Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - GI DYNAMICS, INC. | d130164d8k.htm |
Exhibit 99.1
GI Dynamics, Inc. Q1 16 Quarterly Shareholder Brief 5/12/16
|
|
Forward-Looking Statements Currency References Financial amounts in this presentation are expressed in US Dollars, except where specifically noted. Forward-Looking Statements This presentation contains forward-looking statements concerning: our development and commercialization plans; our potential revenues and revenue growth, costs, excess inventory, profitability and financial performance; our ability to obtain reimbursement for our products; our clinical trials, and associated regulatory submissions and approvals; the number and location of commercial centers offering the EndoBarrier; and our intellectual property position. These forward-looking statements are based on the current estimates and expectations of future events by the management of GI Dynamics, Inc. as of the date of this presentation and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those indicated in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks associated with the consequences of terminating the ENDO Trial and the possibility that future clinical trials will not be successful or confirm earlier results; risks associated with obtaining funding from third parties; risks relating to the timing and costs of clinical trials, the timing of regulatory submissions, the timing, receipt and maintenance of regulatory approvals, the timing and amount of other expenses, and the timing and extent of third-party reimbursement; risks associated with commercial product sales, including product performance; competition; risks related to market acceptance of products; intellectual property risks; risks related to excess inventory; risks related to assumptions regarding the size of the available market, benefits of our products, product pricing, timing of product launches, future financial results and other factors including those described in our filings with the U.S. Securities and Exchange Commission. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law. Disclaimer This presentation and any supplemental materials have been prepared by GI Dynamics, Inc. based on available information. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness, or correctness of such information and opinions and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of GI Dynamics, Inc., or any of its members, directors, officers, employees, or agents or advisors, nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of GI Dynamics, Inc. or any of its directors, officers, employees or agents. 5/12/2016 GI Dynamics, Inc. 2
|
|
Conference Call Agenda Shareholder update Corporate priorities ENDO data Financial update ABCD data Additional clinical information 5/12/2016 GI Dynamics, Inc. 3
|
|
