Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - LA JOLLA PHARMACEUTICAL CO | pr07sep16b.htm |
| EX-99.1 - EXHIBIT 99.1 - LA JOLLA PHARMACEUTICAL CO | pr07sep16a.htm |
| 8-K - 8-K - LA JOLLA PHARMACEUTICAL CO | a8-k07sep2016.htm |

LJPC-401 Phase 1 Results and
Development Update
September 7, 2016

Forward-Looking Statements
These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation
Reform Act of 1995. These statements may be identified by the use of forward-looking terminology such
as "anticipate", "believe", "continue", "could", "estimate", "expect", "intend", "may", "might", "plan",
"potential", "predict", "should" or "will" and include statements regarding La Jolla’s product candidates
and clinical trial progress and results. These forward-looking statements are based on our current
expectations and beliefs, speak only as of the date of this presentation and involve risks and
uncertainties, many of which are outside of our control, that can cause actual results to differ materially
from those anticipated in the forward-looking statements. Potential risks and uncertainties include, but
are not limited to: our ability to commence and complete clinical studies within projected time periods,
the degree to which initial clinical study results are indicative of expected results for future studies,
anticipated regulatory and patent exclusivity periods, the ability to manufacture clinical or commercial
products successfully, the ability to resolve regulatory issues, the ability to out-license programs,
estimated market sizes and anticipated pricing levels for drug candidates, anticipated rates of physician
adoption, if our drug candidates are approved, the ability to successfully develop our product
candidates, including the results of ongoing and future clinical trials (including product safety issues and
efficacy results), the ability to successfully prosecute patents and whether such patents will confer
protection for our product candidates, and the expected duration of the Company’s operating runway
based on current cash resources. Further information regarding these and other risks that could affect
our future results of operations are included in La Jolla’s most recently filed Annual Report on Form 10-
K and subsequent Quarterly Reports on Form 10-Q under the caption “Risk Factors,” as filed with the
U.S. Securities and Exchange Commission at www.sec.gov. We disclaim any intent to update any
forward-looking statements to reflect actual events that occur after the date of this presentation.
2

LJPC-401: Overview
• LJPC-401 is a novel formulation of hepcidin, a naturally occurring
regulator of iron absorption and distribution
• Primary iron overload
Hereditary hemochromatosis (HH) is characterized by a genetic deficiency of
hepcidin resulting in excessive iron accumulation
– Most common genetic disease in Caucasians
– Causes liver cirrhosis, liver cancer, heart disease and/or failure, and diabetes
• Secondary iron overload
Patients with thalassemia (including beta thalassemia), sickle cell disease (SCD)
and myelodysplasia (MDS) have physiologically low hepcidin levels and are
treated with blood transfusions, resulting in acquired iron overload
• LJPC-401 has been shown to be effective at reducing serum iron levels in
preclinical testing
• Scalable manufacturing process capable of producing a pure, properly
folded, stable hepcidin formulation developed
3

