Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REGENERON PHARMACEUTICALS, INC. | a16-1714_18k.htm |
| EX-99.1 - EX-99.1 - REGENERON PHARMACEUTICALS, INC. | a16-1714_1ex99d1.htm |
Exhibit 99.2
2016 Financial Overview Robert landry, svp of finance - cfo JANUARY 2016
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND NON-GAAP FINANCIAL MEASURES 2 This presentation includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of Regeneron's products, product candidates, and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, Praluent® (alirocumab) Injection, sarilumab, dupilumab, fasinumab, REGN 2222, and the immuno-oncology program; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron's late-stage product candidates and new indications for marketed products, including without limitation EYLEA, Praluent, sarilumab, dupilumab, fasinumab, and REGN 2222; ongoing regulatory obligations and oversight impacting Regeneron’s marketed products (such as EYLEA and Praluent), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron's ability to continue to develop or commercialize Regeneron's products and product candidates; competing drugs and product candidates that may be superior to Regeneron's products and product candidates; uncertainty of market acceptance and commercial success of Regeneron's products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the assumptions underlying those projections or guidance, including without limitation those relating to EYLEA U.S. net sales and the Company’s expectations regarding non-GAAP unreimbursed R&D, non-GAAP SG&A, cash tax payments, non-GAAP pre-tax income, and capital expenditures; the potential for any license or collaboration agreement, including Regeneron's agreements with Sanofi and Bayer HealthCare LLC, to be cancelled or terminated without any further product success; and risks associated with third party intellectual property and pending or future litigation relating thereto. A more complete description of these and other material risks can be found in Regeneron's filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2014 and its Form 10-Q for the quarterly period ended September 30, 2015, in each case including in the sections thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management's current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise. This presentation uses non-GAAP unreimbursed R&D, non-GAAP SG&A, and cash tax as a percentage of non-GAAP pre-tax income, which are financial measures that are not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). Regeneron believes that the presentation of these non-GAAP measures is useful to investors because they exclude, as applicable, (i) non-cash share-based compensation expense, which fluctuates from period to period based on factors that are not within the Company's control, such as the Company's stock price on the dates share-based grants are issued, (ii) non-cash interest expense related to the Company's convertible senior notes, since this is not deemed useful in evaluating the Company's operating performance, (iii) loss on extinguishment of debt, since this non-cash charge is based on factors that are not within the Company’s control, and (iv) estimate of income tax expense that is not payable in cash, as there is a significant difference between the Company’s effective tax rate and actual cash income taxes paid or payable. Non-GAAP unreimbursed R&D represents non-GAAP R&D expenses reduced by R&D expense reimbursements from the Company's collaboration partners. Non-GAAP pre-tax income represents GAAP pre-tax income less non-GAAP adjustments. Management uses these non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides forecasts to investors on this basis. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company's non-GAAP financial measures may not be comparable with non-GAAP information provided by other companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP.

