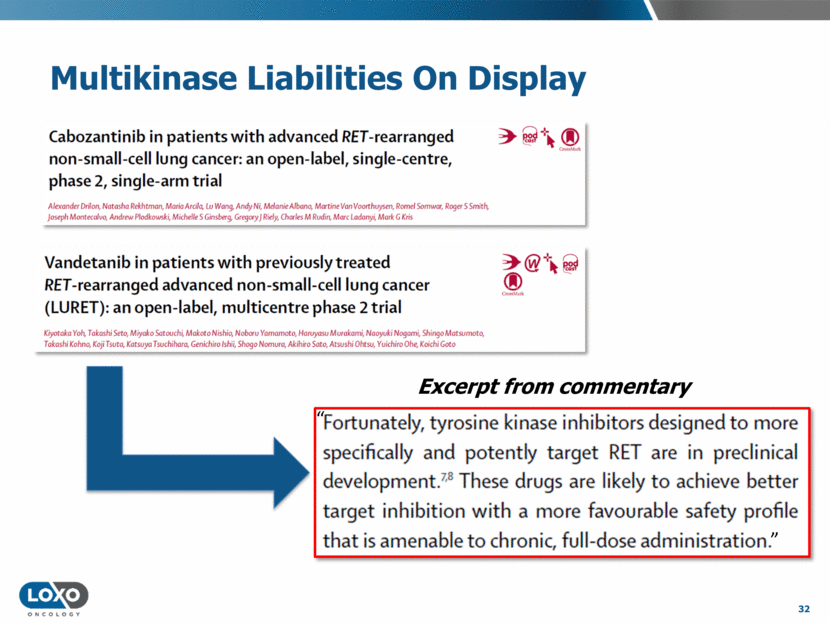
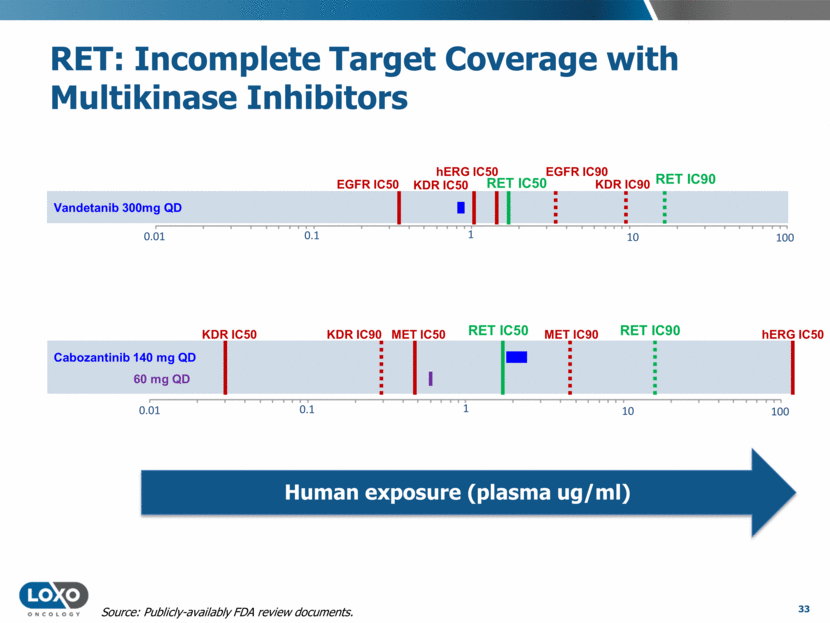
hERG IC50 100 10 1 0.1 0.01 Vandetanib 300mg QD RET IC50 RET IC90 KDR IC50 EGFR IC90 EGFR IC50 KDR IC90 100 10 1 0.1 0.01 RET IC50 hERG IC50 MET IC50 RET IC90 KDR IC50 MET IC90 KDR IC90 Cabozantinib 140 mg QD 60 mg QD RET: Incomplete Target Coverage with Multikinase Inhibitors Human exposure (plasma ug/ml) 33 Source: Publicly-availably FDA review documents.
