Exhibit 99.1


This presentation includes forward-looking statements that are subject to many risks and uncertainties. These forward-looking statements, such as statements about Nemaura’s short-term and long-term growth strategies, can sometimes be identified by use of terms such as “intend,” “expect,” “plan,” “estimate,” “future,” “strive,” and similar words. These statements involve many risks and uncertainties that may cause actual results to differ from what may be expressed or implied in these statements. These risks are discussed in Nemaura’s filings with the Securities and Exchange Commission (the “Commission”), including the risks identified under the section captioned “Risk Factors” in Nemaura’s Annual Report on Form 10-K filed with the Commission on June 13, 2015 and in Nemaura’s Registration Statement on Form S-3 filed with the Commission on March 18, 2016. Nemaura disclaims any obligation to update information contained in these forward-looking statements whether as a result of new information, future events, or otherwise.

vNemaura Medical is a UK-based innovator of unique wearable-tech health tracking and diagnostics products that have evolved out of our core area of expertise in transdermal drug delivery.
vWe believe our proprietary technology offers material advantages over the competition that will dramatically improve end user compliance and optimize the benefits associated with continuous physiologic monitoring.
vWe have engineered a highly sensitive skin patch that utilizes state-of-the-art sensors capable of continuously reading any analyte or prescription medicine present in interstitial fluid. Our products are differentiated by being:
oNon-Invasive
oNeedle-Free
oDaily-Disposable
oCost Effective
vOur pipeline of applications include:
oContinuous Glucose Monitoring (“CGM”) for Diabetics
oAthletic Performance (lactate)
oIntensive Care (oxygen depletion)
Prescription Therapeutic Drug Monitoring (“TDM”) for numerous molecules
One platform technology, multiple product applications
Our proprietary technology platform, known as BEAT™, consists of a daily adhesive disposable skin-patch integrated with a reusable Bluetooth-enabled sensor that collects and quantifies a wide range of analytes present within interstitial fluid.
vThe BEAT™patch passes a mild, non-perceptible electrical current across the skin
