Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
Mark One
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File No. 001-33093
LIGAND PHARMACEUTICALS INCORPORATED
(Exact name of registrant as specified in its charter)
| Delaware | 77-0160744 | |
| (State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) | |
| 11085 North Torrey Pines Rd., Suite 300 San Diego, CA |
92121 | |
| (Address of Principal Executive Offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (858) 550-7500
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Each Exchange on Which Registered | |
| Common Stock, par value $.001 per share |
The NASDAQ Global Market of The NASDAQ Stock Market LLC | |
| Preferred Share Purchase Rights |
The NASDAQ Global Market of The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934. Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large Accelerated Filer ¨ | Accelerated Filer x | Non-accelerated Filer ¨ | Smaller reporting company ¨ | |||
| (Do not check if a smaller | ||||||
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the Registrant’s voting and non-voting stock held by non-affiliates was approximately $288.6 million based on the last sales price of the Registrant’s Common Stock on the NASDAQ Global Market of the NASDAQ Stock Market LLC on June 30, 2009. For purposes of this calculation, shares of Common Stock held by directors, officers and 10% stockholders known to the Registrant have been deemed to be owned by affiliates which should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant or that such person is controlled by or under common control with the Registrant.
As of February 11, 2010, the Registrant had 117,335,286 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Proxy Statement for the Registrant’s 2010 Annual Meeting of Stockholders to be filed with the Commission on or before April 29, 2010 are incorporated by reference in Part III of this Annual Report on Form 10-K. With the exception of those portions that are specifically incorporated by reference in this Annual Report on Form 10-K, such Proxy Statement shall not be deemed filed as part of this Report or incorporated by reference herein.
Table of Contents
| Item 1. |
1 | |||
| Item 1A. |
17 | |||
| Item 1B. |
28 | |||
| Item 2. |
28 | |||
| Item 3. |
28 | |||
| Item 4. |
29 | |||
| Item 5. |
30 | |||
| Item 6. |
32 | |||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
34 | ||
| Item 7A. |
50 | |||
| Item 8. |
51 | |||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
100 | ||
| Item 9A. |
100 | |||
| Item 9B. |
102 | |||
| Item 10. |
102 | |||
| Item 11. |
102 | |||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
102 | ||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
103 | ||
| Item 14. |
103 | |||
| Item 15. |
104 | |||
| 119 | ||||
AVAILABLE INFORMATION:
We file electronically with the Securities and Exchange Commission (or SEC) our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and, as necessary, amendments to these reports, pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended. The public may read or copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is <http://www.sec.gov>.
You may obtain a free copy of our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports which are posted as soon as reasonably practicable after filing on our website at <http://www.ligand.com>, by contacting the Investor Relations Department at our corporate offices by calling (858) 550-7500 or by sending an e-mail message to investors@ligand.com. You may also request information via the Investor Relations page of our website.
Table of Contents
| Item 1. | Business |
Caution: This discussion and analysis may contain predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed in Item 1A. “Risk Factors.” This outlook represents our current judgment on the future direction of our business. These statements include those related to our AVINZA and PROMACTA royalty revenues, collaborative revenues and milestones and product development. Actual events or results may differ materially from Ligand’s expectations. For example, there can be no assurance that our revenues or expenses will meet any expectations or follow any trend(s), that we will be able to retain our key employees or that we will be able to enter into any strategic partnerships or other transactions. We cannot assure you that we will receive expected AVINZA and PROMACTA royalties or other revenues to support our ongoing business or that our internal or partnered pipeline products will progress in their development, gain marketing approval or achieve success in the market. In addition, future arbitration, litigation or disputes with third parties may have a material adverse effect on us. Such risks and uncertainties, and others, could cause actual results to differ materially from any future performance suggested. We undertake no obligation to release publicly the results of any revisions to these forward-looking statements to reflect events or circumstances arising after the date of this annual report. This caution is made under the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934, as amended.
References to “Ligand Pharmaceuticals Incorporated”, “Ligand”, the “Company”, “we” or “our” include our wholly owned subsidiaries—Ligand Pharmaceuticals International, Inc.; Seragen, Inc., or Seragen; Pharmacopeia, LLC; Neurogen Corporation and Nexus Equity VI LLC, or Nexus.
We were incorporated in Delaware in 1987. Our principal executive offices are located at 11085 North Torrey Pines Road, Suite 300, San Diego, California, 92121. Our telephone number is (858) 550-7500.
Overview
We are a biotechnology company that focuses on drug discovery and early-stage development of pharmaceuticals that address critical unmet medical needs or that are more effective and/or safer than existing therapies, more convenient to administer and are cost effective. Our goal is to build a profitable company by generating income from research, milestone, and royalty revenues resulting from our collaborations with pharmaceutical partners.
Our business strategy includes targeted internal drug research and early-stage development capabilities. We believe we have promising product candidates throughout our internal development programs. We also have research and development collaborations for our product candidates with numerous global pharmaceutical companies. These collaborations include ongoing clinical programs at Bristol-Myers Squibb, or BMS, GlaxoSmithKline, or GSK, Pfizer, Merck & Co., or Merck, Roche, Cephalon and Celgene. These partnered product candidates are being studied for the treatment of large market indications such as thrombocytopenia, rheumatoid arthritis, chronic obstructive pulmonary disease, or COPD, asthma, osteoporosis, menopausal symptoms and Alzheimer’s disease as summarized in the following tables.
Table 1: Pipeline Overview
| Marketed |
Under FDA/EU Review | Phase III | ||
| ITP – Eltrombopag/PROMACTA (GSK) | Osteoporosis – Bazedoxifene (Pfizer) |
Hepatitis C – Eltrombopag (GSK) | ||
| Chronic Pain – Avinza (King) | Osteoporosis – Lasofoxifene (Pfizer) |
Menopausal symptoms – Bazedoxifene+Premarin (Pfizer) | ||
| Acadesine – Coronary Artery Bypass Graft (CABG) (Pericor and Merck) | ||||
1
Table of Contents
| Phase II |
Phase I |
Preclinical/Research | ||
| Leukemia (AML & ALL) – Dinaciclib (Merck) | Advanced Myelodysplastic Syndrome (MDS) or Secondary Acute Myeloid Leukemia After MDS – Eltrombopag (GSK) | HepDirect nucleoside for hepatitis C (Roche) | ||
| Mantle Cell Lymphoma or B-Cell Chronic Lymphocytic Leukemia – Dinaciclib (Merck) | Sarcoma – Eltrombopag (GSK) | Hematological-Erythropoietin receptor small molecule agonist | ||
| Advanced Breast and Lung Cancers – Dinaciclib (Merck) | Alzheimer’s – Beta secretase (BACE) inhibitor (Merck) | Diabetes – Glucagon receptor antagonist | ||
| COPD and Asthma – PS291822 (Merck) | Muscle wasting – LGD-4033 (unpartnered) | Hyperlipidemia – Thyroid receptor beta agonist | ||
| RA, psoriasis and atherosclerosis –PS540446 (BMS) | Inflammation – PS873266 (Celgene) | VR1antagonist for pain and cough (Merck) | ||
| ITP-LGD-4665 (GSK) | Atherosclerosis – XL-652 (Exelixis) | Inflammation – JAK-3 inhibitor (Pfizer) | ||
| Eight collaboration programs with undisclosed targets | ||||
Business Strategy
We aim to create value for shareholders by advancing our internally developed programs through early clinical development and then entering licensing agreements with larger pharmaceutical and biotechnology companies with substantially greater development and commercialization infrastructure. In addition to advancing our R&D programs, we expect to collect licensing fees and royalties from existing and future license agreements. We aim to build a profitable company by generating income from our corporate licenses. The principal elements of our strategy are set forth below.
Leverage Proprietary Gene Expression and Combinatorial Chemistry Platform Technologies Related to Multiple Novel Drug Discovery Programs. Our technology applies the most advanced cell-based assays, gene-expression tools, ultra-high throughput screening and one of the world’s largest chemical libraries to discover new and important medicines:
| • | Intracellular Technology: We pioneered the field of Intracellular receptor (IR) drug discovery using cell-based assays of nuclear receptors, cell signaling enzymes and membrane receptors. Intracellular receptors are families of transcription factors that change cell function by selectively turning on or off specific genes in response to circulating signals that act on cells. Our ability to harness these processes through IR technology has enabled the development of novel, small-molecule drugs that act through intracellular receptors, potentially resulting in more targeted drugs with greater specificity than those currently available. |
| • | Chemical Library: In December 2008, we acquired high quality combinatorial libraries and proprietary ultra-high throughput screening technology as a result of our acquisition of Pharmacopeia. Our Encoded Combinatorial Library on Polymeric Support, or ECLiPS™ technology, combinatorial library technology provides the power of one of the world’s largest chemical collections to identifying drugs for novel receptor and enzyme drug targets. We use a proprietary combinatorial compound collection wedded to a unique ultra-high throughput screening platform to drive lead generation for us and our pharma partners. Our collection of drug-like molecules is built by our chemists on polystyrene beads and ‘encoded’ with molecular tags that can be easily ‘decoded’ for hit identification. This ECLiPS forms the basis for one of the largest compound collections in the industry. Our proprietary tagging technology obviates the usual deconvolution process and facilitates both accurate and rapid hit |
2
Table of Contents
| identification. This combinatorial chemistry collection is built for chemical diversity and drug-like properties. In this way our hits combine the desired target activity with appropriate physicochemical properties that support continued drug discovery. |
| • | Ultra-High Throughput Screening: We have married this large proprietary compound collection with industry leading ultra-high throughput screening (UHTS) capacity and capability. More than 70% of our screens are in 1536-well plate formats with well volumes of 1 to 9 microliters. We have developed nanovolume liquid dispensing to deliver reagent volumes as low as 50 nL to 1536 plates with exceptional accuracy. Numerous types of screening and detection capabilities are employed, including cell-free and cell-based, functional or binding, fluorescent or radioactive, and many others. |
Discover and Develop Targeted Modulators that are Promising Drug Candidates. We discover, synthesize and test numerous compounds to identify those that are most promising for clinical development. We perform extensive target profiling and base our selection of promising development candidates on product characteristics such as initial indications of safety and efficacy. We believe that this focused strategy allows us to eliminate unpromising candidates from consideration sooner without incurring substantial clinical costs.
License Drug Candidates to Other Parties. We generally plan to advance drug candidates through initial and/or early-stage drug development. For larger disease indications requiring complex clinical trials, our strategy is to license drug candidates to pharmaceutical or biotechnology partners for final development and global marketing. We believe partnerships are a source of development payments, license fees, future milestone payments and royalties. They also may provide considerable resources for late-stage product development, regulatory activities, manufacturing and marketing. We believe that focusing on discovery and early-stage drug development while benefiting from our partners’ proven development and commercialization expertise will reduce our internal expenses and allow us to have a larger number of drug candidates progress to later stages of drug development. However, after establishing a lead product candidate, we are willing to license that candidate during any stage of the development process that we determine to be beneficial to the company and to the ultimate development and commercialization of that drug candidate.
Generate Revenue through Partnerships to Fund Our Business and Drive Future Profitability. We have multiple sources of potential license and royalty revenue from existing corporate agreements, and we may enter additional partnerships that will provide additional revenue opportunities. We have numerous collaborations that have the potential to generate future royalties for us. We believe the revenue generated from these and future potential collaborations will fund our business and potentially provide profits to our shareholders.
Marketed Products
We currently receive royalty revenues from King Pharmaceuticals, or King, and GSK. In February 2007, we completed the sale of our AVINZA product line to King. As a result of the sale, we received the right to future royalties on the net sales of AVINZA through 2017 (see Table 2 below).
In December 2008, the U.S. Food and Drug Administration, or FDA, granted accelerated approval of GSK’s PROMACTA for the treatment of thrombocytopenia in patients with chronic immune (idiopathic) thrombocytopenic purpura, or ITP, who have had an insufficient response to corticosteroids, immunoglobulins or splenectomy. PROMACTA is also approved under the trade name Revolade(R) in Venezuela, Kuwait, Chile and Russia. GSK also filed a regulatory application for PROMACTA in Japan in September 2009. PROMACTA is the first oral thrombopoietin, or TPO, receptor agonist therapy for the treatment of adult patients with chronic ITP. In December 2009, GSK received a positive opinion for Revolade (eltrombopag/PROMACTA) from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) for the oral treatment of thrombocytopenia (reduced platelet count) in adults with the blood disorder chronic ITP. As a result of the regulatory approvals of PROMACTA, we are entitled to receive tiered royalties on annual net sales of PROMACTA (Table 2). As part of a settlement agreement and mutual release we entered into on February 11, 2009 with The Rockefeller University, or Rockefeller, we agreed to pay a share of such royalties to Rockefeller. See “Item 3. Legal Proceedings.”
3
Table of Contents
Near-term potential royalties: Products under FDA/EU review and in Phase III
We also have the potential to receive near-term royalties on product candidates resulting from our research and development collaboration arrangements with third party pharmaceutical companies if and when any such product candidate is ultimately approved by the FDA and successfully marketed. Our near-term product candidates are discussed below.
In addition to the accelerated approval granted for GSK’s PROMACTA for the treatment of thrombocytopenia in patients with chronic ITP, GSK also reported new phase III results for PROMACTA in chronic ITP at the 2009 14th Congress of European Hematology meeting and completed enrollment of two Phase III trials in patients with hepatitis C in the fourth quarter of 2009. A Phase I/II study in patients with oncology-related thrombocytopenia is ongoing and a Phase I study is ongoing in patients with sarcoma receiving the adriamycin and ifosfamide regimen.
Bazedoxifene (Viviant) is a product candidate that resulted from one of our collaborations with Wyeth (now Pfizer). Bazedoxifene is a synthetic drug that was specifically designed to reduce the risk of osteoporotic fractures while at the same time protecting breast and uterine tissue. Regarding Viviant, the FDA has advised that it expects to convene an advisory committee to review the pending NDAs for both the treatment and prevention indications. Approvable letters were received for each of these NDAs in which, among other things, the FDA requested further analyses and discussion concerning the incidence of stroke and venous thrombotic events, identified certain issues concerning data collection and reporting, and requested additional source documents. An FDA-requested advisory committee meeting is expected to be scheduled following submission of the complete response to the approvable letters. In April 2009, Pfizer received approval in the EU for CONBRIZA (the EU trade name for Viviant) for the treatment of postmenopausal osteoporosis in women at increased risk of fracture. We expect CONBRIZA to be launched in the EU in 2010.
Wyeth (now Pfizer) is also developing bazedoxifene in combination with PREMARIN (Aprela) which is a tissue selective estrogen complex under development for menopausal symptoms and osteoporosis. Two Phase III studies with bazedoxifene/conjugated estrogens (Aprela) showed a reduced number and severity of hot flashes in symptomatic postmenopausal women by up to 80 percent, when compared with placebo. Pfizer expects to file an initial NDA no earlier than the first half of 2010. We are entitled to receive tiered royalties on these products (see Table 2 below).
Lasofoxifene (FABLYN®) is a product candidate that resulted from our collaboration with Pfizer. Pfizer submitted an NDA and an MAA for FABLYN for osteoporosis treatment in December 2007 and January 2008, respectively. The FDA Advisory Committee in September 2008 voted 9-3 in favor of approving this drug. In January 2009, Pfizer received a complete response letter from the FDA requesting additional information for FABLYN. In February 2009, FABLYN received approval in the EU for the treatment of osteoporosis. Pfizer reported that following a strategic review, it decided to explore strategic options for FABLYN, including out-licensing or sale. Under the terms of our agreement with Pfizer, we are entitled to receive royalty payments on worldwide net sales of lasofoxifene for any indication (see Table 2 below). few expect ABLYN to be launched in the EU in 2010.
Advanced R&D Programs
PS291822 is a CXCR2 antagonist that resulted from our collaboration with Schering-Plough (now Merck). PS291822 entered Phase II clinical trials in the fourth quarter of 2006 for COPD and asthma. A Phase II study in patients with COPD was completed in October 2008. Phase II studies in asthma were completed in February 2009. Merck has recently initiated two Phase IIb studies in COPD and asthma with 500 patients each.
PS540446 is an orally active p-38 mitogen-activated protein (MAP) kinase inhibitor that resulted from our collaboration with BMS. Phase II studies for PS540446 were completed in April 2009 for the treatment of moderate to severe psoriasis and in September 2009 for rheumatoid arthritis (RA). Phase II studies in atherosclerosis are ongoing. Positive Phase I results in healthy subjects and in patients with stable RA were reported at the 2008 ACR meeting.
4
Table of Contents
Recent Acquisitions
Neurogen
On December 23, 2009, we acquired all of the outstanding common shares of Neurogen Corporation, or Neurogen. As consideration, we issued approximately 4.2 million shares of our common stock to Neurogen stockholders, or approximately 0.061 of a share of our common stock for each outstanding Neurogen share, as well as approximately $0.6 million in cash. Security holders of Neurogen also received contingent value rights, under which they could receive cash payments under certain circumstances. Neurogen was a drug development company historically focusing on small-molecule drugs to improve the lives of patients suffering from psychiatric and neurological disorders with significant unmet medical needs. Neurogen has conducted its drug development independently and, when advantageous, collaborated with world-class pharmaceutical companies to access additional resources and expertise.
Primary Acquired Assets
| • | Fully Funded Partnership with Merck for Vanilloid Receptor Subtype 1 (VR1) Antagonists in development for pain and cough. |
| • | H3 Antagonist Program for the potential treatment of sleep disorders (e.g. narcolepsy), attention deficit hyperactivity disorder (ADHD), and cognitive deficits (e.g. schizophrenia and Alzheimer’s) in preclinical development. |
| • | Oral Erythropoietin (EPO) Research Program—we have been conducting internal research on orally active erythropoietin agonists. Neurogen conducted its own drug discovery efforts in the area and provided novel chemical scaffolds and additional know-how that could further enhance our oral EPO program. |
| • | Cash and net operating loss carryforwards—we gained approximately $7.4 million in cash from this transaction. Neurogen has more than $180 million in net operating loss carryforwards. While there will be significant limitation to the utilization of the net operating losses over time, the net operating losses may be usable to some extent by us, should the combined companies become profitable. |
Metabasis
On January 27, 2010, we completed the acquisition of Metabasis Therapeutics, Inc., or Metabasis, following approval of the transaction by Metabasis stockholders. As a result, we gained a fully funded partnership with Hoffman-La Roche Inc., or Roche, additional pipeline assets and drug discovery technologies and resources. We paid $1.6 million in cash or about $0.046 per Metabasis share to Metabasis’ stockholders. In addition, Metabasis stockholders received four tradable Contingent Value Rights (CVRs), one CVR from each of four respective series of CVRs, for each Metabasis share. The CVRs will entitle Metabasis stockholders to cash payments as frequently as every six months as cash is received by us from proceeds from Metabasis’ partnership with Roche or the sale or partnering of any of the Metabasis drug development programs, among other triggering events.
Primary Acquired Assets
| • | Fully funded partnership with Roche to develop new treatments for hepatitis C viral infection utilizing the proprietary HepDirect® liver-targeting technology. |
| • | Glucagon Receptor Antagonist Program for diabetes in pre-clinical development. |
| • | Thyroid Receptor Beta Agonist Program for hyperlipidemia in Phase I and preclinical development. |
| • | PeriCor Therapeutics—a common stock ownership position in privately-held PeriCor Therapeutics, Inc. PeriCor licensed acadesine to Schering-Plough Corporation (now Merck & Co.) and the compound is in a Phase III clinical trial for the prevention of adverse cardiovascular and cerebrovascular outcomes in patients undergoing coronary artery bypass graft surgery. |
5
Table of Contents
| • | HepDirect Technology—HepDirect technology supplements our core drug discovery technology platform of ligand-dependent gene expression and ultra-high throughput combinatorial chemistry screening. HepDirect is a prodrug technology that targets delivery of certain drugs to the liver by using a proprietary chemical modification that renders a drug biologically inactive until cleaved by a liver-specific enzyme. |
| • | Other Product Candidates and R&D Programs—other product candidates, including MB07803 for diabetes and pradefovir for hepatitis B, which have been evaluated in clinical trials and early stage R&D programs including glucokinase activators for diabetes and DGAT-1 inhibitors for obesity. |
Collaborative Research and Development Programs
We have entered into multiple research and development collaboration arrangements with third party pharmaceutical companies. The commercial terms of such arrangements typically include some combination of the following types of fees: exclusivity fees, technology access fees, technology development fees and research support payments, as well as milestone payments, license or commercialization fees. We may also receive royalties on product candidates resulting from our research and development collaboration arrangements if and to the extent any such product candidate is ultimately approved by the FDA and successfully marketed (see Table 2 for certain royalties).
Table 2: Royalties*
| Product/Program |
Partner |
Royalty | ||||
| Rate |
Tier | |||||
| Eltrombopag** (PROMACTA™) |
GSK | 4.7% 6.6% 7.5% 9.4% 9.3% |
Less than $100M annual sales On portion of sales in range of $100M - $200M On portion of sales in range of $200M - $400M On portion of sales greater than $400M On portion of sales greater than $1.5B | |||
| LGD-4665** |
GSK | 14.5% | All sales (6.5% for first year sales) | |||
| Various ongoing GSK research collaborations |
GSK | 6%*** 7% 8% 10% |
Less than $500M annual sales On portion of sales in range of $500M - $1B On portion of sales in range of $1B - $3B On portion of sales greater than $3B | |||
| Avinza |
King | 5% | If sales are less than $200M annually Higher royalties paid if sales exceed $200M | |||
| Bazedoxifene (VIVIANT™) Basedoxifene (APRELA™) |
Wyeth (now Pfizer) | 0.5% 1.5% 2.5% |
Less than $400M annual sales On portion of sales in range of $400M - $1.0B annually On portion of sales greater than $1B annually | |||
| Lasofoxifene (FABLYN®) |
Pfizer | 3% | All sales | |||
| JAK-3 inhibitor |
Pfizer | Tiered double digit royalties | ||||
| PS873266 |
Celgene | 2% | All sales | |||
| * | Royalties from other partnered products not listed are either single or double digit royalties as described under collaborative research and development programs. Not all royalties are disclosed due to confidentiality requirements. |
| ** | Net of payments due to The Rockefeller University |
| *** | If GSK exercises its Proof of Concept (PoC) Option for a particular Target, we may continue the development until PoC and receive stepped up royalties ranging from 10% to 14% under the categories of annual sales described above. |
6
Table of Contents
Our collaborative research and development programs are discussed below.
GlaxoSmithKline Collaboration
PROMACTA and LGD-4665
In December 2008, the FDA granted accelerated approval of GSK’s PROMACTA® for the treatment of thrombocytopenia in patients with chronic immune (idiopathic) thrombocytopenic purpura (ITP) who have had an insufficient response to corticosteroids, immunoglobulins or a splenectomy. In December 2009, GSK received a positive opinion for Revolade(R) (eltrombopag/PROMACTA) from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) for the oral treatment of thrombocytopenia (reduced platelet count) in adults with the blood disorder chronic ITP. Revolade is expected to be launched in the EU in the first half of 2010. PROMACTA is the first oral TPO receptor agonist therapy for the treatment of adult patients with chronic ITP. As a result of the FDA’s approval of PROMACTA, we are entitled to receive tiered royalties on annual net sales of PROMACTA (Table 2). As part of a settlement agreement and mutual release we entered into on February 11, 2009 with Rockefeller, we agreed to pay a share of such royalties to Rockefeller. See “Item 3. Legal Proceedings.”
In December 2008, we entered into an exclusive, worldwide license agreement with SmithKline Beecham Corporation, doing business as GSK. Pursuant to the terms of the GSK agreement, we granted GSK the exclusive right to develop, manufacture and commercialize our LGD-4665 product candidate, as well as all other TPO-related molecules discovered by us. Under the terms of the GSK agreement, GSK paid us $5.0 million as an upfront license fee and agreed to pay us up to $158.0 million in development and commercial milestones and a royalty on net sales. In the first year of sales, royalties will be one-half of the regular royalty rate. GSK will direct all product development and commercialization and will be responsible for all costs going forward for development, patent maintenance and prosecution, and commercialization. The term of the license agreement expires ten years from the date of the first commercial sale of the first licensed product in any country worldwide or until the expiration of the last licensed patent with a valid claim, whichever term is longer, although some obligations servive termination. Prior to the expiration of the license agreement, GSK has the right to terminate the agreement upon a specified number of days notice and we may not terminate the agreement unless GSK provides its prior written consent. Any such termination will not relieve the terminating party from obligations that have accrued prior to such termination or that expressly survive such termination. No termination will require us to refund to GSK any or all payments made to us by GSK under the agreement. In the event a party is in breach of any of its material obligations under the license agreement, the other party will have the right to seek damages and such other remedies as may be available to it.
Agreement with Pharmacopeia
In connection with our merger with Pharmacopeia, we assumed a product development and commercialization agreement, or the GSK Agreement, with SmithKlineBeecham Corporation and Glaxo Group Limited (together “GSK”), which was originally entered into in March 2006. Our role in the alliance with GSK is to identify and advance molecules in chosen therapeutic programs to development stage and, subject to certain provisions in the GSK Agreement, further develop the candidates to clinical “proof of concept” (a demonstration of efficacy in humans). We have agreed not to screen our compound library for other collaborators, or for our own account, against any target we screen under the GSK Agreement for a specified period.
The GSK Agreement provides GSK an exclusive option to license the program which is exercisable at specified points of the development process for each program (up to the point of clinical Proof of Concept). Upon licensing a program, GSK is obligated to conduct preclinical development and/or clinical trials and to commercialize pharmaceutical products resulting from such licensed programs on a worldwide basis. We are entitled to receive success-based milestone payments from GSK, starting in the preclinical research stage, for each drug development program under the alliance. If GSK exercises its Candidate Selection Option for a
7
Table of Contents
particular target, GSK is obligated to pay a tiered royalty on the annual net sales of products resulting from a particular target (Table 2). If GSK exercises its Proof of Concept Option for a particular target, we may receive stepped up royalties under the categories of annual sales described in Table 2.
In the event that GSK does not exercise its option to license a program, we retain all rights to such program and may continue to develop the program and commercialize any products resulting from the program, or we may elect to discontinue the program and/or seek other partners for further development and commercialization. Should we develop or partner such a program and commercialize any products resulting from that program, we are obligated to make success-based milestone payments to GSK and pay royalties to GSK ranging from 3% to 7% of net sales upon the successful commercialization of such products.
We and GSK each have the right to terminate the GSK Agreement in our sole discretion under certain specified circumstances at any time during the term of the GSK Agreement. If we exercise our discretionary termination right at any time during the first five years of the term of the GSK Agreement, under certain circumstances we could be required to refund to GSK a portion of the $15.0 million GSK paid to Pharmacopeia for certain initial discovery activities. Pursuant to the terms of the GSK Agreement, the amount of any such refund will be calculated based upon the date upon which such termination occurs. The initial research term of the GSK agreement expires in March 2011.
Pfizer Collaborations
Bazedoxifene Program
Bazedoxifene (Viviant) is a product candidate that resulted from one of our collaborations with Wyeth (now Pfizer). Bazedoxifene is a synthetic drug that was specifically designed to reduce the risk of osteoporotic fractures while at the same time protecting breast and uterine tissue. Regarding Viviant, the FDA has advised that it expects to convene an advisory committee to review the pending NDAs for both the treatment and prevention indications. Approvable letters were received for each of these NDAs in which, among other things, the FDA requested further analyses and discussion concerning the incidence of stroke and venous thrombotic events, identified certain issues concerning data collection and reporting, and requested additional source documents. An FDA-requested advisory committee meeting is expected to be scheduled following submission of the complete response to the approvable letters. In April 2009, Pfizer received approval in the EU for CONBRIZA (the EU trade name for Viviant) for the treatment of postmenopausal osteoporosis in women at increased risk of fracture. We expect CONBRIZA to be launched in the EU in 2010.
Pfizer is also developing bazedoxifene in combination with PREMARIN (Aprela) which is a tissue selective estrogen complex under development for menopausal symptoms and osteoporosis, Two Phase III studies with bazedoxifene/conjugated estrogens (Aprela), showed reduced number and severity of hot flashes in symptomatic postmenopausal women by up to 80 percent, when compared with placebo. Pfizer expects to file an initial NDA no earlier than the first half of 2010. We are entitled to receive tiered royalties on these products (see Table 2).
We previously sold to Royalty Pharma AG, or Royalty Pharma, the rights to a total of 3.0% of net sales of bazedoxifene for a period of ten years following the first commercial sale of each product. After giving effect to the royalty sale, we will receive tiered royalties on annual net sales as described in Table 2. Additionally, the royalty owed to Royalty Pharma may be reduced by one third if net product sales exceed certain thresholds across all indications.
Lasofoxifene Program
Lasofoxifene (FABLYN®) is a product candidate that resulted from our collaboration with Pfizer. Pfizer submitted an NDA and an MAA for FABLYN for osteoporosis treatment in December 2007 and January 2008, respectively. The FDA Advisory Committee in early September 2008 voted 9-3 in favor of approving this drug.
8
Table of Contents
In January 2009, Pfizer received a complete response letter from the FDA requesting additional information for FABLYN. Pfizer is reviewing the letter and will work with the FDA to determine the appropriate next steps regarding its application. In February 2009, FABLYN received approval in the EU for the treatment of osteoporosis.
Under the terms of our agreement with Pfizer, we are entitled to receive royalty payments on worldwide net sales of lasofoxifene for any indication. We previously sold to Royalty Pharma the rights to a total of 3% of net sales of lasofoxifene for a period of ten years following the first commercial sale of lasofoxifene. The amount of net royalties we will receive on annual net sales after giving effect to the royalty sale is described in Table 2.
JAK3 Program
In connection with the completion of our acquisition of Pharmacopeia, we assumed a research and license agreement with Wyeth (now Pfizer), acting through its Wyeth Pharmaceuticals Division, providing for the formation of a new alliance based on our Janus Kinase-3, or JAK3, inhibitor program. The alliance’s goal is to identify, develop and commercialize therapeutic products for the treatment of certain immunological conditions in humans. The agreement was originally entered into in December 2006.
Pursuant to the Agreement, we and Pfizer each have certain exclusive rights to develop and commercialize products resulting from the JAK3 program and the alliance. In November 2009, Pfizer extended its research collaboration with us for JAK3 by one year. The Research and License Agreement entered into in December 2006 with Wyeth provided for an initial three year research term. Under this extension, we will receive $3.1 million in research payments to continue conducting drug discovery and lead candidate optimization. Under the original agreement, we are entitled to receive up to $175 million in milestone payments for the successful development and commercialization of multiple products. In addition, we will receive royalties on product sales.
Schering-Plough Collaboration (now Merck)
1998 Collaboration
In connection with our acquisition of Pharmacopeia, we assumed collaboration and license agreements with Schering-Plough Ltd. (now Merck) and Schering Corporation (collectively “Schering-Plough”) that were originally entered into in October of 1998. These agreements produced a CXCR2 antagonist that entered Phase II clinical trials in the fourth quarter of 2006 for COPD and asthma, an enzyme inhibitor that entered Phase II clinical trials in November 2008 for oncology, a candidate for inflammatory diseases that entered Phase I clinical trials in March 2007, a candidate for respiratory diseases that entered Phase I clinical trials in September 2007 and a BACE inhibitor for Alzheimer’s disease that entered Phase I clinical trials in early 2009.
PS 291822 (SCH-527123), the lead in a series of CXCR2 antagonists, is being developed for the potential oral treatment of chronic obstructive pulmonary disorder (COPD) and asthma. Merck has completed phase II trials in COPD, neutrophilic asthma, mild allergen-induced asthma and psoriasis. In January 2010, Merck initiated two large Phase II dose-ranging studies with 500 patients each in COPD and severe asthma.
Dinaciclib (SCH-727965, PS-095760), a pro-apoptotic inhibitor of cyclin-dependent kinases is under clinical development for the potential treatment of cancer. Three Phase II trials are ongoing;
| • | A phase II trial in patients with advanced breast cancer and non-small cell lung cancer (NSCLC) |
| • | A phase II trial in acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL) |
| • | A phase II trial in mantle cell lymphoma (MCL) and B-cell chronic lymphocytic leukemia (B-CLL) |
A Beta-Secretase inhibitor is in clinical development for Alzheimer’s disease. We received a milestone payment of $1.0 million from Merck for lead selection and Phase I initiation. Merck reported the completion of Phase I single dose trial study with a 58% reduction in A-Beta peptide in cerebral/spinal fluid. Phase I multi-dose trial is ongoing, and a Phase II trial initiation is projected to start in 2010.
9
Table of Contents
Under the terms of these agreements with Merck, while our research activities have ceased, the cessation of those research activities did not affect other aspects of those agreements, including the ongoing Phase II and Phase I clinical trials and preclinical programs that Merck is conducting. We continue to be entitled to payments resulting from the successful achievement by Merck of clinical and regulatory milestones, as well as royalty payments at various rates depending on the origin of collaboration products from discovery and optimization libraries at Ligand and Merck, and on net sales of products resulting from compounds being developed by Merck under those agreements.
2007 Collaboration
In connection with our acquisition of Pharmacopeia, we also assumed an amended and restated collaboration and license agreement with N.V. Organon, entered into in February 2007. In November 2007, Organon was acquired by, and is now a part of, Schering-Plough (now Merck). We mutually terminated the collaboration and license agreement with Merck in July 2009. As part of the termination, Merck continued to fund research through a wind-down period ending December 31, 2009. In addition, we are entitled to receive future royalties and milestones as a result of Merck’s successful advancement through clinical development of therapeutic candidates discovered as a result of the collaboration which result in commercial sales. Merck is solely responsible for the further development and commercialization of all collaboration products after programs are handed over by us, and for all development and commercialization costs.
We received a total of $4.0 million in milestone payments at termination of the collaboration agreement and are entitled to receive further milestones and royalties on programs with identified leads.
Bristol-Myers Squibb Collaborations
Discovery Collaboration Agreement Dated October 11, 2007
On December 9, 2009, we entered into an amendment to the discovery collaboration agreement dated October 11, 2007 between Pharmacopeia and BMS. Pursuant to the terms of the amendment, the research term under the collaboration agreement terminated on December 31, 2009 and the research program under the collaboration agreement was transferred to BMS. We are no longer obligated to provide research support to BMS after December 31, 2009, other than providing certain data and compound transfer services to BMS through June 30, 2010. In connection with the amendment, we paid $1.0 million to BMS, and BMS is no longer required to make milestone payments to us under the collaboration agreement.
SARM
On November 6, 2009, we provided notice to BMS, that pursuant to the terms of the license agreement dated October 11, 2007 between BMS and us, we are exercising our right to terminate the license agreement without cause, effective three months following the date of delivery of written notice of such termination (or February 9, 2010). Under the terms of the license agreement, BMS provided us exclusive worldwide development and commercialization rights to a selective androgen receptor modulator, or SARM, program. There is no financial penalty for us associated with the termination of the license agreement.
P-38 Kinase Program
In connection with the merger with Pharmacopeia, we assumed a collaboration and license agreement with BMS which was originally entered into in November 1997. This collaboration has resulted in a compound that entered Phase II clinical trials in September 2007 for psoriasis. BMS has also completed a Phase II study in rheumatoid arthritis and a Phase II trial in atherosclerosis is ongoing. The research collaboration portion of the agreement has expired; however, we will continue to be entitled to payments resulting from the successful achievement by BMS of certain clinical and regulatory milestones, as well as a royalty on net sales of products resulting from compounds already delivered under the agreement.
10
Table of Contents
Roche
Collaboration for Hepatitis C
In connection with our merger with Metabasis in January 2010, we acquired a fully funded partnership with Roche to develop new treatments for hepatitis C viral infection utilizing the proprietary HepDirect(R) liver-targeting technology. The lead HepDirect nucleoside, MB11362, was declared a clinical candidate in the second quarter of 2009. Roche will fund 100% of program costs and will make milestone and royalty payments upon the achievement of certain development events and commercialization of MB11362 and/or other applicable HepDirect compounds.
Merck
Collaboration for VR1
In connection with our merger with Neurogen, we acquired a fully funded partnership with Merck for Vanilloid Receptor Subtype 1 (VR1) Antagonists. Merck will fund 100% of program costs and make milestone and royalty payments upon the achievement of certain development events and commercialization of any applicable VR1 compounds.
Cephalon Collaboration
In connection with the merger with Pharmacopeia, we assumed a collaboration and license agreement, or the Cephalon Agreement, with Cephalon, Inc., or Cephalon, originally entered into in May 2006, which provides for the formation of a new drug discovery, development and commercialization alliance. Under the Cephalon agreement, Pharmacopeia received an up-front, non-refundable payment of $15.0 million in June 2006 to support its research efforts.
We and Cephalon executed an amendment in January 2009 to the collaboration agreement dated May 16, 2006. The agreement provided that we would have no obligation to continue research activities with respect to the two active collaboration programs and were free to redeploy FTEs currently assigned to the collaboration. All licenses granted to Pharmacopeia by Cephalon with respect to the two active collaboration programs terminated as of the date of amendment. We will be entitled to milestone and royalty payments associated with only one of the two active programs. In addition, we agreed to provide certain chemistry services to Cephalon through a third party vendor for a term of nine months from the date of agreement, which ended in September 2009.
Celgene Collaboration
In connection with the merger with Pharmacopeia, we assumed a research and license agreement, or the Celgene Agreement, with Celgene Corporation, or Celgene. Under the Celgene Agreement we have no further research requirements. Our relationship with Celgene produced a compound that led to a clinical candidate currently being evaluated for the treatment of fibrotic and inflammatory diseases that entered a Phase I clinical trial in the first quarter of 2008. We are entitled to receive payments resulting from the successful achievement by Celgene of clinical milestones, as well as royalties on net sales of products resulting from the collaboration (Table 2).
Exelixis Collaboration
We exclusively licensed certain technology to X-Ceptor Therapeutics in 1999. X-Ceptor was subsequently acquired by Exelixis Inc. in October 2004. Exelixis has three partnered programs based on X-Ceptor technologies, including (a) XL-652, a LXR agonist, is in Phase I development with BMS for the potential treatment of atherosclerosis and other coronary artery diseases, (b) FXR-450, a Farnesoid X receptor modulator, is in preclinical development with Pfizer for the potential treatment of hyperlipidemia including hypertriglyceridemia, and (c) Xl-550, a mineralocorticoid receptor modulator, is in preclinical development with Daiichi-Sankyo for the potential treatment of metabolic disorders and cardiovascular diseases. Ligand is entitled to receive royalties on net sales of products.
11
Table of Contents
PeriCorTherapeutics
We assumed a common stock ownership position in privately-held PeriCor Therapeutics, Inc. through our acquisition of Metabasis. PeriCor sublicensed rights from Metabasis to acadesine and three additional Adenosine Regulating Agents in 2005. PeriCor licensed acadesine to Schering-Plough Corporation (now Merck & Co.) and the compound is in a Phase III clinical trial for the prevention of adverse cardiovascular and cerebrovascular outcomes in patients undergoing coronary artery bypass graft surgery.
Trevena Collaboration
In February 2009, we announced the initiation of a joint research and license alliance to screen targets using Trevena’s novel biological platform against our combinatorial library of compounds, to identify active compounds with potential for development as novel G-protein coupled receptor (GPCR) therapeutics.
Under the terms of the agreement, Trevena has been granted exclusive worldwide rights to sublicense active compounds resulting from the collaboration. We expect to screen targets and receive payments triggered by a tiered screening paradigm for each target.
Internal Product Development Programs
As summarized in the table below, we are developing several proprietary products for a variety of indications.
| Program |
Disease/Indication |
Development Phase | ||
| Selective Androgen Receptor Modulators (SARMs) (agonists) | Muscle wasting and frailty | Phase I | ||
| Thyroid receptor beta agonists | Hyperlipidemia | Phase I and Preclinical | ||
| Small molecule Erythropoietin (EPO) receptor agonists | Chemotherapy-induced anemia, anemia due to kidney failure | Preclinical | ||
| Glucagon receptor antagonists | Diabetes | Preclinical | ||
| Histamine 3 (H3) receptor antagonists | Cognitive disorders | Research | ||
Selective Androgen Receptor Modulators (SARM) Research and Development Programs
We are developing tissue selective androgen receptor modulators, or SARMs, a novel class of non-steroidal, orally active molecules that selectively modulate the activity of the androgen receptor in different tissues, providing a wide range of opportunities for the treatment of many diseases and disorders in both men and women. Tissue-selective androgen receptor agonists may provide utility in the treatment of patients with frailty, cachexia, osteoporosis, sexual dysfunction and hypogonadism. LGD-4033, our current lead, is a next-generation SARM designed to provide the benefits of androgen receptor stimulation on skeletal muscle and bone without the side effects of currently marketed androgens.
Preclinical studies conducted with LGD-4033 suggest that the compound may have favorable activity in the treatment of cachexia, frailty, osteoporosis, hypogonadism as well as other disorders. LGD-4033 has anabolic activity in muscle and bone and in animal models of osteoporosis and muscle wasting restores these tissues to normal levels. By comparison, the compound has weak, partial agonist activity on the prostate and has little effect on this tissue at expected therapeutic doses. The tissue selective properties of LGD-4033 are independent of local drug concentration indicating that tissue selectivity is inherent in the compound. We filed an Investigational New Drug (IND) in December 2008 for LGD-4033. Phase I clinical trials began in June 2009. We
12
Table of Contents
completed Phase I single ascending dose trial in the fourth quarter of 2009. LGD-4033 was found to be well absorbed with good pharmacokinetics consistent with a once-a-day dosing and there were no serious or dose dependent adverse events. A Phase I Multiple Ascending Dose clinical trial has been initiated with results expected in the third quarter of 2010.
We have assembled an extensive SARM compound library and, we believe, one of the most experienced androgen receptor drug discovery teams in the pharmaceutical industry. We plan to seek collaborations with major pharmaceutical companies to exploit broader clinical applications.
Erythropoietin (EPO) Research Program
We are developing small molecule agonists for the EPO receptor. EPO stimulates the differentiation of bone marrow stem cells to form red blood cells. Various recombinant human EPO derivatives are marketed for the treatment of anemia due to renal failure or cancer chemotherapy (e.g., Aranesp, Epogen, Eprex, and Procrit). We believe that a small molecule agonist for the EPO receptor would provide additional benefit in the treatment of anemia and the convenience of oral administration compared to recombinant human protein therapeutics. EPO and TPO act on the same bone marrow hematopoietic stem cell to guide the development of blood cells. We expect that our prior experience in developing small molecule TPO mimetic drugs will lead to increased efficiency in discovering small molecule EPO mimetic drugs. Compounds have been discovered that potently and selectively stimulate differentiation by human bone marrow stem cells to form erythrocytes in vitro. Advanced compounds are orally absorbed by animals and demonstrate pharmacokinetic properties consistent with once daily, oral administration to humans.
Glucagon Receptor Antagonist Research Program
We are developing small molecule glucagon receptor antagonists for the treatment of Type 2 diabetes mellitus. Compounds that block the action of glucagon may reduce the hyperglycemia that is characteristic of this disease. Glucagon stimulates the production of glucose by the liver and its release into the blood stream. In diabetic patients, glucagon secretion is abnormally elevated which contributes to hyperglycemia in these patients. Compounds have been discovered that block the action of glucagon on human hepatocytes in vitro. Our advanced glucagon antagonist compounds demonstrate oral bioavailability in rodents.
