Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - Zynerba Pharmaceuticals, Inc. | zyne-20160930ex3129916a3.htm |
| EX-32.2 - EX-32.2 - Zynerba Pharmaceuticals, Inc. | zyne-20160930ex322892e36.htm |
| EX-32.1 - EX-32.1 - Zynerba Pharmaceuticals, Inc. | zyne-20160930ex3212bbc7e.htm |
| EX-31.1 - EX-31.1 - Zynerba Pharmaceuticals, Inc. | zyne-20160930ex311b1ea8b.htm |
| EX-10.2 - EX-10.2 - Zynerba Pharmaceuticals, Inc. | zyne-20160930ex10291f843.htm |
| EX-10.1 - EX-10.1 - Zynerba Pharmaceuticals, Inc. | zyne-20160930ex101e77c85.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended September 30, 2016
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-37526
Zynerba Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
26-0389433 |
|
|
|
|
|
80 W. Lancaster Avenue, Suite 300 |
|
19333 |
(484) 581-7505
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐ |
|
Accelerated filer ☐ |
|
|
|
|
|
Non-accelerated filer ☒ |
|
Smaller reporting company ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of November 11, 2016 there were 9,953,718 shares of Common Stock, $0.001 par value per share, outstanding.
| 4 | ||
|
|
|
|
| 4 | ||
|
|
|
|
|
|
Consolidated Balance Sheets as of September 30, 2016 and December 31, 2015 (Unaudited) |
4 |
|
|
|
|
|
|
5 | |
|
|
|
|
|
|
6 | |
|
|
|
|
|
|
7 | |
|
|
|
|
|
|
8 | |
|
|
|
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
14 | |
|
|
|
|
| 22 | ||
|
|
|
|
| 22 | ||
|
|
|
|
| 23 | ||
|
|
|
|
| 23 | ||
|
|
|
|
| 23 | ||
|
|
|
|
| 23 | ||
|
|
|
|
| 23 | ||
|
|
|
|
| 23 | ||
|
|
|
|
| 23 | ||
|
|
|
|
| 23 | ||
|
|
|
|
|
|
24 | |
2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements made in this Quarterly Report that are not statements of historical or current facts, such as those under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements discuss our current expectations and projections relating to our financial condition, results of operations, plans, objectives, future performance and business. These statements may be preceded by, followed by or include the words “aim,” “anticipate,” “believe,” “estimate,” “expect,” “forecast,” “intend,” “outlook,” “plan,” “potential,” “project,” “projection,” “seek,” “may,” “could,” “would,” “will,” “should,” “can,” “can have,” “likely,” the negatives thereof and other words and terms of similar meaning.
Forward-looking statements are inherently subject to risks, uncertainties and assumptions; they are not guarantees of performance. You should not place undue reliance on these statements. We have based these forward-looking statements on our current expectations and projections about future events. Although we believe that our assumptions made in connection with the forward-looking statements are reasonable, we cannot assure you that the assumptions and expectations will prove to be correct.
You should understand that the following important factors could affect our future results and could cause those results or other outcomes to differ materially from those expressed or implied in our forward-looking statements:
|
· |
our estimates regarding expenses, future revenue, capital requirements and timing and availability of and the need for additional financing; |
|
· |
the success and timing of our preclinical studies and clinical trials; |
|
· |
the potential results of preclinical studies and clinical trials for ZYN002 and ZYN001; |
|
· |
our dependence on third parties in the conduct of our preclinical studies and clinical trials; |
|
· |
the difficulties and expenses associated with obtaining and maintaining regulatory approval of ZYN002 and ZYN001; |
|
· |
our plans and ability to develop and commercialize ZYN002 and ZYN001; |
|
· |
the successful development of our commercialization capabilities, including sales and marketing capabilities; |
|
· |
the size and growth of the potential markets for ZYN002 and ZYN001, the rate and degree of market acceptance of ZYN002 and ZYN001 and our ability to serve those markets; |
|
· |
legal and regulatory developments in the United States and foreign countries; |
|
· |
the success of competing therapies and products that are or become available; |
|
· |
our exposure to additional scrutiny as a public company; |
|
· |
our ability to limit our exposure under product liability lawsuits; |
|
· |
our use of the proceeds from our initial public offering, or IPO and any subsequent offerings, including our current “at-the-market,” or ATM, offerings; |
|
· |
our ability to obtain and maintain intellectual property protection for ZYN002 and ZYN001; |
|
· |
recently enacted and future legislation regarding the healthcare system; |
|
· |
our ability to obtain and maintain third-party manufacturing for our product candidates on commercially reasonable terms; |
|
· |
the performance of third parties upon which we depend, including third-party contract research organizations, or CROs and third-party manufacturers; |
|
· |
our ability to recruit or retain key scientific or management personnel or to retain our executive officers; and |
|
· |
the other risks, uncertainties and factors discussed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, or our 2015 Annual Report, under the caption “Item 1.A Risk Factors”. |
In light of these risks and uncertainties, expected results or other anticipated events or circumstances discussed in this Form 10-Q (including the exhibits hereto) might not occur. Consequently, there can be no assurance that actual results or developments anticipated by us will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, us. Given these uncertainties, you are cautioned not to place undue reliance on such forward-looking statements. We undertake no obligation, and specifically decline any obligation, to publicly update or revise any forward-looking statements, even if experience or future developments make it clear that projected results expressed or implied in such statements will not be realized, except as may be required by law.
3
PART I – FINANCIAL INFORMATION
Item 1. Consolidated Financial Statements (Unaudited)
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
|
|
|
September 30, |
|
December 31, |
|
||
|
|
|
2016 |
|
2015 |
|
||
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
31,780,773 |
|
$ |
41,513,060 |
|
|
Incentive and tax receivables |
|
|
2,281,205 |
|
|
356,718 |
|
|
Prepaid expenses and other current assets |
|
|
1,464,564 |
|
|
1,545,917 |
|
|
Total current assets |
|
|
35,526,542 |
|
|
43,415,695 |
|
|
Property and equipment, net |
|
|
304,141 |
|
|
227,646 |
|
|
Other assets |
|
|
463,600 |
|
|
200 |
|
|
Total assets |
|
$ |
36,294,283 |
|
$ |
43,643,541 |
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,694,470 |
|
$ |
823,401 |
|
|
Accrued expenses |
|
|
2,882,060 |
|
|
2,272,991 |
|
|
Deferred grant revenue |
|
|
370,575 |
|
|
841,225 |
|
|
Total current liabilities |
|
|
4,947,105 |
|
|
3,937,617 |
|
|
Deferred grant revenue, long-term |
|
|
463,400 |
|
|
— |
|
|
Total liabilities |
|
|
5,410,505 |
|
|
3,937,617 |
|
|
Stockholders' equity: |
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 10,000,000 shares authorized; no shares issued or outstanding |
|
|
— |
|
|
— |
|
|
Common stock, $0.001 par value; 200,000,000 shares authorized; 9,628,278 shares issued and outstanding at September 30, 2016 and 9,199,919 shares issued and outstanding at December 31, 2015 |
|
|
9,628 |
|
|
9,200 |
|
|
Additional paid-in capital |
|
|
69,946,049 |
|
|
62,276,779 |
|
|
Accumulated deficit |
|
|
(39,071,899) |
|
|
(22,580,055) |
|
|
Total stockholders' equity |
|
|
30,883,778 |
|
|
39,705,924 |
|
|
Total liabilities and stockholders' equity |
|
$ |
36,294,283 |
|
$ |
43,643,541 |
|
See accompanying notes to unaudited consolidated financial statements.