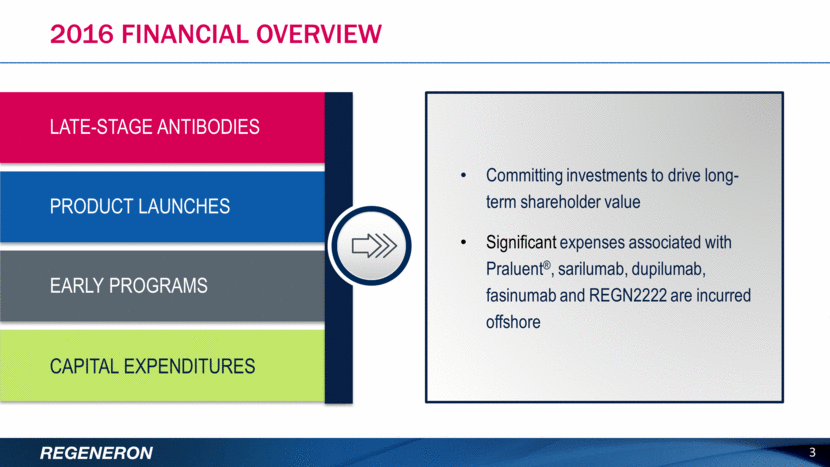
2016 FINANCIAL Overview 3 Committing investments to drive long-term shareholder value Significant expenses associated with Praluent®, sarilumab, dupilumab, fasinumab and REGN2222 are incurred offshore LATE-STAGE ANTIBODIES PRODUCT LAUNCHES EARLY PROGRAMS CAPITAL EXPENDITURES
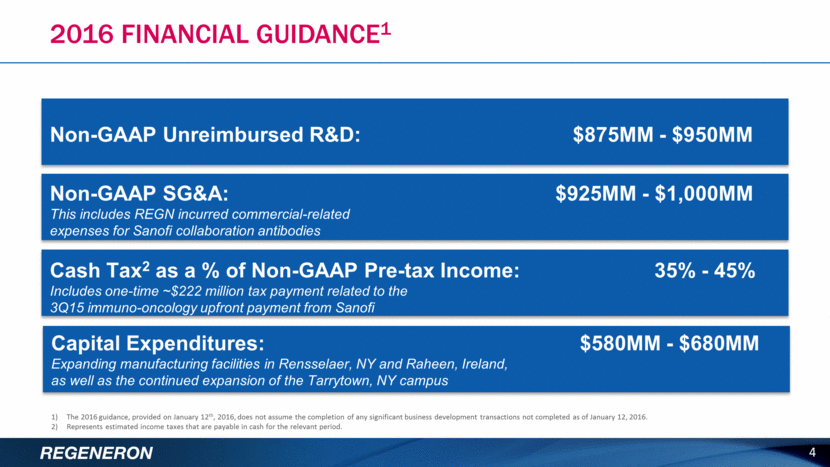
2016 FINANCIAL GUIDANCE1 4 Non-GAAP Unreimbursed R&D: $875MM - $950MM Non-GAAP SG&A: $925MM - $1,000MM This includes REGN incurred commercial-related expenses for Sanofi collaboration antibodies Cash Tax2 as a % of Non-GAAP Pre-tax Income: 35% - 45% Includes one-time ~$222 million tax payment related to the 3Q15 immuno-oncology upfront payment from Sanofi Capital Expenditures: $580MM - $680MM Expanding manufacturing facilities in Rensselaer, NY and Raheen, Ireland, as well as the continued expansion of the Tarrytown, NY campus 1) The 2016 guidance, provided on January 12th, 2016, does not assume the completion of any significant business development transactions not completed as of January 12, 2016. 2) Represents estimated income taxes that are payable in cash for the relevant period.
2016 FINANCIAL Overview 5 Sanofi collaboration antibodies: Regeneron funds 20% of an antibody’s Phase 3 costs after the first positive results in a Phase 3 study for that antibody: Praluent®: OUTCOMES study Sarilumab: MONARCH and EXTEND studies Dupilumab: Atopic Dermatitis and Asthma Phase 3 programs Unpartnered antibodies: Regeneron incurs 100% of the costs for unpartnered antibodies: Fasinumab* and REGN2222 LATE-STAGE ANTIBODIES EARLY PROGRAMS PRODUCT LAUNCHES CAPITAL EXPENDITURES *Partnered with MTPC in Japan and certain other Asian countries
