Attached files
| file | filename |
|---|---|
| 8-K - ASTERIAS BIOTHERAPEUTICS, INC 8-K 8-22-2016 - Asterias Biotherapeutics, Inc. | form8k.htm |
Exhibit 99.1

Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE: ASTAugust 2016 14219863Text 04698Text 230237246Text

Statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ Registration Statement on Form S-3 and Prospectus, as well as its other periodic reports, filed with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements.Asterias has filed with the Securities and Exchange Commission ("SEC") a registration statement (including a prospectus) and has filed or will file with the SEC a prospectus supplement to the prospectus for the offering to which this presentation relates. Before you invest, you should read the prospectus supplement and the accompanying prospectus in that registration statement and the documents incorporated by reference or filed as exhibits to the registration statement for more complete information about Asterias and this offering. You may get these documents and other documents for free by visiting EDGAR on the SEC web site www.sec.gov. Forward-Looking Statements

Strong Leadership Team with Proven Track Record Name Experience Steve CarttPresident and CEO Former COO of Questcor PharmaceuticalsLed Questcor’s 2007-2014 commercial strategy, turnaround and multiple expansionsGrew company revenue from $10 million to $1 billionOver 30 years of experience in pharma and biotech; at Questcor, Elan, ALZA Pharmaceuticals and ALZA Cor Don BaileyChairman of the Board Former CEO of Questcor Pharmaceuticals, led Questcor’s corporate turnaround Russell SkibstedCFO Also serves as CFO of BioTime, Inc.Over 14 years experience as CFO of life science companies; prior executive positions at Proove, Aeolus, Spectrum, and Hana Jane Lebkowski, Ph.D.CSO Former CSO of GeronOver 30 years experience in R&D of cell and gene therapies at Geron, Applied Immune Sciences, and Rhône Poulenc Rorer Katy Spink, Ph.D.COO Former SVP, Cell Therapy Program Operations at GeronPrior to Geron, Dr. Spink was a management consultant at McKinsey Ed Wirth, M.D., Ph.D.CMO Former CSO of InVivo Therapeutics25 years experience translational research at the University of Chicago, Geron and InVivo Ryan ChavezEVP Finance & GC Former General Counsel for Mallinckrodt ARD divisionAssociate General Counsel at Questcor; financial positions at GE During Mr. Cartt’s and Mr. Bailey’s seven year tenure, Questor’s stock price went from $0.35/share in August 2007 to the acquisition price of $93.58/share in 2014During this period, Questcor’s valuation increased over 200x, from $25 million to the company’s acquisition by Mallinckrodt for $5.8 billion 14219863Text 04698Text 230237246Text
