Attached files
| file | filename |
|---|---|
| EX-99.4 - EX-99.4 - Loxo Oncology, Inc. | a16-23351_1ex99d4.htm |
| EX-99.3 - EX-99.3 - Loxo Oncology, Inc. | a16-23351_1ex99d3.htm |
| EX-99.1 - EX-99.1 - Loxo Oncology, Inc. | a16-23351_1ex99d1.htm |
| 8-K - 8-K - Loxo Oncology, Inc. | a16-23351_18k.htm |
Exhibit 99.2
Clinical safety and activity from a Phase 1 study of LOXO-101, a selective TRKA/B/C inhibitor, in solid-tumor patients with NTRK gene fusions David S. Hong1, Afshin Dowlati2, Howard A. Burris, III3, James J. Lee4, Marcia S. Brose5, Anna F. Farago6, Todd M. Bauer3, Matthew Taylor7, Alice T. Shaw6, Steve Smith8, Nisha Nanda8, Scott Cruickshank8, Michael C. Cox8, Robert C. Doebele9 1MD Anderson Cancer Center, Houston, TX 2University Hospitals Case Medical Center, Cleveland, OH 3Sarah Cannon Research Institute, Tennessee Oncology, PLLC, Nashville, TN 4University of Pittsburgh Medical Center, Pittsburgh, PA 5University of Pennsylvania, Philadelphia, PA 6Massachusetts General Hospital, Boston, MA 7Oregon Health & Science University, Portland, OR 8Loxo Oncology, South San Francisco, CA 9University of Colorado, Aurora, CO
DISCLOSURE SLIDE Travel and accommodations expenses supported by Loxo Oncology I am an investigator on numerous Phase 1 studies of molecularly targeted agents, including other NTRK-targeting compounds
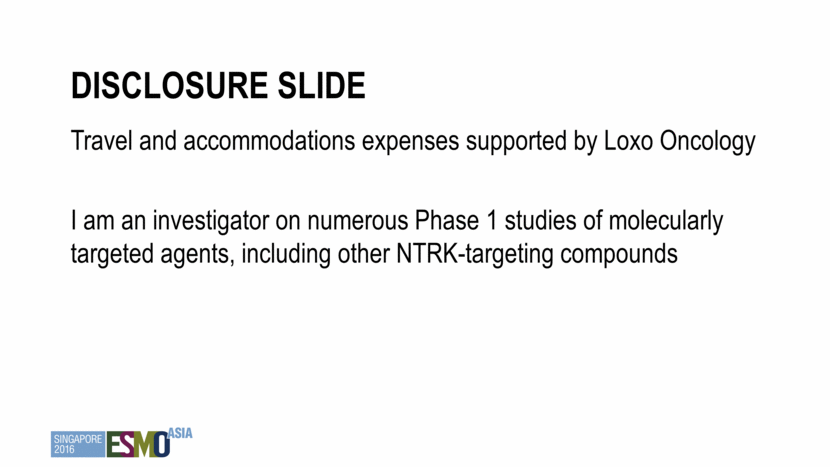
