Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REGENERON PHARMACEUTICALS, INC. | a15-6672_28k.htm |
| EX-99.1 - EX-99.1 - REGENERON PHARMACEUTICALS, INC. | a15-6672_2ex99d1.htm |
Exhibit 99.2
|
|
Peter Jones,1 Harold Bays,2 Umesh Chaudhari,3 Robert Pordy,4 Christelle Lorenzato,5 Kathryn Miller,6 Jennifer Robinson7 1Baylor College of Medicine, Houston, TX, USA; 2Louisville Metabolic and Atherosclerosis Research Center (L-MARC), Louisville, KY, USA; 3Sanofi, Bridgewater, NJ, USA; 4Regeneron., Tarrytown, NY, USA; 5Sanofi, Chilly-Mazarin, France; 6Regeneron, Basking Ridge, NJ, USA; 7University of Iowa, Iowa City, IA, USA Pooled Safety and Adverse Events in Nine Randomized, Placebo-controlled, Phase 2 and 3 Clinical Trials of Alirocumab This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. |
|
|
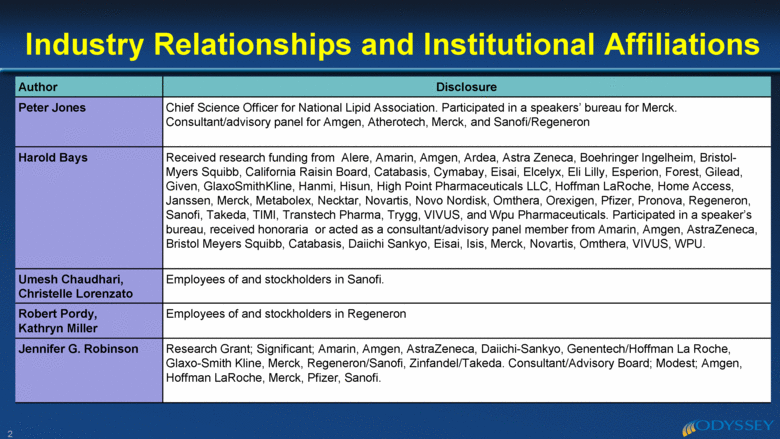
Author Disclosure Peter Jones Chief Science Officer for National Lipid Association. Participated in a speakers’ bureau for Merck. Consultant/advisory panel for Amgen, Atherotech, Merck, and Sanofi/Regeneron Harold Bays Received research funding from Alere, Amarin, Amgen, Ardea, Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, California Raisin Board, Catabasis, Cymabay, Eisai, Elcelyx, Eli Lilly, Esperion, Forest, Gilead, Given, GlaxoSmithKline, Hanmi, Hisun, High Point Pharmaceuticals LLC, Hoffman LaRoche, Home Access, Janssen, Merck, Metabolex, Necktar, Novartis, Novo Nordisk, Omthera, Orexigen, Pfizer, Pronova, Regeneron, Sanofi, Takeda, TIMI, Transtech Pharma, Trygg, VIVUS, and Wpu Pharmaceuticals. Participated in a speaker’s bureau, received honoraria or acted as a consultant/advisory panel member from Amarin, Amgen, AstraZeneca, Bristol Meyers Squibb, Catabasis, Daiichi Sankyo, Eisai, Isis, Merck, Novartis, Omthera, VIVUS, WPU. Umesh Chaudhari, Christelle Lorenzato Employees of and stockholders in Sanofi. Robert Pordy, Kathryn Miller Employees of and stockholders in Regeneron Jennifer G. Robinson Research Grant; Significant; Amarin, Amgen, AstraZeneca, Daiichi-Sankyo, Genentech/Hoffman La Roche, Glaxo-Smith Kline, Merck, Regeneron/Sanofi, Zinfandel/Takeda. Consultant/Advisory Board; Modest; Amgen, Hoffman LaRoche, Merck, Pfizer, Sanofi. Industry Relationships and Institutional Affiliations 2 |
|
|