Key Highlights AST-OPC1 (Pluripotent stem cell platform)Ongoing Phase 1/2a trial in spinal cord injury (SCI)Positive safety profile to-date in two safety cohorts (thoracic SCI trial and cervical SCI trial) Early signs of possible efficacy - one motor level improvement in all three patients in very low dose 2m cell safety cohortFirst efficacy cohort of 10m cells dosed; 1st readout January 2017, additional readouts 2017-2018AST-VAC1 (Cancer Immunotherapy platform – autologous)12-month process development effort underway (key critical path item for program progression)Positive data from completed Phase 2 studyCompleted positive end-of-Phase 2 FDA meetingAST-VAC2 (Cancer immunotherapy platform – allogeneic)Currently finalizing protocol with CRUK; Planned MHRA submission Q4 2016; trial starting Q1 2017 Planning Phase 1/2a trial in non-small cell lung cancer (NSCLC)Fully sponsored by Cancer Research UK (CRUK) 14219863Text 04698Text 230237246Text
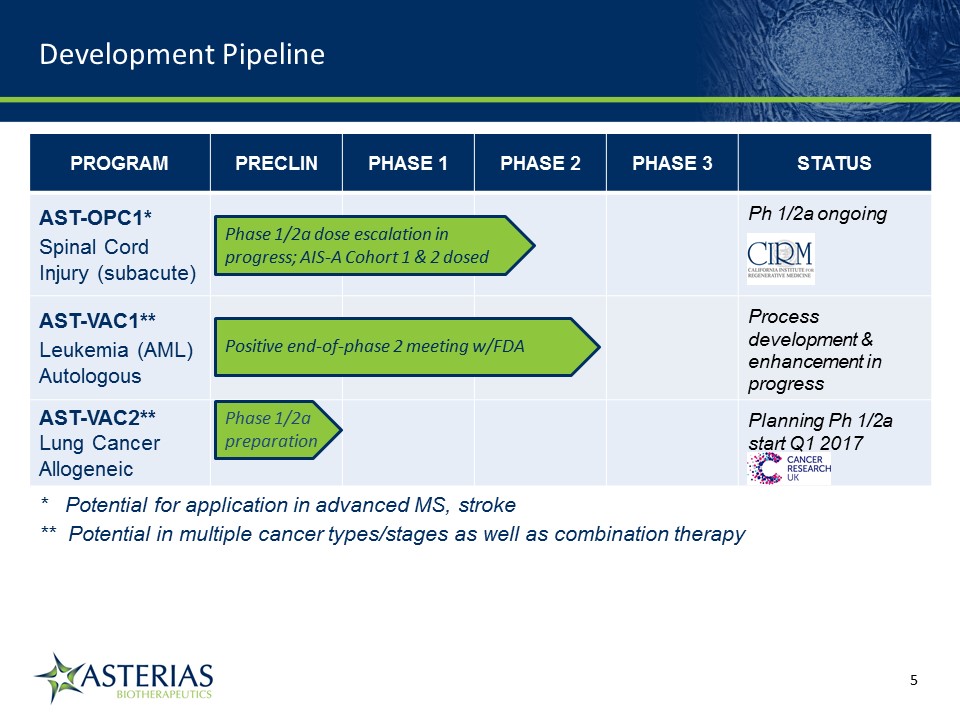
PROGRAM PRECLIN PHASE 1 PHASE 2 PHASE 3 STATUS AST-OPC1*Spinal Cord Injury (subacute) Ph 1/2a ongoing AST-VAC1**Leukemia (AML) Autologous Process development & enhancement in progress AST-VAC2**Lung Cancer Allogeneic Planning Ph 1/2a start Q1 2017 * Potential for application in advanced MS, stroke** Potential in multiple cancer types/stages as well as combination therapy Phase 1/2a dose escalation in progress; AIS-A Cohort 1 & 2 dosed Positive end-of-phase 2 meeting w/FDA Phase 1/2a preparation Development Pipeline 14219863Text 04698Text 230237246Text
Pluripotent Stem Cells Scalable source from well-established stem cell lines to produce desired cell types resulting in “off the shelf” therapeutics to restore functional tissueAST-OPC1: Neural cell type (oligodendrocyte progenitor cells, OPCs); multiple MOAs to restore lost function in neurodegenerative diseasesDoes not utilize any fetal tissue or adult cellsCancer Immunotherapy - AutologousPatient-specific treatment to stimulate patients’ immune system to attack cells which express telomerase, a protein that is expressed in over 95% of cancersAST-VAC1: Patient-specific (autologous) based on a patient’s own cells Cancer Immunotherapy - AllogeneicStem cell derived treatment designed to stimulate patients’ immune system to attack cells which express telomeraseAST-VAC2: Non-patient specific (allogeneic) based on pluripotent stem cells Clinical-Stage Regenerative Platform Technologies 14219863Text 04698Text 230237246Text

Pluripotent stem cell platformSignificant grant funding from California Institute of Regenerative Medicine (CIRM)Phase 1/2a clinical trial in spinal cord injury in progress AST-OPC1 14219863Text 04698Text 230237246Text
Spinal Cord Injury: High Unmet Medical Need, Substantial Commercial Opportunity Spinal cord injury (SCI) affects 17,000 patients per year in U.S.(1)Primarily affects young/healthy males in their 20s and 30s at time of injuryCan be devastating to quality of life and ability to engage in activities of daily livingNo currently approved therapiesLifetime direct healthcare costs for a 25 year old patient can reach $5 million(1)63% of cervical injury patients are unemployed even 8 years post-injurySignificant commercial opportunity in helping restore some motor function, increasing independence and lessening daily care burden(2)AST-OPC1 may be potentially applied to other neurodegenerative disorders (e.g. MS, stroke) 14219863Text 04698Text 230237246Text (1) National Spinal Cord Injury Statistical Center, Facts and Figures 2016(2) Internal estimates
