Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REGENERON PHARMACEUTICALS, INC. | a16-1714_18k.htm |
| EX-99.2 - EX-99.2 - REGENERON PHARMACEUTICALS, INC. | a16-1714_1ex99d2.htm |
Exhibit 99.1
J.P. MORGAN 34TH ANNUAL HEALTHCARE CONFERENCE JANUARY 2016
Note Regarding Forward-Looking Statements and Non-GAAP Financial Measures This presentation includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of Regeneron's products, product candidates, and research and clinical programs now underway or planned, including without limitation EYLEA®(aflibercept) Injection, Praluent®(alirocumab) Injection, sarilumab, dupilumab, fasinumab, REGN2222, and the immuno-oncology program; unforeseen safety issues resulting from the administration of products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron's late-stage product candidates and new indications for marketed products, including without limitation EYLEA, Praluent, sarilumab, dupilumab, fasinumab, and REGN2222; ongoing regulatory obligations and oversight impacting Regeneron’s marketed products (such as EYLEA and Praluent), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron's ability to continue to develop or commercialize Regeneron's products and product candidates; competing drugs and product candidates that may be superior to Regeneron's products and product candidates; uncertainty of market acceptance and commercial success of Regeneron's products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the assumptions underlying those projections or guidance, including without limitation those relating to EYLEA U.S. net sales and the Company’s expectations regarding non-GAAP unreimbursed R&D, non-GAAP SG&A, cash tax payments, non-GAAP pre-tax income, and capital expenditures; the potential for any license or collaboration agreement, including Regeneron's agreements with Sanofi and Bayer HealthCare LLC, to be cancelled or terminated without any further product success; and risks associated with third party intellectual property and pending or future litigation relating thereto. A more complete description of these and other material risks can be found in Regeneron's filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2014 and its Form 10-Q for the quarterly period ended September 30, 2015, in each case including in the sections thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management's current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise. This presentation uses non-GAAP unreimbursed R&D, non-GAAP SG&A, and cash tax as a percentage of non-GAAP pre-tax income, which are financial measures that are not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). Regeneron believes that the presentation of these non-GAAP measures is useful to investors because they exclude, as applicable, (i) non-cash share-based compensation expense, which fluctuates from period to period