Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SELLAS Life Sciences Group, Inc. | gale-201511098xk.htm |
| EX-99.1 - EXHIBIT 99.1 - SELLAS Life Sciences Group, Inc. | gale-20151109ex991.htm |

Q3, 2015 Earnings Report & Business Update

FORWARD LOOKING STATEMENT This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about our 2015 revenue from the sale of Abstral®, our launch of Zuplenz®, the divestiture of the commercial operations including the two commercial products, the issuance and exclusivity of patents, and the progress of development of Galena’s product candidates, including patient enrollment in our clinical trials. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those identified under “Risk Factors” in Galena’s Annual Report on Form 10-K for the year ended December 31, 2014 and most recent Quarterly Reports on Form 10-Q filed with the SEC. Actual results may differ materially from those contemplated by these forward-looking statements. Galena does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this press release. 2

EARNINGS CALL PARTICIPANTS Presenters Mark W. Schwartz, Ph.D. President & Chief Executive Officer Bijan Nejadnik, M.D. Executive Vice President, Chief Medical Officer Gavin Choy, PharmD Senior Vice President, Clinical Sciences and Operations Ryan Dunlap, CPA Vice President & Chief Financial Officer Other Participants Remy Bernarda, MBA SVP, Investor Relations & Corporate Communications Tom Knapp, Esq Interim General Counsel 3

OVERVIEW Mark W. Schwartz, Ph.D. President & Chief Executive Officer

NOVEMBER 2012 DEVELOPMENT PIPELINE Product Therapeutic Area Phase 1 Phase 2 Phase 3 BLA / NDA NeuVax™ Node-positive HER2 IHC 1+/2+ NeuVax™ + Herceptin® Node-positive or node negative/triple negative HER2 IHC 1+/2+ GALE-301 Ovarian & Endometrial PRESENT *NeuVax is an investigational product. Efficacy has not been established. Herceptin is a registered trademark of Genentech. Ongoing Planned 5

NOVEMBER 2015 DEVELOPMENT PIPELINE Product Therapeutic Area Phase 1 Phase 2 Phase 3 BLA / NDA Immunotherapy: Breast Cancer NeuVax™ Node-positive HER2 IHC 1+/2+ NeuVax™ + Herceptin® Node-positive or node negative/triple negative HER2 IHC 1+/2+ NeuVax™ + Herceptin® High risk, node-positive or negative, HER2 IHC 3+ NeuVax™ Ductal Carcinoma in Situ (DCIS) Immunotherapy: Gastric Cancer NeuVax™ Gastric, HER2 IHC 1+/2+/3+ Immunotherapy: Gynecological Cancer GALE-301 Ovarian & Endometrial GALE-301 + GALE-302 Ovarian & Breast Hematology GALE-401 (Anagrelide CR) MPN-related thrombocytosis PRESENT *NeuVax is an investigational product. Efficacy has not been established. Herceptin is a registered trademark of Genentech. Ongoing Planned VADIS 6

FINANCE Ryan Dunlap, CPA Vice President & CFO 7

CONDENSED CONSOLIDATED STATEMENT OF COMPREHENSIVE LOSS (in 000s)(unaudited) 8

Q3 2015 CASH ROLL-FORWARD (000s rounded) Beginning Cash, January 1, 2015 $23,700 Financing Activities $47,600 Operating Burn – Continuing Operations ($27,800) Operating Burn – Discontinued Operations ($5,000) Investing Burn – Continuing Operations ($300) Investing Burn – Discontinued Operations ($500) Debt Service ($2,900) Ending Cash, September 30, 2015 $34,800 9