Scott Schorer: President & CEO Employment Senior consultant working with CEOs and Boards to resolve strategic, operational, finance issues Systagenix Wound Management: President Americas IST / Innovative Spinal Technologies: CEO CentriMed ? Global Healthcare Exchange: CEO US Army Infantry: 82nd Airborne Division: Platoon Leader Experience Raised >$120m private equity, public equity, debt financings Multiple distressed company leadership roles Multiple product approvals & product launches 5/12/2016 GI Dynamics, Inc. 4
|
|
Corporate Priorities 1.Modify Cost Structure. We will implement a leaner, more efficient cost structure by cutting expenses to extend our cash runway. 2.Rebuild Team. I will appoint a new chief financial officer and chief compliance officer (responsible for clinical, regulatory, and quality), in addition to adding other experienced team members. 3.Develop Clinical Data and Core Science. We will continue to support investigator-initiated studies around the world along with internal analyses of the safety and efficacy of EndoBarrier Therapy. 4.Focus Revenue Efforts. We will focus on strategic commercial centers outside the United States. 5.Improve Regulatory Relationships. We will work with the FDA to review lessons learned from the ENDO Trial to design our next EndoBarrier Therapy trial, and collaborate with our European Notified Body and the TGA in Australia to refine systems and post-market surveillance. In process / partially complete: Reduced staff 30%, restructuring expense across entire organization Initial phase complete: CFO Murphy, CCO Callahan, local quality and regulatory professionals In process: Multiple activities to understand root cause for hepatic abscess and means to mitigate risk; refining approach to new IDE protocol In process: Multiple activities to focus commercial activities, drive sales force effectiveness In process: New CCO Callahan initiating dialogue with all regulatory bodies & assessing legacy issues 5/12/2016 GI Dynamics, Inc. 5
|
|
ENDO Trial Efficacy 5/12/2016 GI Dynamics, Inc. 6 Measure Top-Line Results Primary Efficacy Endpoint: Mean Change in HbA1c from Baseline to 12 Months 0.71% greater with EndoBarrier Therapy than with sham control (absolute HbA1c) Posterior Probability of Super-Superiority 92.8% (pre-specified criterion was > 96.5%)
|
|
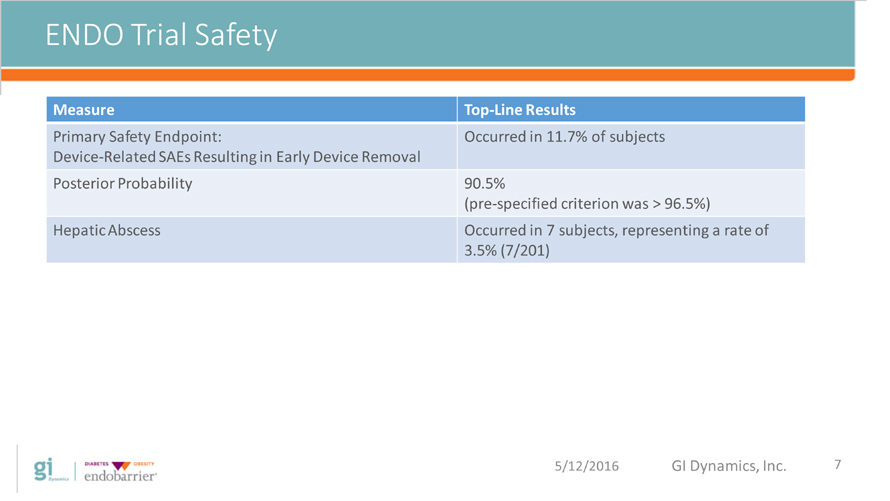
ENDO Trial Safety 5/12/2016 GI Dynamics, Inc. 7 Measure Top-Line Results Primary Safety Endpoint: Device-Related SAEs Resulting in Early Device Removal Occurred in 11.7% of subjects Posterior Probability 90.5% (pre-specified criterion was > 96.5%) Hepatic Abscess Occurred in 7 subjects, representing a rate of 3.5% (7/201)
|
|
Jim Murphy: CFO Employment International Accounting firm Arthur Andersen ? Boston office Life Sciences since 1990: Specialty Pharma, Biotech instrumentation and consumables, Drug development, Med device Predominantly public companies Experience > $350M in capital raised ?private & public Multiple stressed company situations Strong operational experience International M&A on buy & sell side 5/12/2016 GI Dynamics, Inc. 8
|
|
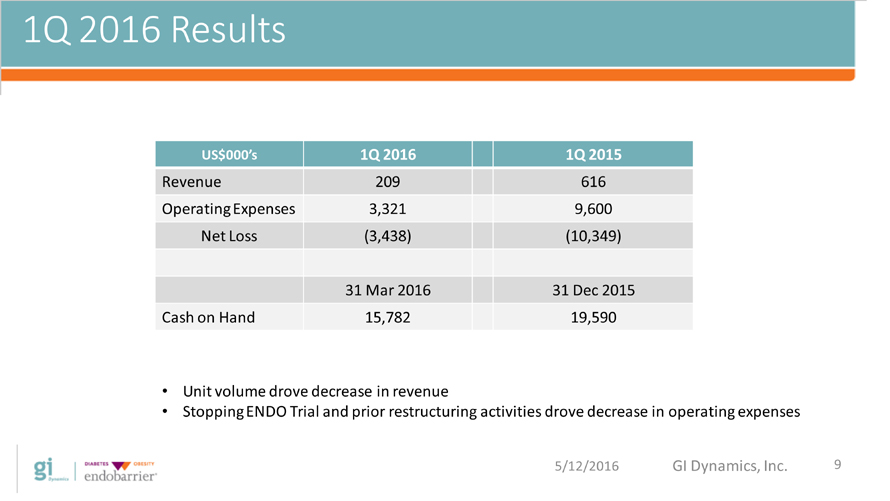