Thyroid Hormone Receptor Beta Agonist Research Program
Thyroid hormone acts throught two distinct receptors, referred to as TR-alpha and TR-beta. TR-beta receptor agonists have been shown to reduce plasma concentration of cholesterol and lipoprotein a, or Lp(a). These actions may be of benefit to patients with dyslipidemia at increased increased risk of cardiovascular disease. Our compounds are designed for preferential drug delivery to the liver where they upregulate LDL uptake and excretion. Tissue-selective drug distribution may improve the safety of these compounds compared to thyroid hormone. Advanced compounds bind TR-beta receptors with high affinity, demonstrate oral absorption, and reduce the plasma cholesterol concentration in animal models of dyslipidemia.
Histamine H3 Receptor Inverse Agonist Research Program
Histamine acts through four distinct receptors, H1-H4. Highly successful drugs have been developed that target the H1 receptor (e.g., CLARITINTM, and ZYRTECTM) and H2 receptors (e.g., ZANTACTM and PEPCIDTM). No H3 receptor blockers are currently approved. An H3-selective inverse agonist will block the action of histamine at the H3 receptor when its endogenous levels are elevated. H3 receptors are widely distributed in the brain where they regulate the secretion of neurotransmitters such as histamine, norepinephrine, dopamine, and serotonin. Our H3 inverse agonists regulate sleep/wakefulness cycles in rodents indicating they may be useful for daytime sleepiness-associated disorders. Published results from a variety of sources suggest that H3 antagonists/inverse agonists may also be useful to improve cognitive dysfunction in schizophrenia, Alzeheimer’s disease, and attention deficit disorder.
13
Table of Contents
Technology
We employ various modern research laboratory methods to discover and conduct preclinical development of new chemical entities. These methods are performed either in our own laboratories or in those of contract research organizations under our direction.
In our efforts to discover new and important medicines, we have concentrated on certain technologies and acquired special expertise related to intracellular receptors and the receptors for hematopoietic growth factors. Intracellular receptors are involved in the actions of non-peptide hormones and drugs such as selective estrogen receptor modulators, or SERMs, and SARMs. Hematopoietic growth factor receptors are involved in the differentiation and proliferation of blood cell progenitors, the formation of new blood cells, and the action of drugs such as PROMACTA, Epogen and Neumega. We use and have developed particular expertise in co-transfection assays, which measure gene transcription in response to the activation of a target receptor, and gene expression in cells selected for expression of particular receptors or transfected with cDNA for particular receptors. Some of these methods are covered by patents issued to or licensed by us, are trade secrets, or are methods that are in the public domain, but that we may use in novel ways to improve our efficiency in identifying promising leads and developing new chemical entities.
Our drug discovery approach is further supported by our proprietary combinatorial chemistry encoding technology, Encoded Combinatorial Libraries on Polymeric Support, or ECLiPS®, our proprietary collection of chemical compounds, assay technology, production automation, information systems and quality assurance programs. We have employed ECLiPS®, together with other technologies to assemble what we believe is the largest group of compound libraries held by one company in the pharmaceutical industry. Our small molecule libraries have been engineered to be both drug-like and diverse. Our compound collection and high throughput screening technologies have been proven to be effective against a wide variety of biological targets. Importantly, we have achieved success against some of our collaborators’ most difficult targets, often after our partners’ internal drug discovery efforts were unsuccessful.
Our tagging technology used in ECLiPS® has been licensed exclusively from the Trustees of Columbia University, or Columbia, and Cold Spring Harbor Laboratory, or Cold Spring, since 1993. We are obligated to pay a minimum annual license fee of $100,000 to Columbia and Cold Spring. The term of the agreement is the later of (i) July 16, 2013 or (ii) the expiration of the last patent relating to the technology, at which time we will have a fully paid license to the technology. The license granted to us under the agreement can be terminated by Columbia and Cold Spring (i) upon 30 days written notice to us if we materially breach the agreement and we fail to cure such material breach in accordance with the agreement or (ii) if we commit any act of bankruptcy, become insolvent, file a petition under any bankruptcy or insolvency act or have any such petition filed against us that is not dismissed within 60 days. We are also obligated to pay royalties to Columbia and Cold Spring based on net sales of pharmaceutical products we develop, or a percentage of all other revenue we recognize from collaborators that is derived from the technology licensed from Columbia and Cold Spring.
In connection with our merger with Metabasis, we acquired certain HepDirect Technology. HepDirect technology supplements our core drug discovery technology platform of ligand-dependent gene expression and ultra-high throughput combinatorial chemistry screening. HepDirect is a prodrug technology that targets delivery of certain drugs to the liver by using a proprietary chemical modification that renders a drug biologically inactive until cleaved by a liver-specific enzyme.
Manufacturing
We currently have no manufacturing facilities and, accordingly, rely on third parties, including our collaborative partners, for clinical production of any products or compounds.
For further discussion of these items, see below under “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
14
Table of Contents
Research and Development Expenses
Research and development expenses from continuing operations were $39.9 million, $30.8 million, and $44.6 million in 2009, 2008 and 2007, respectively, of which 47%, 100%, and 100%, respectively, were sponsored by us.
There were no research and development expenses from discontinued operations in 2009 and 2008. Research and development expenses from discontinued operations were $0.1 million in 2007.
Competition
Some of the drugs we are developing may compete with existing therapies or other drugs in development by other companies. A number of pharmaceutical and biotechnology companies are pursuing IR-related approaches to drug discovery and development. Furthermore, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competing products or technologies and may establish collaborative arrangements with our competitors.
Many of our existing or potential competitors, particularly large pharmaceutical companies, have greater financial, technical and human resources than we do and may be better equipped to develop, manufacture and market products. Many of these companies also have extensive experience in preclinical testing and human clinical trials, obtaining FDA and other regulatory approvals and manufacturing and marketing pharmaceutical products.
Our competitive position also depends upon our ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary products or processes, and secure sufficient capital resources for the often substantial period between technological conception and commercial sales. For a discussion of the risks associated with competition, see below under “Item 1A. Risk Factors.”
Government Regulation
The manufacturing and marketing of our products, our ongoing research and development activities and products being developed by our collaborative partners are subject to regulation for safety and efficacy by numerous governmental authorities in the United States and other countries. In the United States, pharmaceuticals are subject to rigorous regulation by federal and various state authorities, including the FDA. The Federal Food, Drug and Cosmetic Act and the Public Health Service Act govern the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of our products. There are often comparable regulations that apply at the state level. Product development and approval within this regulatory framework takes a number of years and involves the expenditure of substantial resources.
The steps required before a pharmaceutical agent may be marketed in the United States include (1) preclinical laboratory tests, (2) the submission to the FDA of an IND, which must become effective before human clinical trials may commence, (3) adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug, (4) the submission of an NDA to the FDA and (5) the FDA approval of the NDA prior to any commercial sale or shipment of the drug. In addition to obtaining FDA approval for each product, each domestic drug-manufacturing establishment must be registered with the FDA and, in California, with the Food and Drug Branch of California. Domestic manufacturing establishments are subject to pre-approval inspections by the FDA prior to marketing approval, then to biennial inspections, and must comply with current Good Manufacturing Practices (cGMP). To supply products for use in the United States, foreign manufacturing establishments must comply with cGMP and are subject to periodic inspection by the FDA or by regulatory authorities in such countries under reciprocal agreements with the FDA.
For both currently marketed and future products, failure to comply with applicable regulatory requirements after obtaining regulatory approval can, among other things, result in the suspension of regulatory approval, as well as possible civil and criminal sanctions. In addition, changes in existing regulations could have a material adverse effect to us.
15
Table of Contents
For marketing outside the United States before FDA approval to market, we must submit an export permit application to the FDA. We also are subject to foreign regulatory requirements governing human clinical trials and marketing approval for drugs. The requirements relating to the conduct of clinical trials, product licensing, pricing and reimbursement vary widely from country to country and there can be no assurance that we or any of our partners will meet and sustain any such requirements.
We are also increasingly subject to regulation by the states. A number of states now regulate, for example, pharmaceutical marketing practices and the reporting of marketing activities, controlled substances, clinical trials and general commercial practices. We have developed and are developing a number of policies and procedures to ensure our compliance with these state laws, in addition to the federal regulations described above. Significant resources are now required on an ongoing basis to ensure such compliance. For a discussion of the risks associated with government regulations, see below under “Item 1A. Risk Factors.”
Patents and Proprietary Rights
We believe that patents and other proprietary rights are important to our business. Our policy is to file patent applications to protect technology, inventions and improvements to our inventions that are considered important to the development of our business. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position.
Royalties we currently receive from King on AVINZA represent a significant portion of our ongoing revenue. The United States patent on AVINZA expires in November 2017; however, applications for generic forms of AVINZA have been submitted to the FDA. The United States patents relating to PROMACTA do not expire until December 2021. Subject to compliance with the terms of the respective agreements, our rights under our licenses with our exclusive licensors extend for the life of the patents covering such developments. For a discussion of the risks associated with patent and proprietary rights, see below under “Item 1A. Risk Factors.”
Human Resources
As of February 1, 2010, we had 72 full-time employees, of whom 53 are involved directly in scientific research and development activities. Of these employees, 29 hold Ph.D. or M.D. degrees.
16
Table of Contents
| ITEM 1A. | RISK FACTORS |
The following is a summary description of some of the many risks we face in our business. You should carefully review these risks in evaluating our business, including the businesses of our subsidiaries. You should also consider the other information described in this report.
Risks Related To Us and Our Business.
Royalties based on sales of AVINZA and PROMACTA represent a substantial portion of our revenues.
King Pharmaceuticals, or King, is obligated to pay us royalties based on its sales of AVINZA and GlaxoSmithKline, or GSK, is obligated to pay us royalties on its sales of PROMACTA. These royalties represented 21% and 74% of total revenues for the years ended December 31, 2009 and 2008, respectively, and will continue to be a substantial portion of our ongoing revenues for some time. We also receive milestones and collaborative revenue from our partners in various collaborations, but the amount of such revenue is unknown and highly uncertain. As a result, any setback that may occur with respect to AVINZA or PROMACTA could significantly impair our operating results and/or reduce the market price of our stock, as could any reduction in our expected milestone and collaborative revenue. Setbacks for AVINZA and PROMACTA could include problems with shipping, distribution, manufacturing, product safety, marketing, government licenses and approvals, intellectual property rights, competition with existing or new products and physician or patient acceptance of the products, as well as higher than expected total rebates, returns or discounts.
King and GSK’s sales efforts for AVINZA and PROMACTA, respectively, could be affected by a number of factors and decisions regarding their organizations, operations, and activities as well as events both related and unrelated to AVINZA or PROMACTA, including sales force reorganizations and lower than expected sales calls and prescription volumes. AVINZA and PROMACTA could also face stiffer competition from existing or future products. The negative impact on the sales of AVINZA or PROMACTA will negatively affect our royalties, revenues and earnings.
Sales of AVINZA and PROMACTA may also be negatively impacted by higher than expected discounts (especially pharmacy benefit management/group purchasing organization rebates and Medicaid rebates, which can be substantial), returns and chargebacks and/or slower than expected market penetration. Other setbacks that AVINZA could face in the sustained-release opioid market include abuse issues and the inability to obtain sufficient quotas of morphine from the Drug Enforcement Agency to support production requirements.
AVINZA or PROMACTA could also face regulatory action and product safety issues. For example, the FDA previously requested expanded warnings on the AVINZA label to alert doctors and patients to the dangers of using AVINZA with alcohol. Changes were subsequently made to the label. The FDA also requested clinical studies to investigate the risks associated with taking AVINZA with alcohol. Any additional warnings, studies and any further regulatory action could have significant adverse effects on AVINZA sales.
On September 10, 2007, King reported that Actavis, a manufacturer of generic pharmaceutical products headquartered in Iceland, had filed with the FDA an Abbreviated New Drug Application, or ANDA, with a Paragraph IV Certification pertaining to AVINZA, the rights to which were acquired by King from us in February 2007. According to the report, Actavis’s Paragraph IV Certification sets forth allegations that U.S. Patent No. 6,066,339, or the 339 patent, which pertains to AVINZA, and which is listed in the FDA’s Approved Drug Products With Therapeutic Equivalence Evaluations, will not be infringed by Actavis’s manufacture, use, or sale of the product for which the ANDA was submitted. The expiration date for this patent is November 2017. King, King Pharmaceuticals Research and Development, Inc., Elan Corporation, plc and Elan Pharma International Ltd. jointly filed suit in federal district court in New Jersey on October 18, 2007 against Actavis, Inc. and Actavis Elizabeth LLC for patent infringement under the 339 patent. The lawsuit seeks a judgment that would, among other things, prevent Actavis from commercializing its proposed morphine product until after expiration of the 339 patent.
17
Table of Contents
On July 21, 2009, King, King Pharmaceuticals Research and Development, Inc., Elan Corporation, plc and Elan Pharma International Ltd. jointly filed suit in federal district court in New Jersey against Sandoz Inc., or Sandoz, for patent infringement under the 339 patent. According to the complaint, Sandoz filed an ANDA for morphine sulfate extended release capsules and, in connection with the ANDA filing, Sandoz provided written certification to the FDA alleging that the claims of the 339 patent are invalid, unenforceable and/or will not be infringed by the manufacture, use or sale of Sandoz’s proposed morphine product. Similar to the lawsuit against Actavis, this lawsuit seeks a judgment that would, among other things, prevent Sandoz from commercializing its proposed morphine product until after expiration of the 339 patent.
AVINZA was licensed from Elan Corporation, or Elan, which is its sole manufacturer. Any problems with Elan’s manufacturing operations or capacity could reduce sales of AVINZA, as could any licensing or other contract disputes with Elan, raw materials suppliers, or others.
Further, pursuant to the agreement with King, we may no longer receive AVINZA royalties on a quarterly basis, but will collect royalties on an annual basis, which may adversely impact our cash flows.
Our product candidates face significant development and regulatory hurdles prior to marketing which could delay or prevent sales and/or milestone revenue.
Before we obtain the approvals necessary to sell any of our potential products, we must show through preclinical studies and human testing that each product is safe and effective. We and our partners have a number of products moving toward or currently awaiting regulatory action, including bazedoxifene and lasofoxifene. Failure to show any product’s safety and effectiveness could delay or prevent regulatory approval of a product and could adversely affect our business. The clinical trials process is complex and uncertain. For example, the results of preclinical studies and initial clinical trials may not necessarily predict the results from later large-scale clinical trials. In addition, clinical trials may not demonstrate a product’s safety and effectiveness to the satisfaction of the regulatory authorities. Recently, a number of companies have suffered significant setbacks in advanced clinical trials or in seeking regulatory approvals, despite promising results in earlier trials. The FDA may also require additional clinical trials after regulatory approvals are received. Such additional trials may be expensive and time-consuming, and failure to successfully conduct those trials could jeopardize continued commercialization of a product.
The rate at which we complete our clinical trials depends on many factors, including, but are not limited to, our ability to obtain adequate supplies of the products to be tested and patient enrollment. Patient enrollment is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites and the eligibility criteria for the trial. Delays in patient enrollment for our trials may result in increased costs and longer development times. For example, the trial entitled “Eltrombopag To Reduce The Need For Platelet Transfusion In Subjects With Chronic Liver Disease And Thrombocytopenia Undergoing Elective Invasive Procedures (ELEVATE)” was suspended in October 2009 in accordance with an IDMC Recommendation. GSK terminated the ELEVATE study and the program is under review. In addition, our collaborative partners have rights to control product development and clinical programs for products developed under the collaborations. As a result, these collaborative partners may conduct these programs more slowly or in a different manner than expected. Moreover, even if clinical trials are completed, we or our collaborative partners still may not apply for FDA approval in a timely manner or the FDA still may not grant approval.
We rely heavily on collaborative relationships, and any disputes or litigation with our collaborative partners or termination or breach of any of the related agreements could reduce the financial resources available to us, including milestone payments and future royalty revenues.
Our strategy for developing and commercializing many of our potential products, including products aimed at larger markets, includes entering into collaborations with corporate partners and others. These collaborations have provided us with funding and research and development resources for potential products for the treatment of
18
Table of Contents
a variety of diseases. These agreements also give our collaborative partners significant discretion when deciding whether or not to pursue any development program. Our existing collaborations may not continue or be successful, and we may be unable to enter into future collaborative arrangements to develop and commercialize our product candidates.
In addition, our collaborators may develop drugs, either alone or with others that compete with the types of drugs they are developing with us. This would result in increased competition for our programs. If products are approved for marketing under our collaborative programs, revenues we receive will depend on the manufacturing, marketing and sales efforts of our collaborative partners, who generally retain commercialization rights under the collaborative agreements. Generally, our current collaborative partners also have the right to terminate their collaborations under specified circumstances. If any of our collaborative partners breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully, our product development under these agreements will be delayed or terminated. Disputes or litigation may also arise with our collaborators, including disputes or litigation over ownership rights to intellectual property, know-how or technologies developed with our collaborators. Such disputes or litigation could adversely affect our rights to one or more of our product candidates. Any such dispute or litigation could delay, interrupt or terminate the collaborative research, development and commercialization of certain potential products, create uncertainty as to ownership rights of intellectual property, or could result in litigation or arbitration. The occurrence of any of these problems could be time-consuming and expensive and could adversely affect our business.
If we consume cash more quickly than expected, and if we are unable to raise additional capital, we may be forced to curtail operations.
Our operations have consumed substantial amounts of cash since inception. Clinical and preclinical development of drug candidates is a long, expensive and uncertain process. Also, we may acquire companies, businesses or products and the consummation of such acquisitions may consume additional cash. For example, as part of the consideration for our recent acquisition of Pharmacopeia, we distributed approximately $9.3 million in cash to Pharmacopeia stockholders. Security-holders of Pharmacopeia also received contingent value rights under which we could be required to make an aggregate cash payment of $15.0 million to such security-holders under certain circumstances. Security holders of Neurogen and Metabasis also received contingent value rights under which we could be required to make unspecified payments under certain circumstances.
In December 2009, the Internal Revenue Service, or IRS, issued to us a Notice of Proposed Adjustment, or NOPA, seeking an increase to our taxable income for the 2007 fiscal year of $71.5 million and a $4.1 million penalty for substantial underpayment of tax in fiscal 2007. We responded to the NOPA in February 2010, disagreeing with the conclusions reached by the IRS in the NOPA. We recorded a FIN 48 liability of $25.1 million related to the income tax effect of the NOPA and $3.0 million related to estimated interest due on the proposed underpayment of tax. We also recorded deferred income tax assets of $25.1 million associated with the ability to carry back losses from 2008 and 2009 to offset the NOPA. In addition, we recorded an income tax receivable of $4.5 million associated with changes in income tax law in relation to prior AMT taxes paid on carry back periods. We have not recorded the penalties proposed by the IRS in our financial statements as we believe that we met the appropriate standard for the tax position on our 2007 tax return. If we are unsuccessful in our negotiations with the IRS, we may be required to pay the $4.1 million penalty and utilize a significant amount of our net operating loss carryforwards.
We believe that our capital resources, including our currently available cash, cash equivalents, and short-term investments as well as our current and future royalty revenues, will be adequate to fund our operations at their current levels at least for the next twelve months. However, changes may occur that would cause us to consume available capital resources before that time. Examples of relevant potential changes that could impact our capital resources include:
| • | the costs associated with our drug research and development activities, and additional costs we may incur if our development programs are delayed or are more expensive to implement than we currently anticipate; |
19
Table of Contents
| • | changes in existing collaborative relationships, including the funding we receive in connection with those relationships; |
| • | the progress of our milestone and royalty producing activities; |
| • | our ability to reach a favorable resolution with the IRS with respect to their audit of our fiscal 2007 federal tax return, or to other potential tax assessments; |
| • | acquisitions of other businesses or technologies; |
| • | the termination of our lease agreements; |
| • | the purchase of additional capital equipment; |
| • | cash payments or refunds we may be required to make pursuant to certain agreements with third parties; |
| • | competing technological and market developments; and |
| • | the cost of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights, and the outcome of related litigation. |
Additional capital may not be available on favorable terms, or at all. If additional capital is not available, we may be required to curtail operations significantly or to obtain funds by entering into arrangements with partners or other third parties that may require us to relinquish rights to certain of our technologies, products or potential markets that we would not otherwise relinquish.
If, as the result of a merger, or otherwise, our collaborative partners were to change their strategy or the focus of their development and commercialization efforts with respect to our alliance products, the success of our alliance products could be adversely affected.
Our collaborative partners may change the focus of their development and commercialization efforts as the result of a merger. Pharmaceutical and biotechnology companies have historically re-evaluated their priorities from time to time, including following mergers and consolidations which are common in these industries, and two of our collaborative partners have recently entered into merger agreements. In October 2009, Wyeth, a collaborative partner of ours, and Pfizer announced that Pfizer had completed its acquisition of Wyeth in a cash and stock transaction. Furthermore, in November 2009, Schering-Plough Corporation, another of our collaborative partners, and Merck & Co., Inc., or Merck, announced that Merck and Schering-Plough had combined, under the name Merck, in a stock and cash transaction. As a result of the consummation of these mergers, our collaborative partners may develop and commercialize, either alone or with others, products and services that are similar to or competitive with our alliance products. Furthermore, the ability of our alliance products to reach their potential could be limited if our collaborative partners reduce or fail to increase spending related to such products as a result of these mergers.
If our collaborative partners terminate their collaborations with us or do not commit sufficient resources to the development, manufacture, marketing or distribution of our alliance products, we could be required to devote additional resources to our alliance products, seek new collaborative partners or abandon such alliance products, all of which could have an adverse effect on our business.
We may not be successful in entering into additional out-license agreements on favorable terms, which may adversely affect our liquidity or require us to alter development plans on our products.
We have entered into several out-licensing agreements for the development and commercialization of our products. Although we expend considerable resources on internal research and development for our proprietary programs, we may not be successful in entering into additional out-licensing agreements under favorable terms due to several factors including:
| • | the difficulty in creating valuable product candidates that target large market opportunities; |
| • | research and spending priorities of potential licensing partners; |
20
Table of Contents
| • | willingness of and the resources available to pharmaceutical and biotechnology companies to in-license product candidates for their clinical pipelines; or |
| • | differences of opinion with potential partners on the valuation of products we are seeking to out-license. |
The inability to enter into out-licensing agreements under favorable terms and to earn milestone payments, license fees and/or upfront fees may adversely affect our liquidity and may force us to curtail or delay development of some or all of our proprietary programs, which in turn may harm our business and the value of our stock.
Third party intellectual property may prevent us or our partners from developing our potential products and we may owe a portion of any payments we receive from our collaborative partners to one or more third parties.
Our success will depend on our ability and the ability of our collaborative partners to avoid infringing the proprietary rights of others, both in the United States and in foreign countries. In addition, disputes with licensors under our license agreements may arise which could result in additional financial liability or loss of important technology and potential products and related revenue, if any. Further, the manufacture, use or sale of our potential products or our collaborative partners’ products or potential products may infringe the patent rights of others. This could impact AVINZA, PROMACTA, bazedoxifene, lasofoxifene, LGD-4665, and any other products or potential products.
Several drug companies and research and academic institutions have developed technologies, filed patent applications or received patents for technologies that may be related to our business. Others have filed patent applications and received patents that conflict with patents or patent applications we have licensed for our use, either by claiming the same methods or compounds or by claiming methods or compounds that could dominate those licensed to us. In addition, we may not be aware of all patents or patent applications that may impact our ability to make, use or sell any of our potential products. For example, US patent applications may be kept confidential while pending in the United States Patent and Trademark Office and patent applications filed in foreign countries are often first published six months or more after filing.
On March 4, 2008, Rockefeller filed suit in the United States District Court for the Southern District of New York, against us alleging, among other things, a breach by us of our September 30, 1992 license agreement with Rockefeller, as well as other causes of action for unjust enrichment, quantum meruit, specific performance to perform an audit and declaratory relief. In February 2009 we reached a settlement with Rockefeller whereby the parties resolved all disputes that have arisen between them, including Rockefeller’s primary claim relating to the development of PROMACTA as well our counterclaims.
Other possible disagreements or litigation with our collaborative partners could delay our ability and the ability of our collaborative partners to achieve milestones or our receipt of other payments. In addition, other possible disagreements or litigation could delay, interrupt or terminate the research, development and commercialization of certain potential products being developed by either our collaborative partners or by us. The occurrence of any of the foregoing problems could be time-consuming and expensive and could adversely affect our business.
Third parties have not directly threatened an action or claim against us, although we do periodically receive other communications or have other conversations with the owners of other patents or other intellectual property. If others obtain patents with conflicting claims, we may be required to obtain licenses to those patents or to develop or obtain alternative technology. We may not be able to obtain any such licenses on acceptable terms, or at all. Any failure to obtain such licenses could delay or prevent us from pursuing the development or commercialization of our potential products.
21
Table of Contents
In general, litigation claims can be expensive and time consuming to bring or defend against and could result in settlements or damages that could significantly impact our results of operations and financial condition. We cannot predict or determine the outcome of these matters or reasonably estimate the amount or range of amounts of any fines or penalties that might result from a settlement or an adverse outcome. However, a settlement or an adverse outcome could have a material adverse effect on our financial position, liquidity and results of operations.
Challenges to or failure to secure patents and other proprietary rights may significantly hurt our business.
Our success will depend on our ability and the ability of our licensors to obtain and maintain patents and proprietary rights for our potential products both in the United States and in foreign countries. Patents may not be issued from any of these applications currently on file, or, if issued, may not provide sufficient protection. Our patent position, like that of many biotechnology and pharmaceutical companies, is uncertain and involves complex legal and technical questions for which important legal principles are unresolved. We may not develop or obtain rights to products or processes that are patentable. Even if we do obtain patents, such patents may not adequately protect the technology we own or have licensed. In addition, others may challenge, seek to invalidate, infringe or circumvent any patents we own or license and rights we receive under those patents may not provide competitive advantages to us.
Any conflicts resulting from the patent rights of others could significantly reduce the coverage of our patents and limit our ability to obtain meaningful patent protection. We have had and will continue to have discussions with our current and potential collaborative partners regarding the scope and validity of our patents and other proprietary rights. If a collaborative partner or other party successfully establishes that our patent rights are invalid, we may not be able to continue our existing collaborations beyond their expiration. Any determination that our patent rights are invalid also could encourage our collaborative partners to seek early termination of our agreements. Such invalidation could adversely affect our ability to enter into new collaborations.
We may also need to initiate litigation, which could be time-consuming and expensive, to enforce our proprietary rights or to determine the scope and validity of others’ rights. If litigation occurs, a court may find our patents or those of our licensors invalid or may find that we have infringed on a competitor’s rights. In addition, if any of our competitors have filed patent applications in the United States which claim technology we also have invented, the United States Patent and Trademark Office may require us to participate in expensive interference proceedings to determine who has the right to a patent for the technology.
We also rely on unpatented trade secrets and know-how to protect and maintain our competitive position. We require our employees, consultants, collaborative partners and others to sign confidentiality agreements when they begin their relationship with us. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our competitors may independently discover our trade secrets.
Our product development involves a number of uncertainties, and we may never generate sufficient collaborative payments and royalties from the development of products to become profitable.
We were founded in 1987. We have incurred significant losses since our inception. As of December 31, 2009, our accumulated deficit was $681.6 million.
Most of our products in development will require extensive additional development, including preclinical testing and human studies, as well as regulatory approvals, before they can be marketed. We cannot predict if or when any of the products we are developing or those being developed with our partners will be approved for marketing. There are many reasons why we or our collaborative partners may fail in our efforts to develop our potential products, including the possibility that: preclinical testing or human studies may show that our potential products are ineffective or cause harmful side effects; the products may fail to receive necessary regulatory
22
Table of Contents
approvals from the FDA or foreign authorities in a timely manner, or at all; the products, if approved, may not be produced in commercial quantities or at reasonable costs; the products, if approved, may not achieve commercial acceptance; regulatory or governmental authorities may apply restrictions to our products, which could adversely affect their commercial success; or the proprietary rights of other parties may prevent us or our partners from marketing the products.
Any product development failures for these or other reasons, whether with our products or our partners’ products, may reduce our expected revenues, profits, and stock price.
We may not be able to hire and/or retain key employees.
If we are unable to hire and/or retain key employees, we may not have sufficient resources to successfully manage our assets or our business, and we may not be able to perform our obligations under various contracts and commitments. Furthermore, there can be no assurance that we will be able to retain all of our key management and scientific personnel. If we fail to retain such key employees, we may not realize the anticipated benefits of our mergers. Either of these could have substantial negative impacts on our business and our stock price.
We will have continuing obligations to indemnify the buyers of our commercial product lines, and may be subject to other liabilities related to the sale of our commercial product lines.
We agreed to indemnify Eisai and King under certain circumstances pursuant to the asset purchase agreements we entered into with Eisai and King in connection with the sale of our commercial product lines. Some of our indemnification obligations still remain and our potential liability in certain circumstances is not limited to specific dollar amounts. We cannot predict the liabilities that may arise as a result of these matters. Any claims related to our indemnification obligations to King or Eisai could materially and adversely affect our financial condition.
In addition, King assumed our obligation to make payments to Organon based on net sales of AVINZA (the fair value of which was $40.8 million as of December 31, 2009). We remain liable to Organon in the event King defaults on this obligation. Any requirement to pay a material amount to Organon, could adversely affect our business and the price of our securities.
The sale of our commercial product lines does not relieve us of exposure to product liability risks on products we sold prior to divesting these product lines. A successful product liability claim or series of claims brought against us may not be insured and could result in payment of significant amounts of money and divert management’s attention from running our business.
If our partners do not reach the market with our alliance products before our competitors offer products for the same or similar uses, or if our partners are not effective in marketing our alliance products, our revenues from product sales, if any, will be reduced.
We face intense competition in our development activities. Our competitors might succeed in obtaining regulatory approval for competitive products more rapidly than our partners can for our products. In addition, competitors might develop technologies and products that are less expensive and perceived to be safer or more effective than those being developed by us or our partners, which could impair our product development and render our technology obsolete.
We use hazardous materials, which may expose us to significant liability.
In connection with our research and development activities, we handle hazardous materials, chemicals and various radioactive compounds. To properly dispose of these hazardous materials in compliance with environmental regulations, we are required to contract with third parties. We believe that we carry reasonably
23
Table of Contents
adequate insurance for toxic tort claims. However, we cannot eliminate the risk or predict the exposure of accidental contamination or injury from the handling and disposing of hazardous materials, whether by us or our third-party contractors. Any accident in the handling and disposing of hazardous materials may expose us to significant liability.
Our shareholder rights plan and charter documents may hinder or prevent change of control transactions.
Our shareholder rights plan and provisions contained in our certificate of incorporation and bylaws may discourage transactions involving an actual or potential change in our ownership. In addition, our Board of Directors may issue shares of preferred stock without any further action by the stockholders. Such restrictions and issuances may have the effect of delaying or preventing a change in our ownership. If changes in our ownership are discouraged, delayed or prevented, it would be more difficult for our current Board of Directors to be removed and replaced, even if you or our other stockholders believe that such actions are in the best interests of us and our stockholders.
We may lose some or all of the value of some of our short-term investments.
We engage one or more third parties to manage some of our cash consistent with an investment policy that allows a range of investments and maturities. The investments are intended to maintain safety of principal while providing liquidity adequate to meet projected cash requirements. Risks of principal loss are to be minimized through diversified short and medium term investments of high quality, but the investments are not in every case guaranteed or fully insured. As a result of changes in the credit market, one of our short-term investments in commercial paper is in default. We intend to pursue collection efforts, but we might not recoup some or all of our investment in the commercial paper. In addition, from time to time we may suffer other losses on our short-term investment portfolio.
We may require additional money to run our business and may be required to raise this money on terms which are not favorable to us or which reduce our stock price.
We may need to complete additional equity or debt financings to fund our operations. Our inability to obtain additional financing could adversely affect our business. Financings may not be available at all or on terms favorable to us. In addition, these financings, if completed, may not meet our capital needs and could result in substantial dilution to our stockholders.
If adequate funds are not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or drug development programs. We may also be required to liquidate our business or file for bankruptcy protection. Alternatively, we may be forced to attempt to continue development by entering into arrangements with collaborative partners or others that require us to relinquish some or all of our rights to technologies or drug candidates that we would not otherwise relinquish.
Our drug development programs will require substantial additional future funding which could hurt our operational and financial condition.
Our drug development programs require substantial additional capital to successfully complete them, arising from costs to: conduct research, preclinical testing and human studies; establish pilot scale and commercial scale manufacturing processes and facilities; and establish and develop quality control, regulatory, marketing, sales and administrative capabilities to support these programs.
Our future operating and capital needs will depend on many factors, including: the pace of scientific progress in our research and development programs and the magnitude of these programs; the scope and results of preclinical testing and human studies; the time and costs involved in obtaining regulatory approvals; the time and costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims; competing
24
Table of Contents
technological and market developments; our ability to establish additional collaborations; changes in our existing collaborations; the cost of manufacturing scale-up; and the effectiveness of our commercialization activities.
We expect our research and development expenditures over the next three years to continue to be significant. However, we base our outlook regarding the need for funds on many uncertain variables. Such uncertainties include regulatory approvals, the timing of events outside our direct control such as product launches by partners and the success of such product launches, negotiations with potential strategic partners, possible sale of assets or other transactions and other factors. Any of these uncertain events can significantly change our cash requirements.
While we expect to fund our research and development activities from cash generated from AVINZA and PROMACTA royalties and royalties and milestones from our partners in various past and future collaborations to the extent possible, if we are unable to do so, we may need to complete additional equity or debt financings or seek other external means of financing. These financings could depress our stock price. If additional funds are required to support our operations and we are unable to obtain them on terms favorable to us, we may be required to cease or reduce further development or commercialization of our products, to sell some or all of our technology or assets or to merge with another entity.
Significant returns of products we sold prior to selling our commercial businesses could harm our operating results.
Under our agreements to sell our commercial businesses, we remain financially responsible for returns of our products sold before those businesses were transferred to their respective buyers. Consequently, if returns of those products are higher than expected, we could incur substantial expenses for processing and issuing refunds for those returns which, in turn, could negatively impact our financial results. The amount of returns could be affected by a number of factors including, but not limited to, ongoing product demand, product rotation at distributors and wholesalers, and product stability issues.
Our results of operations and liquidity needs could be materially negatively affected by market fluctuations and economic downturn.
Our results of operations could be materially negatively affected by economic conditions generally, both in the U.S. and elsewhere around the world. Continuing concerns over inflation, energy costs, geopolitical issues, the availability and cost of credit, the U.S. mortgage market and a declining residential real estate market in the U.S. have contributed to increased volatility and diminished expectations for the economy and the markets going forward. These factors, combined with volatile oil prices, declining business and consumer confidence and increased unemployment, have precipitated an economic recession and fears of a possible depression. Domestic and international equity markets continue to experience heightened volatility and turmoil. These events and the continuing market upheavals may have an adverse effect on us. In the event of a continuing market downturn, our results of operations could be adversely affected by those factors in many ways, including making it more difficult for us to raise funds if necessary, and our stock price may further decline.
Our investment securities consist primarily of money market funds, corporate debt obligations and U.S. government agency securities. We do not have any auction rate securities. Recently, there has been concern in the credit markets regarding the value of a variety of mortgage-backed securities and the resultant effects on various securities markets. We cannot provide assurance that our investments are not subject to adverse changes in market value. If our investments experience adverse changes in market value, we may have less capital to fund our operations.
25
Table of Contents
We may be unable to successfully integrate the businesses of Neurogen, Metabasis and/or Pharmacopeia and realize the anticipated benefits of the mergers.
In December 2008, we completed our merger with Pharmacopeia. In 2009, we completed our merger with Neurogen and in January 2010, we completed our merger with Metabasis. The success of these mergers will depend, in part, on our ability to realize the anticipated synergies, growth opportunities and cost savings from integrating Pharmacopeia’s, Neurogen’s and/or Metabasis’ business with our business. Our success in realizing these benefits and the timing of this realization depend upon the successful integration of the operations of Pharmacopeia, Neurogen and/or Metabasis. The integration of independent companies is a complex, costly and time-consuming process. It is possible that the integration processes could result in the loss of key employees, diversion of each company’s management’s attention, the disruption or interruption of, or the loss of momentum in, each company’s ongoing business or inconsistencies in standards, controls, procedures and policies, any of which could adversely affect either company’s ability to maintain relationships with licensors, collaborators, partners, suppliers and employees or our ability to achieve the anticipated benefits of the merger, or could reduce our earnings or otherwise adversely affect the business and financial results of the combined company and, as a result, adversely affect the market price of our common stock.
We expect to incur significant costs and commit significant management time integrating Pharmacopeia’s, Neurogen’s and Metabasis’ business operations, technology, development programs, products and personnel with those of ours. If we do not successfully integrate the business of Pharmacopeia, Neurogen and Metabasis, the expenditure of these costs will reduce our cash position.
Our stock price has been volatile and could experience a sudden decline in value.
Our common stock has experienced significant price and volume fluctuations and may continue to experience volatility in the future. As a result, you may not be able to sell your shares quickly or at the latest market price if trading in our stock is not active or the volume is low. Many factors may have a significant impact on the market price of our common stock, including, but not limited to, the following factors: results of or delays in our preclinical studies and clinical trials; the success of our collaboration agreements; publicity regarding actual or potential medical results relating to products under development by us or others; announcements of technological innovations or new commercial products by us or others; developments in patent or other proprietary rights by us or others; comments or opinions by securities analysts or major stockholders; future sales of our common stock by existing stockholders; regulatory developments or changes in regulatory guidance; litigation or threats of litigation; economic and other external factors or other disaster or crises; the departure of any of our officers, directors or key employees; period-to-period fluctuations in financial results; and limited daily trading volume.
The Financial Industry Regulatory Authority, or FINRA, (formerly the National Association of Securities Dealers, Inc.) and the Securities and Exchange Commission, or SEC, have adopted certain new rules. If we were unable to continue to comply with the new rules, we could be delisted from trading on the NASDAQ Global Market, or Nasdaq, and thereafter trading in our common stock, if any, would be conducted through the over-the-counter market or on the Electronic Bulletin Board of FINRA. As a consequence of such delisting, an investor would likely find it more difficult to dispose of, or to obtain quotations as to the price of, our common stock. Delisting of our common stock could also result in lower prices per share of our common stock than would otherwise prevail.
Any future material weaknesses or deficiencies in our internal control over financial reporting could harm stockholder and business confidence on our financial reporting, our ability to obtain financing and other aspects of our business.
While no material weaknesses were identified as of December 31, 2009, we cannot assure you that material weaknesses will not be identified in future periods. The existence of one or more material weakness or significant deficiency could result in errors in our consolidated financial statements. Substantial costs and resources may be
26
Table of Contents
required to rectify any internal control deficiencies. If we fail to achieve and maintain the adequacy of our internal controls in accordance with applicable standards, we may be unable to conclude on an ongoing basis that we have effective internal controls over financial reporting. If we cannot produce reliable financial reports, our business and financial condition could be harmed, investors could lose confidence in our reported financial information, or the market price of our stock could decline significantly. In addition, our ability to obtain additional financing to operate and expand our business, or obtain additional financing on favorable terms, could be materially and adversely affected, which, in turn, could materially and adversely affect our business, our financial condition and the market value of our securities. Moreover, our reputation with customers, lenders, investors, securities analysts and others may be adversely affected.
Impairment charges pertaining to goodwill, identifiable intangible assets or other long-lived assets from our mergers could have an adverse impact on our results of operations and the market value of our common stock.
The total purchase price pertaining to our mergers with Pharmacopeia and Neurogen have been allocated to net tangible assets, identifiable intangible assets, in process research and development and goodwill. To the extent the value of goodwill or identifiable intangible assets or other long-lived assets become impaired, we will be required to incur material charges relating to the impairment. Any impairment charges could have a material adverse impact on our results of operations and the market value of our common stock.
We may undertake strategic acquisitions in the future and any difficulties from integrating such acquisitions could adversely affect our stock price, operating results and results of operations.
We may acquire companies, businesses and products that complement or augment our existing business. We may not be able to integrate any acquired business successfully or operate any acquired business profitably. Integrating any newly acquired business could be expensive and time-consuming. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources and could prove to be more difficult or expensive than we predict. The diversion of our management’s attention and any delay or difficulties encountered in connection with any future acquisitions we may consummate could result in the disruption of our on-going business or inconsistencies in standards and controls that could negatively affect our ability to maintain third-party relationships. Moreover, we may need to raise additional funds through public or private debt or equity financing, or issue additional shares, to acquire any businesses or products, which may result in dilution for stockholders or the incurrence of indebtedness.
As part of our efforts to acquire companies, business or product candidates or to enter into other significant transactions, we conduct business, legal and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. If we fail to realize the expected benefits from acquisitions we may consummate in the future, whether as a result of unidentified risks, integration difficulties, regulatory setbacks and other events, our business, results of operations and financial condition could be adversely affected. If we acquire product candidates, we will also need to make certain assumptions about, among other things, development costs, the likelihood of receiving regulatory approval and the market for such product candidates. Our assumptions may prove to be incorrect, which could cause us to fail to realize the anticipated benefits of these transactions.
In addition, we will likely experience significant charges to earnings in connection with our efforts, if any, to consummate acquisitions. For transactions that are ultimately not consummated, these charges may include fees and expenses for investment bankers, attorneys, accountants and other advisors in connection with our efforts. Even if our efforts are successful, we may incur, as part of a transaction, substantial charges for closure costs associated with elimination of duplicate operations and facilities and acquired in-process research and development charges. In either case, the incurrence of these charges could adversely affect our results of operations for particular quarterly or annual periods.
27
Table of Contents
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
We currently occupy approximately 30,000 square feet of office and laboratory facility in San Diego, California leased through December 2011. We lease approximately 99,000 square feet in three facilities in Cranbury, New Jersey under leases that expire in 2016. We believe these facilities are adequate to meet our space requirements for the foreseeable future.
We also lease a 52,800 square foot facility in San Diego that is leased through July 2015. In January 2008, we began subleasing the 52,800 square foot facility under a sublease through July 2015. We fully vacated this facility in February 2008.
Neurogen Corporation conducted its operations in laboratory and administrative facilities on a single site located in Branford, Connecticut. The total facilities, which were owned by Neurogen comprised approximately 142,000 square feet, of which approximately 21,000 square feet was leased by another company month to month. On February 2, 2010, we sold the facilities, which included approximately 120,000 square feet of laboratory and office space, approximately 40,000 square feet of warehouse space, and the surrounding land for approximately $3.5 million in cash, less expenses.
| Item 3. | Legal Proceedings |
Other Matters
We and Seragen, Inc., our subsidiary, were named parties to Sergio M. Oliver, et al. v. Boston University, et al., a shareholder class action filed on December 17, 1998 in the Court of Chancery in the State of Delaware. We and Seragen were dismissed from the action, but such dismissal is subject to appeal and we and Seragen may have possible indemnification obligations with respect to certain defendants. On December 21, 2009, the remaining parties entered into a Stipulation and Agreement of Compromise, Settlement and Release, or the Stipulation. The Stipulation is subject to Court approval and a hearing to consider approval of the stipulation has been scheduled for March 15, 2010. As of December 31, 2009, we have not accrued an indemnification obligation based on our assessment that our responsibility for any such obligation is not probable or estimable.