4
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
|
|
|
Three months ended |
|
Nine months ended |
|
||||||||
|
|
|
September 30, |
|
September 30, |
|
||||||||
|
|
|
2016 |
|
2015 |
|
2016 |
|
2015 |
|
||||
|
Revenue |
|
$ |
— |
|
$ |
199,407 |
|
$ |
7,250 |
|
$ |
229,625 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
4,504,097 |
|
|
2,271,968 |
|
|
11,880,264 |
|
|
4,136,659 |
|
|
General and administrative |
|
|
1,493,461 |
|
|
1,922,755 |
|
|
4,649,948 |
|
|
3,208,003 |
|
|
Total operating expenses |
|
|
5,997,558 |
|
|
4,194,723 |
|
|
16,530,212 |
|
|
7,344,662 |
|
|
Loss from operations |
|
|
(5,997,558) |
|
|
(3,995,316) |
|
|
(16,522,962) |
|
|
(7,115,037) |
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
22,747 |
|
|
1,572 |
|
|
53,243 |
|
|
2,948 |
|
|
Foreign exchange loss |
|
|
(6,270) |
|
|
— |
|
|
(49,668) |
|
|
— |
|
|
Total other income (expense) |
|
|
16,477 |
|
|
1,572 |
|
|
3,575 |
|
|
2,948 |
|
|
Loss before income taxes |
|
|
(5,981,081) |
|
|
(3,993,744) |
|
|
(16,519,387) |
|
|
(7,112,089) |
|
|
Income tax benefit |
|
|
— |
|
|
— |
|
|
(27,543) |
|
|
— |
|
|
Net loss |
|
$ |
(5,981,081) |
|
$ |
(3,993,744) |
|
$ |
(16,491,844) |
|
$ |
(7,112,089) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share basic and diluted |
|
$ |
(0.67) |
|
$ |
(0.66) |
|
$ |
(1.86) |
|
$ |
(2.37) |
|
|
Basic and diluted weighted average shares outstanding |
|
|
8,912,508 |
|
|
6,045,211 |
|
|
8,865,854 |
|
|
2,998,480 |
|
See accompanying notes to unaudited consolidated financial statements.
5
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
(UNAUDITED)
|
|
|
|
|
||||||||||||
|
|
|
Stockholders' equity |
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
Common stock |
|
Additional |
|
Accumulated |
|
stockholders' |
|
||||||
|
|
|
Shares |
|
Amount |
|
paid-capital |
|
deficit |
|
equity |
|
||||
|
Balance at December 31, 2015 |
|
9,199,919 |
|
$ |
9,200 |
|
$ |
62,276,779 |
|
$ |
(22,580,055) |
|
$ |
39,705,924 |
|
|
Issuance of common stock, net of issuance costs |
|
428,359 |
|
|
428 |
|
|
5,287,328 |
|
|
— |
|
|
5,287,756 |
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
2,381,942 |
|
|
— |
|
|
2,381,942 |
|
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(16,491,844) |
|
|
(16,491,844) |
|
|
Balance at September 30, 2016 |
|
9,628,278 |
|
$ |
9,628 |
|
$ |
69,946,049 |
|
$ |
(39,071,899) |
|
$ |
30,883,778 |
|
See accompanying notes to unaudited consolidated financial statements
6
ZYNERBA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
|
|
|
Nine months ended September 30, |
||||
|
|
|
2016 |
|
2015 |
||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
Net loss |
|
$ |
(16,491,844) |
|
$ |
(7,112,089) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
Depreciation |
|
|
53,294 |
|
|
12,772 |
|
Stock-based compensation |
|
|
2,381,942 |
|
|
836,824 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
Incentive and tax receivables |
|
|
(1,924,487) |
|
|
— |
|
Prepaid expenses and other assets |
|
|
(239,652) |
|
|
(873,220) |
|
Deferred grant revenue |
|
|
(7,250) |
|
|
(229,625) |
|
Accounts payable |
|
|
687,449 |
|
|
1,289,307 |
|
Accrued expenses |
|
|
609,069 |
|
|
(516,637) |
|
Net cash used in operating activities |
|
|
(14,931,479) |
|
|
(6,592,668) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(86,726) |
|
|
(176,096) |
|
Net cash used in investing activities |
|
|
(86,726) |
|
|
(176,096) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
Proceeds from the issuance of common stock, net of offering costs |
|
|
5,285,918 |
|
|
42,181,305 |
|
Proceeds from the exercise of stock options |
|
|
— |
|
|
63,509 |
|
Net cash provided by financing activities |
|
|
5,285,918 |
|
|
42,244,814 |
|
Net (decrease) increase in cash and cash equivalents |
|
|
(9,732,287) |
|
|
35,476,050 |
|
Cash and cash equivalents at beginning of period |
|
|
41,513,060 |
|
|
9,330,681 |
|
Cash and cash equivalents at end of period |
|
$ |
31,780,773 |
|
$ |
44,806,731 |
|
|
|
|
|
|
|
|
|
Supplemental disclosures of cash flow information: |
|
|
|
|
|
|
|
Deferred offering costs included in accounts payable |
|
$ |
140,557 |
|
$ |
59,301 |
|
Property and equipment acquired but not yet paid |
|
|
43,064 |
|
|
— |
See accompanying notes to unaudited consolidated financial statements
7
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS
(1) Nature of Business and Liquidity
Zynerba Pharmaceuticals, Inc. and its subsidiary (the “Company”, “we”) is a clinical stage specialty pharmaceutical company dedicated to developing and commercializing innovative transdermal cannabinoid treatments for patients with high unmet needs. The Company was incorporated on January 31, 2007 under the laws of the State of Delaware as AllTranz, Inc. and changed its name to Zynerba Pharmaceuticals, Inc. in August 2014.
The Company has incurred losses and negative cash flows from operations since inception and has an accumulated deficit of $39.1 million as of September 30, 2016. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant revenue from its product candidates currently in development. The Company's primary source of liquidity has been the issuance of convertible promissory notes and equity securities.
In August 2015, the Company completed its Initial Public Offering (“IPO”) of common stock selling 3,450,000 shares at an offering price of $14.00 per share, resulting in net proceeds of $42.1 million. In September 2016, the Company entered into an Open Market Sales Agreement (the “Sales Agreement”) with Jefferies LLC (“Jefferies”) pursuant to which, as of November 11, 2016, it sold and issued 753,799 shares of its common stock in the open market at a weighted average selling price of $13.38 per share, for net proceeds of $9.5 million. As of September 30, 2016, the Company sold and issued 428,359 shares of its common stock pursuant to the Sales Agreement in the open market at a weighted-average selling price of $13.16, for net proceeds of $5.3 million. From October 1, 2016 through November 11, 2016, the Company sold and issued 325,440 shares of its common stock in the open market at a weighted average selling price of $13.67 per share, for $4.2 million of net proceeds. See Note 7, Common Stock. Management believes that the Company’s available funds, including proceeds from the sale of common stock received through November 11, 2016, are sufficient to develop five Phase 3 ready programs and are sufficient to fund operations and capital requirements into 2018. Substantial additional financings will be needed by the Company to fund its operations, to complete clinical development of and to commercially develop its product candidates. There is no assurance that such financing will be available when needed or on acceptable terms.
The Company is subject to those risks associated with any clinical stage pharmaceutical company that has substantial expenditures for research and development. There can be no assurance that the Company's research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, the Company operates in an environment of rapid technological change and is largely dependent on the services of its employees and consultants.
