Attached files
| file | filename |
|---|---|
| 8-K - 8-K - WEST PHARMACEUTICAL SERVICES INC | form8kinvestorconfmay132015.htm |

1 West Pharmaceutical Services, Inc. Investor Conferences May 2015

2 Cautionary Statement Under the Private Securities Litigation Reform Act of 1995 This presentation and any accompanying management commentary contain “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about expected financial results for 2015 and future years. Each of these estimates is based on preliminary information, and actual results could differ from these preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially from those estimated or predicted in the forward-looking statements. You should evaluate any statement in light of these important factors. Except as required by law or regulation, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise. Non-GAAP Financial Measures Certain financial measures included in these presentation materials, or which may be referred to in management’s discussion of the Company’s results and outlook, have not been calculated in accordance with generally accepted accounting principles (“GAAP”), and therefore are referred to as non-GAAP financial measures. Non-GAAP financial measures should not be considered in isolation or as an alternative to such measures determined in accordance with GAAP. Trademarks West and the diamond logo and By your side for a healthier world™, NovaPure®, EnvisionTM, Westar®, ConfiDose®, SelfDose®, and NovaGuard® are trademarks or registered trademark of West Pharmaceutical Services, Inc., in the United States and other jurisdictions. SmartDose® is a registered trademark of Medimop Medical Projects Ltd., a subsidiary of West Pharmaceutical Services, Inc. Daikyo Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd. Daikyo Crystal Zenith® technology is licensed from Daikyo Seiko, Ltd. érisTM is a trademark of Tech Group Europe Limited. The éris safety syringe system is not available for sale in the United States and its territories. Safe Harbor Statement
3 Today’s Presentation West: Long- Established Partner Leading Packaging Systems Franchise Advancing Delivery Systems Businesses Strong Financial Position Positioned for Future Growth
4 90 YEARS OF HISTORY INNOVATION MARKET LEADING SOLUTIONS Why West?

5 CEO Succession Eric M. Green became West’s sixth Chief Executive on April 24, 2015 • Joined West after 22 years at Sigma-Aldrich Most recently as president of $1.4 billion research business unit Donald E. Morel, Jr. PhD remains Chairman through June 2015 • CEO from April 2002 • Chairman since March 2003
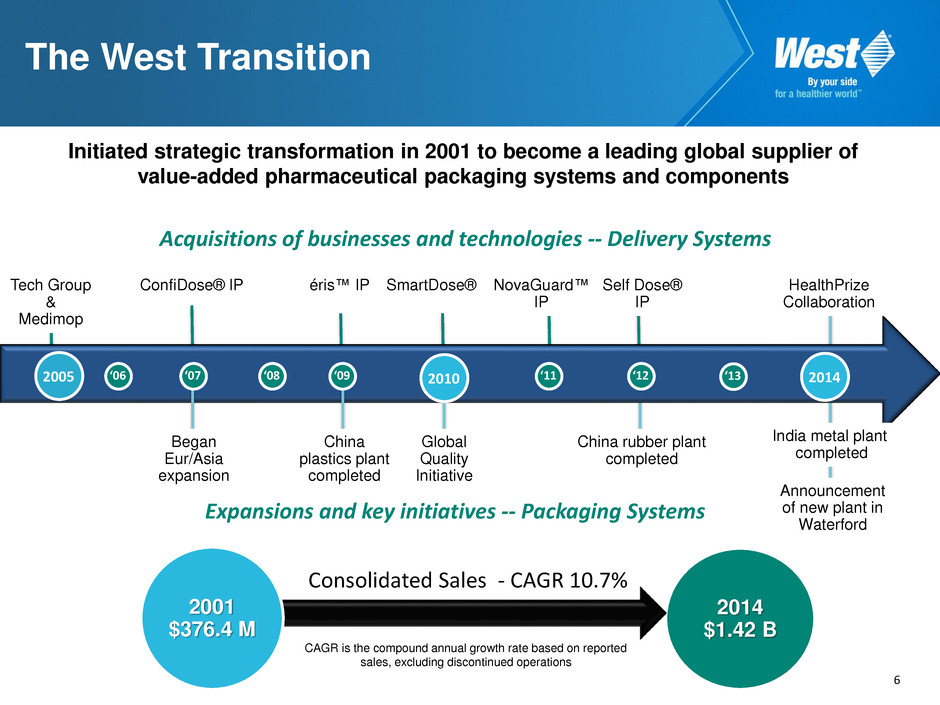