On October 10, 2008, we received notice that a putative class action complaint was filed in the Superior Court of New Jersey, Mercer County (Equity Division) by Allen Heilman, one of Phamacopeia’s stockholders, against Pharmacopeia, the members of its Board of Directors, Ligand and two of Ligand’s wholly owned subsidiaries. The complaint generally alleged that Pharmacopeia’s Board of Directors’ decision to enter into the proposed transaction with Ligand on the terms contained in the merger agreement constitutes a breach of fiduciary duty and gives rise to other unspecified state law claims. The complaint also alleged that Ligand and two of Ligand’s wholly owned subsidiaries aided and abetted Pharmacopeia’s Board of Directors’ breach of fiduciary duty. In addition, the complaint alleged that the named plaintiff sought “equitable relief,” including among other things, an order preliminarily and permanently enjoining the proposed transaction. While management believes that neither Ligand nor Pharmacopeia engaged in any wrongful acts, in an effort to minimize the cost and expense of any litigation, the parties entered into a stipulation of settlement, pursuant to which Pharmacopeia agreed to make certain additional disclosures in its SEC Form 14d-9 and not oppose a fee award to plaintiffs’ attorneys of up to $180,000, which is included in current portion of accrued litigation settlement costs at September 30, 2009. On October 20, 2009, the court granted final approval of the stipulation of settlement and dismissed the class action with prejudice.
On September 9, 2009, we received notice that a class action complaint was filed in the Connecticut Superior Court for the Judicial District of New Haven by Gabriel Guzman, one of Neurogen’s stockholders, against Neurogen, the members of its Board of Directors, Ligand and one of Ligand’s wholly owned subsidiaries.
28
Table of Contents
The amended complaint generally alleged that Neurogen’s Board of Directors’ decision to enter into the transaction with Ligand on the terms contained in the merger agreement constituted a breach of fiduciary duty. The amended complaint also alleges that Ligand and one of Ligand’s wholly owned subsidiaries aided and abetted Neurogen’s Board of Directors’ breach of fiduciary duty. Management believes that neither Ligand nor Neurogen engaged in any wrongful acts and on October 22, 2009, we filed a motion to strike the complaint. The plaintiff filed a Withdrawal of Action to voluntarily dismiss the case in December 2009.
In December 2009, the Internal Revenue Service, or IRS, issued to us a Notice of Proposed Adjustment, or NOPA, seeking an increase to our taxable income for the 2007 fiscal year of $71.5 million and a $4.1 million penalty for substantial underpayment of tax in fiscal 2007. We responded to the NOPA in February 2010, disagreeing with the conclusions reached by the IRS in the NOPA. We recorded a FIN 48 liability of $25.1 million related to the income tax effect of the NOPA and $3.0 million related to estimated interest due on the proposed underpayment of tax. We also recorded deferred income tax assets of $25.1 million associated with the ability to carry back losses from 2008 and 2009 to offset the NOPA. In addition, we recorded an income tax receivable of $4.5 million associated with changes in income tax law in relation to prior AMT taxes paid on carry back periods. We have not recorded the penalties proposed by the IRS in our financial statements as we believe that we met the appropriate standard for the tax position on our 2007 tax return. If we are unsuccessful in our negotiations with the IRS, we may be required to pay the $4.1 million penalty and utilize a significant amount of our net operating loss carryforwards.
In addition, from time to time we are subject to various lawsuits and claims with respect to matters arising out of the normal course of our business. Due to the uncertainty of the ultimate outcome of these matters, the impact on future financial results is not subject to reasonable estimates.
| Item 4. | Reserved |
29
Table of Contents
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities |
Market Information
Our common stock is traded on the NASDAQ Global Market (formerly NASDAQ National Market) under the symbol “LGND”.
The following table sets forth the high and low intraday sales prices for our common stock on the NASDAQ Global Market for the periods indicated:
| Price Range | ||||||
| High | Low | |||||
| Year Ended December 31, 2009: |
||||||
| 1st Quarter |
$ | 3.20 | $ | 1.86 | ||
| 2nd Quarter |
3.18 | 2.51 | ||||
| 3rd Quarter |
3.21 | 2.21 | ||||
| 4th Quarter |
2.43 | 1.63 | ||||
| Year Ended December 31, 2008: |
||||||
| 1st Quarter |
$ | 5.00 | $ | 3.31 | ||
| 2nd Quarter |
4.55 | 2.16 | ||||
| 3rd Quarter |
3.82 | 2.58 | ||||
| 4th Quarter |
2.94 | 1.10 | ||||
As of February 11, 2010, the closing price of our common stock on the NASDAQ Global Market was $1.66.
Holders
As of February 11, 2010, there were approximately 1,648 holders of record of the common stock.
Dividends
On March 22, 2007, we declared a cash dividend on our common stock of $2.50 per share. As we have an accumulated deficit, the dividend was recorded as a charge against additional paid-in capital. The aggregate amount of $252.7 million was paid on April 19, 2007 to shareholders of record as of April 5, 2007. We had previously never declared or paid any cash dividends on our capital stock. We do not intend to pay any additional cash dividends in the foreseeable future. We currently intend to retain future earnings, if any, to finance future growth.
30
Table of Contents
Performance Graph
The graph below shows the five-year cumulative total stockholder return assuming the investment of $100 and the reinvestment of dividends (a one-time dividend of $2.50 was declared on the common stock in April 2007) and is based on the returns of the component companies weighted monthly according to their market capitalizations. The graph compares total stockholder returns of our common stock, of all companies traded on the NASDAQ Stock market, as represented by the NASDAQ Composite® Index, and of the NASDAQ Biotechnology Stock Index, as prepared by The NASDAQ Stock Market Inc. The NASDAQ Biotechnology Stock Index tracks approximately 168 domestic biotechnology stocks.
The stockholder return shown on the graph below is not necessarily indicative of future performance and we will not make or endorse any predictions as to future stockholder returns.
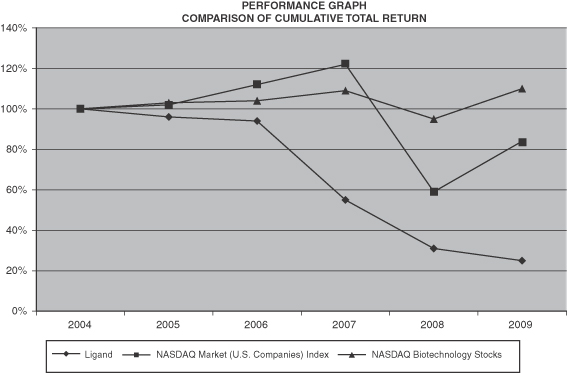
| 12/31/04 | 12/31/05 | 12/31/06 | 12/31/07 | 12/31/08 | 12/31/09 | |||||||||||||
| Ligand |
100 | % | 96 | % | 94 | % | 55 | % | 31 | % | 25 | % | ||||||
| NASDAQ Market (U.S. Companies) Index |
100 | % | 102 | % | 112 | % | 122 | % | 59 | % | 84 | % | ||||||
| NASDAQ Biotechnology Stocks |
100 | % | 103 | % | 104 | % | 109 | % | 95 | % | 110 | % |
31
Table of Contents
| Item 6. | Selected Consolidated Financial Data |
The following selected historical consolidated financial and other data are qualified by reference to, and should be read in conjunction with, our consolidated financial statements and the related notes thereto appearing elsewhere herein and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our selected statement of operations data set forth below for each of the years ended December 31, 2009, 2008, 2007, 2006, and 2005 and the balance sheet data as of December 31, 2009, 2008 2007, 2006, and 2005 are derived from our consolidated financial statements.
| Year Ended December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006(2) | 2005 | ||||||||||||||||
| (in thousands, except share data) | ||||||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||
| Royalties |
$ | 8,334 | $ | 20,305 | $ | 11,409 | $ | — | $ | — | ||||||||||
| Collaborative research and development and other revenues |
30,606 | 7,000 | 1,485 | 3,977 | 10,217 | |||||||||||||||
| Research and development expenses |
39,870 | 30,770 | 44,623 | 41,546 | 30,710 | |||||||||||||||
| General and administrative expenses |
15,211 | 23,785 | 30,410 | 43,908 | 23,134 | |||||||||||||||
| Lease termination costs |
15,235 | — | — | — | — | |||||||||||||||
| Write-off of acquired in-process research and development |
442 | 72,000 | — | — | — | |||||||||||||||
| Gain on sale leaseback |
21,851 | 1,964 | 1,964 | 3,397 | — | |||||||||||||||
| Loss from operations |
(9,967 | ) | (97,276 | ) | (60,175 | ) | (78,080 | ) | (43,627 | ) | ||||||||||
| Loss from continuing operations |
(8,337 | ) | (97,460 | ) | (34,759 | ) | (56,590 | ) | (36,035 | ) | ||||||||||
| Discontinued operations (1) |
6,389 | (654 | ) | 316,447 | 24,847 | (364 | ) | |||||||||||||
| Net income (loss) |
(1,948 | ) | (98,114 | ) | 281,688 | (31,743 | ) | (36,399 | ) | |||||||||||
| Basic per share amounts: |
||||||||||||||||||||
| Income (loss) from continuing operations |
$ | (0.09 | ) | $ | (1.02 | ) | $ | (0.35 | ) | $ | (0.70 | ) | $ | (0.49 | ) | |||||
| Discontinued operations (1) |
0.07 | (0.01 | ) | 3.22 | 0.31 | — | ||||||||||||||
| Net income (loss) |
$ | (0.02 | ) | $ | (1.03 | ) | $ | 2.87 | $ | (0.39 | ) | $ | (0.49 | ) | ||||||
| Weighted average number of common shares |
113,176,511 | 95,505,421 | 98,124,731 | 80,618,528 | 74,019,501 | |||||||||||||||
| Diluted per share amounts: |
||||||||||||||||||||
| Income (loss) from continuing operations |
$ | (0.08 | ) | $ | (1.02 | ) | $ | (0.35 | ) | $ | (0.70 | ) | $ | (0.49 | ) | |||||
| Discontinued operations (1) |
0.06 | (0.01 | ) | 3.22 | 0.31 | — | ||||||||||||||
| Net income (loss) |
$ | (0.02 | ) | $ | (1.03 | ) | $ | 2.87 | $ | (0.39 | ) | $ | (0.49 | ) | ||||||
| Weighted average number of common shares |
113,176,511 | 95,505,421 | 98,124,731 | 80,618,528 | 74,019,501 | |||||||||||||||
32
Table of Contents
| December 31, | ||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents, short-term investments and restricted cash and investments |
$ | 54,694 | $ | 82,012 | $ | 95,819 | $ | 212,488 | $ | 88,756 | ||||||||||
| Working capital (deficit) (3) |
15,994 | 23,315 | 58,975 | 64,747 | (102,244 | ) | ||||||||||||||
| Total assets |
141,807 | 171,448 | 173,278 | 326,053 | 314,619 | |||||||||||||||
| Current portion of deferred revenue, net |
4,989 | 10,301 | — | 57,981 | 157,519 | |||||||||||||||
| Current portion of deferred gain |
1,702 | 1,964 | 1,964 | 1,964 | — | |||||||||||||||
| Long-term obligations (excludes long-term portions of deferred revenue, net and deferred gain) |
72,350 | 58,743 | 53,048 | 85,780 | 173,280 | |||||||||||||||
| Long-term portion of deferred revenue, net |
3,495 | 16,819 | 2,546 | 2,546 | 4,202 | |||||||||||||||
| Long-term portion of deferred gain |
1,702 | 23,292 | 25,256 | 27,220 | — | |||||||||||||||
| Common stock subject to conditional redemption |
8,344 | 12,345 | 12,345 | 12,345 | 12,345 | |||||||||||||||
| Accumulated deficit |
(681,574 | ) | (679,626 | ) | (581,512 | ) | (862,802 | ) | (831,059 | ) | ||||||||||
| Total stockholders’ equity (deficit) |
3,744 | (10,365 | ) | 29,115 | 27,352 | (110,419 | ) | |||||||||||||
| (1) | We sold our Oncology Product Line (“Oncology”) on October 25, 2006 and our AVINZA Product Line (“AVINZA”) on February 26, 2007. The operating results for Oncology and AVINZA have been presented in our consolidated statements of operations as “Discontinued Operations.” |
| (2) | Effective January 1, 2006, we adopted ASC 718, Compensation—Stock Compensation, or ASC 718, using the modified prospective transition method. The implementation of ASC 718 resulted in additional employee stock compensation expense of $4.8 million in 2006. |
| (3) | Working capital (deficit) includes deferred product revenue recorded under the sell-through revenue recognition method. |
33
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Caution: This discussion and analysis may contain predictions, estimates and other forward-looking statements that involve a number of risks and uncertainties, including those discussed in Item 1A. “Risk Factors.” This outlook represents our current judgment on the future direction of our business. These statements include those related to our AVINZA royalty revenues, product returns, and product development. Actual events or results may differ materially from our expectations. For example, there can be no assurance that our revenues or expenses will meet any expectations or follow any trend(s), that we will be able to retain our key employees or that we will be able to enter into any strategic partnerships or other transactions. We cannot assure you that we will receive expected AVINZA royalties to support our ongoing business or that our internal or partnered pipeline products will progress in their development, gain marketing approval or achieve success in the market. In addition, ongoing or future arbitration, or litigation or disputes with third parties may have a material adverse effect on us. Such risks and uncertainties, and others, could cause actual results to differ materially from any future performance suggested. We undertake no obligation to release publicly the results of any revisions to these forward-looking statements to reflect events or circumstances arising after the date of this annual report. This caution is made under the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934, as amended.
Our trademarks, trade names and service marks referenced herein include Ligand. Each other trademark, trade name or service mark appearing in this annual report belongs to its owner.
References to “Ligand Pharmaceuticals Incorporated”, “Ligand”, the “Company”, “we” or “our” include our wholly owned subsidiaries—Ligand Pharmaceuticals International, Inc.; Seragen, Inc., or Seragen; Pharmacopeia, LLC; Neurogen Corporation; and Nexus Equity VI LLC, or Nexus.
Overview
We are a biotechnology company that focuses on drug discovery and early-stage development of pharmaceuticals that address critical unmet medical needs or that are more effective and/or safer than existing therapies, more convenient to administer and are cost effective. Our goal is to build a profitable company by generating income from research, milestone, and royalty revenues resulting from our collaborations with pharmaceutical partners.
On September 7, 2006, we announced the sale of ONTAK, Targretin capsules, Targretin gel, and Panretin gel to Eisai, Inc., or Eisai, and the sale of AVINZA to King Pharmaceuticals, Inc., or King. The Eisai sales transaction subsequently closed on October 25, 2006. The AVINZA sale transaction subsequently closed on February 26, 2007. Accordingly, the results for the Oncology and AVINZA Product Lines have been presented in our consolidated statements of operations as “Discontinued Operations.”
On December 23, 2008, we acquired all of the outstanding common shares of Pharmacopeia, Inc., or Pharmacopeia. Pharmacopeia was a clinical development stage biopharmaceutical company dedicated to discovering and developing novel small molecule therapeutics to address significant medical needs. Pharmacopeia’s strategy was to retain the rights to product candidates at least to clinical validation, and to continue with (i) development on its own New Drug Application, or NDA, filings and (ii) commercialization for selected indications. Pharmacopeia had a broad portfolio of clinical and preclinical candidates under development internally or by partners.
On December 23, 2009, we acquired all of the outstanding common shares of Neurogen Corporation, or Neurogen. As consideration, we issued approximately 4.2 million shares of our common stock to Neurogen stockholders, or approximately 0.061 shares of our common stock for each outstanding Neurogen share, as well as approximately $0.6 million in cash. Security holders of Neurogen also received contingent value rights, under which they could receive cash payments under certain circumstances. Neurogen was a drug development
34
Table of Contents
company historically focusing on small-molecule drugs to improve the lives of patients suffering from psychiatric and neurological disorders with significant unmet medical needs. Neurogen has conducted its drug development independently and, when advantageous, collaborated with world-class pharmaceutical companies to access additional resources and expertise.
On January 27, 2010, we completed the acquisition of Metabasis Therapeutics, Inc., or Metabasis, following approval of the transaction by Metabasis stockholders. As a result, we gained a fully funded partnership with Hoffman-La Roch, or Roche, additional pipeline assets and drug discovery technologies and resources. We paid $1.6 million in cash or about $0.046 per Metabasis share to Metabasis’ stockholders. In addition, Metabasis stockholders received four tradable Contingent Value Rights (CVRs), one CVR from each of four respective series of CVRs, for each Metabasis share. The CVRs will entitle Metabasis stockholders to cash payments as frequently as every six months as cash is received by us from proceeds from Metabasis’ partnership with Roche or the sale or partnering of any of the Metabasis drug development programs, among other triggering events.
Our business strategy includes targeted internal drug research and early-stage development capabilities. We believe that we have promising product candidates throughout our internal development programs. We also have research and development collaborations for our product candidates with numerous global pharmaceutical companies. These collaborations include ongoing clinical programs at Bristol-Myers Squibb, or BMS, GlaxoSmithKline, or GSK, Pfizer, Merck & Co., Inc., or Merck, Roche, Cephalon and Celgene. We aim to create value for shareholders by advancing our internally developed programs through early clinical development and then entering licensing agreements with larger pharmaceutical and biotechnology companies with substantially greater development and commercialization infrastructure. In addition to advancing our R&D programs, we expect to collect licensing fees and royalties from existing and future license agreements. We aim to build a profitable company by generating income from our corporate licenses.
We currently receive royalty revenues from King Pharmaceuticals, or King, and GSK. In February 2007, we completed the sale of our AVINZA product line to King. As a result of the sale, we received the right to future royalties on the net sales of AVINZA through 2017.
In December 2008, the U.S. Food and Drug Administration, or FDA, granted accelerated approval of GSK’s PROMACTA for the treatment of thrombocytopenia in patients with chronic immune (idiopathic) thrombocytopenic purpura, or ITP, who have had an insufficient response to corticosteroids, immunoglobulins or splenectomy. PROMACTA is also approved under the trade name Revolade(R) in Venezuela, Kuwait, Chile and Russia. GSK also filed a regulatory application for PROMACTA in Japan in September 2009. PROMACTA is the first oral thrombopoietin, or TPO, receptor agonist therapy for the treatment of adult patients with chronic ITP. In December 2009, GSK received a positive opinion for Revolade from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) for the oral treatment of thrombocytopenia (reduced platelet count) in adults with the blood disorder chronic ITP. As a result of the regulatory approvals of PROMACTA, we are entitled to receive tiered royalties based on annual net sales of PROMACTA. As part of a settlement agreement and mutual release we entered into on February 11, 2009 with The Rockefeller University, or Rockefeller, we agreed to pay a share of such royalties to Rockefeller. See “Item 3. Legal Proceedings.”
We also have the potential to receive near-term royalties on product candidates resulting from our research and development collaboration arrangements with third party pharmaceutical companies if and when any such product candidate is ultimately approved by the FDA and successfully marketed. Our near-term product candidates are discussed below.
In addition to the accelerated approval granted for GSK’s PROMACTA for the treatment of thrombocytopenia in patients with chronic ITP, GSK also reported new phase III results for PROMACTA in chronic ITP at the 2009 14th Congress of European Hematology meeting and initiated two Phase III trials in patients with hepatitis C in the fourth quarter of 2007. A Phase II study in patients with oncology-related thrombocytopenia is ongoing and a Phase I study is ongoing in patients with sarcoma receiving the adriamycin and ifosfamide regimen.
35
Table of Contents
Bazedoxifene (Viviant) is a product candidate that resulted from one of our collaborations with Wyeth (now Pfizer). Bazedoxifene is a synthetic drug that was specifically designed to reduce the risk of osteoporotic fractures while at the same time protecting breast and uterine tissue. Regarding Viviant, the FDA has advised that it expects to convene an advisory committee to review the pending NDAs for both the treatment and prevention indications. Approvable letters were received for each of these NDAs, in which, among other things, the FDA requested further analyses and discussion concerning the incidence of stroke and venous thrombotic events, identified certain issues concerning data collection and reporting, and requested additional source documents. An FDA-requested advisory committee meeting is expected to be scheduled following submission of the complete response to the approvable letters. In April 2009, Pfizer received approval in the EU for CONBRIZA (the EU trade name for Viviant) for the treatment of postmenopausal osteoporosis in women at increased risk of fracture. We expect CONBRIZA to be launched in the EU in 2010.
Pfizer is also developing bazedoxifene in combination with PREMARIN (Aprela) as a tissue selective estrogen complex under development for menopausal symptoms and osteoporosis. Two Phase III studies with bazedoxifene/conjugated estrogens (Aprela) showed reduced number and severity of hot flashes in symptomatic postmenopausal women by up to 80 percent when compared with placebo. Pfizer expects to file an initial NDA no earlier than the first half of 2010. We are entitled to receive tiered royalties on these products.
Lasofoxifene (FABLYN®) is a product candidate that resulted from our collaboration with Pfizer. Pfizer submitted an NDA and an MAA for FABLYN for osteoporosis treatment in December 2007 and January 2008, respectively. The FDA Advisory Committee in early September 2008 voted 9-3 in favor of approving this drug. In January 2009, Pfizer received a complete response letter from the FDA requesting additional information for FABLYN. In February 2009 FABLYN received approval in the EU for the treatment of osteoporosis. Pfizer reported that following a strategic review, it decided to explore strategic options for FABLYN, including out-licensing or sale. Under the terms of our agreement with Pfizer, we are entitled to receive royalty payments on worldwide net sales of lasofoxifene for any indication.
Results of Operations
Total revenues for 2009 were $38.9 million, compared to $27.3 million in 2008 and $12.9 million in 2007. Our loss from continuing operations for 2009 was $9.9 million, or $0.09 per share, compared to $97.5 million, or $1.02 per share, in 2008 and $34.8 million, or $0.35 per share, in 2007.
Royalty Revenue
Royalty revenues were $8.3 million in 2009 compared to $20.3 million in 2008 and $11.4 million in 2007. The decrease in royalty revenues of $12.0 million for the year ended December 31, 2009 is primarily due to a reduction in the contractual royalty rate from 15% to 5% in October 2008 under our agreement with King for AVINZA sales, partially offset by PROMACTA royalties. The increase in royalty revenues of $8.9 million for the year ended December 31, 2008 is a result of the first full year of AVINZA royalties following our sale of AVINZA to King Pharmaceuticals in Febuary 2007.
Collaborative Research and Development and Other Revenue
Collaborative research and development and other revenues for 2009 were $30.6 million compared to $7.0 million in 2008 and $1.5 million in 2007. Collaborative research and development and other revenues include reimbursement for ongoing research activities, earned milestones, and recognition of prior years’ up-front fees previously deferred.
36
Table of Contents
A comparison of collaborative research and development and other revenues is as follows (in thousands):
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Collaborative research and development |
$ | 23,316 | $ | — | $ | — | |||
| License fees |
525 | 5,000 | — | ||||||
| Milestones and other |
6,765 | 2,000 | 1,485 | ||||||
| $ | 30,606 | $ | 7,000 | $ | 1,485 | ||||
Collaborative Research and Development. The increase of $23.3 million for the year ended December 31, 2009 is due to collaboration revenues resulting from agreements acquired from Pharmacopeia in December 2008.
License fees. License fees decreased $4.5 million for the year ended December 31, 2009 as, during 2008, we received a $5.0 million up-front license fee from an agreement with GSK under which we licensed worldwide exclusive rights to our LGD-4665 product candidate and our other thrombopoietin (TPO)-related molecules to GSK.
Milestones and Other. Milestones in 2009 reflect $4.0 million received from Merck in connection with lead identification and transferred programs, $1.3 million received from GSK for lead identification, and $1.5 million from Pfizer related to NDA filings. Milestones in 2008 reflect $2.0 million received from GSK as a result of FDA approval of eltrombopag. Milestones in 2007 reflect $1.0 million received from GSK in connection with the filing of an NDA for eltrombopag and $0.5 million earned from Wyeth.
Research and Development Expenses
Research and development expenses were $39.9 million in 2009 compared to $30.8 million in 2008 and $44.6 million in 2007. The major components of research and development expenses are as follows (in thousands):
| Years Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Research performed under collaboration agreements |
$ | 21,194 | $ | — | $ | — | |||
| Internal research programs |
12,963 | 21,626 | 21,954 | ||||||
| Total research |
34,157 | 21,626 | 21,954 | ||||||
| Development costs |
5,713 | 9,144 | 22,669 | ||||||
| Total research and development |
$ | 39,870 | $ | 30,770 | $ | 44,623 | |||
The increase in research and development expenses of $9.1 million for the year ended December 31, 2009 was primarily due to $21.2 million of costs associated with servicing our collaboration agreements, partially offset by a $7.0 million reduction in litigation settlement costs as the result of a settlement agreement and mutual release we entered into with The Rockefeller University, or Rockefeller, in 2008, $2.0 million in reduced consulting and outside service costs associated with internal research programs, and a $3.0 million reduction in costs associated with clinical trials.
The decrease in research and development costs of $13.9 million for the year ended December 31, 2008 was primarily due to $13.6 million of reduced development expenses as a result of lower headcount-related expenses in connection with our restructuring and reduced outside service costs associated with our thrombopoietin (TPO) agonists program and a reduction of $7.4 million in restructuring costs as 2007 included one-time severance benefits and stock compensation charges of $6.6 million incurred in connection with our restructuring and one-time stock compensation charges of $0.8 million incurred in connection with the equitable adjustment of stock options, partially offset by $7.0 million related to a settlement agreement and mutual release we entered into with Rockefeller in 2008.
37
Table of Contents
A summary of our significant internal research and development programs as of December 31, 2009 is as follows:
| Program |
Disease/Indication |
Development Phase | ||
| Selective Androgen Receptor Modulators (SARMs) (agonists) | Muscle wasting and frailty | Phase I | ||
| Thyroid receptor beta agonists | Hyperlipidemia | Phase I and Preclinical | ||
| Small molecule Erythropoietin (EPO) receptor agonists | Chemotherapy-induced anemia, anemia due to kidney failure | Preclinical | ||
| Glucagon receptor antagonists | Diabetes | Preclinical | ||
| Histamine 3 (H3) receptor antagonists | Cognitive disorders | Research | ||
We do not provide forward-looking estimates of costs and time to complete our ongoing research and development projects, as such estimates would involve a high degree of uncertainty. Uncertainties include our inability to predict the outcome of complex research, our inability to predict the results of clinical studies, regulatory requirements placed upon us by regulatory authorities such as the FDA and EMEA, our inability to predict the decisions of our collaborative partners, our ability to fund research and development programs, competition from other entities of which we may become aware of in future periods, predictions of market potential from products that may be derived from our research and development efforts, and our ability to recruit and retain personnel or third-party research organizations with the necessary knowledge and skills to perform certain research. Refer to “Item 1A. Risks Factors” for additional discussion of the uncertainties surrounding our research and development initiatives.
General and Administrative Expenses
General and administrative expenses were $15.2 million for 2009, compared to $23.8 million for 2008 and $30.4 million for 2007. The decrease in general and administrative expenses of $8.6 million for the year ended December 31, 2009 was primarily due to $4.3 million of expenses incurred during 2008 as a result of exiting a facility, reduced legal expenses of $3.3 million and lower headcount costs of $0.6 million.
The decrease in general and administrative costs of $6.6 million for the year ended December 31, 2008 was primarily due to lower headcount related expenses of $7.1 million (which included one-time severance benefits of $3.9 million in connection with our restructuring, and stock compensation charges of $1.0 million incurred in connection with the equitable adjustment of stock options), reduced outside consulting and audit fees of $3.6 million and reduced occupancy cost of $1.7 million. These reductions were partially offset by a $4.1 million charge for exit costs when we fully ceased use of one of our leased facilities in the first quarter of 2008 and increased legal expenses of $1.1 million primarily related to litigation with The Salk Institute for Biological Studies, or Salk, and Rockefeller.
Lease Termination Costs
In August 2009, we entered into a lease termination agreement for our corporate facility in San Diego. Under the terms of the agreement, we will pay a termination fee of $14.3 million as follows: $4.5 million was paid upon signing, $4.5 million in July 2010 and $5.3 million in April 2011. As a result, during the year ended December 31, 2009, we recorded lease termination costs of $15.2 million, which includes the net present value of the lease termination payments of $14.3 million and $0.9 million of other costs associated with the lease termination.
38
Table of Contents
Write-off of in-process research and development
For acquisitions prior to January 1, 2009, the fair value of acquired In-Process Research and Development (IPR&D) projects, which have no alternative future use and which have not reached technological feasibility at the date of acquisition, were immediately expensed. We wrote-off the estimated fair value of $72.0 million of acquired in-process research and development related to the acquisition of Pharmacopeia, Inc. in 2008. The estimated fair value relates to specific internal and partnered product candidates targeting a variety of indications which are currently in various stages of development ranging from preclinical to Phase II. Due to the nature of our internal development programs and our collaborative partnerships, management does not expect to incur significant costs related to these programs. The estimated fair value is driven by future milestones and royalties. Management anticipates potential milestones in the near-term and the possibility of significant royalties beginning in 2015. However, as these potential products have not reached commercialization, we or our partners face risks inherent in the development of products in the human health care market and will continue to face significant risks as no assurance can be given that: (1) product development efforts will be successful, (2) required regulatory approvals for any indication will be obtained, (3) any products, if introduced, will be capable of being produced in commercial quantities at reasonable costs or, (4) patient and physician acceptance of these products will be achieved. These risks may cause significant delays in the timing or potential success of commercialization of these products, which could materially impact estimated future cash flows. Of the total fair value, $29.0 million relates to product candidates currently in the preclinical stage of development as follows: $13.0 million related to various candidates under our collaboration with GSK, $8.0 million related to the JAK-3 program with Wyeth, and $8.0 related to our internal CCR1 program; $9.0 million relates to product candidates currently in Phase I clinical trials as follows: $7.5 million related to Schering Plough oncology-related product candidates and $1.5 million related to a product candidate being developed by Celgene targeting inflammation; and $34.0 million relates to product candidates currently in Phase II clinical trials as follows: $19.0 million related to Schering Plough’s CXCR2 program targeting COPD and asthma and $15.0 million related to a P38 MAPK inhibitor program targeting rheumatoid arthritis and psoriasis being developed by BMS.
We used the “Income Method” to determine the estimated fair values of acquired in-process research and development, which uses a discounted cash flow model and applies a probability weighting based on estimates of successful product development and commercialization to estimated future net cash flows resulting from projected revenues and related costs. These success rates take into account the stages of completion and the risks surrounding successful development and commercialization of the underlying product candidates. These cash flows were then discounted to present value using a discount rate of 40% for product candidates in the preclinical stage, 35% for product candidates currently in Phase I clinical trials and 30% for product candidates currently in Phase II clinical trials.
The above assumptions were used solely for the purposes of estimating fair values of these product candidates as of the date of their acquisition. However, we cannot provide assurance that the underlying assumptions used to forecast the cash flows or the timely and successful completion of development and commercialization will materialize, as estimated. Consequently, the eventual realized value of the acquired inprocess research and development may vary from its estimated value at the date of acquisition.
As a result of adjustments to our purchase price allocation related to our acquisition of Pharmacopeia, we wrote-off an additional $0.4 million of acquired in-process research and development during the year ended December 31, 2009.
Accretion of Deferred Gain on Sale Leaseback
In October 2006, we entered into an agreement with Slough for the sale of our real property located in San Diego, California for a purchase price of $47.6 million. This property, with a net book value of $14.5 million, included one building totaling approximately 82,500 square feet, the land on which the building is situated, and two adjacent vacant lots. As part of the sale transaction, we agreed to lease back the building for a period of 15 years.
39
Table of Contents
We recognized an immediate pre-tax gain on the sale transaction of $3.1 million in 2006 and deferred a gain of $29.5 million on the sale of the building. The deferred gain was being recognized as an offset to operating expense on a straight-line basis over the 15 year term of the lease at a rate of approximately $2.0 million per year.
In August 2009, we entered into a lease termination agreement for this building. As a result, we recognized an additional $20.4 million of accretion of deferred gain during the quarter ended September 30, 2009, and will recognize the remaining balance of the deferred gain of $3.1 million through the term of our new building lease, which expires in December 2011. The amount of the deferred gain recognized for the years ended December 31, 2009, 2008 and 2007 was $21.9 million, $2.0 million and $2.0 million, respectively.
Interest Income
Interest income was $0.6 million for 2009, compared to $2.1 million for 2008 and $8.7 million for 2007. The decrease from 2008 to 2009 is due to lower invested balances and lower interest rates. The decrease from 2007 to 2008 is due to lower invested balances as a result of the $252.7 million cash dividend paid on April 19, 2007, as well as lower interest rates.
Income Taxes
During 2009, we recorded an income tax benefit of $1.5 million. In December 2009, the Internal Revenue Service, or IRS, issued to us a Notice of Proposed Adjustment, or NOPA, seeking an increase to our taxable income for the 2007 fiscal year of $71.5 million and a $4.1 million penalty for substantial underpayment of tax in fiscal 2007. We responded to the NOPA in February 2010, disagreeing with the conclusions reached by the IRS in the NOPA. We recorded a FIN 48 liability of $25.1 million related to the income tax effect of the NOPA and $3.0 million related to estimated interest due on the proposed underpayment of tax. We also recorded deferred income tax assets of $25.1 million associated with the ability to carry back losses from 2008 and 2009 to offset the NOPA. In addition, we recorded an income tax receivable of $4.5 million associated with changes in income tax law in relation to prior AMT taxes paid on carry back periods. We have not recorded the penalties proposed by the IRS in our financial statements as we believe that we met the appropriate standard for the tax position on our 2007 tax return. If we are unsuccessful in our negotiations with the IRS, we may be required to pay the $4.1 million penalty and utilize a significant amount of our net operating loss carryforwards.
During 2008, we had losses from continuing operations and discontinued operations. We recorded an income tax benefit from continuing operations of $0.1 million for the year ended December 31, 2008 related to tax refunds. We recorded an income tax benefit from continuing operations of $18.7 million for the year ended December 31, 2007 related to the use of losses from continuing operations to offset income from discontinued operations.
At December 31, 2009, we have federal net operating loss carryforwards of $513.8 million, $205.7 million of state net operating loss carryforwards and $17.1 million of federal research and development credit carryforwards. Federal research and development credit carryforwards of $1.2 million expired at the beginning of 2010 with the remainder expiring through 2027, and we have $12.9 million of California and New Jersey research and development credit carryforwards that have no expiration date.
Pursuant to Internal Revenue Code Sections 382 and 383, use of net operating loss and credit carryforwards may be limited if there were changes in ownership of more than 50%. We have completed a Section 382 study for Ligand through 2007. As a result of these ownership changes, utilization of Ligand’s net operating losses and credits are subject to limitations under Internal Revenue Code Sections 382 and 383.
40
Table of Contents
Discontinued Operations
Oncology Product Line
In 2006, we and Eisai entered into the Oncology purchase agreement pursuant to which Eisai agreed to acquire all of our worldwide rights in and to our oncology products, or Oncology product line, including, among other things, all related inventory, equipment, records and intellectual property, and assume certain liabilities as set forth in the Oncology purchase agreement. The Oncology product line included our four marketed oncology drugs: ONTAK, Targretin capsules, Targretin gel and Panretin gel. Pursuant to the Oncology purchase agreement, at closing on October 25, 2006, we received $185.0 million in net cash proceeds, which is net of $20.0 million that was funded into an escrow account to support any potential indemnification claims made by Eisai following the closing of the sale. In 2007, we recognized a $20.8 million pre-tax gain resulting from the release of funds from the escrow account partially offset by a $2.8 million pre-tax loss due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. In 2008, we recognized a $10.6 million pre-tax loss resulting from the Salk settlement for $13.0 million partially offset by a $2.4 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. In 2009, we recognized a $1.0 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date.
Additionally, $38.6 million of the proceeds received from Eisai were deposited into an escrow account to repay a loan received from King Pharmaceuticals, Inc., or King, the proceeds of which were used to pay our co-promote termination obligation to Organon in October 2006. The escrow amounts were released and the loan repaid to King in January 2007.
In connection with the Oncology purchase agreement with Eisai, we entered into a transition services agreement whereby we agreed to perform certain transition services for Eisai, in order to effect, as rapidly as practicable, the transition of purchased assets from us to Eisai. In exchange for these services, Eisai paid us a monthly service fee through June 25, 2007. Fees earned under the transition services agreement during 2007, which were recorded as an offset to operating expenses, were $2.7 million.
Prior to the Oncology sale, we recorded accruals for rebates, chargebacks, and other discounts related to Oncology products when product sales were recognized as revenue under the sell-through method. Upon the Oncology sale, we accrued for rebates, chargebacks, and other discounts related to Oncology products in the distribution channel which had not sold-through at the time of the Oncology sale and for which we retained the liability subsequent to the Oncology sale. These products expired at various dates through July 31, 2008. Our accruals for Oncology rebates, chargebacks, and other discounts total $7,000 and $0.4 million as of December 31, 2009 and 2008, respectively.
Additionally, and pursuant to the terms of the Oncology purchase agreement, we retained the liability for returns of product from wholesalers that had been sold by us prior to the close of the transaction. Accordingly, as part of the accounting for the gain on the sale of the Oncology Product Line, we recorded a reserve for Oncology product returns. Under the sell-through revenue recognition method, we previously did not record a reserve for returns from wholesalers. Oncology products sold by us may be returned through a specified period subsequent to the product expiration date, but no later than July 31, 2009. Our reserve for Oncology returns was zero and $0.9 million as of December 31, 2009 and 2008, respectively.
AVINZA Product Line
In 2006, we and King entered into the AVINZA purchase agreement pursuant to which King agreed to acquire all of our rights in and to AVINZA in the United States, its territories and Canada, including, among other things, all AVINZA inventory, records and related intellectual property, and assume certain liabilities as set forth in the AVINZA purchase agreement, which we collectively refer to as the Transaction.
41
Table of Contents
Pursuant to the AVINZA purchase agreement, at Closing on February 26, 2007, or Closing Date, we received $280.4 million in net cash proceeds, which is net of $15.0 million that was funded into an escrow account to support any potential indemnification claims made by King following the Closing. Of the escrowed amount, $7.5 million was released to us on August 26, 2007, and the remaining $7.5 million, plus interest of $0.5 million, was released to us on February 26, 2008.
The net cash received also includes reimbursement of $47.8 million for co-promote termination payments which had previously been paid to Organon, $0.9 million of interest we paid King on a loan that was repaid in January 2007 and $0.5 million of severance expense for AVINZA sales representatives not offered positions with King. A summary of the final net cash proceeds, exclusive of $6.6 million in transaction costs and adjusted to reflect the final results of the retail inventory study, is as follows (in thousands):
| Purchase price |
$ | 265,000 | ||
| Reimbursement of Organon payments |
47,750 | |||
| Repayment of interest on King loan |
883 | |||
| Reimbursement of sales representative severance costs |
453 | |||
| 314,086 | ||||
| Less retail pharmacy inventory adjustment |
(11,225 | ) | ||
| Less cost of goods manufacturing adjustment |
(6,000 | ) | ||
| Net cash proceeds |
$ | 296,861 | ||
King also assumed our co-promote termination obligation to make payments to Organon based on net sales of AVINZA ($40.8 million and $58.5 million as of December 31, 2009 and 2008, respectively). As Organon has not consented to the legal assignment of the co-promote termination obligation from us to King, we remain liable to Organon in the event of King’s default of this obligation. In 2007, we recorded a pre-tax gain on the sale of $310.1 million, a $7.5 million pre-tax gain resulting from the release of funds from the escrow account and a $0.6 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. These gains were partially offset by a $3.0 million adjustment to investment banking fees. In 2008, we recognized an $8.1 million pre-tax gain resulting from the release of funds from the escrow account and a $1.5 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. In 2009, we recognized a $5.4 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date.
Also on September 6, 2006, we entered into a contract sales force agreement, or sales call agreement, with King, pursuant to which King agreed to conduct a sales detailing program to promote the sale of AVINZA for an agreed upon fee, subject to the terms and conditions of the sales call agreement. Pursuant to the Sales Call Agreement, King agreed to perform certain minimum monthly product details (i.e. sales calls), which commenced effective October 1, 2006 and continued until the Closing Date. Co-promotion expense recognized under the sales call agreement for 2007 was $2.8 million.
Prior to the AVINZA sale, we recorded accruals for rebates, chargebacks, and other discounts related to AVINZA products when product sales were recognized as revenue under the sell-through method. Upon the AVINZA sale, we accrued for rebates, chargebacks, and other discounts related to AVINZA products in the distribution channel which had not sold-through at the time of the AVINZA sale and for which we retained the liability subsequent to the sale. These products expired at various dates through June 30, 2009. Our accruals for AVINZA rebates, chargebacks, and other discounts total $6,000 and $0.1 million as of December 31, 2009 and 2008, respectively.
Additionally, and pursuant to the terms of the AVINZA purchase agreement, we retained the liability for returns of product from the distribution channel that had been sold by us prior to the close of the transaction.
42
Table of Contents
Accordingly, as part of the accounting for the gain on the sale of AVINZA, we recorded a reserve for AVINZA product returns. Under the sell-through revenue recognition method, we previously did not record a reserve for returns. AVINZA products sold by us may be returned through a specified period subsequent to the product expiration date, but no later than December 31, 2009. Our reserve for AVINZA returns was $18,000 and $8.2 million as of December 31, 2009 and 2008, respectively.
Income Taxes
For the year ended December 31, 2009, we recorded no income tax provision or benefit on discontinued operations.
For the year ended December 31, 2008, we recorded an income tax benefit on discontinued operations of $0.4 million, which related to state tax refunds for taxes paid in 2007.
For the year ended December 31, 2007, we recorded income tax expense on discontinued operations of $22.8 million, which related to the income and gain on sale of our AVINZA product line.
Summary of Results from Discontinued Operations
There were no activities related to discontinued operations in 2009 and 2008. Income from discontinued operations before income taxes was $6.0 million in 2007.
The following table summarizes the 2007 results from discontinued operations included in the 2007 consolidated statement of operations (in thousands):
| AVINZA Product Line | |||
| Product sales |
$ | 18,256 | |
| Operating costs and expenses: |
|||
| Cost of products sold |
3,608 | ||
| Research and development |
120 | ||
| Selling, general and administrative |
3,709 | ||
| Co-promotion |
2,814 | ||
| Co-promote termination charges |
2,012 | ||
| Total operating costs and expenses |
12,263 | ||
| Income before income taxes |
$ | 5,993 | |
Co-promotion expense of $2.8 million in 2007 represents fees paid to King for contract sales expenses incurred under the sales call agreement prior to the closing of the Transaction on February 26, 2007.
In 2007, we recognized $2.0 million of co-promote termination expense which represents the accretion of the termination liability to fair value as of February 26, 2007 and the closing of the AVINZA Product Line sale Transaction.