(2) Summary of Significant Accounting Policies
a. Basis of Presentation
The accompanying unaudited interim consolidated financial statements of the Company and its subsidiary have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X. In the opinion of management, the accompanying consolidated financial statements of the Company include all normal and recurring adjustments (which consist primarily of accruals, estimates and assumptions that impact the financial statements) considered necessary to present fairly the Company's financial position as of September 30, 2016 and its results of operations and cash flows for the nine months ended September 30, 2016 and 2015. Operating results for any interim period are not necessarily indicative of results for any future interim period or for the entire year. The accompanying unaudited interim financial statements should be read in conjunction with the financial statements and related notes included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2015 (“2015 Annual Report”), filed with the Securities and Exchange Commission (“SEC”).
b. Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and reported amounts of revenue and expenses during the reporting period. Actual
8
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS (Continued)
results could differ from such estimates.
c. Incentive and Tax Receivables
The Company’s subsidiary, Zynerba Pharmaceuticals Pty Ltd (the “Subsidiary”), is incorporated in Australia. The Subsidiary is eligible to participate in an Australian research and development tax incentive program. As part of this program, the Subsidiary is eligible to receive a cash refund from the Australian Taxation Office for a percentage of the research and development costs expended by the Subsidiary in Australia. During the three months ended September 30, 2016, the Company received $0.4 million from the Australian Taxation Office related to 2015 eligible spending under this incentive program. The Company’s estimate of the amount of cash refund it expects to receive related to the Australian research and development tax incentive program is included in “Incentive and tax receivables” on the accompanying consolidated balance sheets. As of September 30, 2016, the Company estimates it will collect $2.0 million in 2017 for 2016 eligible spending as part of this incentive program.
In addition, the Subsidiary incurs Goods and Services Tax (“GST”) on services provided by Australian vendors. As an Australian entity, the Subsidiary is entitled to a refund of the GST paid. The Company’s estimate of the amount of cash refund it expects to receive related to GST paid is included in “Incentive and tax receivables” on the accompanying consolidated balance sheets. During the three months ended September 30, 2016, the Company received a refund of $0.2 million for GST paid to Australian vendors through June 30, 2016, and the Company estimates it will collect an additional $0.3 million for GST paid to Australian vendors for the three months ended September 30, 2016.
d. Other Assets
Other assets as of September 30, 2016 consists primarily of noncurrent research and grant revenue remitted to third-party research organizations.
e. Revenue
Revenue consists of state and federal research grants. Revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred and services have been rendered, the price is fixed or determinable and collectability is reasonably assured. Grant revenue received is deferred until the related expenditures are incurred.
f. Research and Development
Research and development costs are expensed as incurred and are primarily comprised of external research and development expenses incurred under arrangements with third parties, such as contract research organizations (“CROs”), consultants and employee-related expenses including salaries and benefits. At the end of each reporting period, the Company compares the payments made to each service provider to the estimated progress towards completion of the related project. Factors that the Company considers in preparing these estimates include the number of patients enrolled in studies, milestones achieved and other criteria related to the efforts of its vendors. These estimates will be subject to change as additional information becomes available. Depending on the timing of payments to vendors and estimated services provided, the Company will record net prepaid or accrued expenses related to these costs. Beginning in the fourth quarter of 2015, research and development costs are reduced by the Australian research and development incentive and GST recorded in the respective period.
g. Net Loss per Share
Basic loss per share is computed by dividing net loss applicable to common stockholders by the weighted average number of shares of common stock outstanding during each period. Diluted loss per share includes the effect, if any, from the potential exercise or conversion of securities, such as convertible preferred stock, restricted stock, and stock options, which would result in the issuance of incremental shares of common stock. In computing the basic and diluted net loss per share applicable to common stockholders, the weighted average number of shares remains the same for both calculations due to the fact that when a net loss exists, dilutive shares are not included in the calculation as the impact is anti-dilutive.
9
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The following potentially dilutive securities have been excluded from the computation of diluted weighted average shares outstanding, as they would be anti-dilutive:
|
|
|
Three and nine months ended |
||
|
|
|
September 30, |
||
|
|
|
2016 |
|
2015 |
|
Stock options |
|
1,793,493 |
|
1,637,399 |
|
Unvested restricted stock |
|
289,942 |
|
434,914 |
|
|
|
2,083,435 |
|
2,072,313 |
h. Reclassification
Certain amounts in the prior year financial statements have been reclassified to conform to the current-year presentation.
i. Recent Accounting Pronouncements
In August 2014, the Financial Accounting Standard Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, which defines management’s responsibility to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures if there is substantial doubt about its ability to continue as a going concern. The pronouncement is effective for annual reporting periods ending after December 15, 2016 with early adoption permitted. The adoption of this guidance is not expected to have a material impact on the Company’s consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases, which requires that lease arrangements longer than 12 months result in an entity recognizing an asset and liability. The pronouncement is effective for interim and annual periods beginning after December 15, 2018 with early adoption permitted. The Company is currently evaluating the impact this guidance is expected to have on its consolidated financial statements.
In March 2016, the FASB issued ASU No. 2016-09, Improvements to Employee Share-Based Payment Accounting, which is intended to simplify the accounting and reporting for employee share-based payment transactions. The pronouncement is effective for interim and annual periods beginning after December 31, 2016 with early adoption permitted. The adoption of this guidance is not expected to have a material impact on the Company’s consolidated financial statements.
In August 2016, the FASB issued ASU No. 2016-15, Classification of Certain Cash Receipts and Cash Payments, which provides specific guidance related to eight cash flow classification issues. The pronouncement is effective for interim and annual periods beginning after December 15, 2017 with early adoption permitted. The adoption of this guidance is not expected to have a material impact on the Company’s consolidated financial statements.
(3) Fair Value Measurements
The Company utilizes a valuation hierarchy that prioritizes fair value measurements based on the types of inputs used for the various valuation techniques related to its financial assets and financial liabilities. The three levels of inputs used to measure fair value are described as follows:
Level 1 — Unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs and quoted prices in active markets for similar assets and liabilities.
Level 3 — Unobservable inputs and models that are supported by little or no market activity.
10
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In accordance with the fair value hierarchy described above, the following table sets forth the Company's financial assets measured at fair value on a recurring basis:
|
|
|
|
|
|
Fair Value Measurement |
|
|||||
|
|
|
|
|
|
as of September 30, 2016 |
|
|||||
|
|
|
Carrying value |
|
|
|
|
|
|
|
|
|
|
|
|
as of September 30, 2016 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
||
|
Cash equivalents (money market accounts) |
|
$ |
28,308,233 |
|
$ |
28,308,233 |
$ |
— |
$ |
— |
|
|
Certificate of deposit (included in prepaid expenses and other current assets) |
|
|
20,000 |
|
|
20,000 |
|
— |
|
— |
|
|
|
|
$ |
28,328,233 |
|
$ |
28,328,233 |
$ |
— |
$ |
— |
|
|
|
|
|
|
|
Fair Value Measurement |
|
|||||
|
|
|
|
|
|
as of December 31, 2015 |
|
|||||
|
|
|
Carrying value |
|
|
|
|
|
|
|
|
|
|
|
|
as of December 31, 2015 |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
||
|
Cash equivalents (money market accounts) |
|
$ |
41,032,351 |
|
$ |
41,032,351 |
$ |
— |
$ |
— |
|
|
Certificate of deposit (included in prepaid expenses and other current assets) |
|
|
20,000 |
|
|
20,000 |
|
— |
|
— |
|
|
|
|
$ |
41,052,351 |
|
$ |
41,052,351 |
$ |
— |
$ |
— |
|
(4) Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following as September 30, 2016 and December 31, 2015:
|
|
|
September 30, |
|
December 31, |
|
||
|
|
|
2016 |
|
2015 |
|
||
|
Prepaid development expenses |
|
$ |
872,051 |
|
$ |
1,211,668 |
|
|
Prepaid insurance |
|
|
424,172 |
|
|
282,440 |
|
|
Other |
|
|
168,341 |
|
|
51,809 |
|
|
Total prepaid expenses and other current assets |
|
$ |
1,464,564 |
|
$ |
1,545,917 |
|
Included in prepaid development expenses above is research and grant revenue remitted to third-party research organizations of $0.4 million and $0.8 million as of September 30, 2016 and December 31, 2015, respectively, that will be recognized as research projects progress and expenses are incurred.