based on factors that are not within the Company's control, such as the Company's stock price on the dates share-based grants are issued, (ii) non-cash interest expense related to the Company's convertible senior notes, since this is not deemed useful in evaluating the Company's operating performance, (iii) loss on extinguishment of debt, since this non-cash charge is based on factors that are not within the Company’s control, and (iv) estimate of income tax expense that is not payable in cash, as there is a significant difference between the Company’s effective tax rate and actual cash income taxes paid or payable. Non-GAAP unreimbursed R&D represents non-GAAP R&D expenses reduced by R&D expense reimbursements from the Company's collaboration partners. Non-GAAP pre-tax income represents GAAP pre-tax income less non-GAAP adjustments. Management uses these non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides forecasts to investors on this basis. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company's non-GAAP financial measures may not be comparable with non-GAAP information provided by other companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP.

Dr. Alfred G. Gilman: 1941--2015 3
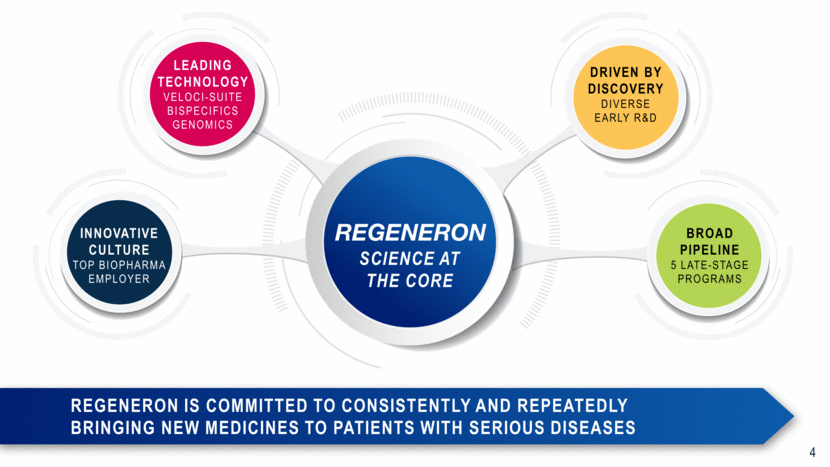
RegenEron is committed to consistently and repeatedly bringing new medicines to patients with serious diseases LEADING TECHNOLOGY VELOCI-SUITE BISPECIFICS GENOMICS INNOVATIVE CULTURE TOP BIOPHARMA EMPLOYER BROAD PIPELINE 5 LATE-STAGE Programs DRIVEN BY DISCOVERY DIVERSE EARLY R&D SCIENCE AT THE CORE 4
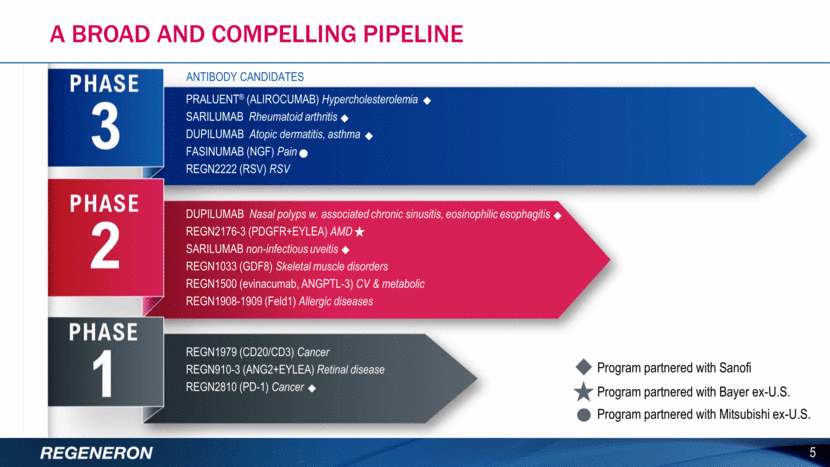
A broad and compelling pipeline 5 Program partnered with Sanofi Program partnered with Bayer ex-U.S. ANTIBODY CANDIDATES PRALUENT® (ALIROCUMAB) Hypercholesterolemia SARILUMAB Rheumatoid arthritis DUPILUMAB Atopic dermatitis, asthma FASINUMAB (NGF) Pain REGN2222 (RSV) RSV DUPILUMAB Nasal polyps w. associated chronic sinusitis, eosinophilic esophagitis REGN2176-3 (PDGFR+EYLEA) AMD SARILUMAB non-infectious uveitis REGN1033 (GDF8) Skeletal muscle disorders REGN1500 (evinacumab, ANGPTL-3) CV & metabolic REGN1908-1909 (Feld1) Allergic diseases REGN1979 (CD20/CD3) Cancer REGN910-3 (ANG2+EYLEA) Retinal disease REGN2810 (PD-1) Cancer Program partnered with Mitsubishi ex-U.S.
EYLEA is the market-leading product among FDA-approved anti-VEGF agents EYLEA full year U.S. net sales of $2.68 billion* EYLEA global sales of over $4 billion* Phase 3 study of EYLEA in Diabetic Retinopathy to begin in 1Q16 Diabetic Retinopathy Clinical Research Network’s Protocol-W study in DR expected to begin in early 2016 EYLEA + PDGFR-beta topline data from Phase 2 expected by year end Fast Track designation in wet age-related macular degeneration (wet AMD) EYLEA + ANG2 Phase 2 studies in wet AMD and Diabetic Macular Edema (DME) expected to begin in 1H16 U.S. Eylea®: leadership in the retinal franchise 6 *2015 unaudited, preliminary numbers
