Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - LA JOLLA PHARMACEUTICAL CO | exhibit321fy201910-k.htm |
| EX-31.1 - EXHIBIT 31.1 - LA JOLLA PHARMACEUTICAL CO | exhibit311fy201910-k.htm |
| EX-23.1 - EXHIBIT 23.1 - LA JOLLA PHARMACEUTICAL CO | exhibit231fy2019.htm |
| EX-21.1 - EXHIBIT 21.1 - LA JOLLA PHARMACEUTICAL CO | exhibit211fy2019.htm |
| EX-10.5 - EXHIBIT 10.5 - LA JOLLA PHARMACEUTICAL CO | exhibit105fy201910-k.htm |
| EX-10.1 - EXHIBIT 10.1 - LA JOLLA PHARMACEUTICAL CO | exhibit101fy201910-k.htm |
| EX-4.2 - EXHIBIT 4.2 - LA JOLLA PHARMACEUTICAL CO | exhibit42fy201910-k.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2019
OR
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 1-36282
LA JOLLA PHARMACEUTICAL COMPANY
(Exact name of registrant as specified in its charter)
California | 33-0361285 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
4550 Towne Centre Court, San Diego, CA | 92121 | |
(Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (858) 207-4264
Securities registered pursuant to Section 12(b) of the Act: | ||||||
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||
Common Stock, par value $0.0001 per share | LJPC | The Nasdaq Capital Market | ||||
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | o | Accelerated filer | x | |
Non-accelerated filer | o | Smaller reporting company | x | |
Emerging growth company | o | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of voting and non-voting shares of common stock held by non-affiliates of the Company as of June 28, 2019 was approximately $190.2 million, based on the closing price on the Nasdaq Capital Market reported for such date. Shares of common stock held by each officer and director and by each person who is known to own 10% or more of the outstanding shares of common stock have been excluded in that such persons may be deemed to be affiliates of the Company. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 3, 2020, there were 27,215,201 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information required to be disclosed in Part III of this report is incorporated by reference from the registrant’s Definitive Proxy Statement for the 2020 Annual Meeting of Shareholders, which proxy statement is expected to be filed no later than 120 days after the end of the fiscal year covered by this report.
TABLE OF CONTENTS
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of the federal securities laws. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements can be identified by words such as “intends,” “believes,” “anticipates,” “indicates,” “plans,” “expects,” “suggests,” “may,” “should,” “potential,” “designed to,” “will” and similar expressions that predict or indicate future events and trends that do not relate to historical matters. You should not unduly rely on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, some of which are beyond our control. These risks, uncertainties and other factors may cause our actual results, performance or achievements to be materially different from the anticipated future results, performance or achievements expressed or implied by the forward-looking statements.
These forward-looking statements include, but are not limited to, statements regarding:
• | our ability to grow net sales of GIAPREZATM (angiotensin II); |
• | our ability to maintain an effective sales and marketing organization; |
• | the potential market size for GIAPREZA; |
• | our ability to obtain an uninterrupted supply of GIAPREZA from our contract manufacturers; |
• | GIAPREZA’s market exclusivity period as a result of the enforcement of regulatory exclusivity and the validity and enforceability of issued and pending patents covering GIAPREZA; |
• | our ability to obtain U.S. Food and Drug Administration (“FDA”) approval of LJPC-0118 (I.V. artesunate); |
• | our ability to obtain an uninterrupted supply of LJPC-0118 from our contract manufacturers; |
• | our ability to receive a tropical disease Priority Review Voucher (“PRV”) for LJPC-0118; |
• | our ability to hire and retain key employees; |
• | our overall financial performance, including but not limited to net product sales and net cash used in operating activities; and |
• | our capital requirements and our potential need for, and ability to obtain, additional financing. |
These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results to differ materially from the anticipated future results, performance or achievements expressed or implied by any forward-looking statements, including the factors described under the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” You should evaluate all forward-looking statements made in this Annual Report on Form 10-K, including the documents we incorporate by reference, in the context of these risks, uncertainties and other factors.
We caution you that the risks, uncertainties and other factors referred to above may not contain all of the risks, uncertainties and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will affect us or our business in the way expected. All forward-looking statements in this Annual Report on Form 10-K apply only as of the date made and are expressly qualified in their entirety by the cautionary statements included in this Annual Report on Form 10-K. We undertake no obligation to publicly update or revise any forward-looking statements to reflect subsequent events or circumstances.
1
PART I
In this Annual Report on Form 10-K, all references to “we,” “our,” “us,” “La Jolla” and “the Company” refer to La Jolla Pharmaceutical Company, a California corporation, and our subsidiaries, including La Jolla Pharma, LLC, on a consolidated basis.
Item 1. Business
Overview
La Jolla Pharmaceutical Company is dedicated to the development and commercialization of innovative therapies that improve outcomes in patients suffering from life-threatening diseases. In December 2017, GIAPREZATM (angiotensin II) was approved by the U.S. Food and Drug Administration (“FDA”) as a vasoconstrictor indicated to increase blood pressure in adults with septic or other distributive shock. GIAPREZA U.S. net sales were $23.1 million in 2019 compared to $10.1 million in 2018, an increase of 129%. In August 2019, GIAPREZA was approved by the European Commission (“EC”) for the treatment of refractory hypotension in adults with septic or other distributive shock who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies. LJPC-0118 (I.V. artesunate) is La Jolla’s investigational product for the treatment of severe malaria.
Product Portfolio
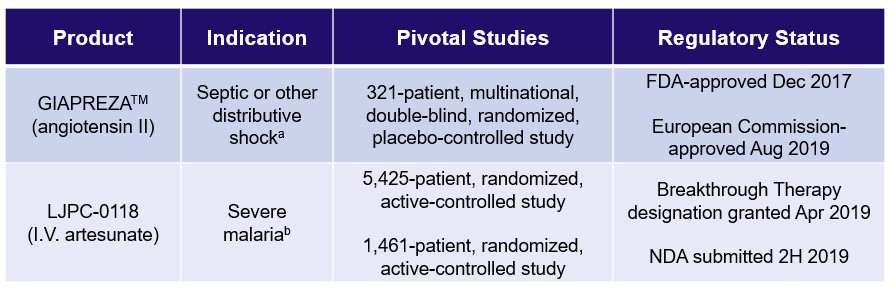
a U.S.: GIAPREZA is a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. European Union: GIAPREZA is indicated for the treatment of refractory hypotension in adults with septic or other distributive shock who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies. |
b This is a proposed indication. LJPC-0118 (I.V. artesunate) is investigational and not approved by any regulatory authority. |
GIAPREZATM (angiotensin II)
In December 2017, GIAPREZATM (angiotensin II) was approved by the FDA as a vasoconstrictor indicated to increase blood pressure in adults with septic or other distributive shock. GIAPREZA U.S. net sales were $23.1 million in 2019 compared to $10.1 million in 2018, an increase of 129%. In August 2019, GIAPREZA was approved by the EC for the treatment of refractory hypotension in adults with septic or other distributive shock who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies. GIAPREZA mimics the body’s endogenous angiotensin II peptide, which is central to the renin-angiotensin-aldosterone system, which in turn regulates blood pressure.
2
Distributive shock is the most common form of shock, as shown in the figure below.
Types of Shocka
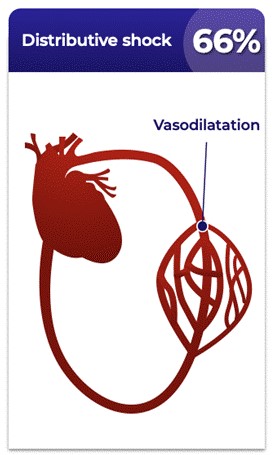
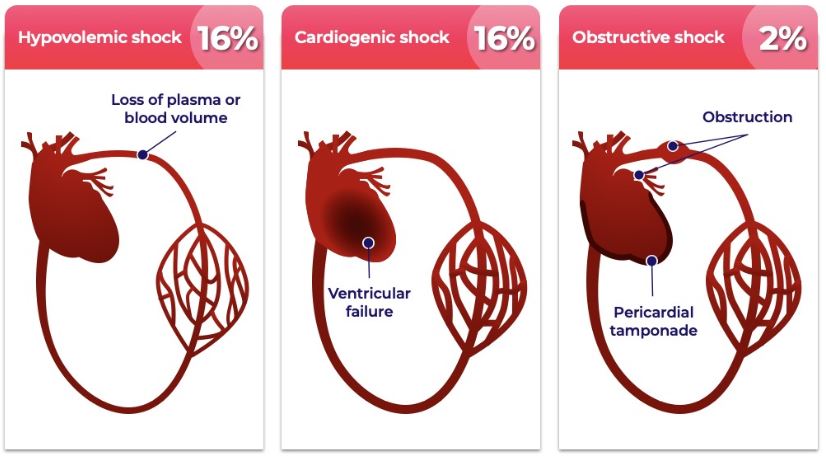
a Vincent et al, New England Journal of Medicine 2013; 369(18):1726−1734 |
3
Distributive shock is a leading cause of death in hospitalized patients, and shock affects one-third of patients in the intensive care unit (“ICU”) (Sakr et al, Critical Care Medicine 2006; 34:589−597). The mortality rate of distributive shock exceeds that of most acute conditions requiring hospitalization, as shown in the figure below.
Mortality Rate
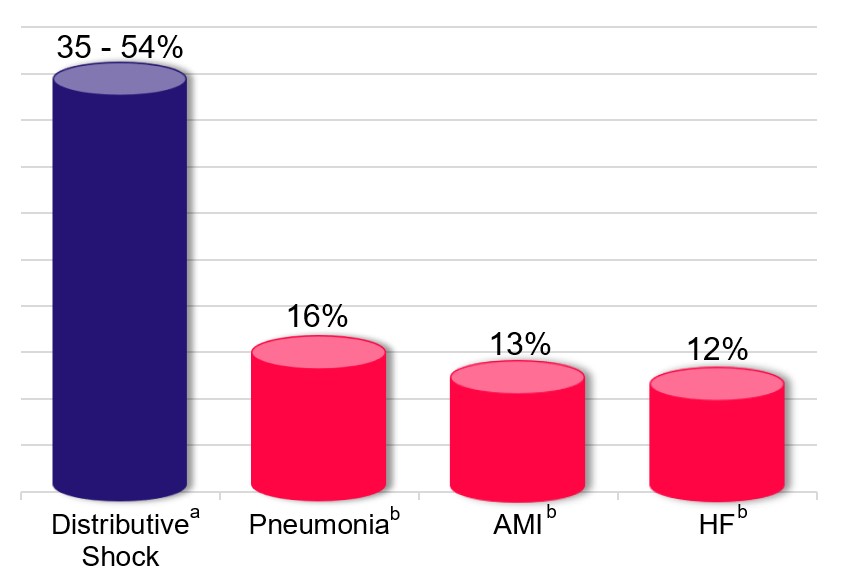
AMI=acute myocardial infraction; HF=heart failure a Based on the 28-day mortality rates of: (i) 35% from the vasopressin arm of Russell et al, New England Journal of Medicine 2008; 358:877-87; (ii) 49% from the norepinephrine arm of De Backer et al, New England Journal of Medicine 2010; 362:779-89; and (iii) 54% from the placebo arm (high-dose norepinephrine or equivalent) of Khanna et al, New England Journal of Medicine 2017; 377:419-430 b 30-day mortality rate from Medicare.gov |
4
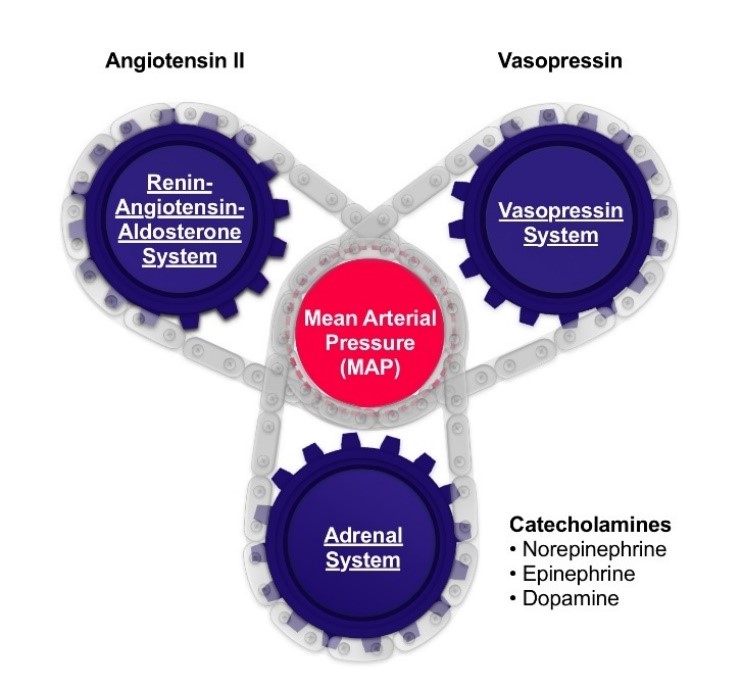
The renin-angiotensin-aldosterone system is one of three systems that work in harmony to regulate blood pressure, as shown in the figure below. GIAPREZA regulates blood pressure through the renin-angiotensin-aldosterone system. Other therapeutic options regulate blood pressure through the adrenal system and vasopressin system.
In Healthy Individuals, Three Systems Work in Harmony to Regulate Blood Pressure
5
Angiotensin II is a powerful vasoconstrictor, acting on blood vessels to cause vasoconstriction and stimulating the release of aldosterone and vasopressin, which increase blood volume and blood pressure, as shown in the figure below.
Renin-Angiotensin-Aldosterone Systema,b,c
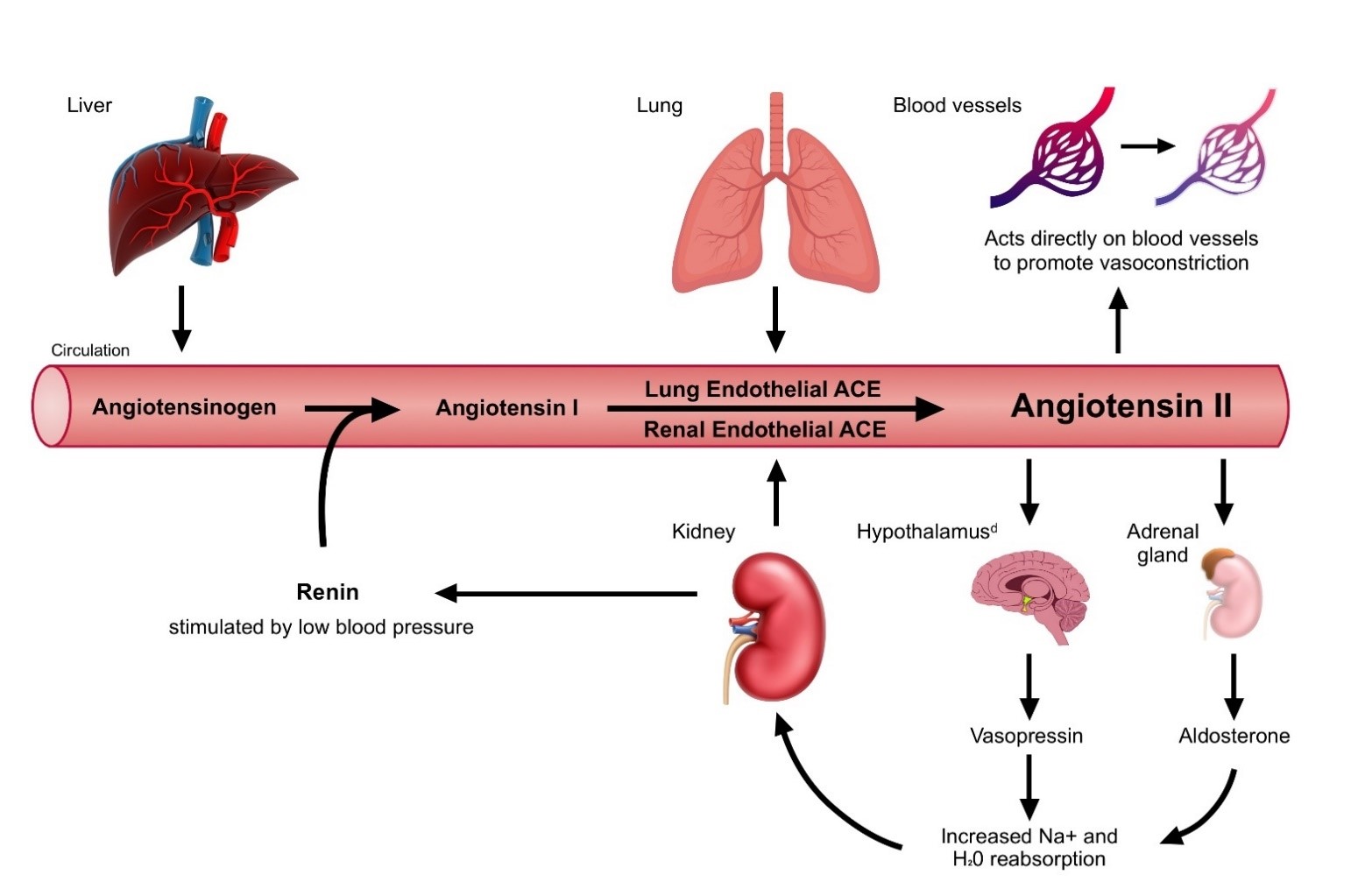
ACE=angiotensin-converting enzyme a Image adapted from Chow et al, Anesthesia & Analgesia Practice 2018; 11(7):175−180 b Hendry et al, Nursing Standard 2012; 27(11):35−40 c Harrison-Bernard, Advances in Physiology Education 2009; 33: 270-274 d Treschan et al, Anesthesiology 2006; 105:599−612 |
6
Annually in the U.S., approximately 100,000−150,000 patients fail to respond to current vasopressor options, as shown in the figure below.