INTRODUCTION Bijan Nejadnik, M.D. Executive Vice President, Chief Medical Officer 10
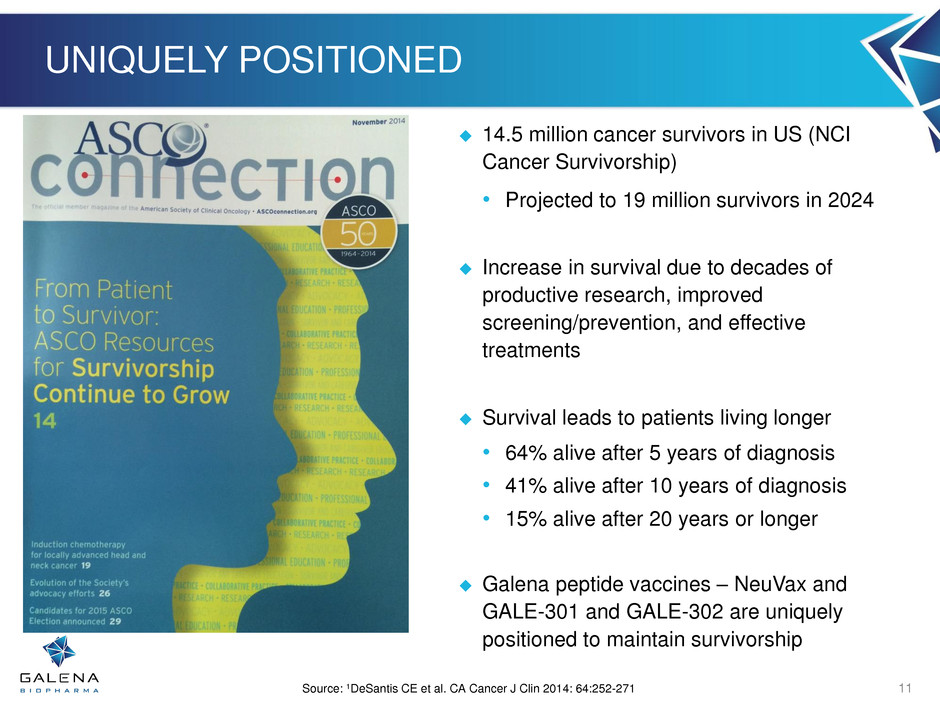
UNIQUELY POSITIONED 14.5 million cancer survivors in US (NCI Cancer Survivorship) • Projected to 19 million survivors in 2024 Increase in survival due to decades of productive research, improved screening/prevention, and effective treatments Survival leads to patients living longer • 64% alive after 5 years of diagnosis • 41% alive after 10 years of diagnosis • 15% alive after 20 years or longer Galena peptide vaccines – NeuVax and GALE-301 and GALE-302 are uniquely positioned to maintain survivorship Source: 1DeSantis CE et al. CA Cancer J Clin 2014: 64:252-271 11

CLINICAL DEVELOPMENT Gavin Choy, PharmD Senior Vice President, Clinical Sciences and Operations 12
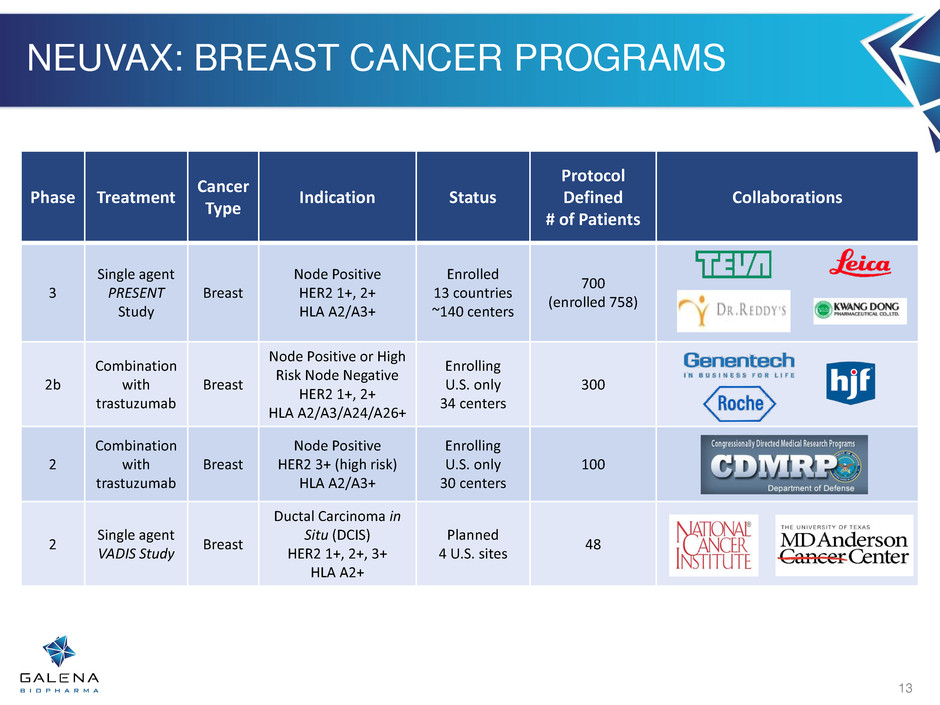
NEUVAX: BREAST CANCER PROGRAMS Phase Treatment Cancer Type Indication Status Protocol Defined # of Patients Collaborations 3 Single agent PRESENT Study Breast Node Positive HER2 1+, 2+ HLA A2/A3+ Enrolled 13 countries ~140 centers 700 (enrolled 758) 2b Combination with trastuzumab Breast Node Positive or High Risk Node Negative HER2 1+, 2+ HLA A2/A3/A24/A26+ Enrolling U.S. only 34 centers 300 2 Combination with trastuzumab Breast Node Positive HER2 3+ (high risk) HLA A2/A3+ Enrolling U.S. only 30 centers 100 2 Single agent VADIS Study Breast Ductal Carcinoma in Situ (DCIS) HER2 1+, 2+, 3+ HLA A2+ Planned 4 U.S. sites 48 13