Praluent®: Approved in the U.S. and EU in high cardiovascular risk hypercholesterolemic patients* 7 Approved in EU on September 25, 2015 Indicated in adults with primary hypercholesterolemia (HeFH and non-familial) or mixed dyslipidaemia, as an adjunct to diet in patients unable to reach their LDL-C goals with a maximally-tolerated statin and patients who are statin intolerant, or for whom a statin is contraindicated *The effect of Praluent® on CV morbidity and mortality has not been determined FDA approval granted on July 24, 2015 Indicated as adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease, who require additional lowering of LDL cholesterol (LDL-C)
Praluent® Launch underway 8 Physician and patient education ongoing through field force efforts, medical conferences, publications, advertising Two approved doses—75 mg and 150 mg—resonating well with physicians Education Access Outcomes Study Global launch Building awareness and education 75 mg dose 150 mg dose
Praluent® Launch underway 9 Sampling, bridging, and patient assistance programs through launch period Praluent coverage for ~150 MM lives in the U.S. Stringent utilization management criteria Contract rebating occurring Parity access with Express Scripts, Prime Therapeutics* Exclusive status with United HealthCare Contract negotiations ongoing with additional payers, expect all coverage decisions to be made by mid-2016 >~47 MM lives under negotiation Medicare coverage decisions expected by April 2016 Ensuring broad access for appropriate patients Education Access Outcomes Study Global launch *ESRX includes commercial and Medicare, Prime Therapeutics includes only commercial lives
Praluent® Launch underway 10 Drug is available in Germany, UK, and Nordic Countries* Expect Italy, Spain, France, Canada, and Japan to launch in 2016 Pricing negotiations are ongoing in many EU countries Education Access Outcomes Study Global launch Global launch ongoing *Not yet on national formularies
Praluent® Launch underway 11 ODYSSEY OUTCOMES study fully enrolled In 2016, expect interim futility analysis when approximately 50% of events have occurred and potentially also when 75% (for futility and overwhelming efficacy) of the targeted number of primary events have occurred Additional details on ODYSSEY OUTCOMES can be found at American Heart Journal Volume 168, Issue 5, November 2014, Pages 682–689.e1 Education Access Outcomes Study Global launch Outcomes study likely to have impact on uptake
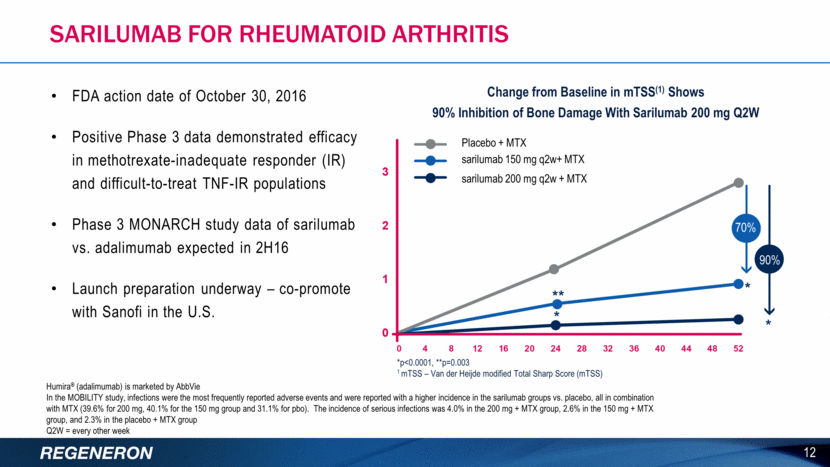
