Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zynerba Pharmaceuticals, Inc. | a16-23198_18k.htm |
Exhibit 99.1
Clinical Research Day December 15, 2016 A clinical-stage specialty pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs

Disclaimer The statements in this presentation may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements, among other things relate to the future operations, opportunities or financial performance of Zynerba Pharmaceuticals, Inc. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. These and other risks are described in our filings with the Securities and Exchange Commission, available at www.sec.gov. Any forward-looking statements that the Company makes in this presentation speak only as of the date of this PRESENTATION. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this presentation. 2 © 2016 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a registered trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners.

Company Highlights 3 A clinical-stage specialty pharmaceutical company pioneering the development of patent-protected synthetic cannabinoid therapeutics for transdermal delivery Experienced team with deep expertise and proven track record of success in patch and gel transdermal delivery, regulatory approval and commercialization Global ownership of two proprietary product candidates intended to treat diseases with significant unmet medical need and market potential ZYN002 – CBD Gel: epilepsy, osteoarthritis and Fragile X syndrome ZYN001 – THC Pro-Drug Patch: fibromyalgia and peripheral neuropathic pain No royalty obligations owed on either product candidate Cash as of September 30, 2016 = $31.8 million; expected runway into 2018 to fund five phase 3 ready programs
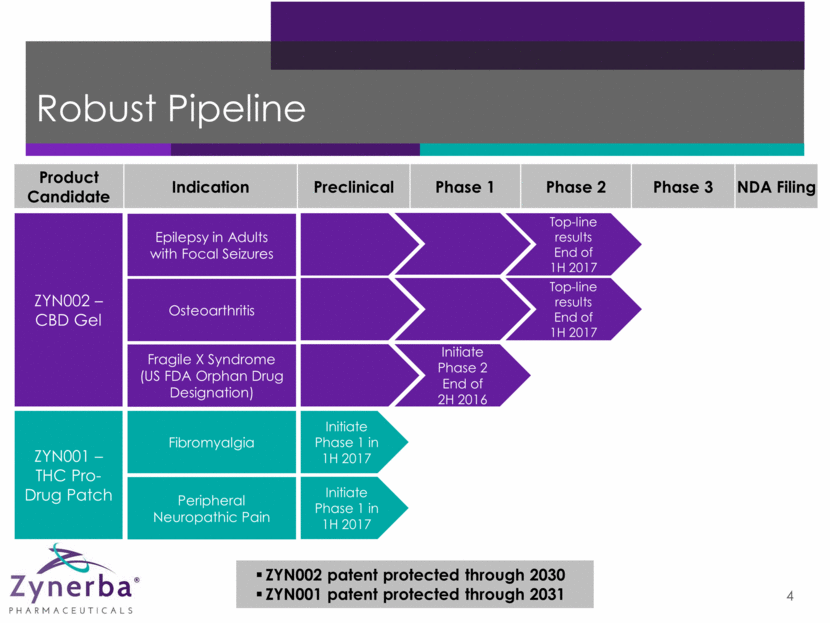
Robust Pipeline 4 ZYN002 patent protected through 2030 ZYN001 patent protected through 2031 Product Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 NDA Filing ZYN001 – THC Pro-Drug Patch ZYN002 – CBD Gel Epilepsy in Adults with Focal Seizures Osteoarthritis Fragile X Syndrome (US FDA Orphan Drug Designation) Fibromyalgia Peripheral Neuropathic Pain Top-line results End of 1H 2017 Top-line results End of 1H 2017 Initiate Phase 2 End of 2H 2016 Initiate Phase 1 in 1H 2017 Initiate Phase 1 in 1H 2017
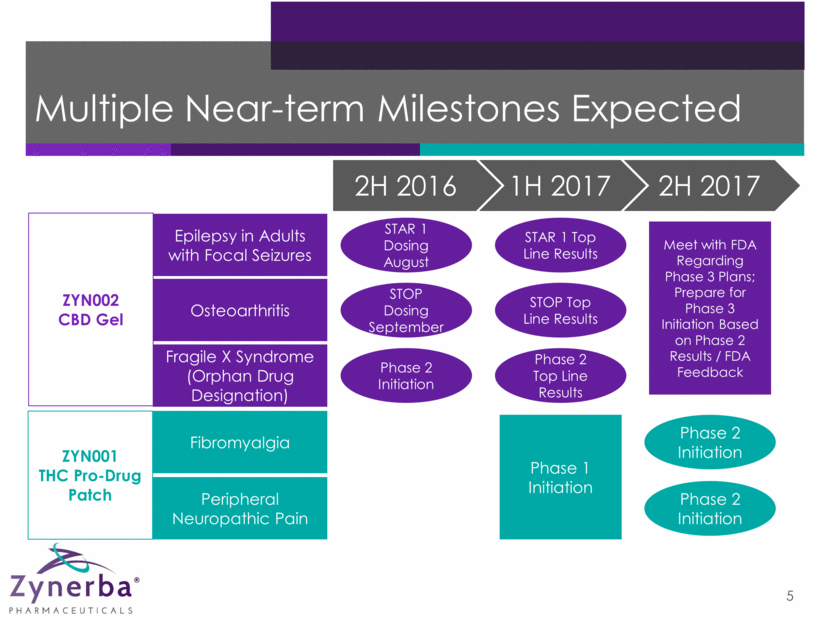
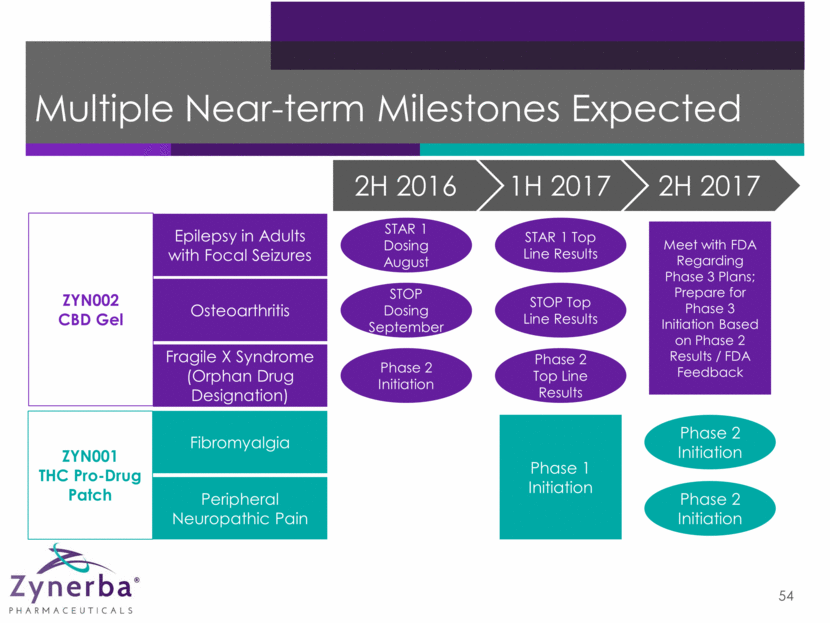
Multiple Near-term Milestones Expected 5 ZYN001 THC Pro-Drug Patch ZYN002 CBD Gel Epilepsy in Adults with Focal Seizures Osteoarthritis Fragile X Syndrome (Orphan Drug Designation) Fibromyalgia Peripheral Neuropathic Pain STOP Dosing September Phase 2 Initiation Phase 2 Top Line Results STOP Top Line Results STAR 1 Top Line Results Phase 1 Initiation STAR 1 Dosing August Phase 2 Initiation Phase 2 Initiation Meet with FDA Regarding Phase 3 Plans; Prepare for Phase 3 Initiation Based on Phase 2 Results / FDA Feedback 2H 2016 1H 2017 2H 2017
