Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - NovoCure Ltd | d105694d8k.htm |
| EX-99.1 - EX-99.1 - NovoCure Ltd | d105694dex991.htm |
| EX-99.2 - EX-99.2 - NovoCure Ltd | d105694dex992.htm |

EF-14 Publication in JAMA December 15, 2015 Exhibit 99.3

Introduction Ashley Cordova Senior Director, Investor Relations and Treasury EF-14 Publication in JAMA

Forward Looking Statements Discussions during this conference call will include forward-looking statements, and actual results could differ materially from those projected in these statements. These statements involve a number of risks and uncertainties, some of which are beyond our control, including those risks and uncertainties described from time-to-time in our SEC filings. We do not intend to update publicly any forward-looking statement, except as required by law. EF-14 Publication in JAMA

Opening Remarks Bill Doyle Executive Chairman EF-14 Publication in JAMA

EF-14 Publication on December 15 EF-14 Publication in JAMA Copyright © 2015 American Medical Association. All Rights Reserved.

EF-14 Publication Review Eilon Kirson Chief Science Officer and Head of Research and Development EF-14 Publication in JAMA
Importance of EF-14 Data GBM is the most common and aggressive form of primary brain cancer Most patients die within 1 to 2 years of diagnosis Tumor Treating Fields, or TTFields, are an antimitotic treatment that selectively disrupts cell division by delivering low-intensity, intermediate-frequency, alternating electric fields EF-14 Publication in JAMA