Sarilumab For Rheumatoid Arthritis FDA action date of October 30, 2016 Positive Phase 3 data demonstrated efficacy in methotrexate-inadequate responder (IR) and difficult-to-treat TNF-IR populations Phase 3 MONARCH study data of sarilumab vs. adalimumab expected in 2H16 Launch preparation underway – co-promote with Sanofi in the U.S. 12 Humira® (adalimumab) is marketed by AbbVie In the MOBILITY study, infections were the most frequently reported adverse events and were reported with a higher incidence in the sarilumab groups vs. placebo, all in combination with MTX (39.6% for 200 mg, 40.1% for the 150 mg group and 31.1% for pbo). The incidence of serious infections was 4.0% in the 200 mg + MTX group, 2.6% in the 150 mg + MTX group, and 2.3% in the placebo + MTX group Q2W = every other week 70% 90% Placebo + MTX sarilumab 150 mg q2w+ MTX sarilumab 200 mg q2w + MTX Change from Baseline in mTSS(1) Shows 90% Inhibition of Bone Damage With Sarilumab 200 mg Q2W *p<0.0001, **p=0.003 1 mTSS – Van der Heijde modified Total Sharp Score (mTSS)
Dupilumab: A Pipeline in a Product DUPILUMAB Nasal polyps (Phase 2) Eosinophilic esophagitis (Phase 2) Other allergic, IL4/13 mediated diseases Asthma Phase 3 Atopic dermatitis Phase 3 Additional Indications 13
Dupilumab: major unmet medical need in Atopic Dermatitis (AD) Approximately 1 million adults estimated to have uncontrolled, moderate-to-severe atopic dermatitis in the U.S. Only topical therapies approved by FDA (topical glucocorticoids, calcineurin inhibitors) Systemic immuno-suppressants (e.g. cyclosporine) are used off-label but have significant side effects Burden of disease for moderate-to-severe adult patients is high Patients have secondary infections1, increased sleep disturbance2, decreased work/school productivity2, decreased self-esteem3, increase in depression and suicidal ideation4 FDA Breakthrough Designation granted in adult AD indication Moderate-to-severe pediatric patients have a significant unmet medical need March 9, 2015 FDA advisory committee highlighted unmet need and encouraged pediatric drug development5 Phase 2 pediatric study fully enrolled, data expected in 1H16. Phase 3 pediatric study to begin in 1H16 14 Pugliarello S, Cozzi A, Girolomoni G. Phenotypes of atopic dermatitis. JDDG 2011; 9:12-20 Murota H, Kitaba S, Tani M, Wataya-Kaneda M, Azukizawa H, Tanemura A, et al. Impact of sedative and non-sedative antihistamines on the impaired productivity and quality of life in patients with pruritic skin diseases. Allergol Int 2010 Dec;59(4):345-54 Torrelo et al. Atopic Dermatitis: impact on quality of life and patients’ attitudes toward its management. Eur J Dermatol 2012;22(1):97-105 Kimata H. Prevalence of suicidal ideation in patients with atopic dermatitis. Suicide Life Threat Behav 2006 Feb;36(1):120-4 http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DermatologicandOphthalmicDrugsAdvisoryCommittee/ucm431514.htm
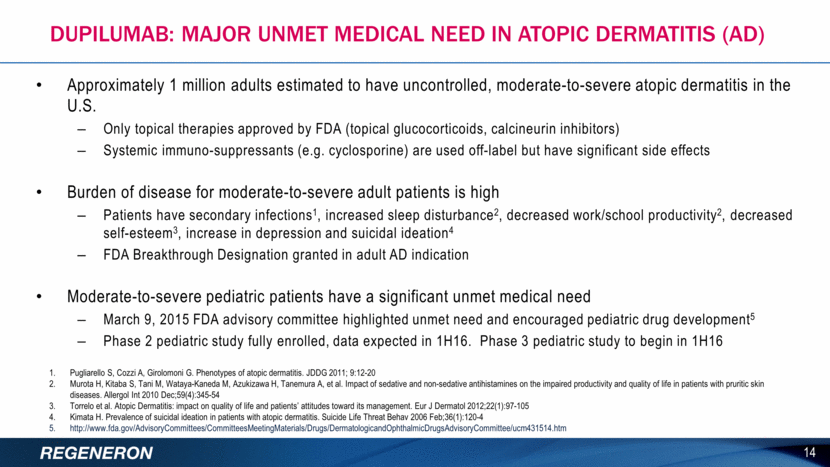