TRK fusions are found across human cancer TRK fusion frequency <5% 5–25% >75% CNS Astrocytoma Brain low-grade glioma Glioblastoma GI Colorectal cancer Cholangiocarcinoma GIST Pancreatic cancer Head and neck Squamous cell carcinoma Lung Adenocarcinoma Large cell neuroendocrine Other Acute myeloid leukemia Breast invasive carcinoma Melanoma Sarcoma Congenital mesoblastic nephroma Papillary thyroid cancer Pontine glioma Spitz tumors Mammary analogue secretory carcinoma (MASC) of the salivary glands Secretory breast carcinoma Infantile fibrosarcoma
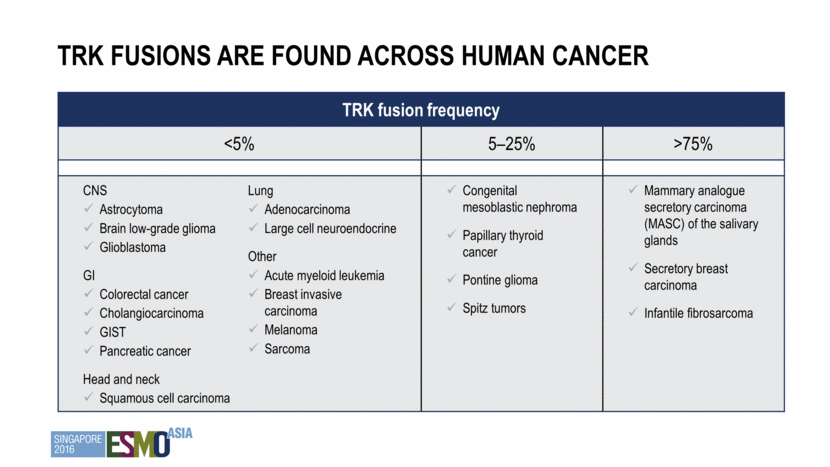
First and only selective TRK inhibitor Highly potent against TRKA, TRKB, TRKC (5-11 nM IC50) Highly selective: limited inhibition of other kinases and >1,000x selective over non-kinase off targets Highly soluble and bioavailable Moderately protein-bound Two clinical formulations: capsule and liquid Larotrectinib (loxo-101) TRK TRK
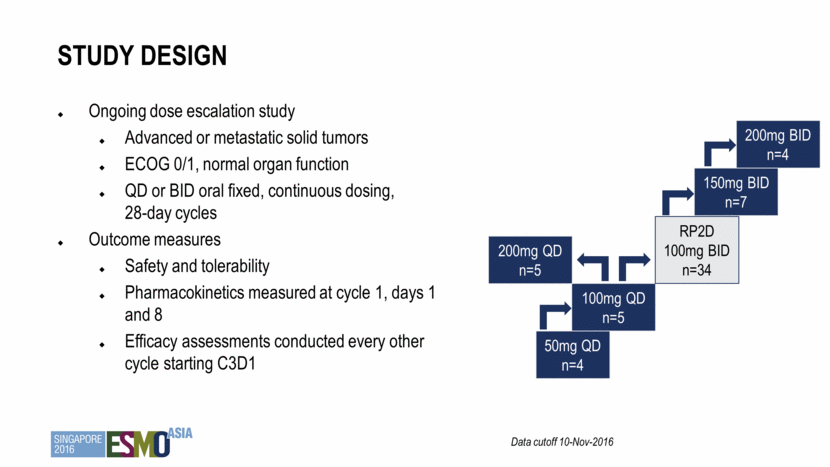
Ongoing dose escalation study Advanced or metastatic solid tumors ECOG 0/1, normal organ function QD or BID oral fixed, continuous dosing, 28-day cycles Outcome measures Safety and tolerability Pharmacokinetics measured at cycle 1, days 1 and 8 Efficacy assessments conducted every other cycle starting C3D1 Study design Data cutoff 10-Nov-2016 50mg QD n=4 100mg QD n=5 RP2D 100mg BID n=34 200mg QD n=5 150mg BID n=7 200mg BID n=4
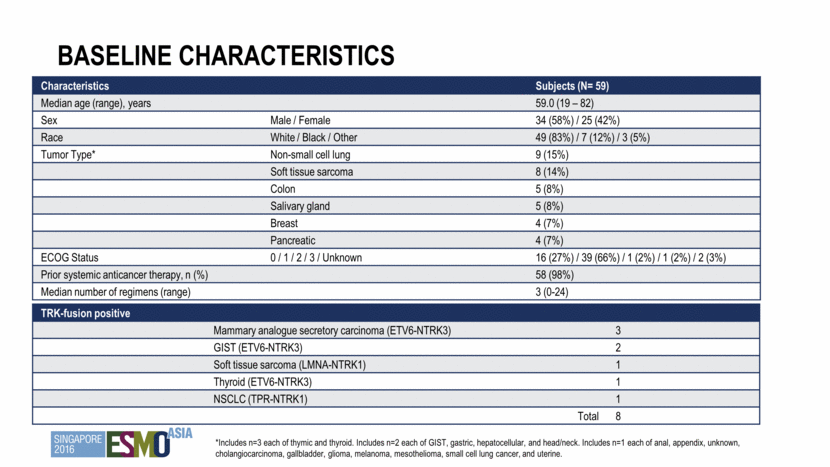
Baseline characteristics Characteristics Subjects (N= 59) Median age (range), years 59.0 (19 – 82) Sex Male / Female 34 (58%) / 25 (42%) Race White / Black / Other 49 (83%) / 7 (12%) / 3 (5%) Tumor Type* Non-small cell lung 9 (15%) Soft tissue sarcoma 8 (14%) Colon 5 (8%) Salivary gland 5 (8%) Breast 4 (7%) Pancreatic 4 (7%) ECOG Status 