(5) Property and Equipment
Property and equipment consisted of the following:
|
|
|
Estimated |
|
|
|
|
|
|
|
|
|
|
useful life |
|
September 30, |
|
December 31, |
|
||
|
|
|
(in years) |
|
2016 |
|
2015 |
|
||
|
Equipment |
|
5 |
|
$ |
260,223 |
|
$ |
139,526 |
|
|
Computer equipment |
|
3 |
|
|
27,111 |
|
|
23,632 |
|
|
Furniture and fixtures |
|
5 |
|
|
99,731 |
|
|
94,118 |
|
|
Total cost |
|
|
|
|
387,065 |
|
|
257,276 |
|
|
Less accumulated depreciation |
|
|
|
|
(82,924) |
|
|
(29,630) |
|
|
Property and equipment, net |
|
|
|
$ |
304,141 |
|
$ |
227,646 |
|
Depreciation expense was $25,844 and $8,230 for the three months ended September 30, 2016 and 2015, respectively. Depreciation expense was $53,294 and $12,772 for the nine months ended September 30, 2016 and 2015, respectively.
11
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) Accrued Expenses
Accrued expenses consisted of the following:
|
|
|
September 30, |
|
December 31, |
|
||
|
|
|
2016 |
|
2015 |
|
||
|
Accrued compensation |
|
$ |
892,122 |
|
$ |
1,047,530 |
|
|
Accrued research and development |
|
|
1,746,010 |
|
|
943,295 |
|
|
Other |
|
|
243,928 |
|
|
282,166 |
|
|
Total accrued expenses |
|
$ |
2,882,060 |
|
$ |
2,272,991 |
|
(7) Common Stock
On September 1, 2016, the Company entered into the Sales Agreement pursuant to which the Company may issue and sell under its Form S-3 shelf registration statement, which was declared effective on September 12, 2016, shares of its common stock having an aggregate offering price up to $30.0 million, subject to certain limitations, from time to time, with Jefferies acting as agent. During the three months ended September 30, 2016, the Company sold and issued 428,359 shares of common stock pursuant to the Sales Agreement in the open market at a weighted-average selling price of $13.16 per share, resulting in net proceeds of $5.3 million. From October 1, 2016 through November 11, 2016, the Company sold and issued 325,440 shares of common stock pursuant to the Sales Agreement in the open market at a weighted average selling price of $13.67 per share, for $4.2 million of net proceeds. Aggregating these transactions through November 11, 2016, the Company sold and issued a total of 753,799 shares of its common stock pursuant to the Sales Agreement in the open market at a weighted-average selling price of $13.38, per share for $9.5 million of net proceeds.
(8) Stock-Based Compensation
The Company maintains the Amended and Restated 2014 Omnibus Incentive Compensation Plan, as amended (“2014 Plan”), which allows for the granting of incentive stock options, nonqualified stock options, stock appreciation rights, stock awards, stock units, performance units and other stock-based awards to purchase an aggregate of 2,450,000 shares of the Company’s common stock to employees, officers, directors, consultants, and advisors, subject to automatic annual increases in the number of shares authorized for issuance under the 2014 Plan on the first trading day of January each year, commencing on January 1, 2017, equal to the lesser of 1.5 million shares and 10% of the number of shares of common stock outstanding on the last trading day of December of the preceding year. In addition, the 2014 Plan provides selected executive employees with the opportunity to receive bonus awards that are considered qualified performance-based compensation. As of September 30, 2016, 210,668 shares are available for issuance under the 2014 Plan.
Options issued under the 2014 Plan have a contractual life of 10 years and may be exercisable in cash or as otherwise determined by the board of directors. The Company has granted options to employees and non-employee directors.
The Company recorded stock-based compensation expense related to its stock option grants and restricted stock awards, as follows:
|
|
|
Nine Months Ended September 30, 2016 |
|
Nine Months Ended September 30, 2015 |
|
||||||||||||||
|
|
|
Research and Development |
|
General and Administrative |
|
Total |
|
Research and Development |
|
General and Administrative |
|
Total |
|
||||||
|
Stock option grants |
|
$ |
811,937 |
|
$ |
1,390,125 |
|
$ |
2,202,062 |
|
$ |
180,022 |
|
$ |
379,813 |
|
$ |
559,835 |
|
|
Restricted stock awards |
|
|
104,099 |
|
|
75,781 |
|
|
179,880 |
|
|
117,147 |
|
|
159,842 |
|
|
276,989 |
|
|
|
|
$ |
916,036 |
|
$ |
1,465,906 |
|
$ |
2,381,942 |
|
$ |
297,169 |
|
$ |
539,655 |
|
$ |
836,824 |
|
12
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Vesting of the stock option grants and restricted stock awards issued prior to the Company’s IPO in August 2015 was contingent upon the closing of the Company’s IPO. Accordingly, prior to the Company’s IPO in August 2015, no expense had been recorded for the stock option grants and restricted stock awards.
The following table summarizes the stock option activity for the nine-month period ended September 30, 2016:
|
|
|
Nine Months Ended September 30, 2016 |
|
||||||
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
|
|
|
Average |
|
Weighted |
|
||
|
|
|
|
|
Grant Date |
|
Average |
|
||
|
|
|
Options |
|
Fair Value |
|
Exercise Price |
|
||
|
Outstanding as of December 31, 2015 |
|
1,637,399 |
|
$ |
6.47 |
|
$ |
10.49 |
|
|
Granted under the 2014 Plan |
|
120,000 |
|
$ |
5.50 |
|
$ |
8.35 |
|
|
Granted as an inducement grant |
|
150,000 |
|
$ |
6.95 |
|
$ |
10.23 |
|
|
Forfeited |
|
(113,906) |
|
$ |
8.12 |
|
$ |
12.67 |
|
|
Outstanding as of September 30, 2016 |
|
1,793,493 |
|
$ |
6.34 |
|
$ |
10.19 |
|
During the nine months ended September 30, 2016, the Company granted a total of 120,000 stock options to non-management members of the Company’s Board of Directors and a new employee. The stock options granted to the non-management directors vest on the earlier of the one-year anniversary of the grant date, or the date of the Company’s 2017 annual stockholders’ meeting. The stock options granted to the Company’s new employee vest 25% upon the first anniversary of the grant date and quarterly over three years thereafter.
The Company entered into an Employment Agreement, effective September 13, 2016, with James Fickenscher to serve as chief financial officer and vice president of corporate development of the Company. Mr. Fickenscher received a grant of stock options to purchase an aggregate of 150,000 shares of the Company’s common stock at an exercise price per share equal to $10.23, which was the closing price of the Company’s common stock on September 13, 2016. These options have a ten-year term and will vest and become exercisable as follows: 25% of such options on September 13, 2017 (one year after the date of grant), with the balance vesting in 12 equal quarterly installments thereafter until September 13, 2020. The options have an aggregate fair value of $1.0 million that will be recognized as expense over the vesting term of the options. The options were granted as an inducement grant pursuant to NASDAQ Listing Rule 5635(c)(4) and are outside of the 2014 Plan.
The weighted average grant date fair value of all stock options granted during the nine months ended September 30, 2016 was estimated using the Black-Scholes option pricing model with the following ranges of assumptions: expected volatility of 77%, risk free interest rate of 1.43% to 1.62%, expected term of 5.5 years to 6.25 years and 0% expected dividend yield.
As of September 30, 2016, there was $7.9 million of unrecognized stock-based compensation expense related to stock options, which is expected to be recognized over a weighted-average period of 2.74 years. As of September 30, 2016, 577,426 stock options with a weighted average grant date fair value of $4.96 were vested and exercisable, and the Company expects all 1,216,067 unvested stock options to vest.