PRIMARY ENDPOINT
Safety and tolerability
via review of: Treatment
Emergent Adverse Events
(TEAEs), changes in clinical
lab values, ECGs, vital sign
and physical exam data
SECONDARY ENDPOINT
Serum iron
LJPC-401: Phase 1 Study Design
• Population: Adult
patients at risk of iron
overload (e.g., HH,
thalassemia, SCD)
• Design: Phase 1, open-
label, dose-escalation,
study
• Study Duration: Single
SC dose, 7-day
observation
Escalating dose levels
3 to 6 subjects at each
dose level
4
Data Monitoring Committee (DMC)
made dose-escalation decisions
LJPC-401: Phase 1 Results Overview
• Fifteen patients dosed at escalating dose levels from 1 mg to 20 mg
Patient subtypes treated: HH = 10; SCD = 3; and thalassemia = 2
• Safety observations
No dose-limiting toxicities at any dose level
1 SAE at 1 mg dose level unrelated to study drug
– Hospitalization for acute sickle cell crisis; fully resolved
9 injection-site reactions – all were mild or moderate in severity, self-limiting,
and fully resolved
No significant changes in serum chemistries or hematology other than serum
iron parameters
• Pharmacodynamic results
Dose-dependent, statistically significant reduction in serum iron (p=0.008)
Maximum serum iron reduction observed at 8 hours post-dose
Durable effect observed through last observation on Day 7
5
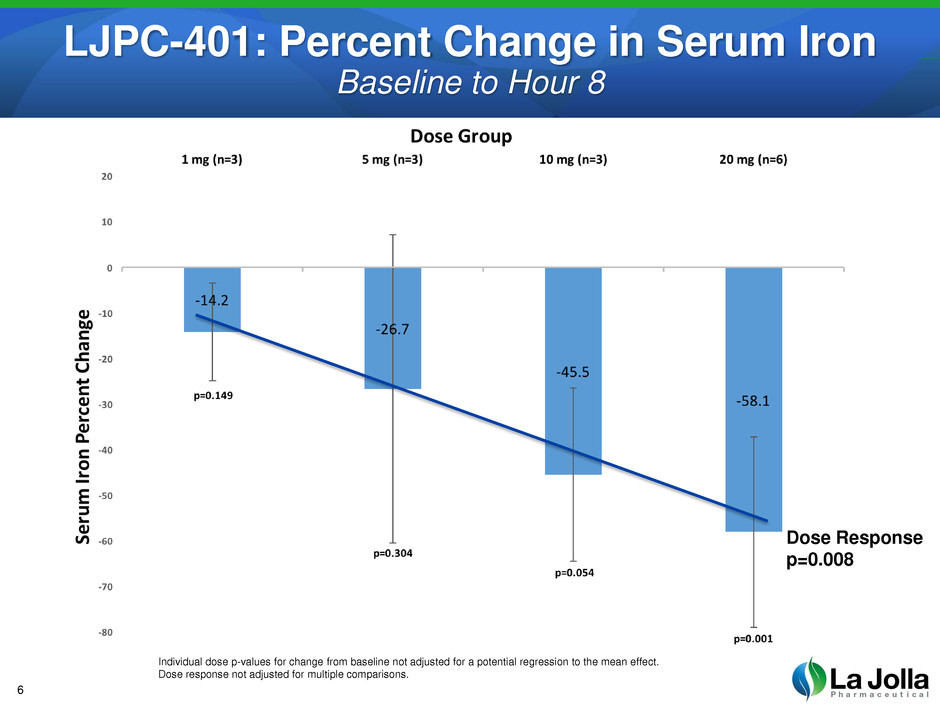
Dose Response
p=0.008
Individual dose p-values for change from baseline not adjusted for a potential regression to the mean effect.
Dose response not adjusted for multiple comparisons.
LJPC-401: Percent Change in Serum Iron
Baseline to Hour 8
6

Nominal Time from Treatment (hours)
Pe
rce
nt
C
ha
ng
e
fro
m
Ba
se
lin
e
(%
)
0 8 24 48 168
-100
-75
-50
-25
0
25
Serum Iron: Percent Change from Baseline
Change from baseline through day 7: -21%
LJPC-401: Percent Change in Serum Iron
Baseline through Day 7 for 20 mg Dose
7
LJPC-401: Phase 1 Results Summary
• Fifteen patients received a single dose ranging from 1 mg to 20 mg
Patient subtypes treated: HH = 10; SCD = 3; and thalassemia = 2
• Well tolerated with no dose-limiting toxicities
Mild to moderate, transient and self-limiting injection-site reactions
• Profound and durable reduction in serum iron observed
Statistically significant dose response (p=0.008)
A single 20 mg dose resulted in a 58% reduction at hour 8, with levels still not
returning to baseline through day 7 (21% reduction)
Iron effect consistent with that observed in preclinical models
8

• Agreement reached with European Medicines Agency (EMA) on pivotal
study design
• Randomized, controlled, multi-center study in beta thalassemia patients
suffering from iron overload
A major unmet medical need in an orphan patient population
• Primary endpoint is a clinically relevant measurement directly related to
iron overload
• Plan to initiate study mid-2017
LJPC-401: Update on Registration Plan
Agreement Reached on Pivotal Study
9
