Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AVADEL PHARMACEUTICALS PLC | flamelform8-kjefferiespres.htm |

November 2016 1
November 2016 2
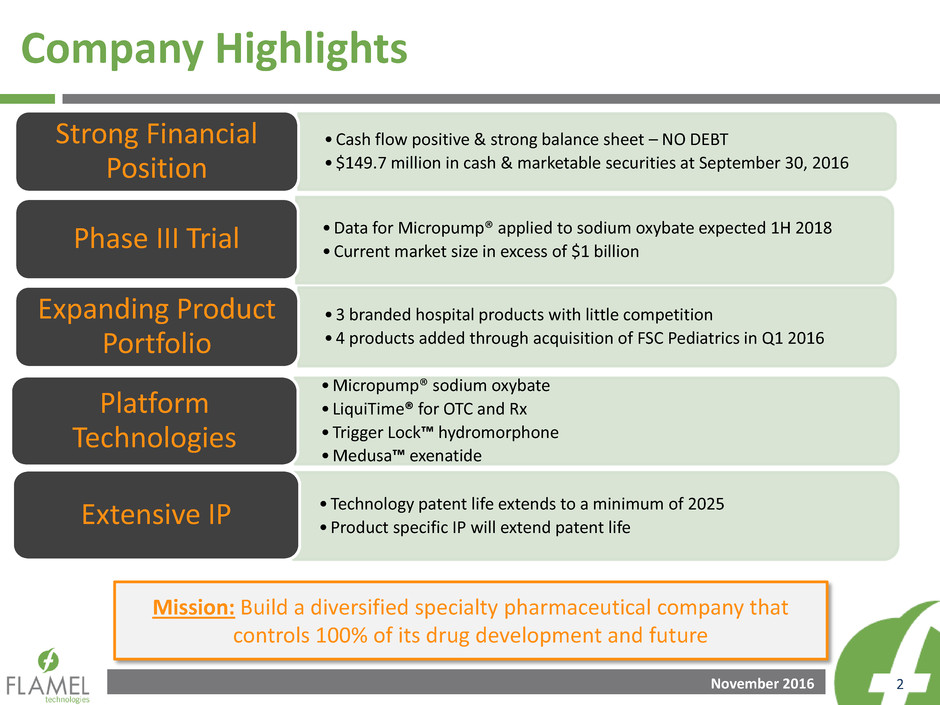
Company Highlights
Mission: Build a diversified specialty pharmaceutical company that
controls 100% of its drug development and future
•Cash flow positive & strong balance sheet – NO DEBT
•$149.7 million in cash & marketable securities at September 30, 2016
Strong Financial
Position
•Data for Micropump® applied to sodium oxybate expected 1H 2018
•Current market size in excess of $1 billion Phase III Trial
•3 branded hospital products with little competition
•4 products added through acquisition of FSC Pediatrics in Q1 2016
Expanding Product
Portfolio
•Micropump® sodium oxybate
• LiquiTime® for OTC and Rx
•Trigger Lock™ hydromorphone
•Medusa™ exenatide
Platform
Technologies
•Technology patent life extends to a minimum of 2025
•Product specific IP will extend patent life Extensive IP
November 2016 3
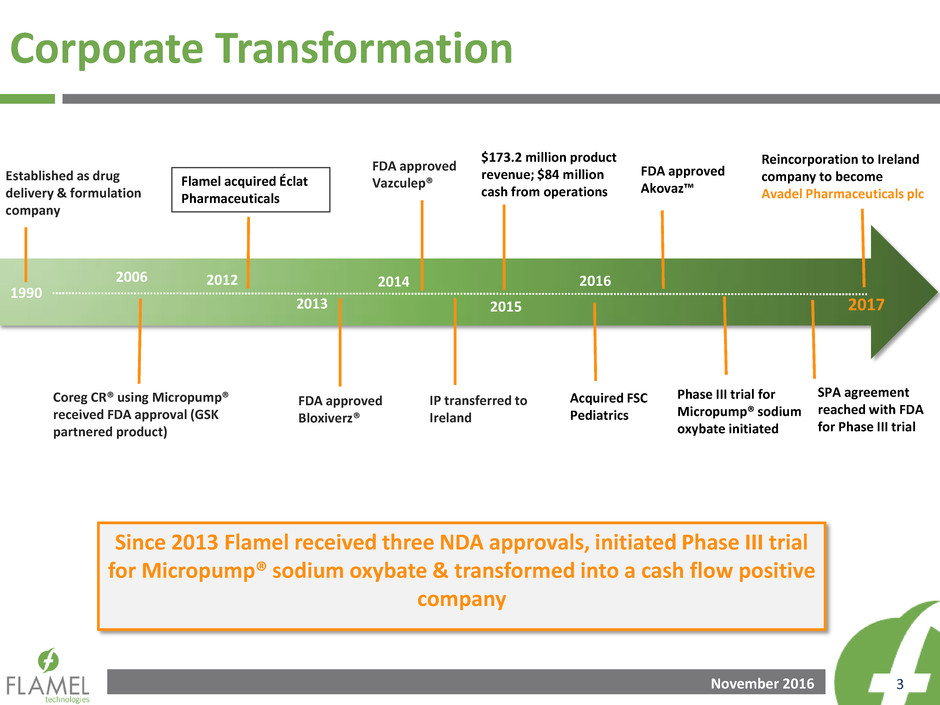
Corporate Transformation
Established as drug
delivery & formulation
company
1990
2006
Coreg CR® using Micropump®
received FDA approval (GSK
partnered product)
2012
Flamel acquired Éclat
Pharmaceuticals
2013
FDA approved
Bloxiverz®
2014
FDA approved
Vazculep®
IP transferred to
Ireland
2015
$173.2 million product
revenue; $84 million
cash from operations
2016
Acquired FSC
Pediatrics
SPA agreement
reached with FDA
for Phase III trial
Since 2013 Flamel received three NDA approvals, initiated Phase III trial
for Micropump® sodium oxybate & transformed into a cash flow positive
company
FDA approved
Akovaz™
Phase III trial for
Micropump® sodium
oxybate initiated
2017
Reincorporation to Ireland
company to become
Avadel Pharmaceuticals plc

November 2016 4
Becoming Avadel Pharmaceuticals plc
1990
Avadel Pharmaceuticals plc (NASDAQ: AVDL)
“AdVAnced DELivery”
Cross-border merger from France to Ireland effective January
1, 2017
No changes to capital structure or share count
November 2016 5
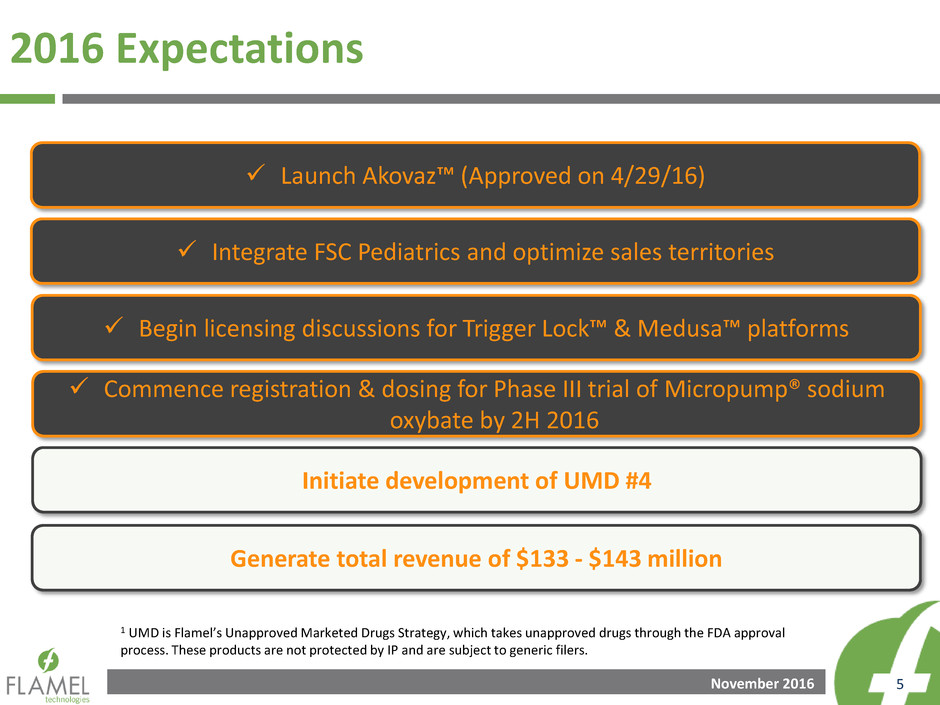
2016 Expectations
1 UMD is Flamel’s Unapproved Marketed Drugs Strategy, which takes unapproved drugs through the FDA approval
process. These products are not protected by IP and are subject to generic filers.