The following table summarizes the restricted stock award activity under the 2014 Plan for the nine-month period ended September 30, 2016:
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
Grant Date |
|
|
|
|
|
Shares |
|
Fair Value |
|
|
|
Unvested as of December 31, 2015 |
|
398,671 |
|
$ |
1.65 |
|
|
Vested |
|
(108,729) |
|
$ |
1.65 |
|
|
Unvested as of September 30, 2016 |
|
289,942 |
|
$ |
1.65 |
|
|
|
|
|
|
|
|
|
As of September 30, 2016, there was $0.4 million of unrecognized stock-based compensation expense related to unvested restricted stock awards as of September 30, 2016, which is expected to be recognized over a weighted-average period of 1.84 years. The Company expects all 289,942 unvested restricted stock awards to vest.
13
Item 2. Management's Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and related notes appearing elsewhere in this quarterly report and the audited financial statements and notes thereto for the year ended December 31, 2015 and the related Management’s Discussion and Analysis of Financial Condition and Results of Operations, both of which are contained in our 2015 Annual Report. The following discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results and the timing of certain events could differ materially from those anticipated in these forward-looking statements as a result of many factors. We discuss factors that we believe could cause or contribute to these differences below and elsewhere in this quarterly report, including those set forth under “Cautionary Note Regarding Forward-looking Statements” and “Risk Factors” in this quarterly report and our 2015 Annual Report.
Overview
Company Overview
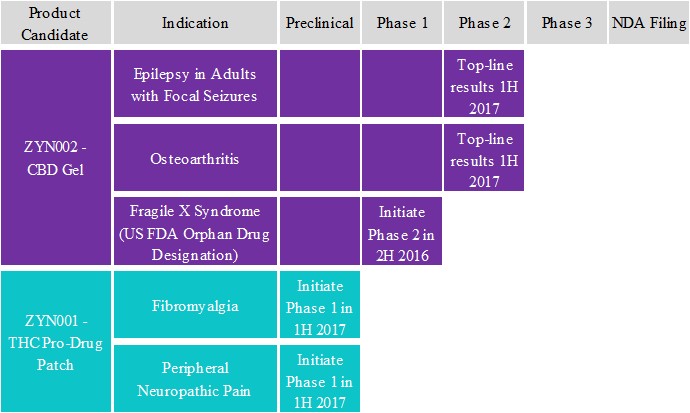
We are a clinical stage specialty pharmaceutical company dedicated to developing and commercializing innovative transdermal synthetic cannabinoid treatments for patients with high unmet needs. We are evaluating two patent protected product candidates, ZYN002 and ZYN001, in five indications. We are studying ZYN002 in adult patients with refractory epileptic focal seizures (formerly known as complex partial seizures) and osteoarthritis, or OA, and intend to study ZYN002 in patients with fragile X syndrome, or FXS. We intend to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain. We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently receiving adequate relief from current treatment regimens. In June 2016, we completed two Phase 1 clinical trials for ZYN002 in healthy volunteers and patients with epilepsy, and initiated a third Phase 1 clinical trial to evaluate different concentrations of cannabidiol, or CBD, in the ZYN002 gel in healthy volunteers, which was completed in July 2016. In June 2016, we also initiated a Phase 2 clinical trial for ZYN002 in adult patients with refractory epileptic focal seizures. In August 2016, we initiated a Phase 2 clinical trial for ZYN002 in patients with OA. We expect to initiate a Phase 2 clinical trial for ZYN002 in patients with FXS before the end of 2016. We expect to initiate Phase 1 clinical trials for ZYN001 in the first half of 2017.
Cannabinoids are a class of compounds derived from Cannabis plants. The two primary cannabinoids contained in Cannabis are CBD and ∆9-tetrahydrocannabinol, or THC. Clinical and preclinical data suggest that CBD has positive effects on treating epilepsy, arthritis and FXS, and THC has positive effects on treating pain. We believe ZYN002 and ZYN001 potentially offer first‑line therapies to patients suffering from epilepsy, OA, FXS, fibromyalgia and peripheral neuropathic pain.
ZYN002 is the first and only synthetic CBD formulated as a permeation‑enhanced gel for transdermal delivery, and is patent‑protected through 2030. CBD is the primary non‑psychoactive component of Cannabis. In preclinical animal studies, ZYN002’s permeation enhancer increased delivery of CBD through the layers of the skin and into the circulatory system. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first‑pass liver metabolism. In addition, an in vitro study published in Cannabis and Cannabinoid Research in April 2016 demonstrated that CBD is degraded to THC in an acidic environment such as the stomach. We believe such degradation may lead to increased psychoactive effects if CBD is delivered orally and may be avoided with the transdermal delivery of ZYN002, which maintains CBD in a neutral pH. ZYN002, which is being developed as a clear gel with once- or twice-daily dosing, is targeting treatment of epilepsy, OA and FXS, which collectively affect millions of patients using treatments that currently comprise a multi‑billion dollar market. We have been granted orphan drug designation from the U.S. Food and Drug Administration, or FDA, for ZYN002 for the treatment of FXS.
ZYN001 is a pro‑drug of THC that enables effective transdermal delivery via a patch and is patent‑protected through 2031. A pro‑drug is a drug administered in an inactive or less active form and designed to enable more effective delivery, which is then converted into an active form through a normal metabolic process. In addition, we expect that ZYN001 will be classified by the FDA as a new chemical entity. We are working with a development partner, LTS LOHMANN Therapie-Systeme AG, or LTS, to optimize the formulation of ZYN001 into a state of the art drug-adhesive matrix transdermal patch.
In our preclinical animal studies, ZYN001 demonstrated effective skin permeation with sustained delivery and rapid conversion of ZYN001 to THC. These preclinical studies suggest increased bioavailability, consistent plasma levels and
14
the avoidance of first‑pass liver metabolism. In addition, preclinical testing conducted has shown no genotoxicity findings and safety pharmacology findings consistent with those seen with THC. ZYN001 is targeting two pain indications, fibromyalgia and peripheral neuropathic pain, which collectively represent multi-billion dollar markets.
In June 2016, we completed two Phase 1 clinical trials for ZYN002 in healthy volunteers and patients with epilepsy. The first Phase 1 single rising dose clinical trial for ZYN002 in healthy human subjects and in patients with epilepsy evaluated the tolerability and pharmacokinetic, or PK, profile of ZYN002. Results from this clinical trial demonstrated that ZYN002 was safe and well-tolerated at all tested dose levels and the incidence of adverse events associated with ZYN002 was similar to placebo for both healthy subjects and epilepsy patients. The second Phase 1 clinical trial was a randomized, double-blind, placebo controlled multiple rising dose clinical trial for ZYN002 in twenty-four healthy volunteers and twelve patients with epilepsy to evaluate the PK profile, pharmacodynamics, or PD, and tolerability of multiple doses (200, 250, and 500 mg) of ZYN002. Each volunteer and patient received seven days of either ZYN002 or placebo. Results from this clinical trial demonstrated that ZYN002 was safe and well-tolerated at all dose levels. The twice daily dosing provided a more favorable PK profile with comparable results between healthy volunteers and epilepsy patients. Transdermal application of ZYN002 was very well tolerated with minimal skin erythema. Skin dryness at the application site was common for both ZYN002 and placebo gel. Overall, the incidence of adverse events associated with ZYN002 was similar to placebo in both healthy volunteers and adult epilepsy patients. There were no reports of somnolence or fatigue and a very low incidence of gastrointestinal events was observed. There were no serious adverse events or discontinuations for healthy volunteers and epilepsy patients receiving ZYN002. One healthy volunteer receiving placebo gel developed a serious adverse event suspected to be a catheter infection and was discontinued from the study. In addition, healthy volunteers and epilepsy patients had no drug related changes in performance on the Trail Making Test, a test of visual attention, psychomotor ability, and task switching; a divided attention task; and the Paced Auditory Serial Addition Task, or PASAT, a test that measures working memory and focused attention. These results indicate that ZYN002 did not produce impairment in critical areas of cognitive functioning often impacted by central nervous system drugs. No changes in mood symptoms as accessed by the Inventory of Depression and Anxiety Symptoms, or IDAS, and the Positive and Negative Affect Schedule, or PANAS were observed for ZYN002 suggesting that ZYN002 is not associated with declines in psychological health.