Liquidity and Capital Resources
We have financed our operations through offerings of our equity securities, issuance of convertible notes, product sales and the subsequent sales of our commercial assets, royalties, collaborative research and development and other revenues, capital and operating lease transactions, accounts receivable factoring and equipment financing arrangements and investment income.
43
Table of Contents
Working capital was $16.0 million at December 31, 2009 compared to $23.3 million at December 31, 2008. Available cash, cash equivalents and short-term investments totaled $53.2 million as of December 31, 2009 compared to $80.7 million as of December 31, 2008. We primarily invest our cash in certificates of deposit and United States government and investment grade corporate debt securities.
In April 2009, we received notification from the SEC that it had completed its investigation and will not recommend enforcement action against us relating to the previously disclosed SEC investigation in connection with the restatement of our financial statements as of and for the years ended December 31, 2002 and 2003 and for the first three quarters of 2004. As a result, in April 2009, we received $10.3 million from a restricted indemnity account which had been established in a trust account with Dorsey & Whitney LLP, counsel to our independent directors and to the Audit Committee of our Board of Directors, to support our indemnification obligations to continuing and departing directors in connection with the SEC investigation and related matters.
In August 2009, we entered into a lease termination agreement for our corporate facility in San Diego. Under the terms of the agreement, we will pay a termination fee of $14.3 million as follows: $4.5 million was paid upon signing, $4.5 million in July 2010 and $5.3 million in April 2011. In addition, we entered into a new lease for a period of 27 months commencing October 2009, for premises consisting of office and lab space located in San Diego to serve as our new corporate headquarters.
In December 2009, the Internal Revenue Service, or IRS, issued to us a Notice of Proposed Adjustment, or NOPA, seeking an increase to our taxable income for the 2007 fiscal year of $71.5 million and a $4.1 million penalty for substantial underpayment of tax in fiscal 2007. We responded to the NOPA in February 2010, disagreeing with the conclusions reached by the IRS in the NOPA. We recorded a FIN 48 liability of $25.1 million related to the income tax effect of the NOPA and $3.0 million related to estimated interest due on the proposed underpayment of tax. We also recorded deferred income tax assets of $25.1 million associated with the ability to carry back losses from 2008 and 2009 to offset the NOPA. In addition, we recorded an income tax receivable of $4.5 million associated with changes in income tax law in relation to prior AMT taxes paid on carry back periods. We have not recorded the penalties proposed by the IRS in our financial statements as we believe that we met the appropriate standard for the tax position on our 2007 tax return. If we are unsuccessful in our negotiations with the IRS, we may be required to pay the $4.1 million penalty and utilize a significant amount of our net operating loss carryforwards.
Our future operating and capital requirements will depend on many factors, including, but not limited to: the pace of scientific progress in our research and development programs; the magnitude of these programs; the scope and results of preclinical testing and clinical trials; the time and costs involved in obtaining regulatory approvals; the costs involved in preparing, filing, prosecuting, maintaining and enforcing patent claims; competing technological and market developments; the amount of royalties on sales of AVINZA and PROMACTA; the efforts of our collaborative partners; obligations under our operating lease agreements and lease termination agreement; and the capital requirements of any companies we may acquire, including Neurogen and Metabasis.
Operating Activities
Operating activities used cash of $33.8 million, $20.6 million, and $97.7 million in 2009, 2008 and 2007, respectively. The use of cash in 2009 reflects a net loss of $1.9 million, adjusted by $7.9 million of gain from discontinued operations and $4.0 million of non-cash items to reconcile the net loss to net cash used in operations. These reconciling items primarily reflect the accretion of deferred gain on the sale leaseback of the building of $21.9 million, non-cash development milestone revenue of $0.9 million and gain on investments of $0.2 million, partially offset by non-cash lease costs of $9.8 million, a write-off of acquired in-process research and development of $0.4 million, non-cash exit and restructuring costs of $0.3 million, the recognition of $3.4 million of stock-based compensation expense, depreciation of assets of $3.1 million, impairment and amortization of acquired intangible assets of $1.5 million, and the write-off of assets of $0.5 million. The use of cash in 2009 is further impacted by changes in operating assets and liabilities due primarily to decreases in
44
Table of Contents
accounts payable and accrued liabilities of $11.0 million, a decrease in deferred revenue of $14.3 million, a decrease in other liabilities of $2.3 million and an increase in accounts receivable, net of $0.6 million. These increases were partially offset by decreases in other current assets of $1.1 million and the release of the restricted indemnity account of $10.3 million. Net cash used in operating activities of discontinued operations was $3.2 million in 2009.
The use of cash in 2008 reflects a net loss of $98.1 million, adjusted by $0.7 million of loss from discontinued operations and $82.7 million of non-cash items to reconcile the net loss to net cash used in operations. These reconciling items primarily reflect the write-off of acquired in-process research and development of $72.0 million, non-cash exit and restructuring costs of $5.3 million, the recognition of $3.6 million of stock-based compensation expense, depreciation of assets of $1.1 million, realized loss on investment of $2.0 million, and the write-off of assets of $0.7 million, partially offset by the accretion of deferred gain on the sale leaseback of the building of $2.0 million. The use of cash in 2008 is further impacted by changes in operating assets and liabilities due primarily to decreases in accounts payable and accrued liabilities of $7.3 million partially offset by decreases in other current assets of $4.9 million and an increase in other liabilities of $1.3 million. Net cash used in operating activities of discontinued operations was $4.6 million in 2008.
The use of cash in 2007 reflects net income of $281.7 million, adjusted by $316.4 million of gain from discontinued operations and $11.0 million of non-cash items to reconcile net income to net cash used in operations. These reconciling items primarily reflect deferred gain on the sale leaseback of the building of $2.0 million, the recognition of $7.6 million of stock-based compensation expense, depreciation and amortization of assets of $2.6 million, a realized loss on investment of $1.3 million, and the write-off of assets of $1.0 million. The use of cash in 2007 is further impacted by changes in operating assets and liabilities due primarily to decreases in accounts payable and accrued liabilities of $54.5 million and to deferred revenue of $8.7 million and an increase in the restricted indemnity account of $10.1 million, partially offset by decreases in accounts receivable, net of $11.5 million, other current assets of $1.4 million, and inventories, net of $0.9 million. The increase in the restricted indemnity account in 2007 was primarily due to the funding of $10.0 million to support our indemnification obligations to continuing and departing directors in connection with the SEC investigation and related matters. Net cash used in operating activities from discontinued operations was $15.6 million in 2007.
Investing Activities
Investing activities provided cash of $24.8 million in 2009, used cash of $24.4 million in 2008 and provided cash of $343.8 million 2007. Cash provided by investing activities in 2009 primarily reflects the net sales of short-term investments of $15.0 million and $9.8 million of net cash acquired from our merger with Neurogen. None of the cash provided by investing activities for 2009 related to discontinued operations.
Cash used in investing activities in 2008 primarily reflects the net purchases of short-term investments of $36.4 million partially offset by $4.1 million of net cash acquired from our merger with Pharmacopeia. Net cash provided by investing activities of discontinued operations was $8.1 million in 2008.
Cash provided by investing activities in 2007 primarily reflects the net purchases of short-term investments of $5.4 million partially offset by the decrease in restricted cash and investments of $1.5 million. Net cash provided by investing activities of discontinued operations was $347.9 million in 2007.
Financing Activities
Financing activities used cash of $3.7 million, $3.0 million and $327.7 million in 2009, 2008 and 2007, respectively. Cash used in financing activities in 2009 primarily reflects payments under equipment financing obligations of $0.5 million and the repayment of debt of $3.4 million related to an equipment line of credit acquired from Pharmacopeia that was paid off in January 2009, partially offset by proceeds from the issuance of common stock of $0.2 million. None of the cash used in financing activities for 2009 related to discontinued operations.
45
Table of Contents
Cash used in financing activities in 2008 primarily reflects repurchase of our common stock of $1.6 million and payments under equipment financing obligations of $1.5 million. None of the cash used in financing activities for 2008 related to discontinued operations.
Cash used in financing activities in 2007 primarily reflects a $252.7 million cash dividend payment, $39.6 million in repurchases of our common stock and payments under equipment financing obligations of $2.2 million. These amounts are partially offset by proceeds from the issuance of common stock, related primarily to the exercise of employee stock options, of $4.4 million. Net cash used in financing activities of discontinued operations was $37.8 million in 2007.
Other
As part of our alliances with GSK, Pfizer (formerly Wyeth), Cephalon and Merck (formerly Schering-Plough) and our discovery collaboration agreement with BMS, we have received up-front cash payments and licenses to certain product candidates. In connection with these agreements, we are obligated to perform significant research and development activities over multiple years and as such, expect to incur significant costs performing such activities. The following table provides the period over which these research and development activities are to be provided, as well as the deferred revenue currently recorded for each agreement as of December 31, 2009:
| Collaborative Agreement |
Expiration of Initial Research Term |
Deferred Revenue | |||
| Merck (formerly Schering-Plough) Agreement |
December 2009 | $ | 438 | ||
| BMS Discovery Collaboration Agreement |
December 2009 | 264 | |||
| Pfizer (formerly Wyeth) Agreement |
December 2010 | 893 | |||
| Trevena Agreement |
January 2011 | 605 | |||
| GSK Agreement |
March 2011 | 3,738 | |||
On March 22, 2007, we announced a return of cash on our common stock in the form of a $2.50 per share special cash dividend. The aggregate amount of $252.7 million was paid on April 19, 2007 to shareholders of record as of April 5, 2007. In addition to the cash dividend, the Board of Directors authorized up to $100.0 million in share repurchases over the subsequent 12 months. In 2007, we repurchased 6.2 million shares of our common stock totaling $39.6 million. Subsequent to December 31, 2007 and through February 28, 2008, we repurchased an additional 0.3 million shares of our common stock totaling $1.6 million. We currently have no plans of issuing any dividends or repurchasing additional shares of our common stock in the near future.
Certain of our property and equipment is pledged as collateral under various equipment financing arrangements. As of December 31, 2009, $0.1 million was outstanding under such arrangements and classified as current. During January 2009, we paid off the remaining $3.4 million of financing obligations acquired through our acquisition of Pharmacopeia.
On July 19, 2007, we purchased $5.0 million of commercial paper issued by Golden Key Ltd. While the investment was highly-rated and within our investment policy at the time of purchase, during the third quarter of 2007, large credit rating agencies downgraded the quality of this security. In addition, as a result of not meeting certain liquidity covenants, the assets were assigned to a trustee who established a committee of the largest senior credit holders to determine the next steps. Subsequently, Golden Key defaulted on its obligation to settle the security on the stated maturity date of October 10, 2007. Based on available information, we estimate that we will be able to recover approximately $1.9 million on this security. As a result of changes in the estimated fair value, we adjusted the carrying value by recording an unrealized gain of $0.2 million in 2009 and an impairment loss of $2.0 million in 2008. Further, liquidity in the capital markets has continued to be volatile. Accordingly, we may be exposed to additional impairment for this investment until it is fully recovered.
46
Table of Contents
In connection with the acquisition of Pharmacopeia on December 23, 2008, Pharmacopeia security holders received a contingent value right that entitles them to an aggregate cash payment of $15.0 million under certain circumstances.
In connection with the acquisition of Neurogen Corporation on December 23, 2009, Neurogen security holders received CVRs under four CVR agreements. The CVRs entitle Neurogen shareholders to cash payments upon the sale or licensing of certain assets and upon the achievement of a specified clinical milestone.
Leases and Off-Balance Sheet Arrangements
We lease our office and research facilities under operating lease arrangements with varying terms through November 2021. The agreements provide for increases in annual rents based on changes in the Consumer Price Index or fixed percentage increases ranging from 3% to 7%. Commencing January 2008, we also sublease a portion of our facilities through July 2015. The sublease agreement provides for a 3% increase in annual rents. We had no off-balance sheet arrangements at December 31, 2009 and 2008.
Contractual Obligations
As of December 31, 2009, future minimum payments due under our contractual obligations are as follows (in thousands):
| Payments Due by Period | |||||||||||||||
| Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years | |||||||||||
| Equipment financing obligations (1) |
$ | 93 | $ | 93 | $ | — | $ | — | $ | — | |||||
| Operating lease obligations (2) |
32,200 | 5,936 | 10,860 | 9,847 | 5,557 | ||||||||||
| Consulting / License Agreements |
343 | 343 | — | — | — | ||||||||||
| Co-promote termination liability (3) |
— | — | — | — | — | ||||||||||
| Total contractual obligations |
$ | 32,636 | $ | 6,372 | $ | 10,860 | $ | 9,847 | $ | 5,557 | |||||
|
(1) Includes interest payments as follows: |
$ | 3 | $ | 3 | $ | — | $ | — | $ | — | |||||
| (2) | We lease an office and research facility under an operating lease arrangement through July 2015. Commencing January 2008, we sublet this facility through July 2015. The sublease agreement provides for a 3% increase in annual rents. As of December 31, 2009, we expect to receive aggregate future minimum lease payments totaling $4.9 million (nondiscounted) over the duration of the sublease agreement as follows and not included in the table above: less than one year, $0.8 million; one to three years, $1.7 million; three to five years, $1.8 million; and more than five years, $0.5 million. |
| (3) | Our co-promote termination obligation to Organon was assumed by King pursuant to the AVINZA purchase agreement. However, as Organon did not consent to the legal assignment of the obligation to King, we remain liable to Organon in the event of King’s default of the obligation. As of December 31, 2009, the total estimated amount of the obligation is $67.1 million on an undiscounted basis. We do not expect to make any cash payments related to this obligation. |
| (4) | Excludes contingent payments to selling security holders of Pharmacopeia and Neurogen. |
As of December 31, 2009, we have net open purchase orders (defined as total open purchase orders at year end less any accruals or invoices charged to or amounts paid against such purchase orders) totaling approximately $4.7 million. We plan to spend approximately $0.5 million on capital expenditures in 2010.
47
Table of Contents
Critical Accounting Policies
Certain of our policies require the application of management judgment in making estimates and assumptions that affect the amounts reported in the consolidated financial statements and disclosures made in the accompanying notes. Those estimates and assumptions are based on historical experience and various other factors deemed to be applicable and reasonable under the circumstances. The use of judgment in determining such estimates and assumptions is by nature, subject to a degree of uncertainty. Accordingly, actual results could differ materially from the estimates made. Our critical accounting policies are as follows:
Revenue Recognition
Royalties on sales of AVINZA and PROMACTA are recognized in the quarter reported by the respective partner. PROMACTA royalties are recorded net of amounts due to other parties.
Revenue from research funding under our collaboration agreements is earned and recognized on a percentage of completion basis as research hours are incurred in accordance with the provisions of each agreement.
Nonrefundable, up-front license fees and milestone payments with standalone value that are not dependent on any future performance by us under our collaboration agreements are recognized as revenue upon the earlier of when payments are received or collection is assured, but are deferred if we have continuing performance obligations. Amounts received under multiple-element arrangements requiring ongoing services or performance by us are recognized over the period of such services or performance.
Revenue from milestones is recognized when earned, as evidenced by written acknowledgement from the collaborator, provided that (i) the milestone event is substantive, its achievability was not reasonably assured at the inception of the agreement, and we have no further performance obligations relating to that event, and (ii) collectibility is reasonably assured. If these criteria are not met, the milestone payment is recognized over the remaining period of our performance obligations under the arrangement.
Co-Promote Termination Accounting
As part of the termination and return of co-promotion rights agreement that we entered into with Organon in January 2006, we agreed to make quarterly payments to Organon, effective for the fourth quarter of 2006, equal to 6.5% of AVINZA net sales through December 31, 2012 and thereafter 6% through patent expiration, currently anticipated to be November 2017. The estimated fair value of the amounts to be paid to Organon after the termination ($95.2 million as of January 2006), based on the future estimated net sales of the product, was recognized as a liability and expensed as a cost of the termination as of the effective date of the agreement, January 2006.
In connection with the AVINZA sale transaction, King assumed our obligation to make payments to Organon based on net sales of AVINZA (the fair value of which approximated $40.8 million as of December 31, 2009). As Organon has not consented to the legal assignment of the co-promote termination obligation from us to King, we remain liable to Organon in the event of King’s default of this obligation. Therefore, we recorded an asset on February 26, 2007 to recognize King’s assumption of the obligation, while continuing to carry the co-promote termination liability in our consolidated financial statements to recognize our legal obligation as primary obligor to Organon. This asset represents a non-interest bearing receivable for future payments to be made by King and is recorded at its fair value. As of December 31, 2009 and thereafter, the receivable and liability will remain equal and adjusted each quarter for changes in the fair value of the obligation. On a quarterly basis, management reviews the carrying value and assesses the co-promote termination receivable for impairment (e.g. in the event King defaults on the assumed obligation to pay Organon). Annually management also reviews the carrying value of the co-promote termination liability. Due to assumptions and judgments inherent in
48
Table of Contents
determining the estimates of future net AVINZA sales through November 2017, the actual amount of net AVINZA sales used to determine the amount of the asset and liability for a particular period may be materially different from current estimates. Any resulting changes to the co-promote termination liability will have a corresponding impact on the co-promote termination payments receivable. As of December 31, 2009 and 2008, the fair value of the co-promote termination liability (and the corresponding receivable) was determined using a discount rate of 15%.
Impairment of Long-Lived Assets
We review long-lived assets for impairment annually or whenever events or circumstances indicate that the carrying amount of the assets may not be recoverable. We measure the recoverability of assets to be held and used by comparing the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the assets exceeds the fair value of the assets. Fair value of our long-lived assets is determined using the expected cash flows discounted at a rate commensurate with the risk involved. As of December 31, 2009, we believe that the future undiscounted cash flows to be received from our long-lived assets will exceed the assets’ carrying value.
Income Taxes
Income taxes are accounted for under the liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of differences between the tax basis of assets or liabilities and their carrying amounts in the consolidated financial statements. A valuation allowance is provided for deferred tax assets if it is more likely than not that these items will either expire before we are able to realize their benefit or if future deductibility is uncertain. In December 2009, the IRS issued to us a NOPA seeking an increase to our taxable income for the 2007 fiscal year of $71.5 million and a $4.1 million penalty for substantial underpayment of tax in fiscal 2007. We responded to the NOPA in February 2010, disagreeing with the conclusions reached by the IRS in the NOPA. We recorded a FIN 48 liability of $25.1 million related to the income tax effect of the NOPA and $3.0 million related to estimated interest due on the proposed underpayment of tax. We also recorded deferred income tax assets of $25.1 million associated with the ability to carry back losses from 2008 and 2009 to offset the NOPA. In addition, we recorded an income tax receivable of $4.5 million associated with changes in income tax law in relation to prior AMT taxes paid on carry back periods. We have not recorded the penalties proposed by the IRS in our financial statements as we believe that we met the appropriate standard for the tax position on our 2007 tax return. If we are unsuccessful in our negotiations with the IRS, we may be required to pay the $4.1 million penalty and utilize a significant amount of our net operating loss carryforwards. As of December 31, 2008, we have provided a full valuation allowance against the deferred tax asset as recoverability was uncertain. Developing the provision for income taxes requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and, if necessary, any valuation allowances that may be required for deferred tax assets. Our judgments and tax strategies are subject to audit by various taxing authorities. While we believe we have provided adequately for our income tax liabilities in our consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on our consolidated financial condition and results of operations.
Stock-Based Compensation
Stock-based compensation cost for awards to employees and non-employee directors is recognized on a straight-line basis over the vesting period until the last tranche vests. Compensation cost for consultant awards is recognized over each separate tranche’s vesting period. We recognized compensation expense of $3.4 million, $3.6 million and $7.6 million for 2009, 2008 and 2007, respectively, associated with option awards, restricted stock and an equitable adjustment of employee stock options. Of the total compensation expense associated with the option awards for 2007, $1.8 million related to the $2.50 equitable adjustment of the exercise price for all options outstanding as of April 3, 2007 that was measured for financial reporting purposes effective March 28, 2007, the date our Compensation Committee of our Board of Directors approved the adjustment.
49
Table of Contents
The fair-value for options that were awarded to employees and directors was estimated at the date of grant using the Black-Scholes option valuation model with the following weighted average assumptions:
| Years Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Risk-free interest rate |
2.1 | % | 3.0 | % | 4.9 | % | |||
| Dividend yield |
— | — | — | ||||||
| Expected volatility |
74 | % | 65 | % | 66 | % | |||
| Expected term |
6 years | 6 years | 6 years | ||||||
The expected term of the employee and non-employee director options is the estimated weighted-average period until exercise or cancellation of vested options (forfeited unvested options are not considered) based on historical experience. The expected term for consultant awards is the remaining period to contractual expiration.
Volatility is a measure of the expected amount of variability in the stock price over the expected life of an option expressed as a standard deviation. In selecting this assumption, we used the historical volatility of our stock price over a period equal to the expected term. Changes in the assumptions used to estimate the fair value of stock-based compensation would impact the amount of compensation expenses recognized during the period.
New Accounting Pronouncements
The FASB established the FASB Accounting Standards Codification™, or Codification, as the source of authoritative U.S. generally accepted accounting principles, or U.S. GAAP, recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements issued for interim and annual periods ending after September 15, 2009. The Codification has changed the manner in which U.S. GAAP guidance is referenced, but did not have an impact on our consolidated financial position, results of operations or cash flows.
In October 2009, the FASB issued ASU No. 2009-13, “Multiple-Deliverable Revenue Arrangements,” or ASU 2009-13, which amends existing revenue recognition accounting pronouncements that are currently within the scope of ASC 605. This guidance eliminates the requirement to establish the fair value of undelivered products and services and instead provides for separate revenue recognition based upon management’s estimate of the selling price for an undelivered item when there is no other means to determine the fair value of that undelivered item. ASU 2009-13 is effective for us prospectively for revenue arrangements entered into or materially modified beginning January 1, 2011. We are currently evaluating the impact, if any, that the adoption of this amendment will have on our consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
At December 31, 2009, our investment portfolio included fixed-income securities of $38.5 million. These securities are subject to interest rate risk and will decline in value if interest rates increase. However, due to the short duration of our investment portfolio, an immediate 10% change in interest rates would have no material impact on our financial condition, results of operations or cash flows. At December 31, 2009, we also have certain equipment financing arrangements with variable rates of interest. Due to the relative insignificance of such arrangements, however, an immediate 10% change in interest rates would have no material impact on our financial condition, results of operations, or cash flows. Declines in interest rates over time will, however, reduce our interest income, while increases in interest rates over time will increase our interest expense.
We do not have a significant level of transactions denominated in currencies other than U.S. dollars and as a result we have very limited foreign currency exchange rate risk. The effect of an immediate 10% change in foreign exchange rates would have no material impact on our financial condition, results of operations or cash flows.
50
Table of Contents
| Item 8. | Consolidated Financial Statements and Supplementary Data |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
51
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Ligand Pharmaceuticals Incorporated
We have audited the accompanying consolidated balance sheets of Ligand Pharmaceuticals Incorporated and subsidiaries (the Company) as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ equity (deficit) and comprehensive income (loss) and cash flows for the years then ended. Our audits of the basic consolidated financial statements included the financial statement schedule listed in the index appearing under Item 15(4)(d). These consolidated financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Ligand Pharmaceuticals Incorporated and subsidiaries as of December 31, 2009 and 2008, and the results of their operations and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note 2, the Company adopted new accounting guidance on January 1, 2009 related to the accounting for business combinations.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Ligand Pharmaceuticals Incorporated and subsidiaries’ internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 3, 2010 expressed an unqualified opinion.
/s/ Grant Thornton LLP
San Diego, California
March 3, 2010
52
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Ligand Pharmaceuticals Incorporated
San Diego, California
We have audited the accompanying consolidated statement of operations, statement of stockholders’ equity (deficit) and comprehensive income (loss), and statement of cash flows of Ligand Pharmaceuticals Incorporated and subsidiaries (the “Company”) for the year ended December 31, 2007. We have also audited Schedule II—Valuation and Qualifying Accounts for the year ended December 31, 2007. These consolidated financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and schedule based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements and schedule are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement and schedule presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated results of operations and cash flows of Ligand Pharmaceuticals Incorporated and subsidiaries for the year ended December 31, 2007, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, Schedule II—Valuation and Qualifying Accounts presents fairly, in all material respects, the information set forth therein for the year ended December 31, 2007.
/s/ BDO Seidman, LLP
San Diego, California
February 28, 2008
53
Table of Contents
LIGAND PHARMACEUTICALS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS (in thousands, except share data)
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 16,032 | $ | 28,753 | ||||
| Short-term investments |
37,200 | 51,918 | ||||||
| Accounts receivable, net |
618 | — | ||||||
| Assets held for sale |
3,170 | — | ||||||
| Other current assets |
1,364 | 2,300 | ||||||
| Current portion of co-promote termination payments receivable |
9,782 | 10,958 | ||||||
| Total current assets |
68,166 | 93,929 | ||||||
| Restricted cash and investments |
1,462 | 1,341 | ||||||
| Property and equipment, net |
8,522 | 12,903 | ||||||
| Goodwill and other identifiable intangible assets |
2,515 | 5,375 | ||||||
| Long-term portion of co-promote termination payments receivable |
30,993 | 47,524 | ||||||
| Restricted indemnity account |
— | 10,232 | ||||||
| Deferred income taxes |
25,068 | — | ||||||
| Other assets |
5,081 | 144 | ||||||
| Total assets |
$ | 141,807 | $ | 171,448 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 16,945 | $ | 14,627 | ||||
| Accrued liabilities |
9,375 | 12,665 | ||||||
| Payable to Neurogen stockholders |
3,770 | — | ||||||
| Allowances for loss on returns, rebates and chargebacks related to discontinued operations |
31 | 9,590 | ||||||
| Accrued litigation settlement costs |
1,000 | 8,680 | ||||||
| Current portion of deferred gain |
1,702 | 1,964 | ||||||
| Current portion of co-promote termination liability |
9,782 | 10,958 | ||||||
| Current portion of lease termination payments |
4,487 | — | ||||||
| Current portion of equipment financing obligations |
91 | 1,829 | ||||||
| Current portion of deferred revenue |
4,989 | 10,301 | ||||||
| Total current liabilities |
52,172 | 70,614 | ||||||
| Long-term portion of co-promote termination liability |
30,993 | 47,524 | ||||||
| Long-term portion of equipment financing obligations |
— | 2,178 | ||||||
| Long-term portion of deferred revenue, net |
3,495 | 16,819 | ||||||
| Long-term portion of deferred gain |
1,702 | 23,292 | ||||||
| Long-term portion of lease termination payments |
5,281 | — | ||||||
| Income tax payable |
28,108 | — | ||||||
| Other long-term liabilities |
7,968 | 9,041 | ||||||
| Total liabilities |
129,719 | 169,468 | ||||||
| Commitments and contingencies |
||||||||
| Common stock subject to conditional redemption; 674, 230 and 997,568 shares issued and outstanding at December 31, 2009 and 2008, respectively |
8,344 | 12,345 | ||||||
| Stockholders’ equity (deficit): |
||||||||
| Convertible preferred stock, $0.001 par value; 5,000,000 shares authorized; none issued |
— | — | ||||||
| Common stock, $0.001 par value; 200,000,000 shares authorized; 123,269,008 and 118,562,748 shares issued at December 31, 2009 and 2008, respectively |
123 | 119 | ||||||
| Additional paid-in capital |
726,816 | 711,195 | ||||||
| Accumulated other comprehensive income |
513 | 81 | ||||||
| Accumulated deficit |
(681,574 | ) | (679,626 | ) | ||||
| Treasury stock, at cost; 6,607,905 shares at December 31, 2009 and 2008 |
(42,134 | ) | (42,134 | ) | ||||
| Total stockholders’ equity (deficit) |
3,744 | (10,365 | ) | |||||
| $ | 141,807 | $ | 171,448 | |||||
See accompanying notes to these consolidated financial statements.
54
Table of Contents
LIGAND PHARMACEUTICALS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share data)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Revenues: |
||||||||||||
| Royalties |
$ | 8,334 | $ | 20,315 | $ | 11,409 | ||||||
| Collaborative research and development and other revenues |
30,606 | 7,000 | 1,485 | |||||||||
| Total revenues |
38,940 | 27,315 | 12,894 | |||||||||
| Operating costs and expenses: |
||||||||||||
| Research and development |
39,870 | 30,770 | 44,623 | |||||||||
| General and administrative |
15,211 | 23,785 | 30,410 | |||||||||
| Lease termination costs |
15,235 | — | — | |||||||||
| Write-off of acquired in-process research and development |
442 | 72,000 | — | |||||||||
| Total operating costs and expenses |
70,758 | 126,555 | 75,033 | |||||||||
| Accretion of deferred gain on sale leaseback |
21,851 | 1,964 | 1,964 | |||||||||
| Loss from operations |
(9,967 | ) | (97,276 | ) | (60,175 | ) | ||||||
| Other income (expense): |
||||||||||||
| Interest income |
586 | 2,161 | 8,655 | |||||||||
| Interest expense |
(270 | ) | (202 | ) | (735 | ) | ||||||
| Other, net |
(221 | ) | (2,198 | ) | (1,201 | ) | ||||||
| Total other income (expense), net |
95 | (239 | ) | 6,719 | ||||||||
| Loss from continuing operations before income tax benefit |
(9,872 | ) | (97,515 | ) | (53,456 | ) | ||||||
| Income tax benefit from continuing operations |
1,535 | 55 | 18,697 | |||||||||
| Loss from continuing operations |
(8,337 | ) | (97,460 | ) | (34,759 | ) | ||||||
| Discontinued operations: |
||||||||||||
| Income (loss) from discontinued operations before income taxes |
— | — | 5,993 | |||||||||
| Gain on sale of AVINZA Product Line before income taxes |
5,434 | 9,584 | 315,184 | |||||||||
| Gain (loss) on sale of Oncology Product Line before income taxes |
955 | (10,630 | ) | 18,037 | ||||||||
| Income tax benefit (expense) on discontinued operations |
— | 392 | (22,767 | ) | ||||||||
| Income (loss) from discontinued operations |
6,389 | (654 | ) | 316,447 | ||||||||
| Net income (loss) |
$ | (1,948 | ) | $ | (98,114 | ) | $ | 281,688 | ||||
| Basic and diluted per share amounts: |
||||||||||||
| Loss from continuing operations |
$ | (0.08 | ) | $ | (1.02 | ) | $ | (0.35 | ) | |||
| Income (loss) from discontinued operations |
0.06 | (0.01 | ) | 3.22 | ||||||||
| Net income (loss) |
$ | (0.02 | ) | $ | (1.03 | ) | $ | 2.87 | ||||
| Weighted average number of common shares |
113,176,511 | 95,505,421 | 98,124,731 | |||||||||
See accompanying notes to these consolidated financial statements.
55
Table of Contents
LIGAND PHARMACEUTICALS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT) AND COMPREHENSIVE INCOME (LOSS)
(in thousands, except share data)
| Common stock | Additional paid-in capital |
Accumulated other comprehensive income (loss) |
Accumulated deficit |
Treasury stock | Total stockholders’ equity (deficit) |
Comprehensive income (loss) |
||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||
| Balance at December 31, 2006 |
99,553,504 | $ | 100 | $ | 891,446 | $ | (481 | ) | $ | (862,802 | ) | (73,842 | ) | $ | (911 | ) | $ | 27,352 | ||||||||||||||
| Effect of adopting FIN 48 |
— | — | — | — | (398 | ) | — | — | (398 | ) | ||||||||||||||||||||||
| Balance at January 1, 2007 |
99,553,504 | 100 | 891,446 | (481 | ) | (863,200 | ) | (73,842 | ) | (911 | ) | 26,954 | ||||||||||||||||||||
| Issuance of common stock under employee stock compensation plans |
989,866 | 1 | 4,569 | — | — | — | — | 4,570 | ||||||||||||||||||||||||
| Repurchase of Company common stock |
— | — | — | — | — | (6,189,309 | ) | (39,610 | ) | (39,610 | ) | |||||||||||||||||||||
| Unrealized net gain on available-for-sale securities |
— | — | — | 14 | — | — | — | 14 | $ | 14 | ||||||||||||||||||||||
| Stock-based compensation |
— | — | 7,580 | — | — | — | — | 7,580 | ||||||||||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | 476 | — | — | — | 476 | 476 | |||||||||||||||||||||||
| Cash dividend paid, net |
— | — | (252,557 | ) | — | — | — | — | (252,557 | ) | ||||||||||||||||||||||
| Net income |
— | — | — | — | 281,688 | — | — | 281,688 | 281,688 | |||||||||||||||||||||||
| Balance at December 31, 2007 |
100,543,370 | 101 | 651,038 | 9 | (581,512 | ) | (6,263,151 | ) | (40,521 | ) | 29,115 | $ | 282,178 | |||||||||||||||||||
| Issuance of common stock under employee stock compensation plans |
22,339 | — | 130 | — | — | — | — | 130 | ||||||||||||||||||||||||
| Repurchase of Company common stock |
— | — | — | — | — | (344,754 | ) | (1,613 | ) | (1,613 | ) | |||||||||||||||||||||
| Unrealized net gain on available-for-sale securities |
— | — | — | 72 | — | — | — | 72 | $ | 72 | ||||||||||||||||||||||
| Stock-based compensation |
— | — | 3,607 | — | — | — | — | 3,607 | ||||||||||||||||||||||||
| Issuance of common stock for acquisition of Pharmacopeia. |
17,997,039 | 18 | 56,420 | — | — | — | — | 56,438 | ||||||||||||||||||||||||
| Net loss |
— | — | — | — | (98,114 | ) | — | — | (98,114 | ) | (98,114 | ) | ||||||||||||||||||||
| Balance at December 31, 2008 |
118,562,748 | 119 | 711,195 | 81 | (679,626 | ) | (6,607,905 | ) | (42,134 | ) | (10,365 | ) | $ | (98,042 | ) | |||||||||||||||||
| Issuance of common stock under employee stock compensation plans |
506,260 | — | 228 | — | — | — | — | 228 | ||||||||||||||||||||||||
| Unrealized net gain on available-for-sale securities |
— | — | — | 432 | — | — | — | 432 | $ | 432 | ||||||||||||||||||||||
| Stock-based compensation |
— | — | 3,365 | — | — | — | — | 3,365 | ||||||||||||||||||||||||
| Shares redeemed in lieu of cash payment for milestone achieved. |
— | — | 3,086 | — | — | — | — | 3,086 | ||||||||||||||||||||||||
| Issuance of common stock for acquisition of Neurogen. |
4,200,000 | 4 | 8,942 | — | — | — | — | 8,946 | ||||||||||||||||||||||||
| Net loss |
— | — | — | — | (1,948 | ) | — | — | (1,948 | ) | (1,948 | ) | ||||||||||||||||||||
| Balance at December 31, 2009 |
123,269,008 | $ | 123 | $ | 726,816 | $ | 513 | $ | (681,574 | ) | (6,607,905 | ) | $ | (42,134 | ) | $ | 3,744 | $ | (1,516 | ) | ||||||||||||
See accompanying notes to these consolidated financial statements.
56
Table of Contents
LIGAND PHARMACEUTICALS INCORPORATED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Operating activities |
||||||||||||
| Net income (loss) |
$ | (1,948 | ) | $ | (98,114 | ) | $ | 281,688 | ||||
| Less: gain (loss) from discontinued operations |
6,389 | (654 | ) | 316,447 | ||||||||
| Loss from continuing operations |
(8,337 | ) | (97,460 | ) | (34,759 | ) | ||||||
| Adjustments to reconcile net income (loss) to net cash used in operating activities, including effects of business acquired: |
||||||||||||
| Write-off of acquired in-process research and development |
442 | 72,000 | — | |||||||||
| Accretion of deferred gain on sale leaseback |
(21,852 | ) | (1,964 | ) | (1,964 | ) | ||||||
| Impairment and amortization of acquired intangible assets |
1,500 | — | 909 | |||||||||
| Depreciation and amortization of property and equipment |
3,134 | 1,052 | 1,706 | |||||||||
| Non-cash lease termination costs |
9,768 | — | — | |||||||||
| Non-cash development milestone revenue |
(915 | ) | — | — | ||||||||
| Loss on asset write-offs |
500 | 746 | 1,029 | |||||||||
| Realized loss (gain) on investment |
(232 | ) | 2,038 | 1,300 | ||||||||
| Stock-based compensation |
3,365 | 3,607 | 7,580 | |||||||||
| Non-cash exit and restructuring costs |
334 | 5,255 | — | |||||||||
| Other |
(17 | ) | (16 | ) | 487 | |||||||
| Changes in operating assets and liabilities, net of acquisition: |
||||||||||||
| Accounts receivable, net |
(618 | ) | — | 11,537 | ||||||||
| Other current assets |
(448 | ) | 4,942 | 2,334 | ||||||||
| Restricted indemnity account and other |
10,346 | (162 | ) | (10,070 | ) | |||||||
| Accounts payable and accrued liabilities |
(10,989 | ) | (7,338 | ) | (54,476 | ) | ||||||
| Other liabilities |
(2,318 | ) | 1,252 | 913 | ||||||||
| Deferred revenue |
(14,302 | ) | — | (8,657 | ) | |||||||
| Net cash used in operating activities of continuing operations |
(30,639 | ) | (16,048 | ) | (82,131 | ) | ||||||
| Net cash used in operating activities of discontinued operations |
(3,162 | ) | (4,577 | ) | (15,596 | ) | ||||||
| Net cash used in operating activities |
(33,801 | ) | (20,625 | ) | (97,727 | ) | ||||||
| Investing activities |
||||||||||||
| Cash acquired from acquisition of Pharmacopeia |
— | 4,135 | — | |||||||||
| Cash acquired from acquisition of Neurogen |
9,796 | — | — | |||||||||
| Purchases of property and equipment |
(522 | ) | (495 | ) | (440 | ) | ||||||
| Proceeds from sale of property and equipment and building |
108 | 92 | 322 | |||||||||
| Purchases of short-term investments |
(32,806 | ) | (68,370 | ) | (25,565 | ) | ||||||
| Proceeds from sale of short-term investments |
47,761 | 32,015 | 20,116 | |||||||||
| Decrease (increase) in restricted cash and investments |
— | 70 | 1,479 | |||||||||
| Other, net |
431 | 71 | 36 | |||||||||
| Net cash provide by (used in) investing activities of continuing operations |
24,768 | (32,482 | ) | (4,052 | ) | |||||||
| Net cash provided by investing activities of discontinued operations |
— | 8,058 | 347,889 | |||||||||
| Net cash provided by (used in) investing activities |
24,768 | (24,424 | ) | 343,837 | ||||||||
| Financing activities |
||||||||||||
| Principal payments on equipment financing obligations |
(473 | ) | (1,527 | ) | (2,169 | ) | ||||||
| Repayment of debt |
(3,443 | ) | — | — | ||||||||
| Net proceeds from issuance of common stock |
228 | 130 | 4,387 | |||||||||
| Dividend paid |
— | — | (252,742 | ) | ||||||||
| Dividend received on treasury stock held by company |
— | — | 185 | |||||||||
| Repurchase of common stock |
— | (1,613 | ) | (39,610 | ) | |||||||
| Net cash used in financing activities of continuing operations |
(3,688 | ) | (3,010 | ) | (289,949 | ) | ||||||
| Net cash provided by (used in) financing activities of discontinued operations |
— | — | (37,750 | ) | ||||||||
| Net cash provided by (used in) financing activities |
(3,688 | ) | (3,010 | ) | (327,699 | ) | ||||||
| Net increase (decrease) in cash and cash equivalents |
(12,721 | ) | (48,059 | ) | (81,589 | ) | ||||||
| Cash and cash equivalents at beginning of year |
28,753 | 76,812 | 158,401 | |||||||||
| Cash and cash equivalents at end of year |
$ | 16,032 | $ | 28,753 | $ | 76,812 | ||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Interest paid |
$ | 270 | $ | 229 | $ | 1,511 | ||||||
| Taxes paid |
14 | 140 | 8,371 | |||||||||
| Supplemental schedule of non-cash investing and financing activities |
||||||||||||
| Conversion of 6% convertible subordinated notes into common stock: |
||||||||||||
| Employee stock option exercises |
— | — | 228 | |||||||||
| Issuance of common stock for acquisition |
8,946 | 56,438 | — | |||||||||
See accompanying notes to these consolidated financial statements.
57
Table of Contents
LIGAND PHARMACEUTICALS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. The Company and Its Business
Ligand Pharmaceuticals Incorporated, a Delaware corporation (the “Company” or “Ligand”), is a biotechnology company that focuses on drug discovery and early-stage development of pharmaceuticals that address critical unmet medical needs or that are more effective and/or safer than existing therapies, more convenient to administer and are cost effective. The consolidated financial statements include the Company’s wholly owned subsidiaries, Ligand Pharmaceuticals International, Inc., Ligand Pharmaceuticals (Canada) Incorporated, Seragen, Inc. (“Seragen”), Nexus Equity VI LLC (“Nexus”), Pharmacopeia LLC (“Pharmacopeia”) and Neurogen Corporation (“Neurogen”). The Company’s principle market is the United States. As further discussed in Note 3, the Company acquired Neurogen on December 23, 2009. As further discussed in Note 5, the Company sold its Oncology Product Line (“Oncology”) and AVINZA Product Line (“AVINZA”) on October 25, 2006 and February 26, 2007, respectively. The operating results for Oncology and AVINZA have been presented in the accompanying consolidated financial statements as “Discontinued Operations”.
The Company’s other potential products are in various stages of development. Potential products that are promising at early stages of development may not reach the market for a number of reasons. Prior to generating revenues from these products, the Company or its collaborative partners must complete the development of the products in the human health care market. No assurance can be given that: (1) product development efforts will be successful, (2) required regulatory approvals for any indication will be obtained, (3) any products, if introduced, will be capable of being produced in commercial quantities at reasonable costs or, (4) patient and physician acceptance of these products will be achieved. The Company faces risks common to companies whose products are in various stages of development. These risks include, among others, the Company’s need for additional financing to complete its research and development programs and commercialize its technologies. The Company has incurred significant losses since its inception. At December 31, 2009, the Company’s accumulated deficit was $681.6 million. Management expects that the Company will continue to incur substantial research and development expenses and may incur additional losses in the future.
2. Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. Intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities, including disclosure of contingent assets and contingent liabilities, at the date of the consolidated financial statements and the reported amounts of revenues and expenses, in-process research and development, goodwill, deferred revenue and income tax net operating losses during the reporting period. The Company’s critical accounting policies are those that are both most important to the Company’s consolidated financial condition and results of operations and require the most difficult, subjective or complex judgments on the part of management in their application, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Because of the uncertainty of factors surrounding the estimates or judgments used in the preparation of the consolidated financial statements, actual results may materially vary from these estimates.
Cash, Cash Equivalents and Short-term Investments
Cash and cash equivalents consist of cash and highly liquid securities with maturities at the date of acquisition of three months or less. Non-restricted equity and debt security investments with a maturity of more
58
Table of Contents
than three months are considered short-term investments and have been classified by management as available-for-sale. Such investments are carried at fair value, with unrealized gains and losses included as a separate component of stockholders’ equity. The Company determines the cost of investments based on the specific identification method.