Parameter Placebo 300mg Q2W 300mg QW % EASI-501 (50% improvement) 29.5% 78.1% 82.5% % EASI-751 (75% improvement) 11.5% 53.1% 60.3% % EASI-901 (90% improvement) 3.3% 29.7% 36.5% % IGA Responders2 1.6% 29.7% 33.3% Phase 2b Study in AD – Responder Analyses at 16 Weeks p < 0.0001 vs placebo for all parameters 300mg QW and 300mg Q2W dose regimens being studied in Phase 3 program EASI = Eczema Area Severity Index Proportion of patients achieving EASI-50/70/90 Proportion of patients achieving IGA < 1 (Investigator’s Global Assessment score of 0 “clear” or 1 “almost clear”); Patients enrolled had IGA >3 QW = weekly, Q2W = every other week 15 Dupilumab AD: Phase 2b Efficacy Primary endpoint of Phase 3 studies
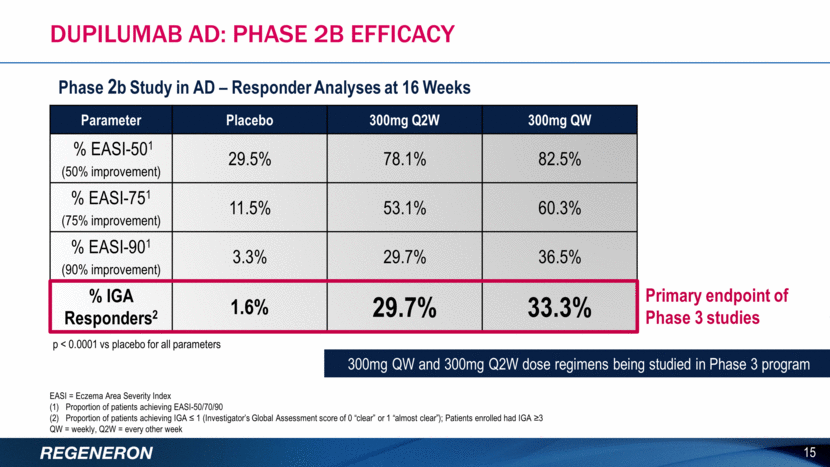
Dupilumab AD: ILLUSTRATIVE EXAMPLE OF AN IGA RESPONDER FROM P2B TRIAL Images of patient before and after receiving dupilumab therapy for atopic dermatitis Images from actual patient who received dupilumab in a Phase 2 clinical study. Results may vary. In this clinical study, all doses of dupilumab showed a dose-dependent improvement in the primary endpoint, the mean percent change in Eczema Area and Severity Index (EASI) score from baseline to week 16. The improvements in EASI score ranged from 74% to 45%, compared to 18% for patients receiving placebo (p<0.0001 for all doses). The most common adverse events in this study were nasopharyngitis. Injection site reactions and headaches were more frequent in the dupilumab group compared to placebo BASELINE 16 Score Grade Definition 0 Clear No Inflammatory signs of atopic dermatitis. 1 Almost Clear Just perceptible erythema and just perceptible papulation induration. 2 Mild Mild erythema and mild papulation induration. No oozing or crusting. 3 Moderate Moderate erythema and moderate papulation induration. Oozing or crusting may be present. 4 Severe Severe erythema and severe papulation induration. Oozing or crusting is present. Investigator Global Assessment Scoring System (IGA)
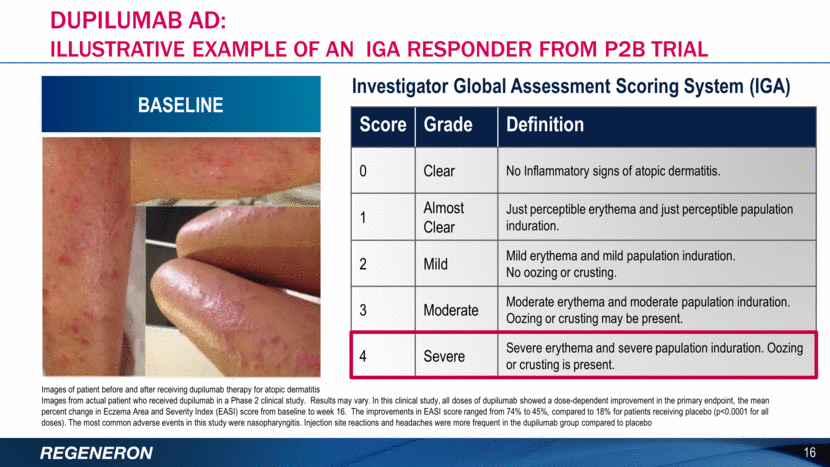
POST-TREATMENT Dupilumab AD: ILLUSTRATIVE EXAMPLE OF AN IGA RESPONDER FROM P2B TRIAL Images of patient before and after receiving dupilumab therapy for atopic dermatitis Images from actual patient who received dupilumab in a Phase 2 clinical study. Results may vary. In this clinical study, all doses of dupilumab showed a dose-dependent improvement in the primary endpoint, the mean percent change in Eczema Area and Severity Index (EASI) score from baseline to week 16. The improvements in EASI score ranged from 74% to 45%, compared to 18% for patients receiving placebo (p<0.0001 for all doses). The most common adverse events in this study were nasopharyngitis. Injection site reactions and headaches were more frequent in the dupilumab group compared to placebo 17 Score Grade Definition 0 Clear No Inflammatory signs of atopic dermatitis. 1 Almost Clear Just perceptible erythema and just perceptible papulation induration. 2 Mild Mild erythema and mild papulation induration. No oozing or crusting. 3 Moderate Moderate erythema and moderate papulation induration. Oozing or crusting may be present. 4 Severe Severe erythema and severe papulation induration. Oozing or crusting is present. Investigator Global Assessment Scoring System (IGA) POST-TREATMENT BASELINE