0 / 1 / 2 / 3 / Unknown 16 (27%) / 39 (66%) / 1 (2%) / 1 (2%) / 2 (3%) Prior systemic anticancer therapy, n (%) 58 (98%) Median number of regimens (range) 3 (0-24) TRK-fusion positive Mammary analogue secretory carcinoma (ETV6-NTRK3) 3 GIST (ETV6-NTRK3) 2 Soft tissue sarcoma (LMNA-NTRK1) 1 Thyroid (ETV6-NTRK3) 1 NSCLC (TPR-NTRK1) 1 Total 8 *Includes n=3 each of thymic and thyroid. Includes n=2 each of GIST, gastric, hepatocellular, and head/neck. Includes n=1 each of anal, appendix, unknown, cholangiocarcinoma, gallbladder, glioma, melanoma, mesothelioma, small cell lung cancer, and uterine.
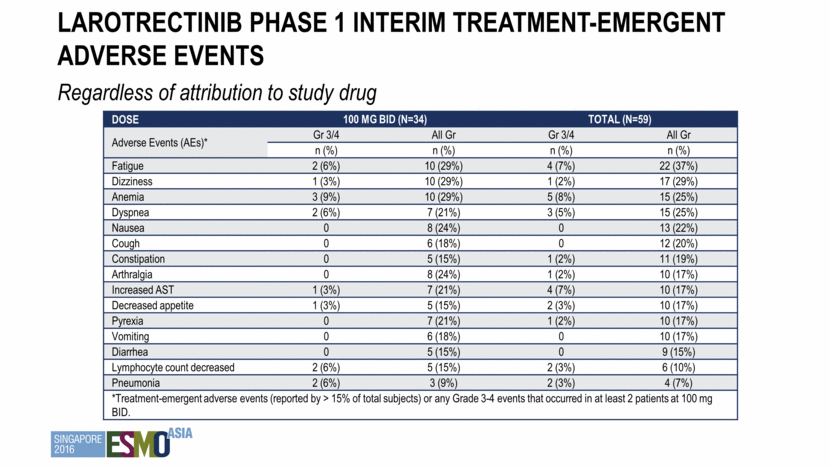
Larotrectinib Phase 1 Interim Treatment-Emergent adverse events Regardless of attribution to study drug Dose 100 mg BID (n=34) Total (n=59) Adverse Events (AEs)* Gr 3/4 All Gr Gr 3/4 All Gr n (%) n (%) n (%) n (%) Fatigue 2 (6%) 10 (29%) 4 (7%) 22 (37%) Dizziness 1 (3%) 10 (29%) 1 (2%) 17 (29%) Anemia 3 (9%) 10 (29%) 5 (8%) 15 (25%) Dyspnea 2 (6%) 7 (21%) 3 (5%) 15 (25%) Nausea 0 8 (24%) 0 13 (22%) Cough 0 6 (18%) 0 12 (20%) Constipation 0 5 (15%) 1 (2%) 11 (19%) Arthralgia 0 8 (24%) 1 (2%) 10 (17%) Increased AST 1 (3%) 7 (21%) 4 (7%) 10 (17%) Decreased appetite 1 (3%) 5 (15%) 2 (3%) 10 (17%) Pyrexia 0 7 (21%) 1 (2%) 10 (17%) Vomiting 0 6 (18%) 0 10 (17%) Diarrhea 0 5 (15%) 0 9 (15%) Lymphocyte count decreased 2 (6%) 5 (15%) 2 (3%) 6 (10%) Pneumonia 2 (6%) 3 (9%) 2 (3%) 4 (7%) *Treatment-emergent adverse events (reported by > 15% of total subjects) or any Grade 3-4 events that occurred in at least 2 patients at 100 mg BID.