Restricted Cash and Investments
Restricted cash and investments consist of certificates of deposit held with a financial institution as collateral under equipment financing and third-party service provider arrangements. The certificates of deposit have been classified by management as held-to-maturity and are accounted for at amortized cost.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash equivalents and investments.
The Company invests its excess cash principally in United States government debt securities, investment grade corporate debt securities and certificates of deposit. The Company has established guidelines relative to diversification and maturities that maintain safety and liquidity. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates. Except as described in Note 6, the Company has not experienced any significant losses on its cash equivalents, short-term investments or restricted investments.
As of December 31, 2009, cash deposits held at financial institutions in excess of FDIC insured amounts of $250,000 were approximately $4.8 million.
Property and Equipment
Property and equipment is stated at cost and consists of the following (in thousands):
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Lab and office equipment |
$ | 24,646 | $ | 27,265 | ||||
| Leasehold improvements |
11,728 | 16,168 | ||||||
| Computer equipment and software |
6,562 | 10,753 | ||||||
| 42,936 | 54,186 | |||||||
| Less accumulated depreciation and amortization |
(34,414 | ) | (41,283 | ) | ||||
| $ | 8,522 | $ | 12,903 | |||||
Depreciation of equipment is computed using the straight-line method over the estimated useful lives of the assets which range from three to ten years. Leasehold improvements are amortized using the straight-line method over their estimated useful lives or their related lease term, whichever is shorter.
Assets Held for Sale
As discussed in Note 3, the Company acquired Neurogen Corporation on December 23, 2009. Neurogen had entered into an agreement with a commercial real estate developer to sell its properties for a gross selling price of $3.5 million. These properties are held for sale on the accompanying consolidated balance sheet at carrying value of $3.2 million net of estimated costs to sell. The sale was completed on February 2, 2010. Net proceeds from the sale will be distributed to Neurogen’s stockholders through a Contingent Value Right (“CVR”) agreement.
59
Table of Contents
Goodwill and Other Identifiable Intangible Assets
Goodwill and other identifiable intangible assets consist of the following (in thousands):
| December 31, | ||||||
| 2009 | 2008 | |||||
| Collaborative research and development with Schering-Plough |
$ | — | $ | 2,000 | ||
| Acquired in-process research and development |
1,815 | — | ||||
| Goodwill |
700 | 3,375 | ||||
| $ | 2,515 | $ | 5,375 | |||
The collaborative research and development with Schering-Plough was being amortized on a straight-line basis over a period of three years. As discussed in Note 14, in July 2009, the Company and N.V. Organon, which was acquired by Schering-Plough in November 2007, mutually agreed to terminate the research collaboration under their collaboration and license agreement. Schering-Plough continued to fund research collaboration activities on those targets currently under investigation through December 2009. As a result of the termination, the Company recorded an impairment charge of $1.1 million and adjusted its remaining useful life to four months. During the year ended December 31, 2009, the Company recorded $0.9 million of amortization expense.
Additionally, during the quarter ended March 31, 2009, the Company adjusted its preliminary purchase price allocation for Pharmacopeia, Inc., which resulted in an increase in transaction costs of $0.3 million and decreases in property and equipment of $1.1 million, liabilities assumed of $4.4 million and goodwill of $3.0 million. During the quarter ended June 30, 2009, the Company further adjusted its purchase price allocation for Pharmacopeia, Inc., which resulted in an increase in the write-off of acquired in-process research and development of $0.4 million and decreases in property and equipment of $0.1 million, acquired intangible assets of $17,000 and goodwill of $0.3 million.
Acquired in-process research and development
Intangible assets related to in-process research and development costs, or IPR&D, are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts. During the period the assets are considered indefinite-lived, they will not be amortized but will be tested for impairment on an annual basis and between annual tests if the Company becomes aware of any events occurring or changes in circumstances that would indicate a reduction in the fair value of the IPR&D projects below their respective carrying amounts. If and when development is complete, which generally occurs if and when regulatory approval to market a product is obtained, the associated assets would be deemed finite-lived and would then be amortized based on their respective estimated useful lives at that point in time.
For acquisitions prior to January 1, 2009, the estimated fair value of IPR&D projects, which had not reached technological feasibility at the date of acquisition and which did not have an alternative future use, were immediately expensed. In 2008, the Company wrote off $72.0 million of acquired IPR&D related to the acquisition of Pharmacopeia, Inc. As a result of subsequent adjustments to the purchase price allocation related to the acquisition of Pharmacopeia, Inc., the Company wrote-off an additional $0.4 million of acquired in-process research and development in 2009.
Impairment of Long-Lived Assets
Management reviews long-lived assets for impairment annually or whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the assets exceeds the fair value of the
60
Table of Contents
assets. Fair value for the Company’s long-lived assets is determined using the expected cash flows discounted at a rate commensurate with the risk involved. As of December 31, 2009, management believes that the future undiscounted cash flows to be received from its long-lived assets will exceed the assets’ carrying value.
Fair Value of Financial Instruments
Fair value is defined as the exit price that would be received to sell an asset or paid to transfer a liability. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. The statement establishes a three-level hierarchy to prioritize the inputs used in measuring fair value. The levels are described in the table below with Level 1 having the highest priority and Level 3 having the lowest.
The following table provides a summary of the assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2009:
| Fair Value Measurements at Reporting Date Using | ||||||||||||
| Total | Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs | |||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||
| Assets: |
||||||||||||
| Fixed income available-for-sale securities |
$ | 37,200 | $ | 35,305 | $ | 1,895 | $ | — | ||||
| Liabilities: |
||||||||||||
| Warrant liability |
$ | 459 | $ | — | $ | — | $ | 459 | ||||
The following table provides a summary of the assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2008:
| Fair Value Measurements at Reporting Date Using | ||||||||||||
| Total | Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs | |||||||||
| (Level 1) | (Level 2) | (Level 3) | ||||||||||
| Assets: |
||||||||||||
| Fixed income available-for-sale securities |
$ | 51,918 | $ | 50,255 | $ | 1,663 | $ | — | ||||
| Liabilities: |
||||||||||||
| Warrant liability |
$ | 670 | $ | — | $ | — | $ | 670 | ||||
The Company’s short-term investments are fixed income available-for-sale securities and include U.S. Government Notes and Corporate Discount Commercial Paper. The fair value of the Company’s short-term investments is determined using quoted market prices in active markets. The fair value of the warrant liability is determined using the Black-Scholes option-pricing model, which uses certain significant unobservable inputs, including stock price (quoted market prices in active market), warrant exercise price (defined in warrant agreement), expected life of warrant (defined in warrant agreement), dividend yields (determined by the Company), and risk-free interest rate (quoted market prices based on expected life assumption).
Revenue Recognition
Royalties on sales of AVINZA and PROMACTA are recognized in the quarter reported by the respective partner.
61
Table of Contents
Revenue from research funding under the Company’s collaboration agreements is earned and recognized on a percentage of completion basis as research hours are incurred in accordance with the provisions of each agreement.
Nonrefundable, up-front license fees and milestone payments with standalone value that are not dependent on any future performance by the Company under the Company’s collaboration agreements are recognized as revenue upon the earlier of when payments are received or collection is assured, but are deferred if the Company has continuing performance obligations. Amounts received under multiple-element arrangements requiring ongoing services or performance by the Company are recognized over the period of such services or performance.
Revenue from milestones is recognized when earned, as evidenced by written acknowledgement from the collaborator, provided that (i) the milestone event is substantive, its achievability was not reasonably assured at the inception of the agreement, and the Company has no further performance obligations relating to that event, and (ii) collectibility is reasonably assured. If these criteria are not met, the milestone payment is recognized over the remaining period of the Company’s performance obligations under the arrangement.
The composition of collaborative research and development and other revenues is as follows (in thousands):
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Collaborative research and development |
$ | 23,316 | $ | — | $ | — | |||
| License fees |
525 | 5,000 | — | ||||||
| Development milestones and other |
6,765 | 2,000 | 1,485 | ||||||
| $ | 30,606 | $ | 7,000 | $ | 1,485 | ||||
Preclinical Study and Clinical Trial Accruals
Substantial portions of the Company’s preclinical studies and all of the Company’s clinical trials have been performed by third-party laboratories, contract research organizations, or other vendors (collectively CROs). Some CROs bill monthly for services performed, while others bill based upon milestone achievement. The Company accrues for each of the significant agreements it has with CROs on a monthly basis. For preclinical studies, accruals are estimated based upon the percentage of work completed and the contract milestones achieved. For clinical studies, accruals are estimated based upon a percentage of work completed, the number of patients enrolled and the duration of the study. The Company monitors patient enrollment, the progress of clinical studies and related activities to the extent possible through internal reviews of data reported to it by the CROs, correspondence with the CROs and clinical site visits. The Company’s estimates are dependent upon the timelines and accuracy of the data provided by its CROs regarding the status of each program and total program spending. The Company periodically evaluates its estimates to determine if adjustments are necessary or appropriate based on information it receives concerning changing circumstances, and conditions or events that may affect such estimates. No material adjustments to preclinical study and clinical trial accrued expenses have been recognized to date.
Warrant Liability
To qualify as permanent equity, an equity derivative, including warrants, must permit the Company to settle in unregistered shares. Under securities law, if the warrants were issued in connection with a public offering and have a cash settlement feature at the holder’s option, the Company does not have the ability to settle in unregistered shares. Therefore, the warrants cannot be classified as permanent equity and are instead classified as a liability. The warrants that the Company issued as part of its equity financing in October 2006 meet this criterion, and their fair value has been recorded as a liability in the accompanying consolidated balance sheets. Other warrants the Company had previously issued qualify as permanent equity and do not require remeasurement.
62
Table of Contents
The Company records its warrant liabilities at fair value using a Black-Scholes option-pricing model and remeasures at each reporting date until the warrants are exercised or have expired. Changes in the fair value of the warrants are reported in the statements of operations as income or expense. The fair value of the warrants is subject to significant fluctuation based on changes in the Company’s stock price, expected volatility, expected life, the risk-free interest rate and dividend yield. The market price for the Company’s common stock has been and may continue to be volatile. Consequently, future fluctuations in the price of the Company’s common stock may cause significant increases or decreases in the fair value of the warrants.
Assets and Liabilities Related to Discontinued Operations
Medicaid Rebates
The Company’s products related to the commercial operations that were sold were subject to state government-managed Medicaid programs whereby discounts and rebates are provided to participating state governments. The Company is still obligated to pay for these rebates for products in the distribution channel that were not sold-through at the time of the sale of the Company’s commercial operations. Medicaid rebates are accounted for by establishing an accrual in an amount equal to the Company’s estimate of Medicaid rebate claims attributable to sales recognized in that period. The estimate of the Medicaid rebates accrual is determined primarily based on historical experience regarding Medicaid rebates, as well as current and historical prescription activity provided by external sources, current contract prices and any expected contract changes. Management additionally considers any legal interpretations of the applicable laws related to Medicaid and qualifying federal and state government programs and any new information regarding changes in the Medicaid programs’ regulations and guidelines that would impact the amount of the rebates. Management adjusts the accrual periodically throughout each period to reflect actual experience, expected changes in future prescription volumes and any changes in business circumstances or trends.
Government Chargebacks
The Company’s products related to the commercial operations that were sold were subject to certain programs with federal government entities and other parties whereby pricing on products is extended below wholesaler list price to participating entities. The Company is still obligated to pay for these chargebacks for products in the distribution channel that were not sold-through at the time of the sale of the Company’s commercial operations. These entities purchase products through wholesalers at the lower vendor price, and the wholesalers charge the difference between their acquisition cost and the lower vendor price back to the Company. Chargebacks are accounted for by establishing an accrual in an amount equal to the estimate of chargeback claims. Management determines estimates of the chargebacks primarily based on historical experience regarding chargebacks and current contract prices under the vendor programs. Management considers vendor payments and claim processing time lags and adjusts the accrual periodically throughout each period to reflect actual experience and any changes in business circumstances or trends.
Managed Health Care Rebates and Other Contract Discounts
The Company previously offered rebates and discounts on certain products related to the commercial operations that were sold to managed health care organizations and to other contract counterparties such as hospitals and group purchasing organizations in the U.S. The Company is still obligated to pay for these rebates and discounts for products in the distribution channel that were not sold-through at the time of the sale of the Company’s commercial operations. Managed health care rebates and other contract discounts are accounted for by establishing an accrual in an amount equal to the estimate of managed health care rebates and other contract discounts. Estimates of the managed health care rebates and other contract discounts accruals are determined primarily based on historical experience regarding these rebates and discounts and current contract prices. Management also considers the current and historical prescription activity provided by external sources, current contract prices and any expected contract changes and adjusts the accrual periodically throughout each period to reflect actual experience and any changes in business circumstances or trends.
63
Table of Contents
Product Returns
In connection with the sale of the Company’s product lines, the Company retained the obligation for returns of product that were shipped to wholesalers prior to the close of the transactions. The accruals for product returns, which were recorded as part of the accounting for the sales transactions, are based on historical experience. Any subsequent changes to the Company’s estimate of product returns are accounted for as a component of discontinued operations.
Costs and Expenses
Collaborative research and development expense consists of the labor, material, equipment and allocated facilities cost of the Company’s scientific staff who are working pursuant to the Company’s collaborative agreements. From time to time, collaborative research and development expense includes costs related to research efforts in excess of those required under certain collaborative agreements. Management has the discretion to set the scope of such excess efforts and may increase or decrease the level of such efforts depending on the Company’s strategic priorities.
Proprietary research and development expense consists of intellectual property in-licensing costs, labor, materials, contracted services, and allocated facility costs that are incurred in connection with internally funded drug discovery and development programs.
Research and development costs are expensed as incurred. Research and development expenses from continuing operations were $39.9 million, $30.8 million and $44.6 million in 2009, 2008, and 2007, respectively, of which 47%, 100% and 100%, respectively, were sponsored by Ligand, and the remainder of which was funded pursuant to collaborative research and development arrangements.
Income Taxes
The Company recognizes liabilities or assets for the deferred tax consequences of temporary differences between the tax bases of assets or liabilities and their reported amounts in the financial statements. These temporary differences will result in taxable or deductible amounts in future years when the reported amounts of the assets or liabilities are recovered or settled. A valuation allowance is established when management determines that it is more likely than not that all or a portion of a deferred tax asset will not be realized. Management evaluates the realizability of its net deferred tax assets on a quarterly basis and valuation allowances are provided, as necessary. During this evaluation, management reviews its forecasts of income in conjunction with other positive and negative evidence surrounding the realizability of its deferred tax assets to determine if a valuation allowance is required. Adjustments to the valuation allowance will increase or decrease the Company’s income tax provision or benefit. Management also applies the relevant guidance to determine the amount of income tax expense or benefit to be allocated among continuing operations, discontinued operations, and items charged or credited directly to stockholders’ equity (deficit).
A tax position must meet a minimum probability threshold before a financial statement benefit is recognized. The minimum threshold is a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The Company recognizes interest and penalties related to uncertain tax positions in income tax expense.
Income (Loss) Per Share
Net income (loss) per share is computed using the weighted average number of common shares outstanding. Basic and diluted income (loss) per share amounts are equivalent for the periods presented as the inclusion of potential common shares in the number of shares used for the diluted computation would be anti-dilutive to loss per share from continuing operations. No potential common shares are included in the computation of any diluted per share amounts, including income (loss) per share from discontinued operations, as the Company reported a net loss
64
Table of Contents
from continuing operations for all periods presented. Potential common shares, the shares that would be issued upon the conversion of convertible notes, the exercise of outstanding warrants and stock options, and the vesting of restricted shares, were 6.3 million, 4.5 million and 2.2 million at December 31, 2009, 2008, and 2007, respectively.
Accounting for Stock-Based Compensation
The Company has employee compensation plans under which various types of stock-based instruments are granted. Share-based payments to employees, including grants of employee stock options, are recognized in the Consolidated Statements of Operations as compensation expense (based on their estimated fair values) generally over the vesting period of the awards using the straight-line method. Compensation expense for consultant awards is recognized over each separate tranche’s vesting period.
Comprehensive Income (Loss)
Comprehensive income (loss) represents net income (loss) adjusted for the change during the periods presented in unrealized gains and losses on available-for-sale securities less reclassification adjustments for realized gains or losses included in net income (loss), as well as foreign currency translation adjustments for the 2007 period. The accumulated unrealized gains or losses and cumulative foreign currency translation adjustments are reported as accumulated other comprehensive income (loss) as a separate component of stockholders’ equity.
Segment Reporting
The Company currently operates in a single operating segment. The Company generates revenue from various sources that result primarily from its underlying research and development activities. In addition, financial results are prepared and reviewed by management as a single operating segment. Management continually evaluates the benefits of operating in distinct segments and will report accordingly when such distinction is made.
New Accounting Pronouncements
ASC 820-10, “Fair Value Measurements and Disclosures” (formerly SFAS No. 157, Fair Value Measurements), with respect to non-financial assets and liabilities was adopted effective January 1, 2009. This standard defines fair value, establishes a framework for measuring fair value and expands disclosures about fair value measurements. This standard did not have an impact on the consolidated financial statements.
ASC 805 (formerly SFAS No. 141(R), Business Combinations), for business combinations was adopted on January 1, 2009. This standard requires that assets acquired, liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date, be measured at fair value as of that date. In a business combination achieved in stages, all identifiable assets and liabilities, including non-controlling interest in the acquiree, are required to be recognized at the full amount of their fair value. It also requires the fair value of IPRD to be recognized as an indefinite-lived intangible asset, contingent consideration to be recognized on the acquisition date, and restructuring and acquisition-related deal costs to be expensed as incurred. In addition, any excess of the fair value of net assets acquired over purchase price and any subsequent changes in estimated contingencies are to be recognized in earnings.
ASC 808-10, “Collaborative Arrangements” (formerly EITF Issue No. 07-1, Accounting for Collaborative Arrangements Related to the Development and Commercialization of Intellectual Property), was adopted on January 1, 2009. This standard defines a collaborative arrangement as one in which the participants are actively involved and are exposed to significant risks and rewards that depend on the ultimate commercial success of the endeavor. Revenues and costs incurred with third parties in connection with collaborative arrangements are presented gross or net based on the criteria in ASC 605-45-45 Overall Considerations of Reporting Revenue Gross as a Principal vs. Net as an Agent (formerly EITF Issue No. 99-19, Reporting Revenue Gross as a
65
Table of Contents
Principal vs. Net as an Agent) and other accounting literature. Payments to or from collaborators are evaluated and presented based on the nature of the arrangement and its terms, the nature of the entity’s business, and whether those payments are within the scope of other accounting literature. The nature and purpose of collaborative arrangements are disclosed along with the accounting policies and the classification of significant financial statement amounts related to the arrangements. Activities in arrangements conducted in a separate legal entity are accounted for under other accounting literature; however, required disclosure applies to the entire collaborative agreement. This standard did not have an impact on the consolidated financial statements.
In October 2009, the FASB issued ASU No. 2009-13, “Multiple-Deliverable Revenue Arrangements,” or ASU 2009-13, which amends existing revenue recognition accounting pronouncements that are currently within the scope of ASC 605. This guidance eliminates the requirement to establish the fair value of undelivered products and services and instead provides for separate revenue recognition based upon management’s estimate of the selling price for an undelivered item when there is no other means to determine the fair value of that undelivered item. ASU 2009-13 is effective for the Company prospectively for revenue arrangements entered into or materially modified beginning January 1, 2011. The Company is currently evaluating the impact, if any, that the adoption of this amendment will have on its consolidated financial statements.
3. Acquisition of Neurogen
On December 23, 2009, the Company completed its acquisition of Neurogen Corporation. Pursuant to the terms of the merger agreement, the Company acquired all of the issued and outstanding shares of Neurogen and in exchange the Company issued to Neurogen stockholders 4.2 million shares of the Company’s common stock and $0.6 million in cash. In connection with the merger, Neurogen’s stockholders will also receive contingent value rights that entitle them to cash and/or shares of third-party stock under certain circumstances. The results of operations of Neurogen have been included in the consolidated financial statements since December 23, 2009 and were not material.
The components of the preliminary purchase price allocation for Neurogen are as follows (in thousands):
| Purchase Consideration: |
|||
| Fair value of common stock issued to Neurogen shareholders |
$ | 8,946 | |
| Cash paid to Neurogen shareholders |
600 | ||
| Fair value of contingent value rights |
3,870 | ||
| Total purchase consideration |
$ | 13,416 | |
| Allocation of Purchase Price: |
||||
| Cash acquired |
$ | 9,796 | ||
| Other current assets |
3,321 | |||
| In-process research and development |
1,815 | |||
| Goodwill |
700 | |||
| Other assets |
324 | |||
| Liabilities assumed |
(2,540 | ) | ||
| $ | 13,416 | |||
There were no acquired identified intangible assets with definite lives from the acquisition with Neurogen.
The Company has allocated $1.8 million of the purchase price of Neurogen to acquired In-Process Research and Development (IPR&D). This amount represents the estimated fair value of various acquired in-process projects that have not yet reached technological feasibility and do not have future alternative use as of the date of the merger. The amount is related to internal and partnered product candidates targeting a variety of indications and currently in the preclinical stage of development. Of the total amount, $1.2 million relates to Neurogen’s fully funded partnership with Merck for Vanilloid Receptor Subtype 1 (VR1) Antagonists. The remaining $0.6 million relates to Neurogen’s internally developed clinical candidates for blockade of the histamine H3 receptor.
66
Table of Contents
Management used the “income method” to determine the estimated fair values of acquired IPR&D, which uses a discounted cash flow model and applies a probability weighting based on estimates of successful product development and commercialization to estimated future net cash flows resulting from projected revenues and related costs. These success rates take into account the stages of completion and the risks surrounding successful development and commercialization of the underlying product candidates. These cash flows were then discounted to present value using a discount rate of 45% for the VR1 program and 50% for the H3 program.
Neurogen had entered into an agreement with a commercial real estate developer to sell its properties for a gross selling price of $3.5 million. These properties are held for sale on the accompanying consolidated balance sheet at carrying value of $3.2 million net of estimated costs to sell. The sale was completed on February 2, 2010. Net proceeds from the sale will be distributed to Neurogen’s stockholders through a CVR.
Had the merger with Neurogen been completed as of the beginning of 2008, the Company’s pro forma results for 2009 and 2008 would have been as follows:
| (in thousands, except per share data) | 2009 | 2008 | ||||||
| Revenue |
$ | 41,590 | $ | 30,315 | ||||
| Operating loss |
(32,969 | ) | (149,040 | ) | ||||
| Net loss |
(24,556 | ) | (132,482 | ) | ||||
| Basic and diluted earnings per share: |
||||||||
| Continuing operations |
$ | (0.28 | ) | $ | (1.32 | ) | ||
| Discontinued operations |
$ | 0.07 | $ | (0.01 | ) | |||
| Net income (loss) |
$ | (0.21 | ) | $ | (1.33 | ) | ||
| Basic and diluted weighted average shares |
117,372 | 99,705 | ||||||
The primary adjustments relate to the loss of interest income due to the timing of transaction related payments. The above pro forma information was determined based on historical results adjusted for the purchase price allocation and estimated related changes in income associated with the merger of Neurogen.
4. Acquisition of Pharmacopeia
On December 23, 2008, the Company completed the acquisition of Pharmacopeia, Inc., a clinical development stage biopharmaceutical company dedicated to discovering and developing novel small molecule therapeutics to address significant medical needs, under which the Company acquired all outstanding shares of Pharmacopeia in a cash and stock transaction. The acquisition was accounted for as a business combination. In connection with the acquisition, the Company issued 17,997,039 shares of common stock to Pharmacopeia stockholders, or 0.5985 shares for each outstanding Pharmacopeia share, as well as $9.3 million in cash. The value of the common stock issued was derived from the number of Ligand common shares issued at a price of $3.14 per share determined by the average closing price of Ligand shares for the two days prior, the day of, and the two days subsequent to the public announcement on September 24, 2008. In addition, Pharmacopeia security holders received a contingent value right (CVR) that entitles each holder the right to receive a proportionate share of an aggregate of $15.0 million if Ligand enters into a license, sale, development, marketing or option agreement with respect to any product candidate from Pharmacopeia’s DARA program (other than any agreement with Bristol-Meyers Squibb or any of its affiliates) on or prior to December 31, 2011. The estimated fair value of the CVRs is not included in the total purchase price as the Company’s management has deemed, based on currently available information, that the likelihood of payment is not probable. The results of Pharmacopeia’s operations have been included in the consolidated financial statements commencing December 23, 2008.
67
Table of Contents
The components of the preliminary purchase price allocation for Pharmacopeia are as follows:
| Purchase Consideration: |
||||
| (in thousands) | ||||
| Fair value of common stock issued to Pharmacopeia shareholders |
$ | 56,439 | ||
| Cash paid to Pharmacopeia shareholders |
9,337 | |||
| Transaction costs |
4,344 | |||
| Total purchase consideration |
$ | 70,120 | ||
| Allocation of Purchase Price: |
||||
| (in thousands) | ||||
| Cash acquired |
$ | 17,754 | ||
| Other current assets |
1,390 | |||
| Property and equipment |
11,500 | |||
| Acquired intangible assets |
2,000 | |||
| In-process research and development |
72,000 | |||
| Goodwill and other identifiable intangible assets |
3,375 | |||
| Other assets |
144 | |||
| Liabilities assumed |
(38,043 | ) | ||
| $ | 70,120 | |||
The acquired identified intangible assets with definite lives from the acquisition with Pharmacopeia are as follows:
| Acquired Intangible Assets |
|||
| (in thousands) | |||
| Collaborative research and development with Schering-Plough |
$ | 2,000 | |
The weighted-average amortization period for the collaborative research and development with Schering Plough is 3 years.
The Company has allocated $72.0 million of the purchase price of Pharmacopeia to acquired In-Process Research and Development (IPR&D). This amount represents the estimated fair value of various acquired in-process projects that have not yet reached technological feasibility and do not have future alternative use as of the date of the merger. The amount is related to internal and partnered product candidates targeting a variety of indications and currently in various stages of development ranging from preclinical to Phase II. Of the total amount, $29.0 million relates to product candidates currently in the preclinical stage of development, $9.0 million relates to product candidates currently in Phase I clinical trials and $34.0 million relates to product candidates currently in Phase II clinical trials.
Management used the “income method” to determine the estimated fair values of acquired IPR&D, which uses a discounted cash flow model and applies a probability weighting based on estimates of successful product development and commercialization to estimated future net cash flows resulting from projected revenues and related costs. These success rates take into account the stages of completion and the risks surrounding successful development and commercialization of the underlying product candidates. These cash flows were then discounted to present value using a discount rate of 40% for product candidates in the preclinical stage, 35% for product candidates currently in Phase I clinical trials and 30% for product candidates currently in Phase II clinical trials.
As discussed in Note 14, in July 2009, the Company and N.V. Organon, which was acquired by Schering-Plough (now Merck) in November 2007, mutually agreed to terminate the research collaboration under their
68
Table of Contents
collaboration and license agreement. Merck continued to fund research collaboration activities on those targets currently under investigation through December 2009. As a result of the termination, the Company recorded an impairment charge of $1.1 million and adjusted its remaining useful life to four months. During the year ended December 31, 2009, the Company recorded $0.9 million of amortization expense. Additionally, during the quarter ended March 31, 2009, the Company adjusted its preliminary purchase price allocation for Pharmacopeia, Inc., which resulted in an increase in transaction costs of $0.3 million and decreases in property and equipment of $1.1 million, liabilities assumed of $4.4 million and goodwill of $3.0 million. During the quarter ended June 30, 2009, the Company further adjusted its purchase price allocation for Pharmacopeia, Inc., which resulted in an increase in the write-off of acquired in-process research and development of $0.4 million and decreases in property and equipment of $0.1 million, acquired intangible assets of $17,000 and goodwill of $0.3 million.
Had the merger with Pharmacopeia been completed as of the beginning of 2008, the Company’s pro forma results for 2008 would have been as follows:
| (in thousands, except per share data) | 2008 | |||
| Revenue |
$ | 51,351 | ||
| Operating loss |
(151,503 | ) | ||
| Net income (loss) |
(145,220 | ) | ||
| Basic and diluted earnings per share: |
||||
| Continuing operations |
$ | (1.27 | ) | |
| Discontinued operations |
$ | (0.01 | ) | |
| Net income (loss) |
$ | (1.28 | ) | |
| Basic and diluted weighted average shares |
113,060 | |||
The primary adjustments relate to the purchase accounting impact of the write-off of IPR&D and the amortization of the acquired collaborative research and development collaboration with Schering-Plough. The above pro forma information was determined based on historical results adjusted for the purchase price allocation and estimated related changes in income associated with the merger of Pharmacopeia.
5. Discontinued Operations
Oncology Product Line
On September 7, 2006, the Company, Eisai Inc., a Delaware corporation and Eisai Co., Ltd., a Japanese company (together with Eisai Inc., “Eisai”), entered into a purchase agreement (the “Oncology Purchase Agreement”) pursuant to which Eisai agreed to acquire all of the Company’s worldwide rights in and to the Company’s oncology products, including, among other things, all related inventory, equipment, records and intellectual property, and assume certain liabilities as set forth in the Oncology Purchase Agreement. The Oncology Product Line included the Company’s four marketed oncology drugs: ONTAK, Targretin capsules, Targretin gel and Panretin gel. Pursuant to the Oncology Purchase Agreement, at closing on October 25, 2006, Ligand received $185.0 million in net cash proceeds, net of $20.0 million that was funded into an escrow account to support any potential indemnification claims made by Eisai following the closing of the sale as further discussed below. In 2007, the Company recognized a $20.8 million pre-tax gain resulting from the release of funds from the escrow account partially offset by a $2.8 million pre-tax loss due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. In 2008, the Company recognized a $10.6 million pre-tax loss resulting from the Salk settlement for $13.0 million partially offset by a $2.4 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. In 2009, the Company recognized a $1.0 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date.
Additionally, $38.6 million of the proceeds received from Eisai were deposited into an escrow account to repay a loan received from King Pharmaceuticals, Inc. (“King”), the proceeds of which were used to pay the Company’s co-promote termination obligation to Organon in October 2006. The escrow amounts were released and the loan repaid to King in January 2007.
69
Table of Contents
In connection with the Oncology Purchase Agreement with Eisai, the Company entered into a transition services agreement whereby the Company agreed to perform certain transition services for Eisai, in order to effect, as rapidly as practicable, the transition of purchased assets from Ligand to Eisai. In exchange for these services, Eisai paid the Company a monthly service fee through June 25, 2007. Fees earned under the transition services agreement during 2007, which were recorded as an offset to operating expenses, were $2.7 million.
The Company agreed to indemnify Eisai, after the closing, for damages suffered by Eisai arising from any breach of any of the Company’s representations, warranties, covenants or obligations in the Oncology Purchase Agreement. The Company’s obligation to indemnify Eisai extends beyond the closing up to, in some cases, 18 months or 36 months and, in other cases, until the expiration of the applicable statute of limitations. In a few instances, the Company’s obligation to indemnify Eisai survives in perpetuity. The Company’s agreement with Eisai required that $20.0 million of the total upfront cash payment be deposited into an escrow account to secure the Company’s indemnification obligations to Eisai after the closing. Of the escrowed amount, $10.0 million was released to the Company on April 25, 2007, and the remaining $10.0 million, plus interest of $0.8 million, was released to the Company on October 25, 2007. The Company’s liability for any indemnification claim brought by Eisai is generally limited to $30.0 million. However, the Company’s obligation to provide indemnification on certain matters is not subject to these indemnification limits. For example, the Company agreed to retain, and provide indemnification without limitation to Eisai for, all liabilities related to certain claims regarding promotional materials for the ONTAK and Targretin drug products. Management cannot estimate the liabilities that may arise as a result of these matters and, therefore, no accrual has been recorded at December 31, 2009 and 2008.
Prior to the Oncology sale, the Company recorded accruals for rebates, chargebacks, and other discounts related to Oncology products when product sales were recognized as revenue under the sell-through method. Upon the Oncology sale, the Company accrued for rebates, chargebacks, and other discounts related to Oncology products in the distribution channel which had not sold-through at the time of the Oncology sale and for which the Company retained the liability subsequent to the sale. These products expired at various dates through July 31, 2008. The Company’s accruals for Oncology rebates, chargebacks, and other discounts total $7,000 and $0.4 million as of December 31, 2009 and 2008, respectively, and are included in accrued liabilities in the accompanying consolidated balance sheets.
Additionally, and pursuant to the terms of the Oncology Purchase Agreement, the Company retained the liability for returns of product from wholesalers that had been sold by the Company prior to the close of the transaction. Accordingly, as part of the accounting for the gain on the sale of the Oncology Product Line, the Company recorded a reserve for Oncology product returns. Under the sell-through revenue recognition method, the Company previously did not record a reserve for returns from wholesalers. Oncology products sold by the Company may be returned through a specified period subsequent to the product expiration date, but no later than July 31, 2009. The Company’s reserve for Oncology returns is zero and $0.9 million as of December 31, 2009 and 2008, respectively, and is included in accrued liabilities in the accompanying consolidated balance sheets.
AVINZA Product Line
On September 6, 2006, Ligand and King Pharmaceuticals, Inc. (King), entered into a purchase agreement (the “AVINZA Purchase Agreement”), pursuant to which King agreed to acquire all of the Company’s rights in and to AVINZA in the United States, its territories and Canada, including, among other things, all AVINZA inventory, records and related intellectual property, and assume certain liabilities as set forth in the AVINZA Purchase Agreement (collectively, the “Transaction”).
Pursuant to the AVINZA Purchase Agreement, at Closing on February 26, 2007 (the “Closing Date”), the Company received $280.4 million in net cash proceeds, which is net of $15.0 million that was funded into an escrow account to support any potential indemnification claims made by King following the Closing. The purchase price reflected a reduction of $12.7 million due to the preliminary estimate of retail inventory levels of AVINZA at the Closing Date exceeding targeted levels. After final studies and review by King, the final retail
70
Table of Contents
inventory-level adjustment was determined to be $11.2 million. The Company received the additional $1.5 million in proceeds in April 2007. The purchase price also reflects a reduction of $6.0 million for anticipated higher cost of goods for King related to the Catalent Pharma Solutions (formerly Cardinal Health PTS, LLC), or Catalent, manufacturing and packaging agreement. At the closing, Ligand agreed to not assign the Catalent agreement to King, wind down the contract, and remain responsible for any resulting liabilities. Subsequent to the closing, on April 30, 2007, the Company entered into a letter agreement with Catalent which terminated, without penalty to either party, the manufacturing and packaging agreement and certain related quality agreements with Catalent. In connection with the termination, the Company and Catalent agreed that certain provisions of the manufacturing and packaging agreement would survive and Catalent would continue to perform limited services. Catalent will also continue to manufacture LGD-4665 capsules for the Company under the terms of a separate agreement. The letter agreement with Catalent also contained a mutual general release of all claims arising from or related to the manufacturing and packaging agreement. The Company paid $0.3 million to a former Ligand executive in connection with the negotiation of the termination of the Catalent manufacturing and packaging agreement.
The net cash received also includes reimbursement of $47.8 million for co-promote termination payments which had previously been paid to Organon, $0.9 million of interest Ligand paid King on a loan that was repaid in January 2007 and $0.5 million of severance expense for AVINZA sales representatives not offered positions with King. A summary of the net cash proceeds received, exclusive of $6.6 million in transaction costs and adjusted to reflect the final results of the retail inventory study, is as follows (in thousands):
| Purchase price |
$ | 265,000 | ||
| Reimbursement of Organon payments |
47,750 | |||
| Repayment of interest on King loan |
883 | |||
| Reimbursement of sales representative severance costs |
453 | |||
| 314,086 | ||||
| Less retail pharmacy inventory adjustment |
(11,225 | ) | ||
| Less cost of goods manufacturing adjustment |
(6,000 | ) | ||
| Net cash proceeds |
$ | 296,861 | ||
King also assumed Ligand’s co-promote termination obligation to make payments to Organon based on net sales of AVINZA ($40.8 million and $58.5 million as of December 31, 2009 and 2008, respectively). As Organon has not consented to the legal assignment of the co-promote termination obligation from Ligand to King, Ligand remains liable to Organon in the event of King’s default of this obligation. In 2007, the Company recorded a pre-tax gain on the sale of $310.1 million, a $7.5 million pre-tax gain resulting from the release of funds from the escrow account and a $0.6 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. These gains were partially offset by a $3.0 million adjustment to investment banking fees. In 2008, the Company recognized an $8.1 million pre-tax gain resulting from the release of funds from the escrow account and a $1.5 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date. In 2009, the Company recognized a $5.4 million pre-tax gain due to subsequent changes in certain estimates of assets and liabilities recorded as of the sale date
In addition to the assumption of existing royalty obligations, King is required to pay Ligand a 15% royalty on AVINZA net sales during the first 20 months after Closing. Subsequent royalty payments will be based upon calendar year net sales. If calendar year net sales are less than $200.0 million, the royalty payment will be 5% of all net sales. If calendar year net sales are greater than $200.0 million, the royalty payment will be 10% of all net sales less than $250.0 million, plus 15% of net sales greater than $250.0 million. Royalty revenues were $7.7 million, $20.3 million and $11.4 million in 2009, 2008 and 2007, respectively.
In connection with the sale, the Company has agreed to indemnify King for a period of 16 months after the closing of the Transaction for a number of specified matters, including any breach of the Company’s
71
Table of Contents
representations, warranties or covenants contained in the asset purchase agreement. In certain defined cases, the Company’s obligation to indemnify King extends for a period of 30 months following the closing of the Transaction. Under the Company’s agreement with King, $15.0 million of the total upfront cash payment was deposited into an escrow account to secure the Company’s indemnification obligations to King following the closing. Of the escrowed amount, $7.5 million was released to the Company on August 26, 2007, and the remaining $7.5 million, plus interest of $0.5 million, was released to the Company on February 26, 2008.
Under certain circumstances, the Company’s liability to King under the indemnification obligations of the asset purchase agreement may be in excess of the amounts deposited in the escrow account. The AVINZA asset purchase agreement also allows King, under certain circumstances, to offset indemnification claims against the royalty payments payable to the Company. Under the asset purchase agreement, the Company’s liability for any indemnification claim brought by King is generally limited to $40.0 million. However, the Company’s obligation to provide indemnification on certain matters is not subject to this indemnification limit. For example, the Company agreed to retain, and provide indemnification without limitation to King for all liabilities arising under certain agreements with Catalent related to the manufacture of AVINZA. The Company cannot predict the liabilities that may arise as a result of these matters. Any liability claims related to these matters or any indemnification claims made by King could materially and adversely affect the Company’s financial condition. No accrual for potential losses under the indemnification has been recorded at December 31, 2009 and 2008.
In connection with the Transaction, King loaned the Company $37.8 million (the “Loan”) which was used to pay the Company’s co-promote termination obligation to Organon due October 15, 2006. This loan was drawn, and the $37.8 million co-promote liability settled in October 2006. Amounts due under the loan were subject to certain market terms, including a 9.5% interest rate. In addition, and as a condition of the loan, $38.6 million of the funds received from Eisai was deposited into a restricted account to be used to repay the loan to King, plus interest. The Company repaid the loan plus interest in January 2007. As noted above, King refunded the interest to the Company on the Closing Date.
Also on September 6, 2006, the Company entered into a contract sales force agreement (the “Sales Call Agreement”) with King, pursuant to which King agreed to conduct a sales detailing program to promote the sale of AVINZA for an agreed upon fee, subject to the terms and conditions of the Sales Call Agreement. Pursuant to the Sales Call Agreement, King agreed to perform certain minimum monthly product details (i.e. sales calls), which commenced effective October 1, 2006 and continued until the Closing Date. Co-promotion expense recognized under the Sales Call Agreement for 2007 was $2.8 million.
Prior to the AVINZA sale, the Company recorded accruals for rebates, chargebacks, and other discounts related to AVINZA products when product sales were recognized as revenue under the sell-through method. Upon the AVINZA sale, the Company accrued for rebates, chargebacks, and other discounts related to AVINZA products in the distribution channel which had not sold-through at the time of the AVINZA sale and for which the Company retained the liability subsequent to the sale. These products expire at various dates through June 30, 2009. The Company’s accruals for AVINZA rebates, chargebacks, and other discounts total $6,000 and $0.1 million as of December 31, 2009 and 2008, respectively, and are included in accrued liabilities in the accompanying consolidated balance sheet.
Additionally, and pursuant to the terms of the AVINZA Purchase Agreement, the Company retained the liability for returns of product from wholesalers that had been sold by the Company prior to the close of the transaction. Accordingly, as part of the accounting for the gain on the sale of AVINZA, the Company recorded a reserve for AVINZA product returns. AVINZA products sold by the Company may be returned through a specified period subsequent to the product expiration date, but no later than December 31, 2009. Under the sell-through revenue recognition method, the Company previously did not record a reserve for returns from wholesalers. The Company’s reserve for AVINZA returns is $18,000 and $8.2 million as of December 31, 2009 and 2008, respectively, and is included in accrued liabilities in the accompanying consolidated balance sheet.
72
Table of Contents
Results from Discontinued Operations
There was no activity related to discontinued operations for the years ended December 31, 2009 and 2008.
The following table summarizes the 2007 results from discontinued operations included in the 2007 consolidated statement of operations (in thousands):
| AVINZA Product Line | |||
| Product sales |
$ | 18,256 | |
| Operating costs and expenses: |
|||
| Cost of products sold |
3,608 | ||
| Research and development |
120 | ||
| Selling, general and administrative |
3,709 | ||
| Co-promotion |
2,814 | ||
| Co-promote termination charges |
2,012 | ||
| Total operating costs and expenses |
12,263 | ||
| Income before income taxes |
$ | 5,993 | |
6. Investments
As of December 31, 2009 and 2008, all of the Company’s investments have a contractual maturity of less than one year. The following table summarizes the various investment categories (in thousands):
| Cost | Gross unrealized gains |
Gross unrealized losses |
Estimated fair value | ||||||||||
| December 31, 2009 |
|||||||||||||
| U.S. government securities |
$ | 19,118 | $ | 51 | $ | (95 | ) | $ | 19,074 | ||||
| Certificates of deposit |
5,784 | 2 | (2 | ) | 5,784 | ||||||||
| Corporate obligations |
11,866 | 486 | (10 | ) | 12,342 | ||||||||
| 36,768 | 539 | (107 | ) | 37,200 | |||||||||
| Certificates of deposit—restricted |
1,341 | — | — | 1,341 | |||||||||
| Total debt securities |
$ | 38,109 | $ | 539 | $ | (107 | ) | $ | 38,541 | ||||
| December 31, 2008 |
|||||||||||||
| U.S. government securities |
$ | 50,174 | $ | 81 | $ | — | $ | 50,255 | |||||
| Corporate obligations |
1,663 | — | — | 1,663 | |||||||||
| 51,837 | 81 | — | 51,918 | ||||||||||
| Certificates of deposit—restricted |
1,341 | — | — | 1,341 | |||||||||
| Total debt securities |
$ | 53,178 | $ | 81 | $ | — | $ | 53,259 | |||||
On July 19, 2007, the Company purchased $5.0 million of commercial paper issued by Golden Key Ltd. While the investment was highly-rated and within the Company’s investment policy at the time of purchase, during the third quarter of 2007, large credit rating agencies downgraded the quality of this security. In addition, as a result of not meeting certain liquidity covenants, the assets were assigned to a trustee who established a committee of the largest senior credit holders to determine the next steps. Subsequently, Golden Key defaulted on its obligation to settle the security on the stated maturity date of October 10, 2007. Based on available information, management estimates that it will be able to recover approximately $1.9 million on this security. Accordingly, management adjusted the carrying value by recording an unrealized gain of $0.2 million in 2009
73
Table of Contents
and impairment losses of $2.0 million $1.3 million in 2008 and 2007, respectively. This impairment is included in other income (expense) in the consolidated statement of operations. Further, liquidity in the capital markets has continued to be volatile. Accordingly, the Company may be exposed to additional impairment for this investment until it is fully recovered. There were no other material realized gains or losses on sales of available-for-sale securities for the years ended December 31, 2009, 2008, and 2007.