GALE-301 & GALE-302: CURRENT CLINICAL DEVELOPMENT 14 Phase Treatment Cancer Type Target Indication Current Status # of Enrolled Patients 1/2a GALE-301 Ovarian, Endometrial HLA A2+ Ovarian Enrolled 51 1b GALE-301 & GALE-302 Ovarian, Breast HLA A2+ Ovarian Enrolled 39
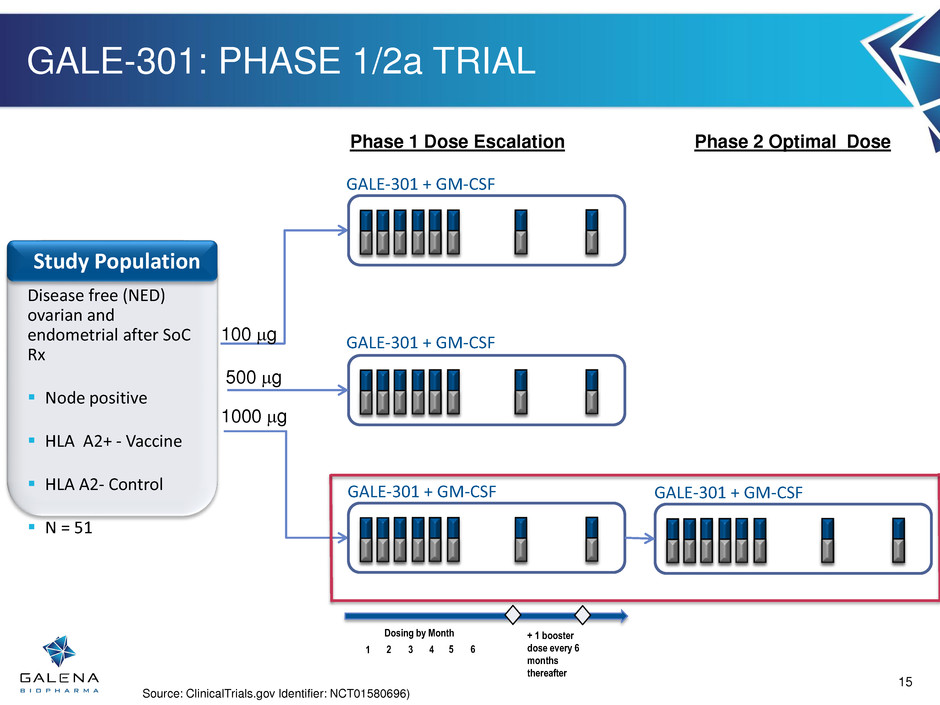
GALE-301: PHASE 1/2a TRIAL 1 2 3 4 Dosing by Month + 1 booster dose every 6 months thereafter 5 6 Disease free (NED) ovarian and endometrial after SoC Rx Node positive HLA A2+ - Vaccine HLA A2- Control N = 51 Study Population GALE-301 + GM-CSF 100 mg GALE-301 + GM-CSF 500 mg GALE-301 + GM-CSF 1000 mg Phase 1 Dose Escalation GALE-301 + GM-CSF Phase 2 Optimal Dose Source: ClinicalTrials.gov Identifier: NCT01580696) 15