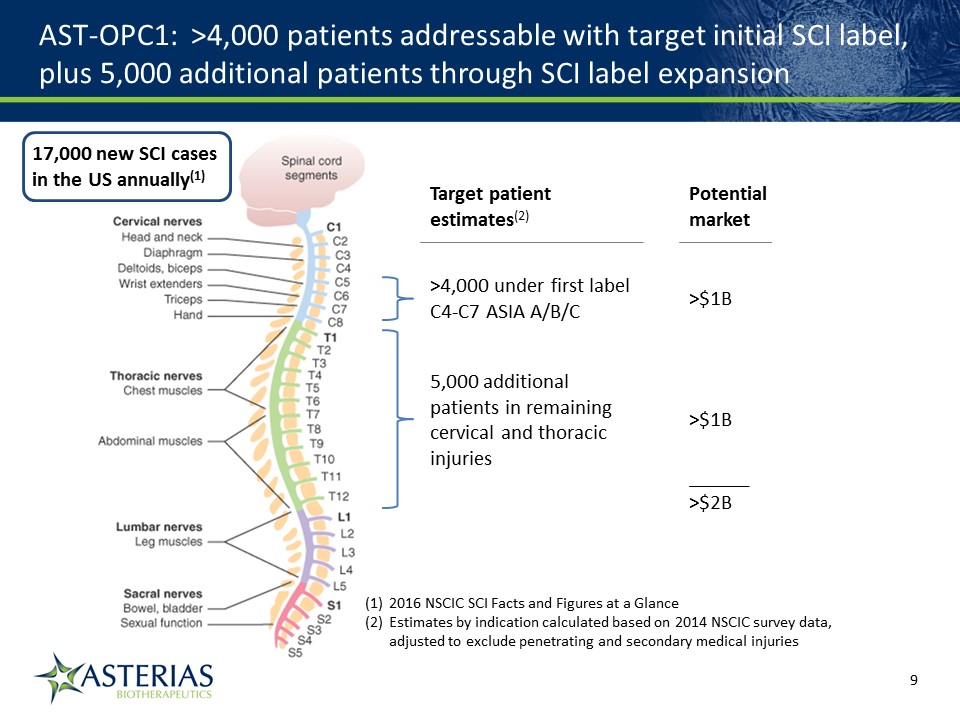
AST-OPC1: >4,000 patients addressable with target initial SCI label, plus 5,000 additional patients through SCI label expansion 17,000 new SCI cases in the US annually(1) >4,000 under first label C4-C7 ASIA A/B/C 2016 NSCIC SCI Facts and Figures at a GlanceEstimates by indication calculated based on 2014 NSCIC survey data, adjusted to exclude penetrating and secondary medical injuries 5,000 additional patients in remaining cervical and thoracic injuries >$1B >$1B Potential market Target patient estimates(2) ______>$2B
AST-OPC1 is a cellular therapy utilizing oligodendrocyte progenitor cells (OPCs) AST-OPC1 is made from a well-established , pluripotent embryonic stem cell line originally created in the 1990s; can serve entire OPC1 product lifecycleImportantly, no fetal tissue or adult cells are usedOPCs, which function to support and myelinate neurons, can be damaged and lost in cases of SCIReplacement of lost OPCs results in:Remyelination of axonsPrevention of cavitationSecretion of neurotrophic factorsImproved motor function AST-OPC1 Addresses the Complex Pathology of SCI 14219863Text 04698Text 230237246Text
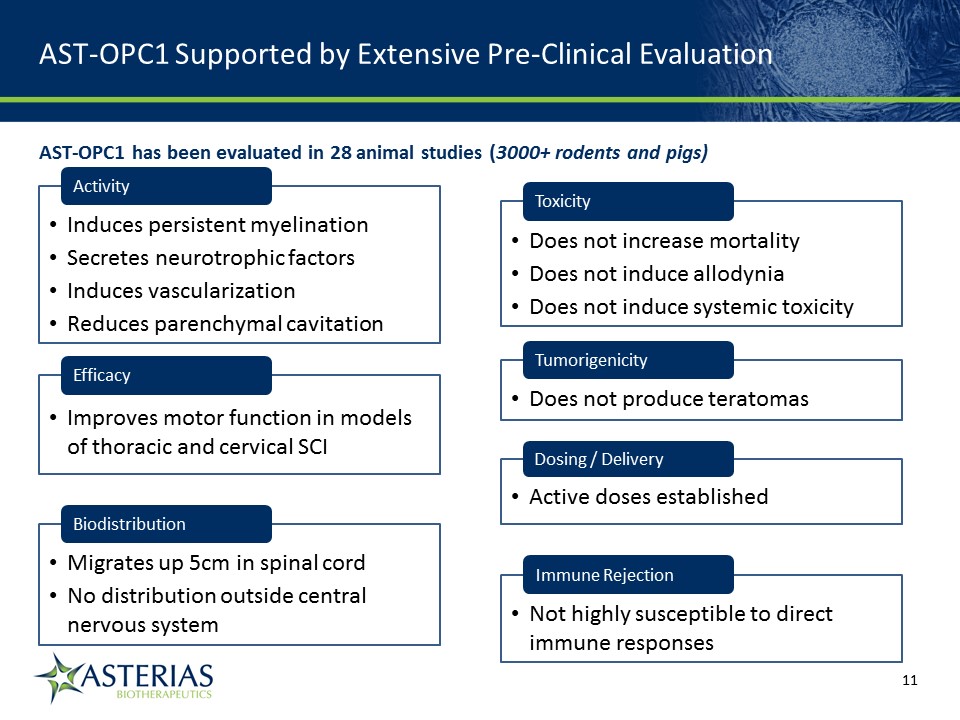
AST-OPC1 Supported by Extensive Pre-Clinical Evaluation 14219863Text 04698Text 230237246Text Activity Induces persistent myelinationSecretes neurotrophic factorsInduces vascularizationReduces parenchymal cavitation Biodistribution Migrates up 5cm in spinal cordNo distribution outside central nervous system Dosing / Delivery Active doses established Toxicity Does not increase mortalityDoes not induce allodyniaDoes not induce systemic toxicity Tumorigenicity Does not produce teratomas Immune Rejection Not highly susceptible to direct immune responses AST-OPC1 has been evaluated in 28 animal studies (3000+ rodents and pigs) Efficacy Improves motor function in models of thoracic and cervical SCI
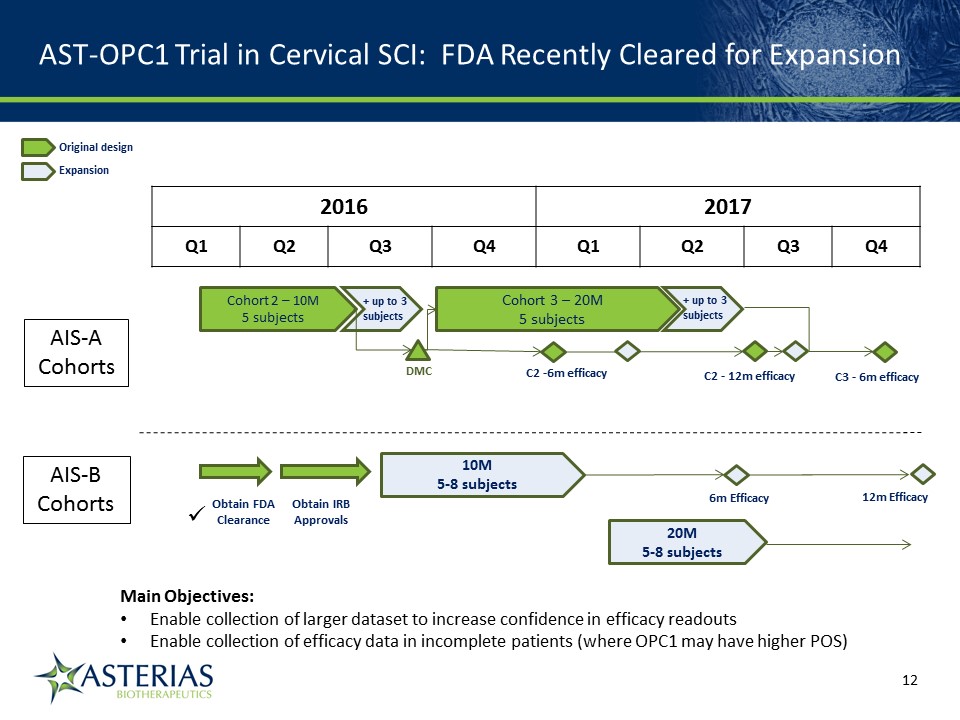
AST-OPC1 Trial in Cervical SCI: FDA Recently Cleared for Expansion 14219863Text 04698Text 230237246Text 2016 2017 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 6m Efficacy DMC Cohort 2 – 10M5 subjects AIS-ACohorts AIS-BCohorts Cohort 3 – 20M5 subjects C2 -6m efficacy C2 - 12m efficacy 10M5-8 subjects 20M5-8 subjects Obtain FDAClearance Obtain IRBApprovals C3 - 6m efficacy + up to 3subjects + up to 3subjects Original design Expansion 12m Efficacy Main Objectives:Enable collection of larger dataset to increase confidence in efficacy readoutsEnable collection of efficacy data in incomplete patients (where OPC1 may have higher POS)
AST-OPC1 Expansion & Acceleration Highlights Addition of two cohorts of AIS-B patients (5-8 subjects each at 10M & 20M cells)On average they have more spared tissue than AIS-A patients, which may lead to greater OPC1-mediated tissue repair and neurological recoveryExpansion to up to 8 AIS-A patients at both the 10M & 20M cell dosesIncrease in the total number of patients from 13 to up to 35 subjectsAdditional changes to the eligibility criteria that will accelerate enrollmentReduction of stagger between cohort enrollmentOverlap of AIS-A and AIS-B enrollment allowedEnrollment of expansion phase projected to complete in Q3 2017 – will provide larger data set that will increase the confidence in the efficacy signal and accelerate a go/no-go decision on advanced clinical development targeting registration 14219863Text 04698Text 230237246Text