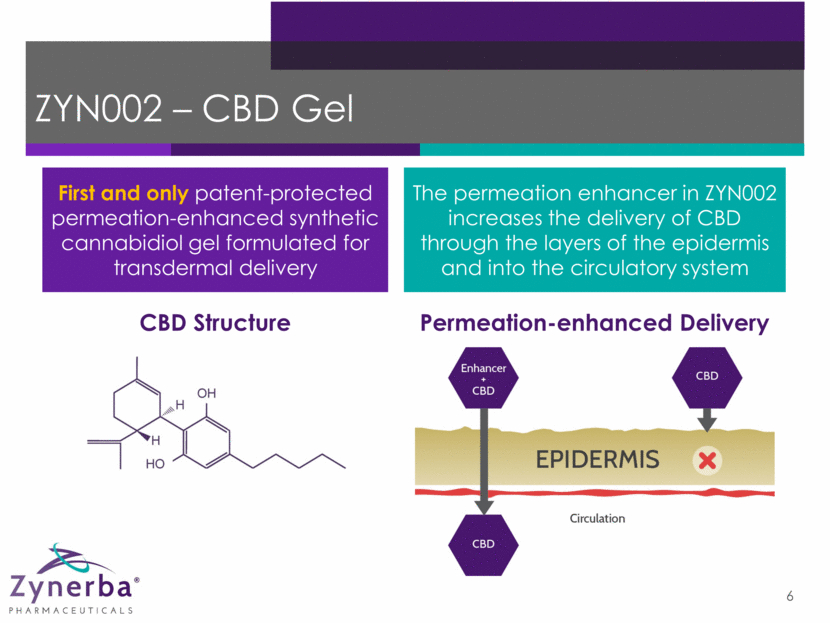
ZYN002 – CBD Gel 6 CBD Structure Permeation-enhanced Delivery First and only patent-protected permeation-enhanced synthetic cannabidiol gel formulated for transdermal delivery The permeation enhancer in ZYN002 increases the delivery of CBD through the layers of the epidermis and into the circulatory system
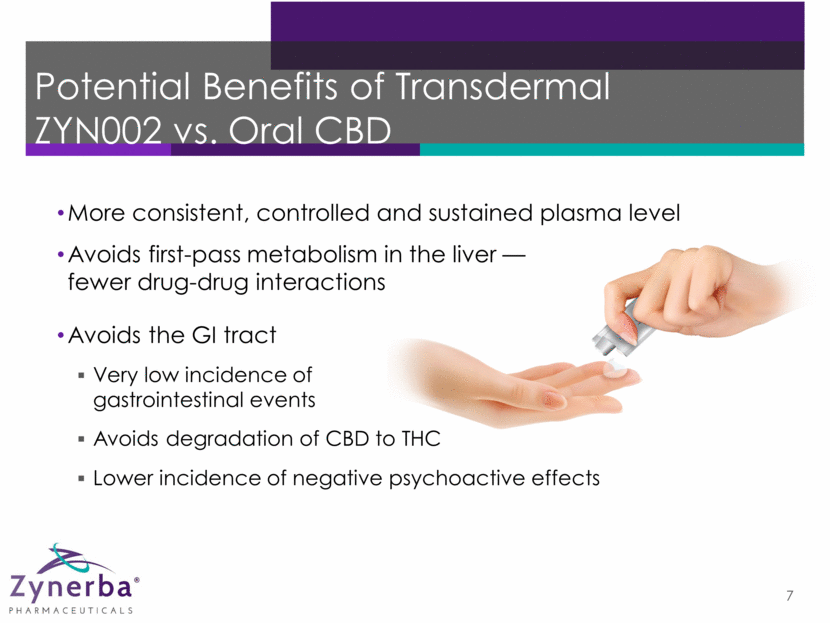
Potential Benefits of Transdermal ZYN002 vs. Oral CBD 7 Avoids the GI tract Very low incidence of gastrointestinal events Avoids degradation of CBD to THC More consistent, controlled and sustained plasma level Avoids first-pass metabolism in the liver — fewer drug-drug interactions Lower incidence of negative psychoactive effects
Objectives for Today 8 “Why CBD in Epilepsy, OA, and Fragile X Syndrome?” Rationale for Deeper Understanding of Opportunity to hear from Understand why Clinical development and status to date Key Opinion Leaders and ask questions ZYNE is a compelling investment opportunity
Agenda 9 Time Topic Presenting 08:00 Opening Comments Armando Anido, Zynerba 08:10 Overview of Cannabinoid Pharmacology Marcel Bonn-Miller, PhD, Zynerba 08:25 Zynerba Clinical Program in Epilepsy Terri Sebree, President, Zynerba 08:40 Epilepsy Jacqueline A. French, MD, NYU Langone Medical Center 08:55 Zynerba Clinical Program in Osteoarthritis Terri Sebree, President, Zynerba 09:10 Osteoarthritis Daniel J. Clauw, MD, University of Michigan Health System 09:25 Zynerba Clinical Program in Fragile X Syndrome Terri Sebree, President, Zynerba 09:40 Fragile X Syndrome Steven Siegel, MD, PhD, University of Southern California 09:55 Closing Comments Armando Anido, Zynerba 10:05 Q & A 10:30 Conclude
10 © 2016 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a registered trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners. Marcel Bonn-Miller, PhD Director of Cannabinoid Research, Zynerba Pharmaceuticals Adjunct Assistant Professor in the Department of Psychiatry at the University of Pennsylvania Perelman School of Medicine Former Research Health Science Specialist at the Center of Excellence in Substance Abuse Treatment and Education at the Philadelphia Veterans Affairs, as well as at the National Center for PTSD, and Center for Innovation to Implementation at the Palo Alto Veterans Affairs Research has focused on the interrelations between cannabis and chronic pain, HIV, PTSD, and sleep disorders Published over 100 peer-reviewed empirical publications and serves on the editorial boards of six scientific journals Dr. Bonn-Miller is a graduate of the University of Vermont with a PhD in clinical psychology and completed a joint postdoctoral fellowship at the Palo Alto Veterans Affairs & Stanford University School of Medicine Dr. Bonn-Miller is an employee of Zynerba Pharmaceuticals. The drugs discussed in this presentation have not been approved by the Food and Drug Agency (FDA). Cannabinoid Pharmacology