Response to Current Vasopressor Options
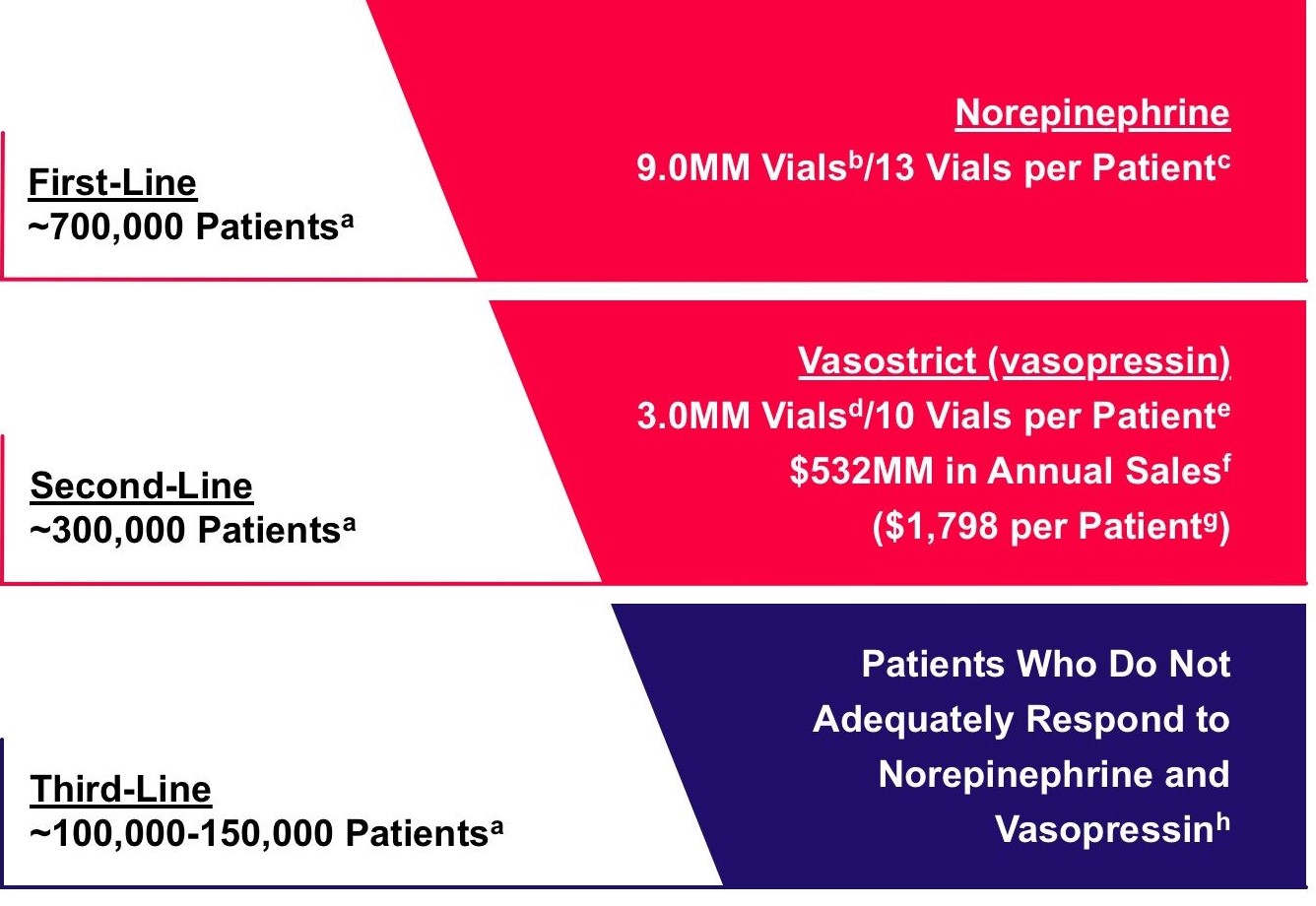
a Annually in the U.S. b Year ended December 31, 2019 per Symphony Health Solutions c Estimate based on Russell et al, New England Journal of Medicine 2008; 358:877−87 and Asfar et al, New England Journal of Medicine 2014; 370:1583−93 d Annual sales per Endo International plc SEC filings, divided by price per vial per Wolters Kluwer PriceRx e Estimate based on Dunser et al, Circulation 2003; 107:2313−2319 and Gordon et al, Critical Care Medicine 2014; 42(6):1325−1333 f Year ended December 31, 2019 per Endo International plc SEC filings g $179.79 per vial per Wolters Kluwer PriceRx, multiplied by 10 vials per patient h Estimate based on: 35.4% 28-day mortality rate in vasopressin arm of Russell et al, New England Journal of Medicine 2008; 358:877-87; 48.5% 28-day mortality rate in norepinephrine arm of De Backer et al, New England Journal of Medicine 2010; 362:779-789; and 54.6% non-responder rate on vasopressin from Sacha et al, Annals of Intensive Care 2018; 8:35 |
7
Angiotensin II for the Treatment of High-Output Shock (“ATHOS-3”)
GIAPREZA was approved by the FDA and the EC based on the results of ATHOS-3, which were published in the New England Journal of Medicine in August 2017. Angiotensin II for the Treatment of High-Output Shock (“ATHOS-3”) was a multinational, randomized, double-blind study in which 321 adults with septic or other distributive shock who remained hypotensive despite fluid and vasopressor therapy received either GIAPREZA or placebo, both in addition to background standard of care therapy. The primary endpoint was mean arterial pressure (“MAP”) response, defined as a MAP of 75 mm Hg or higher or an increase in MAP from baseline of at least 10 mm Hg without an increase in the dose of background vasopressors at Hour 3. The ATHOS-3 study design is shown in the figure below.
ATHOS-3 Study Designa
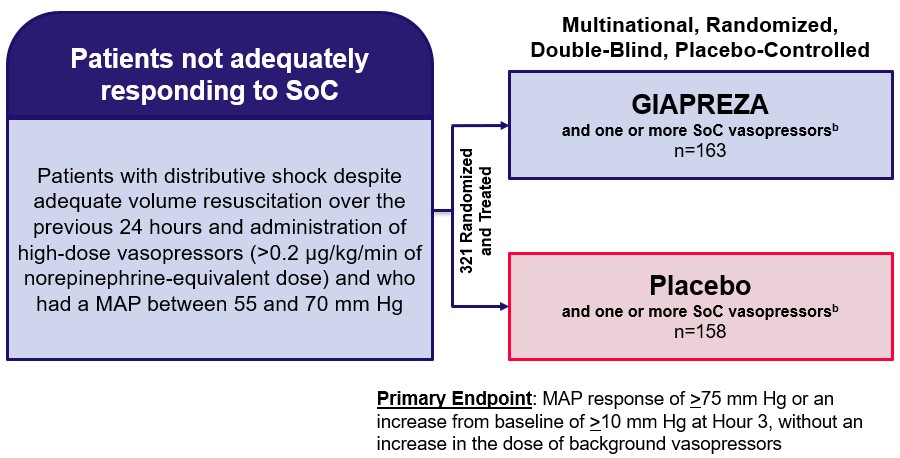
MAP=mean arterial pressure; SoC=standard of care a Khanna et al, New England Journal of Medicine 2017; 377:419-430 b SoC vasopressors included norepinephrine, epinephrine, dopamine and vasopressin |
8
GIAPREZA significantly improved blood pressure response. Specifically, the primary endpoint was achieved by 70% of GIAPREZA-treated patients compared to 23% of placebo-treated patients (P-value <0.0001).
Primary Endpoint: Mean Arterial Pressure (“MAP”) Responsea,b
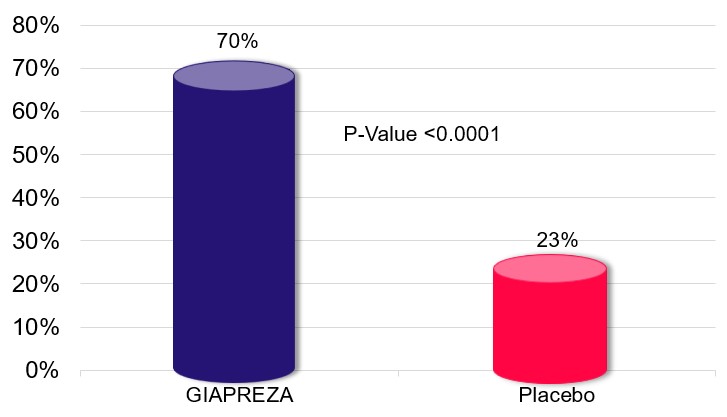
a GIAPREZA FDA prescribing information b MAP response of >75 mm Hg or an increase from baseline of >10 mm Hg at Hour 3, without an increase in the dose of background vasopressors. |
9
In addition, a positive survival trend was observed. Mortality through Day 28 was 46% on GIAPREZA and 54% on placebo (hazard ratio 0.78; 95% confidence interval 0.57−1.07).
Positive Survival Trend Observeda,b
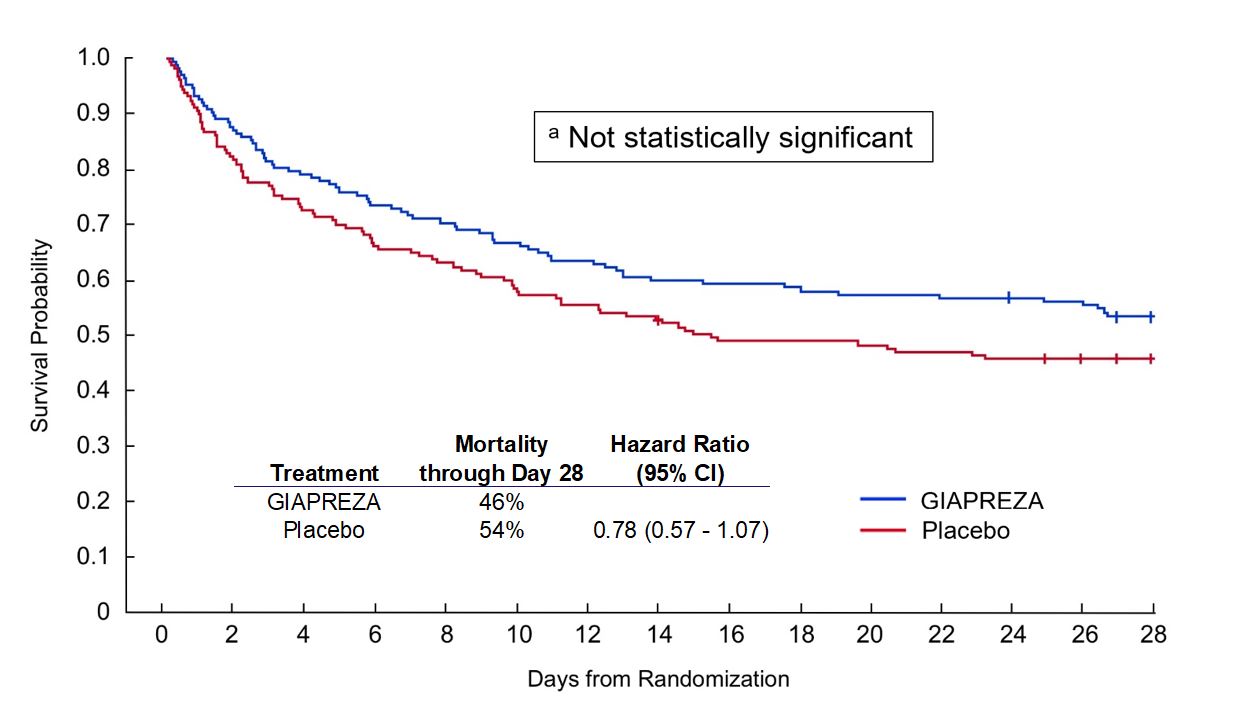
b Curves from Khanna et al, New England Journal of Medicine 2017; 377:419-430; Mortality through Day 28 and Hazard Ratio (95% CI) from GIAPREZA FDA prescribing information |
The most common adverse reactions that were reported in greater than 10% of GIAPREZA-treated patients were thromboembolic events. Adverse reactions occurring in ≥4% of patients treated with GIAPREZA and ≥1.5% more often than in placebo-treated patients are shown in the following table.
Adverse Reactions Occurring in ≥4% of Patients Treated with GIAPREZA and ≥1.5% More Often than
in Placebo-treated Patientsa
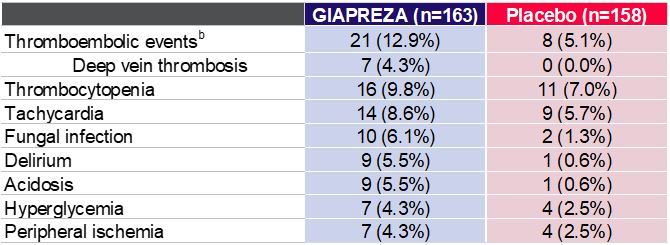
a GIAPREZA FDA prescribing information b Including arterial and venous thrombotic events |
10
The percentage of patients experiencing ≥1 adverse event, the percentage of patients experiencing ≥1 serious adverse event and the percentage of patients discontinuing treatment due to an adverse event are shown in the following table.
Percentage of Patients Experiencing ≥1 Adverse Event, ≥1 Serious Adverse Event and Discontinuing Treatment Due to an Adverse Eventa
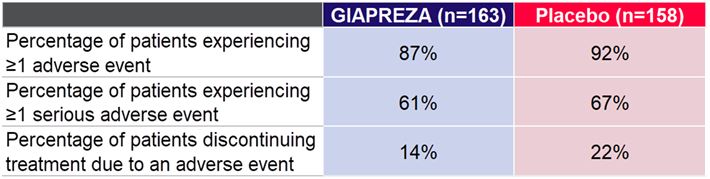
a Khanna et al, New England Journal of Medicine 2017; 377:419-430 |
LJPC-0118 (I.V. artesunate)
LJPC-0118 (I.V. artesunate) is La Jolla’s investigational product for the treatment of severe malaria. The active pharmaceutical ingredient in LJPC-0118, artesunate, was compared to quinine in patients with severe falciparum malaria infection in two randomized, active-controlled, clinical studies. In both studies, in-hospital mortality in the artesunate group was statistically significantly lower than in-hospital mortality in the quinine group. The FDA granted Breakthrough Therapy designation and Orphan Drug designation for LJPC-0118 for the treatment of malaria in April 2019 and July 2019, respectively. La Jolla filed a New Drug Application (“NDA”) with the FDA for LJPC-0118 for the treatment of severe malaria in the second half of 2019.
Severe malaria is a serious and sometimes fatal disease caused by a parasite that commonly infects a certain type of mosquito. Symptoms include: fever, chills, sweating, hypoglycemia and shock. Severe malaria is often complicated by central nervous system infections that may lead to delirium, which may progress to coma. Infections usually occur a few weeks after being bitten. In 2013, an estimated 2 million cases of severe malaria occurred worldwide (World Health Organization), and, in 2018, an estimated 405,000 people died from malaria worldwide (World Health Organization). La Jolla may be eligible to receive a tropical disease Priority Review Voucher (“PRV”) for LJPC-0118, as malaria is defined as a disease qualifying for a tropical disease PRV under Section 524 of the U.S. Federal Food, Drug, and Cosmetic Act (“FDCA”).
11
Two pivotal, randomized, active-controlled studies compared artesunate to quinine in the treatment of severe malaria. 1,461 patients were randomized in the South East Asian Quinine Artesunate Malaria Trial (“SEAQUAMAT”) (Dondorp et al, Lancet 2005; 366: 717-25), and 5,425 patients were randomized in the African Quinine Artesunate Malaria Trial (“AQUAMAT”) (Dondorp et al, Lancet 2010; 376: 1647-57). In-hospital mortality was the primary endpoint in both studies. In both studies, in-hospital mortality in the artesunate group was statistically significantly lower than in-hospital mortality in the quinine group, as shown in the figures below.