2016 FINANCIAL OVERVIEW 6 Regeneron’s ~50% share of commercialization expenses for partnered antibodies: Praluent® Our share of 3Q15 loss was ~$75M First full year launch for Praluent® in 2016 will include additional country launches Sarilumab Anticipated 4Q16 launch requires investment Dupilumab Readying for launch in atopic dermatitis LATE-STAGE ANTIBODIES EARLY PROGRAMS PRODUCT LAUNCHES CAPITAL EXPENDITURES
2016 FINANCIAL OVERVIEW 7 LATE-STAGE ANTIBODIES EARLY PROGRAMS PRODUCT LAUNCHES CAPITAL EXPENDITURES REGENERON GENETICS CENTER REGN1033 REGN1500 EYLEA + PDGFR REGN1979 REGN1908-1909 EYLEA + ANG2 IMMUNO-ONCOLOGY EARLY PROGRAMS / TECHNOLOGIES
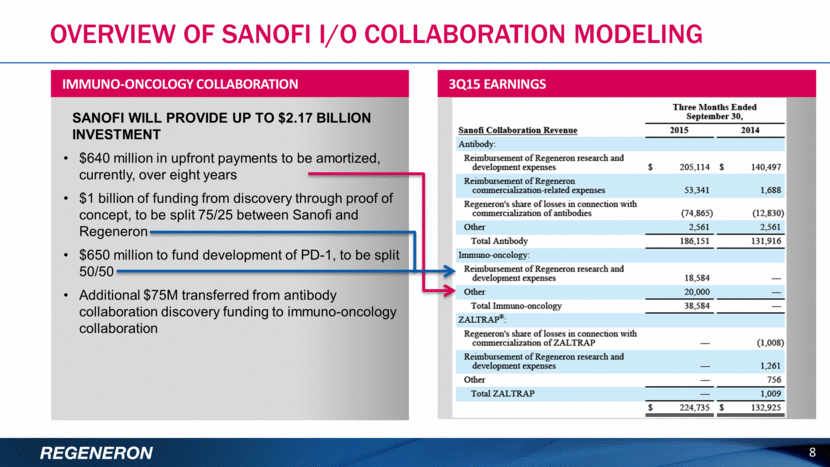
OVERVIEW OF SANOFI I/O COLLABORATION MODELING 8 SANOFI WILL PROVIDE UP TO $2.17 BILLION INVESTMENT $640 million in upfront payments to be amortized, currently, over eight years $1 billion of funding from discovery through proof of concept, to be split 75/25 between Sanofi and Regeneron $650 million to fund development of PD-1, to be split 50/50 Additional $75M transferred from antibody collaboration discovery funding to immuno-oncology collaboration IMMUNO-ONCOLOGY COLLABORATION 3Q15 EARNINGS
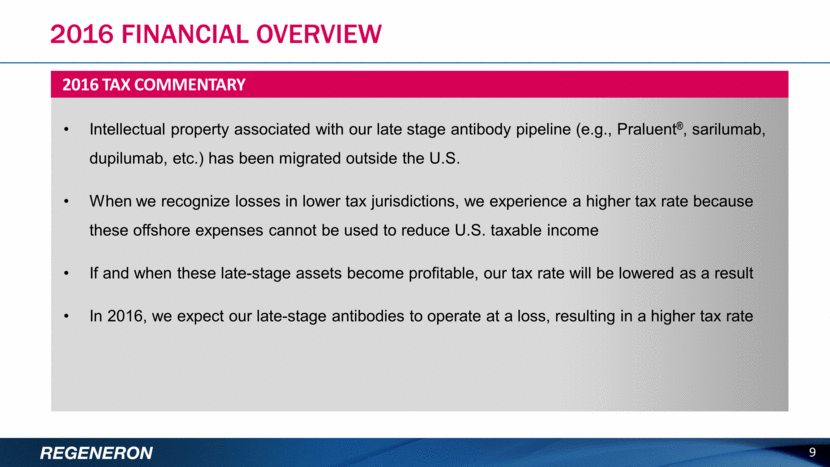
9 Intellectual property associated with our late stage antibody pipeline (e.g., Praluent®, sarilumab, dupilumab, etc.) has been migrated outside the U.S. When we recognize losses in lower tax jurisdictions, we experience a higher tax rate because these offshore expenses cannot be used to reduce U.S. taxable income If and when these late-stage assets become profitable, our tax rate will be lowered as a result In 2016, we expect our late-stage antibodies to operate at a loss, resulting in a higher tax rate 2016 TAX COMMENTARY 2016 FINANCIAL OVERVIEW