6 Initiated strategic transformation in 2001 to become a leading global supplier of value-added pharmaceutical packaging systems and components 2014 $1.42 B CAGR is the compound annual growth rate based on reported sales, excluding discontinued operations Acquisitions of businesses and technologies -- Delivery Systems The West Transition 2005 Tech Group & Medimop ‘06 ‘08 ‘11 ‘12 ‘07 Began Eur/Asia expansion ConfiDose® IP ‘09 éris™ IP China plastics plant completed 2010 SmartDose® IP Global Quality Initiative Self Dose® IP NovaGuard™ IP Expansions and key initiatives -- Packaging Systems 2001 $376.4 M China rubber plant completed ‘13 2014 Announcement of new plant in Waterford India metal plant completed HealthPrize Collaboration Consolidated Sales - CAGR 10.7%

7 PHARMACEUTICAL / BIOTECHNOLOGY GENERIC MEDICAL DEVICE INDUSTRY-LEADING MARKET SHARES IN EUROPE AND NORTH AMERICA A Solid Commercial Base
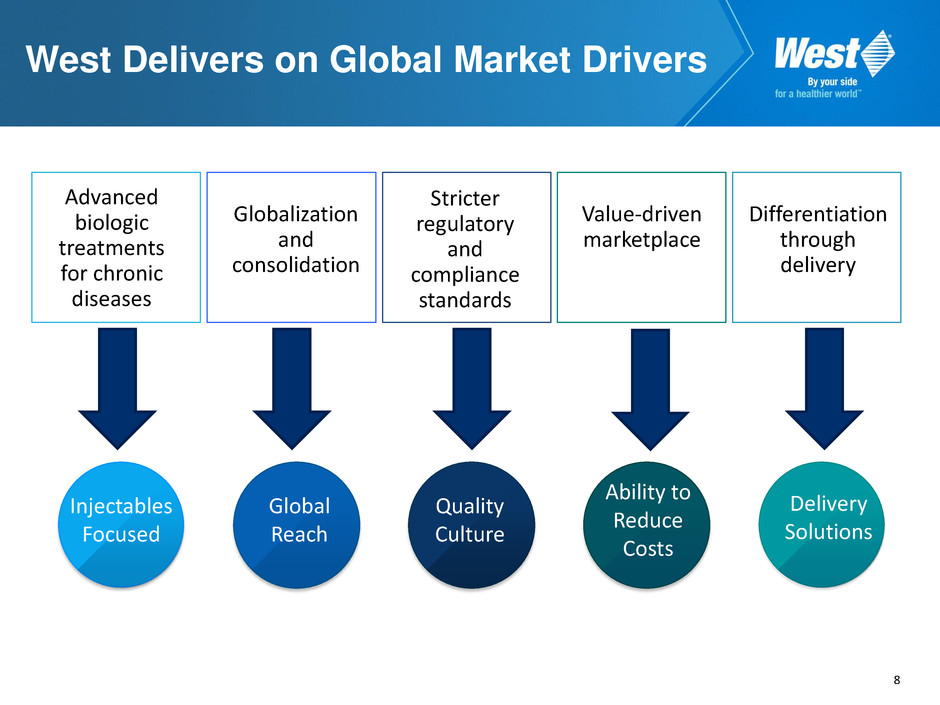
8 West Delivers on Global Market Drivers Injectables Focused Global Reach Quality Culture Ability to Reduce Costs Delivery Solutions Advanced biologic treatments for chronic diseases Globalization and consolidation Stricter regulatory and compliance standards Value-driven marketplace Differentiation through delivery
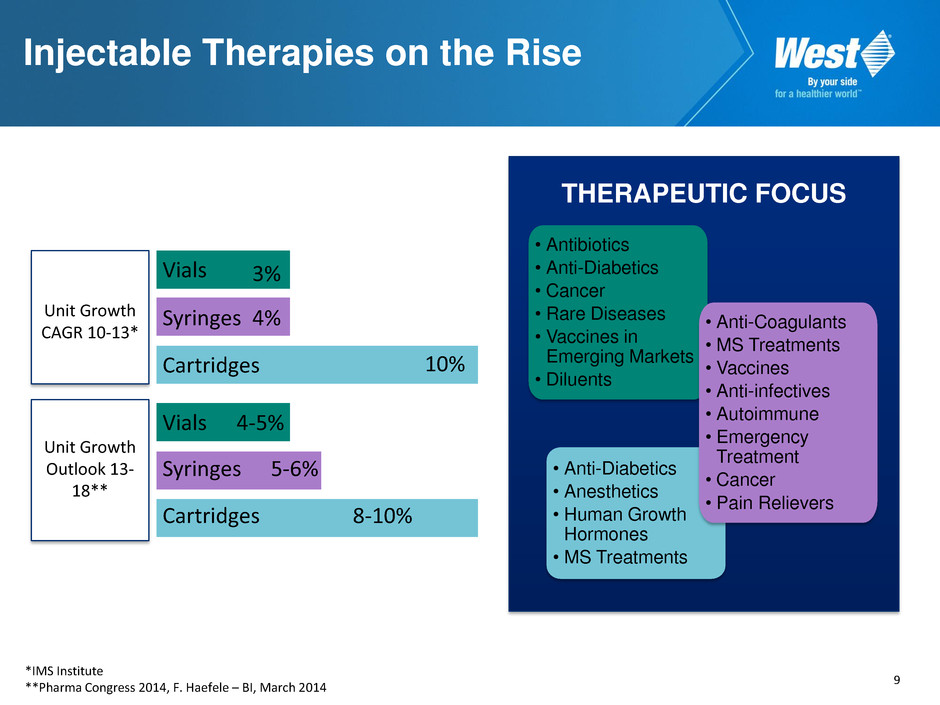