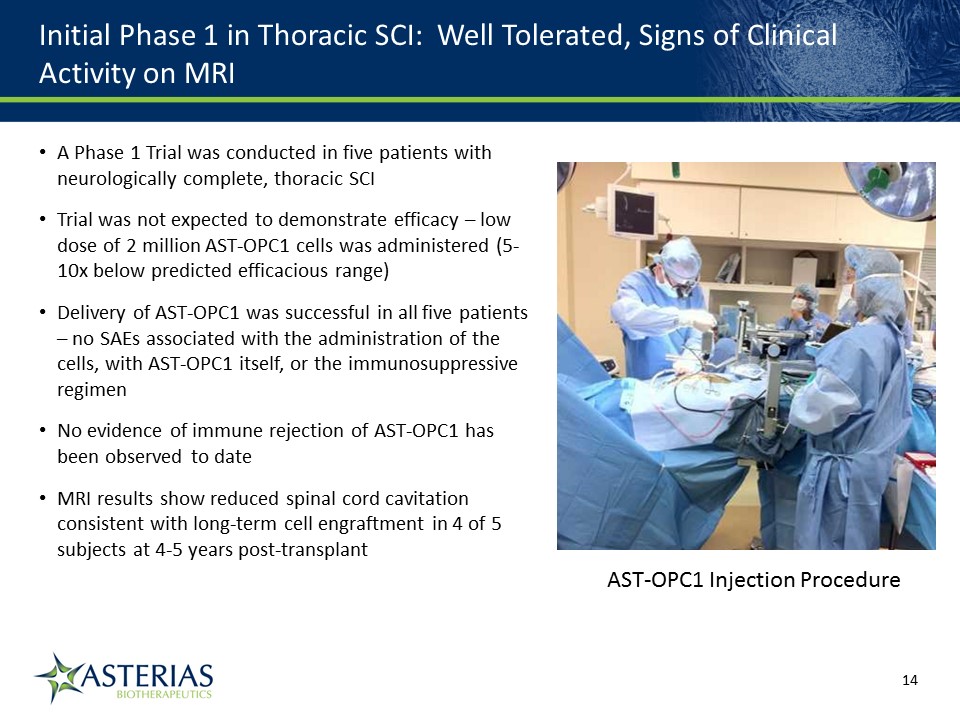
Initial Phase 1 in Thoracic SCI: Well Tolerated, Signs of Clinical Activity on MRI A Phase 1 Trial was conducted in five patients with neurologically complete, thoracic SCITrial was not expected to demonstrate efficacy – low dose of 2 million AST-OPC1 cells was administered (5-10x below predicted efficacious range)Delivery of AST-OPC1 was successful in all five patients – no SAEs associated with the administration of the cells, with AST-OPC1 itself, or the immunosuppressive regimenNo evidence of immune rejection of AST-OPC1 has been observed to dateMRI results show reduced spinal cord cavitation consistent with long-term cell engraftment in 4 of 5 subjects at 4-5 years post-transplant 14219863Text 04698Text 230237246Text AST-OPC1 Injection Procedure

Phase 1 of the Ongoing Phase 1/2a Study in Cervical SCI: Well Tolerated, Measurable Signs of Clinical Activity Phase 1 very low dose (2 million cells), safety-only portion of the ongoing trial was conducted in three patients with neurologically complete, cervical SCINo safety issues observed, confirming favorable safety profile seen in earlier thoracic SCI studyAll three patients have experienced a one motor level improvement in upper extremity motor function compared to baseline – an encouraging early signal particularly given the very low dose administered 14219863Text 04698Text 230237246Text AST-OPC1 Injection Procedure
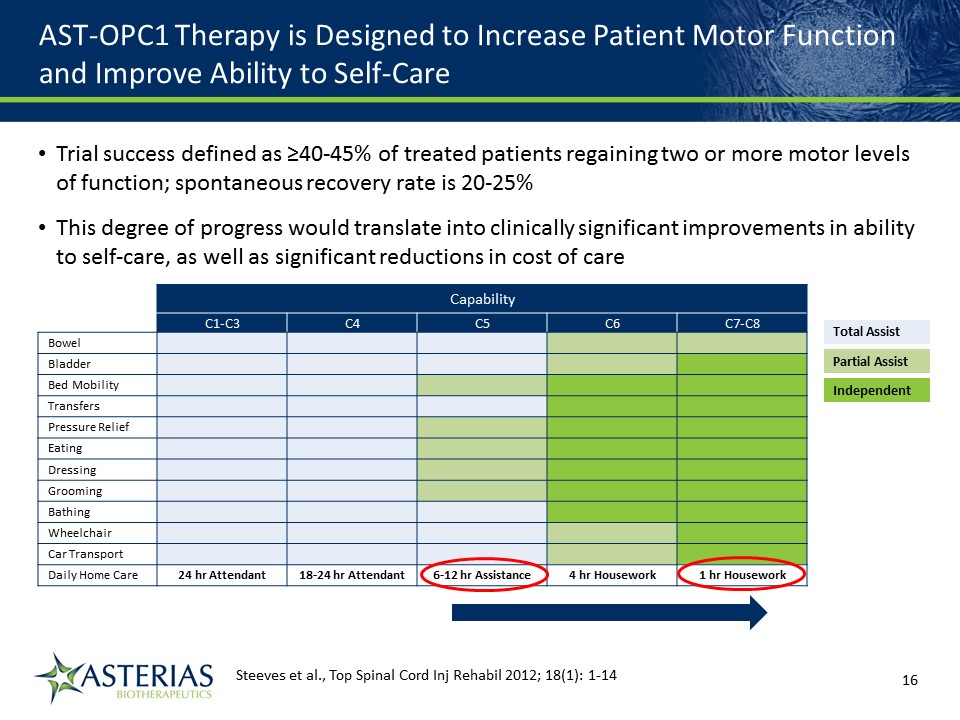
AST-OPC1 Therapy is Designed to Increase Patient Motor Function and Improve Ability to Self-Care Capability C1-C3 C4 C5 C6 C7-C8 Bowel Bladder Bed Mobility Transfers Pressure Relief Eating Dressing Grooming Bathing Wheelchair Car Transport Daily Home Care 24 hr Attendant 18-24 hr Attendant 6-12 hr Assistance 4 hr Housework 1 hr Housework Total Assist Partial Assist Independent Steeves et al., Top Spinal Cord Inj Rehabil 2012; 18(1): 1-14 14219863Text 04698Text 230237246Text Trial success defined as ≥40-45% of treated patients regaining two or more motor levels of function; spontaneous recovery rate is 20-25% This degree of progress would translate into clinically significant improvements in ability to self-care, as well as significant reductions in cost of care
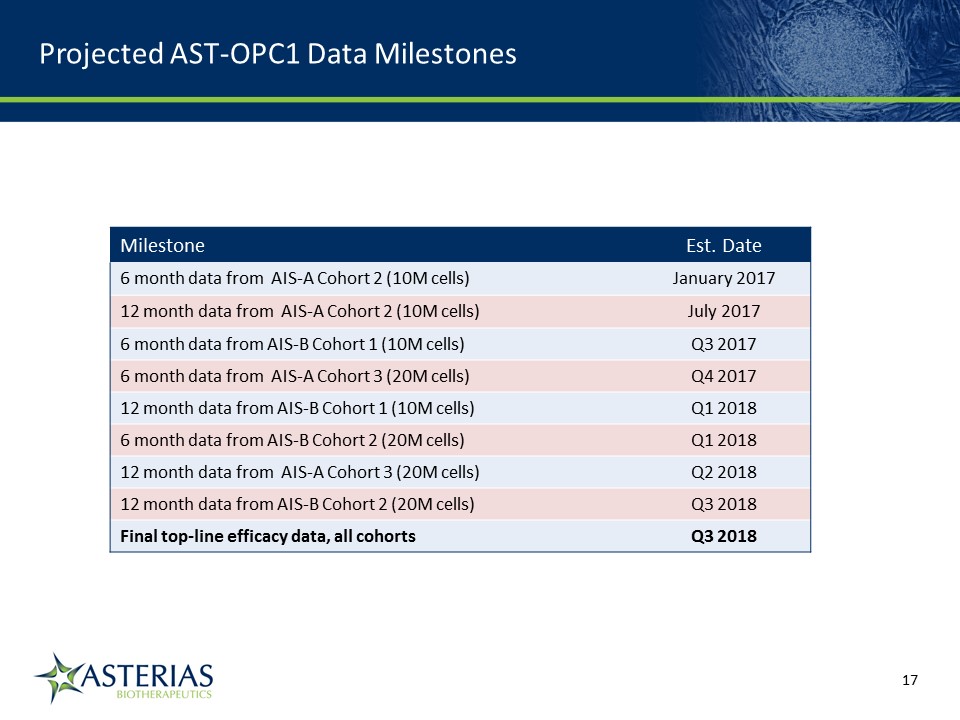
Milestone Est. Date 6 month data from AIS-A Cohort 2 (10M cells) January 2017 12 month data from AIS-A Cohort 2 (10M cells) July 2017 6 month data from AIS-B Cohort 1 (10M cells) Q3 2017 6 month data from AIS-A Cohort 3 (20M cells) Q4 2017 12 month data from AIS-B Cohort 1 (10M cells) Q1 2018 6 month data from AIS-B Cohort 2 (20M cells) Q1 2018 12 month data from AIS-A Cohort 3 (20M cells) Q2 2018 12 month data from AIS-B Cohort 2 (20M cells) Q3 2018 Final top-line efficacy data, all cohorts Q3 2018 Projected AST-OPC1 Data Milestones 14219863Text 04698Text 230237246Text