Primary Endpoint: In-hospital Mortality
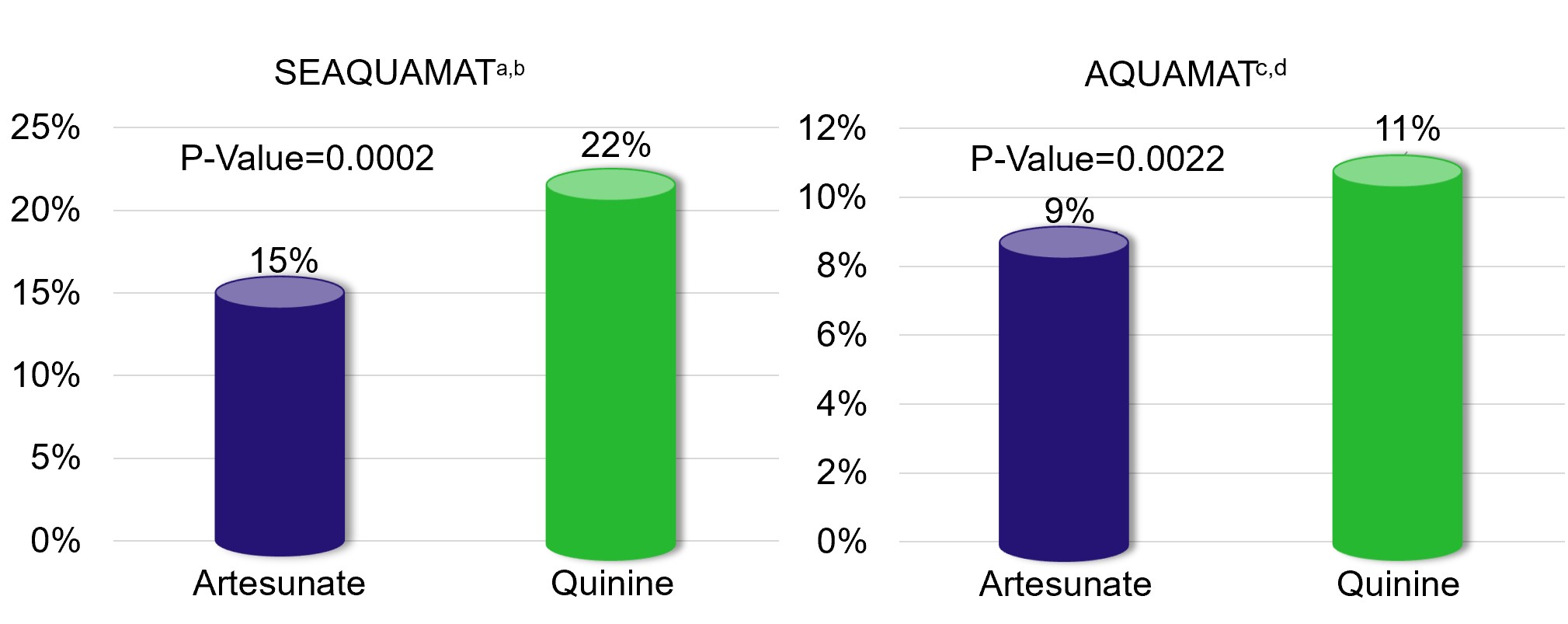
a Dondorp et al, Lancet 2005; 366: 717-25 b South East Asian Quinine Artesunate Malaria Trial c Dondorp et al, Lancet 2010; 376: 1647-57 d African Quinine Artesunate Malaria Trial |
According to the U.S. Center for Disease Control (“CDC”), I.V. artesunate is now the first-line drug for the treatment of severe malaria in the U.S. following the discontinuation of I.V. quinidine and is the recommended World Health Organization first-line treatment for severe malaria, but is neither FDA-approved nor commercially available in the U.S. I.V. artesunate is available from the CDC under an expanded-access investigational new drug (“IND”) protocol for patients with severe malaria.
LJPC-401 (synthetic human hepcidin)
LJPC-401 (synthetic human hepcidin) is La Jolla’s investigational product for the potential treatment of conditions characterized by iron overload. In November 2019, La Jolla announced mixed results from two Phase 2 studies of LJPC-401. Based on these results, the development of LJPC-401 has been de-prioritized, and La Jolla does not expect to invest significant additional resources in this program.
Sales and Marketing Organization
La Jolla employs an experienced sales and marketing team dedicated to the commercialization of GIAPREZA. As of February 24, 2020, this team consists of 40 professionals, including 28 critical care specialists and 3 health systems account directors.
Customers
In 2019, 444 hospitals in the U.S. purchased GIAPREZA. Hospitals purchase our products through a network of specialty and wholesale distributors. We do not believe that the loss of one of these distributors would significantly impact our ability to distribute GIAPREZA, as we expect that sales volume would be absorbed by the remaining distributors. Due to the relatively short lead-time required to fill orders for GIAPREZA, backlog is not material to our business.
12
Competition
Catecholamines (primarily norepinephrine), which are available as generics and inexpensive, are typically used first line to treat distributive shock, while Vasostrict® (Endo International plc) is typically used second line. In the randomized, Phase 3 study ATHOS-3, GIAPREZA demonstrated clinical benefit in patients who were not adequately responding to available vasopressors, including catecholamines and Vasostrict. GIAPREZA’s principal competition as a treatment in patients not adequately responding to available vasopressors is the use of these same vasopressors, particularly norepinephrine, at increased doses. If we are unable to successfully change treatment practices, the commercial prospects for GIAPREZA will be limited.
Manufacturing
We do not currently own or operate manufacturing facilities for the production of GIAPREZA or LJPC-0118. We rely on third-party manufacturers to produce GIAPREZA and LJPC-0118 and expect to continue to do so to meet our development and commercial needs.
In all of our manufacturing agreements, we require that third-party contract manufacturers produce active pharmaceutical ingredients (“APIs”) and drug products in accordance with the FDA’s current Good Manufacturing Practices (“cGMPs”) and all other applicable laws and regulations. We maintain confidentiality agreements with potential and existing manufacturers in order to protect our proprietary rights related to GIAPREZA and LJPC-0118.
The long-term commercial success of GIAPREZA will depend in part on the ability of our contract manufacturers to supply cGMP-compliant API and drug product without interruption.
Regulatory Exclusivity
GIAPREZA is a New Chemical Entity (“NCE”) approved by the FDA. In the U.S., NCEs approved by the FDA are eligible for market exclusivity under the FDCA, which can prevent the approval of generic versions of the NCE for 5 to 7.5 years from the date of the initial approval of the NCE. Specifically, the FDCA provides a 5-year period of marketing exclusivity within the U.S. to the applicant that gains approval of an NDA for an NCE. A drug is an NCE if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an Abbreviated New Drug Application (“ANDA”) or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all of the data required for approval. However, an application may be submitted 4 years after the NDA approval of the NCE if it contains a certification of patent invalidity or non-infringement. This certification will trigger an automatic stay in the approval of any generic competition until the earlier of: (a) 30 months from the certification; or (b) a court ruling of patent invalidity or non-infringement for the relevant patents. In the absence of a court ruling, the 30-month stay will be extended by such amount of time (if any) that is required for 7.5 years to have elapsed from the date of NDA approval of the NCE.
Intellectual Property
Patents and other proprietary rights are important to our business. As of December 31, 2019, we owned or had in-licensed 10 issued U.S. patents, 12 pending U.S. patent applications, 3 issued foreign patents and 45 pending foreign patent applications that cover GIAPREZA. The U.S. patents and patent applications expire between 2029 and 2040, and the foreign patents and patent applications expire between 2034 and 2040. The following table summarizes our issued patents and pending applications for GIAPREZA and our product candidates.
United States | Foreign | |||||||||||
Description | Issued | Pending | Expiration | Issued | Pending | Expiration | ||||||
GIAPREZA | 10 | 12 | 2029−2040 | 3 | 45 | 2034−2040 | ||||||
Other | 19 | 16 | 2022−2040 | 30 | 66 | 2022−2040 | ||||||
We plan to file additional patent applications that, if issued, would provide further protection for GIAPREZA. Although we believe the bases for these patents and patent applications are sound, they are untested, and there is no assurance that they will not be successfully challenged. There can be no assurance that any patent previously
13
issued will be of commercial value, that any patent applications will result in issued patents of commercial value or that our products will not be held to infringe patents held by others.
Material Contracts
In May 2018, we closed a $125.0 million royalty financing agreement (the “Royalty Agreement”) with HealthCare Royalty Partners (“HCR”). Under the terms of the Royalty Agreement, we received $125.0 million in exchange for tiered royalty payments on worldwide net sales of GIAPREZA. HCR is entitled to receive quarterly royalties on worldwide net sales of GIAPREZA beginning April 1, 2018. Quarterly payments to HCR under the Royalty Agreement start at a maximum royalty rate, with step-downs based on the achievement of annual net product sales thresholds. Through December 31, 2021, the royalty rate will be a maximum of 10%. Starting January 1, 2022, the maximum royalty rate may increase by 4% if an agreed-upon, cumulative net product sales threshold has not been met, and, starting January 1, 2024, the maximum royalty rate may increase by an additional 4% if a different agreed-upon, cumulative net product sales threshold has not been met. The Royalty Agreement is subject to maximum aggregate royalty payments to HCR of $225.0 million. The Royalty Agreement expires upon the first to occur of January 1, 2031 or when the maximum aggregate royalty payments have been made. The Royalty Agreement was entered into by our wholly-owned subsidiary, La Jolla Pharma, LLC, and HCR has no recourse under the Royalty Agreement against La Jolla Pharmaceutical Company or any assets other than GIAPREZA.
In December 2014, we entered into a patent license agreement with George Washington University (“GW”), which was amended and restated on March 1, 2016 (the “GW License”) and subsequently assigned to La Jolla Pharma, LLC. Pursuant to the GW License, GW exclusively licensed to us certain intellectual property rights relating to GIAPREZA, including the exclusive rights to certain issued patents and patent applications covering GIAPREZA. Under the GW License, we are obligated to use commercially reasonable efforts to develop, commercialize, market and sell GIAPREZA. We have paid a one-time license initiation fee, annual maintenance fees, an amendment fee, additional payments following the achievement of certain development and regulatory milestones and royalties. As a result of the EC’s approval of GIAPREZA in August 2019, we made a milestone payment to GW in the amount of $0.5 million in the first quarter of 2020. We are obligated to pay a 6% royalty on net sales of GIAPREZA. The patents and patent applications covered by the GW License are expected to expire between 2029 and 2034, and the obligation to pay royalties under this agreement extends through the last-to-expire patent covering GIAPREZA.
Government Regulation
Pharmaceutical products, including GIAPREZA, are subject to extensive government regulation. In the U.S., the FDA regulates pharmaceutical products. FDA regulations govern the testing, research and development activities, manufacturing, quality, storage, advertising, promotion, labeling, sale and distribution of pharmaceutical products. Accordingly, there is a rigorous process for the approval of new drugs and ongoing oversight of marketed products. We may also be subject to foreign regulatory requirements governing clinical studies and drug products if products are tested or marketed abroad. The approval process outside of the U.S. varies from jurisdiction to jurisdiction and the time required may be longer or shorter than that required for FDA approval.
Regulation in the U.S.
The FDA testing and approval process requires substantial time, effort and financial resources. We cannot assure you that any of our product candidates will ever obtain approval. The FDA approval process for new drugs includes, without limitation:
• | preclinical studies; |
• | submission in the U.S. of an IND for clinical studies conducted in the U.S.; |
• | adequate and well-controlled clinical studies to establish safety and efficacy of the product; |
• | review and approval of an NDA in the U.S.; and |
• | inspection of the facilities used in the manufacturing of the drug to assess compliance with the FDA’s cGMP regulations. |
Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain
14
state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain procedural and documentation requirements on us and our third-party manufacturers. Even after regulatory approval is obtained, under certain circumstances, such as later discovery of previously unknown safety risks, the FDA can withdraw approval or subject the drug to additional restrictions.
The FDA closely regulates the marketing and promotion of drugs. Drugs may only be marketed in a manner consistent with their FDA-approved labeling. Approval may be subject to post-marketing surveillance and other record-keeping and reporting obligations. Product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
The failure to comply with FDA’s requirements can result in adverse publicity, warning letters, corrective advertising, restrictions on marketing or manufacturing, refusals to review pending product applications, refusals to permit the import or export of products, seizures, injunctions, and civil and criminal penalties.
Tropical Disease Priority Review Voucher
Under Section 524 of the FDCA, the FDA is authorized to award a tropical disease PRV to sponsors of applications for certain products for the prevention or treatment of certain tropical diseases. A tropical disease PRV may be used by the sponsor that obtains the tropical disease PRV or may be transferred to another sponsor that may use it to obtain Priority Review for a different application. A Priority Review designation means that the FDA’s goal is to take action on an NDA or a Biologics License Application (which need not relate to a tropical disease) within 6 months of its filing (compared to 10 months under standard review), which means that a tropical disease PRV can result in a reduction in FDA review time of up to 4 months. Without a PRV, Priority Review designation is for drugs that, if approved, would be significant improvements in the safety or effectiveness of the treatment or prevention of serious conditions. In order to be eligible for a tropical disease PRV, the application must: (i) be for a tropical disease as defined in Section 524 of the FDCA; (ii) be submitted under Section 505(b)(1) of the FDCA or Section 351 of the Public Health Service Act (“PHSA”); (iii) be for a product that contains no active ingredient that has been approved in any other application submitted under Section 505(b)(1) of the FDCA or Section 351 of the PHSA; and (iv) qualify for Priority Review.
Third-party Payor Coverage and Reimbursement
In the U.S. and most major foreign markets, drugs like GIAPREZA that are administered in the hospital must be purchased by the hospital and generally are not reimbursed by third-party payors. Hospitals instead are reimbursed for patient cases based on patients’ diagnosed conditions under the U.S. Medicare diagnosis-related group (“DRG”) system or other like systems for non-Medicare patients in the U.S. and in most major foreign markets. Adoption of new drugs that are administered in the hospital generally occurs more slowly than adoption of new drugs that are taken on an outpatient basis, which generally are paid for by third-party payors.
U.S. Health Care Fraud and Abuse Laws and Compliance Requirements
We are subject to various federal and state laws targeting fraud and abuse in the health care industry. These laws may impact, among other things, our sales and marketing efforts. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
• | The federal Anti-Kickback Statute, which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, an item or service reimbursable under a federal health care program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value, including for example gifts, cash payments, donations, the furnishing of supplies or equipment, waivers of payment, ownership interests, and providing any item, service or compensation for something other than fair market value. |
• | Federal false claims and civil monetary penalties laws, including the federal civil False Claims Act, which prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent. Although we may not submit claims directly to payors, manufacturers can be held liable under these laws in a variety of ways. These include: providing inaccurate billing or coding information to |
15
customers; improperly promoting a product’s off-label use; violating the federal Anti-Kickback Statute; or misreporting pricing information to government programs.
• | Provisions of the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services. |
• | The federal Physician Payment Sunshine Act requirements, under the Patient Protection and Affordable Care Act (“PPACA”), which require manufacturers of certain drugs and biologics to track and report to U.S. Centers for Medicare & Medicaid Services (“CMS”) payments and other transfers of value they make to U.S. physicians and teaching hospitals as well as physician ownership and investment interests in the manufacturer. |
• | Provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations (“HITECH”), which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information. |
• | Section 1927 of the Social Security Act, which requires that manufacturers of drugs and biological products covered by Medicaid report pricing information to CMS on a monthly and quarterly basis, including the best price available to any customer of the manufacturer, with certain exceptions for government programs, and pay prescription rebates to state Medicaid programs based on a statutory formula derived from reported pricing information. |
• | State law equivalents of each of the above federal laws, such as the recently effective California Consumer Privacy Act, many of which differ from each other in significant ways and may not have the same effect, which complicates our compliance efforts. |
Regulation in Non-U.S. Jurisdictions
In addition to regulations in the U.S., we may be subject to a variety of foreign regulations governing clinical studies and commercial sales and distribution of GIAPREZA or future products. For example, clinical studies conducted in the European Union must be done under a clinical trial application (“CTA”), which is usually supported by an Investigational Medicinal Product Dossier (“IMPD”), and the oversight of ethics committees. If we market GIAPREZA in foreign countries, we also will be subject to foreign regulatory requirements governing marketing approval for pharmaceutical products. The requirements governing the conduct of clinical studies, product approval, pricing and reimbursement vary widely from country to country. Whether or not FDA approval has been obtained, approval of a product by the regulatory authorities of foreign countries must be obtained before marketing the product in those countries. The approval process varies from country to country, and the time required for such approvals may differ substantially from that required for FDA approval. Foreign regulatory approval processes involve many of the risks associated with FDA marketing approval discussed above. There is no assurance that any FDA approval of any of our product candidates will result in similar foreign approvals or vice versa. The process for clinical studies in the European Union and other countries is similar, and studies are heavily scrutinized by the designated ethics committees and regulatory authorities. In addition, foreign regulations may include applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or other transfers of value to health care professionals and entities.
In Europe, the European Union General Data Protection Regulation (2016/679) (“GDPR”) contains provisions specifically directed at the processing of health information. The GDPR provides for potentially significant sanctions and contains extraterritoriality measures intended to bring non-EU companies under the regulation. In addition to the GDPR, individual countries in Europe and elsewhere in the world have enacted similar data privacy legislation. This legislation imposes increased compliance obligations and regulatory risk, including the potential for significant fines for noncompliance.
16
Other Laws and Regulations
We are subject to a variety of financial disclosure and securities trading regulations as a public company in the U.S., including laws relating to the oversight activities of the U.S. Securities and Exchange Commission (“SEC”) and the regulations of the Nasdaq Capital Market, on which our shares of common stock are traded. We are also subject to various laws and regulations relating to safe working conditions, laboratory practices and the experimental use of animals.
Employees
As of February 24, 2020, we employed 91 employees, of which 90 were full time. None of our employees are covered by collective bargaining agreements.
