Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LIGAND PHARMACEUTICALS INC | ligandomt-form8xkmergeragr.htm |
| EX-99.1 - EXHIBIT 99.1 - LIGAND PHARMACEUTICALS INC | ligandtoacquireomtincalead.htm |
| EX-2.1 - EXHIBIT 2.1 - LIGAND PHARMACEUTICALS INC | omttransaction-mergeragree.htm |

OMT, Inc. Acquisition Conference Call December 17, 2015 NASDAQ: LGND

2 The following presentation contains forward-looking statements regarding Ligand’s prospects, plans and strategies, drug development programs and collaborations. Forward-looking statements include the expected completion of the acquisition of OMT and the timing thereof, the benefits of the acquisition to Ligand, the expected revenues from OMT and other financial projections and guidance and Ligand’s expectation that the acquisition will be accretive to revenues and adjusted EPS, the number of partners to be added to Ligand’s portfolio and the potential clinical development of such partners’ antibodies using the OMT platform , intellectual property protection of the OMT platform, expectations regarding our and our partners’ research and development programs, and other statements including words such as “will,“ “should,” “could,” “plan,” etc. Actual events or results may differ from Ligand’s expectations. The forward-looking statements made in the presentation are subject to several risk factors, including, that the various closing conditions for the OMT acquisition may not be satisfied or waived, Ligand’s reliance on OMT’s partners for upfront, milestone, annual maintenance and royalty payments and risks related to OMT’s partners’ ability to terminate their partnership agreements, which may reduce the number of commercial partners Ligand adds to its portfolio, the failure of one OMT partner’s program may impact other OMT partner’s decision to continue using the OMT platform, Ligand’s ability to retain Dr. Buelow after the acquisition is complete, risks inherent in clinical development by OMT’s partners, including through use of the OMT platform, exposure to unknown liabilities as a result of the acquisition, the ability of OMT and its partners to obtain, maintain and successfully enforce adequate patent and other intellectual property protection and the ability to operate their businesses without infringing the intellectual property rights of others, and other risks and uncertainties described in its public filings with the Securities and Exchange Commission, available at www.sec.gov. Additional risks may apply to forward-looking statements made in this presentation. Readers are cautioned not to place undue reliance on these forward-looking statements, which reflect our good faith beliefs (or those of the indicated third parties) and speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and Ligand undertakes no obligation to revise or update this presentation to reflect events or circumstances or update third party research numbers after the date hereof. This caution is made under the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934. Our trademarks, trade names and service marks referenced herein include Ligand and Captisol. Each other trademark, trade name or service mark appearing in this presentation belongs to its owner. The process for reconciliation between non-GAAP financial numbers presented on slide 25 and 26, and the corresponding GAAP figures is explained in the footnotes on that slide and in our earnings press release dated, November 9, 2015. Safe Harbor Statement

Agenda 1) Acquisition Highlights and Value Drivers John Higgins, CEO 2) OMT – Company Overview and Technology Platform Matt Foehr, COO 3) Financial Outlook and Upcoming Events Matt Korenberg, CFO 3

Acquisition Highlights and Value Drivers

OMT – Open Monoclonal Technology, Inc. • Leader in genetically engineered transgenic animals for human antibody discovery — Founded in 2008 by Roland Buelow, PhD, a world-renowned antibody scientist — Financed by Essex Woodlands — Business based on transgenic animal platform licensing to biopharma globally • Three complementary, globally branded platforms — OmniRat®, OmniMouse® and OmniFlic™ - delivering OmniAb™ antibodies — Only rat-based platforms for mono-specific and bispecific antibodies in the industry — Believed to be only company with two species • Licensed the technology to 16 pharma and biotech companies • Virtual operation with lean cost structure — Animal breeding and sourcing done by world-leading vendors in the US and Europe — OmniAb antibody discovery performed by proven CROs in the US, Europe and Asia — Virtual operation with three employees in the US 5

OMT Opportunity • Proven Business Model — Profitable, cash-flow positive company — Potentially highly lucrative portfolio of licenses — Strong science and technology to drive future deal making • Excellent Strategic Fit — Technology licensing business similar to Captisol model — A few employees and low costs to manage the business, partners and licensing — Deal accretive to Ligand's short and long-term financials • Diversifies and Strengthens Ligand — Initially adds 16 shots on goal to portfolio — Existing deals could generate over 30 clinical candidates over next 10 years — Major new technology that will stand next to Captisol for new licensing — Significantly extends Ligand's potential IP horizon for revenue generating assets 6