A clinical-stage specialty pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs Cannabidiol Pharmacology In Epilepsy, Osteoarthritis, and Fragile X Syndrome Marcel O. Bonn-Miller, PhD Director of Cannabinoid Research
Cannabis: Medicine Since 2737 BC Listed in the US Pharmacopeia until 1942 12 74% alcohol

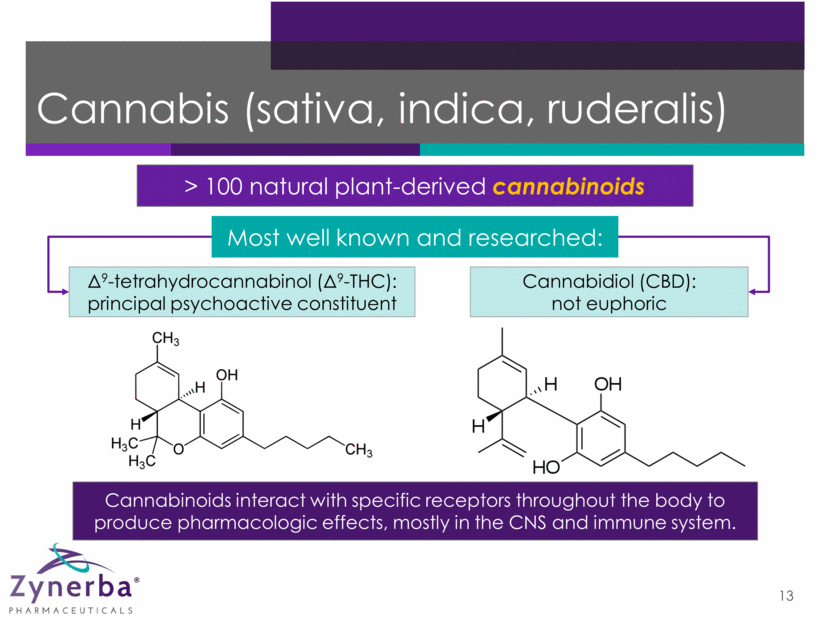
Cannabis (sativa, indica, ruderalis) > 100 natural plant-derived cannabinoids 13 Cannabinoids interact with specific receptors throughout the body to produce pharmacologic effects, mostly in the CNS and immune system. 9-tetrahydrocannabinol (9-THC): principal psychoactive constituent Cannabidiol (CBD): not euphoric Most well known and researched:
Endocannabinoid System Ancient internal signaling system — began evolving 600 million years ago 14 Present in every animal except insects NIMH* (1990) discovers the exact DNA sequence encoding a THC-sensitive receptor in the rat brain *National Institutes of Mental Health Discovery of naturally-occurring transmitters — endocannabinoids — anandamide (1992) and 2-AG (2-arachidonoylglycerol) (1995)
Endocannabinoids 15 Anandamide (N-arachidonoylethanolamine or AEA) Released in response to excitatory synaptic activity In the CNS: Important in synaptic plasticity, cognitive, motor, sensory, and affective processes Neuroprotective in: Acute and chronic neurodegenerative conditions Neuroinflammatory conditions Modulate inflammatory processes Down regulate stress related signals 2-AG (2-arachidonoylglycerol)
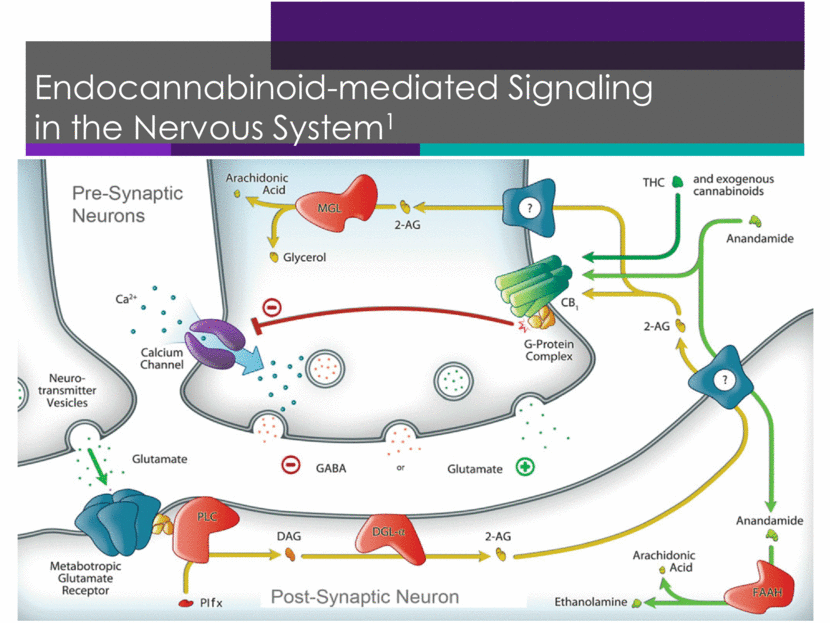
Endocannabinoid-mediated Signaling in the Nervous System1 16
Low affinity for CB1/CB2 receptors Stimulates the release of 2-AG Inhibits anandamide degradation and enhances its signaling3 Agonist TRPV1 receptor: mediates pain perception/inflammation 5-HT1A serotonin receptor Decreases depression Anxiolytic Antagonist at GPR55: modulates blood pressure and bone density Activates adenosine A2A receptor Decreases inflammation Neuroprotection 17 Activity at Multiple Receptors May Account for CBD Effects CBD Pharmacology2
CBD is Effective in a Variety of Acute Seizure Rodent Models5-8 MES, PTZ, audiogenic seizures, penicillin, pilocarpine, transcorneal electroshock and electrical amygdala kindling Increased seizure threshold, reduced seizure severity and reduced mortality in generalized seizures 18 Efficacy in animal models is highly predictive of efficacy in patients with epilepsy MES, maximal electroshock seizure, PTZ, pentylenetetrazole
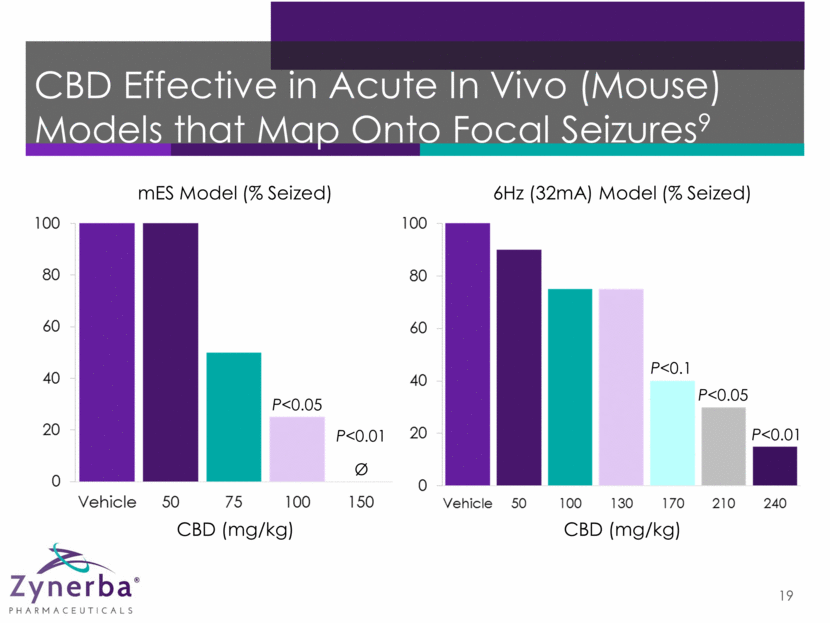
CBD Effective in Acute In Vivo (Mouse) Models that Map Onto Focal Seizures9 19 P<0.1 P<0.05 P<0.01 6Hz (32mA) Model (% Seized) Ø P<0.05 P<0.01 mES Model (% Seized) CBD (mg/kg) CBD (mg/kg)
CBD Pharmacology: Osteoarthritis Recent behavioral and electrophysiological studies have demonstrated that cannabinoids exert anti-nociceptive effects in rodent models of osteoarthritis10 CBD has been shown to have antihyperalgesic effects through its interaction with TRPV1 receptors12 CBD activates the adenosine receptor (A2A), which has broad anti-inflammatory effects throughout the body11 By blocking GPR55 signaling, which promotes osteoclast cell function, CBD may act to decrease bone resorption13 20
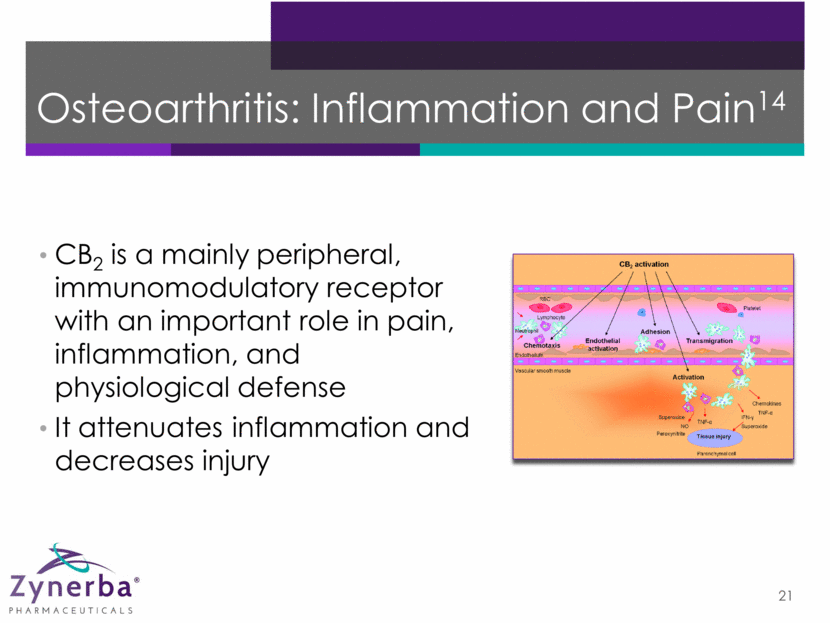
Osteoarthritis: Inflammation and Pain14 21 CB2 is a mainly peripheral, immunomodulatory receptor with an important role in pain, inflammation, and physiological defense It attenuates inflammation and decreases injury
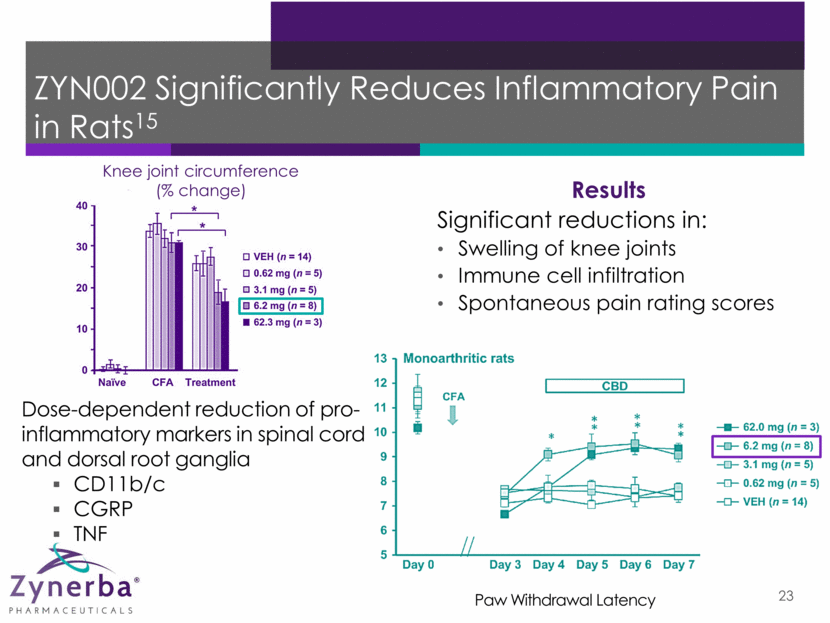
ZYN002 Significantly Reduces Inflammatory Pain in Rats15 22