Corporate and Other Information
The Company was incorporated in Delaware in 1989 and reincorporated in California in 2012. Our principal office is located at 4550 Towne Centre Court, San Diego, CA 92121, and our telephone number is (858) 207-4264. Shares of our common stock trade on the Nasdaq Capital Market under the symbol “LJPC.” Our website address is www.ljpc.com. Information contained on or accessible through our website is not a part of this Annual Report on Form 10-K, and the inclusion of our website address in this Annual Report on Form 10-K is an inactive textual reference only.
We file electronically with the SEC our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our website at www.ljpc.com, free of charge, copies of these reports as soon as reasonably practicable after filing or furnishing these reports with the SEC. The SEC maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
Item 1A. Risk Factors
An investment in shares of our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below and the other information contained in this Annual Report on Form 10-K before deciding to invest in shares of our common stock. The risks described below are not the only ones facing our Company. Additional risks not presently known to us or that we currently consider immaterial may also adversely affect our business. We have attempted to identify below the major factors that could cause differences between actual and planned or expected results, but we cannot assure you that we have identified all of those factors. Our business, financial condition, results of operations and prospects could be materially adversely affected by any of these risks or uncertainties. In such case, the trading price of shares of our common stock could decline, and you could lose all or part of your investment.
RISKS RELATED TO OUR BUSINESS
We are substantially dependent on the commercial success of GIAPREZATM (angiotensin II).
The success of our business is substantially dependent on our ability to successfully commercialize GIAPREZATM (angiotensin II), our only commercial product. Although employees in our Company have prior experience launching pharmaceutical products at prior companies, GIAPREZA is the first product we have launched. Furthermore, the market for effective pharmaceutical sales and marketing professionals is competitive, and maintaining these capabilities is expensive and challenging. If we are unable to maintain an effective sales and marketing organization, GIAPREZA sales could be adversely affected, and our business could suffer.
In the U.S. and most major foreign markets, drugs like GIAPREZA that are administered in the hospital must be purchased by the hospital and generally are not reimbursed by third-party payors. Hospitals instead are reimbursed for patient cases based on patients’ diagnosed conditions under the U.S. Medicare diagnosis-related group (“DRG”) system or other like systems for non-Medicare patients in the U.S. and in most major foreign markets. Adoption of new drugs that are administered in the hospital generally occurs more slowly than adoption of new drugs that are taken on an outpatient basis, which generally are paid for by third-party payors. If we are
17
unsuccessful at convincing hospitals and health care providers to increase their rate of adoption of GIAPREZA, our business will suffer.
Catecholamines (primarily norepinephrine), which are available as generics and inexpensive, are typically used in the first line to treat distributive shock, while Vasostrict® (Endo International plc) is typically used in the second line. In the randomized, Phase 3 study ATHOS-3, GIAPREZA demonstrated clinical benefit in patients who were not adequately responding to available vasopressors, including catecholamines and Vasostrict. GIAPREZA’s principle competition as a treatment in patients not adequately responding to available vasopressors is the use of these same vasopressors, particularly norepinephrine, at increased doses. If we are unable to successfully change treatment practices, the commercial prospects for GIAPREZA will be limited, and our business will suffer.
Our estimate of the potential market size for GIAPREZA is based on prescription and sales data for relevant in-market products, the results of clinical studies, medical literature and other information. If the potential market size for GIAPREZA is smaller than our estimate, the commercial prospects for GIAPREZA may be limited, and our business may suffer.
The commercial success of GIAPREZA will depend on our ability to obtain an uninterrupted supply of GIAPREZA from our contract manufacturers.
We do not currently own or operate manufacturing facilities for the production of GIAPREZA or LJPC-0118 (I.V. artesunate). We rely on sole-source third-party manufacturers to produce GIAPREZA and LJPC-0118 and expect to continue to do so to meet our development and commercial needs. In all of our manufacturing agreements, we require that third-party contract manufacturers produce active pharmaceutical ingredients (“APIs”) and drug products in accordance with the U.S. Food and Drug Administration’s (“FDA’s”) current Good Manufacturing Practices (“cGMPs”) and all other applicable laws and regulations. The long-term commercial success of GIAPREZA will depend in part on the ability of our contract manufacturers to supply cGMP-compliant API and drug product without interruption. If there is an interruption in the supply of GIAPREZA from our contract manufacturers, our business will suffer.
GIAPREZA’s market exclusivity period will depend on the validity and enforceability of issued and pending patents covering GIAPREZA.
As of December 31, 2019, we owned or had in-licensed 10 issued U.S. patents, 12 pending U.S. patent applications, 3 issued foreign patents and 45 pending foreign patent applications that cover GIAPREZA. The U.S. patents and patent applications expire between 2029 and 2040, and the foreign patents and patent applications expire between 2034 and 2040. Although we believe the bases for these patents and patent applications are sound, they are untested, and there is no assurance that they will not be successfully challenged. There can be no assurance that any patent previously issued will protect GIAPREZA from generic competition or that any patent application will result in an issued patent that will protect GIAPREZA from generic competition. Furthermore, there can be no assurance that GIAPREZA will not be held to infringe valid patents held by others. If our owned and in-licensed intellectual property do not protect GIAPREZA from generic competition, GIAPREZA sales will decline, and our business will suffer. If GIAPREZA is held to infringe valid patents held by others, we could be subject to liability, and our business could suffer.
Product liability lawsuits against us could cause us to incur substantial liabilities and reduce GIAPREZA sales.
Patients suffering from distributive shock are gravely ill and have a high mortality rate. Although 28-day mortality in patients treated with GIAPREZA was lower than in patients treated with placebo in the randomized, Phase 3 study ATHOS-3, there was a higher incidence of arterial and venous thrombotic and thromboembolic events in patients treated with GIAPREZA in this study. Some patients who are treated with GIAPREZA will die due to their underlying illness or suffer adverse events (which may or may not be drug related). As such, we may face product liability lawsuits. Although we carry product liability insurance, product liability lawsuits against us could cause us to incur substantial liabilities and reduce GIAPREZA sales. Furthermore, any such lawsuits could impair our business reputation and result in the initiation of investigations by regulators.
18
Our ability to continue commercializing GIAPREZA is dependent on our fulfillment of contractual obligations to certain parties.
In May 2018, we closed a $125.0 million royalty financing agreement (the “Royalty Agreement”) with HealthCare Royalty Partners (“HCR”). Under the terms of the Royalty Agreement, we received $125.0 million in exchange for tiered royalty payments on worldwide net sales of GIAPREZA. HCR is entitled to receive quarterly royalties on worldwide net sales of GIAPREZA beginning April 1, 2018. Quarterly payments to HCR under the Royalty Agreement start at a maximum royalty rate, with step-downs based on the achievement of annual net product sales thresholds. Through December 31, 2021, the royalty rate will be a maximum of 10%. Starting January 1, 2022, the maximum royalty rate may increase by 4% if an agreed-upon, cumulative net product sales threshold has not been met, and, starting January 1, 2024, the maximum royalty rate may increase by an additional 4% if a different agreed-upon, cumulative net product sales threshold has not been met. The Royalty Agreement is subject to maximum aggregate royalty payments to HCR of $225.0 million. The Royalty Agreement expires upon the first to occur of January 1, 2031 or when the maximum aggregate royalty payments have been made. The Royalty Agreement was entered into by our wholly-owned subsidiary, La Jolla Pharma, LLC, and HCR has no recourse under the Royalty Agreement against La Jolla Pharmaceutical Company or any assets other than GIAPREZA. However, under the terms of the Royalty Agreement, La Jolla Pharma, LLC has certain obligations, including the obligation to use commercially reasonable and diligent efforts to commercialize GIAPREZA. If La Jolla Pharma, LLC is held to not have met these obligations, HCR would have the right to terminate the Royalty Agreement and demand payment from La Jolla Pharma, LLC of either $125.0 million or $225.0 million (depending on which obligation La Jolla Pharma, LLC is held to not have met), minus aggregate royalties already paid to HCR. In the event that La Jolla Pharma, LLC fails to timely pay such amount if and when due, HCR would have the right to foreclose on the GIAPREZA-related assets.
In December 2014, we entered into a patent license agreement with George Washington University (“GW”), which was amended and restated on March 1, 2016 (the “GW License”) and subsequently assigned to La Jolla Pharma, LLC. Pursuant to the GW License, GW exclusively licensed to us certain intellectual property rights relating to GIAPREZA, including the exclusive rights to certain issued patents and patent applications covering GIAPREZA. Under the GW License, La Jolla Pharma, LLC is obligated to use commercially reasonable efforts to develop, commercialize, market and sell GIAPREZA and make certain payments to GW, including a 6% royalty on net sales of GIAPREZA. If La Jolla Pharma, LLC is held to not have met its obligations, GW could terminate the GW License and La Jolla Pharma, LLC would no longer have rights to the GW issued patents and patent applications covering GIAPREZA.
Our ability to obtain FDA approval of LJPC-0118 is uncertain.
La Jolla filed a New Drug Application (“NDA”) with the FDA for LJPC-0118 for the treatment of severe malaria in the second half of 2019. There can be no assurance that the FDA will approve LJPC-0118 in a timely manner, or at all.
Our ability to receive a tropical disease Priority Review Voucher is uncertain.
La Jolla may be eligible to receive a tropical disease Priority Review Voucher (“PRV”) for LJPC-0118, as malaria is defined as a disease qualifying for a tropical disease PRV under Section 524 of the FDCA. The FDA is authorized to award a tropical disease PRV to sponsors of applications for certain products for the prevention or treatment of certain tropical diseases. A tropical disease PRV may be used by the sponsor that obtains the tropical disease PRV or may be transferred to another sponsor that may use it to obtain Priority Review for a different application. In order to be eligible for a tropical disease PRV, the application must: (i) be for a tropical disease as defined in Section 524 of the FDCA; (ii) be submitted under Section 505(b)(1) of the FDCA or Section 351 of the Public Health Service Act (“PHSA”); (iii) be for a product that contains no active ingredient that has been approved in any other application submitted under Section 505(b)(1) of the FDCA or Section 351 of the PHSA; and (iv) qualify for Priority Review. Even if LJPC-0118 is approved by the FDA, there can be no assurance that we will receive a tropical disease PRV. Furthermore, even if we receive a tropical disease PRV, there can be no assurance that it will be of any value to us.
Our ability to hire and retain key employees is uncertain.
As of February 24, 2020, we employed 91 employees. The market for effective professionals in the pharmaceutical industry is competitive, and hiring and retaining these professionals are expensive and challenging.
19
If we are unable to hire and retain key employees, we may be unable to effectively execute on our operating plan, and our business could suffer.
Business interruptions resulting from geopolitical actions, natural disasters, public health crises or other catastrophic events could have an adverse impact on our business.
Business interruptions resulting from geopolitical actions, such as war and terrorism, natural disasters, public health crises, such as a pandemic, or other catastrophic events could have an adverse impact on our business. For example, if one of these events were to adversely affect one of our contract manufacturers, our supply of GIAPREZA could be interrupted. Furthermore, in the case of a pandemic, the ability of our critical care specialists to access hospitals and call on physicians may be curtailed, which may adversely affect product sales.
Our overall financial performance, including but not limited to net product sales and net cash used in operating activities, may not meet our expectations.
Our overall financial performance, including but not limited to net product sales and net cash used in operating activities, is difficult to predict and may fluctuate from quarter to quarter and year to year. Historical financial performance may not be indicative of future financial performance. For example, our net product sales may be below expectations, and our costs to operate our business, including cost of product sales, research and development expenses and selling, general and administrative expenses, could exceed our estimates. If our overall financial performance does not meet our expectations, our business could suffer.
Our capital requirements and our potential need for, and ability to obtain, additional financing are uncertain.
As of December 31, 2019, we had cash of $87.8 million. GIAPREZA is our only approved product and our only source of product revenue. To reach the point at which we are able to generate positive cash flow from operations, we may need to raise additional capital. The amount and timing of future funding requirements, if any, will depend on many factors, including the success of our commercialization efforts for GIAPREZA, our ability to receive a tropical disease PRV and our ability to control expenses. If necessary, we will raise additional capital through equity or debt financings or collaboration agreements. We can provide no assurance that additional financing will be available to us on favorable terms, or at all. If we need to raise additional capital and are unable to do so, we may be forced to curtail or cease our operations.
RISKS RELATED TO OUR INDUSTRY
We are subject to various federal, state and foreign laws and regulations governing the health care industry that could result in substantial penalties for noncompliance.
We are subject to various federal, state and foreign laws and regulations governing the health care industry that could result in substantial penalties for noncompliance. These laws and regulations may impact our ability to operate, including our sales and marketing efforts. In addition, we may be subject to patient privacy regulation by federal, state and foreign governments that govern jurisdictions in which we conduct our business. The laws and regulations that may affect our ability to operate include:
• | The federal Anti-Kickback Statute, which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, an item or service reimbursable under a federal health care program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value, including for example gifts, cash payments, donations, the furnishing of supplies or equipment, waivers of payment, ownership interests, and providing any item, service or compensation for something other than fair market value. |
• | Federal false claims and civil monetary penalties laws, including the federal civil False Claims Act, which prohibits anyone from, among other things, knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services that are false or fraudulent. Although we may not submit claims directly to payors, manufacturers can be held liable under these laws in a variety of ways. These include: providing inaccurate billing or coding information to customers; improperly promoting a product’s off-label use; violating the federal Anti-Kickback Statute; or misreporting pricing information to government programs. |
20
• | Provisions of the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services. |
• | The federal Physician Payment Sunshine Act requirements, under the Patient Protection and Affordable Care Act (“PPACA”), which require manufacturers of certain drugs and biologics to track and report to U.S. Centers for Medicare & Medicaid Services (“CMS”) payments and other transfers of value they make to U.S. physicians and teaching hospitals as well as physician ownership and investment interests in the manufacturer. |
• | Various federal, state and foreign data privacy and security laws and regulations. These include provisions of HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations (“HITECH”), which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information in the U.S. and the General Data Protection Regulation (“GDPR”) in the European Union that became effective in May 2018. We may not be directly subject to certain of these laws and regulations, such as privacy and security requirements under HIPAA; however, we may be subject to criminal penalties for knowingly, aiding and embedding these violations. |
• | Section 1927 of the Social Security Act, which requires that manufacturers of drugs and biological products covered by Medicaid report pricing information to CMS on a monthly and quarterly basis, including the best price available to any customer of the manufacturer, with certain exceptions for government programs, and pay prescription rebates to state Medicaid programs based on a statutory formula derived from reported pricing information. |
• | State and/or foreign law equivalents of each of the above federal laws, such as the recently effective California Consumer Privacy Act, many of which differ from each other in significant ways and may not have the same effect, which complicates our compliance efforts. |
If we are found to be in violation of any of the laws or regulations described above or any other laws or regulations that apply to us, we may be subject to substantial penalties, including civil and criminal penalties, damages, fines and possible exclusion from participation in Medicare, Medicaid and other federal health care programs. If we are subjected to substantial penalties, our business will suffer, and we may be forced to curtail or cease our operations.
Drug development involves a lengthy and expensive process with an uncertain outcome.
Drug development involves a lengthy and expensive process with an uncertain outcome. Failure can occur at any time during drug development. The results of nonclinical studies and early clinical studies may not be predictive of the results of later-stage clinical studies. For example, the safety or efficacy results of clinical studies do not ensure that later clinical studies will demonstrate similar results. Even if clinical studies demonstrate the safety and efficacy of the product candidate, there is no assurance that such product candidate will receive regulatory approval.
Drugs approved by the FDA are subject to ongoing regulation.
Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain procedural and documentation requirements on us and our third-party manufacturers. Even after regulatory approval is obtained, under certain circumstances, such as later discovery of previously unknown safety risks, the FDA can withdraw approval or subject the drug to additional restrictions.
Our use of hazardous materials could subject us to liability, fines and sanctions.
Laboratory and clinical testing sometimes involve the use of hazardous, radioactive or otherwise toxic materials. We are subject to federal, state and local laws and regulations governing how we use, manufacture, handle, store and dispose of these materials. Although we believe that our safety procedures for handling and disposing of such materials comply in all material respects with all federal, state and local laws and regulations, there is always the risk of accidental contamination or injury from these materials. If we fail to comply with such laws and regulations, we could be subject to liability, fines and sanctions, and our business may suffer.
21
RISKS RELATED TO OWNERSHIP OF OUR COMMON STOCK
The price per share of our common stock may fluctuate significantly, and you may lose all or part of your investment.
The price per share of our common stock may fluctuate significantly, and you may lose all or part of your investment. These fluctuations could be based on various factors, including factors described elsewhere in this Annual Report on Form 10-K and below:
• | changes in analyst estimates, ratings and price targets; |
• | negative press reports or other negative publicity, whether or not true, about our business; |
• | developments concerning the pharmaceutical and biotechnology industry in general; |
• | market sentiment towards pharmaceutical and biotechnology stocks; |
• | developments concerning the overall economy; and |
• | market sentiment toward equity securities. |
We have never paid a dividend on shares of our common stock, and you should rely on price appreciation of shares of our common stock for return on your investment.
We have never paid a dividend on shares of our common stock. Even if we decide to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on our future results of operations, financial condition, contractual restrictions and other factors. You should not rely on dividend income from shares of our common stock and should rely on price appreciation of shares of our common stock for return on your investment.