9 Injectable Therapies on the Rise Vials Unit Growth CAGR 10-13* Unit Growth Outlook 13- 18** Syringes Cartridges Cartridges 4% 10% 8-10% 4-5% Syringes 5-6% *IMS Institute **Pharma Congress 2014, F. Haefele – BI, March 2014 Vials 3% • Antibiotics • Anti-Diabetics • Cancer • Rare Diseases • Vaccines in Emerging Markets • Diluents • Anti-Diabetics • Anesthetics • Human Growth Hormones • MS Treatments • Anti-Coagulants • MS Treatments • Vaccines • Anti-infectives • Autoimmune • Emergency Treatment • Cancer • Pain Relievers THERAPEUTIC FOCUS
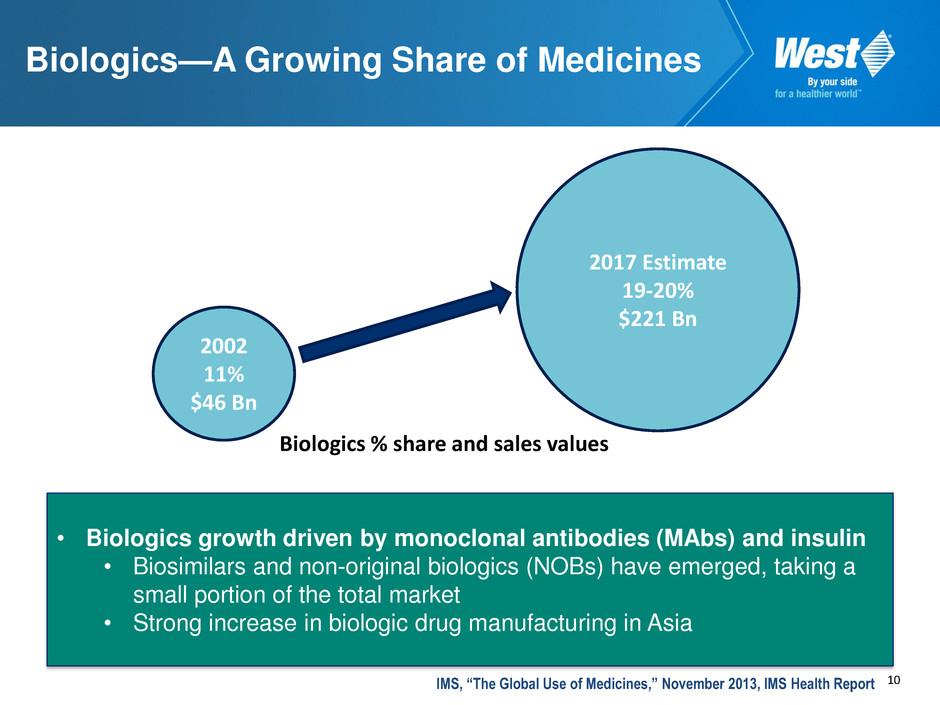
10 Biologics—A Growing Share of Medicines 2002 11% $46 Bn 2017 Estimate 19-20% $221 Bn Biologics % share and sales values • Biologics growth driven by monoclonal antibodies (MAbs) and insulin • Biosimilars and non-original biologics (NOBs) have emerged, taking a small portion of the total market • Strong increase in biologic drug manufacturing in Asia IMS, “The Global Use of Medicines,” November 2013, IMS Health Report
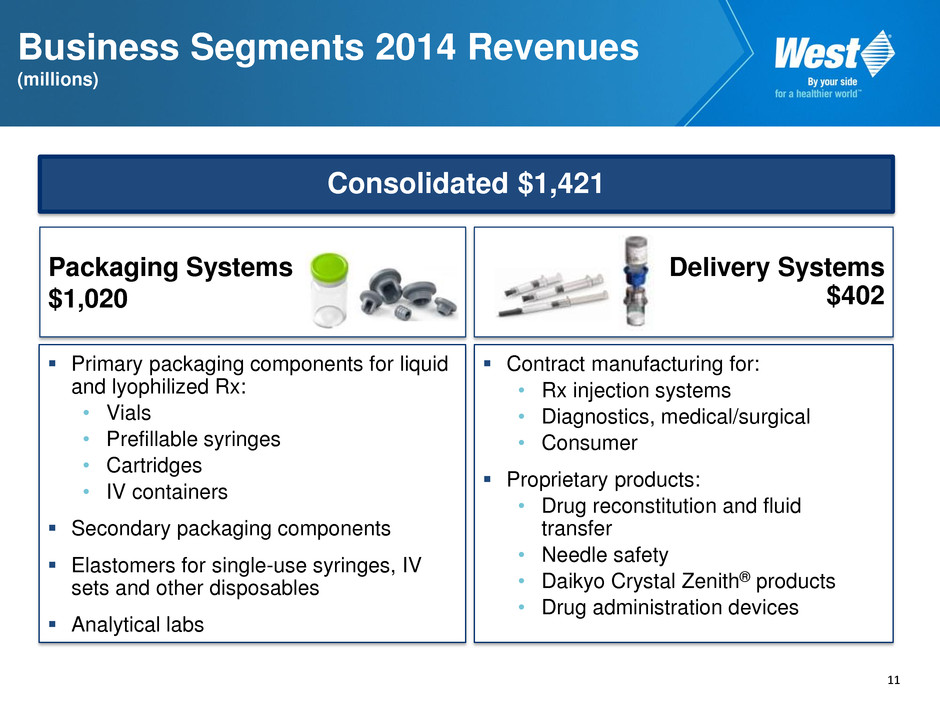
11 Business Segments 2014 Revenues (millions) Consolidated $1,421 Packaging Systems $1,020 Delivery Systems $402 Primary packaging components for liquid and lyophilized Rx: • Vials • Prefillable syringes • Cartridges • IV containers Secondary packaging components Elastomers for single-use syringes, IV sets and other disposables Analytical labs Contract manufacturing for: • Rx injection systems • Diagnostics, medical/surgical • Consumer Proprietary products: • Drug reconstitution and fluid transfer • Needle safety • Daikyo Crystal Zenith® products • Drug administration devices