In July 2016, we completed a third Phase 1 clinical trial for ZYN002 which was randomized, double-blind and placebo controlled in 42 healthy volunteers. The volunteers received a range of CBD doses from 395 mg to 504 mg daily in 2.5% and 4.2% ZYN002 formulations for fourteen days. Results from this clinical trial demonstrated that ZYN002 was very well tolerated with minimal skin erythema. CBD plasma concentrations were dose dependent and did not fluctuate at steady state. The 4.2% formulation demonstrated a comparable PK and tolerability profile to the 2.5% concentration and was easier to use due to the lower volume. There were no serious adverse events or discontinuations from this clinical trial.
In the Phase 1 program, ZYN002 was demonstrated to be safe and well tolerated, provided a favorable CBD PK profile, and no THC was detected in plasma or urine.
In June 2016, we initiated a Phase 2 randomized, multi-center, multi-dose clinical trial designed to evaluate the efficacy and safety of ZYN002 in adult patients with refractory epileptic focal seizures, which we refer to as the STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy) trial. Approximately 180 patients will be randomized in the trial and will be followed for 8 weeks during the baseline phase. After the baseline phase, patients are randomized (1:1:1) to receive one of two doses of CBD gel (195 mg or 97.5 mg CBD in ZYN002 4.2%) or placebo gel every 12 hours for 12 weeks. The primary endpoint of the study is median percentage change in seizure frequency at 12 weeks compared to baseline. In August 2016, we announced that the first patients were randomized and dosed in STAR 1, and we expect to report preliminary top line results in the first half of 2017. In November 2016, we announced that we initiated a 12-month open-label extension clinical trial (STAR 2) for patients who successfully complete the STAR 1 trial.
In August 2016, we initiated a Phase 2 randomized, multi-center, multi-dose clinical trial designed to evaluate the efficacy and safety of ZYN002 in adult patients with knee pain due to OA, which we refer to as the STOP 1 (Synthetic Transdermal Cannabidiol for the Treatment of Knee Pain due to Osteoarthritis) trial. Approximately 300 patients will be enrolled in the clinical trial and will be followed for two weeks during a baseline phase, which includes a one-week washout period. After completion of the baseline phase, patients will be randomized (1:1:1) to receive one of two doses of CBD gel (250 mg or 125 mg CBD in ZYN002 4.2%) or placebo gel every 12 hours for 12 weeks. The primary endpoint of the study is the change from baseline in the weekly mean of the 24-hour average worst pain score. In
15
September 2016, we announced that the first patients were randomized and dosed in the STOP 1 trial, and we expect to report topline results in the first half of 2017.
We plan to evaluate the tolerability and PK profile of ZYN001 in a Phase 1 single rising dose clinical trial in healthy human subjects in the first half of 2017. Subsequent to the single rising dose clinical trials, we intend to conduct a Phase 1 multiple rising dose clinical trial to examine the tolerability, PK and PD of multiple doses of ZYN001 in healthy human subjects and in patients with fibromyalgia.
Our key development programs and expected timelines for the development of ZYN002 and ZYN001 are shown in the chart below:
We have never been profitable and have incurred net losses since inception. Our net losses were $16.5 million for the nine months ended September 30, 2016. We expect to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates. Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or JOBS Act, was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an "emerging growth company." As an "emerging growth company," we have elected not to take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision not to take advantage of the extended transition period is irrevocable.
Subject to certain conditions set forth in the JOBS Act, as an "emerging growth company," we are not required to, among other things, (i) provide an auditor's attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s
16
compensation to median employee compensation. These exemptions will apply until December 31, 2020 or until we no longer meet the requirements for being and “emerging growth company,” whichever occurs first.
Financial Operations Overview
The following discussion sets forth certain components of our consolidated statements of operations as well as factors that impact those items.
Revenue
Our revenue consists of state and federal research grants and, historically, fees received from research services for third-party product development. We recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable and collectability is reasonably assured. Grant revenue received is deferred until the related expenditures are incurred. Revenue in each of the periods presented is related to work performed in connection with grants received.
Research and Development Expenses
Our research and development expenses consist of expenses incurred in development, preclinical studies and clinical trials relating to our product candidates, including:
|
· |
expenses associated with preclinical development and clinical trials; |
|
· |
personnel-related expenses, such as salaries, benefits, travel and other related expenses, including stock-based compensation; |
|
· |
payments to third-party CROs, contractor laboratories and independent contractors; and |
|
· |
depreciation, maintenance and other facility-related expenses. |
We expense all research and development costs as incurred. Preclinical and clinical development expenses for our product candidates are a significant component of our current research and development expenses. Product candidates in later stage clinical development generally have higher research and development expenses than those in earlier stages of development, primarily due to increased size and duration of the clinical trials. We track and record information regarding external research and development expenses for each grant, study or trial that we conduct. From time to time, we use third-party CROs, contractor laboratories and independent contractors in preclinical studies and clinical trials. We recognize the expenses associated with third parties performing these services for us in our preclinical studies and clinical trials based on the percentage of each trial or study completed at the end of each reporting period.
We incurred research and development expenses of $4.5 million and $2.3 million for the three months ended September 30, 2016 and 2015, respectively. Research and development expenses for the three months ended September 30, 2016 are net of a $1.2 million Australian tax incentive and tax refund, which we expect will result in a refund of $1.2 million of certain research and development costs incurred in Australia and Goods and Services Tax, or GST, paid on research and development expenses paid to Australian vendors.
We incurred research and development expenses of $11.9 million and $4.1 million for the nine months ended September 30, 2016 and 2015, respectively. Research and development expenses for the nine months ended September 30, 2016 are net of a $2.5 million Australian tax incentive and tax refund, which we expect will result in a refund of $2.3 million of certain research and development costs incurred in Australia and GST paid on research and development expenses paid to Australian vendors. We received a refund of $0.2 million during the nine months ended September 30, 2016 for GST paid on research and development expenses paid to Australian vendors.
We expect research and development expenses in future years to continue to increase as we continue our clinical trials and begin new phases for each of our product candidates as a result of the work needed for completion of our Phase 2 clinical trials of ZYN002, initiated in June 2016, and the expected initiation of our Phase 1 clinical trials for ZYN001 in the first half of 2017. These expenditures are subject to numerous uncertainties regarding timing and cost to completion. Completion of our preclinical development and clinical trials may take several years or more and the length of time
17
generally varies according to the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
|
· |
the number of sites included in the clinical trials; |
|
· |
the length of time required to enroll suitable patients; |
|
· |
the size of patient populations participating in the clinical trials; |
|
· |
the duration of patient follow-ups; |
|
· |
the development stage of the product candidates; and |
|
· |
the efficacy and safety profile of the product candidates. |
Due to the early stages of our research and development, we are unable to determine the duration or completion costs of our development of ZYN002 and ZYN001. As a result of the difficulties of forecasting research and development costs of ZYN002 and ZYN001 as well as the other uncertainties discussed above, we are unable to determine when and to what extent we will generate revenue from the commercialization and sale of an approved product candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and other related costs, including stock-based compensation, for personnel serving in our executive, finance, accounting, legal and human resource functions. Our general and administrative expenses also include facility and related costs not included in research and development expenses, professional fees for legal services, including patent-related expenses, consulting, tax and accounting services, insurance and general corporate expenses. We expect that our general and administrative expenses will increase with the continued development and potential commercialization of our product candidates.