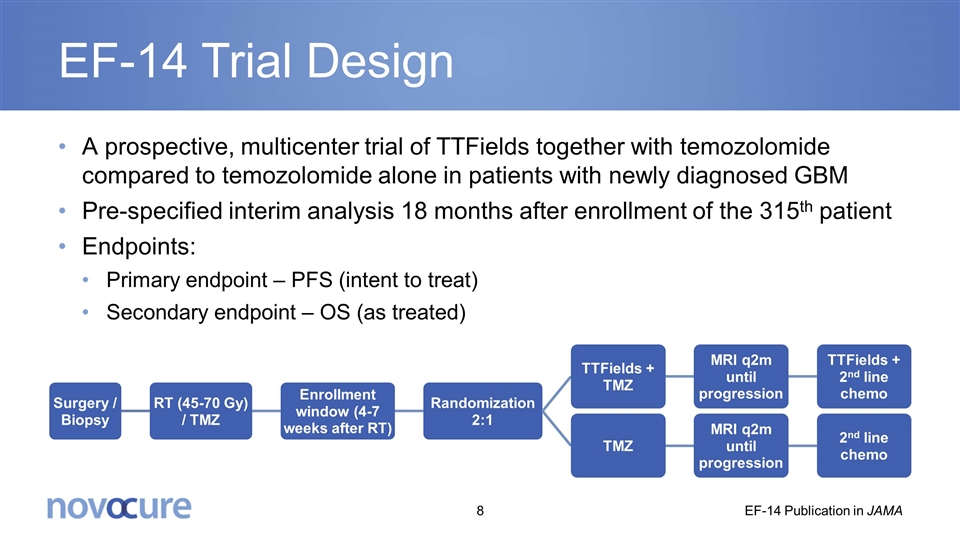
EF-14 Trial Design A prospective, multicenter trial of TTFields together with temozolomide compared to temozolomide alone in patients with newly diagnosed GBM Pre-specified interim analysis 18 months after enrollment of the 315th patient Endpoints: Primary endpoint – PFS (intent to treat) Secondary endpoint – OS (as treated) EF-14 Publication in JAMA Surgery / Biopsy RT (45-70 Gy ) / TMZ Enrollment window (4-7 weeks after RT) Randomization 2:1 TTFields + TMZ TMZ MRI q2m until progression MRI q2m until progression TTFields + 2 nd line chemo 2 nd line chemo
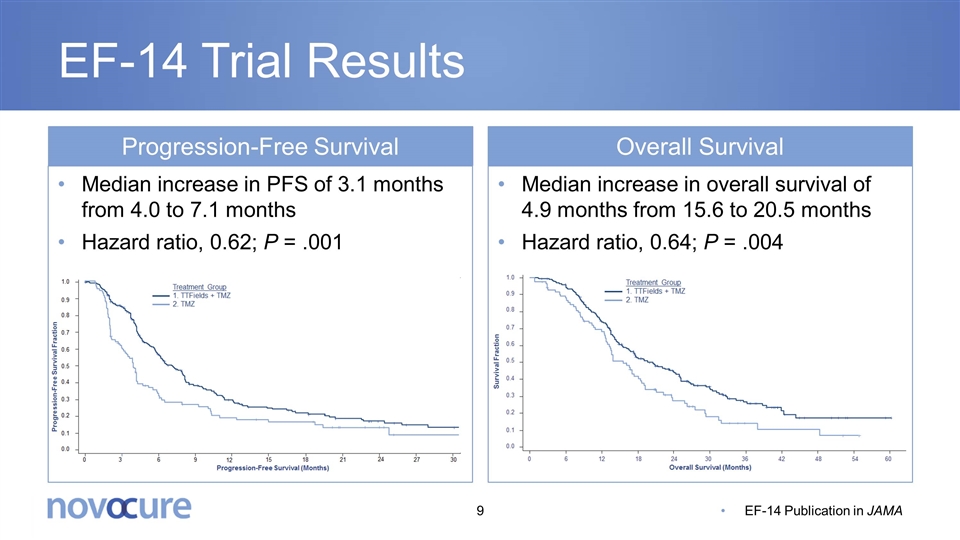
EF-14 Trial Results Progression-Free Survival Overall Survival Median increase in overall survival of 4.9 months from 15.6 to 20.5 months Hazard ratio, 0.64; P = .004 Median increase in PFS of 3.1 months from 4.0 to 7.1 months Hazard ratio, 0.62; P = .001 EF-14 Publication in JAMA
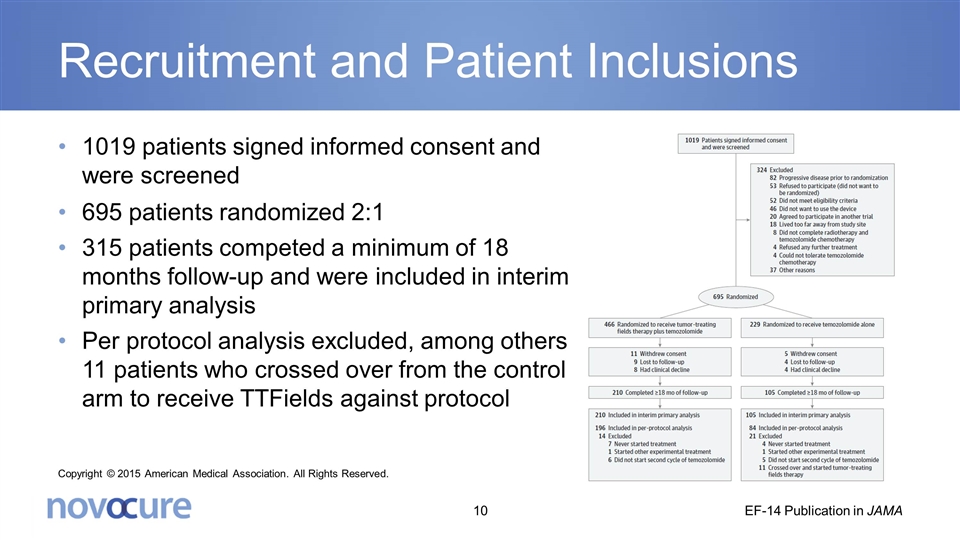
Recruitment and Patient Inclusions 1019 patients signed informed consent and were screened 695 patients randomized 2:1 315 patients competed a minimum of 18 months follow-up and were included in interim primary analysis Per protocol analysis excluded, among others, 11 patients who crossed over from the control arm to receive TTFields against protocol Copyright © 2015 American Medical Association. All Rights Reserved. EF-14 Publication in JAMA