Conversion of our convertible preferred stock would result in substantial dilution for our existing shareholders of common stock.
As of December 31, 2019, there were approximately 27.2 million shares of common stock outstanding. We may be required to issue up to approximately 6.7 million additional shares of common stock upon conversion of existing convertible preferred stock. The issuance of these additional shares would represent approximately 20% dilution to our existing shareholders of common stock.
If we need to obtain additional financing in the future, such financing could result in dilution to your investment, adversely affect the price per share of our common stock and/or create future operating and financial restrictions.
As of December 31, 2019, we had cash of $87.8 million. GIAPREZA is our only approved product and our only source of product revenue. To reach the point at which we are able to generate positive cash flow from operations, we may need to raise additional capital. The amount and timing of future funding requirements, if any, will depend on many factors, including the success of our commercialization efforts for GIAPREZA, our ability to receive a tropical disease PRV and our ability to control expenses. If necessary, we will raise additional capital through equity or debt financings. We can provide no assurance that additional financing will be available to us on favorable terms, or at all. If we issue additional equity securities or securities convertible into equity securities, you will suffer dilution to your investment, and such issuance may adversely affect the price per share of our common stock. Any new debt financing we enter into may involve covenants that restrict our operations, which may include limitations on borrowing and specific restrictions on the use of our assets, as well as prohibitions on our ability to create liens or pay dividends.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
Our corporate headquarters is located at 4550 Towne Centre Court, San Diego, California 92121. We lease 83,008 square feet of office and laboratory space.
22
Item 3. Legal Proceedings
We are not currently a party to any material legal proceedings.
Item 4. Mine Safety Disclosures
Not applicable.
23
PART II
Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities
Market Information
Shares of our common stock are traded on the Nasdaq Capital Market, under the symbol “LJPC.”
Holders of Record
As of February 3, 2020, we had 4 holders of record. Certain shares of common stock are held in “street” name, and, accordingly, the number of beneficial owners of such shares of common stock is not known or included in the foregoing number. This number of holders of record also does not include shareholders whose shares may be held in trust by other entities.
Dividends
We have never paid dividends on shares of our common stock, and we do not have any plans to pay dividends in the foreseeable future.
24
Item 6. Selected Financial Data
We are a smaller reporting company, as defined by Rule 12b-2 under the Securities and Exchange Act of 1934 and in Item 10(f)(1) of Regulation S-K, and are not required to provide the information under this item.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our audited financial statements and the related notes and other financial information included elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, include forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” set forth in this Annual Report on Form 10-K for a discussion of important factors that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Discussion and analysis of our 2017 financial condition and results of operations compared to our 2018 financial condition and results of operations can be found in Item 7 of the Company’s Annual Report on Form 10-K filed on March 4, 2019.
Business Overview
La Jolla Pharmaceutical Company is dedicated to the development and commercialization of innovative therapies that improve outcomes in patients suffering from life-threatening diseases. In December 2017, GIAPREZATM (angiotensin II) was approved by the U.S. Food and Drug Administration (“FDA”) as a vasoconstrictor indicated to increase blood pressure in adults with septic or other distributive shock. GIAPREZA U.S. net sales were $23.1 million in 2019 compared to $10.1 million in 2018, an increase of 129%. In August 2019, GIAPREZA was approved by the European Commission (“EC”) for the treatment of refractory hypotension in adults with septic or other distributive shock who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies. LJPC‑0118 (I.V. artesunate) is La Jolla’s investigational product for the treatment of severe malaria.
Results of Operations
The following table summarizes our results of operations for each of the periods below (in thousands):
Year Ended December 31, | |||||||||||
2019 | 2018 | Change | |||||||||
Net product sales | $ | 23,054 | $ | 10,056 | $ | 12,998 | |||||
Cost of product sales | (2,392 | ) | (1,643 | ) | (749 | ) | |||||
Research and development expense | (85,329 | ) | (117,302 | ) | 31,973 | ||||||
Selling, general and administrative expense | (45,134 | ) | (85,162 | ) | 40,028 | ||||||
Other expense, net | (6,707 | ) | (5,418 | ) | (1,289 | ) | |||||
Net loss | $ | (116,508 | ) | $ | (199,469 | ) | $ | 82,961 | |||
Net Product Sales
Net product sales consist solely of revenue recognized from sales of GIAPREZA to hospitals in the U.S. through a network of specialty and wholesaler distributors (“Customers”). GIAPREZA U.S. net sales were $23.1 million for the year ended December 31, 2019 compared to $10.1 million for the year ended December 31, 2018, an increase of 129%. La Jolla launched GIAPREZA in the U.S. in March 2018.
Cost of Product Sales
Cost of product sales primarily consists of royalties paid or payable to GW and the costs to produce, package and deliver GIAPREZA to our Customers. These costs include raw materials, labor and manufacturing and
25
quality control, as well as shipping and distribution costs. For the year ended December 31, 2019, cost of product sales was $2.4 million compared to $1.6 million for the same period in 2018.
Research and Development Expense
Research and development expense consists of non-personnel and personnel expenses. The following table summarizes these expenses for each of the periods below (in thousands):
Year Ended December 31, | |||||||||||
2019 | 2018 | Change | |||||||||
Non-personnel expenses: | |||||||||||
LJPC-401 | $ | 17,603 | $ | 16,353 | $ | 1,250 | |||||
GIAPREZA | 6,007 | 16,871 | (10,864 | ) | |||||||
LJPC-0118 | 1,746 | 6,352 | (4,606 | ) | |||||||
Other programs | 6,731 | 6,001 | 730 | ||||||||
Facility | 7,318 | 7,574 | (256 | ) | |||||||
Other | 3,991 | 7,464 | (3,473 | ) | |||||||
Total non-personnel expense | $ | 43,396 | $ | 60,615 | $ | (17,219 | ) | ||||
Personnel expenses: | |||||||||||
Salaries, bonuses and benefits | 27,072 | 35,574 | (8,502 | ) | |||||||
Share-based compensation expense | 14,861 | 21,113 | (6,252 | ) | |||||||
Total personnel expense | $ | 41,933 | $ | 56,687 | $ | (14,754 | ) | ||||
Total research and development expense | $ | 85,329 | $ | 117,302 | $ | (31,973 | ) | ||||
During the year ended December 31, 2019, total research and development non-personnel expense decreased primarily as a result of decreases in GIAPREZA- and LJPC-0118-related expenses. GIAPREZA-related expenses decreased as a result of the completion of certain development activities relating to the application for approval to market GIAPREZA in the European Union, which was granted in 2019. LJPC‑0118‑related expenses decreased as a result of the completion of certain development activities relating to the NDA for LJPC-0118, which was filed in 2019. During the year ended December 31, 2019, total research and development personnel expense, including share-based compensation expense, decreased as a result of reduced headcount in 2019 from a Company-wide realignment in October 2018, partially offset by $4.4 million of one-time charges in 2019 resulting from another Company-wide realignment in November 2019. We anticipate research and development expense will decrease significantly in 2020 as a result of the de-prioritization of LJPC-401 and the Company-wide realignment in November 2019.
26
Selling, General and Administrative Expense
Selling, general and administrative expense consists of non-personnel and personnel expenses. The following table summarizes these expenses for each of the periods below (in thousands):
Year Ended December 31, | |||||||||||
2019 | 2018 | Change | |||||||||
Non-personnel expenses: | |||||||||||
Sales and marketing | $ | 7,194 | $ | 23,933 | $ | (16,739 | ) | ||||
Professional fees | 4,398 | 4,939 | (541 | ) | |||||||
Facility | 1,519 | 1,268 | 251 | ||||||||
Other | 2,805 | 2,629 | 176 | ||||||||
Total non-personnel expense | $ | 15,916 | $ | 32,769 | $ | (16,853 | ) | ||||
Personnel expenses: | |||||||||||
Salaries, bonuses and benefits | 20,346 | 38,355 | (18,009 | ) | |||||||
Share-based compensation expense | 8,872 | 14,038 | (5,166 | ) | |||||||
Total personnel expense | $ | 29,218 | $ | 52,393 | $ | (23,175 | ) | ||||
Total selling, general and administrative expense | $ | 45,134 | $ | 85,162 | $ | (40,028 | ) | ||||
During the year ended December 31, 2019, total selling, general and administrative non-personnel expenses decreased primarily as a result of decreases in sales and marketing-related expenses associated with the initial commercial launch of GIAPREZA in the U.S., which occurred in March 2018. During the year ended December 31, 2019, total selling, general and administrative expense, including share-based compensation expense, decreased as a result of reduced headcount in 2019 from a Company-wide realignment in October 2018. We anticipate selling, general and administrative expense will decrease modestly in 2020 as a result of a reduction in general and administrative personnel associated with the Company-wide realignment in November 2019, partially offset by a modest increase in sales and marketing personnel.
Other Expense, Net
Other income (expense), net primarily consists of interest accrued for our deferred royalty obligation, distributions in connection with our non-voting profits interest in a related party and interest income generated from cash held in savings accounts. During the year ended December 31, 2019, other expense, net increased to $6.7 million from $5.4 million for the same period in 2018, an increase of $1.3 million. This increase was primarily due to a $3.5 million increase in interest accrued for our deferred royalty obligation, partially offset by the receipt of distributions of $1.9 million in connection with the Company’s non-voting profits interest in a related party and a $0.2 million increase in interest income generated from cash held in savings accounts.
Liquidity and Capital Resources
Since January 2012, when the Company was effectively restarted, through December 31, 2019, our cash used in operating activities was $425.9 million. As of December 31, 2019, we had an accumulated deficit of $1,037.3 million and have financed our operations through public and private offerings of securities, a royalty financing, revenues from net product sales, interest income on invested cash balances and other income.
As of December 31, 2019 and 2018, we had cash of $87.8 million and $172.6 million, respectively. Based on our current operating plans and projections, we believe that our existing cash will be sufficient to fund operations for at least one year from the date this Annual Report on Form 10-K is filed with the U.S. Securities and Exchange Commission (the “SEC”).
Cash used for operating activities was $85.0 million and $152.4 million for the years ended December 31, 2019 and 2018, respectively. The decrease in cash used for operating activities was a result of the decrease in our net loss, primarily offset by changes in non-cash expenses and working capital.
27
Cash used for investing activities was $0.7 million and $2.3 million for the years ended December 31, 2019 and 2018, respectively. The decrease in cash used in investing activities was the result of purchases of property and equipment in 2018.
Cash provided by financing activities was $0.9 million and $236.4 million for the years ended December 31, 2019 and 2018, respectively. The decrease in cash provided by financing activities was primarily the result of the receipt of $109.8 million of net proceeds from the sale of shares of our common stock in an underwritten public offering in March 2018 and $124.3 million of net proceeds from the Royalty Agreement in May 2018.
Off−Balance Sheet Arrangements
We have no off-balance sheet arrangements that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in our financial condition, expenses, results of operations, liquidity, capital expenditures or capital resources.
Contractual Obligations
We are a smaller reporting company, as defined by Rule 12b-2 under the Securities and Exchange Act of 1934 and in Item 10(f)(1) of Regulation S-K, and are not required to provide the information under this item.
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations are based on our audited consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”). The preparation of these audited consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. We evaluate our estimates on an ongoing basis. We base our estimates on historical experience and on other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our consolidated financial statements included in Item 15 of this Annual Report on Form 10-K, we believe that the following accounting policies and estimates are most critical to understanding and evaluating our reported financial results.
Revenue Recognition
Our revenue solely consists of U.S. net sales from GIAPREZA, which we launched in the U.S. in March 2018. In 2019, 444 hospitals in the U.S. purchased GIAPREZA. Hospitals purchase our products through a network of specialty and wholesale distributors (“Customers”). In addition to distribution agreements with Customers, we enter into arrangements with group purchasing organizations (“GPOs”) and health systems that provide for privately negotiated chargebacks and discounts with respect to the purchase of GIAPREZA.
Revenue from product sales is recorded at the transaction price, net of estimates for variable consideration consisting of chargebacks, discounts, returns and administrative fees. Variable consideration is estimated using the expected-value amount method, which is the sum of probability-weighted amounts in a range of possible consideration amounts. Actual amounts of consideration ultimately received may differ from our estimates. If actual results vary materially from our estimates, we will adjust these estimates, which will affect net sales of GIAPREZA and results from our operations in the period such estimates are adjusted.
Accrued Expenses
As part of the process of preparing the financial statements, we are required to estimate accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed by service providers and estimating the level of service performed and the associated cost incurred for services that have not yet been invoiced. We make estimates of accrued expenses as of each balance sheet date in the financial statements based on facts and circumstances known at that time. We
28
periodically confirm the accuracy of recorded estimates with the service providers and make adjustments, if necessary.
We base our accrued expenses on our estimates of the services received and efforts expended pursuant to our contractual arrangements. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepayment accordingly. The financial terms of our contractual agreements may be subject to interpretation, and the timing of payment relative to the timing of services rendered may vary.
Interest Expense
The deferred royalty obligation royalty from our financing agreement (the “Royalty Agreement”) with HealthCare Royalty Partners, which was entered into by our wholly-owned subsidiary, La Jolla Pharma, LLC, is repaid based on the net sales of GIAPREZA. Interest expense and the amortization of issuance costs related to the deferred royalty obligation are recognized over the expected repayment term using the effective interest method. The assumptions used in determining the expected repayment term of the deferred royalty obligation require us to make estimates that could impact the effective interest rate. Each reporting period, we update our estimate of accrued interest expense under the Royalty Agreement based on actual and forecasted net sales of GIAPREZA. Changes in interest expense resulting from changes in the effective interest rate, if any, are recorded on a prospective basis.
Recent Accounting Pronouncements
Recent accounting pronouncements are disclosed in Note 2 to the accompanying audited consolidated financial statements included in Item 15 of this Annual Report on Form 10-K.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
We are a smaller reporting company, as defined by Rule 12b-2 under the Securities and Exchange Act of 1934 and in Item 10(f)(1) of Regulation S-K, and are not required to provide the information under this item.
Item 8. Financial Statements and Supplementary Data
The financial statements required by this item are set forth at the end of this Annual Report on Form 10-K beginning on page F-1 and are incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a) Disclosure Controls and Procedures; Changes in Internal Control Over Financial Reporting
Our management, with the participation of our principal executive and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities and Exchange Act of 1934 (the “Exchange Act”)) as of December 31, 2019. Based on this evaluation, our principal executive and principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 2019 at the reasonable assurance level. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated,
29
can provide only reasonable assurance of achieving their objectives, and our management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
During the year ended December 31, 2019, we implemented controls in connection with the newly-adopted leases policy as disclosed in Note 2 to our consolidated financial statements included in Item 15 of this Annual Report on Form 10-K.
(b) Management Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) of the Exchange Act. Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive and principal financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
As of December 31, 2019, our management used the Committee of Sponsoring Organizations of the Treadway Commission Internal Control - Integrated Framework (2013) (“COSO Framework”) to evaluate the effectiveness of internal control over financial reporting.
Management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2019 and has concluded that such internal control over financial reporting was effective.
(c) Attestation report of the independent registered public accounting firm
The effectiveness of the Company’s internal control over financial reporting has been audited by Squar Milner LLP, an independent registered public accounting firm, as stated in their attestation report appearing below, which expresses an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting as of December 31, 2019.
30
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of La Jolla Pharmaceutical Company
Opinion on the Internal Control Over Financial Reporting
We have audited La Jolla Pharmaceutical Company's (the Company) internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of La Jolla Pharmaceutical Company as of December 31, 2019 and 2018, and the related consolidated statements of operations, shareholders’ (deficit) equity, and cash flows for each of the two years in the period ended December 31, 2019, and the related notes to the consolidated financial statements of the Company and our report dated March 2, 2020 expressed an unqualified opinion.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting in the accompanying Management Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ SQUAR MILNER LLP
San Diego, California
March 2, 2020
31
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officer and Corporate Governance
The information required by this Item is expected to be in our Definitive Proxy Statement for the 2020 Annual Meeting of Shareholders (the “2020 Proxy Statement”), which we expect to be filed with the U.S. Securities and Exchange Commission (“SEC”) within 120 days of the end of our year ended December 31, 2019 and is incorporated herein by reference.
We have adopted a Code of Business Conduct and Ethics that applies to all of our directors, officers and employees, including our principal executive, principal financial and principal accounting officers, or persons performing similar functions. Our Code of Business Conduct and Ethics is posted on our website located at www.ljpc.com in the Corporate Governance section under “Investor Relations.” We intend to disclose future amendments to certain provisions of the Code of Business Conduct and Ethics, and waivers of the Code of Business Conduct and Ethics granted to executive officers and directors, on our website within 4 business days following the date of the amendment or waiver.
Item 11. Executive Compensation
The information required by this Item is incorporated herein by reference to information in our 2020 Proxy Statement, including under the sections entitled “Executive Compensation,” “Executive Compensation−Director Compensation,” “Executive Compensation−Compensation Committee Interlocks and Insider Participation,” “Executive Compensation−Risk Management and Mitigation” and “Executive Compensation−Compensation Committee Report.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters
The information required by this Item is incorporated herein by reference to information in our 2020 Proxy Statement, including under the sections entitled “Certain Relationships and Related−Person Transactions,” “Corporate Governance” and “Corporate Governance−Board Committees.”