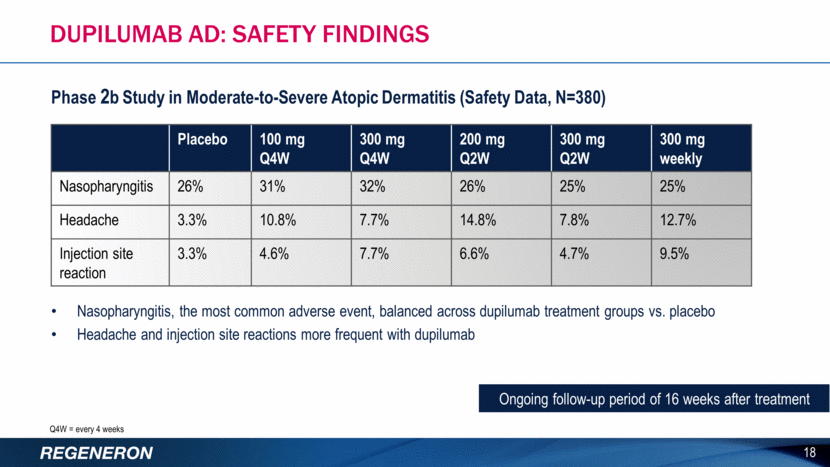
Nasopharyngitis, the most common adverse event, balanced across dupilumab treatment groups vs. placebo Headache and injection site reactions more frequent with dupilumab Ongoing follow-up period of 16 weeks after treatment 18 Phase 2b Study in Moderate-to-Severe Atopic Dermatitis (Safety Data, N=380) Dupilumab AD: SAFETY FINDINGS Placebo 100 mg Q4W 300 mg Q4W 200 mg Q2W 300 mg Q2W 300 mg weekly Nasopharyngitis 26% 31% 32% 26% 25% 25% Headache 3.3% 10.8% 7.7% 14.8% 7.8% 12.7% Injection site reaction 3.3% 4.6% 7.7% 6.6% 4.7% 9.5% Q4W = every 4 weeks
Dupilumab AD: LIBERTY SOLO and CHRONOS Study Design 19 Primary endpoint: Percentage of patients with both IGA 0 to 1 and reduction from baseline of >2 points at week 16 SOLO 1 and SOLO 2 Study Design CHRONOS Study Design Primary endpoint: Percentage of patients with both IGA 0 to 1 and reduction from baseline of >2 points at week 16 Dupilumab 300 mg Q2W + TCS Placebo + TCS Primary efficacy endpoint at 16 weeks Long-term safety and efficacy up to 52 weeks Dupilumab 300 mg QW + TCS N=700 Dupilumab 300 mg Q2W Placebo Primary endpoint at 16 weeks Dupilumab 300 mg QW N=600 Patients eligible to enroll in OLE or SOLO-CONTINUE Data from Phase 3 SOLO studies expected in 1H16

DUPILUMAB ASTHMA: UNMET NEED DESPITE EXISTING THERAPIES Estimated that approximately 26 million people are affected by asthma in the U.S. Despite therapy with ICS/LABA, asthma is not adequately controlled in 5% to 10% of the patients1 Approximately 1.7 million patients have have moderate-to-severe, uncontrolled asthma in the U.S. It is estimated that asthma results in 1.9 million visits to the emergency room each year 479,300 hospital admissions each year 9 deaths each day 20 1http://www.cdc.gov/asthma/asthmadata.htm LABA = long acting beta agonist. ICS = inhaled corticosteroid
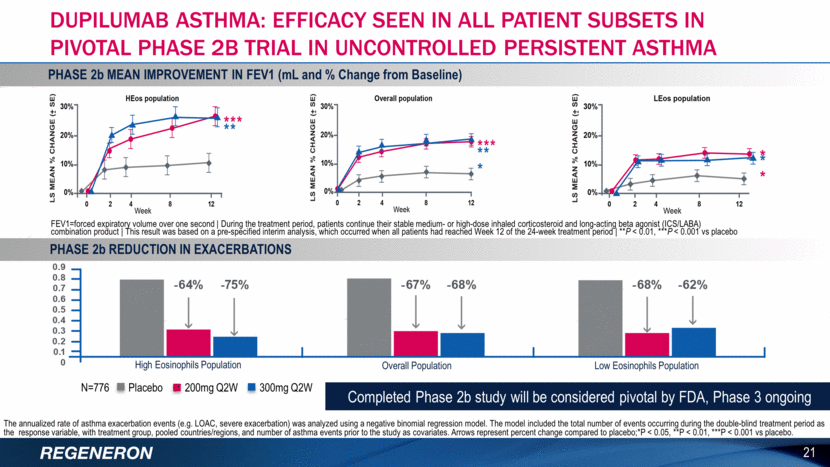
The annualized rate of asthma exacerbation events (e.g. LOAC, severe exacerbation) was analyzed using a negative binomial regression model. The model included the total number of events occurring during the double-blind treatment period as the response variable, with treatment group, pooled countries/regions, and number of asthma events prior to the study as covariates. Arrows represent percent change compared to placebo;*P < 0.05, **P < 0.01, ***P < 0.001 vs placebo. High Eosinophils Population Overall Population Low Eosinophils Population 21 DUPILUMAB ASTHMA: EFFICACY SEEN IN ALL PATIENT SUBSETS IN PIVOTAL PHASE 2B TRIAL IN UNCONTROLLED PERSISTENT ASTHMA PHASE 2b MEAN IMPROVEMENT IN FEV1 (mL and % Change from Baseline) FEV1=forced expiratory volume over one second During the treatment period, patients continue their stable medium- or high-dose inhaled corticosteroid and long-acting beta agonist (ICS/LABA) combination product This result was based on a pre-specified interim analysis, which occurred when all patients had reached Week 12 of the 24-week treatment period **P < 0.01, ***P < 0.001 vs placebo PHASE 2b REDUCTION IN EXACERBATIONS N=776 Placebo 200mg Q2W 300mg Q2W Completed Phase 2b study will be considered pivotal by FDA, Phase 3 ongoing