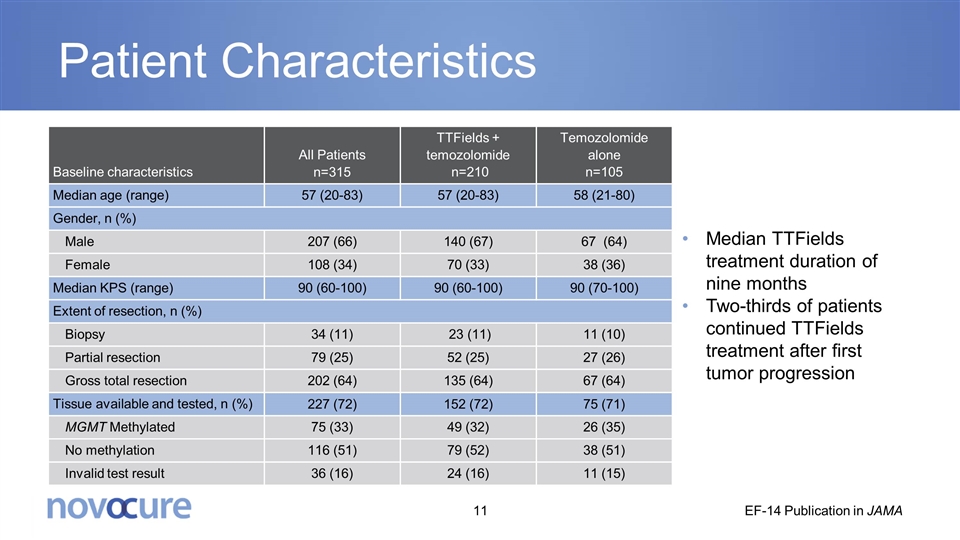
Patient Characteristics Baseline characteristics All Patients n=315 TTFields + temozolomide n=210 Temozolomide alone n=105 Median age (range) 57 (20-83) 57 (20-83) 58 (21-80) Gender, n (%) Male 207 (66) 140 (67) 67 (64) Female 108 (34) 70 (33) 38 (36) Median KPS (range) 90 (60-100) 90 (60-100) 90 (70-100) Extent of resection, n (%) Biopsy 34 (11) 23 (11) 11 (10) Partial resection 79 (25) 52 (25) 27 (26) Gross total resection 202 (64) 135 (64) 67 (64) Tissue available and tested, n (%) 227 (72) 152 (72) 75 (71) MGMT Methylated 75 (33) 49 (32) 26 (35) No methylation 116 (51) 79 (52) 38 (51) Invalid test result 36 (16) 24 (16) 11 (15) Median TTFields treatment duration of nine months Two-thirds of patients continued TTFields treatment after first tumor progression EF-14 Publication in JAMA
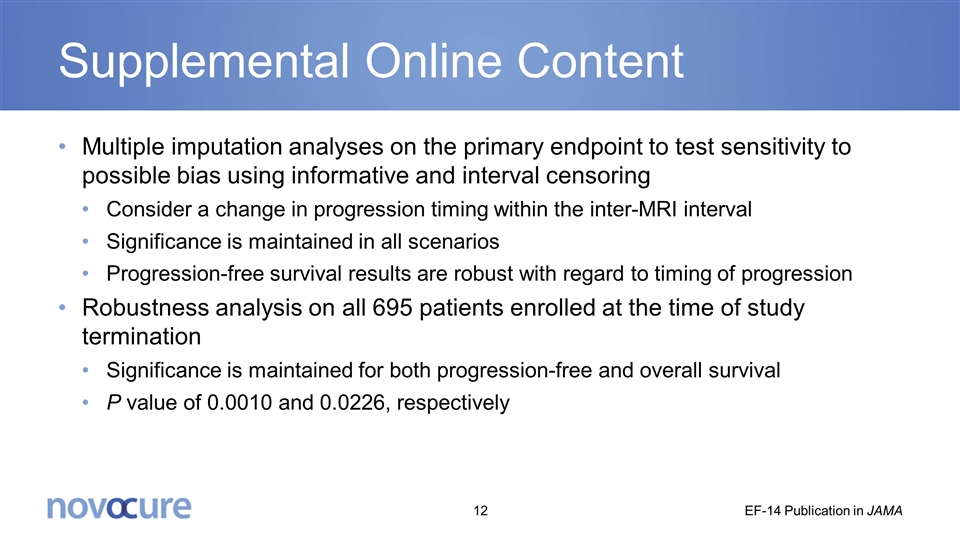
Supplemental Online Content Multiple imputation analyses on the primary endpoint to test sensitivity to possible bias using informative and interval censoring Consider a change in progression timing within the inter-MRI interval Significance is maintained in all scenarios Progression-free survival results are robust with regard to timing of progression Robustness analysis on all 695 patients enrolled at the time of study termination Significance is maintained for both progression-free and overall survival P value of 0.0010 and 0.0226, respectively EF-14 Publication in JAMA