Generate total revenue of $133 - $143 million
Initiate development of UMD #4
Commence registration & dosing for Phase III trial of Micropump® sodium
oxybate by 2H 2016
Begin licensing discussions for Trigger Lock™ & Medusa™ platforms
Integrate FSC Pediatrics and optimize sales territories
Launch Akovaz™ (Approved on 4/29/16)
November 2016 6
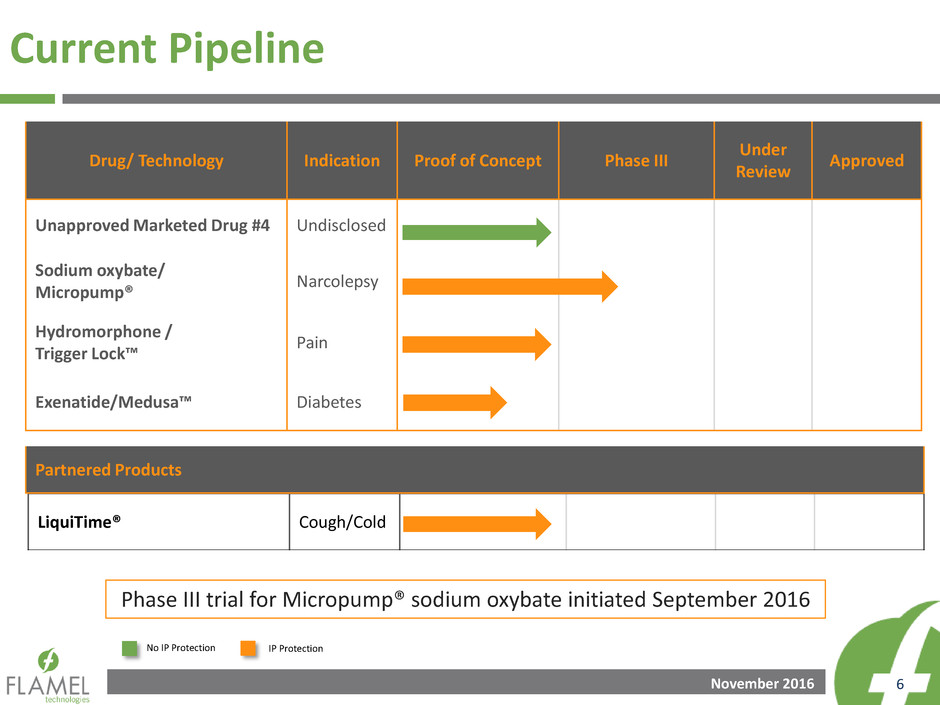
LiquiTime® Cough/Cold
Drug/ Technology Indication Proof of Concept Phase III
Under
Review
Approved
Unapproved Marketed Drug #4 Undisclosed
Sodium oxybate/
Micropump®
Narcolepsy
Hydromorphone /
Trigger Lock™
Pain
Exenatide/Medusa™ Diabetes
Current Pipeline
Partnered Products
Phase III trial for Micropump® sodium oxybate initiated September 2016
No IP Protection IP Protection
November 2016 7
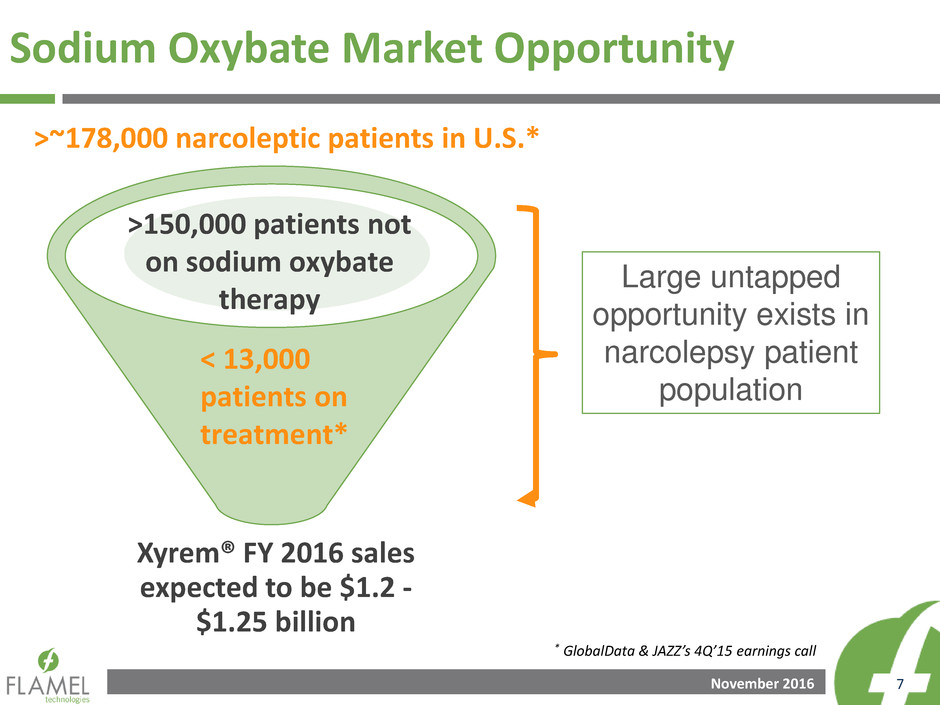
Xyrem® FY 2016 sales
expected to be $1.2 -
$1.25 billion
Sodium Oxybate Market Opportunity
* GlobalData & JAZZ’s 4Q’15 earnings call
>~178,000 narcoleptic patients in U.S.*
< 13,000
patients on
treatment*
Large untapped
opportunity exists in
narcolepsy patient
population
>150,000 patients not
on sodium oxybate
therapy
November 2016 8
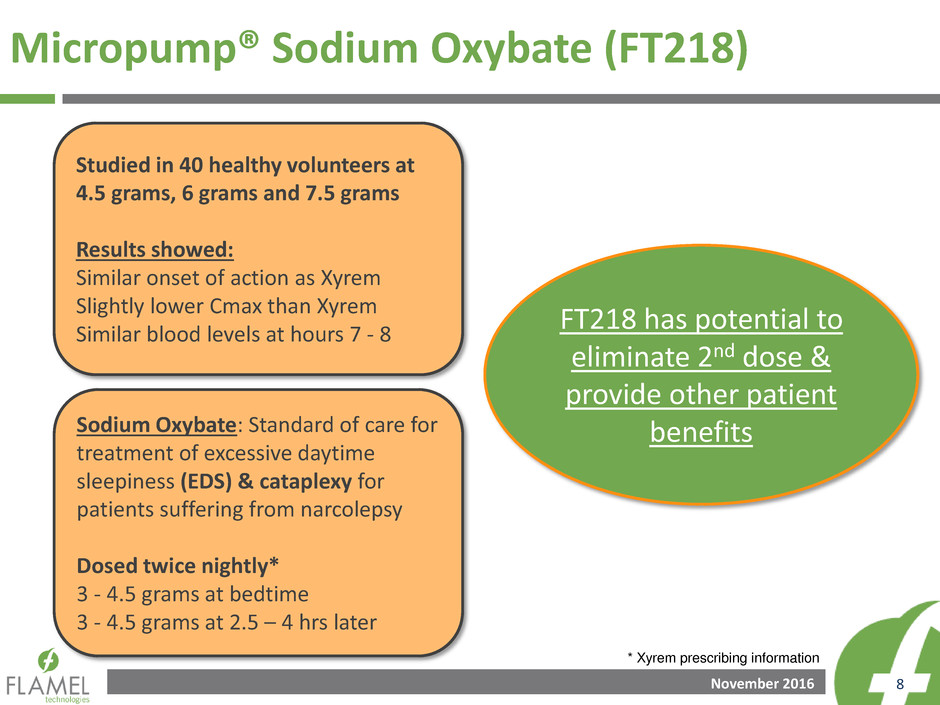
Micropump® Sodium Oxybate (FT218)
* Xyrem prescribing information
Studied in 40 healthy volunteers at
4.5 grams, 6 grams and 7.5 grams
Results showed:
Similar onset of action as Xyrem
Slightly lower Cmax than Xyrem
Similar blood levels at hours 7 - 8
Sodium Oxybate: Standard of care for
treatment of excessive daytime
sleepiness (EDS) & cataplexy for
patients suffering from narcolepsy
Dosed twice nightly*
3 - 4.5 grams at bedtime
3 - 4.5 grams at 2.5 – 4 hrs later
FT218 has potential to
eliminate 2nd dose &
provide other patient
benefits
November 2016 9
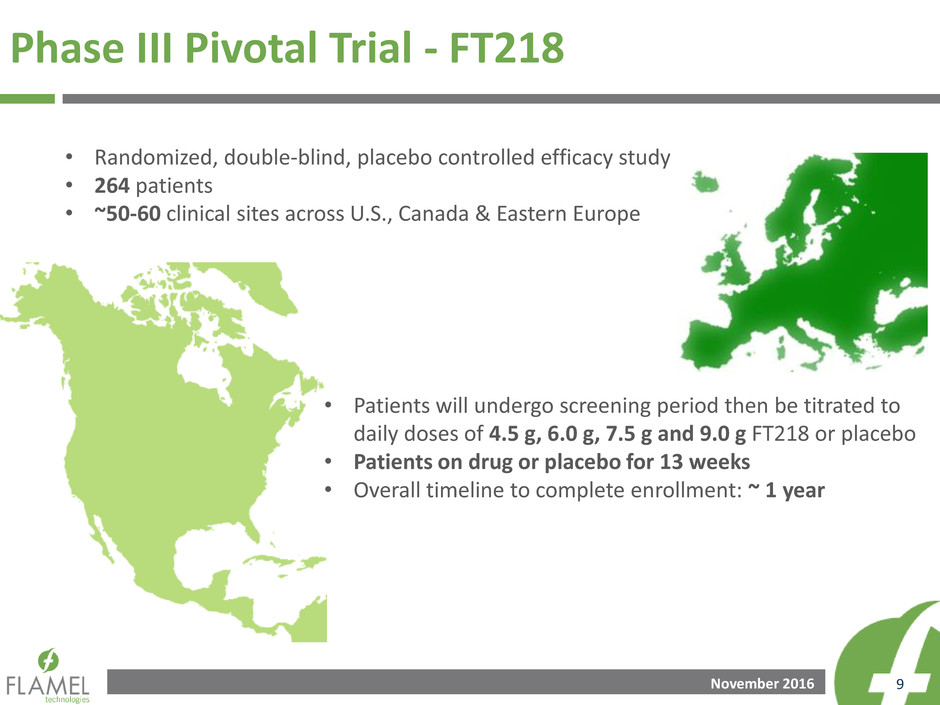
Phase III Pivotal Trial - FT218
• Randomized, double-blind, placebo controlled efficacy study
• 264 patients
• ~50-60 clinical sites across U.S., Canada & Eastern Europe
• Patients will undergo screening period then be titrated to
daily doses of 4.5 g, 6.0 g, 7.5 g and 9.0 g FT218 or placebo
• Patients on drug or placebo for 13 weeks
• Overall timeline to complete enrollment: ~ 1 year