7 Major Value Drivers for OMT Acquisition • Financial Contribution — Projected to be accretive to revenues and adjusted EPS — Potential for significant financial contribution to Ligand through future royalties — A strong platform for Ligand to seek new licenses and partnerships • Portfolio Expansion — Major addition of high quality partners and fully-funded shots on goal — Brings to Ligand 16 new shots on goal — With OMT, Ligand will have more than 140 fully-funded programs and more than 81 partners • Technology Diversification — OmniAb is a broad and robust technology platform and is a key resource used by biopharma companies to discover new biologic drugs — A new technology pillar for Ligand’s business, standing alongside the Captisol drug-formulation technology • Royalty Extenstion — Significant extension of potential patent protection period and royalty terms for Ligand-partnered programs — Patents for OMT technology run through 2033, but each newly discovered antibody may be the basis for its own novel intellectual property, resulting in patents for each antibody on a drug-by- drug basis that could extend past 2040 7

Antibody Prescription Medicines 2014 Global Sales 8 Confidential Drug Therapeutic Area 2014 sales ($ billions) Drug Therapeutic Area 2014 sales ($ billions) Humira Autoimmune $12.9 Humira Autoimmune $12.9 Sovaldi/Harvoni Hepatitis C $10.3 Remicade Autoimmune $9.8 Remicade Autoimmune $9.8 MabThera/Rituxan Cancer $7.5 Enbrel Autoimmune $8.9 Avastin Cancer $7.0 Lantus Diabetes $8.4 Herceptin Cancer $6.9 Abilify CNS Disorders $8.2 Lucentis Opthalmology $4.3 MabThera/Rituxan Cancer $7.5 Soliris Orphan Diseases $2.2 Seretide/Advair Asthma $7.0 Stelara Autoimmune $2.1 Avastin Cancer $7.0 Tysabri Autoimmune $2.0 Herceptin Cancer $6.9 Erbitux Cancer $1.9 5 of Top 10 Selling Drugs are Antibodies Top 10 Antibodies Sold $57 B in 2014 Note: Lucentis is an antibody fragment Source: www.pharmalive.com Antibody-based medicine

0 100 200 300 400 500 2008 2015 150 A n ti b o d ies in c lin ic al d ev el o p me n t 468 • Antibodies are a promising and rapidly growing category of therapeutic research • Number of antibodies in clinical development has tripled since 2008 Antibodies in Clinical Development 9 Sources: Nelson et al., Nature Reviews, 2010. Reichert Biotechnology Consulting LLC, 2015.
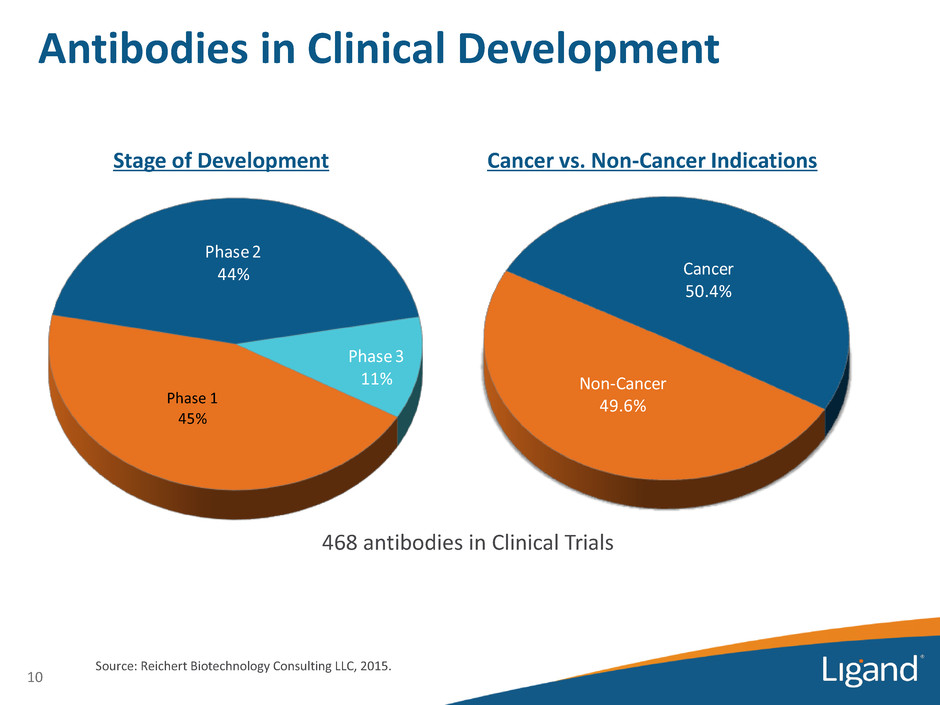
Phase 1 45% Phase 2 44% Phase 3 11% Antibodies in Clinical Development 10 Source: Reichert Biotechnology Consulting LLC, 2015. Non-Cancer 49.6% Cancer 50.4% Stage of Development Cancer vs. Non-Cancer Indications 468 antibodies in Clinical Trials