JAMA Conclusion Conclusions and Relevance In this interim analysis of 315 patients with glioblastoma who had completed standard chemoradiation therapy, adding TTFields to maintenance temozolomide chemotherapy significantly prolonged progression-free and overall survival. EF-14 Publication in JAMA Copyright © 2015 American Medical Association. All Rights Reserved.

Concluding Remarks Bill Doyle Executive Chairman EF-14 Publication in JAMA
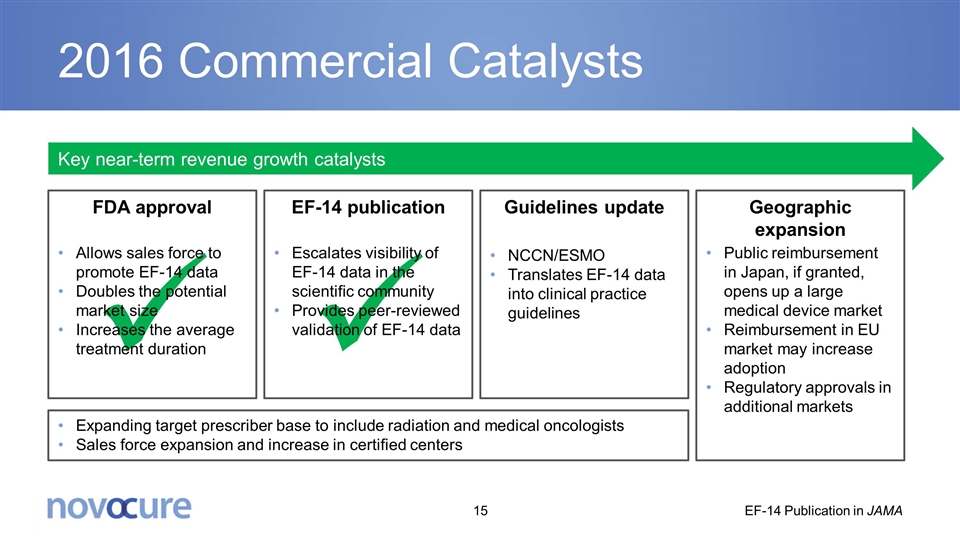
ü ü FDA approval Allows sales force to promote EF-14 data Doubles the potential market size Increases the average treatment duration 2016 Commercial Catalysts EF-14 publication Escalates visibility of EF-14 data in the scientific community Provides peer-reviewed validation of EF-14 data Guidelines update NCCN/ESMO Translates EF-14 data into clinical practice guidelines Geographic expansion Public reimbursement in Japan, if granted, opens up a large medical device market Reimbursement in EU market may increase adoption Regulatory approvals in additional markets Expanding target prescriber base to include radiation and medical oncologists Sales force expansion and increase in certified centers Key near-term revenue growth catalysts EF-14 Publication in JAMA
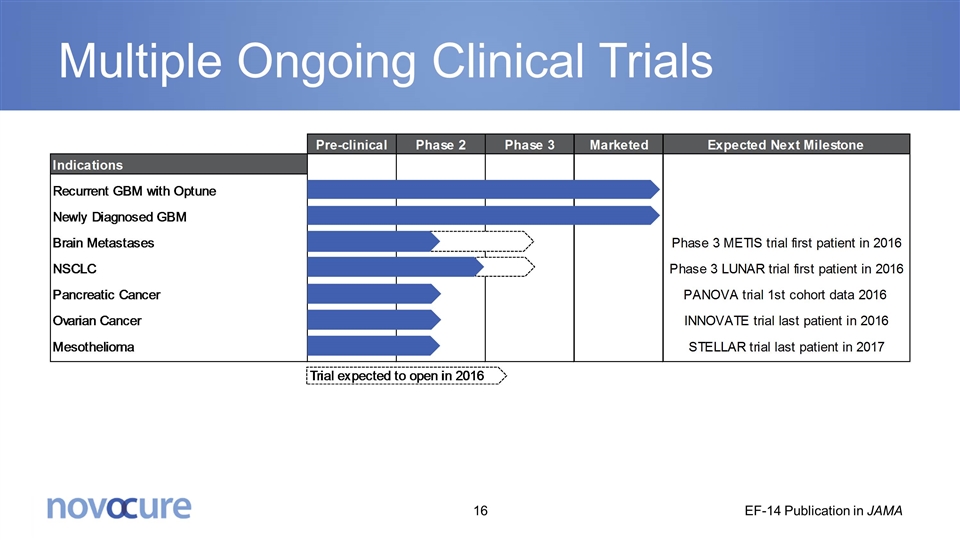
Multiple Ongoing Clinical Trials EF-14 Publication in JAMA

Q&A Session EF-14 Publication in JAMA