November 2016 10
Micropump® Overview
Robust platform technology utilizing microparticles for the
extended/delayed release of drugs in GI tract
Micropump 1st approved in 2006 in Coreg CR (carvedilol)
10 years – no generics
Tailored release profile solves dosing problems related to PK profiles and
drugs with short half lives
Applicable to wide variety of molecules
Patented through 2027 with product specific
patents to extend protection
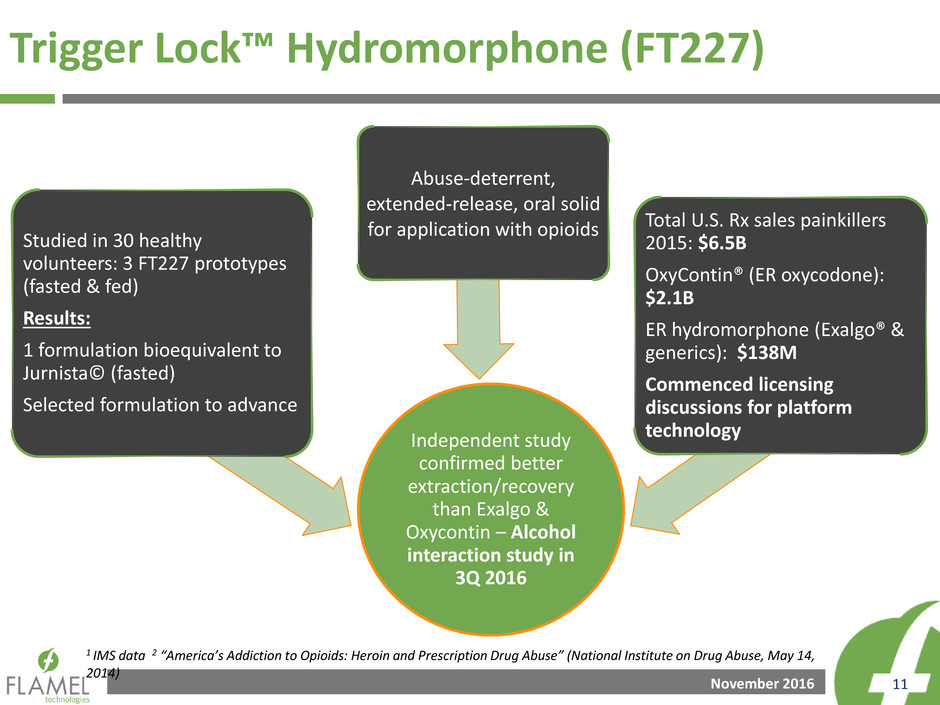
November 2016 11
Independent study
confirmed better
extraction/recovery
than Exalgo &
Oxycontin – Alcohol
interaction study in
3Q 2016
Studied in 30 healthy
volunteers: 3 FT227 prototypes
(fasted & fed)
Results:
1 formulation bioequivalent to
Jurnista© (fasted)
Selected formulation to advance
Abuse-deterrent,
extended-release, oral solid
for application with opioids Total U.S. Rx sales painkillers
2015: $6.5B
OxyContin® (ER oxycodone):
$2.1B
ER hydromorphone (Exalgo® &
generics): $138M
Commenced licensing
discussions for platform
technology
Trigger Lock™ Hydromorphone (FT227)
1 IMS data 2 “America’s Addiction to Opioids: Heroin and Prescription Drug Abuse” (National Institute on Drug Abuse, May 14,
2014)
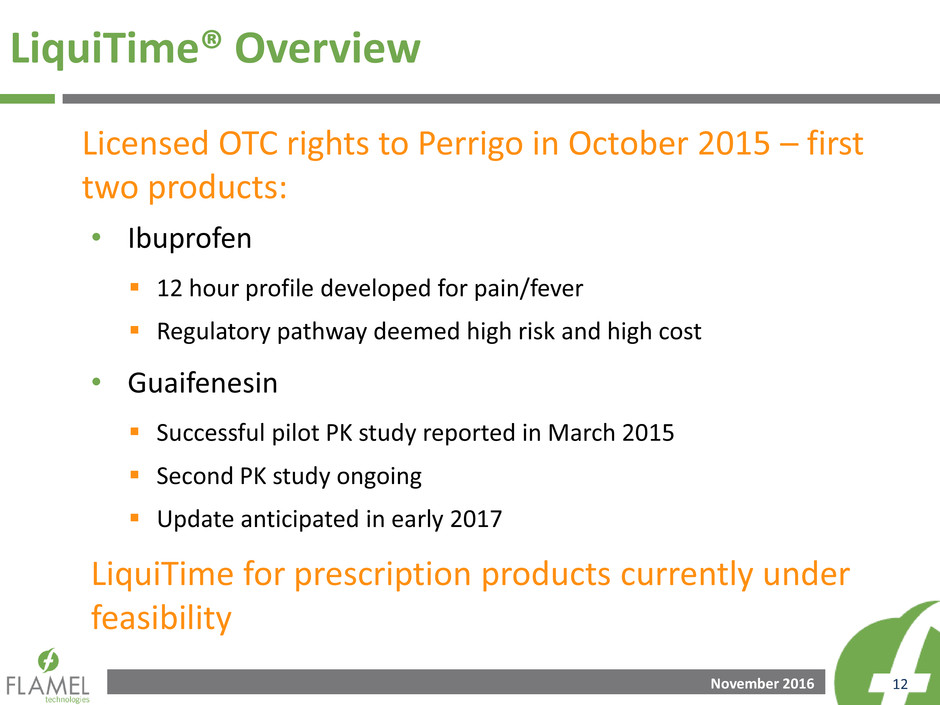
November 2016 12
LiquiTime® Overview
• Ibuprofen
12 hour profile developed for pain/fever
Regulatory pathway deemed high risk and high cost
• Guaifenesin
Successful pilot PK study reported in March 2015
Second PK study ongoing
Update anticipated in early 2017
LiquiTime for prescription products currently under
feasibility
Licensed OTC rights to Perrigo in October 2015 – first
two products:
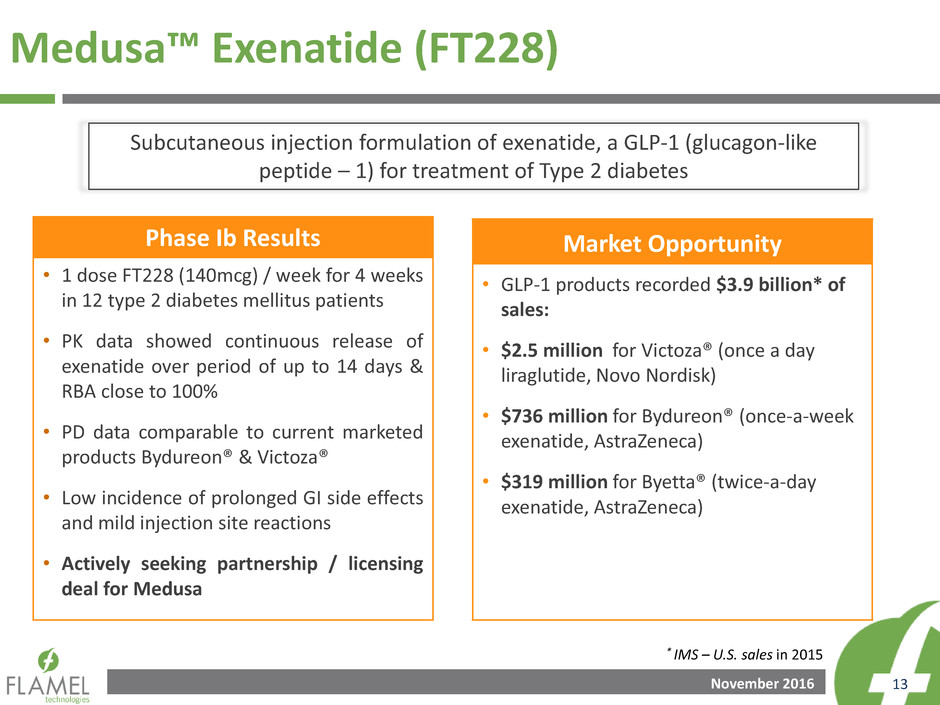
November 2016 13
Phase Ib Results
• 1 dose FT228 (140mcg) / week for 4 weeks
in 12 type 2 diabetes mellitus patients
• PK data showed continuous release of
exenatide over period of up to 14 days &
RBA close to 100%
• PD data comparable to current marketed
products Bydureon® & Victoza®
• Low incidence of prolonged GI side effects
and mild injection site reactions
• Actively seeking partnership / licensing
deal for Medusa
Market Opportunity
• GLP-1 products recorded $3.9 billion* of
sales:
• $2.5 million for Victoza® (once a day
liraglutide, Novo Nordisk)
• $736 million for Bydureon® (once-a-week
exenatide, AstraZeneca)
• $319 million for Byetta® (twice-a-day
exenatide, AstraZeneca)
Medusa™ Exenatide (FT228)
* IMS – U.S. sales in 2015
Subcutaneous injection formulation of exenatide, a GLP-1 (glucagon-like
peptide – 1) for treatment of Type 2 diabetes

November 2016 14
Marketed Products
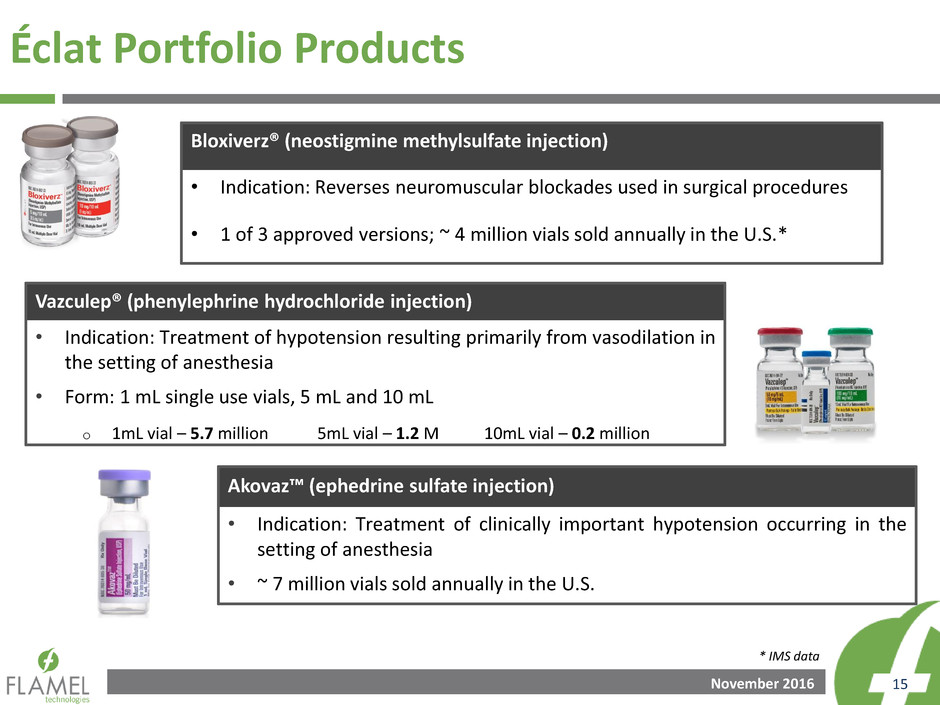
November 2016 15
Éclat Portfolio Products
* IMS data
Bloxiverz® (neostigmine methylsulfate injection)
• Indication: Reverses neuromuscular blockades used in surgical procedures
• 1 of 3 approved versions; ~ 4 million vials sold annually in the U.S.*
Vazculep® (phenylephrine hydrochloride injection)
• Indication: Treatment of hypotension resulting primarily from vasodilation in
the setting of anesthesia
• Form: 1 mL single use vials, 5 mL and 10 mL
o 1mL vial – 5.7 million 5mL vial – 1.2 M 10mL vial – 0.2 million
Akovaz™ (ephedrine sulfate injection)
• Indication: Treatment of clinically important hypotension occurring in the
setting of anesthesia
• ~ 7 million vials sold annually in the U.S.
November 2016 16
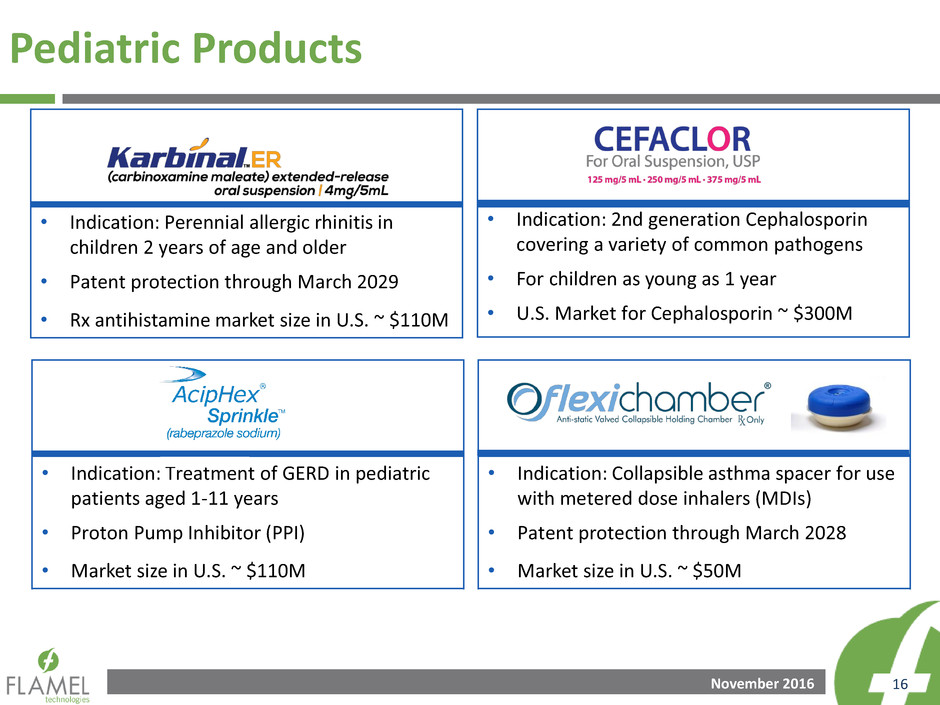
Pediatric Products
• Indication: Perennial allergic rhinitis in
children 2 years of age and older
• Patent protection through March 2029
• Rx antihistamine market size in U.S. ~ $110M
• Indication: 2nd generation Cephalosporin
covering a variety of common pathogens
• For children as young as 1 year
• U.S. Market for Cephalosporin ~ $300M
• Indication: Treatment of GERD in pediatric
patients aged 1-11 years
• Proton Pump Inhibitor (PPI)
• Market size in U.S. ~ $110M
• Indication: Collapsible asthma spacer for use
with metered dose inhalers (MDIs)
• Patent protection through March 2028
• Market size in U.S. ~ $50M
November 2016 17
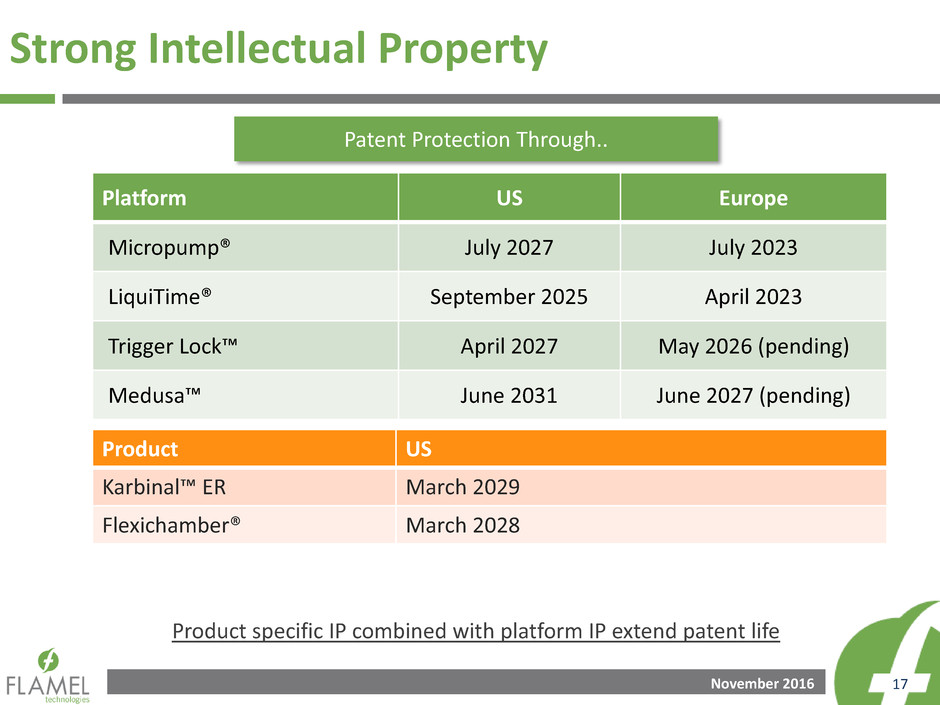
Strong Intellectual Property
Platform US Europe
Micropump® July 2027 July 2023
LiquiTime® September 2025 April 2023
Trigger Lock™ April 2027 May 2026 (pending)
Medusa™ June 2031 June 2027 (pending)
Product US
Karbinal™ ER March 2029
Flexichamber® March 2028
Patent Protection Through..