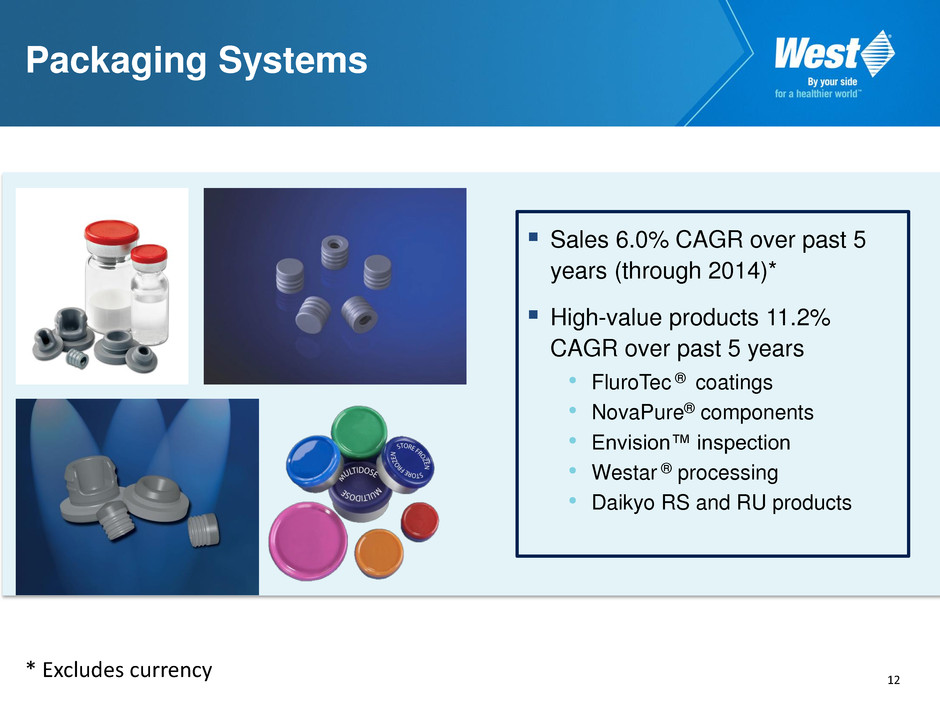
12 Packaging Systems Sales 6.0% CAGR over past 5 years (through 2014)* High-value products 11.2% CAGR over past 5 years • FluroTec ® coatings • NovaPure® components • Envision™ inspection • Westar ® processing • Daikyo RS and RU products * Excludes currency
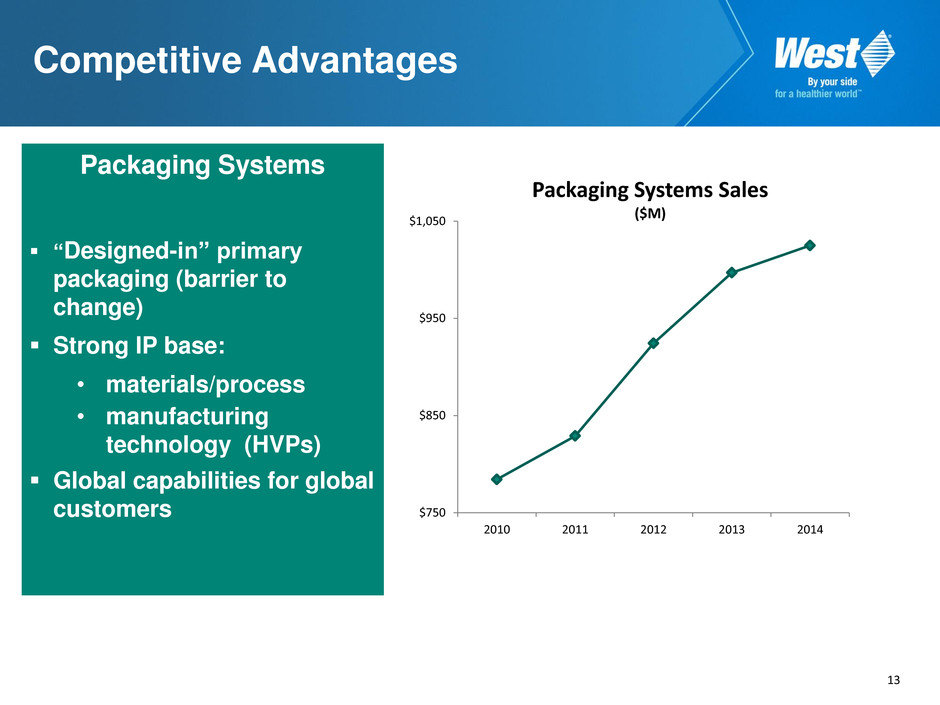
13 Competitive Advantages Packaging Systems “Designed-in” primary packaging (barrier to change) Strong IP base: • materials/process • manufacturing technology (HVPs) Global capabilities for global customers $750 $850 $950 $1,050 2010 2011 2012 2013 2014 Packaging Systems Sales ($M)

14 West and Daikyo components The Top 50 pharma and biotech companies in the world rely on The top 35 injectable biologics rely on West and Daikyo components > 130 million needle safety systems annually > 100 million components and assemblies for pens and auto-injectors annually A Trusted Partner
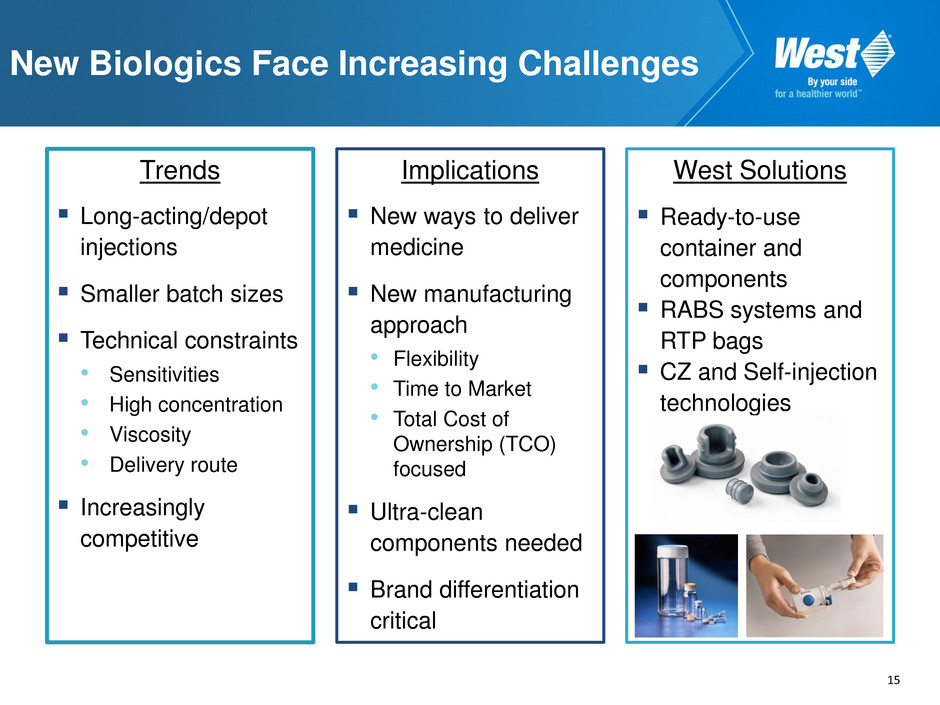
15 New Biologics Face Increasing Challenges Trends Long-acting/depot injections Smaller batch sizes Technical constraints • Sensitivities • High concentration • Viscosity • Delivery route Increasingly competitive Implications New ways to deliver medicine New manufacturing approach • Flexibility • Time to Market • Total Cost of Ownership (TCO) focused Ultra-clean components needed Brand differentiation critical West Solutions Ready-to-use container and components RABS systems and RTP bags CZ and Self-injection technologies