RET: Product Profile Goals Potent against RET fusions and mutations Superior RET inhibition in pharmacodynamic models Tumor regression in in vivo models Activity against predicted acquired resistance mutations, such as V804M and V804L Avoid off-targets such as VEGFR, Aurora kinase, FGFR, EGFR, MET Excellent drug-like properties High GI absorption, low clearance Once daily or BID dosing Low potential for drug-drug interactions 34
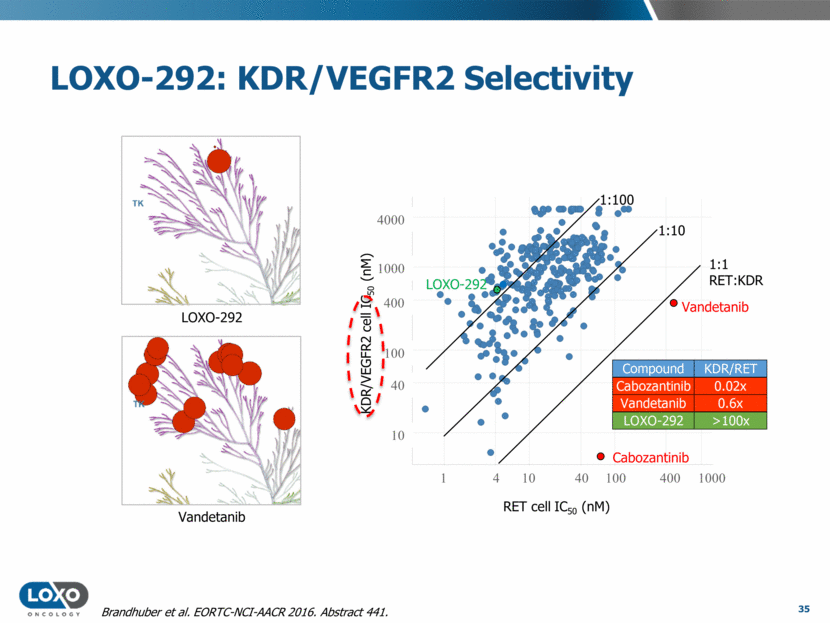
LOXO-292: KDR/VEGFR2 Selectivity 35 Vandetanib LOXO-292 1:10 1:100 1:1 RET:KDR 10 10 100 4000 100 40 40 4 1 RET cell IC50 (nM) 400 1000 1000 400 KDR/VEGFR2 cell IC50 (nM) Cabozantinib Vandetanib LOXO-292 Brandhuber et al. EORTC-NCI-AACR 2016. Abstract 441.
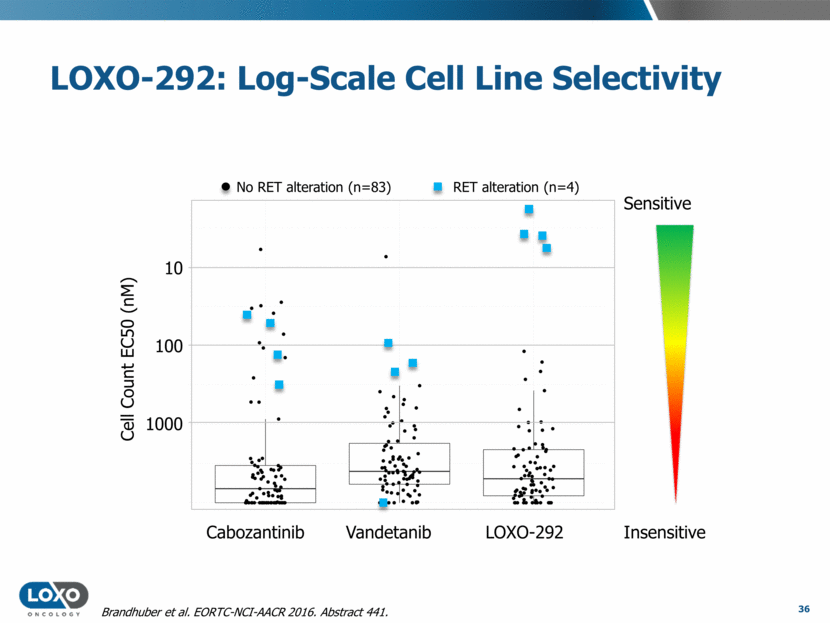
LOXO-292: Log-Scale Cell Line Selectivity 36 No RET alteration (n=83) RET alteration (n=4) Insensitive Sensitive Cabozantinib Vandetanib LOXO-292 10 100 1000 Cell Count EC50 (nM) Brandhuber et al. EORTC-NCI-AACR 2016. Abstract 441.