7. Other Balance Sheet Details
Other current assets consist of the following (in thousands):
| December 31, | ||||||
| 2009 | 2008 | |||||
| Income taxes receivable |
$ | — | $ | 817 | ||
| Prepaid expenses |
848 | 1,147 | ||||
| Other receivables |
516 | 325 | ||||
| Other |
— | 11 | ||||
| $ | 1,364 | $ | 2,300 | |||
Accrued liabilities consist of the following (in thousands):
| December 31, | ||||||
| 2009 | 2008 | |||||
| Warrant liability |
$ | 459 | $ | 670 | ||
| Compensation |
2,808 | 2,686 | ||||
| Legal |
134 | 4,166 | ||||
| Restructuring costs |
61 | 848 | ||||
| Other |
5,913 | 4,295 | ||||
| $ | 9,375 | $ | 12,665 | |||
74
Table of Contents
The following summarizes the activity in the accounts related to allowances for loss on returns, rebates, chargebacks, and other discounts (in thousands):
| Charge- backs and Rebates |
Returns | Total | ||||||||||
| Balance at December 31, 2006 |
6,247 | 8,441 | 14,688 | |||||||||
| Provision |
3,929 | (1,243 | ) (3) | 2,686 | ||||||||
| AVINZA Transaction Provision (1) |
1,953 | 19,355 | 21,308 | |||||||||
| Oncology Transaction Provision (2) |
810 | 3,856 | 4,666 | |||||||||
| Payments |
(10,723 | ) | — | (10,723 | ) | |||||||
| Charges |
— | (15,350 | ) | (15,350 | ) | |||||||
| Balance at December 31, 2007 |
2,216 | 15,059 | 17,275 | |||||||||
| AVINZA Transaction Provision (1) |
(857 | ) | (211 | ) | (1,068 | ) | ||||||
| Oncology Transaction Provision (2) |
(49 | ) | (2,856 | ) | (2,905 | ) | ||||||
| Payments |
(802 | ) | — | (802 | ) | |||||||
| Charges |
— | (2,910 | ) | (2,910 | ) | |||||||
| Balance at December 31, 2008 |
$ | 508 | $ | 9,082 | $ | 9,590 | ||||||
| AVINZA Transaction Provision (1) |
(28 | ) | (5,463 | ) | (5,491 | ) | ||||||
| Oncology Transaction Provision (2) |
(234 | ) | (784 | ) | (1,018 | ) | ||||||
| Payments |
(232 | ) | — | (232 | ) | |||||||
| Charges |
— | (2,818 | ) | (2,818 | ) | |||||||
| Balance at December 31, 2009 |
$ | 14 | $ | 17 | $ | 31 | ||||||
| (1) | The AVINZA transaction provision amounts represent additional accruals recorded in connection with the sale of the AVINZA Product Line to King Pharmaceuticals, Inc. on February 26, 2007. The Company maintains the obligation for returns of product that were shipped to wholesalers prior to the close of the King transaction on February 26, 2007 and chargebacks and rebates associated with product in the distribution channel as of the closing date. |
| (2) | The 2007 Oncology transaction provision amounts represent changes in the estimates of the accruals for chargebacks and rebates recorded in connection with the sale of the Oncology Product Line. |
| (3) | The credit for returns in 2007 primarily consists of a change in the estimate of ONTAK end-customer returns. The accrual for ONTAK end-customer returns is a result of the operations of the Oncology Product Line prior to its sale on October 25, 2006. |
8. AVINZA Co-Promotion
In February 2003, Ligand and Organon Pharmaceuticals USA Inc. (Organon) announced that they had entered into an agreement for the co-promotion of AVINZA. Subsequently in January 2006, Ligand signed an agreement with Organon that terminated the AVINZA co-promotion agreement between the two companies and returned AVINZA co-promotion rights to Ligand. The termination was effective as of January 1, 2006; however, the parties agreed to continue to cooperate during a transition period that ended September 30, 2006 (the “Transition Period”) to promote the product. The Transition Period co-operation included a minimum number of product sales calls per quarter as well as the transition of ongoing promotions, managed care contracts, clinical trials and key opinion leader relationships to Ligand. During the Transition Period, Ligand paid Organon an amount equal to 23% of AVINZA net sales. Ligand also paid and was responsible for the design and execution of all clinical, advertising and promotion expenses and activities.
Additionally, in consideration of the early termination and return of rights under the terms of the agreement, Ligand agreed to and paid Organon $37.8 million in October 2006. Ligand further agreed to and paid Organon $10.0 million in January 2007, in consideration of the minimum sales calls during the Transition Period. In addition, following the Transition Period, Ligand agreed to make quarterly royalty payments to Organon equal to 6.5% of AVINZA net sales through December 31, 2012 and thereafter 6.0% through patent expiration, currently anticipated to be November of 2017.
75
Table of Contents
The unconditional payment of $37.8 million to Organon and the estimated fair value of the amounts to be paid to Organon after the termination ($95.2 million as of January 1, 2006), based on the estimated net sales of the product (currently anticipated to be paid quarterly through November 2017), were recognized as liabilities and expensed as costs of the termination as of the effective date of the agreement, January 1, 2006. Additionally, the conditional payment of $10.0 million, which represents an approximation of the fair value of the service element of the agreement during the Transition Period (when the provision to pay 23% of AVINZA net sales is also considered), was recognized ratably as additional co-promotion expense over the Transition Period.
As more fully described in Note 4, on February 26, 2007, Ligand and King executed an agreement pursuant to which King acquired all of the Company’s rights in and to AVINZA, assumed certain liabilities, and reimbursed Ligand the $47.8 million previously paid to Organon (comprised of the $37.8 million paid in October 2006 and the $10.0 million that the Company paid in January 2007). King also assumed the Company’s co-promote termination obligation to make royalty payments to Organon based on net sales of AVINZA. For the fourth quarter of 2006 and through the closing of the AVINZA sale transaction, amounts owed by Ligand to Organon on net reported sales of AVINZA did not result in current period expense, but instead were charged against the co-promote termination liability. The liability was adjusted at each reporting period to fair value and was recognized, utilizing the interest method, as additional co-promote termination charges for that period at a rate of 15%, the discount rate used to initially value this component of the termination liability.
In connection with King’s assumption of this obligation, Organon did not consent to the legal assignment of the co-promote termination obligation to King. Accordingly, Ligand remains liable to Organon in the event of King’s default of the obligation. Therefore, Ligand recorded an asset as of February 26, 2007 to recognize King’s assumption of the obligation, while continuing to carry the co-promote termination liability in the Company’s consolidated financial statements to recognize Ligand’s legal obligation as primary obligor to Organon. This asset represents a non-interest bearing receivable for future payments to be made by King and is recorded at its fair value. As of December 31, 2007 and thereafter, the receivable and liability will remain equal and adjusted each quarter for changes in the fair value of the obligation including for any changes in the estimate of future net AVINZA product sales. This receivable will be assessed on a quarterly basis for impairment (e.g. in the event King defaults on the assumed obligation to pay Organon). As of December 31, 2007, the fair value of the co-promote termination liability (and the corresponding receivable) was reduced by $36.7 million based on revised estimated future net AVINZA product sales using a discount rate of 15%.
On an annual basis, management reviews the carrying value of the co-promote termination liability. Due to assumptions and judgments inherent in determining the estimates of future net AVINZA sales through November 2017, the actual amount of net AVINZA sales used to determine the current fair value of the Company’s co-promote termination asset and liability may be materially different from current estimates.
76
Table of Contents
A summary of the co-promote termination liability as of December 31, 2009 and 2008 is as follows (in thousands):
| Net present value of payments based on estimated future net AVINZA product sales as of December 31, 2007 |
$ | 59,456 | ||
| Assumed payments made by King or assignee |
(8,803 | ) | ||
| Fair value adjustments due to passage of time |
7,829 | |||
| Total co-promote termination liability as of December 31, 2008 |
58,482 | |||
| Less: remaining current portion of co-promote termination liability as of December 31, 2008 |
(10,958 | ) | ||
| Long-term portion of co-promote termination liability as of December 31, 2008 |
$ | 47,524 | ||
| Net present value of payments based on estimated future net AVINZA product sales as of December 31, 2008 |
$ | 58,482 | ||
| Assumed payments made by King or assignee |
(8,525 | ) | ||
| Fair value adjustments due to passage of time |
(9,182 | ) | ||
| Total co-promote termination liability as of December 31, 2009 |
40,775 | |||
| Less: remaining current portion of co-promote termination liability as of December 31, 2009 |
(9,782 | ) | ||
| Long-term portion of co-promote termination liability as of December 31, 2009 |
$ | 30,993 | ||
9. Note Payable
In December 2006, Pharmacopeia entered into a loan and security agreement (the Line of Credit) with a lending institution to provide up to a total of $5.0 million in funding in the form of term loans, from time to time through December 2008. Term loans secured by laboratory equipment have a fixed term of 48 months. Term loans secured by all other collateral categories have a fixed term of 36 months.
As of December 31, 2008, the aggregate balance of term loans originated under the Line of Credit was approximately $3.4 million, of which approximately $2.1 million was classified as equipment financing obligations, long-term. Interest rates on these term loans range from 10.08% to 10.28%. The Company paid off the Line of Credit in full in January 2009.
10. Warrant Liability
In connection with the acquisition of Pharmacopeia, the Company assumed approximately 867,637 warrants (as adjusted as a result of the merger from the original 1,450,000) to purchase its common stock. To qualify as permanent equity, an equity derivative must permit the issuer to settle in unregistered shares. Under securities law, if the warrants were issued in connection with a public offering and have a cash settlement feature at the holder’s option, a company does not have the ability to settle in unregistered shares. Therefore, the warrants cannot be classified as permanent equity and are instead classified as a liability. The warrants issued as part of Pharmacopeia’s equity financing in October 2006 meet this criterion, and have been recorded as a liability in the accompanying balance sheet. The fair value of the warrants will be remeasured at each reporting date until the warrants are exercised or have expired. Changes in the fair value of the warrants are reported in the statement of operations as income (decreases) or expense (increases).
At December 31, 2009 and 2008, the fair value of the warrants was approximately $0.5 million and $0.7 million, respectively, and included in accrued liabilities.
77
Table of Contents
The fair value of the warrants was calculated using the Black-Scholes option-pricing model with the following assumptions at December 31:
| 2009 | 2008 | |||||
| Risk-free interest rate |
1.1 | % | 1.0 | % | ||
| Dividend yield |
— | — | ||||
| Expected volatility |
98 | % | 78 | % | ||
| Expected term |
2.3 years | 3.3 years |
11. Commitments and Contingencies
ECLiPS® Royalties
Under its license agreement with the Trustees of Columbia (Columbia) University and Cold Spring Harbor Laboratory (Cold Spring) (the “License Agreement”), the Company has an exclusive license for technology used in its proprietary combinatorial chemistry encoding technology, Encoded Combinatorial Libraries on Polymeric Support, or ECLiPS®. The License Agreement obligates the Company to pay a minimum annual license fee of $0.1 million to both Columbia and Cold Spring. The License Agreement expires upon the later of (i) July 16, 2013 or (ii) the expiration of the last patent relating to the technology, at which time the Company will have a fully paid license to the technology. The license granted to the Company under the License Agreement can be terminated by Columbia and Cold Spring (i) upon 30 days written notice to the Company if the Company materially breaches the Agreement and the Company fails to cure such material breach in accordance with the License Agreement or (ii) if the Company commits any act of bankruptcy, becomes insolvent, files a petition under any bankruptcy or insolvency act or has any such petition filed against it that is not dismissed within 60 days. The Company is also obligated to pay royalties to Columbia and Cold Spring based on net sales of pharmaceutical products the Company develops, as well as a percentage of all other revenue the Company recognizes from collaborators that is derived from the technology licensed from Columbia and Cold Spring.
Property Leases
In August 2009, the Company entered into a lease termination agreement for its 82,500 square foot office and laboratory facility in San Diego, California, which had a lease term through November 2021. Under the terms of the termination agreement, the Company will pay a termination fee of $14.3 million as follows: $4.5 million was paid upon signing, $4.5 million in July 2010 and $5.3 million in April 2011. As a result, in 2009, the Company recorded lease termination costs of $15.2 million, which included the net present value of the lease termination payments of $14.3 million and $0.9 million of other direct costs associated with the lease termination. The Company may be required to deliver to the landlord an irrevocable letter of credit for the then-outstanding termination fee if it does not maintain cash and investments of at least $30.0 million prior to the date upon which the second payment is due and cash and investments of at least $20.0 million prior to the date upon which the final payment is due. The Company must also maintain a current ratio of at least 110% measured monthly. In addition, the Company entered into a new lease for a period of 27 months commencing October 2009, for premises consisting of approximately 30,000 square feet of office and lab space located in San Diego to serve as its new corporate headquarters. Under the terms of the new lease, the Company pays a basic annual rent of $1.2 million (subject to an annual fixed percentage increase, as set forth in the agreement), plus other normal and necessary expenses associated with the lease.
The Company also leases an office and research facility in San Diego, California under an operating lease arrangement through July 2015. The Company fully vacated this facility in February 2008. The lease agreement provides for increases in annual rents based on changes in the Consumer Price Index or fixed percentage increases ranging from 3% to 7%. Commencing January 2008, the Company sublet this facility through July 2015. The sublease agreement provides for a 3% increase in annual rents. As of December 31, 2008, the Company expects to receive aggregate future minimum lease payments totaling $5.7 million (nondiscounted)
78
Table of Contents
over the duration of the sublease agreement. The Company recorded a net charge to operating expenses of $4.3 million for exit costs when it fully ceased use of this facility in the first quarter of 2008. The net charge consisted of a $6.5 million charge for future rent payments offset by a $2.3 million reversal of deferred rent.
The Company leases approximately 99,000 square feet in three facilities in Cranbury, New Jersey under leases that expire in 2016. The leases for the New Jersey facilities provide generally for scheduled rent increases, options to extend the leases with certain changes to the terms of the lease agreement, and refurbishment allowances. Commencing September 2009, the Company sublet 5,100 square feet of space through August 2014. As of December 31, 2009, the Company expects to receive $0.4 million in aggregate future lease payments over the duration of the sublease agreement.
As of December 31, 2009, annual minimum payments due under the Company’s office and equipment lease obligations and annual minimum rentals expected to be received by the Company under subleases are as follows (in thousands):
| Year ending December 31, |
Operating leases |
Sublease Income |
Net Payments | ||||||
| 2010 |
$ | 5,936 | $ | 921 | $ | 5,015 | |||
| 2011 |
6,032 | 946 | 5,086 | ||||||
| 2012 |
4,828 | 971 | 3,857 | ||||||
| 2013 |
4,891 | 998 | 3,893 | ||||||
| 2014 |
4,956 | 994 | 3,962 | ||||||
| Thereafter |
5,557 | 479 | 5,078 | ||||||
| $ | 32,200 | $ | 5,309 | $ | 26,891 | ||||
Total rent expense under all office leases for 2009, 2008 and 2007 was $5.1 million, $11.0 million and $5.4 million, respectively. The Company recognizes rent expense on a straight-line basis. Deferred rent at December 31, 2009 and 2008 was $1.6 million and $1.4 million, respectively, and is included in other long-term liabilities.
Equipment Financing
The Company has entered into capital lease and equipment agreements that require monthly payments through September 2010 including interest ranging from 8.36% to 10.11%. The balance under the equipment financing obligations was $0.1 million at December 31, 2009. The cost of equipment under these agreements at December 31, 2009 and 2008 was $0.6 million and $5.5 million, respectively. At December 31, 2009 and 2008, related accumulated amortization was $0.5 million and $4.6 million, respectively. The underlying equipment is used as collateral under the equipment financing.
In addition, as of December 31, 2008, Pharmacopeia had a $3.4 million Line of Credit balance in the form of term loans secured by laboratory and other underlying collateral. The line of credit was paid in full as of January 2009.
Product Liability
The Company’s business exposes it to potential product liability risks. The Company’s products also may need to be recalled to address regulatory issues. A successful product liability claim or series of claims brought against the Company could result in payment of significant amounts of money and divert management’s attention from running the business. Some of the compounds the Company is investigating may be harmful to humans. For example, retinoids as a class are known to contain compounds which can cause birth defects. The Company may not be able to maintain insurance on acceptable terms, or the insurance may not provide adequate protection in
79
Table of Contents
the case of a product liability claim. To the extent that product liability insurance, if available, does not cover potential claims, the Company would be required to self-insure the risks associated with such claims. No reserve for any potential losses under product liability claims has been recorded at December 31, 2009 and 2008.
Litigation
SEC Investigation
The SEC issued a formal order of private investigation dated September 7, 2005, to investigate the circumstances surrounding restatement of our consolidated financial statements for the years ended December 31, 2002 and 2003, and for the first three quarters of 2004. In April 2009, the Company received notification from the SEC that it had completed its investigation and will not recommend enforcement action against the Company.
Other Matters
The Company and Seragen, Inc., a subsidiary, were named parties to Sergio M. Oliver, et al. v. Boston University, et al., a shareholder class action filed on December 17, 1998 in the Court of Chancery in the State of Delaware. The Company and Seragen were dismissed from the action, but such dismissal is subject to appeal and the Company and Seragen may have possible indemnification obligations with respect to certain defendants. On December 21, 2009, the remaining parties entered into a Stipulation and Agreement of Compromise, Settlement and Release, or the Stipulation. The Stipulation is subject to Court approval and a hearing to consider approval of the stipulation has been scheduled for March 15, 2010. As of December 31, 2009, the Company had not accrued an indemnification obligation based on management’s assessment that its responsibility for any such obligation is not probable or estimable.
In July 2007, the Salk Institute for Biological Studies (Salk) filed a demand for arbitration with the American Arbitration Association, seeking damages for alleged breach of contract. In September 2008, the Company reached a settlement with Salk, whereby the parties resolved all disputes that had arisen between them. As part of the settlement, the Company agreed to pay Salk a total of $13.0 million, which was recorded as research and development expense in 2008, of which $9.5 million was due immediately upon settlement and $3.5 million due six months from the date of settlement in return for which Salk acknowledged that no additional payments would be due from Ligand or any sublicensee for any past, present or future conduct, including development of any compound in Ligand’s internal or partnered pipeline, except for any future bazedoxifene related payments. Pursuant to the parties’ agreement, the American Arbitration Association dismissed the proceeding. On March 4, 2008, The Rockefeller University (Rockefeller) filed suit, now proceeding in the United States District Court for the Southern District of New York, against the Company alleging, among other things, a breach by the Company of their September 30, 1992 license agreement with Rockefeller. In February 2009, the Company reached a settlement with Rockefeller whereby the parties resolved all disputes that have arisen between them. As part of the settlement, the Company agreed to pay Rockefeller, $5.0 million immediately upon settlement, $1.0 million on or before February 10, 2010, $1.0 million on or before February 10, 2011, and 50% of any milestone payment and 5.88% to 7.0% of certain royalties, in each case received by the Company pursuant to an agreement with SmithKline Beecham Corporation (now known as GlaxoSmithKline) entered into on December 29, 1994. The Company also agreed to pay Rockefeller 1.5% of world-wide net sales of LGD-4665 as certain payments are received by the Company pursuant to its agreement with SmithKline Beecham Corporation entered into on December 17, 2008. As of December 31, 2009, the Company has recorded a liability of $2.0 million related to the settlement; of which $1.0 million is included in current portion of accrued litigation settlement costs and $1.0 million is included in other long-term liabilities in the accompanying balance sheets.
On October 10, 2008, the Company received notice that a putative class action complaint was filed in the Superior Court of New Jersey, Mercer County (Equity Division) by Allen Heilman, one of Phamacopeia’s stockholders, against Pharmacopeia, the members of its Board of Directors, Ligand and two of Ligand’s wholly owned subsidiaries. The complaint generally alleged that Pharmacopeia’s Board of Directors’ decision to enter
80
Table of Contents
into the proposed transaction with Ligand on the terms contained in the merger agreement constitutes a breach of fiduciary duty and gives rise to other unspecified state law claims. The complaint also alleged that Ligand and two of Ligand’s wholly owned subsidiaries aided and abetted Pharmacopeia’s Board of Directors’ breach of fiduciary duty. In addition, the complaint alleged that the named plaintiff sought “equitable relief,” including among other things, an order preliminarily and permanently enjoining the proposed transaction. While management believes that neither Ligand nor Pharmacopeia engaged in any wrongful acts, in an effort to minimize the cost and expense of any litigation, the parties entered into a stipulation of settlement, pursuant to which Pharmacopeia agreed to make certain additional disclosures in its SEC Form 14d-9 and not oppose a fee award to plaintiffs’ attorneys of up to $180,000, which is included in current portion of accrued litigation settlement costs at September 30, 2009. On October 20, 2009, the court granted final approval of the stipulation of settlement and dismissed the class action with prejudice.
On September 9, 2009, the Company received notice that a class action complaint was filed in the Connecticut Superior Court for the Judicial District of New Haven by Gabriel Guzman, one of Neurogen’s stockholders, against Neurogen, the members of its Board of Directors, Ligand and one of Ligand’s wholly owned subsidiaries. The amended complaint generally alleged that Neurogen’s Board of Directors’ decision to enter into the transaction with Ligand on the terms contained in the merger agreement constituted a breach of fiduciary duty. The amended complaint also alleges that Ligand and one of Ligand’s wholly owned subsidiaries aided and abetted Neurogen’s Board of Directors’ breach of fiduciary duty. Management believes that neither Ligand nor Neurogen engaged in any wrongful acts and on October 22, 2009, the Company filed a motion to strike the complaint. The plaintiff filed a Withdrawal of Action to voluntarily dismiss the case in December 2009.
In addition, from time to time the Company is subject to various lawsuits and claims with respect to matters arising out of the normal course of the Company’s business. Due to the uncertainty of the ultimate outcome of these matters, the impact on future financial results is not subject to reasonable estimates.
Funding of Legacy Director Indemnity Fund
On March 1, 2007 Ligand entered into an Indemnity Fund Agreement (the “Agreement”) with Dorsey & Whitney LLP (“Dorsey”), counsel to Company’s independent directors and to the Audit Committee of the Board of Directors. Under the Agreement, the Company established in a Dorsey trust account a $10 million indemnity fund (the “Fund”) to support the Company’s existing indemnification obligations to continuing and departing directors in connection with the ongoing Securities & Exchange Commission (the “Commission”) investigation and related matters (the “Legacy Liabilities”). Pursuant to the Agreement, any amounts remaining in the Fund, together with interest earned thereon, are to be returned to the Company upon receipt of written communication from the Commission that the investigation initiated by the Commission has been discontinued without any remaining Legacy Liabilities. Accordingly, as a result of the termination of the Commission’s investigation, on April 15, 2009 Dorsey released the balance of the fund, amounting to $10.3 million, to Ligand.
12. Common Stock Subject to Conditional Redemption—Pfizer Settlement Agreement
In April 1996, the Company and Pfizer entered into a settlement agreement with respect to a lawsuit filed in December 1994 by the Company against Pfizer. In connection with a collaborative research agreement the Company entered into with Pfizer in 1991, Pfizer purchased shares of the Company’s common stock. Under the terms of the settlement agreement, at the option of either the Company or Pfizer, milestone and royalty payments owed to the Company can be satisfied by Pfizer by transferring to the Company shares of the Company’s common stock at the exchange ratio of $12.375 per share. The remaining common stock issued and outstanding to Pfizer following the settlement was reclassified as common stock subject to conditional redemption (between liabilities and equity) since Pfizer has the option to settle milestone and royalties payments owed to the Company with the Company’s shares, and such option is not within the Company’s control. In March 2009, the Company earned a milestone from Pfizer, Inc. (Pfizer). In April 2009, pursuant to the Company’s 1991 research agreement
81
Table of Contents
and 1996 settlement agreement with Pfizer, Pfizer elected to pay the milestone by returning 323,338 shares of stock it owns in the Company, which at the date the milestone was earned had a market value of $0.9 million. Ligand retired the tendered shares in May 2009. The difference between the fair value of the shares tendered and the carrying value of such shares based on the contractual exchange ratio, approximately $3.1 million, was credited to additional paid-in capital. The Company is entitled to royalties on future sales from Pfizer, which pursuant to the 1996 settlement agreement, Pfizer may elect to pay by returning shares of stock it owns in Ligand. At December 31, 2009 and 2008, the remaining shares of the Company’s common stock that could be redeemed totaled approximately 674,230 and 997,568, respectively, and are reflected at the exchange ratio price of $12.375.
13. Stockholders’ Equity
Stock Plans
On May 29, 2009, the Company’s stockholders approved the amendment and restatement of the Company’s 2002 Stock Incentive Plan (the “Amended 2002 Plan”). The Company’s 2002 Stock Incentive Plan was amended to (i) increase the number of shares available for issuance under the Amended 2002 Plan by 7,600,000 shares, (ii) revise the list of performance criteria that may be used by the compensation committee for purposes of granting awards under the Amended 2002 Plan that are intended to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code, as amended, and (iii) eliminate the automatic option grant program for non-employee directors, the director fee stock issuance program and the director fee option grant program, which programs have been superseded by the Company’s amended and restated Director Compensation Policy. As of December 31, 2009, there were 8.4 million shares available for future option grants or direct issuance under the Amended 2002 Plan.
The Company grants options to employees, non-employee consultants, and non-employee directors. Only new shares of common stock are issued upon the exercise of stock options. Non-employee directors are accounted for as employees. Options and restricted stock granted to certain directors vest in equal monthly installments over one year from the date of grant. Options granted to employees vest 1/8 on the six month anniversary of the date of grant, and 1/48 each month thereafter for forty-two months. All option awards generally expire ten years from the date of grant.
Stock-based compensation cost for awards to employees and non-employee directors is recognized on a straight-line basis over the vesting period until the last tranche vests. Compensation cost for consultant awards is recognized over each separate tranche’s vesting period. The Company recognized compensation expense of $3.4 million, $3.6 million and $7.6 million for 2009, 2008 and 2007, respectively, associated with option awards, restricted stock and an equitable adjustment of employee stock options. Of the total compensation expense associated with the option awards for 2007, $1.8 million related to the $2.50 equitable adjustment of the exercise price for all options outstanding as of April 3, 2007 that was measured for financial reporting purposes effective March 28, 2007, the date the Compensation Committee of the Company’s Board of Directors approved the adjustment. The compensation expense related to share-based compensation arrangements is recorded as components of research and development expenses ($2.0 million, $1.0 million and $3.4 million) and general and administrative expenses ($1.4 million, $2.6 million and $4.2 million) for the years ended December 31, 2009, 2008 and 2007, respectively. There was no deferred tax benefit recognized in connection with these costs.
The fair-value for options that were awarded to employees and directors was estimated at the date of grant using the Black-Scholes option valuation model with the following weighted average assumptions:
| Year Ended December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Risk-free interest rate |
2.1 | % | 3.0 | % | 4.9 | % | |||
| Dividend yield |
— | — | — | ||||||
| Expected volatility |
74 | % | 65 | % | 66 | % | |||
| Expected term |
6 years | 6 years | 6 years | ||||||
82
Table of Contents
The expected term of the employee and non-employee director options is the estimated weighted-average period until exercise or cancellation of vested options (forfeited unvested options are not considered) based on historical experience. The expected term for consultant awards is the remaining period to contractual expiration.
Volatility is a measure of the expected amount of variability in the stock price over the expected life of an option expressed as a standard deviation. In selecting this assumption, the Company used the historical volatility of the Company’s stock price over a period equal to the expected term.
Following is a summary of the Company’s stock option plan activity and related information:
| Shares | Weighted Average Exercise Price |
Weighted- Average Remaining Contractual Term in Years |
Aggregate Intrinsic Value (In thousands) | |||||||||
| Balance at January 1, 2007 |
5,766,386 | $ | 10.43 | (A) | 6.04 | $ | 4,602 | |||||
| Granted |
843,936 | 7.06 | ||||||||||
| Exercised |
(648,277 | ) | 6.87 | |||||||||
| Forfeited |
(589,893 | ) | 8.25 | |||||||||
| Cancelled |
(3,149,120 | ) | 11.71 | |||||||||
| Balance at December 31, 2007 |
2,223,032 | 8.87 | 5.17 | 304 | ||||||||
| Granted |
1,304,500 | 3.52 | ||||||||||
| Exercised |
(4,438 | ) | 3.41 | |||||||||
| Forfeited |
(107,058 | ) | 6.88 | |||||||||
| Cancelled |
(385,960 | ) | 9.64 | |||||||||
| Balance at December 31, 2008 |
3,030,076 | 6.55 | 6.63 | 81 | ||||||||
| Granted |
1,651,850 | 2.65 | ||||||||||
| Exercised |
(21,250 | ) | 2.03 | |||||||||
| Forfeited |
(315,491 | ) | 4.00 | |||||||||
| Cancelled |
(333,708 | ) | 8.41 | |||||||||
| Balance at December 31, 2009 |
4,011,477 | 5.02 | 6.88 | 31 | ||||||||
| Exercisable at December 31, 2009 |
2,073,647 | 6.62 | 5.42 | 31 | ||||||||
| Options expected to vest as of December 31, 2009 |
3,757,569 | 5.12 | 6.77 | 31 | ||||||||
| (A) | Adjusted to reflect April 2007 equitable adjustment |
The weighted-average grant-date fair value of all stock options granted during 2009 was $1.68 per share. The total intrinsic value of all options exercised during 2009, 2008 and 2007 was approximately $2,000, $3,000 and $1.7 million, respectively. As of December 31, 2009, there was $4.7 million of total unrecognized compensation cost related to nonvested stock options. That cost is expected to be recognized over a weighted average period of 2.6 years.
Cash received from options exercised in 2009, 2008 and 2007 was $43,000, $15,000 and $4.2 million, respectively. There is no current tax benefit related to options exercised because of Net Operating Losses (NOLs) for which a full valuation allowance has been established.
83
Table of Contents
Following is a further breakdown of the options outstanding as of December 31, 2009:
| Options Outstanding | Options exercisable | |||||||||||
| Range of exercise prices |
Options outstanding |
Weighted average remaining life in years |
Weighted average exercise price |
Options exercisable |
Weighted average exercise price | |||||||
| $0.01–$ 2.48 |
288,920 | 6.89 | $ | 2.23 | 114,336 | $ | 2.02 | |||||
| 2.69– 2.69 |
1,220,900 | 9.13 | 2.69 | 243,122 | 2.69 | |||||||
| 2.95– 3.45 |
135,036 | 2.46 | 3.05 | 77,369 | 3.08 | |||||||
| 3.50– 3.50 |
1,039,500 | 8.15 | 3.50 | 510,559 | 3.50 | |||||||
| 4.01– 8.13 |
672,284 | 6.17 | 6.88 | 479,883 | 6.98 | |||||||
| 8.25–14.66 |
654,837 | 2.29 | 11.48 | 648,378 | 11.50 | |||||||
| 0.01–14.66 |
4,011,477 | 6.88 | 5.02 | 2,073,647 | 6.62 | |||||||
Restricted Stock Activity
The following is a summary of the Company’s restricted stock activity and related information:
| Shares | Weighted-Average Grant Date Fair Value | |||||
| Nonvested at January 1, 2007 |
1,297 | $ | 11.56 | |||
| Granted |
320,300 | 9.69 | ||||
| Vested |
(1,297 | ) | 11.56 | |||
| Forfeited |
(24,700 | ) | 7.15 | |||
| Nonvested at December 31, 2007 |
295,600 | 9.90 | ||||
| Granted |
434,000 | 3.38 | ||||
| Vested |
(110,012 | ) | 10.92 | |||
| Forfeited |
(20,916 | ) | 5.43 | |||
| Nonvested at December 31, 2008 |
598,672 | 5.14 | ||||
| Granted |
358,460 | 2.63 | ||||
| Vested |
(298,246 | ) | 6.14 | |||
| Forfeited |
(84,599 | ) | 3.51 | |||
| Nonvested at December 31, 2009 |
574,287 | 3.29 | ||||
Restricted stock awards generally vest over three years. As of December 31, 2009, unrecognized compensation cost related to non-vested stock awards amounted to $0.9 million. That cost is expected to be recognized over a weighted average period of 1.5 years.
Employee Stock Purchase Plan
On May 29, 2009, the Company’s stockholders approved the amendment and restatement of the Company’s Employee Stock Purchase Plan (the “Amended ESPP”). The Amended ESPP was amended to (a) increase the number of shares authorized for issuance under the Employee Stock Purchase Plan by 800,000, (b) extend the term of the Employee Stock Purchase Plan until June 2019, (c) reduce the length of offering periods from twenty-four months to six months and reduce the number of purchase intervals during each offering period from eight to one, (d) eliminate the requirement that an employee have at least three months of employment as a condition to his or her eligibility to participate in the Amended ESPP, (e) provide that a participant will be eligible to purchase up to 7,500 shares of Ligand common stock during each offering period, but in no event may a
84
Table of Contents
participant purchase more than 7,500 shares of common stock during any calendar year, and (f) update the plan to conform it to recently issued Treasury Regulations applicable to employee stock purchase plans.
The Amended ESPP allows employees to purchase a limited amount of common stock at the end of each six month period at a price equal to 85% of the lesser of fair market value on either the start date of the period or the last trading day of the period (the “Lookback Provision”). The 15% discount and the Lookback Provision make the Amended ESPP compensatory. There were 134,660, 46,217 and 29,139 shares of common stock issued under the Amended ESPP in 2009, 2008 and 2007, respectively, resulting in an expense of $0.1 million, $0.03 million and $0.04 million, respectively. For shares purchased under the Company’s Amended ESPP, a weighted-average expected volatility of 27%, 60% and 38% was used for 2009, 2008 and 2007, respectively. The expected term for shares issued under the ESPP is six months. As of December 31, 2009, 597,517 shares of common stock had been issued under the Amended ESPP to employees and 712,731 shares are available for future issuance.
Preferred Stock
The Company has authorized 5,000,000 shares of preferred stock, of which 1,600,000 are designated Series A Participating Preferred Stock (the “Preferred Stock”). The Board of Directors of Ligand has the authority to issue the Preferred Stock in one or more series and to fix the designation, powers, preferences, rights, qualifications, limitations and restrictions of the shares of each such series, including the dividend rights, dividend rate, conversion rights, voting rights, rights and terms of redemption (including sinking fund provisions), liquidation preferences and the number of shares constituting any such series, without any further vote or action by the stockholders. The rights and preferences of Preferred Stock may in all respects be superior and prior to the rights of the common stock. The issuance of the Preferred Stock could decrease the amount of earnings and assets available for distribution to holders of common stock or adversely affect the rights and powers, including voting rights, of the holders of the common stock and could have the effect of delaying, deferring or preventing a change in control of Ligand. As of December 31, 2009 and 2008, there are no preferred shares issued or outstanding.
Shareholder Rights Plan
In October 2006, the Company’s Board of Directors renewed the Company’s stockholder rights plan, which was originally adopted and has been in place since September 2002, and which expired on September 13, 2006, through the adoption of a new 2006 Stockholder Rights Plan (the “2006 Rights Plan”). The 2006 Rights Plan provides for a dividend distribution of one preferred share purchase right (a “Right”) on each outstanding share of the Company’s common stock. Each Right entitles stockholders to buy 1/1000th of a share of Ligand Series A Participating Preferred Stock at an exercise price of $100. The Rights will become exercisable if a person or group announces an acquisition of 20% or more of the Company’s common stock, or announces commencement of a tender offer for 20% or more of the common stock. In that event, the Rights permit stockholders, other than the acquiring person, to purchase the Company’s common stock having a market value of twice the exercise price of the Rights, in lieu of the Preferred stock. In addition, in the event of certain business combinations, the Rights permit the purchase of the common stock of an acquiring person at a 50% discount. Rights held by the acquiring person become null and void in each case. The 2006 Rights Plan expires in 2016.
Cash Dividend
On March 22, 2007, the Company declared a cash dividend on the common stock of the Company of $2.50 per share. As the Company had an accumulated deficit, the dividend was recorded as a charge against additional paid-in capital in the first quarter of 2007. The aggregate amount of $252.7 million was paid on April 19, 2007 to shareholders of record as of April 5, 2007.
85
Table of Contents
Modification to Employee Stock Options
In February 2007, the Company’s shareholders approved a modification to the 2002 Stock Incentive Plan (the “2002 Plan”) to allow equitable adjustments to be made to options outstanding under the 2002 Plan. Effective April 2007, the Company reduced the exercise price by $2.50 (or to the par value of the stock for those options with an exercise price below $2.50 per share), as an equitable adjustment, for all options then outstanding under the 2002 Plan to reflect the special cash dividend. The Company recognized $1.8 million of stock compensation expense in connection with the equitable adjustment effective March 28, 2007, the date the Compensation Committee of the Company’s Board of Directors approved the equitable adjustment.
Shares Issued in Business Combination
On December 23, 2009, in connection with its acquisition of Neurogen Corporation, the Company issued 4,200,000 shares of common stock to Neurogen stockholders, or 0.0608 shares for each outstanding Neurogen share.
On December 23, 2008, in connection with its acquisition of Pharmacopeia, the Company issued 17,997,039 shares of common stock to Pharmacopeia stockholders, or 0.5985 shares for each outstanding Pharmacopeia share.
Warrants
As of December 31, 2009, warrants to purchase 867,637 shares of the Company’s common stock were outstanding with an exercise price of $8.59 per share and an expiration date of April 2012, and warrants to purchase 105,554 shares of the Company’s common stock were outstanding with an exercise price of $9.47 per share and an expiration date of March 2011. The two series of warrants were assumed in the acquisition of Pharmacopeia, Inc.
In addition, as of December 31, 2009, 981,411 warrants with an exercise price of $29.90 per warrant and an expiration date of April 2013 were outstanding to purchase an aggregate of 776,160 shares of the Company’s common stock. If exercised, these warrants are also entitled to receive $0.1 million in cash and 981,411 of each of the Company’s four contingent value rights issued to Neurogen shareholders in December 2009. The series of warrants was assumed in the acquisition of Neurogen Corporation.
Share Repurchases
In March 2007, the Board of Directors authorized up to $100.0 million in share repurchases over the subsequent 12 months. Through February 2008, the Company repurchased 6.5 million shares of its common stock totaling $41.2 million.
Share Reserves
As of December 31, 2009, the Company had 6.8 million shares reserved for future issuance related to stock options, stock awards, stock purchase plan and warrants.
14. Collaboration Agreements
The Company has entered into multiple research and development collaboration arrangements with third party pharmaceutical companies. The commercial terms of such arrangements typically include some combination of the following types of fees: exclusivity fees, technology access fees, technology development fees and research support payments, as well as milestone payments, license or commercialization fees. The Company may also receive royalties on product candidates resulting from its research and development
86
Table of Contents
collaboration arrangements if and to the extent any such product candidate is ultimately approved by the FDA and successfully marketed. The Company’s collaborations are discussed below.
Bristol-Myers Squibb Collaborations
SARM Program
In connection with the Company’s acquisition of Pharmacopeia, the Company assumed an exclusive licensing agreement with BMS, originally entered into in October 2007, which provides the Company exclusive worldwide development and commercialization rights to a SARM program, including PS178990, for which a Phase I single ascending dose study had been completed. PS178990 is a non-steroidal SARM that was designed to provide the benefits of testosterone to patients without unwanted side effects on the prostate.
Under the SARM license agreement, the Company is required to make milestone payments to BMS upon the submission and approval of a therapeutic product for marketing in the United States and certain other jurisdictions and is obligated to make milestone payments to BMS upon achieving certain worldwide annual net sales of products resulting from the SARM program. The Company is also obligated to pay to BMS a stepped royalty on annual net sales on products covered by the SARM License agreement. BMS has a limited right of first negotiation in the event that the Company attempts to license compounds that are the subject of the SARM License agreement to a third party other than BMS.
The Company also assumed a discovery collaboration agreement with BMS to provide a portion of its medicinal chemistry resources to a BMS discovery program unrelated to the SARM program for a period up to three years beginning in October 2007. The discovery collaboration agreement provides that each such year, the Company is required to provide a fixed number of full-time workers for the BMS discovery program, divided between employees located at its facility in Cranbury, New Jersey and contracted headcount located outside the United States.
In addition, the Company agreed to pay milestone payments to BMS associated with the submission and approval of a therapeutic product for marketing and a stepped royalty on net sales of therapeutic products, if any, resulting from the SARM program. BMS has a limited right of first negotiation in the event that the Company desires to license compounds that are the subject of the SARM License agreement to a third party other than BMS.
In December 2009, the Company and BMS entered into an amendment to the discovery collaboration agreement. Pursuant to the terms of the Amendment, the research term under the Collaboration Agreement terminated on December 31, 2009 and the research program under the Collaboration Agreement will be transferred to BMS. The Company will no longer be obligated to provide research support to BMS after December 31, 2009, other than providing certain data and compound transfer services to BMS through June 30, 2010. In connection with the Amendment, the Company paid $1.0 million to BMS in January 2010 and BMS is no longer required to make milestone payments to the Company under the Collaboration Agreement. The Company has included the liability in accrued liabilities in the consolidated balance sheet at December 31, 2009.
P-38 Kinase Program
In connection with the merger with Pharmacopeia, the Company assumed a collaboration and license agreement with BMS which was originally entered into in November 1997. This collaboration has resulted in a compound that entered Phase II clinical trials in September 2007 for psoriasis. BMS has also completed a Phase II study in rheumatoid arthritis and a Phase II trial in atherosclerosis is ongoing. The research collaboration portion of the agreement has expired; however, the Company is entitled to payments resulting from the successful achievement by BMS of certain clinical and regulatory milestones, as well as a royalty on net sales of products resulting from compounds already delivered under the agreement.
87
Table of Contents
As of December 31, 2009 and 2008, the Company had deferred revenue of approximately $0.3 million and $13.0 million, respectively, related to BMS agreements.
GlaxoSmithKline Collaboration
Agreement with Pharmacopeia
In connection with the completion of the Company’s acquisition of Pharmacopeia, the Company assumed a product development and commercialization agreement which Pharmacopeia and SmithKlineBeecham Corporation and Glaxo Group Limited (together GSK) entered into in March 2006. The Company’s role in the collaboration is to identify and advance molecules in chosen therapeutic programs to development stage and, subject to certain provisions in the GSK agreement, further develop the candidates to clinical “proof of concept” (a demonstration of efficacy in humans). The Company agreed that it will not screen its compound library for other collaborators, or for its own account, against any target it screens under the GSK agreement for a specified period.