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated herein by reference to information in our 2020 Proxy Statement, including under the sections entitled “Certain Relationships and Related−Person Transactions,” “Corporate Governance” and “Corporate Governance−Board Committees.”
Item 14. Principal Accountant Fees and Services
The information required by this Item is incorporated herein by reference to information in our 2020 Proxy Statement, including under the section entitled “Proposal 2: Ratification of Selection of Independent Registered Public Accounting Firm.”
32
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Financial Statements
The response to this portion of Item 15 is set forth under Item 8 hereof.
(a)(2) Financial Statements Schedules
No financial statement schedules are provided because the information called for is not required or is shown in the financial statements or the notes thereto.
(a)(3) Exhibits
33
X | ||||||||
X | ||||||||
X | ||||||||
101.INS | XBRL Instance Document | X | ||||||
101.SCH | XBRL Taxonomy Extension Schema Document | X | ||||||
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | X | ||||||
101.LAB | XBRL Taxonomy Extension Label Linkbase Document | X | ||||||
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||
* | This exhibit is a management contract or compensatory plan or arrangement. |
† | Confidential treatment has been requested with respect to certain portions of the exhibit, which portions have been omitted and filed separately with the Securities and Exchange Commission. |
Item 16. Form 10-K Summary
None.
34
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
La Jolla Pharmaceutical Company | ||
Date: | March 2, 2020 | /s/ Dennis Mulroy |
Dennis Mulroy | ||
Chief Financial Officer | ||
(Principal Executive, Principal Financial and Accounting Officer) | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENT, that each individual whose signature appears below hereby constitutes and appoints Dennis Mulroy the true and lawful attorney-in-fact and agent of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, to sign in any and all capacities (including, without limitation, the capacities listed below), with respect to this Annual Report on Form 10-K, and any and all amendments thereto, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the U.S. Securities and Exchange Commission (the “SEC”), and hereby grants to such attorney-in-fact and agent full power and authority to do and perform each and every act and anything necessary to be done to enable La Jolla Pharmaceutical Company to comply with the provisions of the Securities Exchange Act of 1934 (the “Exchange Act”) and all the requirements of the SEC, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Exchange Act, this report has been signed by the following persons in the capacities set forth opposite their names and on the dates indicated below.
Signature | Title | Date | |||
/s/ Dennis Mulroy | Chief Financial Officer (Principal Executive, Principal Financial and Accounting Officer) | March 2, 2020 | |||
Dennis Mulroy | |||||
/s/ Kevin Tang | Chairman of the Board and Director | March 2, 2020 | |||
Kevin Tang | |||||
/s/ Craig Johnson | Director | March 2, 2020 | |||
Craig Johnson | |||||
/s/ Laura Johnson | Director | March 2, 2020 | |||
Laura Johnson | |||||
/s/ David Ramsay | Director | March 2, 2020 | |||
David Ramsay | |||||
/s/ Robert Rosen | Director | March 2, 2020 | |||
Robert Rosen | |||||
35
La Jolla Pharmaceutical Company
Index to Financial Statements
36
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of La Jolla Pharmaceutical Company
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of La Jolla Pharmaceutical Company (the Company) as of December 31, 2019 and 2018, the related consolidated statements of operations, shareholders’ (deficit) equity, and cash flows for each of the two years in the period ended December 31, 2019, and the related notes to the consolidated financial statements (collectively the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control−Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013, and our report dated March 2, 2020 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Change in Accounting Principle
As discussed in Note 2 to the financial statements, the Company has changed its method of accounting for operating leases with terms greater than one year in the year ended December 31, 2019 due to the adoption of ASU 2016-02, Leases (Topic 842).
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ SQUAR MILNER LLP
We have served as the Company's auditor since 2012.
San Diego, California
March 2, 2020
F - 1
LA JOLLA PHARMACEUTICAL COMPANY
Consolidated Balance Sheets
(in thousands, except par value and share amounts)
December 31, 2019 | December 31, 2018 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash | $ | 87,820 | $ | 172,604 | |||
Accounts receivable, net | 2,960 | 1,381 | |||||
Inventory, net | 2,211 | 2,020 | |||||
Prepaid expenses and other current assets | 4,467 | 5,111 | |||||
Total current assets | 97,458 | 181,116 | |||||
Property and equipment, net | 18,389 | 22,267 | |||||
Right-of-use lease asset | 15,491 | — | |||||
Restricted cash | 909 | 909 | |||||
Total assets | $ | 132,247 | $ | 204,292 | |||
LIABILITIES AND SHAREHOLDERS’ (DEFICIT) EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 4,177 | $ | 8,572 | |||
Accrued expenses | 9,312 | 8,485 | |||||
Accrued payroll and related expenses | 8,332 | 7,509 | |||||
Lease liability, current portion | 2,766 | — | |||||
Deferred rent, current portion | — | 1,370 | |||||
Total current liabilities | 24,587 | 25,936 | |||||
Lease liability, less current portion | 26,481 | — | |||||
Deferred rent, less current portion | — | 13,609 | |||||
Deferred royalty obligation, net | 124,379 | 124,323 | |||||
Other noncurrent liabilities | 12,790 | 4,503 | |||||
Total liabilities | 188,237 | 168,371 | |||||
Commitments and contingencies (Note 10) | |||||||
Shareholders’ (deficit) equity: | |||||||
Common Stock, $0.0001 par value; 100,000,000 shares authorized, 27,195,469 and 26,259,254 shares issued and outstanding at December 31, 2019 and December 31, 2018, respectively | 3 | 3 | |||||
Series C-12 Convertible Preferred Stock, $0.0001 par value; 11,000 shares authorized, 3,906 shares issued and outstanding at December 31, 2019 and December 31, 2018; and liquidation preference of $3,906 at December 31, 2019 and 2018 | 3,906 | 3,906 | |||||
Series F Convertible Preferred Stock, $0.0001 par value; 10,000 shares authorized, 0 and 2,737 shares issued and outstanding at December 31, 2019 and December 31, 2018, respectively; and liquidation preference of $0 and $2,737 at December 31, 2019 and 2018, respectively | — | 2,737 | |||||
Additional paid-in capital | 977,432 | 950,258 | |||||
Accumulated deficit | (1,037,331 | ) | (920,983 | ) | |||
Total shareholders’ (deficit) equity | (55,990 | ) | 35,921 | ||||
Total liabilities and shareholders’ (deficit) equity | $ | 132,247 | $ | 204,292 | |||
See accompanying notes to the consolidated financial statements.
F - 2
LA JOLLA PHARMACEUTICAL COMPANY
Consolidated Statements of Operations
(in thousands, except per share amounts)
Year Ended December 31, | |||||||
2019 | 2018 | ||||||
Revenue | |||||||
Net product sales | $ | 23,054 | $ | 10,056 | |||
Total revenue | 23,054 | 10,056 | |||||
Operating expenses | |||||||
Cost of product sales | 2,392 | 1,643 | |||||
Research and development | 85,329 | 117,302 | |||||
Selling, general and administrative | 45,134 | 85,162 | |||||
Total operating expenses | 132,855 | 204,107 | |||||
Loss from operations | (109,801 | ) | (194,051 | ) | |||
Other (expense) income | |||||||
Interest expense | (10,774 | ) | (7,303 | ) | |||
Interest income | 2,128 | 1,885 | |||||
Other income—related party | 1,939 | — | |||||
Total other (expense) income, net | (6,707 | ) | (5,418 | ) | |||
Net loss | $ | (116,508 | ) | $ | (199,469 | ) | |
Net loss per share, basic and diluted | $ | (4.30 | ) | $ | (7.85 | ) | |
Weighted-average common shares outstanding, basic and diluted | 27,112 | 25,422 | |||||
See accompanying notes to the consolidated financial statements.
F - 3
La Jolla Pharmaceutical Company
Consolidated Statements of Shareholders’ (Deficit) Equity
For the Years Ended December 31, 2019 and 2018
(in thousands)
Series C-12 Convertible Preferred Stock | Series F Convertible Preferred Stock | Common Stock | Additional Paid-in Capital | Accumulated Deficit | Total Shareholders’ (Deficit) Equity | ||||||||||||||||||||||||||||
Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||
Balance at December 31, 2017 | 4 | $ | 3,906 | 3 | $ | 2,737 | 22,167 | $ | 2 | $ | 803,071 | $ | (721,514 | ) | $ | 88,202 | |||||||||||||||||
Issuance of common stock for March 2018 financing | — | — | — | — | 3,910 | 1 | 109,808 | — | 109,809 | ||||||||||||||||||||||||
Share-based compensation expense | — | — | — | — | — | — | 35,080 | — | 35,080 | ||||||||||||||||||||||||
Issuance of common stock under 2013 Equity Plan | — | — | — | — | 150 | — | 1,908 | — | 1,908 | ||||||||||||||||||||||||
Issuance of common stock under ESPP | — | — | — | — | 32 | — | 391 | — | 391 | ||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | (199,469 | ) | (199,469 | ) | ||||||||||||||||||||||
Balance at December 31, 2018 | 4 | 3,906 | 3 | 2,737 | 26,259 | 3 | 950,258 | (920,983 | ) | 35,921 | |||||||||||||||||||||||
Share-based compensation expense | — | — | — | — | — | — | 23,733 | — | 23,733 | ||||||||||||||||||||||||
Issuance of common stock under 2013 Equity Plan | — | — | — | — | 5 | — | 31 | — | 31 | ||||||||||||||||||||||||
Issuance of common stock under ESPP | — | — | — | — | 149 | — | 833 | — | 833 | ||||||||||||||||||||||||
Issuance of common stock for conversion of Series F Preferred Stock | — | — | (3 | ) | (2,737 | ) | 782 | — | 2,737 | — | — | ||||||||||||||||||||||
Cumulative-effect adjustment from adoption of ASU 2018-07 | — | — | — | — | — | — | (160 | ) | 160 | — | |||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | (116,508 | ) | (116,508 | ) | ||||||||||||||||||||||
Balance at December 31, 2019 | 4 | $ | 3,906 | — | $ | — | 27,195 | $ | 3 | $ | 977,432 | $ | (1,037,331 | ) | $ | (55,990 | ) | ||||||||||||||||
See accompanying notes to the consolidated financial statements.
F - 4
LA JOLLA PHARMACEUTICAL COMPANY
Consolidated Statements of Cash Flows
(in thousands)
Year Ended December 31, | |||||||
2019 | 2018 | ||||||
Operating activities | |||||||
Net loss | $ | (116,508 | ) | $ | (199,469 | ) | |
Adjustments to reconcile net loss to net cash used for operating activities: | |||||||
Share-based compensation expense | 23,733 | 35,151 | |||||
Depreciation and amortization expense | 4,552 | 4,405 | |||||
Loss on disposal of equipment | 24 | 236 | |||||
Non-cash interest expense | 8,775 | 6,797 | |||||
Non-cash rent expense | 1,307 | — | |||||
Changes in operating assets and liabilities: | |||||||
Accounts receivable, net | (1,579 | ) | (1,381 | ) | |||
Inventory, net | (191 | ) | (2,020 | ) | |||
Prepaid expenses and other current assets | 644 | (1,964 | ) | ||||
Accounts payable | (4,395 | ) | (2,912 | ) | |||
Accrued expenses | 395 | 5,451 | |||||
Accrued payroll and related expenses | 823 | 2,514 | |||||
Lease liability | (2,530 | ) | — | ||||
Deferred rent | — | 824 | |||||
Net cash used for operating activities | (84,950 | ) | (152,368 | ) | |||
Investing activities | |||||||
Purchase of property and equipment | (698 | ) | (2,340 | ) | |||
Net cash used for investing activities | (698 | ) | (2,340 | ) | |||
Financing activities | |||||||
Net proceeds from issuance of common stock under ESPP | 833 | 391 | |||||
Net proceeds from issuance of common stock under 2013 Equity Plan | 31 | 1,908 | |||||
Net proceeds from royalty financing | — | 124,289 | |||||
Net proceeds from the issuance of common stock | — | 109,809 | |||||
Net cash provided by financing activities | 864 | 236,397 | |||||
Net (decrease) increase in cash and restricted cash | (84,784 | ) | 81,689 | ||||
Cash and restricted cash at beginning of period | 173,513 | 91,824 | |||||
Cash and restricted cash at end of period | $ | 88,729 | $ | 173,513 | |||
Supplemental disclosure of non-cash investing and financing activities | |||||||
Conversion of Series F Convertible Preferred Stock into common stock | $ | 2,737 | $ | — | |||
Cumulative-effect adjustment from adoption of ASU 2018-07 | $ | (160 | ) | $ | — | ||
Initial recognition of right-of-use lease asset | $ | 16,798 | $ | — | |||
Interest paid | $ | 1,999 | $ | 506 | |||
Reconciliation of cash and restricted cash to the consolidated balance sheets | |||||||
Cash | $ | 87,820 | $ | 172,604 | |||
Restricted cash | 909 | 909 | |||||
Total cash and restricted cash | $ | 88,729 | $ | 173,513 | |||
See accompanying notes to the consolidated financial statements.
F - 5
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
1. Business
La Jolla Pharmaceutical Company (collectively with its wholly-owned subsidiaries, the “Company”) is dedicated to the development and commercialization of innovative therapies that improve outcomes in patients suffering from life-threatening diseases. In December 2017, GIAPREZATM (angiotensin II) was approved by the U.S. Food and Drug Administration (“FDA”) as a vasoconstrictor indicated to increase blood pressure in adults with septic or other distributive shock. GIAPREZA U.S. net sales were $23.1 million in 2019 compared to $10.1 million in 2018, an increase of 129%. In August 2019, GIAPREZA was approved by the European Commission (“EC”) for the treatment of refractory hypotension in adults with septic or other distributive shock who remain hypotensive despite adequate volume restitution and application of catecholamines and other available vasopressor therapies.
LJPC-0118 (I.V. artesunate) is La Jolla’s investigational product for the treatment of severe malaria.
As of December 31, 2019 and 2018, the Company had cash of $87.8 million and $172.6 million, respectively. Based on the Company’s current operating plans and projections, the Company expects that its existing cash will be sufficient to fund operations for at least one year from the date this Annual Report on Form 10-K is filed with the U.S. Securities and Exchange Commission (the “SEC”).
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The Company’s consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) and include the accounts of the Company and its wholly-owned subsidiaries. All inter-company transactions and balances have been eliminated in consolidation. The preparation of the Company’s consolidated financial statements requires management to make estimates and assumptions that impact the reported amounts of assets, liabilities, revenue and expenses and the disclosure of contingent assets and liabilities in its consolidated financial statements and the accompanying notes. Actual results may differ materially from these estimates.
Certain amounts previously reported in the financial statements have been reclassified to conform to the current year presentation. Such reclassifications did not affect net loss, shareholders’ (deficit) equity or cash flows.
Summary of Significant Accounting Policies
Cash and Restricted Cash
The Company considers all highly liquid investments with maturities of three months or less when purchased as cash equivalents. The Company maintains its cash in checking and savings accounts. Income generated from cash held in savings accounts is recorded as interest income. The carrying value of the Company’s savings accounts is included in cash and approximates the fair value. Cash is classified as restricted cash when certain funds are reserved for a specific purpose and are not available for immediate or general business use.
Accounts Receivable, Net
Accounts receivable are recorded net of customers’ allowances for prompt-pay discounts, chargebacks and doubtful accounts. Allowances for prompt-pay discounts and chargebacks are based on contractual terms. The Company estimates the allowance for doubtful accounts based on existing contractual payment terms, actual payment patterns of its customers and individual customer circumstances. As of December 31, 2019, the Company did not have any allowances for doubtful accounts.
Inventory, Net
Inventory is stated at the lower of cost or estimated net realizable value on a first-in, first-out (“FIFO”) basis. The Company periodically analyzes inventory levels and writes down inventory as cost of product sales when: inventory has become obsolete; inventory has a cost basis in excess of its estimated net realizable value; or inventory quantities are in excess of expected product sales.
F - 6
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist of cash. The Company maintains its cash in checking and savings accounts at federally insured financial institutions in excess of federally insured limits.
In 2019, 444 hospitals in the U.S. purchased GIAPREZA. Hospitals purchase our products through a network of specialty and wholesale distributors (“Customers”). The Company does not believe that the loss of one of these distributors would significantly impact the ability to distribute GIAPREZA, as the Company expects that sales volume would be absorbed by the remaining distributors. The following table includes the percentage of U.S. net product sales and accounts receivable balances for the Company’s three major Customers, each of which comprised 10% or more of its U.S. net product sales:
U.S. Net Product Sales | Accounts Receivable | |||||
Year Ended December 31, 2019 | As of December 31, 2019 | |||||
Customer A | 34 | % | 28 | % | ||
Customer B | 31 | % | 39 | % | ||
Customer C | 30 | % | 33 | % | ||
Total | 95 | % | 100 | % | ||
Property and Equipment, Net
Property and equipment is stated at cost less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, which range from two to seven years. Amortization of leasehold improvements is computed using the straight-line method over the shorter of the lease term or the estimated useful life of the related assets. Maintenance and repairs are charged to operating expense as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts, and any gain or loss is included in operating expense.