ZYN002 Significantly Reduces Inflammatory Pain in Rats15 Dose-dependent reduction of pro-inflammatory markers in spinal cord and dorsal root ganglia CD11b/c CGRP TNF Knee joint circumference (% change) Paw Withdrawal Latency Results Significant reductions in: Swelling of knee joints Immune cell infiltration Spontaneous pain rating scores 23
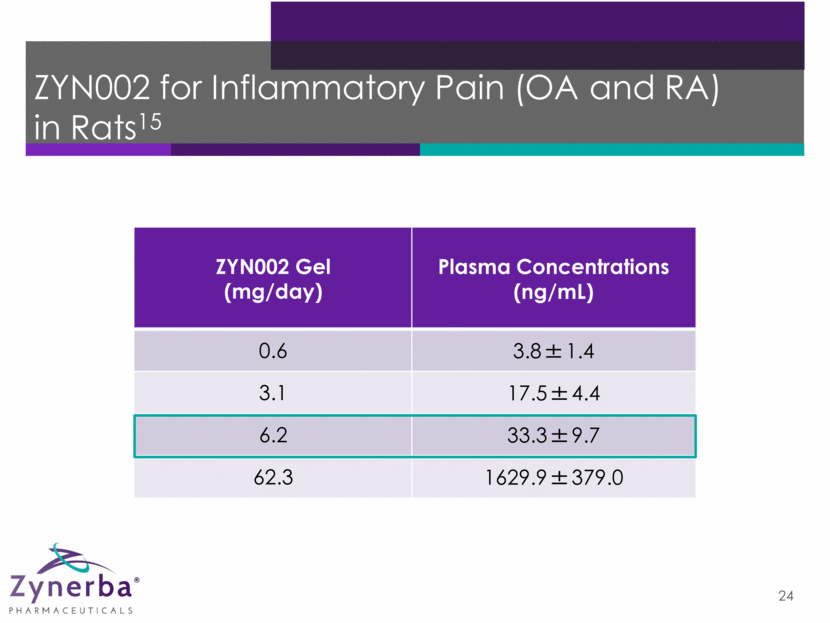
ZYN002 for Inflammatory Pain (OA and RA) in Rats15 24 ZYN002 Gel (mg/day) Plasma Concentrations (ng/mL) 0.6 3.8±1.4 3.1 17.5±4.4 6.2 33.3±9.7 62.3 1629.9±379.0
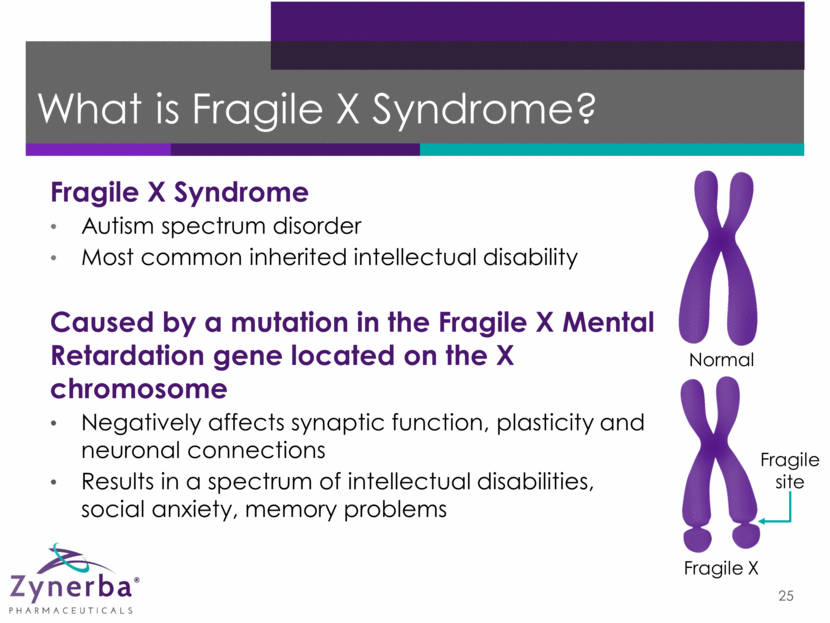
25 Normal Fragile X Fragile site Fragile X Syndrome Autism spectrum disorder Most common inherited intellectual disability Caused by a mutation in the Fragile X Mental Retardation gene located on the X chromosome Negatively affects synaptic function, plasticity and neuronal connections Results in a spectrum of intellectual disabilities, social anxiety, memory problems What is Fragile X Syndrome?
CBD Pharmacology: Fragile X Action Effect Attenuates endocannabinoid signaling May allow bypass of the FMRP protein deficiency Increases 2-AG levels May attenuate/reverse a biological mechanism of abnormal cellular function16 Interacts with receptors that regulate fear and anxiety17 5-HT1A TRPV1 May prove beneficial in patients with FXS TRPV1, Transient receptor potential vanilloid type 1 26
CBD Effects in Fragile X Syndrome 27 CBD may effectively treat Fragile X Syndrome19-21 In mouse knock-out model, inhibition of the metabolism of 2-AG improves Fragile X syndrome symptoms CBD inhibits the metabolism of 2-AG and thereby increasing availability to modulate neurotransmitter release Normal Fragile X Fragile site
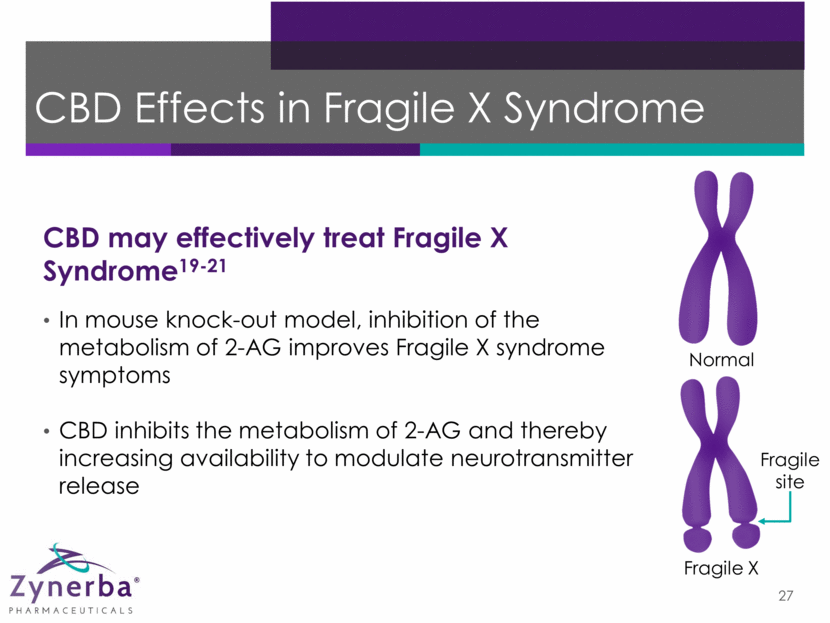
CBD Improves Social Anxiety23 Change from baseline on Visual Analog Mood Scales (n=12) Oral CBD 600 mg Controls Placebo Basal Pretest Adaptation Speech Post-speech Basal Pretest Adaptation Speech Post-speech *P<0.05 versus healthy controls +P<0.05 versus CBD-treated social anxiety patients Anxiety Cognitive Impairment 28
Summary 29 Cannabidiol acts as agonist or antagonist at diverse array of receptors throughout the body, leading to an opportunity of treating a range of conditions Cannabidiol may modulate the endocannabinoid system Theory and preclinical data combine to support the treatment of focal seizures, osteoarthritis, and Fragile X Syndrome with cannabidiol Translatability of examined animal models and early clinical evidence provide confidence that effects will be observed in humans
References Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Comprehensive treatment of chronic pain by medical, interventional, and integrative approaches. Deer TR et al. (eds.) Available at: http://cannabisclinicians.org/wp-content/uploads/2015/05/Russo-Hohmann-Role-of-cannabinoids-in-pain-management-from-Deer-2013.pdf. Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev. 2010;62:588-631. Elmes MW, Kaczocha M, Berger WT, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for 9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015;290:8711-8721. Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452:295-301. Consroe P, Wolkin A. Cannabidiol--antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201:26-32. Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol. 1982;83:293-298. Jones NA, Hill AJ, Smith I, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332:569-577. 30