Patient-specific (autologous)Acute Myelogenous Leukemia (AML)Process development/enhancement in progress AST-VAC1 14219863Text 04698Text 230237246Text

~19,000 new AML cases diagnosed and ~10,500 deaths from AML in U.S. annually(1)AML incidence increases markedly after 60, and older patients do not tolerate AML therapies wellAverage age of an AML patient is 67Patients over 60 often have trouble tolerating intensive treatmentMany are poor candidates/not eligible for hematopoietic cell transplantation (HCT)AST-VAC1 is being developed as a treatment for maintenance of remission in patients >60 years and in high-risk patients, not eligible for transplantsAST-VAC1: has demonstrated a favorable safety profile in Phase 1 and Phase 2 trials conducted to-date AST-VAC1 may also be an attractive alternative to HCT in younger/lower risk patients, and could have utility in myelodysplastic syndromes (MDS) AST-VAC1 Targets AML Need in Older and High-Risk Patients 2014 estimate 14219863Text 04698Text 230237246Text
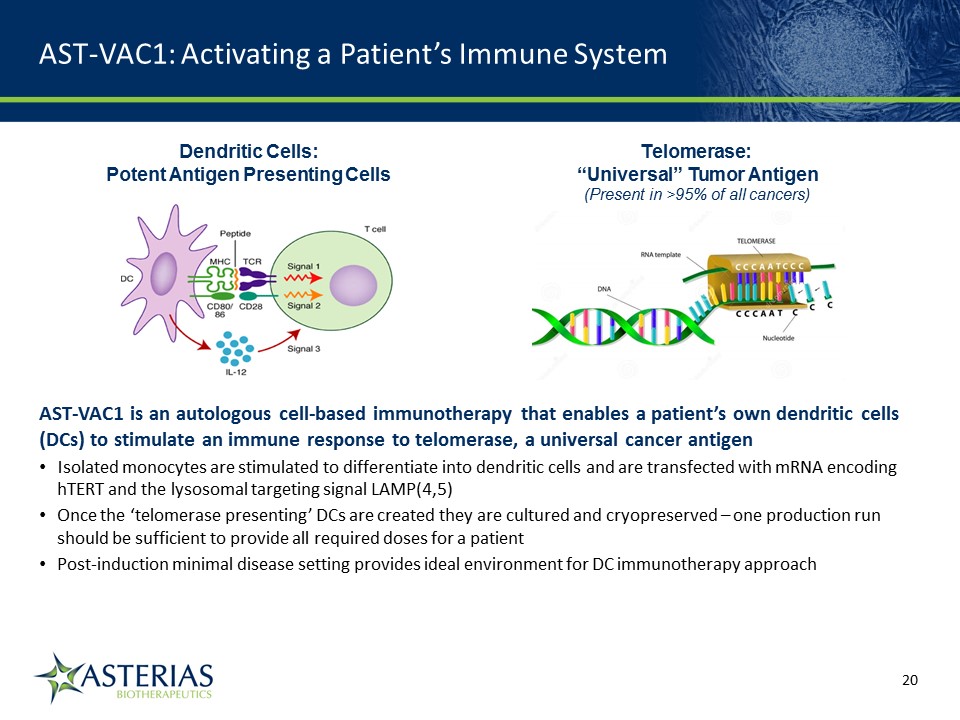
AST-VAC1: Activating a Patient’s Immune System Dendritic Cells: Potent Antigen Presenting Cells AST-VAC1 is an autologous cell-based immunotherapy that enables a patient’s own dendritic cells (DCs) to stimulate an immune response to telomerase, a universal cancer antigenIsolated monocytes are stimulated to differentiate into dendritic cells and are transfected with mRNA encoding hTERT and the lysosomal targeting signal LAMP(4,5)Once the ‘telomerase presenting’ DCs are created they are cultured and cryopreserved – one production run should be sufficient to provide all required doses for a patientPost-induction minimal disease setting provides ideal environment for DC immunotherapy approach Telomerase: “Universal” Tumor Antigen(Present in >95% of all cancers) 14219863Text 04698Text 230237246Text
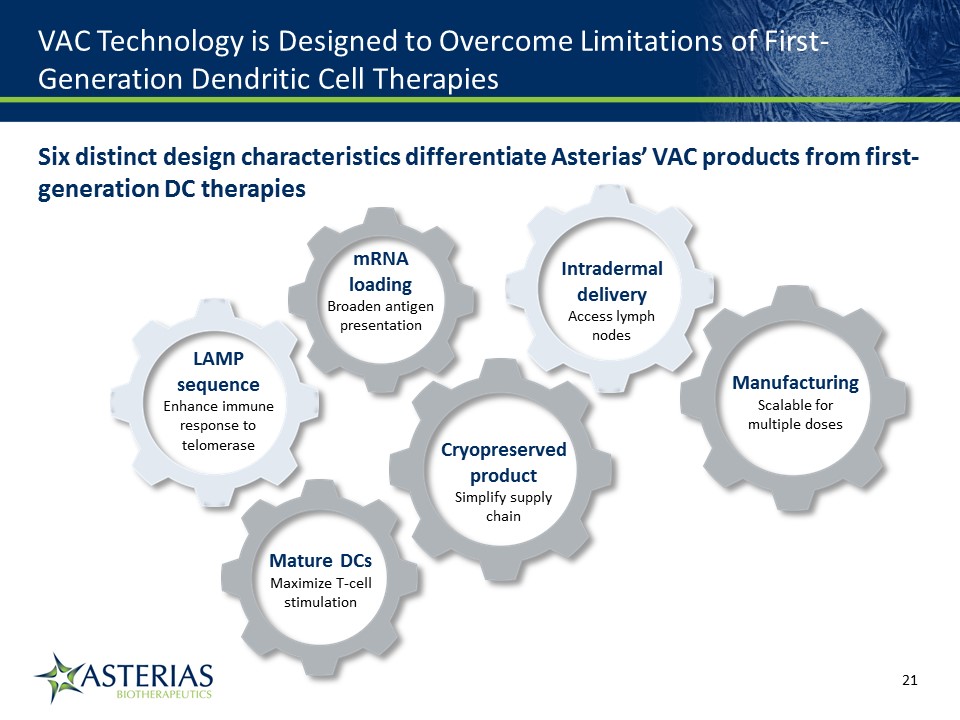
VAC Technology is Designed to Overcome Limitations of First-Generation Dendritic Cell Therapies Six distinct design characteristics differentiate Asterias’ VAC products from first-generation DC therapies LAMP sequenceEnhance immune response to telomerase Cryopreserved productSimplify supply chain Mature DCsMaximize T-cell stimulation ManufacturingScalable for multiple doses mRNA loadingBroaden antigen presentation Intradermal deliveryAccess lymph nodes 14219863Text 04698Text 230237246Text
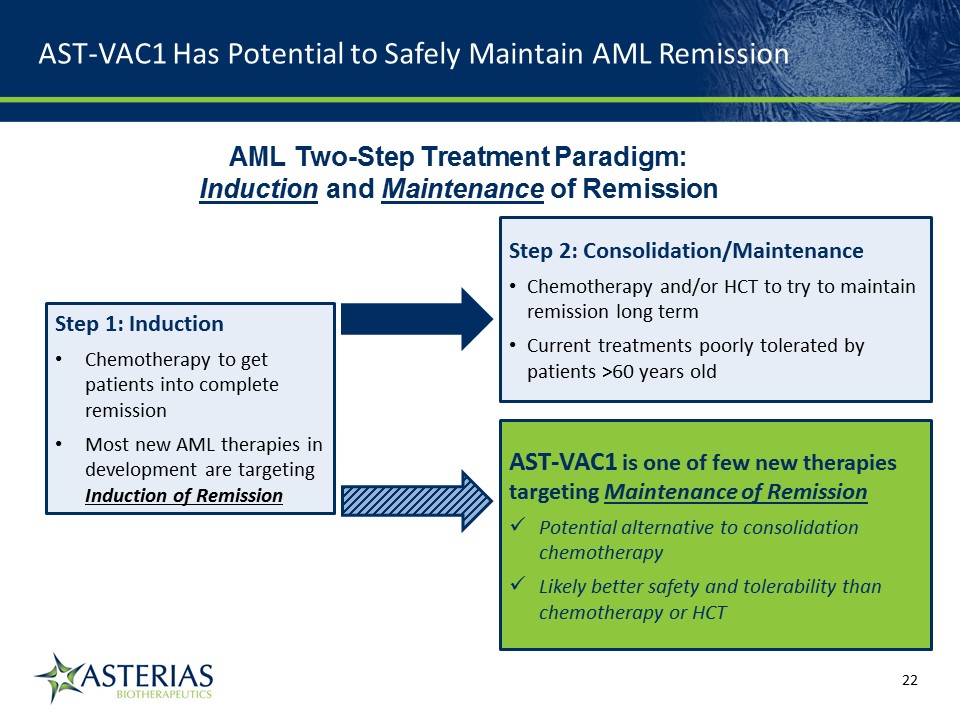
AST-VAC1 Has Potential to Safely Maintain AML Remission AML Two-Step Treatment Paradigm: Induction and Maintenance of Remission Step 1: InductionChemotherapy to get patients into complete remissionMost new AML therapies in development are targeting Induction of Remission Step 2: Consolidation/MaintenanceChemotherapy and/or HCT to try to maintain remission long termCurrent treatments poorly tolerated by patients >60 years old AST-VAC1 is one of few new therapies targeting Maintenance of RemissionPotential alternative to consolidation chemotherapyLikely better safety and tolerability than chemotherapy or HCT 14219863Text 04698Text 230237246Text
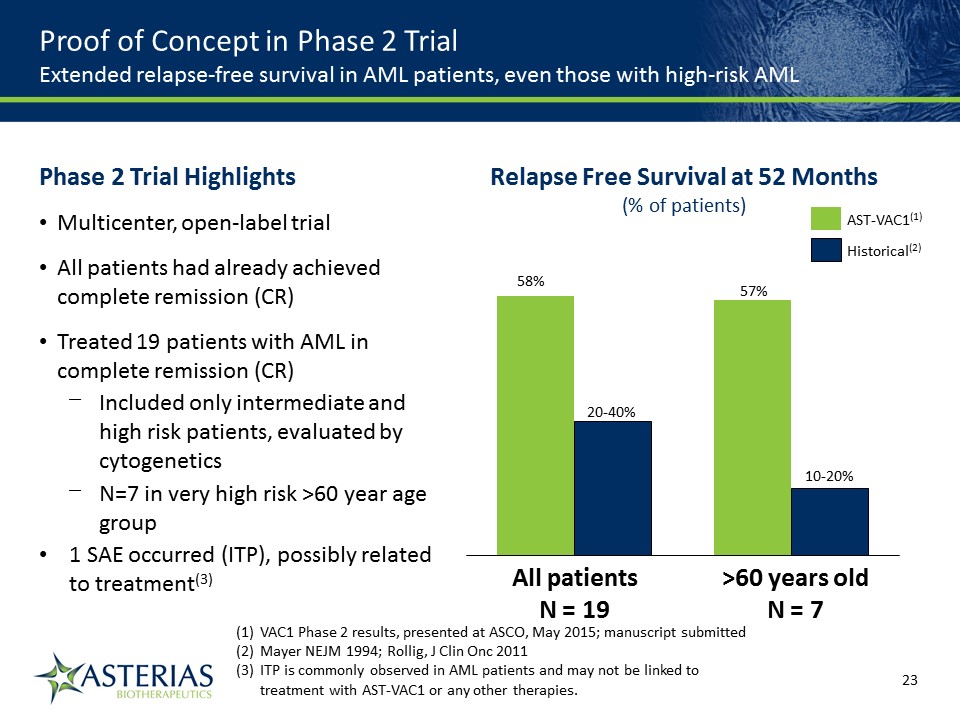