Leases
At lease commencement, the Company records a lease liability based on the present value of lease payments over the expected lease term. The Company calculates the present value of lease payments using the discount rate implicit in the lease, unless that rate cannot be readily determined. In that case, the Company uses its incremental borrowing rate, which is the rate of interest that the Company would have to pay to borrow on a collateralized basis an amount equal to the lease payments over the expected lease term. The Company records a corresponding right-of-use lease asset based on the lease liability, adjusted for any lease incentives received and any initial direct costs paid to the lessor prior to the lease commencement date.
After lease commencement, the Company measures its leases as follows: (i) the lease liability based on the present value of the remaining lease payments using the discount rate determined at lease commencement; and (ii) the right-of-use lease asset based on the remeasured lease liability, adjusted for any unamortized lease incentives received, any unamortized initial direct costs and the cumulative difference between rent expense and amounts paid under the lease agreement. Any lease incentives received and any initial direct costs are amortized on a straight-line basis over the expected lease term. Rent expense is recorded on a straight-line basis over the expected lease term.
Revenue Recognition
The Company adopted the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”) Topic 606 – Revenue from Contracts with Customers (“ASC 606”) at the time of its first commercial shipment of GIAPREZA in the first quarter of 2018. The Company had no revenue from product sales prior to the first quarter of 2018. There have been no contract assets or liabilities recorded to date relating to product sales.
F - 7
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
Under ASC 606, the Company recognizes revenue when its customers obtain control of the Company’s product, which typically occurs on delivery. Revenue is recognized in an amount that reflects the consideration that the Company expects to receive in exchange for those goods. To determine revenue recognition for contracts with customers within the scope of ASC 606, the Company performs the following 5 steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies the relevant performance obligations.
Revenue from product sales is recorded at the transaction price, net of estimates for variable consideration consisting of chargebacks, discounts, returns and administrative fees. Variable consideration is estimated using the expected-value amount method, which is the sum of probability-weighted amounts in a range of possible consideration amounts. Actual amounts of consideration ultimately received may differ from the Company’s estimates. If actual results vary materially from the Company’s estimates, the Company will adjust these estimates, which will affect revenue from product sales and earnings in the period such estimates are adjusted. These items include:
• | Chargebacks—Chargebacks are discounts the Company provides to distributors in the event that the sales prices to end users are below the distributors’ acquisition price. This may occur due to a direct contract with a health system, a group purchasing organization (“GPO”) agreement or a sale to a government facility. Chargebacks are estimated based on known chargeback rates and recorded as a reduction of revenue on delivery to the Company’s customers. |
• | Discounts—The Company offers customers various forms of incentives and consideration, including prompt-pay and other discounts. The Company estimates discounts primarily based on contractual terms. These discounts are recorded as a reduction of revenue on delivery to the Company’s customers. |
• | Returns—The Company offers customers a limited right of return, generally for damaged or expired product. The Company estimates returns based on an internal analysis, which includes actual experience. The estimates for returns are recorded as a reduction of revenue on delivery to the Company’s customers. |
• | Administrative Fees—The Company pays administrative fees to GPOs for services and access to data. Additionally, the Company pays an Industrial Funding Fee as part of the U.S. General Services Administration’s Federal Supply Schedules program. These fees are based on contracted terms and are paid after the quarter in which the product was purchased by the applicable GPO or government agency. |
The Company will continue to assess its estimates of variable consideration as it accumulates additional historical data and will adjust these estimates accordingly.
Shipping and Handling Expense
Shipping and handling expense is included in cost of product sales.
Research and Development Expense
Research and development expense includes salaries and benefits, facilities and other overhead costs, research-related manufacturing costs, contract service and clinical and preclinical-related service costs performed by clinical research organizations, research institutions and other outside service providers. Research and development expense is charged to operations as incurred when the expenditures relate to the Company’s research and development efforts and have no alternative future uses.
In accordance with certain research and development agreements, the Company is obligated to make certain upfront payments upon execution of the agreement. Advance payments, including nonrefundable amounts, for materials or services that will be used or rendered for future research and development activities are deferred and capitalized. Such amounts are recognized as an expense as the related goods are delivered or the related services are performed.
Acquisition or milestone payments that the Company makes in connection with in-licensed technology are expensed as incurred when there is uncertainty in receiving future economic benefits from the licensed technology. The Company considers the future economic benefits from the licensed technology to be uncertain until such
F - 8
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
licensed technology is incorporated into products that are approved for marketing by the FDA or when other significant risk factors are abated. For accounting purposes, management has viewed future economic benefits for all of the Company’s licensed technology to be uncertain.
Patent Costs
Legal costs in connection with approved patents and patent applications are expensed as incurred, as recoverability of such expenditures is uncertain. These costs are recorded in selling, general and administrative expense in the consolidated statements of operations.
Share-based Compensation
The Company accounts for share-based payment arrangements in accordance with ASC 718, Compensation - Stock Compensation (“ASC 718”), which requires the recognition of compensation expense, using a fair-value based method, for all costs related to share-based payments, including stock options and restricted stock awards. These standards require companies to estimate the fair value of share-based payment awards on the date of the grant using an option-pricing model. The Company has elected to account for forfeitures as they occur.
Income Taxes
The Company accounts for income taxes using the asset and liability method. Deferred tax assets and liabilities are determined based on the differences between the financial statement carrying amounts and the income tax basis of assets and liabilities. Deferred tax assets and liabilities are measured using enacted tax rates applicable to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rate is recognized in income in the period that includes the enactment date. A valuation allowance is applied against any deferred tax asset if, based on available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. For uncertain tax positions that meet a “more likely than not” threshold, the Company recognizes the benefit of uncertain tax positions in the consolidated financial statements. The Company’s practice is to recognize interest and penalties, if any, related to uncertain tax positions in income tax expense in the consolidated statements of operations.
Interest Expense
Interest expense and the amortization of issuance costs related to the deferred royalty obligation (see Note 7) are recognized over the expected repayment term of the deferred royalty obligation using the effective interest method. The assumptions used in determining the expected repayment term of the deferred royalty obligation require the Company to make estimates that could impact the effective interest rate. Each reporting period, the Company estimates the expected repayment term of the deferred royalty obligation based on forecasted net sales of GIAPREZA. Changes in interest expense resulting from changes in the effective interest rate, if any, are recorded on a prospective basis.
Net Loss per Share
Basic net loss per share is calculated by dividing net loss by the weighted-average number of common shares outstanding during the period, without consideration of potential common shares. Diluted net loss per share is calculated by dividing net loss by the weighted-average number of common shares outstanding plus potential common shares. Convertible preferred stock, stock options and warrants are considered potential common shares and are included in the calculation of diluted net loss per share using the treasury stock method when their effect is dilutive. Potential common shares are excluded from the calculation of diluted net loss per share when their effect is anti-dilutive. As of December 31, 2019 and 2018, there were 12.4 million and 14.0 million potential common shares, respectively, that were excluded from the calculation of diluted net loss per share because their effect was anti-dilutive.
F - 9
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
Comprehensive Loss
Comprehensive loss is defined as a change in equity during a period from transactions and other events and circumstances from non-owner sources. There have been no items qualifying as other comprehensive loss, and, therefore, comprehensive loss for the periods reported was comprised solely of the Company’s net loss.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment.
Fair Value Measurements
The Company follows the provisions of ASC 820-10, Fair Value Measurements and Disclosures (“ASC 820-10”), which defines fair value, establishes a framework for measuring fair value in GAAP and requires certain disclosures about fair value measurements. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
As a basis for considering such assumptions, ASC 820-10 establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value as follows: Level 1 - observable inputs such as quoted prices in active markets; Level 2 - inputs other than the quoted prices in active markets that are observable either directly or indirectly; and Level 3 - unobservable inputs, in which there is little or no market data, which require the Company to develop its own assumptions. The hierarchy requires the Company to use observable market data, when available, and to minimize the use of unobservable inputs when determining fair value.
The Company’s financial instruments include cash, accounts receivable, inventory, prepaid expenses and other current assets, accounts payable and accrued expenses. The carrying amounts reported in the balance sheets for these financial instruments approximate fair value because of their short-term nature.
In certain cases where there is limited activity or less transparency around inputs to valuation, the related assets or liabilities are classified as Level 3. The Company's deferred royalty obligation is classified as Level 3 in the ASC 820-10, three-tier fair value hierarchy, and its carrying value approximates fair value (see Note 7).
Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
In June 2018, the FASB issued Accounting Standards Update (“ASU”) No. 2018-07, Stock Compensation (Topic 718): Improvements to Nonemployee Share-based Payment Accounting (“ASU 2018-07”). The standard expands the scope of ASC 718 to include share-based payment awards granted to nonemployees in exchange for goods and services. ASU 2018-07 is effective for annual and interim reporting periods beginning after December 15, 2018.
In the first quarter of 2019, the Company adopted ASU 2018-07. Prior to the adoption of ASU 2018-07, share-based payments awards granted to nonemployees were measured at fair value on their grant date, subject to periodic remeasurement, and share-based compensation expense was recognized on a straight-line basis over their vesting terms. After the adoption of ASU 2018-07, the fair value of share-based payment awards granted to nonemployees is not required to be remeasured periodically and share-based compensation expense will continue to be recorded on a straight-line basis over their vesting period, consistent with share-based payment awards granted to employees. As a result of the adoption of ASU 2018-07, the Company remeasured all of its outstanding nonemployee share-based payment awards at fair value and recognized a cumulative-effect adjustment of $0.2 million to accumulated deficit as of January 1, 2019.
F - 10
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
In February 2016, the FASB issued ASU No. 2016-02, Leases (“ASU 2016-02”). This guidance requires lessees to recognize operating leases with a term greater than one year on the balance sheet as a right-of-use asset and corresponding lease liability. ASU 2016-02 is effective for annual and interim reporting periods beginning after December 15, 2018. Although ASU 2016-02 is required to be adopted at the earliest period presented using a modified retrospective approach, the FASB issued ASU No. 2018-11, Leases (Topic 842): Targeted Improvements (“ASU 2018-11”), which allows for an alternative transition method of adoption by recognizing a cumulative-effect adjustment, if any, to the opening balance of retained earnings in the period of adoption.
The Company adopted ASU 2016-02 on January 1, 2019 utilizing the alternative transition method allowed under ASU 2018-11. As a result, the Company recorded a lease liability and right-of-use lease asset of $31.8 million and $16.8 million, respectively, on its balance sheet as of January 1, 2019. The lease liability represents the present value of the remaining lease payments of the Company’s corporate headquarters lease (see Note 10), discounted using the Company’s incremental borrowing rate as of January 1, 2019. The corresponding right-of-use lease asset is recorded based on the lease liability, adjusted for the unamortized lease incentives received and the cumulative difference between rent expense and amounts paid under the corporate headquarters lease. The adoption of ASU 2016-02 did not have a material impact on either the statement of operations or statement of cash flows for the year ended December 31, 2019.
3. Balance Sheet Details
Inventory, Net
Inventory, net consisted of the following (in thousands):
December 31, | |||||||
2019 | 2018 | ||||||
Work-in-process | $ | 1,505 | $ | 1,907 | |||
Finished goods | 706 | 113 | |||||
Total inventory, net | $ | 2,211 | $ | 2,020 | |||
As of December 31, 2019 and December 31, 2018, total inventory is recorded net of $0.1 million and $0.8 million, respectively, of inventory reserves.
Property and Equipment, Net
Property and equipment, net consisted of the following (in thousands):
December 31, | |||||||
2019 | 2018 | ||||||
Lab equipment | $ | 9,665 | $ | 9,047 | |||
Furniture and fixtures | 2,598 | 2,573 | |||||
Computer hardware | 1,296 | 1,296 | |||||
Software | 733 | 733 | |||||
Leasehold improvements | 14,504 | 14,504 | |||||
Total property and equipment, gross | 28,796 | 28,153 | |||||
Accumulated depreciation and amortization | (10,407 | ) | (5,886 | ) | |||
Total property and equipment, net | $ | 18,389 | $ | 22,267 | |||
F - 11
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
Accrued Expenses
Accrued expenses consisted of the following (in thousands):
December 31, | |||||||
2019 | 2018 | ||||||
Accrued clinical study costs | $ | 3,496 | $ | 2,430 | |||
Accrued interest expense | 2,692 | 2,260 | |||||
Accrued manufacturing costs | 1,339 | 1,823 | |||||
Accrued other | 1,785 | 1,972 | |||||
Total accrued expenses | $ | 9,312 | $ | 8,485 | |||
4. George Washington University License
In December 2014, the Company entered into a patent license agreement with George Washington University (“GW”), which was amended and restated on March 1, 2016 (the “GW License”) and subsequently assigned to La Jolla Pharma, LLC. Pursuant to the GW License, GW exclusively licensed to the Company certain intellectual property rights relating to GIAPREZA, including the exclusive rights to certain issued patents and patent applications covering GIAPREZA. Under the GW License, the Company is obligated to use commercially reasonable efforts to develop, commercialize, market and sell GIAPREZA. The Company has paid a one-time license initiation fee, annual maintenance fees, an amendment fee, additional payments following the achievement of certain development and regulatory milestones and royalties. As a result of the EC’s approval of GIAPREZA in August 2019, the Company made a milestone payment to GW in the amount of $0.5 million in the first quarter of 2020. The Company is obligated to pay a 6% royalty on net sales of GIAPREZA. The patents and patent applications covered by the GW License are expected to expire between 2029 and 2034, and the obligation to pay royalties under this agreement extends through the last-to-expire patent covering GIAPREZA.
5. Other Income—Related Party
The Company has a non-voting profits interest in a related party, which provides the Company with the potential to receive a portion of the future distributions of profits, if any. Investment funds affiliated with the Chairman of the Company’s board of directors have a controlling interest in the related party. In the fourth quarter of 2019, the Company received distributions of $1.9 million in connection with this profits interest.
6. Shareholders’ (Deficit) Equity
Common Stock
As of December 31, 2019 and 2018, there were 27,195,469 and 26,259,254 shares of common stock, $0.0001 par value, issued and outstanding, respectively.
In March 2018, the Company sold 3,910,000 shares of common stock in an underwritten public offering at a price of $29.50 per share for gross proceeds of approximately $115.3 million. The Company received proceeds of approximately $109.8 million, net of approximately $5.5 million in underwriting commissions, discounts and other issuance costs.
Preferred Stock
As of December 31, 2019 and 2018, 3,906 shares of Series C-12 Convertible Preferred Stock (“Series C-12 Preferred”) were issued, outstanding and convertible into 6,735,378 shares of common stock. As of December 31, 2019, no shares of Series F Convertible Preferred Stock (“Series F Preferred”) were issued and outstanding. As of December 31, 2018, 2,737 shares of Series F Preferred were issued and outstanding, and, in January 2019, all of the these issued and outstanding shares of Series F Preferred were converted into 782,031 shares of common stock.
F - 12
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
The holders of Series C-12 Preferred and Series F Preferred do not have voting rights, other than for general protective rights required by the California General Corporation Law and are not entitled to special dividends. The Series C-12 Preferred have, and the Series F Preferred had, a liquidation preference in an amount equal to $1,000 per share. As of December 31, 2019 and 2018, the Series C-12 Preferred liquidation preference was approximately $3.9 million. As of December 31, 2018, the Series F Preferred liquidation preference was approximately $2.7 million.
Equity Incentive Plans
2013 Equity Incentive Plan
In September 2013, the Company adopted the 2013 Equity Incentive Plan (the “2013 Equity Plan”). The 2013 Equity Plan is an omnibus equity compensation plan that permits the issuance of various types of share-based compensation awards, including stock options, restricted stock awards, stock appreciation rights and restricted stock units, as well as cash awards, to employees, directors and eligible consultants. The 2013 Equity Plan has a 10-year term and permits the issuance of incentive stock options under Section 422 of the Internal Revenue Code of 1986, as amended (“IRC”). The administrator under the plan has broad discretion to establish the terms of awards, including the size, term, exercise price and vesting conditions. Generally, grants to employees vest over four years, with 25% vesting on the one-year anniversary and the remainder vesting either quarterly or monthly thereafter; grants to non-employee directors generally vest over one year on the one-year anniversary.
A total of 9,600,000 shares of common stock have been reserved for issuance under the 2013 Equity Plan. As of December 31, 2019, 3,769,824 shares of common stock remained available for future grants under the 2013 Equity Plan.
2018 Employee Stock Purchase Plan
In July 2018, the Company adopted the 2018 Employee Stock Purchase Plan (the “ESPP”). Under the ESPP, eligible employees may purchase shares of the Company’s common stock twice per month at a price equal to 85% of the closing price of shares of the Company’s common stock on the date of each purchase. Eligible employees purchasing shares under the ESPP are subject to an annual cap equal to the lesser of $25,000 or 10% of the employee’s annual cash compensation. Shares purchased under the ESPP cannot be sold for a period of one year following the purchase date (or such shorter period of time if the participating employee’s employment terminates before this one-year anniversary).
A total of 750,000 shares of common stock have been reserved for issuance under the ESPP. As of December 31, 2019, 568,728 shares of common stock remained available for future grants under the ESPP.