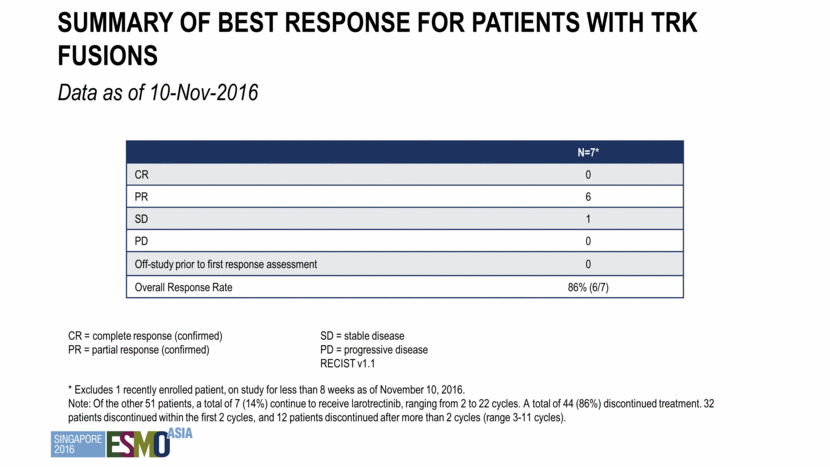
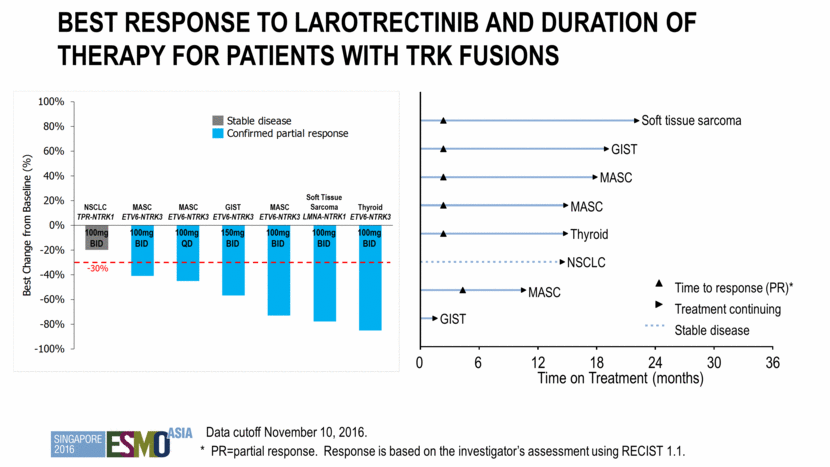
Summary of best response for patients with trk fusions Data as of 10-Nov-2016 N=7* CR 0 PR 6 SD 1 PD 0 Off-study prior to first response assessment 0 Overall Response Rate 86% (6/7) * Excludes 1 recently enrolled patient, on study for less than 8 weeks as of November 10, 2016. Note: Of the other 51 patients, a total of 7 (14%) continue to receive larotrectinib, ranging from 2 to 22 cycles. A total of 44 (86%) discontinued treatment. 32 patients discontinued within the first 2 cycles, and 12 patients discontinued after more than 2 cycles (range 3-11 cycles). CR = complete response (confirmed) PR = partial response (confirmed) SD = stable disease PD = progressive disease RECIST v1.1
Best Response to larotrectinib and duration of therapy for Patients with TRK Fusions Time to response (PR)* Treatment continuing Stable disease GIST MASC NSCLC Thyroid MASC MASC GIST Soft tissue sarcoma Time on Treatment (months) 0 6 12 18 24 30 36 Data cutoff November 10, 2016. * PR=partial response. Response is based on the investigator’s assessment using RECIST 1.1. 100mg BID 150mg BID 100mg BID 100mg BID 100mg BID 100mg QD 100mg BID NSCLC TPR-NTRK1 MASC ETV6-NTRK3 MASC ETV6-NTRK3 MASC ETV6-NTRK3 GIST ETV6-NTRK3 Soft Tissue Sarcoma LMNA-NTRK1 Thyroid ETV6-NTRK3 -100% -80% -60% -40% -20% 0% 20% 40% 60% 80% 100% Best Change from Baseline (%) Stable disease Confirmed partial response - 30%
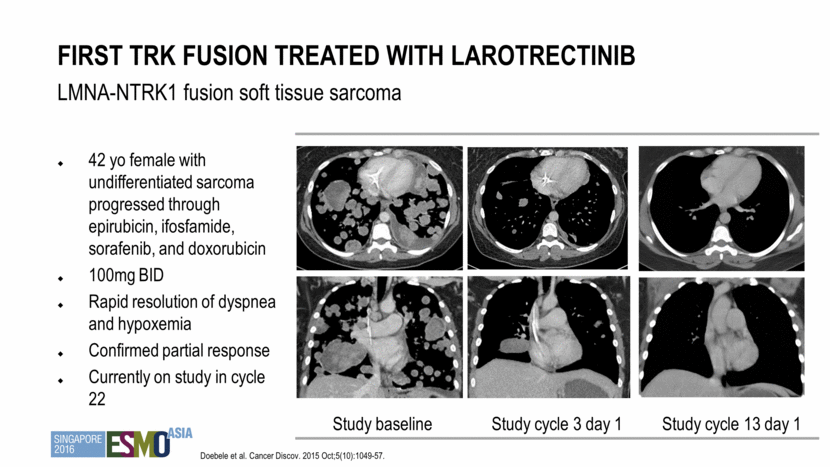