PHASE 1/2a TRIAL RESULTS: OPTIMAL DOSE GROUP PRELIMINARY EFFICACY 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% 30.0% Vaccine Control % of Subj e ct s Recurrence 24.0% 13.3% Source: Greene et al, ECC 2015 16 Phase 1: Determined optimal dose and demonstrated safety and potent immune response Phase 2a: Preliminary data in 1000 mcg dose group: • At 12 months median follow-up: Vaccine group: 2 clinical recurrences (13.3%) n=15 Control group: 12 recurrences (55%) n=22 • 2 year DFS estimate in 1000 mcg dose group is 85.7% vaccine vs. 33.6% control (p<0.02) • GALE-301 plus GM-CSF is well tolerated and elicits a strong in vivo immune response with primarily Grade 1 and Grade 2 toxicities
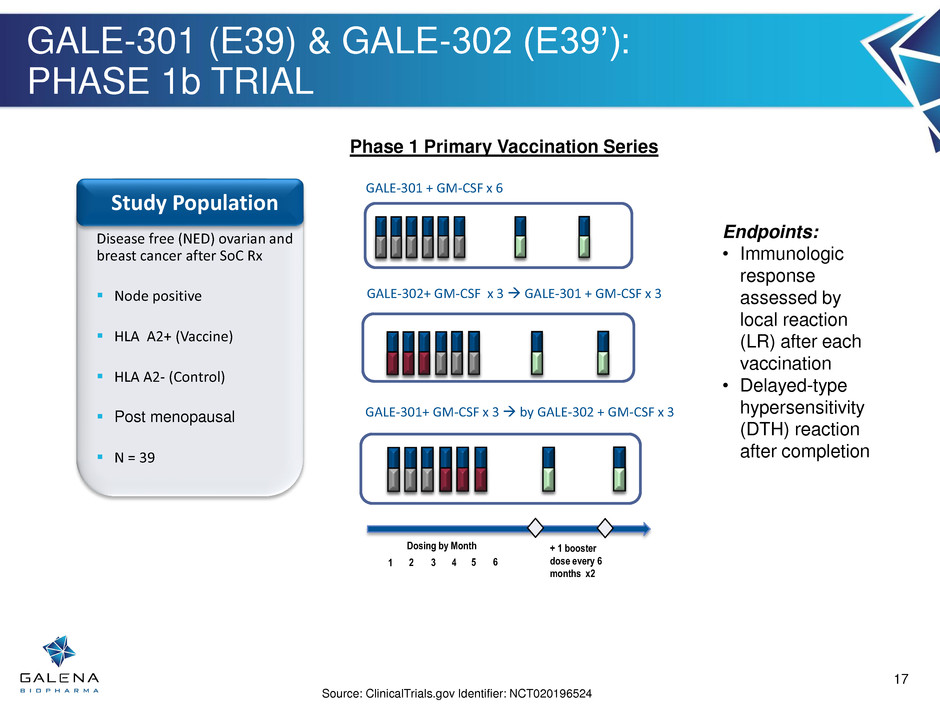
GALE-301 (E39) & GALE-302 (E39’): PHASE 1b TRIAL 1 2 3 4 Dosing by Month + 1 booster dose every 6 months x2 5 6 Disease free (NED) ovarian and breast cancer after SoC Rx Node positive HLA A2+ (Vaccine) HLA A2- (Control) Post menopausal N = 39 Study Population GALE-301 + GM-CSF x 6 Phase 1 Primary Vaccination Series Endpoints: • Immunologic response assessed by local reaction (LR) after each vaccination • Delayed-type hypersensitivity (DTH) reaction after completion Source: ClinicalTrials.gov Identifier: NCT020196524 GALE-302+ GM-CSF x 3 GALE-301 + GM-CSF x 3 GALE-301+ GM-CSF x 3 by GALE-302 + GM-CSF x 3 17