Equity Awards
The activity related to equity awards, which are comprised of stock options and inducement grants, during the year ended December 31, 2019 is summarized as follows:
Equity Awards | Weighted- average Exercise Price per Share | Weighted- average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||
Outstanding at December 31, 2018 | 6,466,214 | $ | 23.26 | |||||||||
Granted(1) | 2,126,023 | $ | 6.33 | |||||||||
Exercised | (5,211 | ) | $ | 6.00 | ||||||||
Cancelled/forfeited | (2,970,186 | ) | $ | 18.29 | ||||||||
Outstanding at December 31, 2019 | 5,616,840 | $ | 19.50 | 4.33 years | $ | — | ||||||
Exercisable at December 31, 2019 | 3,645,726 | $ | 22.76 | 4.48 years | $ | — | ||||||
F - 13
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
(1) In March 2019, the Company issued a stock option grant to the Company’s recently appointed Chief Commercial Officer to purchase 80,000 shares of common stock. The grant was awarded as an inducement grant outside of the 2013 Equity Plan. On the first anniversary of the grant date, 25% of the underlying shares become exercisable with the remaining shares vesting on a monthly basis over the subsequent three years, subject to continued service during that time.
The total intrinsic value of equity awards exercised during the years ended December 31, 2019 and 2018 were less than $0.1 million and $1.4 million, respectively. The total grant-date fair value of equity awards vested during the years ended December 31, 2019 and 2018 was $25.9 million and $38.0 million, respectively.
Share-based Compensation Expense
For the years ended December 31, 2019 and 2018, respectively, the weighted-average grant date fair value per stock option was $4.99 and $20.52, respectively. The Company estimates the fair value of each stock option grant on the grant date using the Black-Scholes option-pricing model (the “Black-Scholes model”) with the following assumptions:
Year Ended December 31, | |||||
2019 | 2018 | ||||
Volatility | 97 | % | 114 | % | |
Expected life (years) | 6.05 | 6.07 | |||
Risk-free interest rate | 2.5 | % | 2.8 | % | |
Dividend yield | — | — | |||
Expected volatility is based on the historical volatility of shares of the Company’s common stock. In determining the expected life of employee stock options, the Company uses the “simplified” method. The expected life assumptions for stock options granted to nonemployees, other than nonemployee directors, are based upon the contractual term of the stock options. The risk-free interest rate is based on the U.S. Treasury yield for a period consistent with the expected term of the stock options in effect at the time of the grants. The dividend yield assumption is based on the expectation of no future dividend payments by the Company.
In addition to assumptions used in the Black-Scholes model, the Company reduces share-based compensation expense based on actual forfeitures in the period that each forfeiture occurs.
Under the ESPP, eligible employees may purchase shares of the Company’s common stock twice per month at a price equal to 85% of the closing price of shares of the Company’s common stock on the date of each purchase. The benefit received by the employees, which is equal to a 15% discount on the shares of the Company’s common stock purchased, is recognized as share-based compensation expense on the date of each purchase. The Company recorded $0.1 million and $0.2 million of share-based compensation related to the ESPP for the years ended December 31, 2019 and 2018, respectively. As of December 31, 2019, there was no unrecognized share-based compensation expense related to shares of common stock issued under the ESPP.
The classification of share-based compensation expense is summarized as follows (in thousands):
Year Ended December 31, | |||||||
2019 | 2018 | ||||||
Research and development | $ | 14,861 | $ | 21,113 | |||
Selling, general and administrative | 8,872 | 14,038 | |||||
Total share-based compensation expense | $ | 23,733 | $ | 35,151 | |||
F - 14
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
As of December 31, 2019, $18.4 million of total unrecognized share-based compensation expense related to unvested stock options remains and is expected to be recognized over a weighted-average period of approximately 2.3 years.
Third-party Share-based Compensation Expense
The Company estimates the fair value of stock options and warrants issued to nonemployees, other than nonemployee directors, on the grant date using the Black-Scholes model.
In December 2014, the Company granted warrants to purchase 51,000 shares of common stock to two outside third-parties at an exercise price equal to the fair market value of the stock on the grant dates. One grant vested 25% on each anniversary date over four years. The other grant vested 100% on the one-year anniversary of the grant. In January 2016, the Company granted warrants to purchase 17,000 shares of common stock to an outside third-party at an exercise price equal to the fair market value of the stock on the date of each grant. The grant vested 100% on the one-year anniversary of the grant. In January 2017, the Company granted warrants to purchase 25,013 shares of common stock to an outside third-party at an exercise price equal to the fair market value of the stock on the date of each grant. The grant vested 100% on the one-year anniversary of the grant.
In March 2018, the Company issued 43,056 shares of common stock in a cashless exercise of 83,013 warrants to a third-party warrant holder. As of December 31, 2019, the Company had outstanding warrants to purchase 10,000 shares of common stock and did not recognize share-based compensation expense for these outstanding warrants for the years ended December 31, 2019 and 2018.
7. Deferred Royalty Obligation
In May 2018, the Company closed a $125.0 million royalty financing agreement (the “Royalty Agreement”) with HealthCare Royalty Partners (“HCR”). Under the terms of the Royalty Agreement, the Company received $125.0 million in exchange for tiered royalty payments on worldwide net sales of GIAPREZA. HCR is entitled to receive quarterly royalties on worldwide net sales of GIAPREZA beginning April 1, 2018. Quarterly payments to HCR under the Royalty Agreement start at a maximum royalty rate, with step-downs based on the achievement of annual net product sales thresholds. Through December 31, 2021, the royalty rate will be a maximum of 10%. Starting January 1, 2022, the maximum royalty rate may increase by 4% if an agreed-upon, cumulative net product sales threshold has not been met, and, starting January 1, 2024, the maximum royalty rate may increase by an additional 4% if a different agreed-upon, cumulative net product sales threshold has not been met. The Royalty Agreement is subject to maximum aggregate royalty payments to HCR of $225.0 million. The Royalty Agreement expires upon the first to occur of January 1, 2031 or when the maximum aggregate royalty payments have been made. The Royalty Agreement was entered into by the Company’s wholly-owned subsidiary, La Jolla Pharma, LLC, and HCR has no recourse under the Royalty Agreement against La Jolla Pharmaceutical Company or any assets other than GIAPREZA.
On receipt of the $125.0 million payment from HCR, the Company recorded a deferred royalty obligation of $125.0 million, net of issuance costs of $0.7 million. For the years ended December 31, 2019 and 2018, the Company recognized interest expense, including amortization of the obligation discount, of $10.8 million and $7.3 million, respectively. The carrying value of the deferred royalty obligation as of December 31, 2019 was $124.4 million, net of unamortized obligation discount of $0.6 million, and was classified as noncurrent. The related accrued interest expense liability was $15.5 million and $6.8 million as of December 31, 2019 and December 31, 2018, respectively, of which $12.8 million and $4.5 million was classified as other noncurrent liabilities, respectively. For the years ended December 31, 2019 and 2018, the Company made royalty payments to HCR of $2.0 million and $0.5 million, respectively, and, as of December 31, 2019, the Company recorded royalty obligations payable of $0.7 million in accrued expenses. The deferred royalty obligation is classified as Level 3 in the ASC 820-10, three-tier fair value hierarchy, and its carrying value approximates fair value.
Under the terms of the Royalty Agreement, La Jolla Pharma, LLC has certain obligations, including the obligation to use commercially reasonable and diligent efforts to commercialize GIAPREZA. If La Jolla Pharma, LLC is held to not have met these obligations, HCR would have the right to terminate the Royalty Agreement and demand payment from La Jolla Pharma, LLC of either $125.0 million or $225.0 million (depending on which
F - 15
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
obligation La Jolla Pharma, LLC is held to not have met), minus aggregate royalties already paid to HCR. In the event that La Jolla Pharma, LLC fails to timely pay such amount if and when due, HCR would have the right to foreclose on the GIAPREZA-related assets. The Company concluded that certain of these contract provisions that could result in an acceleration of amounts due under the Royalty Agreement are embedded derivatives that require bifurcation from the deferred royalty obligation and fair value recognition. The Company determined the fair value of each derivative by assessing the probability of each event occurring, as well as the potential repayment amounts and timing of such repayments that would result under various scenarios. As a result of this assessment, the Company determined that the fair value of the embedded derivatives is immaterial as of December 31, 2019. Each reporting period, the Company estimates the fair value of the embedded derivatives until the features lapse and/or the termination of the Royalty Agreement. Any change in the fair value of the embedded derivatives will be recorded as either a gain or loss on the consolidated statements of operations.
8. Defined Contribution Plan
The Company has a defined contribution plan (the “401(k) Plan”) covering substantially all of the Company’s employees. The 401(k) Plan is a tax-qualified retirement saving plan, pursuant to which all employees are able to contribute the lesser of 50% of their annual compensation (as defined) or the limit prescribed by the Internal Revenue Service to the 401(k) Plan on a before-tax basis. The Company matches employee contributions to the 401(k) Plan based on each participant’s contribution during the plan year, up to 3.5% of each participant’s annual compensation.
For the years ended December 31, 2019 and 2018, the Company made matching contributions to the 401(k) Plan of $0.9 million and $1.4 million, respectively.
9. Income Taxes
For the years ended December 31, 2019 and 2018, the Company did not record a provision for income taxes, as the Company recorded a full valuation allowance against its deferred tax assets.
Deferred tax assets are as follows (in thousands):
December 31, | |||||||
2019 | 2018 | ||||||
Deferred tax assets: | |||||||
Net operating loss carryforwards | $ | 81,406 | $ | 60,105 | |||
Research and development credits | 24,011 | 21,262 | |||||
Deferred royalty obligation | 30,460 | 31,937 | |||||
Share-based compensation expense | 9,581 | 11,904 | |||||
Depreciation and amortization expense | — | 1,033 | |||||
Lease liability | 7,127 | — | |||||
Other | 1,263 | 1,004 | |||||
Total gross deferred tax assets | 153,848 | 127,245 | |||||
Deferred tax liabilities: | |||||||
Depreciation and amortization | (1,612 | ) | — | ||||
Right-of-use lease asset | (3,775 | ) | — | ||||
Valuation allowance | (148,461 | ) | (127,245 | ) | |||
Net deferred tax assets | $ | — | $ | — | |||
F - 16
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
The difference between income taxes computed using the U.S. federal income effective tax rate and the provision for income taxes is as follows (in thousands):
Year Ended December 31, | |||||||
2019 | 2018 | ||||||
Federal statutory rate | $ | (24,467 | ) | $ | (41,888 | ) | |
State tax benefit | (3,825 | ) | (14,449 | ) | |||
Change in valuation allowance | 102,583 | 55,167 | |||||
Share-based compensation expense | 7,532 | 4,041 | |||||
State rate true-up | 2,513 | — | |||||
Section 382 limited tax attributes/expired | — | 1,936 | |||||
Establishment of NOLs and credits, post-Section 382 ownership change | (81,368 | ) | — | ||||
Research and development credits | (2,599 | ) | (5,164 | ) | |||
Foreign rate differential | (62 | ) | (58 | ) | |||
Other permanent differences | (307 | ) | 415 | ||||
Provision for income taxes | $ | — | $ | — | |||
As of December 31, 2019 and 2018, the Company established a full valuation allowance against its federal and state deferred tax assets due to the uncertainty surrounding the realization of such assets.
Pursuant to Section 382 and 383 of the IRC, utilization of the Company’s federal net operating loss (“NOL”)
carryforwards and research and development credit carryforwards may be subject to annual limitations in the event of any significant changes in its ownership structure. These annual limitations may result in the expiration of net operating loss carryforwards and research and development credit carryforwards, unless utilized. The Company has completed an IRC Section 382 and 383 analysis regarding the limitation of net operating loss carryforwards and research and development credit carryforwards through March 31, 2019. As a result of a Section 382 ownership change in September 2013, the Company recorded a reduction to its federal and state net operating loss carryforwards of $341.3 million and $170.9 million, respectively. In addition, the Company recorded a reduction to its federal research and development credit carryforwards of $15.5 million.
As of December 31, 2019, the Company had federal and state net operating loss carryforwards of $305.6 million and $227.2 million, respectively. In addition, the Company had estimated federal and California research and development credit carryforwards of $10.8 million and $16.7 million, respectively. Federal net operating loss carryforwards of $183.8 million, state net operating loss carryforwards of $227.2 million and federal research and development credit carryforwards of $10.8 million will begin to expire in 2034, unless utilized. Federal net operating loss carryforwards of $121.8 million and California research and development credit carryforwards of $16.7 million will carry forward indefinitely, unless utilized.
There were no unrecognized tax benefits as of December 31, 2019 and 2018. The Company does not anticipate there will be a significant change in unrecognized tax benefits within the next 12 months.
The Company had no accrual for interest or penalties on the Company’s consolidated balance sheets as of December 31, 2019 or December 31, 2018, and has not recognized interest and/or penalties in the consolidated statements of operations for the years ended December 31, 2019 and 2018.
The Company is subject to taxation in the U.S. and various state jurisdictions. The Company’s tax returns since inception are subject to examination by the U.S. and various state tax authorities. The Company is not currently undergoing a tax audit in any federal or state jurisdiction.
10. Commitments and Contingencies
Leases
On December 29, 2016, the Company entered into an agreement with BMR-Axiom LP to lease office and laboratory space as its corporate headquarters located at 4550 Towne Centre Court, San Diego, California (the
F - 17
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
“Lease”) for a period of 10 years commencing on October 30, 2017 (the “Initial Lease Term”). The Company has an option to extend the Lease for an additional 5 years at the end of the Initial Lease Term.
The Company provided a standby letter of credit for $0.9 million in lieu of a security deposit. This amount will decrease to $0.6 million after year two of the Initial Lease Term and decrease to $0.3 million after year 5 of the Initial Lease Term. As of December 31, 2019, $0.9 million was pledged as collateral for such letter of credit and recorded as restricted cash. The annual rent under the Lease is subject to escalation during the term. In addition to rent, the Lease requires the Company to pay certain taxes, insurance and operating costs relating to the leased premises. The Lease contains customary default provisions, representations, warranties and covenants. The Lease is classified as an operating lease.
Future minimum lease payments under the Lease as of December 31, 2019 are as follows (in thousands):
2020 | $ | 4,058 | |
2021 | 4,174 | ||
2022 | 4,294 | ||
2023 | 4,417 | ||
2024 | 4,544 | ||
Thereafter | 13,590 | ||
Total future minimum lease payments | $ | 35,077 | |
Less: discount | (5,830 | ) | |
Total lease liability | $ | 29,247 | |
The Lease provided an allowance for tenant improvements of $13.7 million, which is classified as leasehold improvements on the Company’s consolidated balance sheet and is being amortized on a straight-line basis over the Initial Lease Term.
The Company recorded a lease liability for the Lease based on the present value of the Lease payments over the Initial Lease Term, discounted using the Company’s incremental borrowing rate. The Company recorded a corresponding right-of-use lease asset based on the lease liability, adjusted for incentives received prior to the Lease commencement date. The option to extend the Initial Lease Term was not recognized as a part of either the Company’s lease liability or right-of-use lease asset. Lease expense was $2.8 million for the years ended December 31, 2019 and 2018. Amortization for the right-of-use lease asset was $1.3 million for the year ended December 31, 2019.
Licensing Agreements
In the normal course of business, the Company enters into licensing agreements under which the Company commits to certain annual maintenance payments. Annual future minimum licensing payments under the Company’s agreements as of December 31, 2019 are as follows (in thousands):
2020 | $ | 99 | |
2021 | 99 | ||
2022 | 99 | ||
2023 | 99 | ||
2024 | 99 | ||
Total future minimum license payments | $ | 495 | |
Supply Agreements
In the normal course of business, the Company enters into agreements for the manufacturing and supply of GIAPREZA and LJPC-0118. In 2017, the Company entered into agreements arranging for the manufacture and supply of GIAPREZA through 2022. During this time, the Company is obligated to make certain minimum
F - 18
La Jolla Pharmaceutical Company
Notes to the Consolidated Financial Statements
purchases. Annual future minimum payments for manufacturing and supply agreements as of December 31, 2019 are as follows (in thousands):
2020 | $ | 1,976 | |
2021 | 1,976 | ||
2022 | 611 | ||
Total future minimum manufacturing and supply agreement payments | $ | 4,563 | |
11. Company-wide Realignment and Restructuring Plan
On October 18, 2018, the Company effected a Company-wide realignment. For the year ended December 31, 2018, total expense for these activities was $4.0 million, with $1.6 million included in research and development expense and $2.4 million included in selling, general and administrative expense. Total expense was comprised of $7.7 million for severance costs, offset by a $3.7 million reversal of non-cash, share-based compensation expense related to forfeited, unvested equity awards. As of March 31, 2019, all severance costs had been paid. No expense for these activities was recorded for the year ended December 31, 2019.
On December 2, 2019, the Board of Directors of the Company approved a restructuring plan that reduced the Company’s headcount (the “2019 Realignment”). The 2019 Realignment did not result in any reductions in headcount in the Company’s commercial organization supporting GIAPREZA. For the year ended December 31, 2019, total expense for these activities was $4.9 million, $4.4 million of which is included in research and development expense and $0.5 million of which is included in general and administrative expense. Total expense was comprised of $5.8 million for one-time termination benefits to the affected employees, including severance and health care benefits, offset by a $0.9 million reversal of non-cash, share-based compensation expense related to forfeited, unvested equity awards. As of December 31, 2019, the Company had paid $0.9 million of the $5.8 million cash severance and health care benefits charges, and the remaining $4.9 million of the cash severance and health care benefits charges were included in accrued payroll and related expenses. The Company expects to make substantially all of the payments resulting from the 2019 Realignment in the first half of 2020.
F - 19