57% 10-20% >60 years oldN = 7 20-40% 58% All patientsN = 19 Relapse Free Survival at 52 Months(% of patients) Historical(2) AST-VAC1(1) VAC1 Phase 2 results, presented at ASCO, May 2015; manuscript submittedMayer NEJM 1994; Rollig, J Clin Onc 2011ITP is commonly observed in AML patients and may not be linked to treatment with AST-VAC1 or any other therapies. Proof of Concept in Phase 2 TrialExtended relapse-free survival in AML patients, even those with high-risk AML Phase 2 Trial HighlightsMulticenter, open-label trialAll patients had already achieved complete remission (CR) Treated 19 patients with AML in complete remission (CR)Included only intermediate and high risk patients, evaluated by cytogeneticsN=7 in very high risk >60 year age group1 SAE occurred (ITP), possibly related to treatment(3) 14219863Text 04698Text 230237246Text

Optimizing Path Forward for AST-VAC1 Development Critical path item is development/enhancement of the AST-VAC1 production process; effort underway, projected completion mid-2017Key objectives: reduce cycle time, COGSPositive “end-of Phase 2” meeting with FDA; FDA would support proceeding directly to Phase 3 (w/SPA)FDA would support relapse-free survival as surrogate endpoint for accelerated approval; overall survival data would be required for full approvalCurrently evaluating optimal clinical/regulatory path forward; confirmatory Phase 2b or directly to Phase 3Single Phase 3 placebo-controlled trial, if successful, could potentially support an accelerated regulatory pathway and BLA filingFirst conducting a confirmatory Phase 2b would better inform trial design, eliminate risk, potentially allow for non-dilutive funding and strengthen position for partnering 14219863Text 04698Text 230237246Text

Non-patient specific (allogeneic), “ready-for-use”Non-small cell lung cancer (NSCLC)Development partnership with Cancer Research UKPlanning Phase 1/2a clinical trial to start Q1 2017 AST-VAC2 14219863Text 04698Text 230237246Text
AST-VAC2 Targets an Unmet Need in NSCLC, a Very Large Population of Cancer Patients with High Mortality Rate Not Staged3% Lung cancer is the most common type of cancer(1)Estimated 1.8M new cases and 1.6M lung cancer deaths worldwide in 2012~220,000 new cases and ~160,000 deaths annually in the U.S.80% of cases are non-small cell lung cancer (NSCLC)NSCLC has the highest death rate of any cancer(2)Overall 5-year survival rate for NSCLC is only 18%AST-VAC2 may also have applicability in other cancers (e.g. bladder, renal cell, melanoma), since telomerase expression is present in many cancer typesAST-VAC2 also has potential for use in combination with immune checkpoint inhibitors American Lung AssociationNCI SEER Cancer Statistics Review 14219863Text 04698Text 230237246Text