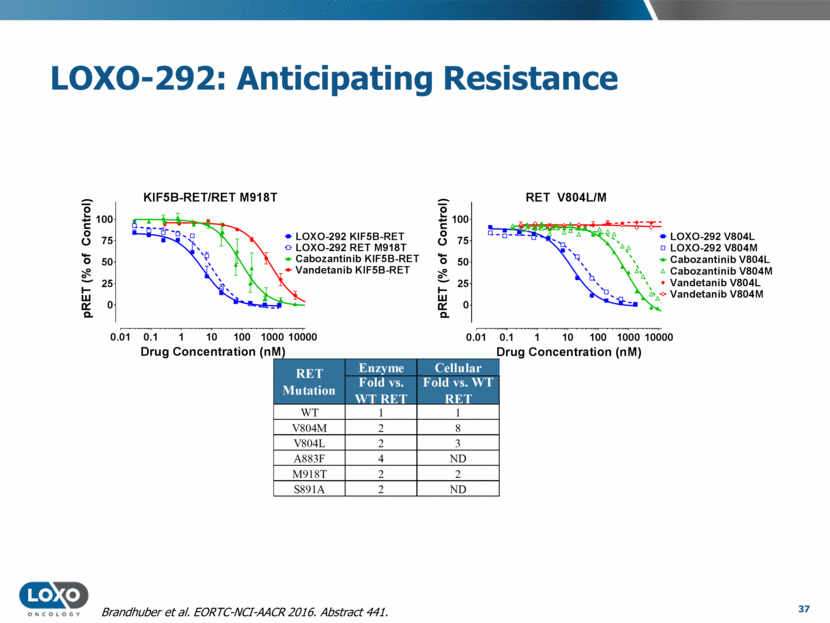
LOXO-292: Anticipating Resistance 37 Brandhuber et al. EORTC-NCI-AACR 2016. Abstract 441. RET Mutation WT V804M V804L A883F M918T S891A KIF5B-RET/RET M918T 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p R E T ( % o f C o n t r o l ) LOXO-292 KIF5B-RET Cabozantinib KIF5B-RET Vandetanib KIF5B-RET LOXO-292 RET M918T RET V804L/M 0.01 0.1 1 10 100 1000 10000 0 25 50 75 100 Drug Concentration (nM) p R E T ( % o f C o n t r o l ) Vandetanib V804M Cabozantinib V804L LOXO-292 V804L Vandetanib V804L Cabozantinib V804M LOXO-292 V804M
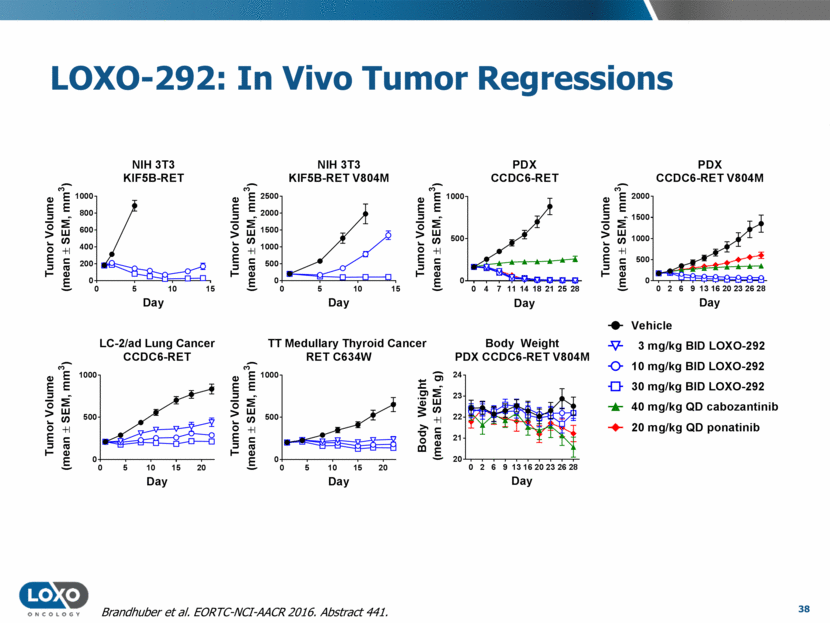
LOXO-292: In Vivo Tumor Regressions 38 Brandhuber et al. EORTC-NCI-AACR 2016. Abstract 441. 0 5 10 15 0 200 400 600 800 1000 NIH 3T3 KIF5B-RET Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 5 10 15 0 500 1000 1500 2000 2500 NIH 3T3 KIF5B-RET V804M Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 4 7 11 14 18 21 25 28 0 500 1000 PDX CCDC6-RET Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 2 6 9 13 16 20 23 26 28 0 500 1000 1500 2000 PDX CCDC6-RET V804M Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 5 10 15 20 0 500 1000 LC-2/ad Lung Cancer CCDC6-RET Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 