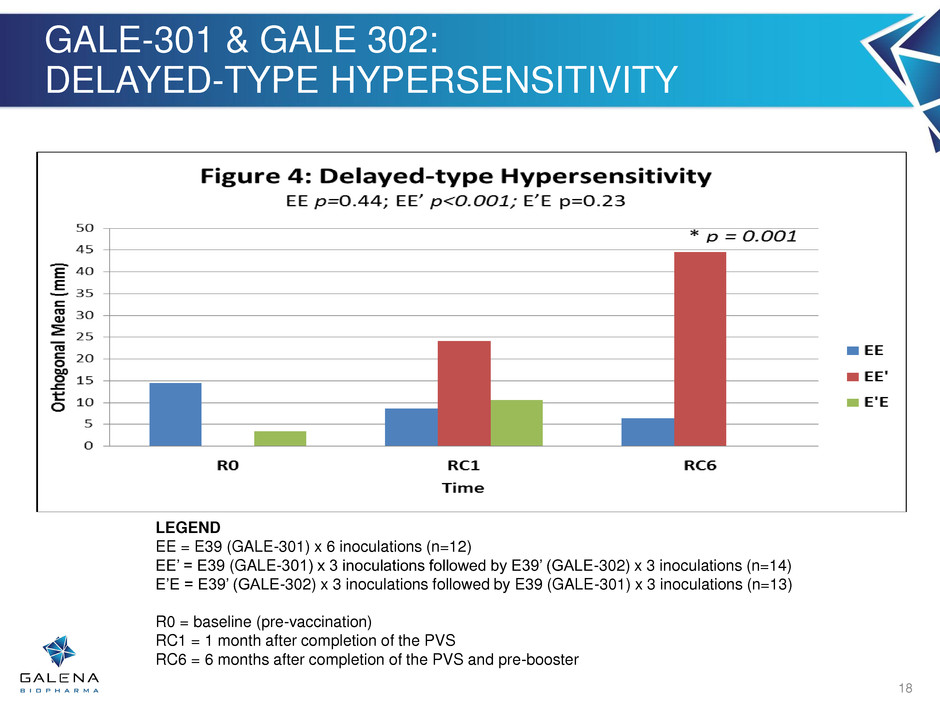
GALE-301 & GALE 302: DELAYED-TYPE HYPERSENSITIVITY 18 LEGEND EE = E39 (GALE-301) x 6 inoculations (n=12) EE’ = E39 (GALE-301) x 3 inoculations followed by E39’ (GALE-302) x 3 inoculations (n=14) E’E = E39’ (GALE-302) x 3 inoculations followed by E39 (GALE-301) x 3 inoculations (n=13) R0 = baseline (pre-vaccination) RC1 = 1 month after completion of the PVS RC6 = 6 months after completion of the PVS and pre-booster

THANK YOU Mark W. Schwartz, Ph.D. President and Chief Executive Officer 19

UPCOMING MILESTONES NeuVax™ Enroll N=700 into PRESENT trial Complete enrollment in Phase 3 PRESENT trial • Initiate DCIS trial (1Q16) • PRESENT: reach 70 events (1Q16) • PRESENT: interim analysis (2Q16) GALE-301 GALE- 302 Report Top-Line Phase 2a clinical data Report 1-Year Phase 2a analysis Report GALE-301 + GALE-302 Phase 1b data GALE-401 (anagrelide CR) Report Top-Line efficacy and safety data • Report Final Phase 2 data 20

WHY WE’RE HERE Source: E75 vaccine's final tests start in S.A. By Don Finley, January 22, 2012; Photo credit: Kin Man Hui/San Antonio Express-News/ZUMAPress “I've had several friends who've had (breast cancer) and then…it came back and they had to go through treatment again. So this would be wonderful, not to have to come back.” – First NeuVax Phase 3 patient 21