OMT Overview and Technology Platform

OmniAb Antibody Platforms • Key advantages: — Human antibodies have reduced immunogenicity — Using transgenic rodents avoids the need for genetic engineering to “humanize” antibodies and accelerates antibody discovery — Broad diversity of high-quality antibodies • OMT’s genetically engineered novel, transgenic rodents produce fully human antibodies OMT Transgenic Animal Human AntibodiesImmunization 12

OmniAb Facilitates Rapid Antibody Discovery • Groundbreaking research by OMT led to Science publication, creating scientific community visibility for the antibody generation technology • OmniAb underlying technologies : An industry leading patented, validated human antibody rat 2nd species with different immune response genes yields additional antibodies and increased epitope coverage for partners Rat with single common light chain, designed for bispecific human antibodies 13 Source: Geurts AM, et al, Science. 2009.

The OMT Licensing and Partnering Model • OMT enters into Platform License Agreements with partners/licensees that provide access to the OmniAb platform (OmniRat, OmniMouse and/or OmniFlic) for use in identifying antibodies for development ‒ Generates revenue from upfront payments, annual maintenance fees, potential development /commercial milestones and/or potential royalties ‒ Royalties rates in the low-to-mid single digits • OmniAb colony maintenance and supply handled by Charles River Labs and Taconic ‒ Sell directly to OMT partners/licensees ‒ Reduces operational risk and required partnership/license maintenance • Similar to Captisol, scientific and technical support form strong relationship foundation ‒ Roland Buelow, Ph.D., founder of OMT, brings 30 years of extensive R&D experience in transgenic animal generation and antibody development to Ligand 14

OMT Partners/Licensees 16 Partnerships and Licensees with Potential to Grow • Impressive and growing roster of currently active partners/licensees validates the utility of the technology. Disclosed partners/licensees include: 15 • Ligand projects up to three antibodies from the OMT platform will be in human Phase 1 trials by the end of 2017 and as many as 15 antibodies could be in Phase 1 or more advanced trials by 2020