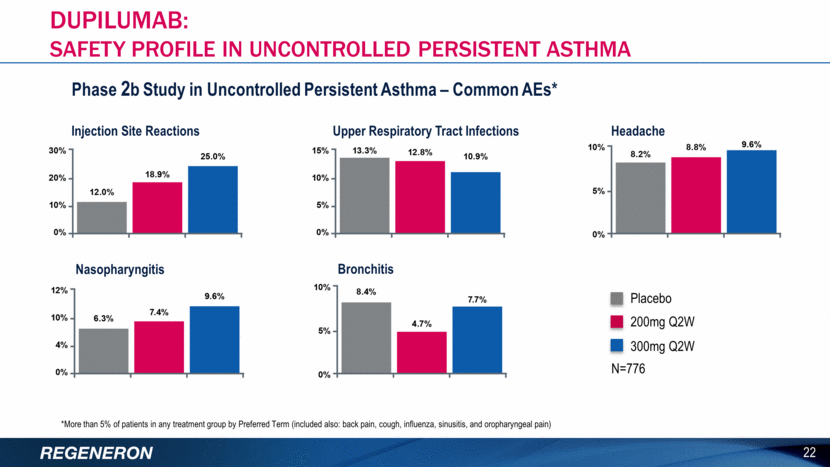
Injection Site Reactions Upper Respiratory Tract Infections Headache Nasopharyngitis Bronchitis Phase 2b Study in Uncontrolled Persistent Asthma – Common AEs* *More than 5% of patients in any treatment group by Preferred Term (included also: back pain, cough, influenza, sinusitis, and oropharyngeal pain) N=776 Placebo 200mg Q2W 300mg Q2W 22 DUPILUMAB: SAFETY PROFILE IN UNCONTROLLED PERSISTENT ASTHMA
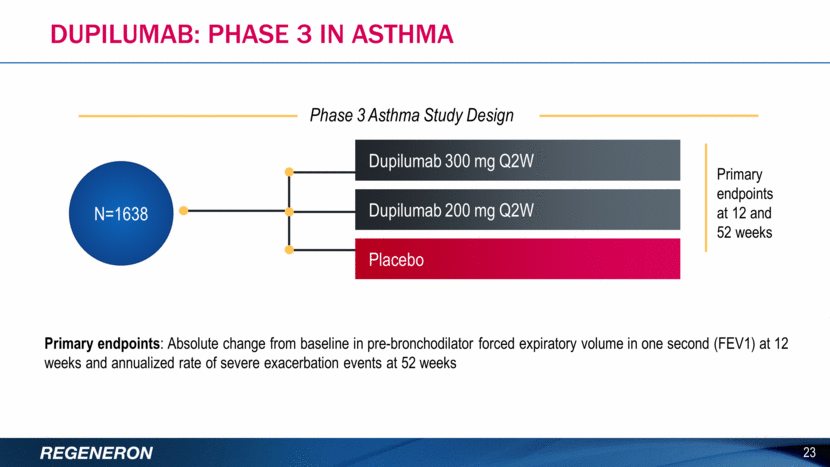
DUPILUMAB: PHASE 3 IN ASTHMA 23 Primary endpoints: Absolute change from baseline in pre-bronchodilator forced expiratory volume in one second (FEV1) at 12 weeks and annualized rate of severe exacerbation events at 52 weeks Dupilumab 300 mg Q2W Placebo N=1638 Dupilumab 200 mg Q2W Primary endpoints at 12 and 52 weeks Phase 3 Asthma Study Design
Fasinumab (NGF mAb) presents a novel, non-opioid approach to addressing chronic pain Osteoarthritis (OA) estimated to affect about 25 million adults in the U.S., with many inadequately served by current therapies* Based on discussions with the FDA, Phase 3 trials (>16 weeks) expected to begin in 1H16 Data from 16 week Phase 2/3 in OA pain anticipated in 1H16 Partnered with Mitsubishi Tanabe Pharma (MTPC) in Japan, Korea, and nine other Asian countries, excluding China FASINUMAB: PHASE 3 STUDY IN OSTEOARTHRITIS PAIN 24 * http://www.cdc.gov/arthritis/basics/osteoarthritis.htm
REGN2222: PHASE 3 IN RESPIRATORY SYNCYTIAL VIRUS (RSV) 25 1 in 5 infants <6 months will require medical attention for RSV infection Hospitalization, emergency room or clinic visits Current guidelines restrict use of only approved prophylactic to infants born before 29 weeks gestation, infants with bronchopulmonary dysplasia, or congenital heart disease First Phase 3 study, NURSERY-Pre-term underway in infants <35 weeks gestation* Enrollment expected to be completed in 2017 25 * Patients must be 6-months or less in age
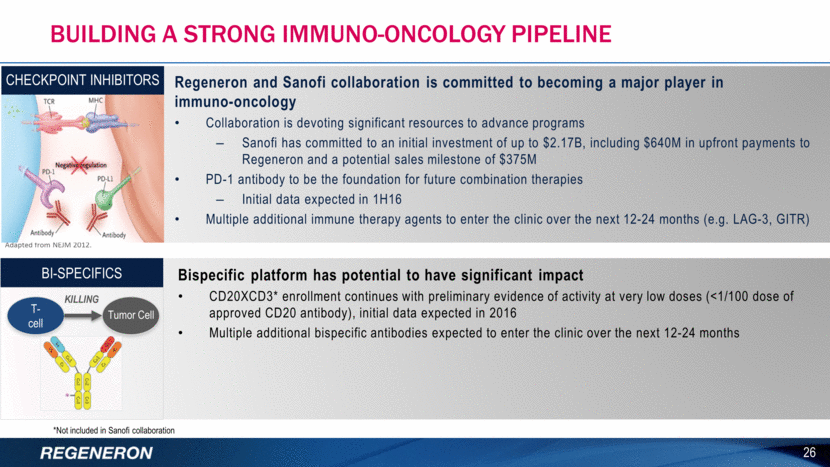
BUILDING A STRONG IMMUNO-ONCOLOGY PIPELINE Bispecific platform has potential to have significant impact CD20XCD3* enrollment continues with preliminary evidence of activity at very low doses (<1/100 dose of approved CD20 antibody), initial data expected in 2016 Multiple additional bispecific antibodies expected to enter the clinic over the next 12-24 months 26 *Not included in Sanofi collaboration BI-SPECIFICS Adapted from NEJM 2012. T-cell Regeneron and Sanofi collaboration is committed