vThis current pulls a defined analyte, for example glucose, out of interstitial fluid, which is found just below the top layer of skin, into a reservoir in the patch
vThe Bluetooth-enabled sensor measures and transmits glucose data to a proprietary application pre-downloaded on user’s own smartwatch / smartphone
vA proprietary algorithm within the application converts this data to a glucose concentration value
vThis value is displayed on the smartwatch /smartphone with sound and/or buzzer alert, and can also be forwarded on to cloud based health care team
vAn optional hand held reader can be used instead of smartphone/smartwatch
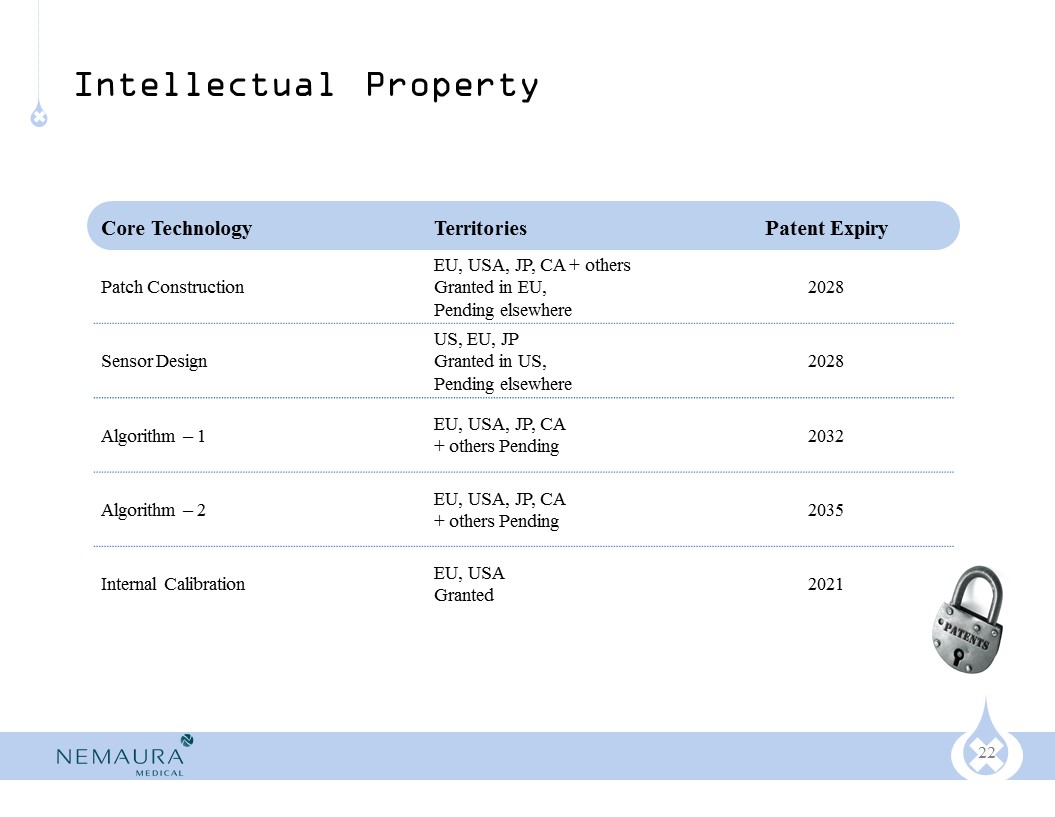
The patch construction and the algorithm that processes the signal to determine glucose levels are our proprietary patented/patent pending technology
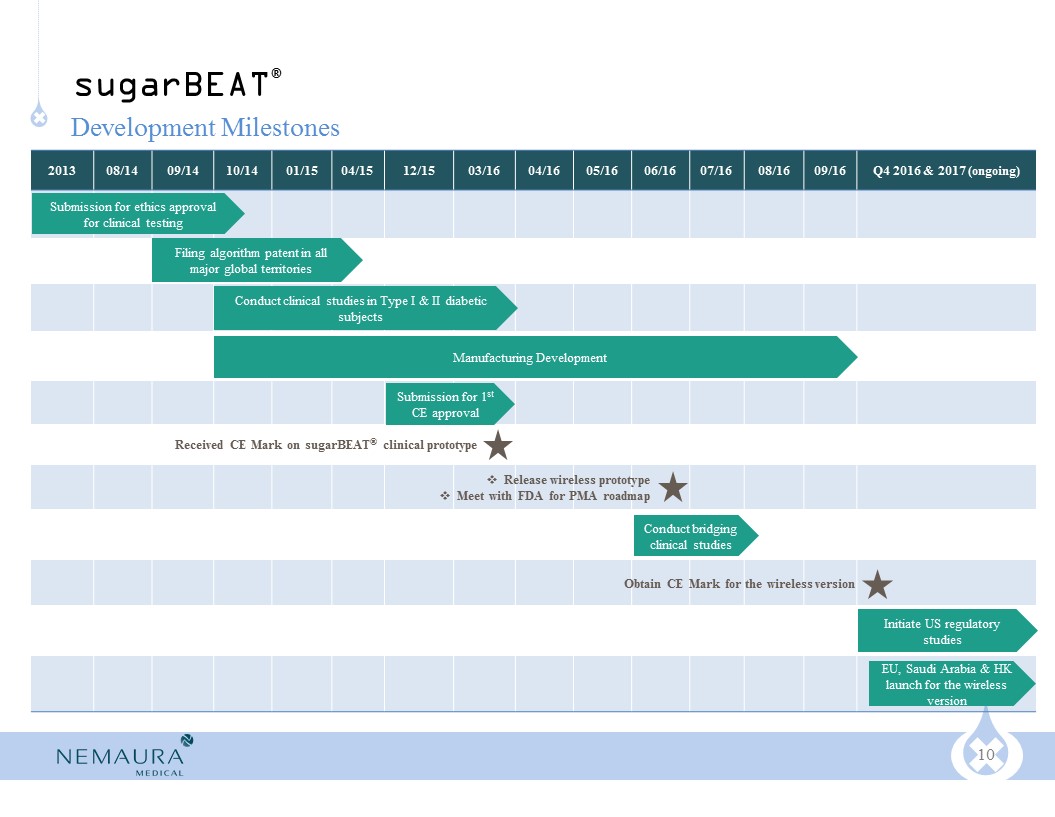
vsugarBEAT® commercial launch expected by end of Q4 2016 (CE Mark issued during Q1 2016)
vInterim clinical data on increased measurement frequency for sugarBEAT® CGM released in April 2016
vsugarBEAT® U.S. clinical trials pending regulatory approval through the PMA route
vAdditional products using BEAT™ platform to be commercialized in 2017
Smartphone, Tablet,
Smartwatch or
Optional hand-held reader
Providing full color, high-resolution images of the data, rapidly converted using an algorithm, and displayed in various formats including tabular and graphical
Daily-disposable adhesive skin-patch integrated with reusable Bluetooth-enabled sensor
Patch applied to the skin, attached to a slim electronics module containing a highly-sensitive sensor array (combined approximate size 4cm x 3cm x 1cm, with disposable skin-patch measuring 1.5cm x 2cm x 1mm)
vCleanse and prep skin
vApply patch to arm, leg or abdomen
vApproximate 30 minutes warm-up period
vSensor measures glucose within extracted interstitial fluid
vSensor records a reading every 5 minutes and transmits via Bluetooth to pre-downloaded App on user’s smart device
vUser’s smart device can be connected via cloud to virtual GP
vEach disposable skin-patch lasts up to 24 hours
vFinger prick calibration required whenever new patch is affixed
vSecond-generation patch expected to eliminate need for routine finger prick calibration
vExpected sensor shelf-life is 2 years