5 10 15 20 0 500 1000 TT Medullary Thyroid Cancer RET C634W Day T u m o r V o l u m e ( m e a n ± S E M , m m 3 ) 0 2 6 9 13 16 20 23 26 28 20 21 22 23 24 Body Weight PDX CCDC6-RET V804M Day B o d y W e i g h t ( m e a n ± S E M , g ) 0 25 50 75 100 1000 10000 PK-PD Relationship Drug Plasma Concentration (ng/mL) p R E T i n T u m o r ( P e r c e n t o f C o n t r o l ) 10 mg/kg 30 mg/kg 100 mg/kg 60 mg/kg 0 Veh 10 mg/kg 30 mg/kg 10 mg/kg 30 mg/kg 3000 30000 Cabozantinib KIFB-RET LOXO-292 KIF5B-RET LOXO-292 close analog KIF5B-RET V804M Cabozantinib KIF5B-RET V804M LOXO-292 close analog KIF5B-RET Vehicle 3 mg/kg BID LOXO-292 10 mg/kg BID LOXO-292 30 mg/kg BID LOXO-292 40 mg/kg QD cabozantinib 20 mg/kg QD ponatinib
LOXO-292 Clinical Development LOXO-292 Phase 1 study expected to start in early 2017 Opportunity for patient enrichment in Phase 1 Growing target awareness Detection technology is relatively straightforward Expect single agent activity at biologically relevant doses Potential for initial clinical data by YE 2017 39

summary 40

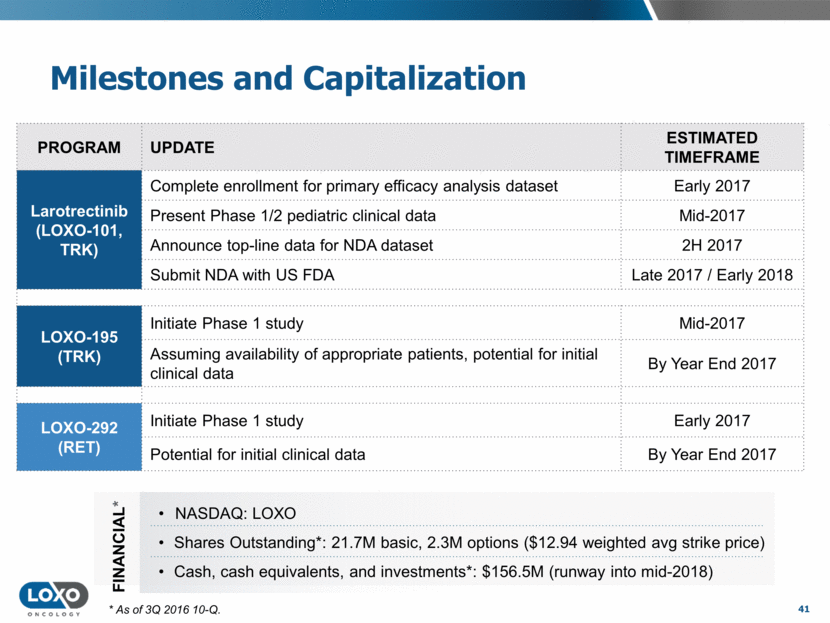