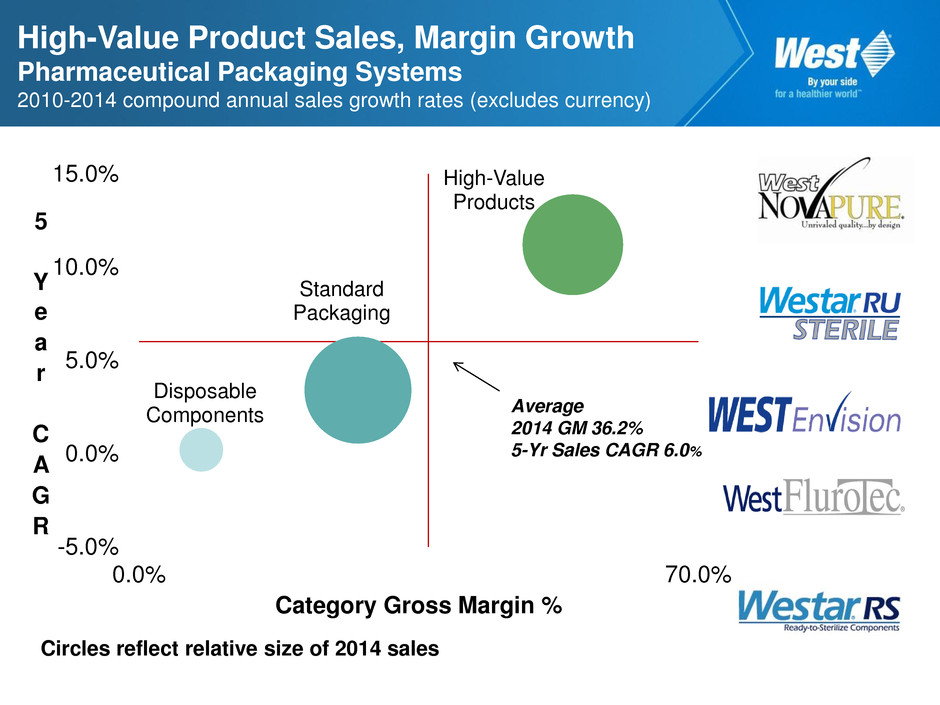
High-Value Product Sales, Margin Growth Pharmaceutical Packaging Systems 2010-2014 compound annual sales growth rates (excludes currency) Circles reflect relative size of 2014 sales Disposable Components Standard Packaging High-Value Products -5.0% 0.0% 5.0% 10.0% 15.0% 0.0% 70.0% 5 Y e a r C A G R Category Gross Margin % Average 2014 GM 36.2% 5-Yr Sales CAGR 6.0%

17 Delivery Systems Sales CAGR of 7.4% over past 5 years (through 2014)* Proprietary products grew 20% per annum over past 5 years • Daikyo Crystal Zenith® vials and syringes • SmartDose® electronic wearable bolus injector • Mix2Vial administration device • érisTM safety system Tech Group * Excludes currency

18 Contract Manufacturing
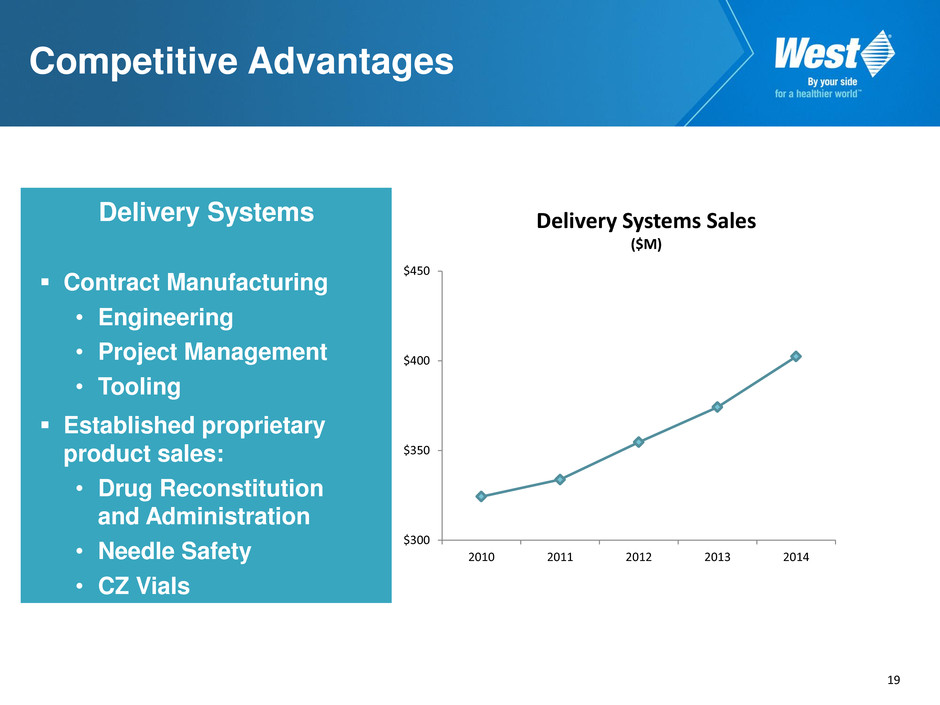
19 Competitive Advantages Delivery Systems Contract Manufacturing • Engineering • Project Management • Tooling Established proprietary product sales: • Drug Reconstitution and Administration • Needle Safety • CZ Vials $300 $350 $400 $450 2010 2011 2012 2013 2014 Delivery Systems Sales ($M)
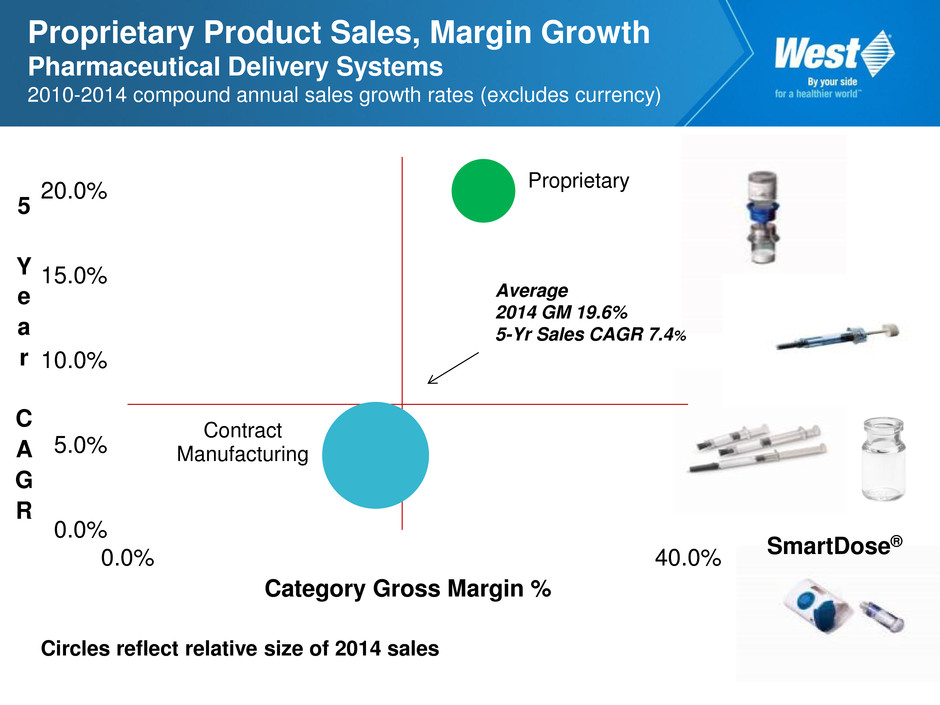