Product specific IP combined with platform IP extend patent life
November 2016 18
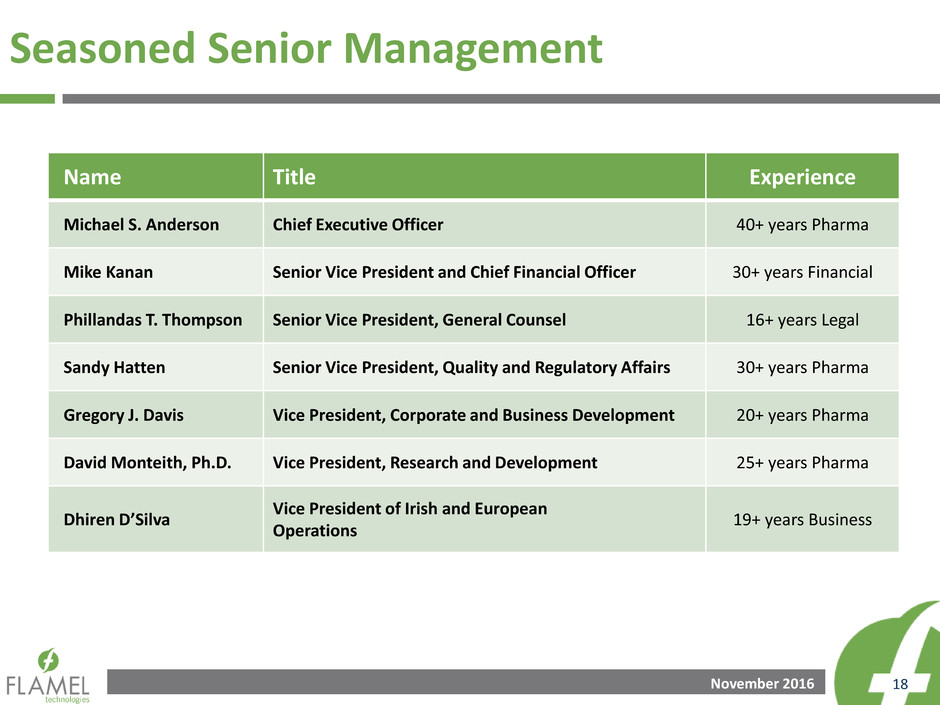
Seasoned Senior Management
Name Title Experience
Michael S. Anderson Chief Executive Officer 40+ years Pharma
Mike Kanan Senior Vice President and Chief Financial Officer 30+ years Financial
Phillandas T. Thompson Senior Vice President, General Counsel 16+ years Legal
Sandy Hatten Senior Vice President, Quality and Regulatory Affairs 30+ years Pharma
Gregory J. Davis Vice President, Corporate and Business Development 20+ years Pharma
David Monteith, Ph.D. Vice President, Research and Development 25+ years Pharma
Dhiren D’Silva
Vice President of Irish and European
Operations
19+ years Business
November 2016 19
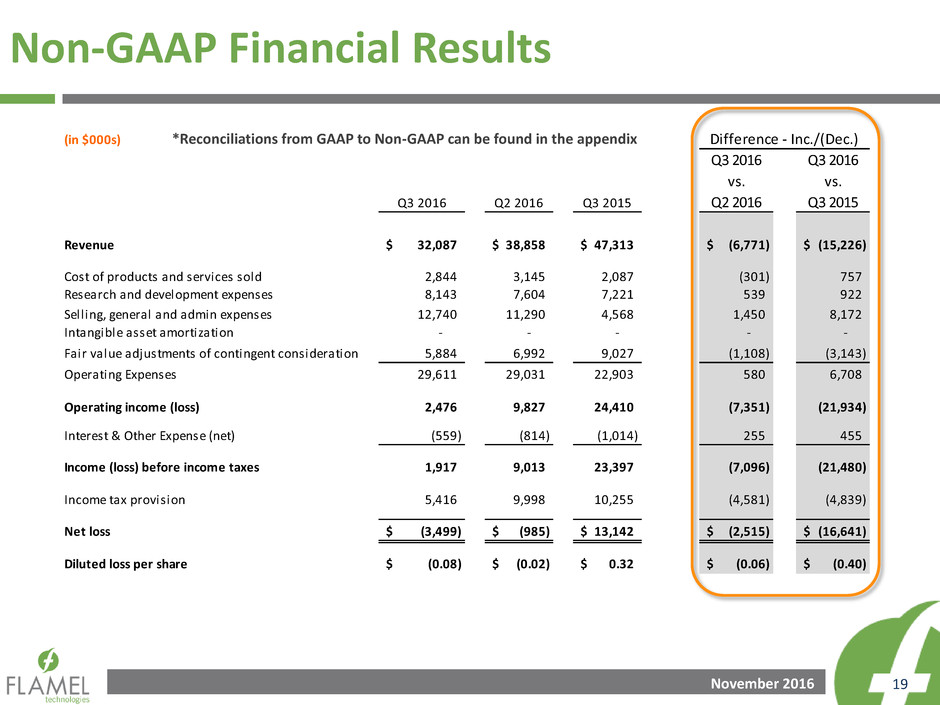
Non-GAAP Financial Results
*Reconciliations from GAAP to Non-GAAP can be found in the appendix
(in $000s)
Q3 2016 Q2 2016 Q3 2015
Q3 2016
vs.
Q2 2016
Q3 2016
vs.
Q3 2015
Revenue 32,087$ 38,858$ 47,313$ (6,771)$ (15,226)$
Cost of products and services sold 2,844 3,145 2,087 (301) 757
Research and development expenses 8,143 7,604 7,221 539 922
Selling, general and admin expenses 12,740 11,290 4,568 1,450 8,172
Intangible asset amortization - - - - -
Fair value adjustments of contingent consideration 5,884 6,992 9,027 (1,108) (3,143)
Operating Expenses 29,611 29,031 22,903 580 6,708
Operating income (loss) 2,476 9,827 24,410 (7,351) (21,934)
Interest & Other Expense (net) (559) (814) (1,014) 255 455
Income (loss) before income taxes 1,917 9,013 23,397 (7,096) (21,480)
Income tax provision 5,416 9,998 10,255 (4,581) (4,839)
Net loss (3,499)$ (985)$ 13,142$ (2,515)$ (16,641)$
Diluted loss per share (0.08)$ (0.02)$ 0.32$ (0.06)$ (0.40)$
Difference - Inc./(Dec.)
November 2016 20
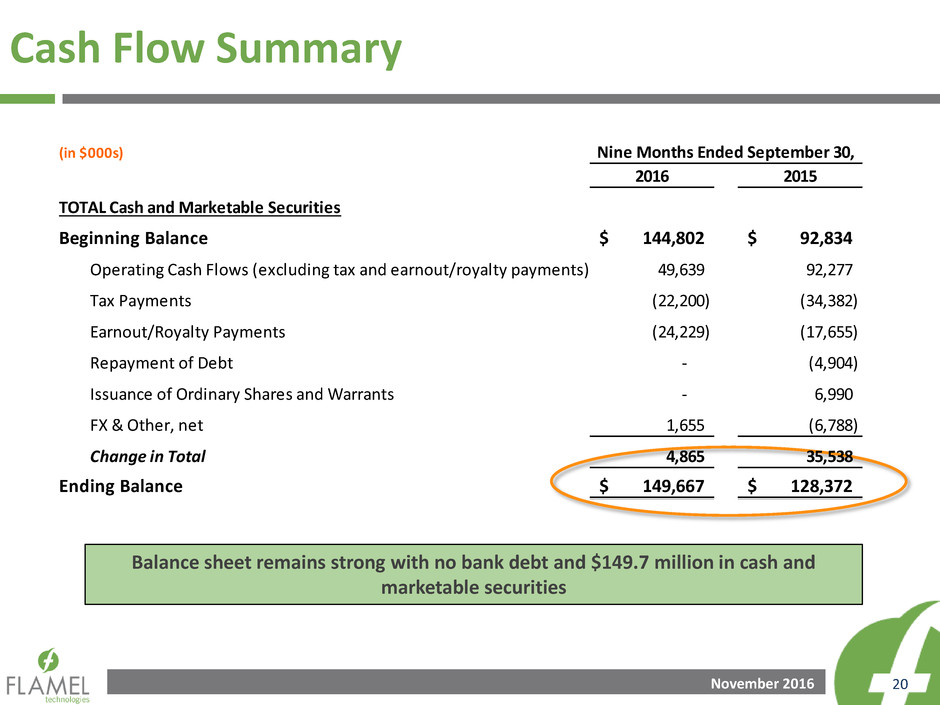
Cash Flow Summary
Balance sheet remains strong with no bank debt and $149.7 million in cash and
marketable securities
(in $000s)
2016 2015
TOTAL Cash and Marketable Securities
Beginning Balance 144,802$ 92,834$
Operating Cash Flows (excluding tax and earnout/royalty payments) 49,639 92,277
Tax Payments (22,200) (34,382)
Earnout/Royalty Payments (24,229) (17,655)
Repayment of Debt - (4,904)
Issuance of Ordinary Shares and Warrants - 6,990
FX & Other, net 1,655 (6,788)
Change in Total 4,865 35,538
Ending Balance 149,667$ 128,372$
Nine Months Ended September 30,
November 2016 21
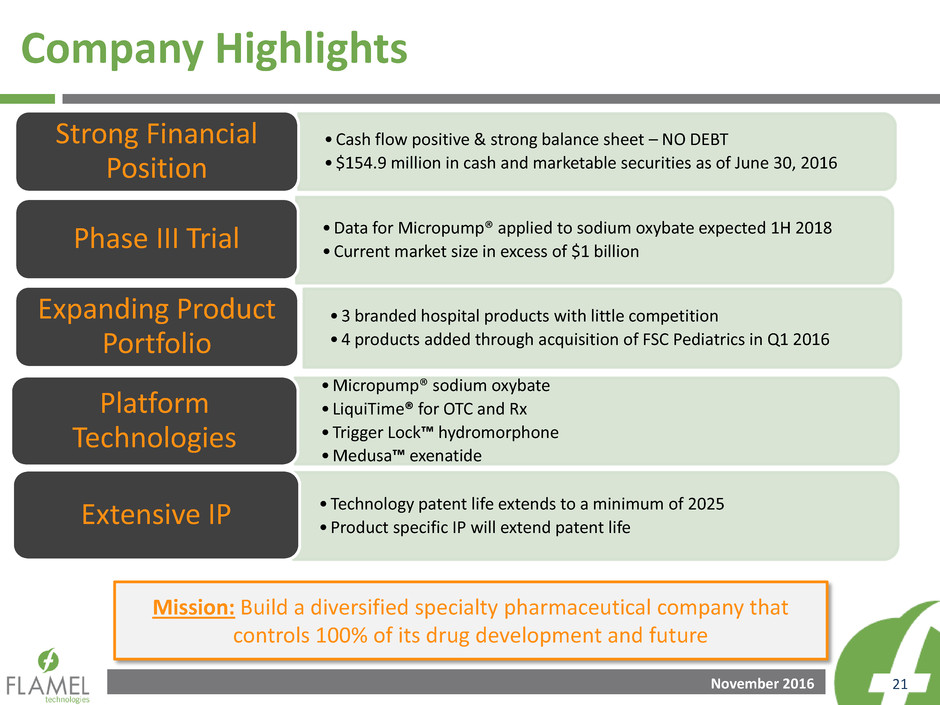
Company Highlights
Mission: Build a diversified specialty pharmaceutical company that
controls 100% of its drug development and future
•Cash flow positive & strong balance sheet – NO DEBT
•$154.9 million in cash and marketable securities as of June 30, 2016
Strong Financial
Position
•Data for Micropump® applied to sodium oxybate expected 1H 2018
•Current market size in excess of $1 billion Phase III Trial
•3 branded hospital products with little competition
•4 products added through acquisition of FSC Pediatrics in Q1 2016
Expanding Product
Portfolio
•Micropump® sodium oxybate
• LiquiTime® for OTC and Rx
•Trigger Lock™ hydromorphone
•Medusa™ exenatide
Platform
Technologies
•Technology patent life extends to a minimum of 2025
•Product specific IP will extend patent life Extensive IP
November 2016 22
Appendix
November 2016 23
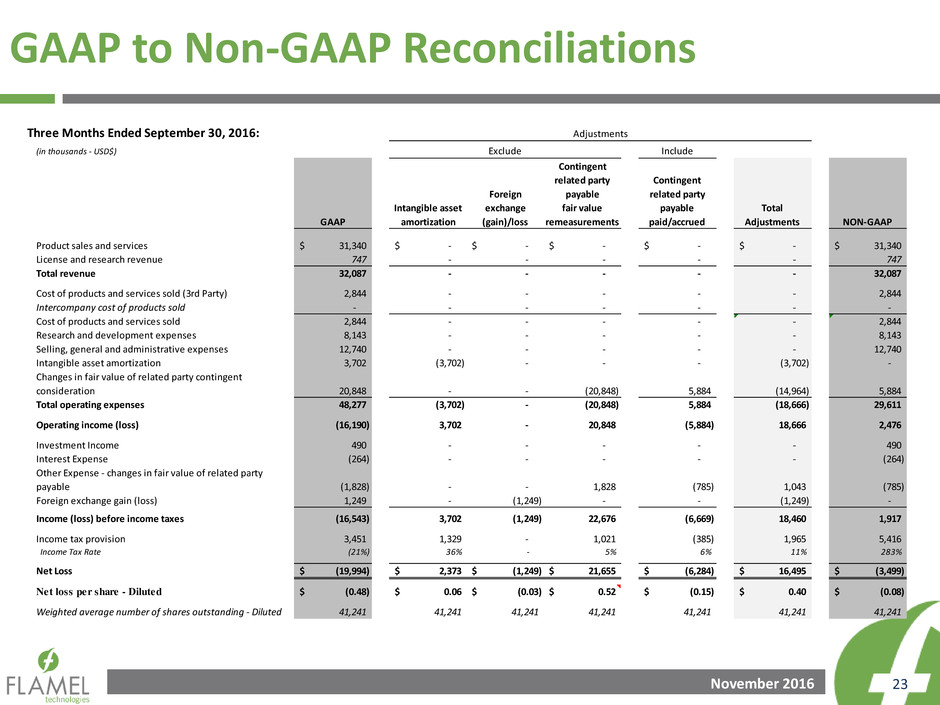