OMT is a Leader in a Highly-Specialized Antibody Landscape 16 • OMT’S platform meets seven important standards 1) Fully Human 5) Bispecific format 2) Eukaryotic System 6) Open platform 3) Transgenic Mouse 7) Optimized Ab production 4) Transgenic Rat • Only antibody discovery platform that provides all seven
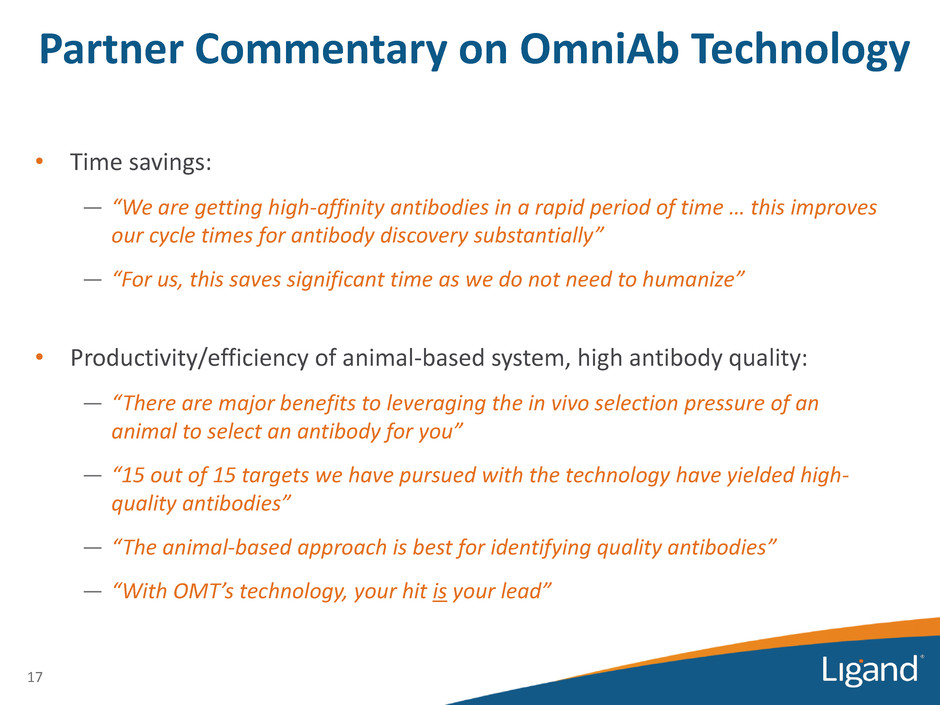
Partner Commentary on OmniAb Technology • Time savings: — “We are getting high-affinity antibodies in a rapid period of time … this improves our cycle times for antibody discovery substantially” — “For us, this saves significant time as we do not need to humanize” • Productivity/efficiency of animal-based system, high antibody quality: — “There are major benefits to leveraging the in vivo selection pressure of an animal to select an antibody for you” — “15 out of 15 targets we have pursued with the technology have yielded high- quality antibodies” — “The animal-based approach is best for identifying quality antibodies” — “With OMT’s technology, your hit is your lead” 17

• Freedom to operate: — “Freedom to operate was very clear to us, because OMT has a rat” — “Having a rat and a mouse available was a big selling point for us” • As compared to competition or other technologies: — “We ran OmniRat head-to-head versus phage display and OmniRat won … the OmniRat will beat phage display any day” — “OmniRat gets us there in half the time as compared to another technology we’ve used” • Promise of next generation: — “I would be very surprised if OmniFlic doesn’t deliver on expectations for bispecific antibodies” 18 Partner Commentary on OmniAb Technology

Major Expansion of Licensing Opportunities Furthering Technology Diversification Solving solubility and stability challenges predominantly for small molecules Allows for higher and more stable expression of recombinant proteins SUREtechnology Platform™ Proven, unrestricted Fully Human Antibody technology enabling drug discovery in infectious diseases, cancer and autoimmunity Selectively delivers broad range of pharmaceutical agents to the liver LTP Technology™ 19

Underlying IP Diversification and Expansion Addition of OmniAb Broadens and Extends Portfolio Pioneering platform patent to 2023 in all major countries (except China) Proprietary vector patent to 2026 in all major countries SUREtechnology Platform™ Multiple patent families provide broad global coverage Potential patent term to 2033 EU: 2025 US: 2029 BUT, post-antibody discovery, partner/ licensee IP creates much longer patent and royalty tail from the 2030s to early 2040s Initial patents: EU: 2028 US: 2029 & 2033 Four distinct composition-of- matter patent families Patent term to 2036 LTP Technology™ 20

Biotech 52% Big Pharma 19% Generic 11% Spec Pharma 18% > 83 Partners Proforma Partner Portfolio Expansion of Partner Base Biotech 44% Big Pharma 28% Generic 16% Spec Pharma 10% > 74 Partners Current Ligand Pro Forma 21