Proprietary Product Sales, Margin Growth Pharmaceutical Delivery Systems 2010-2014 compound annual sales growth rates (excludes currency) Circles reflect relative size of 2014 sales SmartDose® Proprietary Contract Manufacturing 0.0% 5.0% 10.0% 15.0% 20.0% 0.0% 40.0% 5 Y e a r C A G R Category Gross Margin % Average 2014 GM 19.6% 5-Yr Sales CAGR 7.4%

Packaging Systems Delivery Systems Build on Delivery Systems capabilities and proprietary products: Daikyo Crystal Zenith® Drug administration aids Self-dosing devices/combination products Our Growth Initiatives 2015-2019 High-value products: modest unit growth, increasing ASP* and margin Geographic & HVP manufacturing expansion: China, India sourcing and end-markets, Kinston & Jersey Shore Global operating efficiencies Zero Defect Quality Initiative *Average Selling Price 21
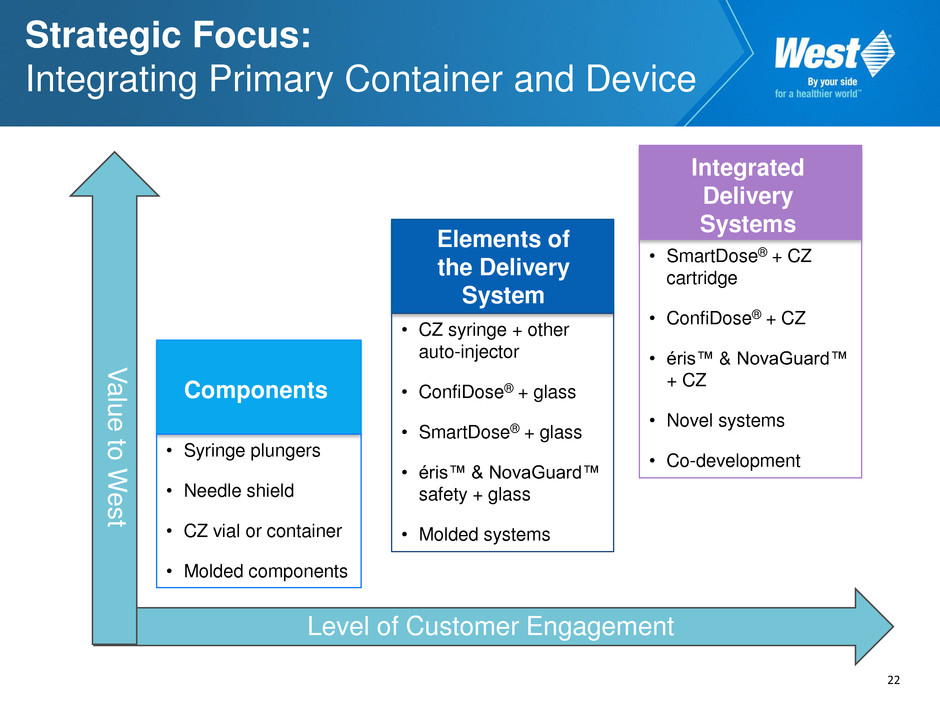
22 Level of Customer Engagement V al u e to W e s t Strategic Focus: Integrating Primary Container and Device Elements of the Delivery System • CZ syringe + other auto-injector • ConfiDose® + glass • SmartDose® + glass • éris™ & NovaGuard™ safety + glass • Molded systems Integrated Delivery Systems • SmartDose® + CZ cartridge • ConfiDose® + CZ • éris™ & NovaGuard™ + CZ • Novel systems • Co-development Components • Syringe plungers • Needle shield • CZ vial or container • Molded components