We expect that our general and administrative expenses in 2016 and for the next several years will be higher than in past years as we increase our headcount. We also anticipate increased expenses relating to our operations as a public company, including increased costs for the hiring of additional personnel, and for payment to outside consultants, including lawyers and accountants, to comply with additional regulations, corporate governance, internal control and similar requirements applicable to public companies, as well as increased costs for insurance.
Interest Income
Interest income consists primarily of interest earned on our money market bank account.
Foreign Exchange Loss
Foreign exchange loss relates to the effect of exchange rates on transactions at our Australian subsidiary.
Results of Operations
Comparison of the Three Months Ended September 30, 2016 and 2015
Revenue
Revenue decreased by $199,407, or 100%, and was zero for the three months ended September 30, 2016, compared to $199,407 for the three months ended September 30, 2015. Revenue in the 2015 period was entirely related to work performed in connection with grants received. The decrease from 2015 reflected reduced research activities associated with our remaining grant.
Research and Development Expenses
Research and development expenses increased by $2.2 million or 98%, to $4.5 million for the three months ended September 30, 2016 from $2.3 million for the three months ended September 30, 2015. The increase was primarily related to an increase in the number and size of our non-clinical studies and clinical trials for ZYN002 and ZYN001.
18
General and Administrative Expenses
General and administrative expenses decreased by $0.4 million, or 22%, to $1.5 million for the three months ended September 30, 2016 from $1.9 million for the three months ended September 30, 2015. The decrease was primarily related to expenses incurred during the 2015 period in preparation for our 2015 IPO.
Other Income (Expense)
During the three months ended September 30, 2016 and 2015, the Company recognized $22,747 and $1,572, respectively, in interest income. During the three-month period ended September 30, 2016, the Company recognized foreign currency losses of $6,270. There was no foreign currency gain or loss for the same period in 2015.
Comparison of the Nine Months Ended September 30, 2016 and 2015
Revenue
Revenue decreased by $222,375, or 97%, to $7,250 for the nine months ended September 30, 2016, compared to $229,625 for the nine months ended September 30, 2015. The decrease from 2015 reflected reduced research activities associated with our remaining grants.
Research and Development Expenses
Research and development expenses increased by $7.8 million, or 187% to $11.9 million for the nine months ended September 30, 2016 from $4.1 million for the nine months ended September 30, 2015. The increase was primarily related to an increase in the number and size of our non-clinical studies and clinical trials for ZYN002 and ZYN001.
General and Administrative Expenses
General and administrative expenses increased by $1.4 million, or 45% to $4.6 million for the nine months ended September 30, 2016 from $3.2 million for the nine months ended September 30, 2015. The increase primarily relates to increases in personnel costs, primarily stock-based compensation expense. No stock-based compensation expense was recorded prior to our IPO in August 2015 since the vesting of the stock-based compensation awards prior to the IPO was contingent on the closing of the IPO. Stock-based compensation expense was recorded for the full nine-month 2016 period.
Other Income (Expense)
During the nine months ended September 30, 2016, the Company recognized interest income of $53,243, compared to interest income of $2,948 for the nine months ended September 30, 2015. During the nine months ended September 30, 2016, the Company also recognized a loss of $49,668 associated with foreign currency transactions related to our clinical trials in Australia. There was no foreign currency gain or loss in 2015 for the same period.
Income Tax Benefit
During the nine months ended September 30, 2016, we reversed $27,543 of income tax expense associated with our Australian subsidiary.
Liquidity and Capital Resources
Since our inception in 2007, we have devoted most of our cash resources to research and development and general and administrative activities. We have financed our operations primarily with the proceeds from the sale of equity securities (most notably our recent IPO, which raised $42.1 million of net proceeds, and sales under our “at-the-market” offering, which raised $5.3 million of net proceeds through September 30, 2016 and $4.2 million of proceeds from October 1, 2016 through November 11, 2016) and convertible promissory notes, state and federal grants and research services.
To date, we have not generated any revenue from the sale of products, and we do not anticipate generating any revenue from the sales of products for the foreseeable future. We have incurred losses and generated negative cash flows from
19
operations since inception. As of September 30, 2016, our principal sources of liquidity were our cash and cash equivalents, which totaled $31.8 million. Our working capital was $30.6 million as of September 30, 2016.
Based on our current operating plans, we believe that the net proceeds from our recent equity financings, including net proceeds received after September 30, 2016 of $4.2 million, and our existing cash and cash equivalents, will be sufficient to develop five Phase 3 ready programs for different indications, and these resources are sufficient to fund operations and capital requirements into 2018. However, it is difficult to predict our spending for our product candidates prior to obtaining FDA approval. Moreover, changing circumstances may cause us to expend cash significantly faster than we currently anticipate, and we may need to spend more cash than currently expected because of circumstances beyond our control.
Equity Financings
In August 2015, we completed our IPO, selling 3,450,000 shares of common stock at an offering price of $14.00 per share, resulting in gross proceeds of $48.3 million. Net proceeds received after deducting underwriting discounts and commissions and offering expenses were $42.1 million. In connection with the closing of the IPO, all outstanding shares of our Series 1 convertible preferred stock were converted into an aggregate of 3,704,216 shares of common stock.
In September 2016, we entered into an Open Market Sales Agreement (the “Sales Agreement”) with Jefferies LLC (“Jefferies”) pursuant to which, as of November 11, 2016, we sold and issued 753,799 shares of our common stock in the open market at a weighted average selling price of $13.38 per share, for net proceeds of $9.5 million. Of the 753,799 shares sold pursuant to the Sales Agreement, as of September 30, 2016, we sold and issued 428,359 shares of our common stock in the open market at a weighted-average selling price of $13.16, for net proceeds of $5.3 million and from October 1, 2016 through November 11, 2016, we sold and issued 325,440 shares of our common stock in the open market at a weighted average selling price of $13.67 per share, for $4.2 million of net proceeds.
Debt
We had no debt outstanding as of September 30, 2016 or December 31, 2015.
Future Capital Requirements
During the nine months ended September 30, 2016, net cash used in operating activities was $14.9 million, and our accumulated deficit as of September 30, 2016 was $39.1 million. Our expectations regarding future cash requirements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we make in the future. We have no current understandings, agreements or commitments for any material acquisitions or licenses of any products, businesses or technologies. We may need to raise substantial additional capital in order to engage in any of these types of transactions.
We expect to continue to incur substantial additional operating losses for at least the next several years as we continue to develop our product candidates and seek marketing approval and, subject to obtaining such approval, the eventual commercialization of our product candidates. If we obtain marketing approval for either of our product candidates, we will incur significant sales, marketing and manufacturing expenses. In addition, we expect to incur additional expenses to add operational, financial and information systems and personnel, including personnel to support our planned product commercialization efforts. We also expect to continue to incur significant costs to comply with corporate governance, internal controls and similar requirements applicable to us as a public company.
Our future use of operating cash and capital requirements will depend on many forward-looking factors, including the following:
|
· |
the initiation, progress, timing, costs and results of preclinical studies and clinical trials for our product candidates; |
|
· |
the clinical development plans we establish for these product candidates; |
|
· |
the number and characteristics of product candidates that we develop or may in-license; |
20
|
· |
the terms of any collaboration agreements we may choose to execute; |
|
· |
the outcome, timing and cost of meeting regulatory requirements established by the United States Drug Enforcement Agency, the FDA, the European Medicines Agency or other comparable foreign regulatory authorities; |
|
· |
the cost of filing, prosecuting, defending and enforcing our patent claims and other intellectual property rights, including patent infringement actions brought by third parties against us; |
|
· |
costs and timing of the implementation of commercial scale manufacturing activities; and |
|
· |
the cost of establishing, or outsourcing, sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own. |
To the extent that our capital resources are insufficient to meet our future operating and capital requirements, we will need to finance our cash needs through public or private equity offerings, debt financings, collaboration and licensing arrangements or other financing alternatives. We have no committed external sources of funds. Additional equity or debt financing or collaboration and licensing arrangements may not be available on acceptable terms, if at all.