AST-VAC2 is Allogeneic, ‘Ready for Use,’ Non-Patient Specific AST-VAC2 cells are mature allogeneic dendritic cells that have been transfected with hTERT and LAMP(4,5), similarly to AST-VAC1Not patient-specific: no need to produce individualized vaccines for each patientAST-VAC2 is ready to use on demand, shortening the time to administrationAST-VAC2 dendritic cells (DCs) are manufactured from Asterias embryonic stem cellsFunctionality comparable to monocyte-derived DCsProcess has been transferred to CRUK in support of Phase 1/2a studyProduct to be supplied from a single master cell bank, allowing scalability/consistencyThousands of patient treatments can be manufactured in a single batch 14219863Text 04698Text 230237246Text Centralized Production Facility Point of Care Distributed Frozen Inventory

Anticipate MHRA clearance for Phase 1/2a NSCLC trial by end 2016/early 2017Clinical trial enrollment projected to begin Q1 2017Partnership with Cancer Research UK (CRUK) funds full cost of GMP manufacturing, regulatory filing, and Phase 1/2a trialCRUK partnership saves Asterias an estimated ~$30MFollowing a successful Phase 1/2a trial, Asterias can continue development in exchange for modest milestones and royalties to CRUK Phase 1/2a Trial to be Funded Through CRUK Partnership 14219863Text 04698Text 230237246Text

Key Highlights AST-OPC1Ongoing Phase 1/2a trial in spinal cord injury (SCI)Positive safety profile to-date in two safety cohorts (thoracic SCI trial and cervical SCI trial) Early signs of possible efficacy - one motor level improvement in all three patients in very low dose 2m cell safety cohortFirst efficacy cohort of 10m cells dosed; 1st readout January 2017, additional readouts 2017-2018AST-VAC112-month process development effort underway (key critical path item for program progression)Completed positive end-of-Phase 2 FDA meeting in February 2016Potential for accelerated development pathway and BLA filingAST-VAC2Currently finalizing protocol with CRUK; Planned MHRA submission Q4 2016; trial starting Q1 2017 Planning Phase 1/2a trial in non-small cell lung cancer (NSCLC)Fully sponsored by Cancer Research UK (CRUK) 14219863Text 04698Text 230237246Text

APPENDIX 14219863Text 04698Text 230237246Text

Asterias: a publicly traded, independently operated company that is presently <48% owned by Biotime (BTX) September 2012 – BioTime (BTX) creates Asterias to acquire assets in the field of regenerative medicineOctober 2013 – Asterias acquires cell therapy assets from Geron, including INDs for the clinical stage AST-OPC1 and AST-VAC1 programsOctober 2014 – Asterias lists its common stock on the NYSE MKTFebruary 2016 – Asterias appoints Steve Cartt as CEO and Don Bailey as Chairman of the Board of DirectorsMay 2016 – completed capital raise which netted $18.5 million and reduced BTX ownership stake to less than 48% Corporate History 14219863Text 04698Text 230237246Text
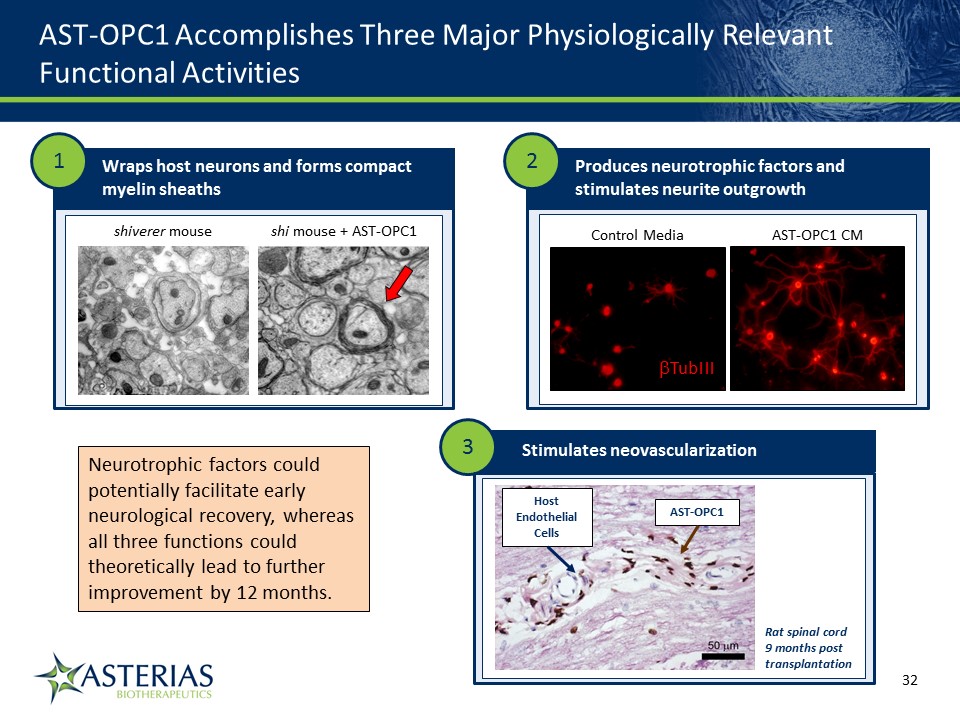
AST-OPC1 Accomplishes Three Major Physiologically Relevant Functional Activities 14219863Text 04698Text 230237246Text shiverer mouse shi mouse + AST-OPC1 Wraps host neurons and forms compact myelin sheaths AST-OPC1 CM Control Media TubIII Produces neurotrophic factors and stimulates neurite outgrowth 1 2 Stimulates neovascularization 3 Host Endothelial Cells AST-OPC1 Rat spinal cord 9 months post transplantation Neurotrophic factors could potentially facilitate early neurological recovery, whereas all three functions could theoretically lead to further improvement by 12 months.