References cont. Jones NA, Glyn SE, Akiyama S, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21:344-352. Jones N, Hill T, Stott C, Wright S. Assessment of the anticonvulsant effects and tolerability of GW Pharmaceuticals’ cannabidiol in the anticonvulsant screening program. Poster presented at the American Epilepsy Society Conference Dec 4-8, 2015. La Porta C, Bura SA, Negrete R, Maldonado R. Involvement of the endocannabinoid system in osteoarthritis pain. Eur J Neurosci. 2014;39:485-500. Mecha M, Feliú A, Iñigo PM, Mestre L, Carrillo-Salinas FJ, Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013;59:141-150. Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247-250. Whyte LS, Ryberg E, Sims NA, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16511-16516. Zubrzycki M, Liebold A, Janecka A, Zubrzycka M. A new face of endocannabinoids in pharmacotherapy. Part I: protective role of endocannabinoids in hypertension and myocardial infarction. J Physiol Pharmacol. 2014;65:171-181. 31

References cont. Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948. Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012;3:1080. Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12:825-836. Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001;3: 359-371. Di Marzo V1, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci. 2008;29:229-233. Bisogno T1, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845-852. Grotenhermen F. Pharmacology of cannabinoids. Neuro Endocrinol Lett. 2004;25:14-23. Crippa JA, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121-30. Bergamaschi MM, Queiroz RH, Chagas MH, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36:1219-1226. 32

Zynerba Clinical Development Update Terri Sebree President A clinical-stage specialty pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs

Trials 1 day, 7 day, and 14 day trials Subjects 98 healthy volunteers (n=70 ZYN002; n=28 placebo) and 22 epilepsy patients (n=16 ZYN002; n=6 placebo) Dose Daily doses levels – 50 to 504 mg CBD in 1%, 2.5% and 4.2% concentrations Safety & Tolerability ZYN002 was safe and well-tolerated at all dose levels and concentrations Transdermal application well-tolerated with minimal erythema No somnolence or fatigue reported Very low incidence of GI events observed Adverse events with ZYN002 in healthy volunteers and epilepsy patients were similar to placebo Pharmacokinetics Favorable CBD pharmacokinetic properties, no differences between healthy volunteers and epilepsy patients CBD plasma concentrations are dose dependent and at steady state do not fluctuate No THC detected in plasma or urine Cognition/Mood Demonstrated no impairment in cognitive function based on Trail Making, Divided Attention, & PASAT No changes in mood as accessed by IDAS and PANAS ZYN002 Phase 1 Results 34
Zynerba Clinical Trials Program 35 Investigating CBD in 3 major disease states with high unmet clinical needs Osteoarthritis — STOP Epilepsy — STAR Fragile X Syndrome
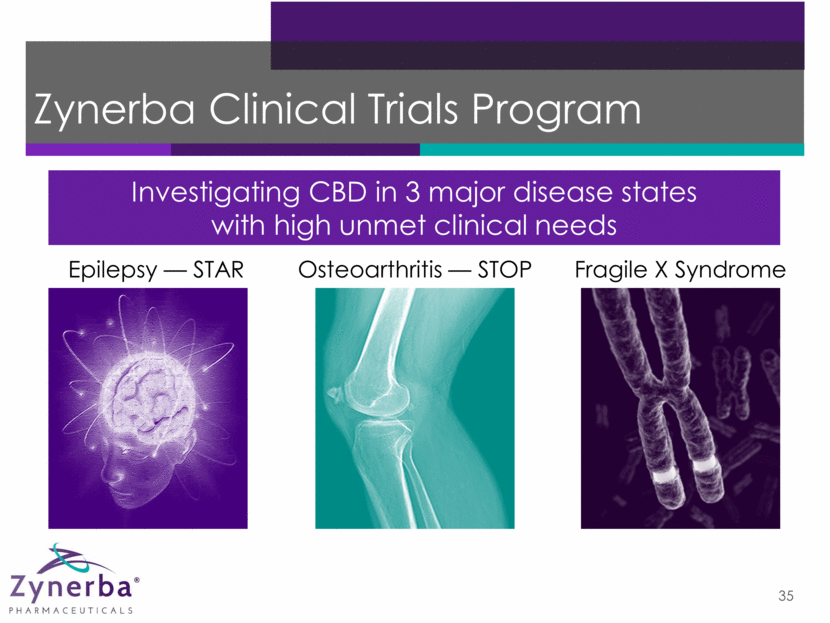
Epilepsy Phase 2 Clinical Trial – STAR 36 ZYN002 High-Dose CBD 195 mg* ZYN002 Low-Dose CBD 97.5 mg* Placebo Baseline Assess seizure frequency/type Open-Label Extension Synthetic Transdermal CAnnabidiol for the TReatment of Epilepsy *In 4.2% gel ZYN002 High-Dose CBD 195 mg* ZYN002 Low-Dose CBD 97.5 mg* STAR 2: ZYN2-CL-004 STAR 1: ZYN2-CL-03 As needed, dose reduced to: BID for 12 months BID for 12 weeks 8 weeks Randomized (1:1:1), double-blind, placebo-controlled — 180 adults
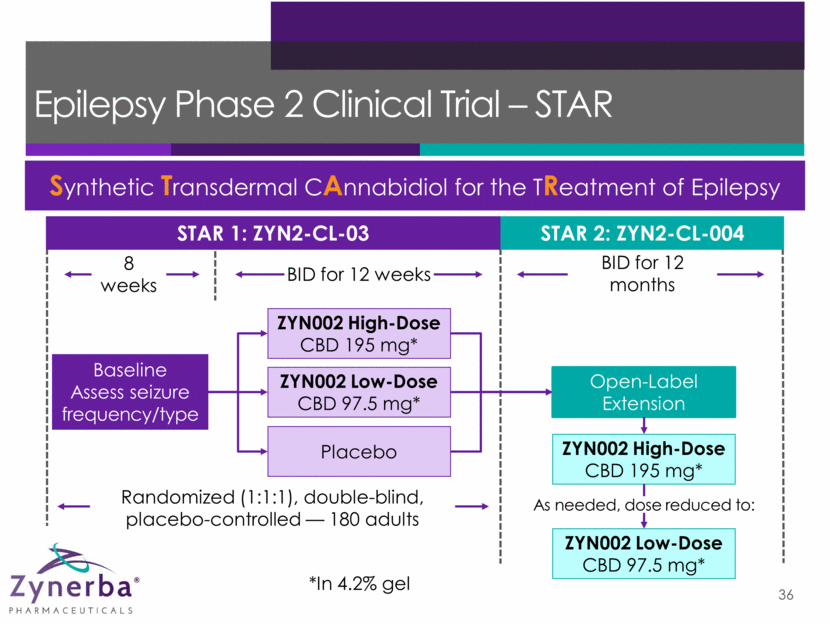
ZYN2-CL-03: STAR 1 Efficacy Endpoints 37 Primary Endpoint: Median reduction in seizure frequency per 28-day period comparing baseline with the maintenance period via daily seizure diary Secondary Endpoints Proportion of patients with 50% reduction from baseline in seizure frequency Percent change from baseline in seizure frequency Change from baseline in seizure frequency Seizure-free days 100% seizure-free
ZYN2-CL-03: STAR 1 Safety Assessments Adverse events Skin irritation examination Physical and neurological examinations 12-lead ECG Clinical labs 38
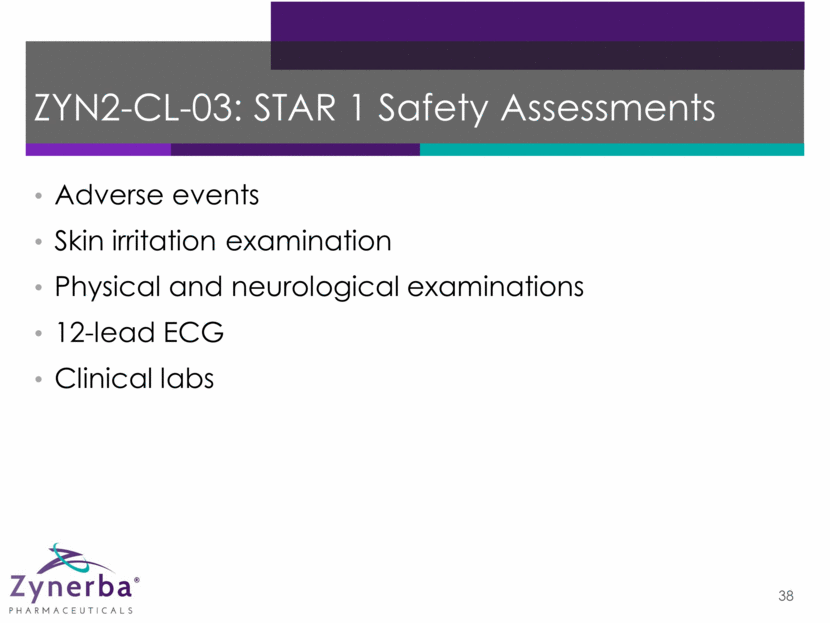
39 ZYN2-CL-03: STAR 1 Demographics* Age, years (mean, range) 39 (18-68) Gender (%) Male Female 44 56 Race(%) White Asian Other 87 5 8 *As of 12 December 2016
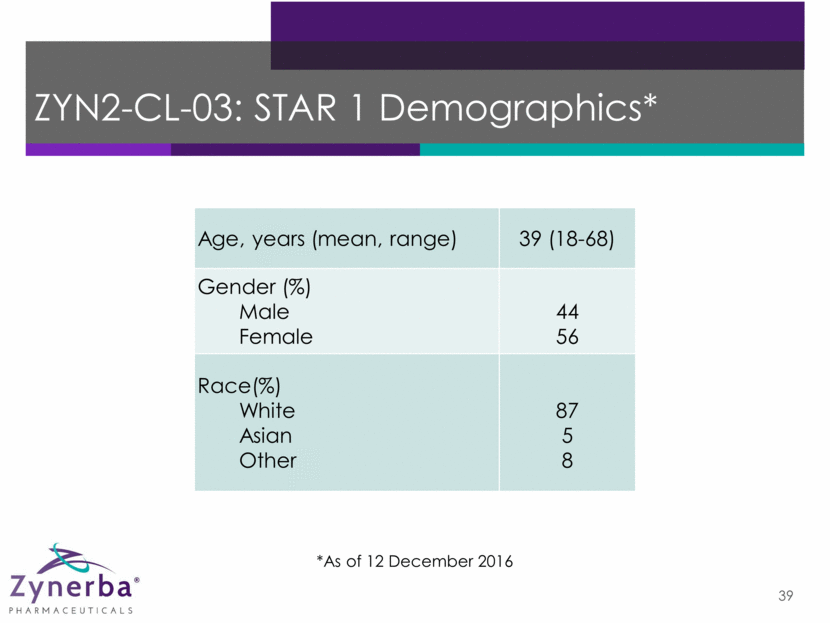
40 ZYN2-CL-03: STAR 1 Baseline Information* Anti-Epileptic Drug Patients Anti-Epileptic Drug Patients Levetiracetam (Keppra) 39 Clonazepam 11 Carabamazepine 38 Zonisamide (Zonegran) 10 Lamotrigine 32 Oxcarbazepine 10 Perampanel (Fycompa) 29 Valproate 8 Lacosamide (Vimpat) 24 Phenytoin 3 Topiramate 16 Phenobarbitone 2 AED Use Average=2.6 AEDs Median=3.0 AEDs Median Seizure Frequency† 10.5 (3-330) †Median Monthly *As of 7 December 2016
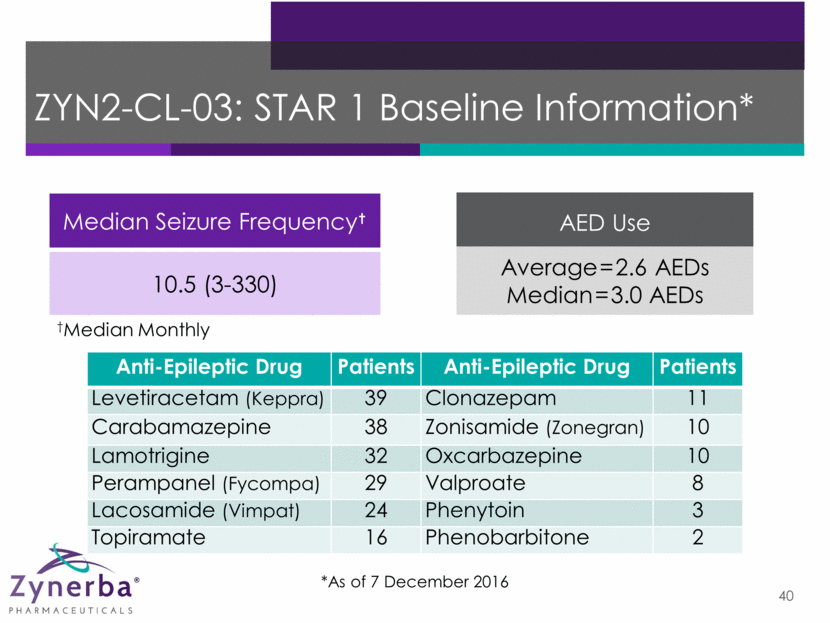
41 ZYN2-CL-03: STAR 1 Enrollment* *As of 14 December 2016 Patients n Screened 185 Randomized 105 Completed 37 Enrolled into Star 2 36
42 © 2016 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a registered trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners. Jacqueline A. French, MD, Director of Clinical Trials at the NYU Langone Medical Center Comprehensive Epilepsy Center & Professor in the Department of Neurology at NYU Langone Director of the Epilepsy Study Consortium and CSO of the Epilepsy Foundation Certified by the American Board of Psychiatry & Neurology in Neurology Published research featured in Lancet Neurology and The New England Journal of Medicine, among other respected publications; research focuses on finding new therapeutic interventions for people with epilepsy and on clinical trial methodology. Worked with the U.S. Food and Drug Administration to develop new trial designs for antiepileptic drug approval Dr. French is a graduate of Brown University’s School of Medicine, trained in neurology at Mount Sinai Hospital in New York and completed fellowships in EEG and epilepsy at Mount Sinai Hospital and Yale University Dr. French is not an employee of Zynerba Pharmaceuticals and has been compensated for transportation and meals in relation with this event. The drugs discussed in this presentation have not been approved by the Food and Drug Agency (FDA). Dr. French is a member of Zynerba Pharmaceutical’s Scientific Advisory board. All opinions expressed in this presentation belong to Dr. French and do not reflect the opinions of the NYU Langone Medical Center or its Comprehensive Epilepsy Center. Epilepsy