If we raise additional funds by issuing equity securities, our stockholders will experience dilution. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us.
Cash Flows
The following table summarizes our cash flows from operating, investing and financing activities for the nine months ended September 30, 2016 and 2015.
|
|
|
Nine Months Ended September 30, |
|
||||
|
|
|
2016 |
|
2015 |
|
||
|
Statement of Cash Flows Data: |
|
|
|
|
|
|
|
|
Total net cash (used in) provided by: |
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(14,931,479) |
|
$ |
(6,592,668) |
|
|
Investing activities |
|
|
(86,726) |
|
|
(176,096) |
|
|
Financing activities |
|
|
5,285,918 |
|
|
42,244,814 |
|
|
Net (decrease) increase in cash and cash equivalents |
|
$ |
(9,732,287) |
|
$ |
35,476,050 |
|
Operating Activities
For the nine months ended September 30, 2016, cash used in operating activities was $14.9 million compared to $6.6 million for the nine months ended September 30, 2015. The increase from the comparable 2015 period was primarily the result of increased research and development activities related to the non-clinical studies and clinical trials of ZYN002 and ZYN001, as well as an increase in the number of employees hired to support our research and development and general and administrative activities, and an increase in receivables, related to an incentive associated with research and development costs in Australia and the expected refund of GST paid to Australian vendors.
We expect cash used in operating activities to continue to increase in 2016 as compared to 2015 due to an expected increase in our operating losses associated with ongoing development of our product candidates.
Investing Activities
For the nine months ended September 30, 2016 cash used in investing activities represented the cost of research and development equipment located at one of our vendors and computer equipment and furniture and fixtures associated
21
with our corporate headquarters. For the nine months ended September 30, 2015, cash used in investing activities represented the cost of computer equipment and furniture and fixtures associated with our corporate headquarters.
Financing Activities
Cash provided by financing activities for the nine months ended September 30, 2016 represented proceeds from sales of our shares of common stock under the Sales Agreement, net of related offering costs. Cash provided by financing activities for the nine months ended September 30, 2015 represented proceeds from our IPO and stock option exercises in the 2015 period.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, except for operating leases.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to various market risks, which may result in potential losses arising from adverse changes in market rates, such as interest rates and foreign exchange rates. We do not enter into derivatives or other financial instruments for trading or speculative purposes and do not believe we are exposed to material market risk with respect to our cash and cash equivalents.
We have contracted with third parties to manufacture our product candidates in Canada and to conduct Phase 1 and Phase 2 clinical trials in Australia. Manufacturing and research costs related to these activities are paid for in a combination of U.S. dollars and local currencies. Accordingly, we are subject to limited foreign currency exchange rate risk. For example, for the nine months ended September 30, 2016, we recognized a loss of $49,668 related to foreign currency transactions. We do not believe foreign currency exchange rate risk is a material risk at this time due to the limited extent of our operations, however, as we continue to conduct clinical trials and seek to manufacture a more significant portion of our product candidates outside of the United States in the future, we could incur significant foreign currency exchange rate risk.
As of September 30, 2016, we had cash and cash equivalents of $31.8 million consisting primarily of cash and money market accounts. We do not engage in any hedging activities against changes in interest rates or foreign currency exchange rates. Because of the short-term maturities of our cash and cash equivalents, we do not believe that an immediate 10% increase in interest rates would have any material impact on the realized value of our investments.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of September 30, 2016. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as of September 30, 2016, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control Over Financial Reporting
No change in our internal control over financial reporting occurred during the fiscal quarter ended September 30, 2016 that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
22
We are not currently a party to any legal proceedings.
You should carefully consider the risk factors described in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015 (“2015 Annual Report”) under the caption “Item 1.A “Risk Factors”. There have been no material changes to the risk factors disclosed in our 2015 Annual Report.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
Recent Sales of Unregistered Securities
None.
Use of Proceeds
Our IPO was effected through a Registration Statement on Form S-1 (File No. 333-205355) that was declared effective by the SEC on August 4, 2015, which registered an aggregate of 3,450,000 shares of our common stock. On August 10, 2015, we received net proceeds from the IPO of $42.1 million.
As of September 30, 2016, we have used approximately $15.7 million of the net offering proceeds from our IPO to fund the development efforts of ZYN002 (including funding of our Phase 1 and Phase 2 clinical trials), development efforts of ZYN001, working capital, research and development and general corporate purposes. None of the net proceeds have been paid directly or indirectly to (i) our directors, officers or any of their associates; (ii) persons owning 10% or more of our common stock; or (iii) our affiliates, other than payments in the ordinary course of business to our wholly-owned subsidiary, to officers for salaries and bonuses and to non-employee directors as compensation for board service.
Our use of the net proceeds to date is consistent with the use of proceeds described in our prospectus filed with the SEC pursuant to Rule 424(b)(4) on August 5, 2015, or the prospectus, and there has been no material change in our planned use of the balance of the net proceeds from the IPO described in the prospectus.
Purchase of Equity Securities
We did not purchase any of our registered equity securities during the period covered by this Quarterly Report on Form 10-Q.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
None.
Not applicable
The exhibits filed as part of this Quarterly Report on Form 10-Q are set forth on the Exhibit Index, which Exhibit Index is incorporated herein by reference.
23
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
ZYNERBA PHARMACEUTICALS, INC. |
|
|
|
|
|
|
Date: November 14, 2016 |
By: |
/s/ ARMANDO ANIDO |
|
|
|
Armando Anido |
|
|
|
Chief Executive Officer |
|
|
|
(Principal executive officer) |
|
|
|
|
|
Date: November 14, 2016 |
By: |
/s/ JAMES E. FICKENSCHER |
|
|
|
James E. Fickenscher |
|
|
|
Chief Financial Officer |
|
|
|
(Principal financial and accounting officer) |
24
EXHIBIT INDEX
| 10.1 |
|
Separation Agreement, dated August 10, 2016, between and among Zynerba Pharmaceuticals, Inc. and Richard A. Baron (filed herewith).* |
| 10.2 |
|
Employment Agreement, dated August 11, 2016, by and between Zynerba Pharmaceuticals, Inc. and James E. Fickenscher (filed herewith).* |
| 10.3 |
|
Open Market Sale Agreement, dated September 1, 2016, by and between Zynerba Pharmaceuticals, Inc. and Jefferies LLC (incorporated herein by reference to Exhibit 1.2 to the Company’s Registration Statement on Form S-3 filed on September 1, 2016 (File No. 333-213430). |
| 31.1 |
|
Certification of Chief Executive Officer pursuant to Rules 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). |
| 31.2 |
|
Certification of Chief Financial Officer pursuant to Rules 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). |
| 32.1 |
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (furnished herewith). |
| 32.2 |
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (furnished herewith). |
| 101 |
|
The following financial information from this Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2016, formatted in XBRL (Extensible Business Reporting Language) and furnished electronically herewith: (i) the Consolidated Balance Sheets as of September 30, 2016 and December 31, 2015 (Unaudited); (ii) the Consolidated Statements of Operations for the Three and Nine Months Ended September 30, 2016 and 2015 (Unaudited); (iii) the Consolidated Statement of Stockholders’ Equity for the Nine Months Ended September 30, 2016, (iv) the Consolidated Statements of Cash Flows for the Nine Months Ended September 30, 2016 and 2015 (Unaudited) and (v) the Notes to Unaudited Consolidated Financial Statements, tagged in detail. |
|
|
|
|
|
* |
|
Indicates management contract or compensatory plan or arrangement. |
25