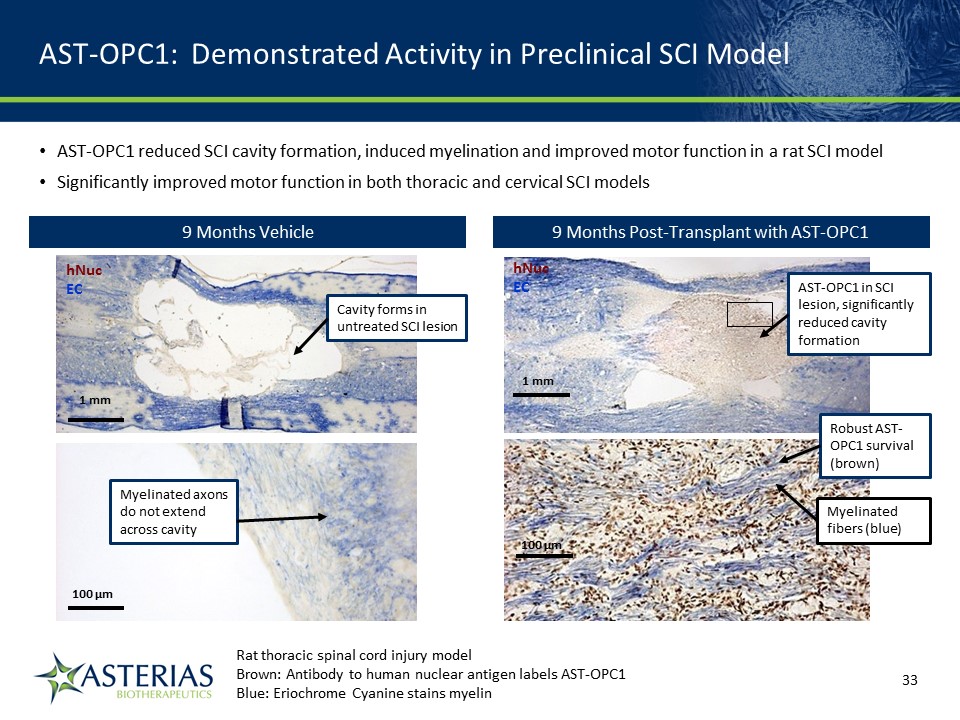
AST-OPC1: Demonstrated Activity in Preclinical SCI Model hNucEC 1 mm 100 µm AST-OPC1 in SCI lesion, significantly reduced cavity formation Robust AST-OPC1 survival (brown) Myelinated fibers (blue) 9 Months Vehicle hNucEC 1 mm 100 µm Cavity forms in untreated SCI lesion Myelinated axons do not extend across cavity Rat thoracic spinal cord injury modelBrown: Antibody to human nuclear antigen labels AST-OPC1 Blue: Eriochrome Cyanine stains myelin 14219863Text 04698Text 230237246Text AST-OPC1 reduced SCI cavity formation, induced myelination and improved motor function in a rat SCI modelSignificantly improved motor function in both thoracic and cervical SCI models 9 Months Post-Transplant with AST-OPC1
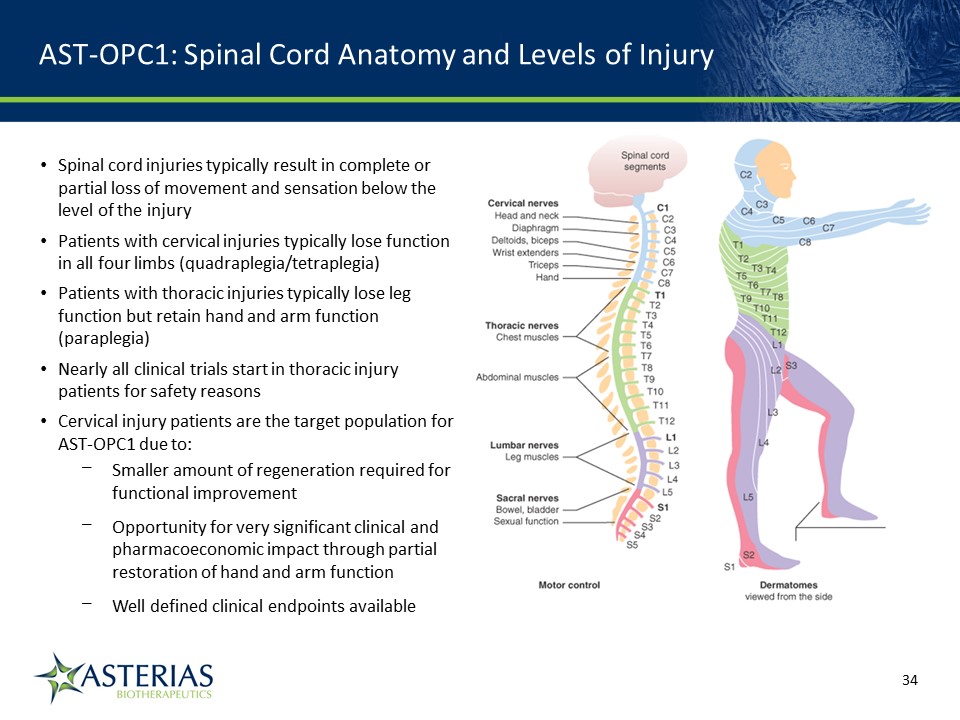
AST-OPC1: Spinal Cord Anatomy and Levels of Injury 14219863Text 04698Text 230237246Text Spinal cord injuries typically result in complete or partial loss of movement and sensation below the level of the injuryPatients with cervical injuries typically lose function in all four limbs (quadraplegia/tetraplegia)Patients with thoracic injuries typically lose leg function but retain hand and arm function (paraplegia)Nearly all clinical trials start in thoracic injury patients for safety reasonsCervical injury patients are the target population for AST-OPC1 due to:Smaller amount of regeneration required for functional improvementOpportunity for very significant clinical and pharmacoeconomic impact through partial restoration of hand and arm functionWell defined clinical endpoints available
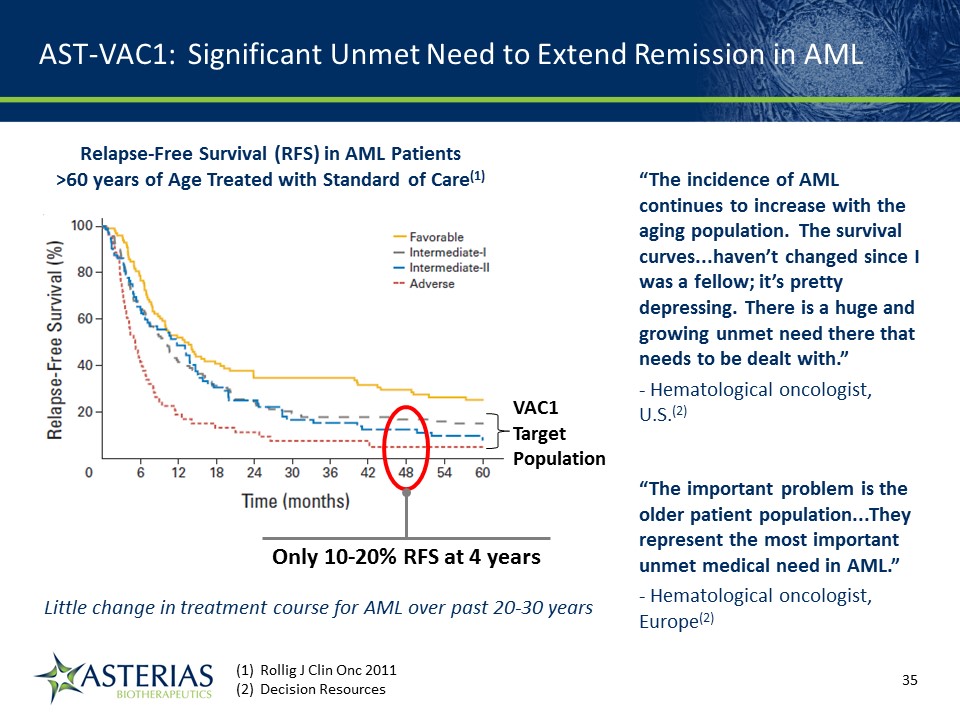
Rollig J Clin Onc 2011 Decision Resources Relapse-Free Survival (RFS) in AML Patients >60 years of Age Treated with Standard of Care(1) Only 10-20% RFS at 4 years AST-VAC1: Significant Unmet Need to Extend Remission in AML “The incidence of AML continues to increase with the aging population. The survival curves...haven’t changed since I was a fellow; it’s pretty depressing. There is a huge and growing unmet need there that needs to be dealt with.”- Hematological oncologist, U.S.(2) “The important problem is the older patient population...They represent the most important unmet medical need in AML.”- Hematological oncologist, Europe(2) 14219863Text 04698Text 230237246Text VAC1TargetPopulation Little change in treatment course for AML over past 20-30 years
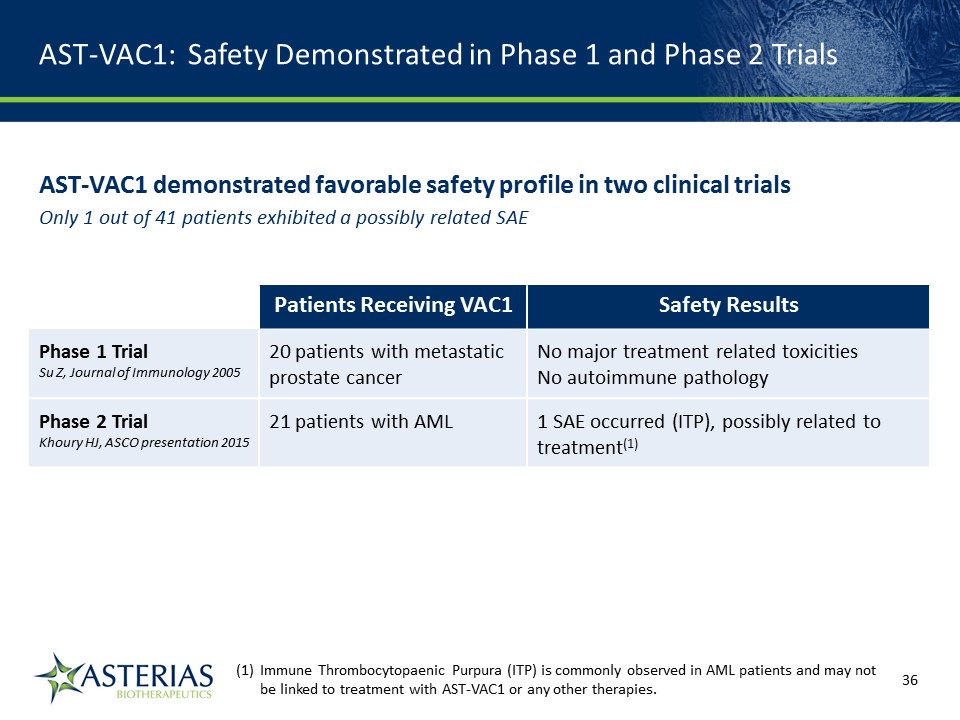
AST-VAC1: Safety Demonstrated in Phase 1 and Phase 2 Trials AST-VAC1 demonstrated favorable safety profile in two clinical trialsOnly 1 out of 41 patients exhibited a possibly related SAE Patients Receiving VAC1 Safety Results Phase 1 TrialSu Z, Journal of Immunology 2005 20 patients with metastatic prostate cancer No major treatment related toxicitiesNo autoimmune pathology Phase 2 TrialKhoury HJ, ASCO presentation 2015 21 patients with AML 1 SAE occurred (ITP), possibly related to treatment(1) 14219863Text 04698Text 230237246Text Immune Thrombocytopaenic Purpura (ITP) is commonly observed in AML patients and may not be linked to treatment with AST-VAC1 or any other therapies.