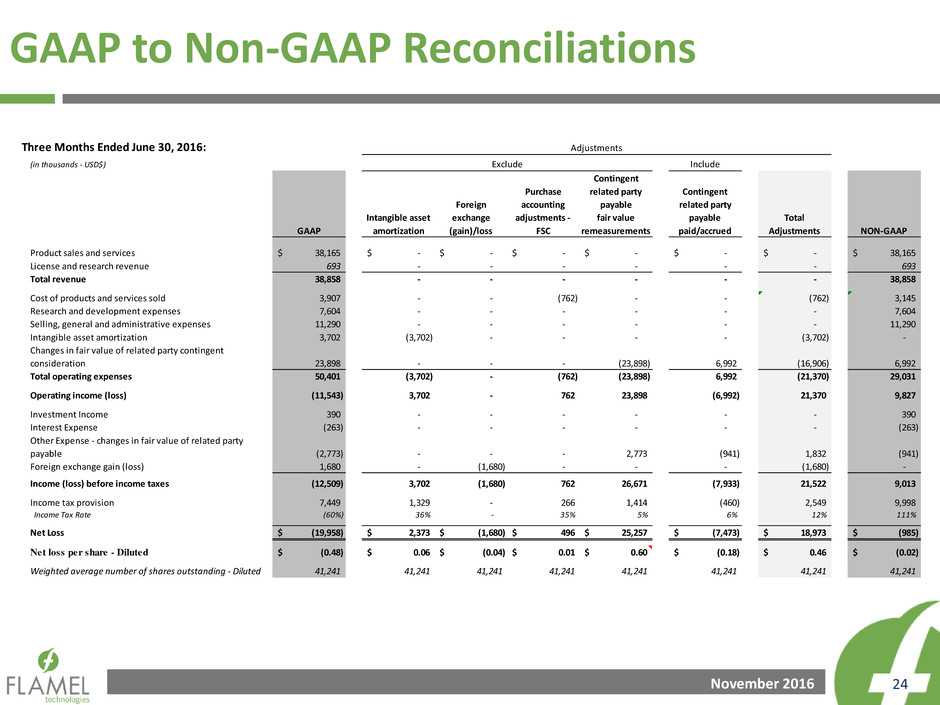
GAAP to Non-GAAP Reconciliations
Three Months Ended September 30, 2016:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Contingent
related party
payable
fair value
remeasurements
Contingent
related party
payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 31,340$ -$ -$ -$ -$ -$ 31,340$
License and research revenue 747 - - - - - 747
Total revenue 32,087 - - - - - 32,087
Cost of products and services sold (3rd Party) 2,844 - - - - - 2,844
Intercompany cost of products sold - - - - - - -
Cost of products and services sold 2,844 - - - - - 2,844
Research and development expenses 8,143 - - - - - 8,143
Selling, general and administrative expenses 12,740 - - - - - 12,740
Intangible asset amortization 3,702 (3,702) - - - (3,702) -
Changes in fair value of related party contingent
consideration 20,848 - - (20,848) 5,884 (14,964) 5,884
Total operating expenses 48,277 (3,702) - (20,848) 5,884 (18,666) 29,611
Operating income (loss) (16,190) 3,702 - 20,848 (5,884) 18,666 2,476
Investment Income 490 - - - - - 490
Interest Expense (264) - - - - - (264)
Other Expense - changes in fair value of related party
payable (1,828) - - 1,828 (785) 1,043 (785)
Foreign exchange gain (loss) 1,249 - (1,249) - - (1,249) -
Income (loss) before income taxes (16,543) 3,702 (1,249) 22,676 (6,669) 18,460 1,917
I come tax provision 3,451 1,329 - 1,021 (385) 1,965 5,416
Income Tax Rate (21%) 36% - 5% 6% 11% 283%
Net Loss (19,994)$ 2,373$ (1,249)$ 21,655$ (6,284)$ 16,495$ (3,499)$
Net loss per share - Diluted (0.48)$ 0.06$ (0.03)$ 0.52$ (0.15)$ 0.40$ (0.08)$
Weighted average number of shares outstanding - Diluted 41,241 41,241 41,241 41,241 41,241 41,241 41,241
Adjustments
Exclude
November 2016 24
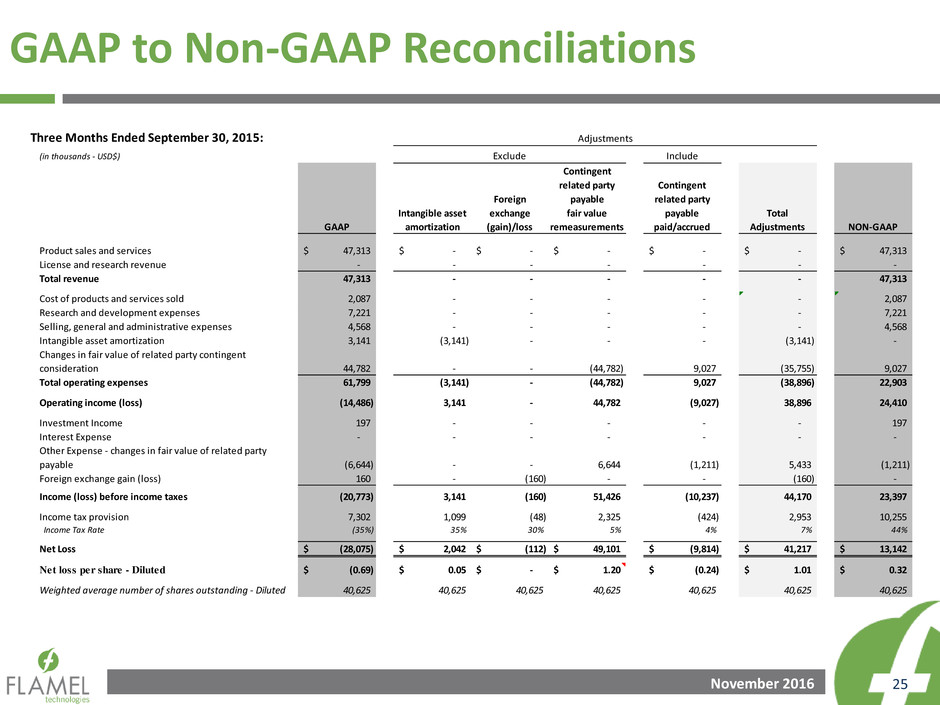
GAAP to Non-GAAP Reconciliations
Three Months Ended June 30, 2016:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Purchase
accounting
adjustments -
FSC
Contingent
related party
payable
fair value
remeasurements
Contingent
related party
payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 38,165$ -$ -$ -$ -$ -$ -$ 38,165$
License and research revenue 693 - - - - - - 693
Total revenue 38,858 - - - - - - 38,858
Cost of products and services sold 3,907 - - (762) - - (762) 3,145
Research and development expenses 7,604 - - - - - - 7,604
Selling, general and administrative expenses 11,290 - - - - - - 11,290
Intangible asset amortization 3,702 (3,702) - - - - (3,702) -
Changes in fair value of related party contingent
consideration 23,898 - - - (23,898) 6,992 (16,906) 6,992
Total operating expenses 50,401 (3,702) - (762) (23,898) 6,992 (21,370) 29,031
Operating income (loss) (11,543) 3,702 - 762 23,898 (6,992) 21,370 9,827
Investment Income 390 - - - - - - 390
Interest Expense (263) - - - - - - (263)
Other Expense - changes in fair value of related party
payable (2,773) - - - 2,773 (941) 1,832 (941)
Foreign exchange gain (loss) 1,680 - (1,680) - - - (1,680) -
Income (loss) before income taxes (12,509) 3,702 (1,680) 762 26,671 (7,933) 21,522 9,013
I come tax provision 7,449 1,329 - 266 1,414 (460) 2,549 9,998
Income Tax Rate (60%) 36% - 35% 5% 6% 12% 111%
Net Loss (19,958)$ 2,373$ (1,680)$ 496$ 25,257$ (7,473)$ 18,973$ (985)$
Net loss per share - Diluted (0.48)$ 0.06$ (0.04)$ 0.01$ 0.60$ (0.18)$ 0.46$ (0.02)$