The GSK agreement provides GSK an exclusive option, exercisable at defined points during the development process for each program up to “proof of concept,” to license that program. Upon licensing a program, GSK is obligated to conduct preclinical development and/or clinical trials and commercialize pharmaceutical products, if any, resulting from such licensed programs on a worldwide basis. The Company is entitled to receive success-based milestone payments, starting in preclinical research, from GSK for each drug development program under the alliance and the potential for double-digit royalties upon the successful commercialization by GSK of any product resulting therefrom.
In the event that GSK does not exercise its option to license a program, the Company will retain all rights to that program and may continue to develop the program and commercialize any products resulting from the program, or the Company may elect to cease progressing the program and/or seek other partners for further development and commercialization. Should the Company develop or partner such a program and commercialize any products resulting from that program, it will be obligated to pay GSK success-based milestone payments and royalties upon successful commercialization, if any.
Pharmacopeia received $15.0 million in connection with initial discovery activities which the Company is obligated to perform under the GSK agreement. The Company recognizes revenue on a percentage of completion basis as it performs the required discovery activities in an amount from time to time less than or equal to the non-refundable portion of payments received in connection with the GSK agreement. The initial research term of the GSK agreement expires in March 2011. As of December 31, 2009 and 2008, the Company had deferred revenue of approximately $3.7 million and $6.3 million, respectively, related to GSK agreements.
The Company and GSK each have the right to terminate the GSK agreement in their sole discretion under certain specified circumstances at any time during the term of the GSK agreement. In addition, the Company and GSK each have the right to terminate the GSK agreement under other circumstances that are customary in these types of agreements. If the Company exercises its discretionary termination right at any time during the first five years of the term, under certain circumstances, the Company could be required to refund to GSK a portion of the $15.0 million referred to above which it received related to its initial discovery activities. The amount of any such refund will be calculated based upon when during the term of the GSK agreement that termination occurs and the amount of research funding the Company had received prior to such termination. However, there are no instances where the deferred revenue would be amortized below the amount that could be potentially refundable pursuant to the terms of the GSK agreement. Further, should GSK exercise its discretionary termination rights, there are no provisions in the GSK agreement that would require the Company to refund payments received relating to its performance of initial discovery activities or milestone payments received under the GSK agreement.
88
Table of Contents
PROMACTA and TPO
In December 2008, the FDA granted accelerated approval of GSK’s PROMACTA® for the treatment of thrombocytopenia in patients with chronic immune (idiopathic) thrombocytopenic purpura (ITP) who have had an insufficient response to corticosteroids, immunoglobulins or splenectomy. In December 2009, GSK received a positive opinion for Revolade(R) (eltrombopag/PROMACTA) from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) for the oral treatment of thrombocytopenia (reduced platelet count) in adults with the blood disorder chronic ITP. Revolade is expected to be launched in the EU in the first half of 2010. PROMACTA is the first oral TPO receptor agonist therapy for the treatment of adult patients with chronic ITP. As a result of the FDA’s approval of PROMACTA, the Company will be entitled to receive tiered royalties in the range of 4.7%-9.4% on annual net sales of PROMACTA, net of payments due to Rockefeller as part of a settlement agreement and mutual release the Company entered into on February 11, 2009 with Rockefeller.
In December 2008, the Company entered into an exclusive, worldwide license agreement with SmithKline Beecham Corporation, doing business as GSK. Pursuant to the terms of the GSK agreement, the Company granted GSK the exclusive right to develop, manufacture and commercialize its LGD-4665 product candidate, as well as all other TPO-related molecules discovered by the Company. Under the terms of the GSK agreement, GSK paid the Company $5.0 million as an upfront license fee and agreed to pay up to $158.0 million in development and commercial milestones and a royalty on net sales. In the first year of sales, royalties will be one-half of the regular royalty rate. GSK will direct all product development and commercialization and will be responsible for all costs going forward for development, patent maintenance and prosecution, and commercialization. The term of the license agreement expires ten years from the date of the first commercial sale of the first licensed product in any country worldwide or until the expiration of the last licensed patent with a valid claim, whichever term is longer, although some obligations servive termination. Prior to the expiration of the license agreement, GSK has the right to terminate the agreement upon a specified number of days notice and the Company may not terminate the agreement unless GSK provides its prior written consent. Any such termination will not relieve the terminating party from obligations that have accrued prior to such termination or that expressly survive such termination. No termination will require the Company to refund to GSK any or all payments made to the Company by GSK under the agreement. In the event a party is in breach of any of its material obligations under the license agreement, the other party will have the right to seek damages and such other remedies as may be available to it.
Pfizer Collaborations
JAK3 Program
In connection with the completion of the Company’s acquisition of Pharmacopeia, the Company assumed a research and license agreement with Pfizer (formerly Wyeth), acting through its Wyeth Pharmaceuticals Division, providing for the formation of a new alliance based on Pharmacopeia’s Janus Kinase-3, or JAK3, inhibitor program. The alliance’s goal is to identify, develop and commercialize therapeutic products for the treatment of certain immunological conditions in humans.
Each of the companies has certain exclusive rights to develop and commercialize products resulting from the JAK3 program and the alliance. The Company retains the right to develop and commercialize therapeutic products for the treatment of dermatological and ocular diseases employing topical administration, and Pfizer has the right to develop human therapeutic products for all other indications and routes of delivery. Under the terms of the Pfizer agreement, Pharmacopeia received an up-front cash payment and will receive quarterly research funding through December 2009. In addition, the Company may receive up to $175.0 million if Pfizer achieves preclinical and clinical development and regulatory and commercialization milestones, as well as double-digit royalties on the net sales of any products commercialized by Pfizer under the alliance. Each company is responsible for all development, regulatory, manufacturing and commercialization activities for any products it develops and commercializes in its field.
89
Table of Contents
The revenue for this research is recognized on a percentage of completion basis, which is expected to approximate straight-line recognition of revenue over the initial three year term of the alliance. As of December 31, 2009 and 2008, the Company had deferred revenue of approximately $0.9 million and $1.5 million, respectively, related to Pfizer agreements.
Each of the companies has the right to terminate the Pfizer agreement under certain specified circumstances at any time during the term of the Pfizer agreement. In addition, Pfizer has the right, upon providing the Company six months’ prior written notice, to terminate the research collaboration and/or the Pfizer agreement in its entirety or in part. Such right to termination would not apply to Pfizer’s obligations with respect to any program developed by the collaboration and licensed by Pfizer. Termination will not require the Company to refund to Pfizer any or all of the cash payments described above.
In November 2009, Pfizer exercised its right under the contract and extended the research term and related quarterly research funding through December 2010.
Bazedoxifene Program
Bazedoxifene (Viviant) is a product candidate that resulted from the Company’s collaboration with Wyeth (now Pfizer). Bazedoxifene is a synthetic drug that was specifically designed to reduce the risk of osteoporotic fractures while at the same time protecting breast and uterine tissue. Regarding Viviant, the FDA has advised that it expects to convene an advisory committee to review the pending NDAs for both the treatment and prevention indications. Approvable letters were received for each of these NDAs, in which, among other things, the FDA requested further analyses and discussion concerning the incidence of stroke and venous thrombotic events, identified certain issues concerning data collection and reporting, and requested additional source documents. An FDA-requested advisory committee meeting is expected to be scheduled following submission of the complete response to the approvable letters, which was targeted for the second half of 2009. In April 2009, Pfizer received approval in the EU for CONBRIZA (the EU trade name for Viviant) for the treatment of postmenopausal osteoporosis in women at increased risk of fracture. The Company expects CONBRIZA to be launched in the EU in 2010.
Pfizer is also developing bazedoxifene in combination with PREMARIN (Aprela) is a tissue selective estrogen complex under development for menopausal symptoms and osteoporosis, Two Phase III studies with bazedoxifene/conjugated estrogens (Aprela), showed reduced number and severity of hot flashes in symptomatic postmenopausal women by up to 80 percent, when compared with placebo. Pfizer expects to file an initial NDA no earlier than the first half of 2010. The Company is entitled to receive tiered royalties on these products.
The Company previously sold to Royalty Pharma AG, or Royalty Pharma, the rights to a total of 3.0% of net sales of bazedoxifene for a period of ten years following the first commercial sale of each product. After giving effect to the royalty sale, the Company will receive 0.5% of the first $400.0 million in net annual sales. If net annual sales are between $400.0 million and $1.0 billion, the Company will receive a net royalty of 1.5% on the portion of net sales between $400.0 million and $1.0 billion, and if annual sales exceed $1.0 billion, the Company will receive a net royalty of 2.5% on the portion of net sales exceeding $1.0 billion. Additionally, the royalty owed to Royalty Pharma may be reduced by one third if net product sales exceed certain thresholds across all indications.
Lasofoxifene Program
Lasofoxifene (FABLYN®) is a product candidate that resulted from the Company’s collaboration with Pfizer. Pfizer submitted an NDA and an MAA for FABLYN for osteoporosis treatment in December 2007 and January 2008, respectively. The FDA Advisory Committee in early September 2008 voted 9-3 in favor of approving this drug. In January 2009, Pfizer received a complete response letter from the FDA requesting additional information for FABLYN. In February 2009, FABLYN received approval in the EU for the treatment
90
Table of Contents
of osteoporosis. Pfizer reported that following a strategic review, it decided to explore strategic options for FABLYN, including out-licensing or sale. Under the terms of the Company’s agreement with Pfizer, the Company is entitled to receive royalty payments on worldwide net sales of lasofoxifene for any indication.
Under the terms of its agreement with Pfizer, the Company is entitled to receive royalty payments equal to 6% of worldwide net sales of lasofoxifene for any indication. The Company previously sold to Royalty Pharma the rights to a total of 3% of net sales of lasofoxifene for a period of ten years following the first commercial sale of lasofoxifene. Accordingly, the Company will receive approximately 3% of worldwide net annual sales of lasofoxifene.
Cephalon Collaboration
In connection with the Company’s acquisition of Pharmacopeia, the Company assumed a collaboration and license agreement with Cephalon, Inc., or Cephalon, providing for the formation of a new drug discovery, development and commercialization alliance. Under the Cephalon agreement, Pharmacopeia received an up-front, non-refundable payment of $15.0 million in June 2006 to support its research efforts.
Cephalon is responsible for identifying hit and lead compounds, and the Company and Cephalon agreed to work collaboratively to advance the lead compounds to clinical candidates. The Company is principally responsible for medicinal chemistry research and Cephalon provides biology support, including preclinical disease models, as required by the Cephalon agreement. The Company has agreed that, for a specified period, it will not screen its compound library for other collaborators, or for its own account, against any target it works on under the Cephalon agreement.
Upon the nomination of any clinical candidates by the alliance, Cephalon will be primarily responsible for their development and commercialization. The Company will retain an option to develop certain candidates from the alliance, subject to Cephalon’s agreeing to such development. For any preclinical development candidate advanced under the alliance, the developing company will make clinical, regulatory and sales milestone payments to the non-developing company. In addition, the company commercializing any resulting product will pay the non-commercializing company up to double-digit royalties based on the sales level achieved.
As stated above, under the Cephalon agreement, Pharmacopeia received a non-refundable payment of $15.0 million and was principally responsible for performing medicinal chemistry research. The revenue for this research is recognized on a percentage of completion basis. As of December 31, 2008, the Company had deferred revenue of approximately $0.3 million related to the Cephalon agreement. The initial research term of the Cephalon agreement expired in May 2009.
Merck (formerlySchering-Plough Collaboration)
2007 Collaboration
In connection with the completion of the Company’s acquisition of Pharmacopeia, the Company also assumed an amended and restated collaboration and license agreement with N.V. Organon, entered into in February 2007. In November 2007, Organon was acquired by, and is now a part of, Merck (formerly Schering-Plough). Under the agreement, Pharmacopeia agreed to work collaboratively with Merck to generate lead compounds at targets in mutual therapeutic areas selected by Merck and agreed upon by a joint research committee. The purpose of the agreement is to produce development-ready compounds, the potential development of which will be handled primarily by Merck. The agreement provided that the Company would receive up to $4.0 million per year from Merck in research funding over the remaining portion of the five-year term of the agreement.
Pursuant to the agreement the Company has the option to purchase the right to co-develop and co-commercialize certain therapeutic candidates of mutual interest discovered through the alliance. For the therapeutic candidates that the Company does not elect to co-develop and co-commercialize, Schering-Plough
91
Table of Contents
will retain exclusive development and commercialization rights, and the Company will receive milestone payments as a result of Merck’s successful advancement, if any, of each candidate through clinical development. The Company will also receive up to double-digit royalties on net sales, if any, of pharmaceutical products resulting from the collaboration when the lead optimization was conducted by the Company, and lower royalties when the lead optimization was conducted by Merck.
On July 29, 2009, the Company and Merck mutually agreed to terminate the research collaboration under their collaboration and license agreement pursuant to which the parties agreed to work collaboratively to discover, develop and commercialize therapeutic products across a broad range of indications. As a result of the termination, Merck continued to fund research collaboration activities on those targets currently under investigation through December 2009, and the Company is eligible to receive potential milestone payments and royalties under certain circumstances.
1998 Collaboration
In connection with our acquisition of Pharmacopeia, the Company assumed collaboration and license agreements with Schering-Plough Ltd. (now Merck) and Schering Corporation (collectively “Schering-Plough”) that were originally entered into in October of 1998. These agreements produced a CXCR2 antagonist that entered Phase II clinical trials in the fourth quarter of 2006 for COPD and asthma, an enzyme inhibitor that entered Phase II clinical trials in November 2008 for oncology, a candidate for inflammatory diseases that entered Phase I clinical trials in March 2007, a candidate for respiratory diseases that entered Phase I clinical trials in September 2007 and a BACE inhibitor for Alzheimer’s disease that entered Phase I clinical trials in early 2009.
PS 291822 (SCH-527123), the lead in a series of CXCR2 antagonists, is being developed for the potential oral treatment of chronic obstructive pulmonary disorder (COPD) and asthma. Merck has completed phase II trials in COPD, neutrophilic asthma, mild allergen-induced asthma and psoriasis. In January 2010, Merck initiated two large Phase II dose-ranging studies with 500 patients each in COPD and severe asthma.
Dinaciclib (SCH-727965, PS-095760), a pro-apoptotic inhibitor of cyclin-dependent kinases is under clinical development for the potential treatment of cancer. Three Phase II trials are ongoing;
| • | A phase II trial in patients with advanced breast cancer and non-small cell lung cancer (NSCLC) |
| • | A phase II trial in acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL) |
| • | A phase II trial in mantle cell lymphoma (MCL) and B-cell chronic lymphocytic leukemia (B-CLL) |
A Beta-Secretase inhibitor is in clinical development for Alzheimer’s disease. Merck reported the completion of Phase I single dose trial study with a 58% reduction in A-Beta peptide in cerebral/spinal fluid. Phase I multi-dose trial is ongoing, and a Phase II trial initiation is projected to start in 2010.
Under the terms of these agreements with Merck, while the Company’s research activities have ceased, the cessation of those research activities did not affect other aspects of those agreements, including the ongoing Phase II and Phase I clinical trials and preclinical programs that Merck is conducting. The Company is entitled to payments resulting from the successful achievement by Merck of clinical and regulatory milestones, as well as royalty payments at different rates depending on the origin of collaboration products from discovery and optimization libraries at Ligand and Merck, and on net sales of products resulting from compounds being developed by Merck under those agreements.
Collaboration for VR1
In connection with the Company’s merger with Neurogen Corporation, the Company acquired a fully funded partnership with Merck for Vanilloid Receptor Subtype 1 (VR1) Antagonists. Merck will fund 100% of program costs and make milestone and royalty payments upon the achievement of certain development events and commercialization of any applicable VR1 compounds.
92
Table of Contents
As of December 31, 2009 and 2008, the Company had deferred revenue of approximately $0.4 million and $3.5 million, respectively, related to Merck.
Cephalon Collaboration
In connection with the merger with Pharmacopeia, the Company assumed a collaboration and license agreement, or the Cephalon Agreement, with Cephalon, Inc., or Cephalon, originally entered into in May 2006, which provides for the formation of a new drug discovery, development and commercialization alliance. Under the Cephalon agreement, Pharmacopeia received an up-front, non-refundable payment of $15.0 million in June 2006 to support its research efforts.
The Company and Cephalon executed an amendment in January 2009 to the collaboration agreement dated May 16, 2006. The agreement provided for that the Company would have no obligation to continue research activities with respect to the two active collaboration programs and was released to redeploy FTEs currently assigned to the collaboration. All licenses granted to the Company by Cephalon with respect to the two active collaboration programs terminated as of the date of amendment. The Company is entitled to milestone and royalty payments associated with only one of the two active programs. In addition, the Company agreed to provide certain chemistry services to Cephalon through a third party vendor for a term of nine months from the date of agreement, which ended in September 2009.
Celgene Collaboration
In connection with the merger with Pharmacopeia, the Company assumed a research and license agreement, or the Celgene Agreement, with Celgene Corporation, or Celgene. Under the Celgene Agreement the Company has no further research requirements. The collaboration with Celgene produced a compound that led to a clinical candidate currently being evaluated for the treatment of fibrotic and inflammatory diseases that entered a Phase I clinical trial in the first quarter of 2008. The Company is entitled to receive payments resulting from the successful achievement by Celgene of clinical milestones, as well as royalties on net sales of products resulting from the collaboration.
Exelixis Collaboration
The Company exclusively licensed certain technology to X-Ceptor Therapeutics in 1999. X-Ceptor was subsequently acquired by Exelixis Inc. in October 2004. Exelixis has three partnered programs based on X-Ceptor technologies, including (a) XL-652, a LXR agonist, is in Phase I development with BMS for the potential treatment of atherosclerosis and other coronary artery diseases, (b) FXR-450, a Farnesoid X receptor modulator, is in preclinical development with Pfizer for the potential treatment of hyperlipidemia including hypertriglyceridemia, and (c) Xl-550, a mineralocorticoid receptor modulator, is in preclinical development with Daiichi-Sankyo for the potential treatment of metabolic disorders and cardiovascular diseases. The Company is entitled to receive royalties on net sales of products.
PeriCorTherapeutics
The Company assumed a common stock ownership position in privately-held PeriCor Therapeutics, Inc. through its acquisition of Metabasis. PeriCor sublicensed rights from Metabasis to acadesine and three additional Adenosine Regulating Agents in 2005. PeriCor licensed acadesine to Schering-Plough Corporation (now Merck & Co.) and the compound is in a Phase III clinical trial for the prevention of adverse cardiovascular and cerebrovascular outcomes in patients undergoing coronary artery bypass graft surgery.
Trevena Collaboration
In February 2009, the Company announced the initiation of a joint research and license alliance to screen targets using Trevena’s novel biological platform against our combinatorial library of compounds, to identify active compounds with potential for development as novel G-protein coupled receptor (GPCR) therapeutics.
93
Table of Contents
Under the terms of the agreement, Trevena has been granted exclusive worldwide rights to sublicense active compounds resulting from the collaboration. The Company expects to screen targets and receive payments triggered by a tiered screening paradigm for each target.
15. Income Taxes
At December 31, 2009, the Company has federal net operating loss carryforwards of $513.8 million and $205.7 million of state net operating loss carryforwards. The Company also has $17.1 million of federal research and development credit carryforwards. Federal research and development credit carryforwards of $1.2 million expired at the beginning of 2010 with the remainder expiring through 2027, and the Company has $12.9 million of California and New Jersey research and development credit carryforwards that have no expiration date.
Pursuant to Internal Revenue Code Sections 382 and 383, use of net operating loss and credit carryforwards may be limited if there were changes in ownership of more than 50%. The Company has completed a Section 382 study for Ligand through December 31, 2007. As a result of ownership changes, utilization of Ligand’s net operating losses and credits are subject to limitations under Internal Revenue Code Sections 382 and 383. Included in the amounts above are $114.1 million of federal net operating loss carryforwards, $64.5 million of state net operating loss carryforwards, $3.5 million of federal research and development credit carryforwards, and $2.5 million of state research and development credit carryforwards related to Pharmacopeia. The Company has not completed a 382 study for Pharmacopeia. As such, the utilization of Pharmacopeia’s net operating losses and credits may be subject to limitations under Internal Revenue Code Sections 382 and 383. Also included in the amounts above are $11.0 million of federal net operating loss carryforwards, and $11.0 million of state net operating loss carryforwards related to Neurogen. The carryforwards for Neurogen have been limited to the amounts available under Internal Revenue Code Section 382.
The components of the income tax benefit for continuing operations are as follows (in thousands):
| Year Ended December 31, | |||||||||||
| 2009 | 2008 | 2007 | |||||||||
| Current Benefit: |
|||||||||||
| Federal |
$ | (23,533 | ) | $ | 27 | $ | 16,966 | ||||
| State |
— | — | 1,743 | ||||||||
| Foreign |
— | 28 | (12 | ) | |||||||
| (23,533 | ) | 55 | 18,697 | ||||||||
| Deferred Benefit: |
|||||||||||
| Federal |
25,068 | — | — | ||||||||
| State |
— | — | — | ||||||||
| Foreign |
— | — | — | ||||||||
| $ | 1,535 | $ | 55 | $ | 18,697 | ||||||
94
Table of Contents
Significant components of the Company’s deferred tax assets and liabilities as of December 31, 2009 and 2008 are shown below. A valuation allowance has been recognized to offset the net deferred tax assets, other than the net operating losses which will be utilized via carry-back to the 2006 and 2007 income tax years, as management believes realization of such assets is not more-likely-than-not as of December 31, 2009. A valuation allowance has been recognized to fully offset the net deferred tax assets as of December 31, 2008 as realization of such assets is not more-likely-than-not.
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (in thousands) | ||||||||
| Deferred assets: |
||||||||
| Net operating loss carryforwards |
$ | 184,075 | $ | 141,620 | ||||
| Research and AMT credit carryforwards |
29,093 | 35,657 | ||||||
| Capitalized research and development |
— | 300 | ||||||
| Fixed assets and intangibles |
4,785 | 6,255 | ||||||
| Accrued expenses |
269 | 6,042 | ||||||
| Deferred revenue |
1,420 | 8,823 | ||||||
| Litigation settlement reserve |
771 | 2,713 | ||||||
| Present value of AVINZA royalties |
16,633 | 19,703 | ||||||
| Organon termination asset |
(15,727 | ) | (22,128 | ) | ||||
| Organon termination liability |
15,727 | 22,128 | ||||||
| Organon royalty obligation |
569 | 818 | ||||||
| Deferred sale leaseback |
1,313 | 9,787 | ||||||
| Lease termination costs |
5,698 | — | ||||||
| Other |
5,366 | 5,085 | ||||||
| 249,992 | 236,803 | |||||||
| Valuation allowance for deferred tax assets |
(224,924 | ) | (236,803 | ) | ||||
| Net deferred tax assets |
$ | 25,068 | $ | — | ||||
For 2009 and 2008, stock option deductions did not impact the valuation allowance through paid-in capital. Other changes to the valuation allowance allocated directly to accumulated other comprehensive income (loss) are related to unrealized gains and losses on foreign currency transactions of $0.1 million, $0.01 million and $0.02 million for 2009, 2008, and 2007, respectively.
A reconciliation of income tax benefit for continuing operations to the amount computed by applying the statutory federal income tax rate to the loss from continuing operations is summarized as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Amounts computed at statutory federal rate |
$ | 3,387 | $ | 33,155 | $ | 18,174 | ||||||
| State taxes net of federal benefit |
234 | (2,293 | ) | 1,220 | ||||||||
| Effect of foreign operations |
— | 28 | (12 | ) | ||||||||
| Meals & entertainment |
(10 | ) | (7 | ) | (19 | ) | ||||||
| In process R&D from merger |
(136 | ) | (24,480 | ) | — | |||||||
| Stock-based compensation |
(1,144 | ) | (537 | ) | (910 | ) | ||||||
| Adjustment to NOLs and R&D tax credits |
(678 | ) | (678 | ) | — | |||||||
| Federal research and development credits |
(887 | ) | (155 | ) | 1,287 | |||||||
| FIN 48 liability and interest |
(24,116 | ) | ||||||||||
| Carry back claims |
25,651 | |||||||||||
| Change in valuation allowance |
(775 | ) | (5,019 | ) | (1,043 | ) | ||||||
| Other |
9 | 41 | — | |||||||||
| $ | 1,535 | $ | 55 | $ | 18,697 | |||||||
95
Table of Contents
A reconciliation of income tax benefit (expense) for discontinued operations to the amount computed by applying the statutory federal income tax rate to income from discontinued operations is summarized as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Amounts computed at statutory federal rate |
$ | (2,172 | ) | $ | 356 | $ | (115,333 | ) | ||||
| State taxes net of federal benefit |
150 | 219 | 3,109 | |||||||||
| Effect of foreign operations |
— | — | — | |||||||||
| Stock-based compensation |
— | — | (40 | ) | ||||||||
| Release of FIN 48 liability |
— | — | 398 | |||||||||
| Change in valuation allowance |
2,022 | (204 | ) | 89,001 | ||||||||
| Other |
— | 21 | 98 | |||||||||
| $ | — | $ | 392 | $ | (22,767 | ) | ||||||
Tax positions must meet a minimum probability threshold that a tax position must meet before a financial statement benefit is recognized. The minimum threshold is defined as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. As of the date of adoption, the Company’s gross liability for income taxes associated with uncertain tax positions totaled $8.9 million. As a result of the implementation of FIN 48, the Company recognized an increase of $0.4 million to reserve for uncertain tax positions which was recorded as a cumulative effect adjustment to accumulated deficit. The Company’s remaining FIN 48 liabilities are presented net of the deferred tax asset balances on the accompanying consolidated balance sheet.
In December 2009, the Internal Revenue Service, or IRS, issued the Company a Notice of Proposed Adjustment, or NOPA, seeking an increase to its taxable income for the 2007 fiscal year of $71.5 million and a $4.1 million penalty for substantial underpayment of tax in fiscal 2007. The Company responded to the NOPA in February 2010, disagreeing with the conclusions reached by the IRS in the NOPA. As of December 31, 2009, the Company recorded a FIN 48 liability of $25.1 million related to the income tax effect of the NOPA and $3.0 million related to estimated interest due on the proposed underpayment of tax. The Company also recorded deferred income tax assets of $25.1 million associated with the ability to carry back losses from 2008 and 2009 to offset the NOPA. In addition, the Company recorded an income tax receivable of $4.5 million associated with changes in income tax law in relation to prior AMT taxes paid on carry back periods, which is included in other non-current assets at December 31, 2009. The Company has not recorded the penalties proposed by the IRS in its financial statements as the Company believes that it has met the appropriate standard for the tax position on its 2007 tax return. If the Company is unsuccessful in its negotiations with the IRS, it may be required to pay the $4.1 million penalty and utilize a significant amount of its net operating loss carryforwards.
A reconciliation of the amount of unrecognized tax benefits at December 31, 2009 and 2008 is as follows (in thousands):
| Balance at December 31, 2007 |
9,467 | |||
| Additions based on tax positions related to the current year |
322 | |||
| Reductions for tax positions of prior years |
(262 | ) | ||
| Balance at December 31, 2008 |
9,527 | |||
| Additions based on tax positions related to the current year |
25,068 | |||
| Reductions for tax positions of prior years |
(569 | ) | ||
| Balance at December 31, 2009 |
$ | 34,026 | ||
96
Table of Contents
Included in the balance of unrecognized tax benefits at December 31, 2009 is $34.0 million of tax benefits that, if recognized would result in adjustments to the related deferred tax assets and valuation allowance and not affect the Company’s effective tax rate.
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2009, accrued interest related to uncertain tax positions is $3.0 million.
All of the Company’s tax years from 1991-2009 remain open to examination by the major taxing jurisdictions to which the Company is subject.
Management’s estimate of the reasonably possible change in the amount of the unrecognized tax benefits is a decrease of $25.2 million during the next twelve months. This is due to the expiration of the federal carry over period for research credits reported and to expected settlements with the Internal Revenue Service.
16. Summary of Unaudited Quarterly Financial Information
The following is a summary of the unaudited quarterly results of operations for the years ended December 31, 2009 and 2008 (in thousands, except per share amounts).
| Quarter ended | ||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||
| 2009 |
||||||||||||||
| Total revenues |
$ | 9,470 | $ | 7,594 | $ | 7,901 | $ | 13,974 | ||||||
| Total operating costs and expenses |
17,279 | 12,742 | 27,571 | 13,148 | ||||||||||
| Income tax benefit (expense) |
— | — | — | — | ||||||||||
| Income (loss) from continuing operations |
(7,482 | ) | (4,476 | ) | 1,055 | 2,566 | ||||||||
| Discontinued operations |
2,366 | 2,808 | 748 | 467 | ||||||||||
| Net income (loss) |
$ | (5,116 | ) | $ | (1,668 | ) | $ | 1,803 | $ | 3,033 | ||||
| Basic and diluted per share amounts: |
||||||||||||||
| Loss from continuing operations |
(0.07 | ) | (0.04 | ) | 0.01 | 0.02 | ||||||||
| Discontinued operations |
0.02 | 0.03 | 0.01 | 0.01 | ||||||||||
| Net loss |
$ | (0.05 | ) | $ | (0.01 | ) | $ | 0.02 | $ | 0.03 | ||||
| Weighted average shares—basic |
113,118 | 113,148 | 113,007 | 113,382 | ||||||||||
| Weighted average shares—diluted |
113,118 | 113,148 | 113,139 | 113,51 | ||||||||||
| Quarter ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| 2008 |
||||||||||||||||
| Total revenues |
$ | 4,874 | $ | 4,804 | $ | 5,248 | $ | 12,389 | ||||||||
| Total operating costs and expenses |
17,264 | 10,928 | 12,094 | 86,269 | ||||||||||||
| Income tax benefit (expense) |
1,781 | 1,030 | (2,990 | ) | 234 | |||||||||||
| Loss from continuing operations |
(9,717 | ) | (4,889 | ) | (9,124 | ) | (73,730 | ) | ||||||||
| Discontinued operations |
5,784 | (1,540 | ) | (9,001 | ) | 4,103 | ||||||||||
| Net loss |
$ | (3,933 | ) | $ | (6,429 | ) | $ | (18,125 | ) | $ | (69,627 | ) | ||||
| Basic and diluted per share amounts: |
||||||||||||||||
| Loss from continuing operations |
(0.10 | ) | (0.05 | ) | (0.10 | ) | (0.76 | ) | ||||||||
| Discontinued operations |
0.06 | 0.02 | (0.09 | ) | 0.04 | |||||||||||
| Net loss |
$ | (0.04 | ) | $ | (0.07 | ) | $ | (0.19 | ) | $ | (0.72 | ) | ||||
| Weighted average shares—basic and diluted |
95,047 | 99,056 | 95,068 | 96,841 | ||||||||||||
17. Sale Leaseback
In October 2006, the Company, along with its wholly-owned subsidiary Nexus, entered into an agreement with Slough for the sale of its real property located in San Diego, California for a purchase price of $47.6
97
Table of Contents
million. This property, with a net book value of $14.5 million, included one building totaling approximately 82,500 square feet, the land on which the building is situated, and two adjacent vacant lots. As part of the sale transaction, the Company agreed to lease back the building for a period of 15 years.
The Company recognized an immediate pre-tax gain on the sale transaction of $3.1 million in 2006 and deferred a gain of $29.5 million on the sale of the building. The deferred gain was being recognized as an offset to operating expense on a straight-line basis over the 15 year term of the lease at a rate of approximately $2.0 million per year.
In August 2009, the Company entered into a lease termination agreement for this building. As a result, the Company recognized an additional $20.4 million of accretion of deferred gain during the quarter ended September 30, 2009, and will recognize the remaining balance of the deferred gain of $3.1 million through the term of its new building lease, which expires in December 2011. The amount of the deferred gain recognized for the years ended December 31, 2009, 2008 and 2007 was $21.9 million, $2.0 million and $2.0 million, respectively.
18. Reductions in Workforce
In December 2009, the Company announced a reduction in its workforce of twelve positions, six of which were eliminated effective December 31, 2009, five were eliminated in early 2010 and one will be eliminated effective June 30, 2010. Accrued severance costs of $0.4 million were included in the accrued restructuring costs as of December 31, 2009.
In December 2008, Pharmacopeia announced a reduction in its workforce of thirty positions, twenty-two of which were eliminated effective December 31, 2008 and the remaining eight of which were eliminated effective June 30, 2009. Accrued severance costs of $0.7 million was included in the accrued restructuring costs as of December 31, 2008. Also included in accrued restructuring costs was a $0.2 million of costs to exit a leased facility which is comprised of the difference between the remaining lease obligations of the abandoned operating leases, which run through the year 2016, and the Company’s estimate of potential future sublease income, discounted to present value.
In December 2007, the Company entered into a plan to eliminate approximately 27 employee positions, across all functional areas, which were no longer deemed necessary in connection with the Company’s ongoing efforts to be a highly-focused research and development and royalty-driven biotech company. The affected employees were informed of the plan in December 2007 with an effective termination date of December 31, 2007 for the majority of the affected employees. The Company completed the plan by the end of the first quarter of 2008. In connection with the termination plan, the Company recognized expenses of $1.1 million in the fourth quarter of 2007 which were paid in the first quarter of 2008.
In the fourth quarter of 2006, following the sale of the Company’s Oncology Product Line to Eisai, and in the first quarter of 2007, following the sale of AVINZA to King, the Company eliminated nearly 270 employee positions, across all functional areas, which were no longer deemed necessary as a result of the Company’s decision to sell its commercial assets and refocus the Company as a smaller, highly-focused research and development and royalty-driven biotechnology company. As a result, the Company recognized expenses of $11.3 million in 2007.
19. Subsequent Event
On January 27, 2010, the Company completed the acquisition of Metabasis Therapeutics, Inc. (NASDAQ: MBRX), following approval of the transaction by Metabasis stockholders. As a result, the Company gained a fully funded partnership with Roche, additional pipeline assets and drug discovery technologies and resources. The transaction was first announced on October 27, 2009. The Company paid $1.6 million in cash or about $0.046 per Metabasis share to Metabasis’ stockholders. In addition, Metabasis stockholders received four
98
Table of Contents
tradable Contingent Value Rights (CVRs), one CVR from each of four respective series of CVRs, for each Metabasis share. The CVRs will entitle Metabasis stockholders to cash payments as frequently as every six months as cash is received by the Company from proceeds from Metabasis’ partnership with Roche or the sale or partnering of any of the Metabasis drug development programs, among other triggering events. The Company has also committed to spend at least $8 million in new research and development funding on the Metabasis programs within 42 months following the closing of the transaction.
The components of the preliminary purchase price allocation for Metabasis are as follows:
| Purchase Consideration: |
||||
| (in thousands) | ||||
| Cash paid to Metabasis shareholders |
$ | 1,641 | ||
| Fair value of contingent value rights |
8,054 | |||
| Total purchase consideration |
$ | 9,695 | ||
| Allocation of Purchase Price: |
||||
| (in thousands) | ||||
| Cash acquired |
$ | 390 | ||
| Other current assets |
271 | |||
| Goodwill and other identifiable intangible assets |
11,113 | |||
| Liabilities assumed |
(2,079 | ) | ||
| $ | 9,695 | |||
For purposes of the preliminary purchase price allocation, the estimated fair value of the Roche, TR Beta, Glucagon and General Contingent Value Rights is based upon the total estimated fair value of Metabasis of approximately $8 million, which is consistent with Metabasis’ market value as of the closing date of the merger. For purposes of estimating the preliminary purchase price, Ligand’s management assumed that an aggregate of 50% of the total identifiable intangible assets’ estimated fair value would be paid out to Metabasis stockholders under the provisions of the Contingent Value Rights agreements.
99
Table of Contents
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
(a) Disclosure Controls and Procedures
The Company is required to maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in its reports under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to management, including the Company’s Chief Executive Officer (CEO) and Chief Financial Officer (CFO) as appropriate, to allow timely decisions regarding required disclosure.
In connection with the preparation of this Form 10-K for the year ended December 31, 2009, management, under the supervision of the CEO and CFO, conducted an evaluation of disclosure controls and procedures. Based on that evaluation, the CEO and CFO concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2009.
(b) Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of the Company’s financial reporting for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect the Company’s transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of the Company’s financial statements in accordance with generally accepted accounting principles; providing reasonable assurance that receipts and expenditures of the Company are made in accordance with management and directors of the Company; and providing reasonable assurance that unauthorized acquisition, use or disposition of company assets that could have a material effect on the Company’s financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of the Company’s financial statements would be prevented or detected.
Management conducted an evaluation of the effectiveness of the Company’s internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation, management concluded that the Company’s internal control over financial reporting was effective as of December 31, 2009.
On December 23, 2009, the Company completed the acquisition of Neurogen Corporation and, as permitted by SEC guidance, the Company excluded from its assessment of the effectiveness of its internal control over financial reporting as of December 31, 2009, the internal control over financial reporting of this entity. Total assets and revenues constituted twelve and zero percent, respectively, of the Company’s consolidated financial statement amounts as of and for the year ended December 31, 2009. The Company plans to integrate Neurogen Corporation’s historical internal control over financial reporting into its own internal control over financial reporting in 2010. Accordingly, certain changes will be made to the Company’s internal control over financial reporting until such time as this integration is complete.
Grant Thornton LLP, the Company’s independent registered public accountants, has audited the effectiveness of the Company’s internal control over financial reporting as of December 31, 2009, based on the COSO criteria; their report is included in Item 9A.
100
Table of Contents
Report of Independent Registered Public Accounting Firm
Board of Directors and Shareholders
Ligand Pharmaceuticals Incorporated
We have audited Ligand Pharmaceuticals Incorporated and subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Ligand Pharmaceuticals Incorporated’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on Ligand Pharmaceuticals Incorporated and subsidiaries’ internal control over financial reporting based on our audit. Our audit of, and opinion on, the Company’s internal control over financial reporting does not include internal control over financial reporting of Neurogen Corporation, a wholly owned subsidiary, whose financial statements reflect total assets and revenues constituting twelve and zero percent, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2009. As indicated in Management’s Report on Internal Control Over Financial Reporting, Neurogen Corporation was acquired during 2009 and therefore, management’s assertion on the effectiveness of the Company’s internal control over financial reporting excluded internal control over financial reporting of Neurogen Corporation.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Ligand Pharmaceuticals Incorporated and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Ligand Pharmaceuticals Incorporated and subsidiaries as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ equity (deficit) and comprehensive income (loss), and cash flows for the years then ended and our report dated March 3, 2010, expressed an unqualified opinion and contained an explanatory paragraph relating to the change in accounting for business combinations.
/s/ Grant Thornton LLP
San Diego, California
March 3, 2010
101
Table of Contents
| Item 9B. | Other Information |
On February 9, 2010, the Company issued a press release (the “Initial Release”) announcing its financial results for the quarter and year ended December 31, 2009, a copy of which was furnished as Exhibit 99.1 on a Current Report on Form 8-K filed on the same date. In its condensed consolidated statement of operations included in the Initial Release, the Company reported an income tax benefit of approximately $1.5 million as an income tax benefit on discontinued operations and an income tax receivable of approximately $1.5 million which was included in other non-current assets on its condensed consolidated balance sheet. Subsequent to the Company’s earnings release, as a result of continuing analysis of authoritative literature, the Company determined that the income tax benefit should be recorded in continuing operations instead of in discontinued operations. The Company also determined that it should record a FIN 48 liability of approximately $25.1 million related to the income tax effect of a Notice of Proposed Adjustment received from the IRS in December 2009 and a FIN 48 liability of approximately $3.0 million related to estimated interest due on the proposed underpayment of tax, as well as deferred income tax assets of approximately $25.1 million related to its ability to carry back net operating losses and income tax receivables of approximately $4.5 million related to changes in tax laws.
As a result of the foregoing, for the quarter ended December 31, 2009, income from continuing operations increased to approximately $2.6 million, or $0.02 per share, from $1.0 million, or $0.01 per share, and income from discontinued operations decreased to approximately $0.5 million, or $0.01 per share, from $2.0 million, or $0.02 per share. There was no change to total net income for the quarter ended December 31, 2009. For the year ended December 31, 2009, loss from continuing operations decreased to approximately $8.3 million, or $0.08 per share, from $9.9 million, or $0.09 per share, and income from discontinued operations decreased to approximately $6.4 million, or $0.06 per share, from $7.9 million, or $0.07 per share. There was no change to total net income for the year ended December 31, 2009. Additionally, total assets increased to approximately $141.8 million from $113.7 million, and total liabilities increased to approximately $129.7 million from $101.6 million.
| Item 10. | Directors, Executive Officers and Corporate Governance |
Code of Conduct
The Board of Directors has adopted a Code of Conduct and Ethics Policy (“Code of Conduct”) that applies to all officers, directors and employees. The Company will promptly disclose any material amendment or waiver to the Code of Conduct which affects any corporate officer. The Code of Conduct was filed with the SEC as an exhibit to our report on Form 10-K for the year ended December 31, 2003, and can be accessed via our website (http://www.ligand.com), Corporate Overview page. You may also request a free copy by writing to: Investor Relations, Ligand Pharmaceuticals Incorporated, 11085 North Torrey Pines Road, Suite 300, San Diego, CA 92121.
The other information under Item 10 is hereby incorporated by reference from Ligand’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission on or prior to April 29, 2010. See also the identification of the executive officers following Item 4 of this Annual Report on Form 10-K.
| Item 11. | Executive Compensation |
Item 11 is hereby incorporated by reference from Ligand’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission on or prior to April 29, 2010.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Item 12 is hereby incorporated by reference from Ligand’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission on or prior to April 29, 2010.
102
Table of Contents
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Item 13 is hereby incorporated by reference from Ligand’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission on or prior to April 29, 2010.
| Item 14. | Principal Accountant Fees and Services |
Item 14 is hereby incorporated by reference from Ligand’s Definitive Proxy Statement to be filed with the Securities and Exchange Commission on or prior to April 29, 2010.
103
Table of Contents
| Item 15. | Exhibits and Financial Statement Schedule |
(a) The following documents are included as part of this Annual Report on Form 10-K.
(1) Financial statements
| Index to Consolidated Financial Statements |
||
| Report of Independent Registered Public Accounting Firm—Grant Thornton LLP |
||
| Report of Independent Registered Public Accounting Firm—BDO Seidman, LLP |
||
| Consolidated Balance Sheets |
||
| Consolidated Statements of Operations |
||
| Consolidated Statements of Stockholders’ Equity (Deficit) and Comprehensive Income (Loss) |
||
| Consolidated Statements of Cash Flows |
||
| Notes to Consolidated Financial Statements |
(2) Schedules not included herein have been omitted because they are not applicable or the required information is in the consolidated financial statements or notes thereto.