to becoming a major player in immuno-oncology Collaboration is devoting significant resources to advance programs Sanofi has committed to an initial investment of up to $2.17B, including $640M in upfront payments to Regeneron and a potential sales milestone of $375M PD-1 antibody to be the foundation for future combination therapies Initial data expected in 1H16 Multiple additional immune therapy agents to enter the clinic over the next 12-24 months (e.g. LAG-3, GITR) CHECKPOINT INHIBITORS Tumor Cell Killing
REGENERON RAPID RESPONSE: LEVERAGING CORE VELOCISUITE® TECHNOLOGIES Rapid Response enables Regeneron to compress time for discovery and preclinical validation from years to months 27 MERS Identification and validation of Spike-protein blocking antibodies Phase 1 studies planned for 2H16 EBOLA Identification and validation of a novel therapeutic cocktail of three antibodies Phase 1 study in healthy volunteers planned for 1H16
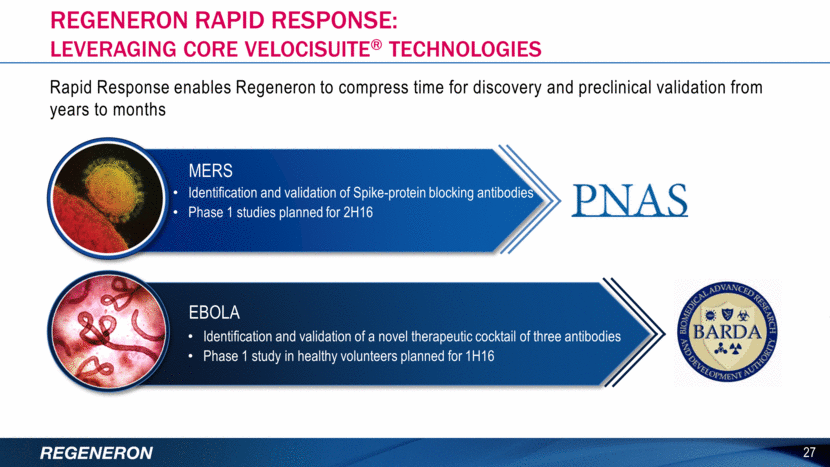
Regeneron genetics center: unprecedented speed, scale & integration 28 100,000 ~100,000 DOZEN >95% EXOMES SEQUENCED TO DATE ADDITIONAL EXOMES TO BE SEQUENCED IN 2016 POTENTIAL NEW DRUG TARGETS IDENTIFIED RESEARCH PARTNERS OF GENES WITH IDENTIFIED LOF CARRIER(S) MULTIPLE EXISTING TARGETS VALIDATED NUMEROUS
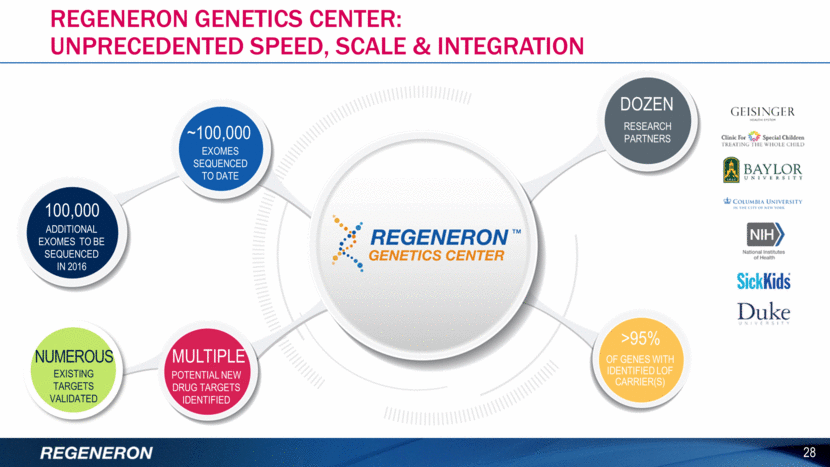
2016 Financial guidance1 29 ACTIVITY GUIDANCE Non-GAAP Unreimbursed R&D $875MM - $950MM Non-GAAP SG&A This includes REGN incurred commercial-related expenses for Sanofi collaboration antibodies $925MM - $1000MM Cash Tax2 as a % of Non-GAAP Pre-tax Income Includes one-time ~$222 million tax payment related to the 3Q15 immuno-oncology upfront payment from Sanofi 35% to 45% Capital Expenditures Expanding manufacturing facilities in Rensselaer, NY and Raheen, Ireland, as well as the continued expansion of the Tarrytown, NY campus $580MM - $680MM 1This financial guidance does not assume the completion of any significant business development transactions not completed as of January 12th, 2016 2Represents estimated income taxes that are payable in cash for the relevant period.
Upcoming Milestones in 2016 30 EYLEA + PDGF: Readout from Phase 2 study EYLEA + ANG2: Initiation of Phase 2 study EYLEA: Initiation of Phase 3 study in diabetic retinopathy Praluent®: Ongoing launches worldwide Praluent®: Interim analyses from ODYSSEY OUTCOMES study Sarilumab: Regulatory review and potential launch in the U.S. Dupilumab: Phase 3 readouts in atopic dermatitis and rolling BLA submission Dupilumab: Initiation of Phase 3 pediatric study in atopic dermatitis Fasinumab: Readout from Phase 2/3 clinical study, initiation of Phase 3 studies >16 weeks duration Immuno-oncology: Data from Phase 1 PD-1 and CD20xCD3 programs Regeneron Rapid Response: MERS and Ebola antibodies to enter clinical development

J.P. MORGAN 34TH ANNUAL HEALTHCARE CONFERENCE JANUARY 2016