Weighted average number of shares outstanding - Diluted 41,241 41,241 41,241 41,241 41,241 41,241 41,241 41,241
Adjustments
Exclude
November 2016 25
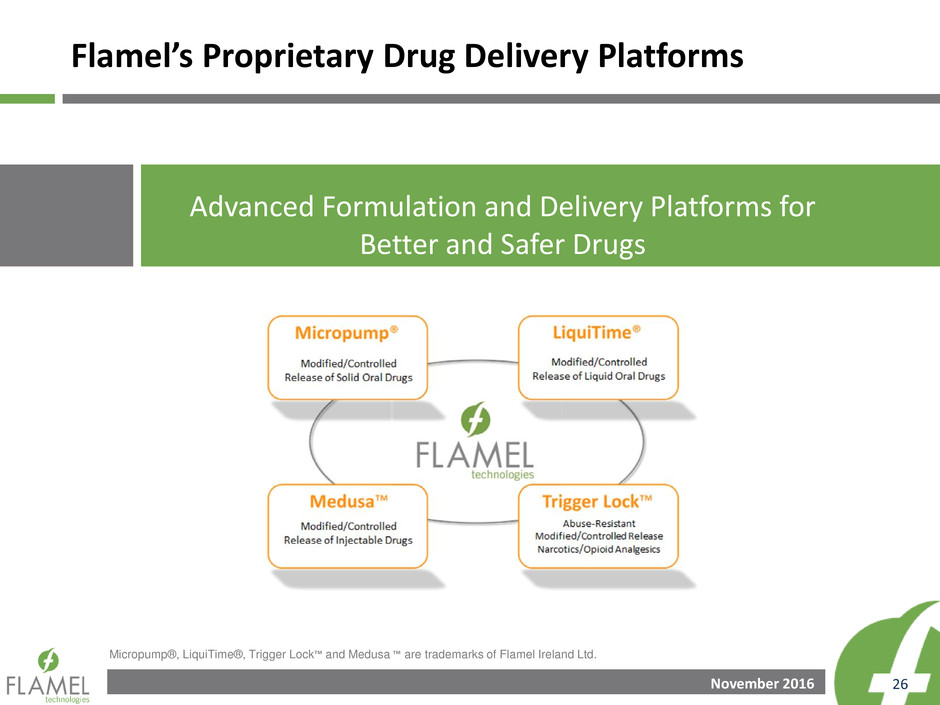
GAAP to Non-GAAP Reconciliations
Three Months Ended September 30, 2015:
(in thousands - USD$) Include
GAAP
Intangible asset
amortization
Foreign
exchange
(gain)/loss
Contingent
related party
payable
fair value
remeasurements
Contingent
related party
payable
paid/accrued
Total
Adjustments NON-GAAP
Product sales and services 47,313$ -$ -$ -$ -$ -$ 47,313$
License and research revenue - - - - - - -
Total revenue 47,313 - - - - - 47,313
Cost of products and services sold 2,087 - - - - - 2,087
Research and development expenses 7,221 - - - - - 7,221
Selling, general and administrative expenses 4,568 - - - - - 4,568
Intangible asset amortization 3,141 (3,141) - - - (3,141) -
Changes in fair value of related party contingent
consideration 44,782 - - (44,782) 9,027 (35,755) 9,027
Total operating expenses 61,799 (3,141) - (44,782) 9,027 (38,896) 22,903
Operating income (loss) (14,486) 3,141 - 44,782 (9,027) 38,896 24,410
Investment Income 197 - - - - - 197
Interest Expense - - - - - - -
Other Expense - changes in fair value of related party
payable (6,644) - - 6,644 (1,211) 5,433 (1,211)
Foreign exchange gain (loss) 160 - (160) - - (160) -
Income (loss) before income taxes (20,773) 3,141 (160) 51,426 (10,237) 44,170 23,397
I come tax provision 7,302 1,099 (48) 2,325 (424) 2,953 10,255
Income Tax Rate (35%) 35% 30% 5% 4% 7% 44%
Net Loss (28,075)$ 2,042$ (112)$ 49,101$ (9,814)$ 41,217$ 13,142$
Net loss per share - Diluted (0.69)$ 0.05$ -$ 1.20$ (0.24)$ 1.01$ 0.32$
Weighted average number of shares outstanding - Diluted 40,625 40,625 40,625 40,625 40,625 40,625 40,625
Adjustments
Exclude
November 2016 26
Advanced Formulation and Delivery Platforms for
Better and Safer Drugs
Micropump®, LiquiTime®, Trigger Lock™ and Medusa ™ are trademarks of Flamel Ireland Ltd.
Flamel’s Proprietary Drug Delivery Platforms
November 2016 27
Micropump®
Drug Delivery Platform
Modified/Controlled Release
of Solid Oral Drugs
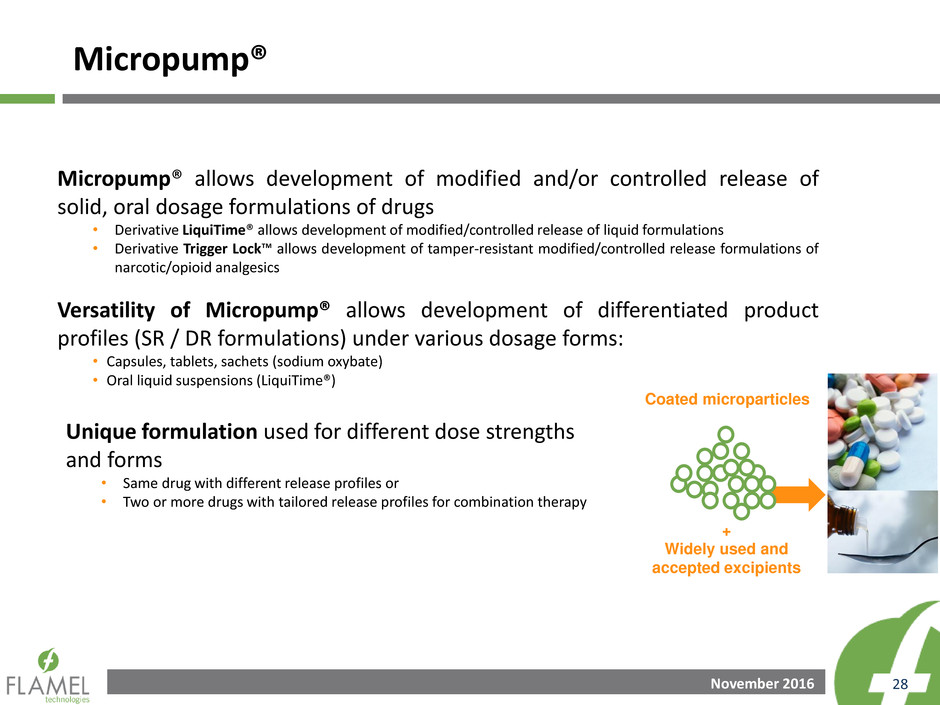
November 2016 28
Micropump®
Micropump® allows development of modified and/or controlled release of
solid, oral dosage formulations of drugs
• Derivative LiquiTime® allows development of modified/controlled release of liquid formulations
• Derivative Trigger Lock™ allows development of tamper-resistant modified/controlled release formulations of
narcotic/opioid analgesics
Versatility of Micropump® allows development of differentiated product
profiles (SR / DR formulations) under various dosage forms:
• Capsules, tablets, sachets (sodium oxybate)
• Oral liquid suspensions (LiquiTime®)
+
Widely used and
accepted excipients
Coated microparticles
Unique formulation used for different dose strengths
and forms
• Same drug with different release profiles or
• Two or more drugs with tailored release profiles for combination therapy
November 2016 29
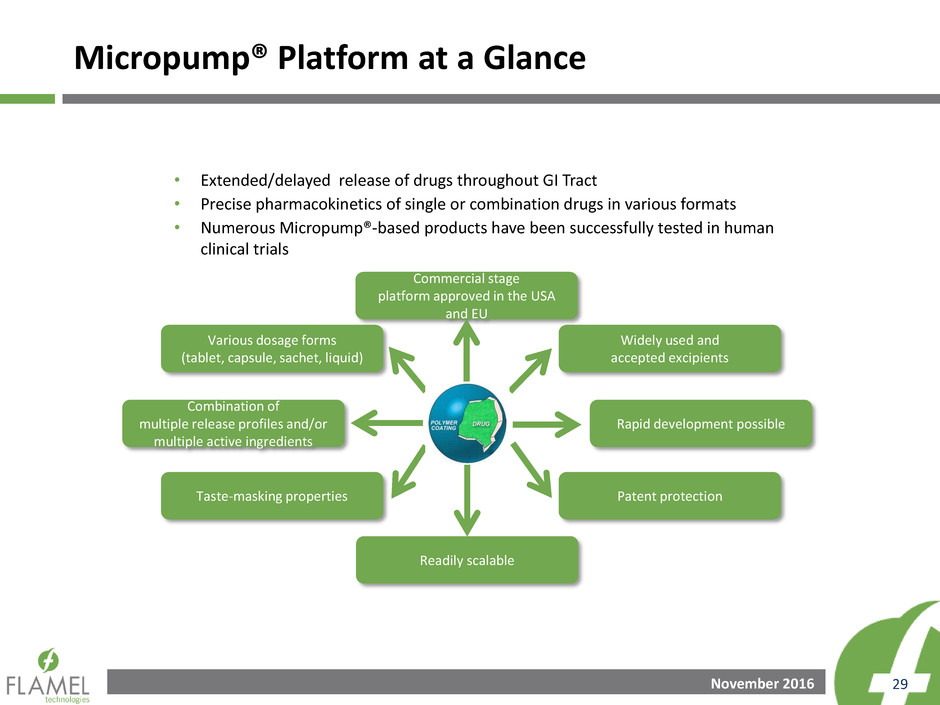
Micropump® Platform at a Glance
• Extended/delayed release of drugs throughout GI Tract
• Precise pharmacokinetics of single or combination drugs in various formats
• Numerous Micropump®-based products have been successfully tested in human