23 Progress on New Proprietary Products SmartDose® Electronic Wearable Injector • 8 active development projects • Phase III clinical trial • Shipped in 2014: > 100,000 units > 800,000 cartridges Daikyo Crystal Zenith® Vials and Syringes • Over 30 products approved in JP, NA, EU • Doubled # of formal stability trials in 2014 • 50 ml vial approved in the US-custom application • 2 ml vial recommended for approval in the US • Clinical studies: 3.5 ml & 2.5 ml cartridges; 1 ml long insert needle We Are Delivering on Our Strategies— Commercial Progress

24 Geographic Expansion Completed India metals facility • Production initiated - 7/14 • Product transfers from Singapore Free-up Singapore for more HVPs Initiated Waterford, Ireland expansion • Phase 1 construction commencing - Q2/15 Expanded Kinston site to include high- value processing for components • Dual sourcing/risk mitigation We Are Delivering on Our Strategies— Geographic Expansion

25 Key partner to pharmaceutical, biotech, and medical device customers “Sticky” core business – significant barriers to entry Strong competitive position • Diversified customer base • Maturing proprietary technology pipeline • Global footprint with strong position in fast growing Asian markets Market drivers support business model High-value products driving margins for future growth Financial strength to invest • Strong balance sheet and operating cash flow Take-Away Messages

26 West Pharmaceutical Services, Inc.