(3) The following exhibits are filed as part of this Form 10-K and this list includes the Exhibit Index.
| Exhibit |
Description | |
| 2.1 (1) | Agreement and Plan of Reorganization dated May 11, 1998, by and among the Company, Knight Acquisition Corp. and Seragen, Inc. (Filed as Exhibit 2.1). | |
| 2.2 (58) | Agreement and Plan of Merger, dated as of September 24, 2008, by and among Ligand Pharmaceuticals Incorporated, Pharmacopeia, Inc., Margaux Acquisition Corp. and Latour Acquisition, LLC. (Exhibit 2.1). | |
| 2.3 (1) | Form of Certificate of Merger for acquisition of Seragen, Inc. (Filed as Exhibit 2.2). | |
| 2.4 (83) | Agreement and Plan of Merger, by and among the Company, Neurogen Corporation and Neon Signal, LLC, dated as of August 23, 2009 (Filed as Exhibit 10.1). | |
| 2.5 (87) | Amendment to Agreement and Plan of Merger, by and among the Company, Neurogen Corporation, and Neon Signal, LLC, dated September 18, 2009 (Filed as Exhibit 10.1). | |
| 2.6 (87) | Amendment No. 2 to Agreement and Plan of Merger, by and among the Company, Neurogen Corporation, and Neon Signal, LLC, dated November 2, 2009 (Filed as Exhibit 10.2). | |
| 2.7 (85) | Amendment No. 3 to Agreement and Plan of Merger, by and among the Company, Neurogen Corporation, and Neon Signal, LLC, dated November 2, 2009 (Filed as Exhibit 10.2). | |
| 2.8 (84) | Certificate of Merger for acquisition of Neurogen Corporation (Filed as Exhibit 2.1). | |
| 2.9 (88) | Agreement and Plan of Merger, dated as of October 26, 2009, by and among Ligand Pharmaceuticals Incorporated, Metabasis Therapeutics, Inc., and Moonstone Acquisition, Inc. (Exhibit 10.1) | |
| 2.10 (86) | Amendment to Agreement and Plan of Merger, by and among Ligand Pharmaceuticals Incorporated, Metabasis Therapeutics, Inc., Moonstone Acquisition, Inc., and David F. Hale as Stockholders’ Representative, dated November 25, 2009 | |
| 3.1 (1) | Amended and Restated Certificate of Incorporation of the Company. (Filed as Exhibit 3.2). | |
104
Table of Contents
| Exhibit |
Description | |
| 3.2 (1) | Bylaws of the Company, as amended. (Filed as Exhibit 3.3). | |
| 3.3 (2) | Amended Certificate of Designation of Rights, Preferences and Privileges of Series A Participating Preferred Stock of the Company. | |
| 3.4 (20) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company dated June 14, 2000. | |
| 3.5 (3) | Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company dated September 30, 2004. | |
| 3.6 (31) | Amendment to the Bylaws of the Company dated November 13, 2005. (Filed as Exhibit 3.1). | |
| 3.7 (56) | Amendment of Bylaws of the Company dated December 4, 2007. (Filed as Exhibit 3.1). | |
| 4.1 (4) | Specimen stock certificate for shares of Common Stock of the Company. | |
| 4.2 (24) | Pledge Agreement dated November 26, 2002, between Ligand Pharmaceuticals Incorporated and J.P. Morgan Trust Company, National Association. (Filed as Exhibit 4.5). | |
| 4.3 (24) | Control Agreement dated November 26, 2002, among Ligand Pharmaceuticals Incorporated, J.P. Morgan Trust Company, National Association and JP Morgan Chase Bank. (Filed as Exhibit 4.6). | |
| 4.4 (44) | 2006 Preferred Shares Rights Agreement, by and between Ligand Pharmaceuticals Incorporated and Mellon Investor Services LLC, dated as of October 13, 2006. (Filed as Exhibit 4.1) | |
| 10.1 (35) | Second Amendment to Non-Qualified Deferred Compensation Plan. | |
| 10.3 (4) | Form of Stock Issuance Agreement. | |
| 10.30 (4) | Form of Proprietary Information and Inventions Agreement. | |
| 10.33 (4) | License Agreement, dated November 14, 1991, between the Company and Rockefeller University (with certain confidential portions omitted). | |
| 10.34 (4) | License Agreement and Bailment, dated July 22, 1991, between the Company and the Regents of the University of California (with certain confidential portions omitted). | |
| 10.35 (4) | Agreement, dated May 1, 1991, between the Company and Pfizer Inc (with certain confidential portions omitted). | |
| 10.38 (4) | License Agreement, dated January 5, 1990, between the Company and the University of North Carolina at Chapel Hill (with certain confidential portions omitted). | |
| 10.41 (4) | License Agreement, dated October 1, 1989, between the Company and Institute Pasteur (with certain confidential portions omitted). | |
| 10.46 (4) | Form of Indemnification Agreement between the Company and each of its directors. | |
| 10.47 (4) | Form of Indemnification Agreement between the Company and each of its officers. | |
| 10.58 (4) | Stock Purchase Agreement, dated September 9, 1992, between the Company and Glaxo, Inc. | |
| 10.59 (4) | Research and Development Agreement, dated September 9, 1992, between the Company and Glaxo, Inc. (with certain confidential portions omitted). | |
| 10.60 (4) | Stock Transfer Agreement, dated September 30, 1992, between the Company and the Rockefeller University. | |
| 10.61 (4) | Stock Transfer Agreement, dated September 30, 1992, between the Company and New York University. | |
105
Table of Contents
| Exhibit |
Description | |
| 10.62 (4) | License Agreement, dated September 30, 1992, between the Company and the Rockefeller University (with certain confidential portions omitted). | |
| 10.73 (14) | Supplementary Agreement, dated October 1, 1993, between the Company and Pfizer, Inc. to Agreement, dated May 1, 1991. | |
| 10.78 (15) | Research, Development and License Agreement, dated July 6, 1994, between the Company and Abbott Laboratories (with certain confidential portions omitted). (Filed as Exhibit 10.75). | |
| 10.83 (15) | Option Agreement, dated September 2, 1994, between the Company and American Home Products Corporation, as represented by its Wyeth-Ayerst Research Division (with certain confidential portions omitted). (Filed as Exhibit 10.80). | |
| 10.93 (5) | Indemnity Agreement, dated June 3, 1995, between the Company, Allergan, Inc. and Allergan Ligand Retinoid Therapeutics, Inc. | |
| 10.97 (5) | Research, Development and License Agreement, dated December 29, 1994, between SmithKline Beecham Corporation and the Company (with certain confidential portions omitted). | |
| 10.98 (5) | Stock and Note Purchase Agreement, dated February 2, 1995, between SmithKline Beecham Corporation, S.R. One, Limited and the Company (with certain confidential portions omitted). | |
| 10.140 (18) | Promissory Notes, General Security Agreements and a Credit Terms and Conditions letter dated March 31, 1995, between the Company and Imperial Bank (Filed as Exhibit 10.101). | |
| 10.148 (16) | Lease, dated July 6, 1994, between the Company and Chevron/Nexus partnership, First Amendment to lease dated July 6, 1994. | |
| 10.150 (6) | Master Lease Agreement, signed May 30, 1996, between the Company and USL Capital Corporation. | |
| 10.151 (17) | Settlement Agreement and Mutual Release of all Claims, signed April 20, 1996, between the Company and Pfizer, Inc. (with certain confidential portions omitted). | |
| 10.152 (17) | Letter Amendment to Abbott Agreement, dated March 14, 1996, between the Company and Abbott Laboratories (with certain confidential portions omitted). | |
| 10.157 (6) | Master Lease Agreement, signed February 13, 1997, between the Company and Lease Management Services. | |
| 10.163 (19) | Extension of Master Lease Agreement between Lease Management Services and Ligand Pharmaceuticals dated July 29, 1997. | |
| 10.167 (7) | Development and License Agreement, dated November 25, 1997, between the Company and Eli Lilly and Company (with certain confidential portions omitted). | |
| 10.168 (7) | Collaboration Agreement, dated November 25, 1997, among the Company, Eli Lilly and Company, and Allergan Ligand Retinoid Therapeutics, Inc. (with certain confidential portions omitted). | |
| 10.169 (7) | Option and Wholesale Purchase Agreement, dated November 25, 1997, between the Company and Eli Lilly and Company (with certain confidential portions omitted). | |
| 10.171 (7) | First Amendment to Option and Wholesale Purchase Agreement dated February 23, 1998, between the Company and Eli Lilly and Company (with certain confidential portions omitted). | |
| 10.172 (7) | Second Amendment to Option and Wholesale Purchase Agreement, dated March 16, 1998, between the Company and Eli Lilly and Company (with certain confidential portions omitted). | |
106
Table of Contents
| Exhibit |
Description | |
| 10.184 (9) | Letter agreement, dated May 11, 1998, by and among the Company, Eli Lilly and Company and Seragen, Inc. (Filed as Exhibit 99.6). | |
| 10.185 (1) | Amendment No. 3 to Option and Wholesale Purchase Agreement, dated May 11, 1998, by and between Eli Lilly and Company and the Company. (Filed as Exhibit 10.6). | |
| 10.186 (1) | Agreement, dated May 11, 1998, by and among Eli Lilly and Company, the Company and Seragen, Inc. (Filed as Exhibit 10.7). | |
| 10.188 (9) | Settlement Agreement, dated May 1, 1998, by and among Seragen, Inc., Seragen Biopharmaceuticals Ltd./Seragen Biopharmaceutique Ltee, Sofinov Societe Financiere D’Innovation Inc., Societe Innovatech Du Grand Montreal, MDS Health Ventures Inc., Canadian Medical Discoveries Fund Inc., Royal Bank Capital Corporation and Health Care and Biotechnology Venture Fund (Filed as Exhibit 99.2). | |
| 10.189 (9) | Accord and Satisfaction Agreement, dated May 11, 1998, by and among Seragen, Inc., Seragen Technology, Inc., Trustees of Boston University, Seragen LLC, Marathon Biopharmaceuticals, LLC, United States Surgical Corporation, Leon C. Hirsch, Turi Josefsen, Gerald S.J. and Loretta P. Cassidy, Reed R. Prior, Jean C. Nichols, Elizabeth C. Chen, Robert W. Crane, Shoreline Pacific Institutional Finance, Lehman Brothers Inc., 520 Commonwealth Avenue Real Estate Corp. and 660 Corporation (Filed as Exhibit 99.4). | |
| 10.191 (8) | Letter of Agreement dated September 28, 1998 among the Company, Elan Corporation, plc and Elan International Services, Ltd. (with certain confidential portions omitted), (Filed as Exhibit 10.5). | |
| 10.198 (10) | Stock Purchase Agreement by and between the Company and Warner-Lambert Company dated September 1, 1999 (with certain confidential portions omitted). (Filed as Exhibit 10.2). | |
| 10.200 (10) | Nonexclusive Sublicense Agreement, effective September 8, 1999, by and among Seragen, Inc., Hoffmann-La Roche Inc. and F. Hoffmann-La Roche Ltd. (with certain confidential portions omitted). (Filed as Exhibit 10.4). | |
| 10.203 (10) | License Agreement effective June 30, 1999 by and between the Company and X-Ceptor Therapeutics, Inc. (with certain confidential portions omitted). (Filed as Exhibit 10.7). | |
| 10.218 (11) | Royalty Stream Purchase Agreement dated as of December 31, 1999 among Seragen, Inc., the Company, Pharmaceutical Partners, L.L.C., Bioventure Investments, Kft, and Pharmaceutical Royalties, LLC. (with certain confidential portions omitted). | |
| 10.220 (12) | Research, Development and License Agreement by and between Organon Company and Ligand Pharmaceuticals Incorporated dated February 11, 2000 (with certain confidential portions omitted). | |
| 10.224 (13) | Research, Development and License Agreement by and between Bristol Myers Squibb Company and Ligand Pharmaceuticals Incorporated dated May 19, 2000 (with certain confidential portions omitted). | |
| 10.230 (20) | Amended and Restated Registration Rights Agreement, dated as of June 29, 2000 among the Company and certain of its investors. | |
| 10.242 (21) | First Addendum to Amended and Restated Registration Rights Agreement dated June 29, 2000, effective as of December 20, 2001. | |
| 10.244 (22) | Second Addendum to Amended and Restated Registration Rights Agreement dated June 29, 2000, effective as of March 28, 2002. | |
| 10.245 (22) | Purchase Agreement, dated March 6, 2002, between the Company and Pharmaceutical Royalties International (Cayman) Ltd. | |
107
Table of Contents
| Exhibit |
Description | |
| 10.246 (23) | Amended and Restated License Agreement Between The Salk Institute for Biological Studies and the Company (with certain confidential portions omitted). | |
| 10.247 (23) | Amendment Number 1 to Purchase Agreement, dated July 29, 2002, between the Company and Pharmaceutical Royalties International (Cayman) Ltd. | |
| 10.250 (25) | Amended and Restated License and Supply Agreement, dated December 6, 2002, between the Company, Elan Corporation, plc and Elan Management Limited (with certain confidential portions omitted). | |
| 10.252 (25) | Amendment Number 1 to Amended and Restated Registration Rights Agreement, dated November 12, 2002, between the Company and Elan Corporation plc and Elan International Services, Ltd. | |
| 10.253 (25) | Second Amendment to Purchase Agreement, dated December 19, 2002, between the Company and Pharmaceuticals Royalties International (Cayman) Ltd. | |
| 10.254 (25) | Amendment Number 3 to Purchase Agreement, dated December 30, 2002, between the Company and Pharmaceuticals Royalties International (Cayman) Ltd. (with certain confidential portions omitted). | |
| 10.255 (25) | Purchase Agreement, dated December 30, 2002, between the Company and Pharmaceuticals Royalties International (Cayman) Ltd. (with certain confidential portions omitted). | |
| 10.256 (26) | Co-Promotion Agreement, dated January 1, 2003, by and between the Company and Organon Pharmaceuticals USA Inc. (with certain confidential portions omitted). | |
| 10.259 (27) | Amendment No. 2 to Amended and Restated Registration Rights Agreement, dated June 25, 2003. | |
| 10.261 (28) | Letter Agreement, dated July 1, 2003, between the Company and Paul V. Maier. | |
| 10.264 (29) | Option Agreement Between Investors Trust & Custodial Services (Ireland) Ltd., as Trustee for Royalty Pharma, Royalty Pharma Finance Trust and the Company, dated October 1, 2003 (with certain confidential portions omitted). | |
| 10.265 (29) | Amendment to Purchase Agreement Between Royalty Pharma Finance Trust and the Company, dated October 1, 2003 (with certain confidential portions omitted). | |
| 10.267 (36) | 2002 Stock Incentive Plan (as amended and restated through March 9, 2006). | |
| 10.268 (29) | 2002 Employee Stock Purchase Plan, dated July 1, 2002 (as amended through June 30, 2003). | |
| 10.269 (29) | Form of Stock Option Agreement. | |
| 10.270 (29) | Form of Employee Stock Purchase Plan Stock Purchase Agreement. | |
| 10.271 (29) | Form of Automatic Stock Option Agreement. | |
| 10.272 (29) | Form of Director Fee Stock Option Agreement. | |
| 10.273 (30) | Letter Agreement, dated as of February 26, 2004, between the Company and Martin Meglasson. | |
| 10.274 (30) | Adoption Agreement for Smith Barney Inc. Execchoice (R) Nonqualified Deferred Compensation Plan. | |
| 10.276 (30) | Manufacturing and Packaging Agreement, dated February 13, 2004 between Cardinal Health PTS, LLC and the Company (with certain confidential portions omitted). | |
| 10.279 (32) | Form of Distribution, Storage, Data and Inventory Management Services Agreement. | |
108
Table of Contents
| Exhibit |
Description | |
| 10.280 (32) | Amendment Number 1 to the Option Agreement between Investors Trust & Custodial Services (Ireland) Ltd., solely in its capacity as Trustee for Royalty Pharma, Royalty Pharma Finance Trust and Ligand Pharmaceuticals Incorporated dated November 5, 2004. | |
| 10.281 (32) | Amendment to Agreement among Ligand Pharmaceuticals Incorporated, Seragen, Inc. and Eli Lilly and Company dated November 8, 2004. | |
| 10.282 (32) | Amendment to Purchase Agreement between Royalty Pharma Finance Trust, Ligand Pharmaceuticals Incorporated & Investors Trust and Custodial Services (Ireland) Ltd., solely in its capacity as Trustee of Royalty Pharma dated November 5, 2004. | |
| 10.283 (34) | Form of Management Lockup Agreement. | |
| 10.287 (36) | Amended and Restated Research, Development and License Agreement dated as of December 1, 2005 between the Company and Wyeth (formerly American Home Products Corporation) (with certain confidential portions omitted). | |
| 10.288 (33) | Settlement Agreement dated as of December 2, 2005 by and among Ligand Pharmaceuticals Incorporated and Third Point LLC, Third Point Offshore Fund, Ltd., Third Point Partners LP, Third Point Ultra Ltd., Lyxor/Third Point Fund Ltd., and Third Point Partners Qualified LP. (Filed as Exhibit 10.1). | |
| 10.289 (36) | Form of Stock Issuance Agreement for non-employee directors. | |
| 10.290 (36) | Form of Amended and Restated Director Fee Stock Option Agreement for 2005 award to Alexander Cross. | |
| 10.291 (36) | Form of Amended and Restated Director Fee Stock Option Agreement for 2005 award to Henry Blissenbach, John Groom, Irving Johnson, John Kozarich, Daniel Loeb, Carl Peck, Jeffrey Perry, Brigette Roberts and Michael Rocca. | |
| 10.292 (37) | Termination and Return of Rights Agreement between Ligand Pharmaceuticals Incorporated and Organon USA Inc. dated as of January 1, 2006 | |
| 10.292A (38) | Form of Letter Agreement between the Company and certain of its officers dated as of March 1, 2006 (Filed as Exhibit 10.292). | |
| 10.293 (40) | First Amendment to the Manufacturing and Packaging Agreement between Cardinal Health PTS, LLC and Ligand Pharmaceuticals Incorporated (with certain confidential portions omitted). | |
| 10.294 (42) | Purchase Agreement, by and between Ligand Pharmaceuticals Incorporated, King Pharmaceuticals, Inc. and King Pharmaceuticals Research and Development, Inc., dated as of September 6, 2006. | |
| 10.295 (43) | Contract Sales Force Agreement, by and between Ligand Pharmaceuticals Incorporated and King Pharmaceuticals, Inc. dated as of September 6, 2006. | |
| 10.296 (42) | Purchase Agreement, by and among Ligand Pharmaceuticals Incorporated, Seragen, Inc., Eisai Inc. and Eisai Co., Ltd., dated as of September 7, 2006. | |
| 10.297 (39) | Separation Agreement dated as of July 31, 2006 by and between the Company and David E. Robinson. | |
| 10.298 (47) | Offer letter/employment agreement by and between the Company and Henry F. Blissenbach, dated as of August 1, 2006. | |
| 10.299 (41) | Form of Letter Agreement (Change of Control Severance Agreement) by and between the Company and certain officers dated as of August 25, 2006. | |
109
Table of Contents
| Exhibit |
Description | |
| 10.300 (41) | Form of Letter Agreement (Ordinary Severance Agreement) by and between the Company and certain officers dated as of August 25, 2006. | |
| 10.301 (53) | Stipulation of Settlement by and among Plaintiffs and Ligand Pharmaceuticals, Inc. et al., In re Ligand Pharmaceuticals Inc. Securities Litigation, United States District Court, District of Southern California, dated as of June 28, 2006, approved by Order dated October 16, 2006. | |
| 10.302 (53) | Stipulation of Settlement by and among Plaintiffs and Ligand Pharmaceuticals, Inc. et al., In re Ligand Pharmaceuticals Inc. Derivative Litigation, Superior Court of California, County of San Diego, dated as of September 19, 2006, approved by Order dated October 12, 2006. | |
| 10.303 (53) | Loan Agreement by and between Ligand Pharmaceuticals Incorporated and King Pharmaceuticals, 303 Inc. dated as of October 12, 2006. | |
| 10.304 (49) | Letter Agreement by and between Ligand and King Pharmaceuticals, Inc. effective as of December 29, 2006. | |
| 10.305 (49) | Amendment Number 1 to Purchase Agreement, Contract Sales Force Agreement and Confidentiality Agreement by and between Ligand and King Pharmaceuticals, Inc. effective as of November 30, 2006. | |
| 10.306 (46) | Purchase Agreement and Escrow Instructions by and between Nexus Equity VI, LLC, a California Limited Liability Company, and Ligand Pharmaceuticals Incorporated, a Delaware Corporation and Slough Estates USA Inc., a Delaware corporation dated October 25, 2006. | |
| 10.307 (48) | Amendment No. 1 to the Stockholders Agreement effective as of December 12, 2006, by and among Ligand Pharmaceutical Incorporated and Third Point LLC, Third Point Offshore Fund, Ltd., Third Point Partners LP, Third Point Ultra Ltd., Lyxor/Third Point Fund Ltd., and Third Point Partners Qualified LP. | |
| 10.308 (53) | 2006 Employee Severance Plan dated as of October 4, 2006. | |
| 10.309 (53) | Form of Letter Agreement regarding Change of Control Severance Benefits between the Company and its officers. | |
| 10.310 (45) | Form of Letter Agreement by and between the Company and Tod G. Mertes dated as of October 19, 2006. | |
| 10.311 (49) | Letter Agreement by and between the Company and John L. Higgins dated as of January 10, 2007. | |
| 10.312 (51) | Amendment Number 2 to Purchase Agreement, by and between the Company and King Pharmaceuticals, Inc. effective as of February 26, 2007. | |
| 10.313 (52) | Indemnity Fund Agreement. | |
| 10.314 (54) | Letter Agreement by and between the Company and John P. Sharp dated as of March 30, 2007. (Filed as Exhibit 10.1). | |
| 10.315 (55) | Form of Executive Officer Change in Control Severance Agreement. (Filed as Exhibit 10.1). | |
| 10.316 (56) | Third Amendment to the Company’s Nonqualified Deferred Compensation Plan effective as of December 4, 2007. (Filed as Exhibit 10.1). | |
| 10.317 (57) | Sublease Agreement between the Company and eBIOSCIENCE, INC., effective as of December 13, 2007. (Filed as Exhibit 10.1). | |
| 10.318 (59) | Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the Company’s 2002 Stock Incentive Plan. (Filed as Exhibit 10.318). | |
110
Table of Contents
| Exhibit |
Description | |
| 10.319 (59) | Form of Amendment to Restricted Stock Agreement for executive officers other than Chief Executive Officer. (Filed as Exhibit 10.319). | |
| 10.320 (59) | Amendment to Restricted Stock Agreement between the Company and John L. Higgins. (Filed as Exhibit 10.320). | |
| 10.321 (60) | Tax Sharing and Indemnification Agreement between Pharmacopeia, Inc. and Pharmacopeia, Inc., dated April 30, 2004 (Filed as Exhibit 10.2). | |
| 10.322 (61) | Pharmacopeia Drug Discovery, Inc. Amended and Restated 2004 Stock Incentive Plan. (Filed as Appendix A). | |
| 10.323(78) | Pharmacopeia, Inc. 2000 Stock Option Plan. | |
| 10.324(78) | Collaboration and License Agreement, dated as of July 9, 2003 and effective August 8, 2003, between Pharmacopeia, Inc. and Schering-Plough Ltd. (with certain confidential portions omitted). | |
| 10.325(78) | Collaboration and License Agreement, dated as of July 9, 2003 and effective August 8, 2003, between Pharmacopeia, Inc. and Schering Corporation (with certain confidential portions omitted). | |
| 10.326 (62) | Amendment No. 1, dated July 27, 2006, to the Collaboration and License Agreements, effective as of July 9, 2003, between (i) Pharmacopeia, Inc. and Schering Corporation and (ii) Pharmacopeia, Inc. and Schering-Plough Ltd. (Filed as Exhibit 10.1). | |
| 10.327(78) | Lease, dated August 20, 2003, between Pharmacopeia, Inc. and Eastpark at 8A (Building 1000). | |
| 10.328 (63) | Amendment to Lease, dated September 10, 2007, between Eastpark at 8A and Pharmacopeia, Inc. (Building 1000). (Filed as Exhibit 10.1). | |
| 10.329(78) | Lease, dated August 20, 2003, between Pharmacopeia, Inc. and Eastpark at 8A (Building 3000). | |
| 10.330 (63) | Amendment to Lease, dated April 18, 2007, between Eastpark at 8A and Pharmacopeia, Inc. (Building 3000). (Filed as Exhibit 10.2). | |
| 10.331 (64) | Product Development and Commercialization Agreement among SmithKlineBeecham Corporation, doing business as GlaxoSmithKline, Glaxo Group Limited and Pharmacopeia, Inc., dated as of March 24, 2006 (Filed as Exhibit 10.1). | |
| 10.332 (65) | Amendment No. 1, dated August 10, 2006, to the Product Development and Commercialization Agreement among the Company, SmithKlineBeecham Corporation, doing business as GlaxoSmithKline, and Glaxo Group Limited. (Filed as Exhibit 10.2). | |
| 10.333 (66) | License Agreement, dated as of March 27, 2006, between Pharmacopeia, Inc. and Bristol-Myers Squibb Company (Filed as Exhibit 10.2). | |
| 10.334 (67) | Collaboration and License Agreement between Pharmacopeia, Inc. and Cephalon, Inc., dated May 18, 2006. (Filed as Exhibit 10.1). | |
| 10.335 (68) | License Agreement, amended and restated as of July 1, 2003, among The Trustees of Columbia University in the City of New York, Cold Spring Harbor Laboratory and Pharmacopeia, Inc. (Filed as Exhibit 10.2). | |
| 10.336 (69) | Form of Purchase Agreement dated July 27, 2005 between Pharmacopeia, Inc. and the Purchasers set forth therein. (Filed as Exhibit 10.1). | |
| 10.337 (70) | Form of Indemnity Agreement between Pharmacopeia, Inc. and its directors and executive officers. (Filed as Exhibit 3.3). | |
111
Table of Contents
| Exhibit |
Description | |
| 10.338 (71) | Research and License Agreement, dated December 22, 2006, between Pharmacopeia, Inc. and Wyeth (Filed as Exhibit 10.43). | |
| 10.339 (72) | Master Security Agreement, dated December 26, 2006, between Oxford Finance Corporation and Pharmacopeia, Inc. (Filed as Exhibit 10.1) | |
| 10.340 (73) | Collaboration and License Agreement, amended and restated effective as of February 8, 2007, between Pharmacopeia, Inc. and N.V. Organon. (Filed as Exhibit 10.1). | |
| 10.341 (74) | License Agreement, dated October 11, 2007, between Bristol-Myers Squibb Company and Pharmacopeia, Inc. (Filed as Exhibit 10.45). | |
| 10.342 (75) | Discovery Collaboration Agreement, dated October 11, 2007, between Bristol-Myers Squibb Company and Pharmacopeia, Inc. (Filed as Exhibit 10.46). | |
| 10.343 (76) | Separation Agreement and General Release, dated May 8, 2008, between Pharmacopeia, Inc. and Leslie Johnston Browne, Ph.D. (Filed as Exhibit 10.1). | |
| 10.343 (60) | Contingent Value Rights Agreement, dated December 23, 2008, among the Company, Pharmacopeia, Inc. and Mellon Investor Services LLC. (Filed as Exhibit 10.1). | |
| 10.344 (59) | Amended and Restated Severance Plan, dated December 20, 2008, of the Company. (Filed as Exhibit 10.2). | |
| 10.345 (77) | Settlement Agreement and Mutual Release of all Claims, by and between the Company and The Salk Institute for Biological Studies, dated as of September 2, 2008 (Filed as Exhibit 10.316). | |
| 10.346 (78) | License Agreement, dated of December 17, 2008, between the Company and SmithKline Beecham Corporation, doing business as GlaxoSmithKline (with certain confidential portions omitted) (Filed as Exhibit 10.346).. | |
| 10.347 (79) | Settlement Agreement and Mutual Release, by and between the Company and The Rockefeller University, dated as of February 11, 2009 (Filed as Exhibit 10.318). | |
| 10.348 (79) | Research and License Agreement, by and between the Company and Trevena, Inc., dated as of February 5, 2009 (Filed as Exhibit 10.319). | |
| 10.349 (79) | Separation Agreement by and between the Company and Zofia Dziewanowska, by and between the Company and Dr. Dziewanowska, dated as of March 27, 2009 (Filed as Exhibit 10.320). | |
| 10.350 (80) | Exclusive Patent License Agreement, by and between Glycomed, Inc., a wholly owned subsidiary of the Company and ParinGenix Inc, dated as of June 18, 2009 (Filed as Exhibit 10.321). | |
| 10.351 (80) | Amended and Restated Director Compensation and Stock Ownership Policy, effective as of April 16, 2009 (Filed as Exhibit 10.322). | |
| 10.352 (81) | Research Collaboration Termination Agreement, between the Company and N.V. Organon, dated as of July 29, 2009 (Filed as Exhibit 10.323). | |
| 10.353 (82) | Lease, between the Company and HCP TPSP, LLC, dated August 7, 2009 (Filed as Exhibit 10.321). | |
| 10.354 (82) | Lease Termination Agreement, between the Company and TPSC IX, LLC, dated August 7, 2009 (Filed as Exhibit 10.322). | |
| 10.359 (83) | Form of Voting Agreement, entered into with the Company by each of Warburg Pincus Private Equity VIII, L.P., Stephen R. Davis, Julian C. Baker, Baker/Tisch Investments, L.P., Baker Bros. Investments, L.P., Baker Bros. Investments II, L.P., Baker Biotech Fund I, L.P., Baker Brothers Life Sciences, L.P. and FBB Associates, dated August 23, 2009 (as to first two persons) or August 22, 2009 (as to last seven persons) (Filed as Exhibit 10.6). | |
112
Table of Contents
| Exhibit |
Description | |
| 10.355 (84) | Aplindore Contingent Value Rights Agreement (Filed as Exhibit 10.2). | |
| 10.356 (84) | H3 Contingent Value Rights Agreement (Filed as Exhibit 10.3). | |
| 10.357 (84) | Merck Contingent Value Rights Agreement (Filed as Exhibit 10.4). | |
| 10.358 (84) | Real Estate Contingent Value Rights Agreement (Filed as Exhibit 10.5). | |
| 10.360 (81) | Amendment No. 2 to Product Development and Commercialization Agreement, by and among SmithKline Beecham Corporation, doing business as GlaxoSmithKline, Glaxo Group Limited, and Pharmacopeia, LLC, a wholly owned subsidiary of the Company, filed herewith (Filed as Exhibit 10.331). | |
| 10.361 | Amendment to Discovery Collaboration Agreement between the Company and Bristol-Myers Squibb Company dated December 9, 2009. | |
| 10.362 (89) | Collaborative Research Agreement and License and Royalty Agreement between Neurogen Corporation and Pfizer Inc, dated as of January 1, 1992 (Filed as Exhibit 10.35) (File No. 000-18311). | |
| 10.363 (90) | Collaborative Research Agreement and License and Royalty Agreement between Neurogen Corporation and Pfizer Inc, dated as of July 1, 1994 (Filed as Exhibit 10.1) (File No. 000-18311). | |
| 10.364 (90) | Stock Purchase Agreement between Neurogen Corporation and Pfizer dated as of July 1, 1994 (Filed as Exhibit 10.2) (File No. 000-18311). | |
| 10.365 (91) | Collaboration and License Agreement and Screening Agreement between Neurogen Corporation and Schering-Plough Corporation (Filed as Exhibit 10.1) (File No. 000-18311). | |
| 10.366 (92) | Collaborative Research Agreement between Neurogen Corporation and Pfizer dated as of November 1, 1995 (Filed as Exhibit 10.1) (File No. 000-18311). | |
| 10.367 (92) | Development and Commercialization Agreement between Neurogen Corporation and Pfizer dated as of November 1, 1995 (Filed as Exhibit 10.2) (File No. 000-18311). | |
| 10.368 (92) | Stock Purchase Agreement between Neurogen Corporation and Pfizer dated as of November 1, 1995 (Filed as Exhibit 10.3) (File No. 000-18311). | |
| 10.369 (93) | Stock Purchase Agreement dated as of November 25, 1996 between American Home Products Corporation, acting through its Wyeth-Ayerst Laboratories Division, and Neurogen Corporation (Filed as Exhibit 10.1) (File No. 000-18311). | |
| 10.370 (94) | Technology agreement between Neurogen Corporation and Pfizer Inc, dated as of June 15, 1999 (Filed as Exhibit 10.27) (File No. 000-18311). | |
| 10.371 (95) | Collaboration and License Agreement dated as of December 11, 2001 between Neurogen Corporation and Aventis Pharmaceuticals Inc. (Filed as Exhibit 10.35) (File No. 000-18311). | |
| 10.371 (96) | Collaboration and License Agreement dated as of November 24, 2003 between Neurogen Corporation and Merck Sharp & Dohme Limited (Filed as Exhibit 10.43) (File No. 000-18311). | |
| 10.373 (96) | Stock Purchase Agreement dated as of November 24, 2003 between Neurogen Corporation and Merck Sharp & Dohme Limited (Filed as Exhibit 10.43) (File No. 000-18311). | |
| 10.374 (97) | Amended and Restated Employment Agreement between Neurogen Corporation and Stephen R. Davis dated as of May 8, 2007 (Filed as Exhibit 10.1) (File No. 000-18311). | |
| 10.375 (98) | Employment Contract between Neurogen Corporation and Thomas A. Pitler dated as of March 30, 2009 (Filed as Exhibit 10.54) (File No. 000-18311). | |
113
Table of Contents
| Exhibit |
Description | |
| 10.376 (98) | Employment Contract between Neurogen Corporation and Kenneth Sprenger dated as of March 30, 2009 (Filed as Exhibit 10.55) (File No. 000-18311). | |
| 10.377 (98) | Employment Contract between Neurogen Corporation and George Maynard dated as of March 30, 2009 (Filed as Exhibit 10.56) (File No. 000-18311). | |
| 14.1 (29) | Code of Business Conduct and Ethics. | |
| 21.1 | Subsidiaries of Registrant. | |
| 23.1 | Consent of independent registered public accounting firm-Grant Thornton LLP | |
| 23.2 | Consent of independent registered public accounting firm-BDO Seidman, LLP | |
| 24.1 | Power of Attorney (See page 121). | |
| 31.1 | Certification by Principal Executive Officer, Pursuant to Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.2 | Certification by Principal Financial Officer, Pursuant to Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1 | Certification by Principal Executive Officer, Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 32.2 | Certification by Principal Financial Officer, Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| (1) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Registration Statement on Form S-4 (No. 333-58823) filed on July 9, 1998. |
| (2) | This exhibit was previously filed as part of and is hereby incorporated by reference to same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 1999. |
| (3) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2004. |
| (4) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Registration Statement on Form S-1 (No. 33-47257) filed on April 16, 1992 as amended. |
| (5) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Registration Statement on Form S-1/S-3 (No. 33-87598 and 33-87600) filed on December 20, 1994, as amended. |
| (6) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the period ended December 31, 1996. |
| (7) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the period ended December 31, 1997. |
| (8) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 1998. |
| (9) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Current Report on Form 8-K of Seragen, Inc. filed on May 15, 1998. |
| (10) | This exhibit was previously filed as part of and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 1999. |
| (11) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the period ended December 31, 1999. |
| (12) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2000. |
| (13) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2000. |
114
Table of Contents
| (14) | This exhibit was previously filed as part of, and are hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 1993. |
| (15) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 1994. |
| (16) | This exhibit was previously filed, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 1995. |
| (17) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly report on Form 10-Q for the period ended June 30, 1996. |
| (18) | This exhibit was previously filed as part of, and are hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly report on Form 10-Q for the period ended September 30, 1995. |
| (19) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 1997. |
| (20) | This exhibit was previously filed as part of, and are hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2000. |
| (21) | This exhibit was previously filed as part of, and are hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2001. |
| (22) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2002. |
| (23) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2002. |
| (24) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Registration Statement on Form S-3 (No. 333-102483) filed on January 13, 2003, as amended. |
| (25) | This exhibit was previously filed as part of, and are hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2002. |
| (26) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2003. |
| (27) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2003. |
| (28) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2003. |
| (29) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2003. |
| (30) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2004. |
| (31) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on November 14, 2005. |
| (32) | This exhibit was previously filed as part of, and are hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2004. |
| (33) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 5, 2005. |
| (34) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2005. |
| (35) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 14, 2005. |
| (36) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Registration Statement on Form S-1 (no. 333-131029) filed on January 13, 2006 as amended. |
| (37) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with an Amendment to the Company’s Registration Statement on Form S-1 (No. 333-1031029) filed on February 10, 2006. |
115
Table of Contents
| (38) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2006. |
| (39) | This exhibit was previously filed as part of, and is being incorporated by reference to the numbered exhibit filed with the Company’s Current Report Form 8-K filed on August 4, 2006. |
| (40) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2006. |
| (41) | This exhibit was previously filed as part of, and is being incorporated by reference to the numbered exhibit filed with the Company’s Current Report Form 8-K filed on August 30, 2006. |
| (42) | This exhibit was previously filed as part of, and is being incorporated by reference to the numbered exhibit filed with the Company’s Current Report Form 8-K filed on September 11, 2006. |
| (43) | This exhibit was previously filed as part of, and is being incorporated by reference to the numbered exhibit filed with the Company’s Current Report Form 8-K filed on September 12, 2006. |
| (44) | This exhibit was previously filed as part of, and is being incorporated by reference to the numbered exhibit filed with the Company’s Current Report Form 8-K filed on October 17, 2006. |
| (45) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on October 20, 2006. |
| (46) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on October 31, 2006. |
| (47) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2006. |
| (48) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 14, 2006. |
| (49) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on January 5, 2007. |
| (50) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on January 16, 2007. |
| (51) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on February 28, 2007. |
| (52) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on March 5, 2007. |
| (53) | This exhibit was previously filed as part of, and are hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. |
| (54) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on May 4, 2007. |
| (55) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on August 22, 2007. |
| (56) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 6, 2007. |
| (57) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 19, 2007. |
| (58) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on September 26, 2008. |
| (59) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the period ended December 31, 2007. |
| (60) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Pharmacopeia, Inc.’s Current Report on Form 8-K filed on May 3, 2004. |
| (61) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered appendix filed with the Pharmacopeia, Inc.’s Form DEF 14A filed on March 26, 2007. |
| (62) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Pharmacopeia, Inc.’s Current Report on Form 8-K filed on August 2, 2006. |
116
Table of Contents
| (63) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended September 30, 2007. |
| (64) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended March 31, 2006. |
| (65) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended September 30, 2006. |
| (66) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended March 31, 2006. |
| (67) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended June 30, 2006. |
| (68) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended June 30, 2005. |
| (69) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Pharmacopeia, Inc.’s Current Report on Form 8-K filed on August 2, 2005. |
| (70) | This exhibit was previously filed as part of, and is hereby incorporated by reference to numbered exhibit filed with the Pharmacopeia, Inc.’s Registration Statement on Form 10 (Reg. No. 000-50523). |
| (71) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2006. |
| (72) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia Inc.’s Quarterly Report on Form 10-Q for the period ended June 30, 2006. |
| (73) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended March 31, 2007. |
| (74) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2007. |
| (75) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2007. |
| (76) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended March 31, 2008. |
| (77) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Pharmacopeia, Inc.’s Quarterly Report on Form 10-Q for the period ended September 30, 2008. |
| (78) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Annual Report on Form 10-K for the period ended December 31, 2008. |
| (79) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended March 31, 2009. |
| (80) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2009. |
| (81) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the same numbered exhibit filed with the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2009. |
117
Table of Contents
| (82) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on August 11, 2009. |
| (83) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on August 24, 2009. |
| (84) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 24, 2009. |
| (85) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 17, 2009. |
| (86) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on December 1, 2009. |
| (87) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on November 6, 2009. |
| (88) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with the Company’s Current Report on Form 8-K filed on October 28, 2009. |
| (89) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Annual Report on Form 10-K for the period ended December 31, 1991. |
| (90) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Quarterly Report on Form 10-Q for the period ended June 30, 1994. |
| (91) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Current Report on Form 8-K filed on July 28, 1995. |
| (92) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Current Report on Form 8-K filed on November 1, 1995. |
| (93) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Current Report on Form 8-K filed on March 31, 1997. |
| (94) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Quarterly Report on Form 10-Q for the period ended June 30, 1999 |
| (95) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Annual Report on Form 10-K for the period ended December 31, 2001. |
| (96) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Annual Report on Form 10-K for the period ended December 31, 2003. |
| (97) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Quarterly Report on Form 10-Q for the period ended March 31, 2007. |
| (98) | This exhibit was previously filed as part of, and is hereby incorporated by reference to the numbered exhibit filed with Neurogen Corporation’s Annual Report on Form 10-K for the period ended March 31, 2009. |
(4)(d) Financial Statement Schedule
Schedules not included herein have been omitted because they are not applicable or the required information is in the consolidated financial statements or notes thereto.
Schedule II—Valuation and Qualifying Accounts (in thousands)
| Balance at Beginning of Period |
Charges | Deductions | Other | Balance at End of Period | |||||||||||
| December 31, 2009: |
|||||||||||||||
| Allowance for doubtful accounts and cash discounts |
$ | 200 | $ | — | $ | — | $ | — | $ | 200 | |||||
| December 31, 2008: |
|||||||||||||||
| Allowance for doubtful accounts and cash discounts |
$ | 200 | $ | — | $ | — | $ | — | $ | 200 | |||||
| December 31, 2007: |
|||||||||||||||
| Allowance for doubtful accounts and cash discounts |
$ | 530 | $ | 569 | $ | 899 | $ | — | $ | 200 | |||||
| (A) | This reserve was adjusted in connection with the accounting for the sale of the AVINZA Product Line on February 26, 2007. |
118
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| LIGAND PHARMACEUTICALS INCORPORATED | ||
| By: | /s/ JOHN L. HIGGINS | |
| John L. Higgins, | ||
| President and Chief Executive Officer | ||
Date: March 3, 2010
POWER OF ATTORNEY
Know all men by these presents, that each person whose signature appears below constitutes and appoints John L. Higgins or John P. Sharp, his or her attorney-in-fact, with power of substitution in any and all capacities, to sign any amendments to this Annual Report on Form 10-K, and to file the same with exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that the attorney-in-fact or his or her substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ JOHN L. HIGGINS John L. Higgins |
President, Chief Executive Officer and Director (Principal Executive Officer) | March 3, 2010 | ||
| /s/ JOHN P. SHARP John P. Sharp |
Vice President, Finance and Chief Financial Officer (Principal Financial and Accounting Officer) | March 3, 2010 | ||
| /s/ JASON M. ARYEH Jason M. Aryeh |
Director | March 3, 2010 | ||
| /s/ STEVEN J. BURAKOFF Steven J. Burakoff |
Director | March 3, 2010 | ||
| /s/ TODD C. DAVIS Todd C. Davis |
Director | March 3, 2010 | ||
| /s/ DAVID M. KNOTT David M. Knott |
Director | March 3, 2010 | ||
| /s/ JOHN W. KOZARICH John W. Kozarich |
Director | March 3, 2010 | ||
| /s/ STEPHEN L. SABBA Stephen L. Sabba |
Director | March 3, 2010 | ||
119