42 yo female with undifferentiated sarcoma progressed through epirubicin, ifosfamide, sorafenib, and doxorubicin 100mg BID Rapid resolution of dyspnea and hypoxemia Confirmed partial response Currently on study in cycle 22 First trk fusion treated with larotrectinib LMNA-NTRK1 fusion soft tissue sarcoma Study baseline Study cycle 3 day 1 Study cycle 13 day 1 Doebele et al. Cancer Discov. 2015 Oct;5(10):1049-57.
Larotrectinib is a purpose-built, oral, selective and potent TRK inhibitor Larotrectinib is well tolerated, with few adverse events likely related to drug Larotrectinib appears broadly active against TRK fusion cancers in this Phase 1 study Confirmed RECIST responses observed in 6 of 7 evaluable patients, with tumor regression in all 7 Encouraging durability signal: All TRK fusion patients remain on study with most past one year Larotrectinib Phase 2 basket trial currently enrolling patients with TRK gene fusions conclusions
Solid tumors, including CNS tumors, with TRK fusion based on local or pre-existing testing Enrolling patients > 12 yo; ECOG 0-3 Dose: 100mg BID Primary endpoint: Best ORR Global study: Active in US, EU, and Asia (South Korea, Singapore, Japan) Patient assistance for trial travel and logistics Navigate trial Larotrectinib Phase 2 Basket Study

Larotrectinib patients and their families and caregivers Co-investigators and study support staffs This trial sponsored and supported by Loxo Oncology Larotrectinib invented by Array BioPharma acknowledgements