Milestones and Capitalization 41 PROGRAM UPDATE ESTIMATED TIMEFRAME Larotrectinib (LOXO-101, TRK) Complete enrollment for primary efficacy analysis dataset Early 2017 Present Phase 1/2 pediatric clinical data Mid-2017 Announce top-line data for NDA dataset 2H 2017 Submit NDA with US FDA Late 2017 / Early 2018 LOXO-195 (TRK) Initiate Phase 1 study Mid-2017 Assuming availability of appropriate patients, potential for initial clinical data By Year End 2017 LOXO-292 (RET) Initiate Phase 1 study Early 2017 Potential for initial clinical data By Year End 2017 FINANCIAL* NASDAQ: LOXO Shares Outstanding*: 21.7M basic, 2.3M options ($12.94 weighted avg strike price) Cash, cash equivalents, and investments*: $156.5M (runway into mid-2018) * As of 3Q 2016 10-Q.
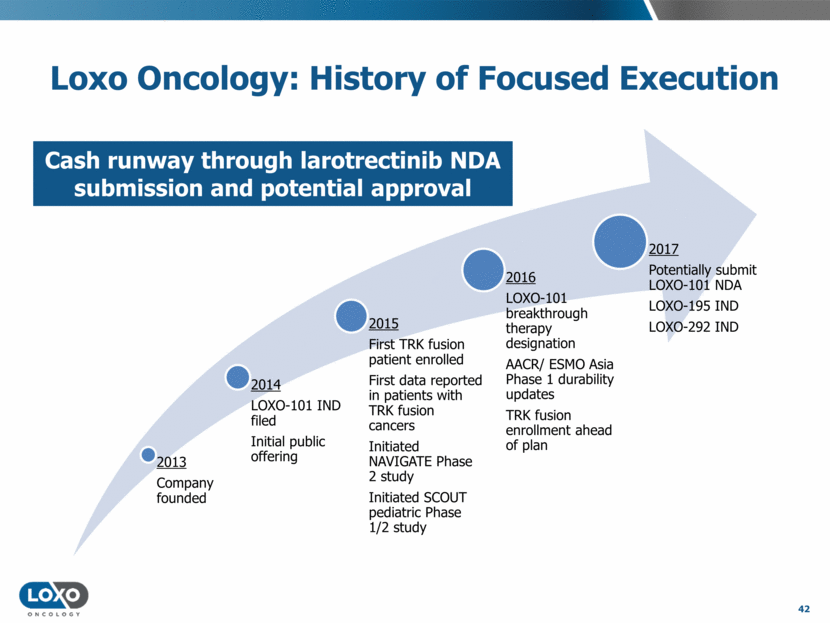
Loxo Oncology: History of Focused Execution 42 Cash runway through larotrectinib NDA submission and potential approval 2013 Company founded 2014 LOXO-101 IND filed Initial public offering 2015 First TRK fusion patient enrolled First data reported in patients with TRK fusion cancers Initiated NAVIGATE Phase 2 study Initiated SCOUT pediatric Phase 1/2 study 2016 LOXO-101 breakthrough therapy designation AACR/ ESMO Asia Phase 1 durability updates TRK fusion enrollment ahead of plan 2017 Potentially submit LOXO-101 NDA LOXO-195 IND LOXO-292 IND