1Q 2016 Results US$000?s 1Q 2016 1Q 2015 Revenue 209 616 Operating Expenses 3,321 9,600 Net Loss (3,438) (10,349) 31 Mar 2016 31 Dec 2015 Cash on Hand 15,782 19,590
|
|
Brian Callahan: CCO and EVP Clinical, Regulatory, Quality Employment Founded EEC & Associates a global compliance-consulting firm for the Medical Device, Pharmaceutical, Biologics Industry: Vertex, Hologic, Johnson & Johnson, Fortress Investments, BMS, Schering Plough, Wyeth, Pliva, MDS Pharma, IST, BioCryst Pharmaceuticals Leadership: Histogenics, Prochon Biotech, Organogenesis, Quintiles R&D, engineering, technical services, compliance US Army nuclear weapons specialist Experience Involved in numerous compliance recovery projects Excellent working relationship with current and former FDA officials, MHRA, IMOH, PMDA, and ANVISA Strong Clinical (Preclinical, Phase I, II, and III), Regulatory (510Ks, PMA, BLA, ANDA and NDAs), and Quality experience (Over 40 FDA inspections) 5/12/2016 GI Dynamics, Inc. 10
|
|
Clinical, Regulatory, Quality Completing Endo Trial Clinical Study Report for FDA submission Updating Clinical Evidence Report for TGA Auditing quality systems Engaging with notified body, TGA and FDA 5/12/2016 GI Dynamics, Inc. 11
|
|
ABCD REVISE Diabesity Study A multi-center, randomized controlled trial initiated and funded by the Association of British Clinical Diabetologists (ABCD). Two-year study aims to evaluate the efficacy and legacy effect of treating obese type 2 diabetes patients with the EndoBarrier gastrointestinal liner combined with a GLP-1 receptor analog (liraglutide), compared to patients treated with either liraglutide alone or EndoBarrier Therapy alone. 5/12/2016 GI Dynamics, Inc. 12
|
|
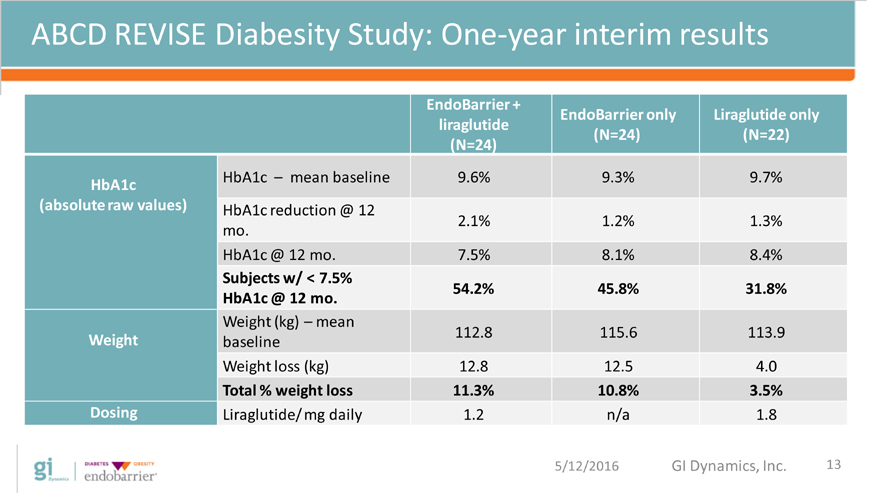
ABCD REVISE Diabesity Study: One-year interim results EndoBarrier + liraglutide (N=24) EndoBarrier only (N=24) Liraglutide only (N=22) HbA1c (absolute raw values) HbA1c ? mean baseline 9.6% 9.3% 9.7% HbA1c reduction @ 12 mo. 2.1% 1.2% 1.3% HbA1c @ 12 mo. 7.5% 8.1% 8.4% Subjects w/ < 7.5% HbA1c @ 12 mo. 54.2% 45.8% 31.8% Weight Weight (kg) ? mean baseline 112.8 115.6 113.9 Weight loss (kg) 12.8 12.5 4.0 Total % weight loss 11.3% 10.8% 3.5% Dosing Liraglutide/ mg daily 1.2 n/a 1.8
|
|
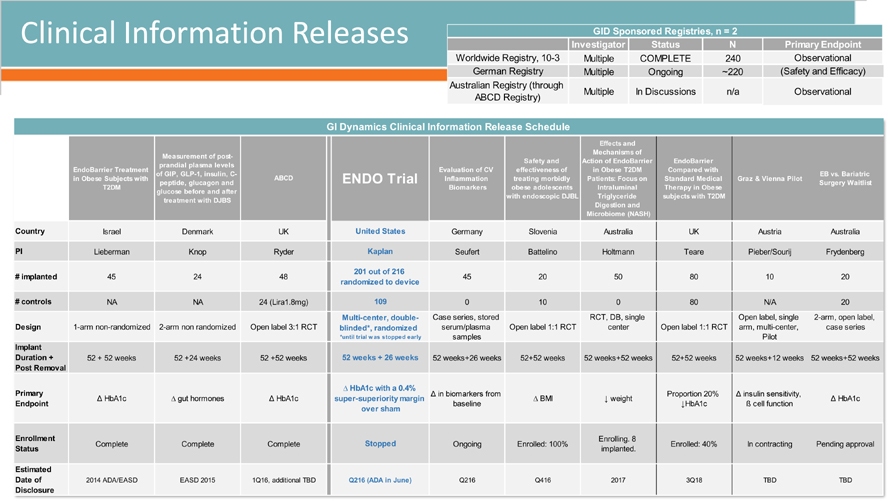
Clinical Information Releases 5/12/2016 GI Dynamics, Inc. 14 Investigator Status N Multiple COMPLETE 240 Multiple Ongoing ~220 Multiple In Discussions n/a Australian Registry (through ABCD Registry) Observational GID Sponsored Registries, n = 2 Primary Endpoint Worldwide Registry, 10-3 Observational German Registry (Safety and Efficacy)
|
|
Thank you 5/12/2016 GI Dynamics, Inc. 15 Investor Inquiries: Media Inquiries: Investor relations Media relations United States: James Murphy Chief Financial Officer +1 (781) 357-3281 United States/Europe/Australia: investor@gidynamics.com +1 (781) 357-3250 Australia: David Allen or John Granger Hawkesbury Partners Pty Limited +61 2 9325 9046 United States/Australia: Catie Corcoran WE Buchan +1 (813) 895-4575