Financial Outlook and Upcoming Events
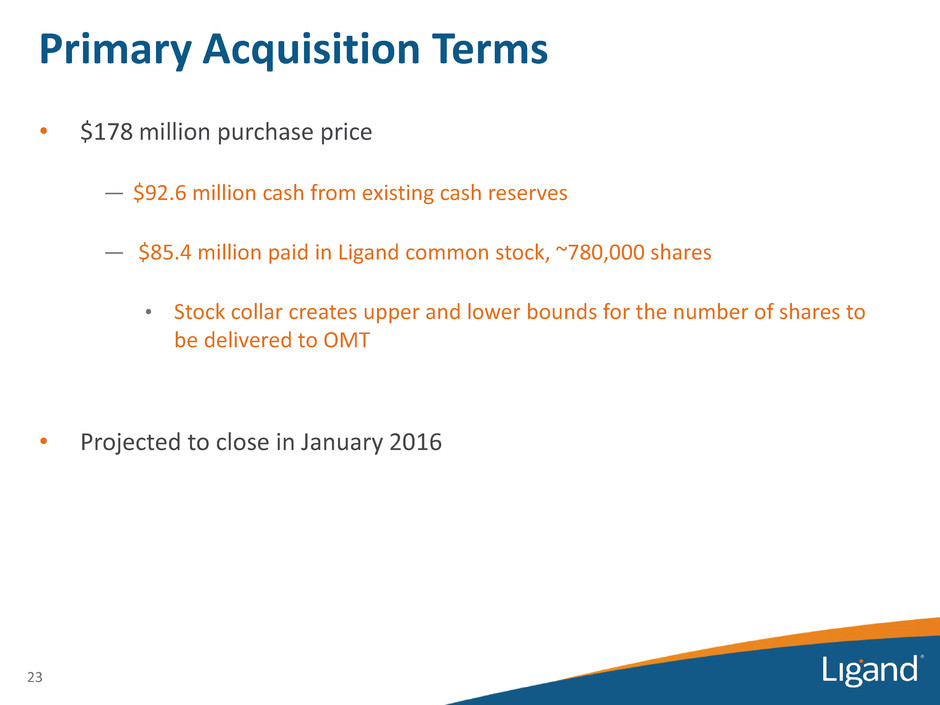
Primary Acquisition Terms • $178 million purchase price — $92.6 million cash from existing cash reserves — $85.4 million paid in Ligand common stock, ~780,000 shares • Stock collar creates upper and lower bounds for the number of shares to be delivered to OMT • Projected to close in January 2016 23

• Revenue — Near-term revenue consists of annual access fees and milestones from existing contracts — Adds $6 million in 2016 and $12 million in 2017 — Adds 7% - 10% annually for the next decade — Initial products potentially launch in 2026 providing possibility for substantial new royalty streams into the 2030s and possibly 2040s • Adjusted EPS — Slightly accretive to adjusted EPS in 2016; $0.20 accretive in 2017 — 4% - 8% accretive to adjusted EPS in 2017 and beyond Projected Financial Impact 24 Note: The adjusted financial measures discussed above exclude changes in contingent liabilities, mark-to-market adjustment for amounts owed to licensors, non-cash stock-based compensation expense, non-cash debt-related costs, pro-rata non-cash net losses of Viking Therapeutics, non-cash OMT purchase price amortization and non-cash tax expense.

• Guidance — 2016 pro forma revenues between $113 million and $117 million, adjusted EPS in the range of $3.33 to $3.38 — 2017 pro forma revenues expected to exceed $158 million, adjusted EPS expected to exceed $4.95 • OMT non-cash amortization expense estimates are expected to be determined in the near-term — Amortization charges will be recognized in GAAP EPS and the non-cash charge will be excluded from adjusted EPS • Ligand projected to have ~$100 million of cash at closing Projected Financial Impact, continued 25 Note: The adjusted financial measures discussed above exclude changes in contingent liabilities, mark-to-market adjustment for amounts owed to licensors, non-cash stock-based compensation expense, non-cash debt-related costs, pro-rata non-cash net losses of Viking Therapeutics, non-cash OMT purchase price amortization and non-cash tax expense.

Upcoming Investor Events Event Location Date CJS New Ideas Conference New York January 13, 2016 Year-End Earnings Call Conference Call February – TBD Deutsche Bank Pharma One-on-One Day Denver March 3, 2016 Annual Roth Conference Dana Point, CA March 13-16, 2016 26

OMT, Inc. Acquisition Conference Call December 17, 2015 NASDAQ: LGND