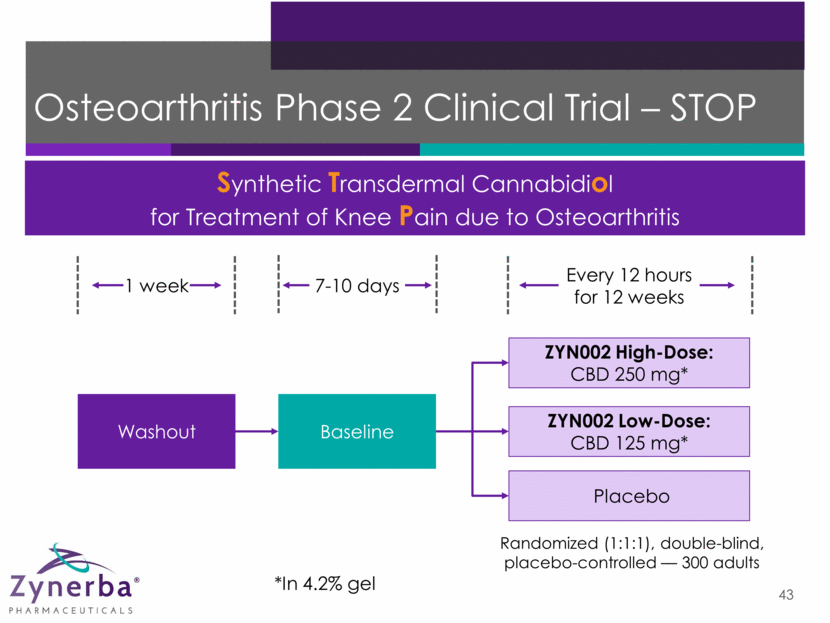
Osteoarthritis Phase 2 Clinical Trial – STOP 43 Synthetic Transdermal Cannabidiol for Treatment of Knee Pain due to Osteoarthritis ZYN002 High-Dose: CBD 250 mg* ZYN002 Low-Dose: CBD 125 mg* Placebo Washout Baseline 1 week Every 12 hours for 12 weeks 7-10 days *In 4.2% gel Randomized (1:1:1), double-blind, placebo-controlled — 300 adults
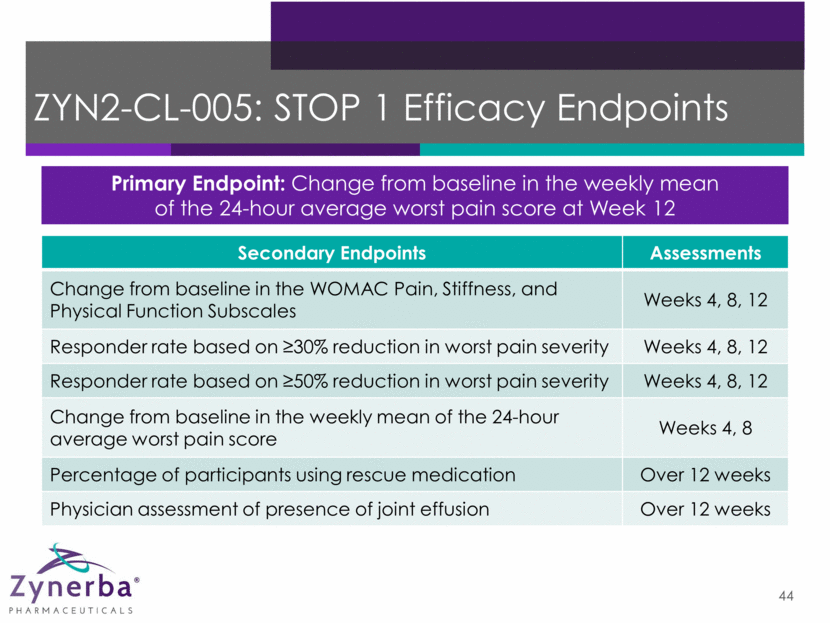
ZYN2-CL-005: STOP 1 Efficacy Endpoints 44 Primary Endpoint: Change from baseline in the weekly mean of the 24-hour average worst pain score at Week 12 Secondary Endpoints Assessments Change from baseline in the WOMAC Pain, Stiffness, and Physical Function Subscales Weeks 4, 8, 12 Responder rate based on 30% reduction in worst pain severity Weeks 4, 8, 12 Responder rate based on 50% reduction in worst pain severity Weeks 4, 8, 12 Change from baseline in the weekly mean of the 24-hour average worst pain score Weeks 4, 8 Percentage of participants using rescue medication Over 12 weeks Physician assessment of presence of joint effusion Over 12 weeks
ZYN2-CL-005: STOP 1 Safety Assessments Adverse events Skin irritation examination Physical and neurological examinations 12-lead ECG Clinical labs 45

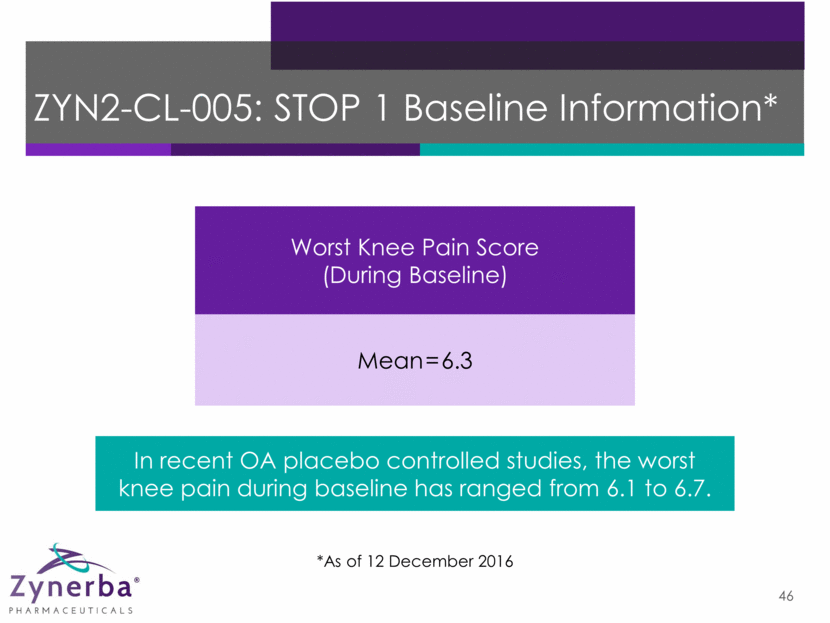
46 ZYN2-CL-005: STOP 1 Baseline Information* In recent OA placebo controlled studies, the worst knee pain during baseline has ranged from 6.1 to 6.7. Worst Knee Pain Score (During Baseline) Mean=6.3 *As of 12 December 2016
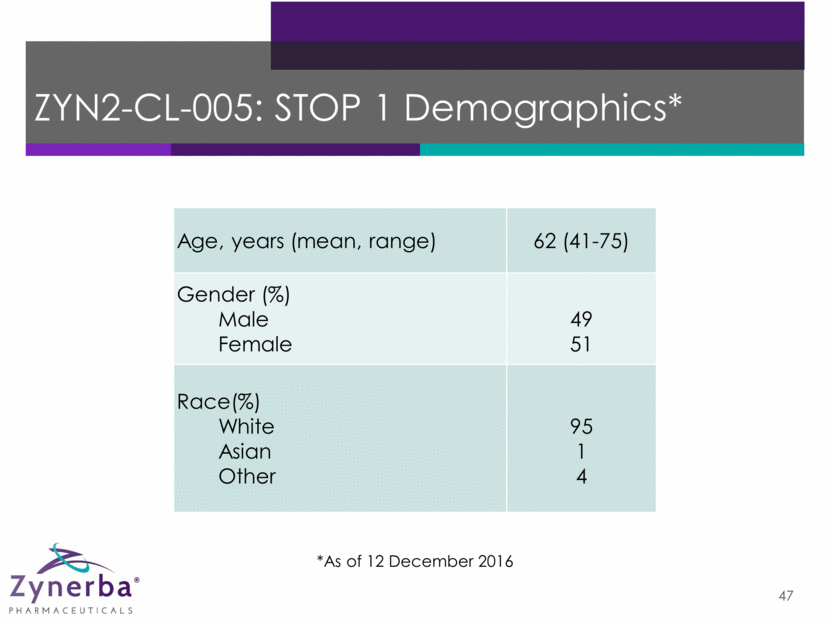
47 ZYN2-CL-005: STOP 1 Demographics* Age, years (mean, range) 62 (41-75) Gender (%) Male Female 49 51 Race(%) White Asian Other 95 1 4 *As of 12 December 2016
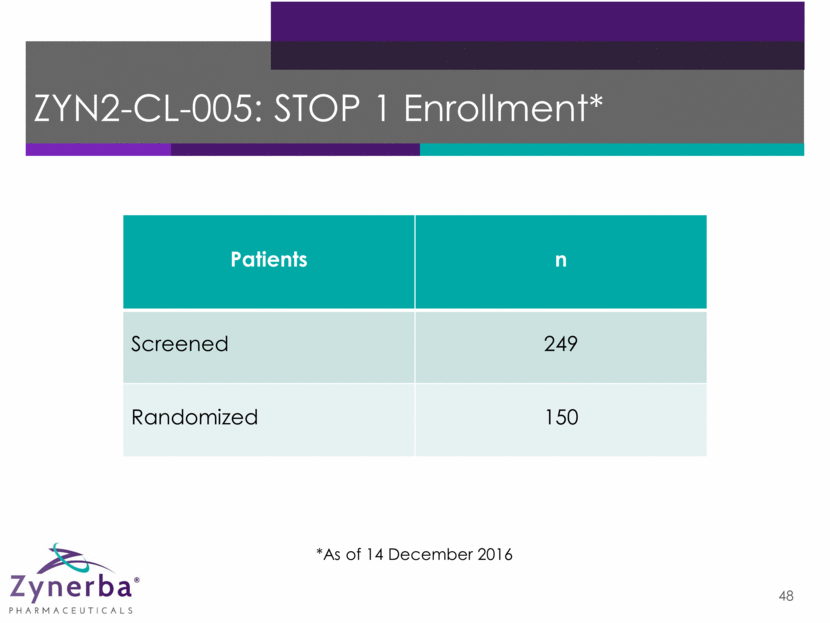
48 ZYN2-CL-005: STOP 1 Enrollment* *As of 14 December 2016 Patients n Screened 249 Randomized 150
49 Daniel J. Clauw, MD Professor of Pain Management and Anesthesiology at the University of Michigan Health System Director of the Chronic Pain and Fatigue Research Center at the University of Michigan Oversees a multidisciplinary group that performs both mechanistic studies and clinical trials in overlapping conditions characterized by chronic pain and fatigue, including fibromyalgia, chronic fatigue syndrome, and Gulf War Illnesses Served as the first Associate Dean for Clinical and Translational Research within the University of Michigan Medical School, and PI of the UM Clinical and Translational Sciences Award (CTSA) until January 2009 Board certified in Internal Medicine and Rheumatology Dr. Clauw completed his M.D. at the University of Michigan’s Medical School and completed his internal medicine residency and rheumatology fellowship at Georgetown University School of Medicine Dr. Clauw is not an employee of Zynerba Pharmaceuticals and has been compensated for transportation and meals in relation with this event. The drugs discussed in this presentation have not been approved by the Food and Drug Agency (FDA). Dr. Clauw is a member of Zynerba Pharmaceutical’s Scientific Advisory board. All opinions expressed in this presentation belong to Dr. Clauw and do not reflect the opinions of the University of Michigan Health System. © 2016 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a registered trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners. Osteoarthritis