In exchange for purchasing $10 million of Nemaura Medical stock (5 million shares), DB Pharma and Nemaura entered into an equal joint venture granting DB an exclusive license to market the sugarBEAT® system across Europe, to commence upon final regulatory approval:
vDB Pharma is a UK-based specialty pharmaceutical company focused on the marketing and distribution of prescription drug and healthcare products throughout Europe
vThe company is headed by Dallas Burston, MBBS, a pharmaceutical entrepreneur with a distinguished track record of M&A in the field, having founded and sold over $150 million worth of assets to date, including:
-Selling a Northampton-based pharmaceutical company, Bartholomew-Rhodes, for £19.8 million
-Selling a 51% stake in Ashbourne Pharmaceuticals for £32 million which was later acquired by Sinclair Pharma
-Founding Dallas Burston Ltd, a developer of specialty pharmaceutical products that was acquired by Cambridge-based SynGenix in an all stock transaction
-Selling DB Ashbourne Ltd, a UK-focused marketer and supplier of prescription medicines, to Ethypharm







Dr. Dewan FazlulHoque Chowdhury CEO and President
§Over 18 years’ experience in the pharmaceutical and medical device industry and holder of over 50 patents across more than 15 patent families
§2009-Present: President, Chief Executive Officer and Board Director at Nemaura in charge of research and development of core technologies, product development, innovation and commercialization; coordinates and oversees legal compliance; development of the company mission; policy and planning
§2005- 2009: Founder and CEO of Microneedle Technologies and Nemaura Pharma Limited; developed and launched a microneedle device used in skin clinics; responsible for negotiating licensing deals for a transdermal patch to treat Alzheimer's disease currently under FDA review
§MSc in Microsystem and nanotechnology, from Cranfield University and PhD in Nanomedicine from Oxford University
Bashir Timol Director of Business Strategy §Over 10 years’ experience as a business entrepreneur §10 years experience as angel investor in life-science and bio-tech firms §Led initial angel-consortium that provided start-up funding for Nemaura Medical §2013-Present: Director of Business Strategy at Nemaura responsible for financial planning, business and market development and corporate strategies §2007-Present: Director of Nemaura Pharma Ltd §2013-Present: Director of Dermal Diagnostics §2009- Present: Director at SABT 1 Ltd §2009-Present: Director at OneE Group Ltd §Bachelor degree in Economics from the University of Central Lancashire, UK

Madhavi Saharawi Medical Device Advisor §Over 16 years of experience in Quality Assurance and Assurance and Regulatory Affairs (QA & RA) including 14 years plus in Class I to Class III Medical devices, and a former Notified Body Technical File Reviewer §2015-2016: UK &US Director of Regulatory Affairs at Nikon (Optos Plc) §2011 – 2015: Senior Director of QA and RA at Becon and Dickinson (formerly Cardinal Health &CareFusion) §2010 – 2011: Europe Sr. Manager of QA and RA at CareFusion UK (formerly Cardinal Health & Jaeger) §2009 – 2010: Global medical device auditor and QA lead at SGS Notified Body (UK) §2008 – 2009: Manager of QA and RA at t+Medical Limited, UK (a J & J Subsidiary) §2007 – 2008: QA Sr. Engineer at Elekta Oncology Ltd. §2002 – 2006: various positions at Abbott Diabetes UK §Bachelor and Master’s degree from OsmainaUniversity and member of Chartered Quality Institute
Kathryn Farrar Director of Finance §2014-Present: Director of Finance at Nemaura Medical §2010-Present: Financial Manager for Nemaura Pharma Limited, an affiliated company, and is responsible for preparation and management of the accounts of Nemaura Pharma and related companies §1998-2009: KPMG UK §Chartered accountant since 2001 §Received Degree of Masters in Chemistry from University of Oxford