clinical trials
Various dosage forms
(tablet, capsule, sachet, liquid)
Commercial stage
platform approved in the USA
and EU
Widely used and
accepted excipients
Rapid development possible
Combination of
multiple release profiles and/or
multiple active ingredients
Taste-masking properties
Readily scalable
Patent protection
November 2016 30
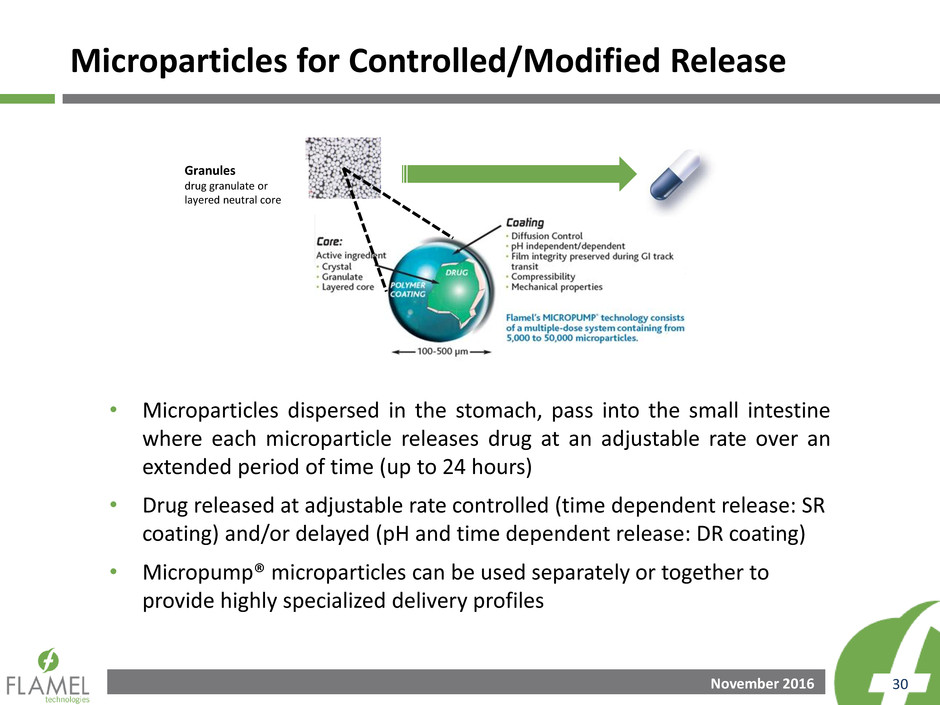
• Microparticles dispersed in the stomach, pass into the small intestine
where each microparticle releases drug at an adjustable rate over an
extended period of time (up to 24 hours)
• Drug released at adjustable rate controlled (time dependent release: SR
coating) and/or delayed (pH and time dependent release: DR coating)
• Micropump® microparticles can be used separately or together to
provide highly specialized delivery profiles
Microparticles for Controlled/Modified Release
Granules
drug granulate or
layered neutral core
November 2016 31
LiquiTime®
Drug Delivery Platform
Modified/Controlled Release
of Liquid Oral Drugs
November 2016 32
LiquiTime®
• Allows development of modified/controlled release liquid formulations for
patients having issues swallowing tablets/capsules
• Not limited to working solely with ionic drugs as with resin-complex based
technologies
• Readily scalable to commercial quantities
• Easy to swallow, good mouth feel, taste masked - dose flexibility while
maintaining accuracy and safety
Pediatric1
• US population younger than 18 years old =
76 million in 2019
• 75% of households with children under 12
purchased an OTC pain reliever over the past 12
months
• Sales of OTC pediatric product in the US
= $1.6 B in 2013 ($1.9 B estimated in 2018)
Geriatric
• 810 million people > 60 years in 2012
2 billion expected in 20502
• In 2010 approximately 45-50% of the
prescriptions were written for people aged 60
and above and one in three patients took at least
5 drugs or more on a daily basis in the United
States3
Applicable to:
1 “OTC Pediatrics – US” (March 2014, Mintel) 2 World
Health Organization
3 “Geriatric Medicine Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013 – 2019”
(Transparency Market Research)
November 2016 33
Trigger Lock™
Platform
Abuse Deterrent Extended Release
of Opioids
November 2016 34
• Drug loaded Micropump® microparticles: Sustained Release (SR) microparticles
individually polymer coated which are resistant to crushing
• Viscosifying ingredient(s): To prevent abuse by injection after extraction in a
small volume of solvent
• Quenching ingredient(s): To prevent extraction in large volumes of liquid
(forming a complex with the opioid preventing its solubilization in
aqueous/alcoholic media)
Trigger Lock™ for Abuse Deterrence
Each microparticle retains its polymer coating
Trigger Lock™ is virtually impervious to
crushing
November 2016 35
Medusa™
Drug Delivery Platform
Modified/Controlled Release
of Injectable Drugs
November 2016 36
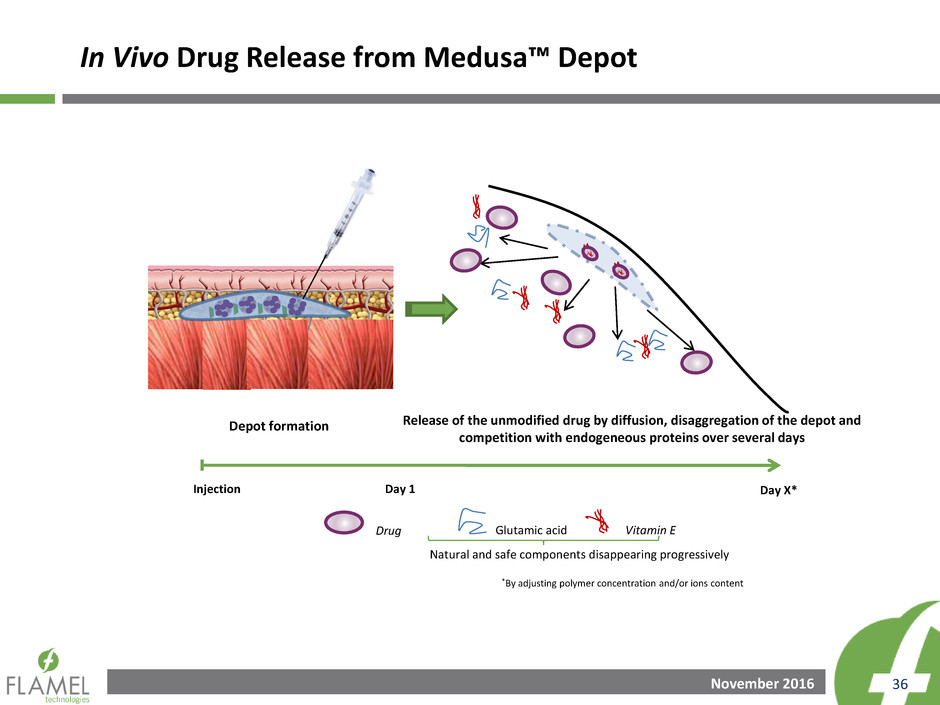
In Vivo Drug Release from Medusa™ Depot
Release of the unmodified drug by diffusion, disaggregation of the depot and
competition with endogeneous proteins over several days
Depot formation
Drug Vitamin E Glutamic acid
Day 1 Injection Day X*
Natural and safe components disappearing progressively
*By adjusting polymer concentration and/or ions content