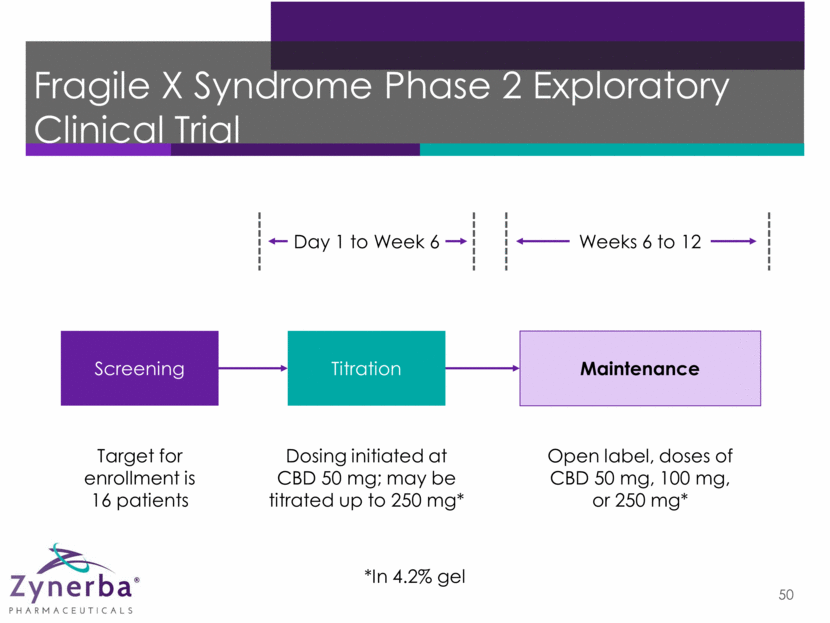
Fragile X Syndrome Phase 2 Exploratory Clinical Trial 50 Maintenance Dosing initiated at CBD 50 mg; may be titrated up to 250 mg* Screening Titration Weeks 6 to 12 Day 1 to Week 6 Open label, doses of CBD 50 mg, 100 mg, or 250 mg* *In 4.2% gel Target for enrollment is 16 patients
51 © 2016 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a registered trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners. Steven Siegel, MD, PhD Chair of the Department of Psychiatry and Behavioral Sciences at the Keck School of Medicine, University of Southern California Held a number of faculty, hospital and administrative appointments during a 20 year tenure at the University of Pennsylvania Noted physician and researcher with an expertise in schizophrenia and psychosis and has published more than 130 manuscripts spanning topics related to drug abuse, basic research in animal models of schizophrenia and autism, as well as clinical aspects of schizophrenia Serves on the editorial board of several leading journals related to psychiatry and neurobiology Dr. Siegel received his M.D. and Ph.D. in Neurobiology at the Mount Sinai School of Medicine, completed an undergraduate degree in Neuroscience at Colgate University and finished his residency in Psychiatry and a Fellowship in Neuropsychiatry at the University of Pennsylvania before joining the faculty Dr. Siegel is not an employee of Zynerba Pharmaceuticals and has been compensated for transportation and meals in relation with this event. The drugs discussed in this presentation have not been approved by the Food and Drug Agency (FDA). Dr. Siegel is a member of Zynerba Pharmaceutical’s Scientific Advisory board. All opinions expressed in this presentation belong to Dr. Siegel and do not reflect the opinions of the University of Southern California or the Keck School of Medicine. Fragile X Syndrome
A clinical-stage specialty pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs Closing Comments Armando Anido Chairman and CEO

A Compelling Investment Opportunity – Why Zynerba Could Be the Next Explosive Growth Cannabinoid Company 53 We believe ZYN002 (CBD gel) for focal seizures is a significantly de-risked lead candidate Reliable animal models show efficacy of CBD in epilepsy Third party studies have shown activity of CBD in reducing focal seizures STAR 1 trial is well powered to show clinical benefit versus placebo Zynerba’s transdermal approach has significant benefits Patent protection Synthetic manufacturing process can be scaled for large volume markets Attractive COGS and no royalty obligations should result in high gross margins Consistent, controlled blood levels of drug and potentially a better safety profile than oral formulations Focused on valuable unmet medical need markets ~ 1.5 million adult epilepsy patients in the US with focal seizures; ~ 31 million OA patients; and Orphan designation for Fragile X Syndrome Major clinical milestones with ZYN002 anticipated over next 6 months

Multiple Near-term Milestones Expected 54 ZYN001 THC Pro-Drug Patch ZYN002 CBD Gel Epilepsy in Adults with Focal Seizures Osteoarthritis Fragile X Syndrome (Orphan Drug Designation) Fibromyalgia Peripheral Neuropathic Pain STOP Dosing September Phase 2 Initiation Phase 2 Top Line Results STOP Top Line Results STAR 1 Top Line Results Phase 1 Initiation STAR 1 Dosing August Phase 2 Initiation Phase 2 Initiation Meet with FDA Regarding Phase 3 Plans; Prepare for Phase 3 Initiation Based on Phase 2 Results / FDA Feedback 2H 2016 1H 2017 2H 2017
Closing Summary 55 “Why CBD in Epilepsy, OA, and Fragile X Syndrome?” Rationale for Deeper Understanding of Opportunity to hear from Understand why Clinical development and status to date Key Opinion Leaders and ask questions ZYNE is a compelling investment opportunity
A clinical-stage specialty pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs Questions and Answers

A clinical-stage specialty pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs Clinical Research Day December 15, 2016