Inhibition of PCSK9 may provide an effective means to lower LDL-C Alirocumab is a fully human monoclonal antibody to PCSK9 Individual alirocumab trials have shown robust LDL-C reductions and AEs generally comparable with placebo1-12 Pooled safety data are reported from 9 placebo-controlled, randomized Phase 2 and 3 trials (of 8 to 78 weeks duration) in patients with hypercholesterolemia on stable background statin Background 3 1. McKenney JM et al. JACC. 2012;59:2344–2353; Roth EM et al. NEJM 2012;367:1891–1900; Stein EA et al. Lancet. 2012;380:29–36; Roth EM et al. Int J Cardiol 2014;176:55–61; Kereiakes DJ et al. Circulation. 2014;130:2119; Cannon CP et al. Eur Heart J. 2015; Bays H et al. Circulation. 2014;130:A16194; Robinson JG et al. NEJM, in press; Moriarty P et al. Circulation. 2014;130:2108; Kastelein JJP et al. ESC 2014; Ginsberg H et al. Circulation. 2014;130:2119; Teramoto T et al. Circulation. 2014;130:A13651. |
|
|
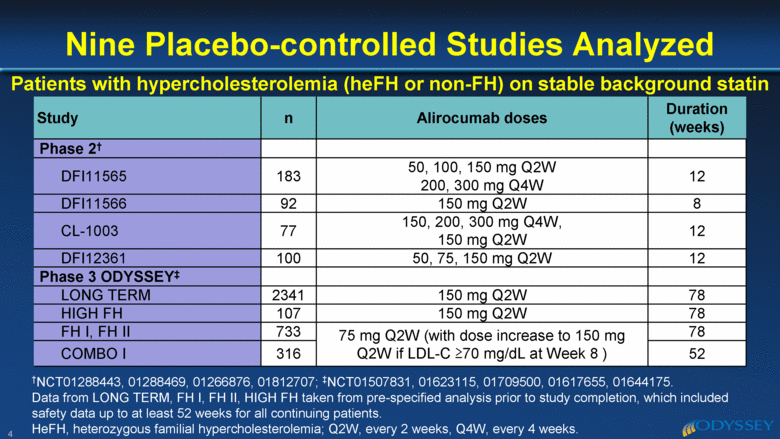
4 Nine Placebo-controlled Studies Analyzed †NCT01288443, 01288469, 01266876, 01812707; ‡NCT01507831, 01623115, 01709500, 01617655, 01644175. Data from LONG TERM, FH I, FH II, HIGH FH taken from pre-specified analysis prior to study completion, which included safety data up to at least 52 weeks for all continuing patients. HeFH, heterozygous familial hypercholesterolemia; Q2W, every 2 weeks, Q4W, every 4 weeks. Patients with hypercholesterolemia (heFH or non-FH) on stable background statin Study n Alirocumab doses Duration (weeks) Phase 2† DFI11565 183 50, 100, 150 mg Q2W 200, 300 mg Q4W 12 DFI11566 92 150 mg Q2W 8 CL-1003 77 150, 200, 300 mg Q4W, 150 mg Q2W 12 DFI12361 100 50, 75, 150 mg Q2W 12 Phase 3 ODYSSEY‡ LONG TERM 2341 150 mg Q2W 78 HIGH FH 107 150 mg Q2W 78 FH I, FH II 733 75 mg Q2W (with dose increase to 150 mg Q2W if LDL-C >70 mg/dL at Week 8 ) 78 COMBO I 316 52 |
|
|
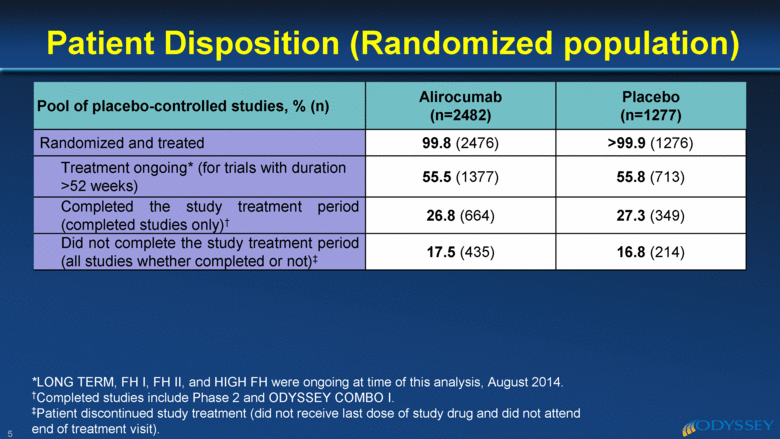
Patient Disposition (Randomized population) 5 Pool of placebo-controlled studies, % (n) Alirocumab (n=2482) Placebo (n=1277) Randomized and treated 99.8 (2476) >99.9 (1276) Treatment ongoing* (for trials with duration >52 weeks) 55.5 (1377) 55.8 (713) Completed the study treatment period (completed studies only)† 26.8 (664) 27.3 (349) Did not complete the study treatment period (all studies whether completed or not)‡ 17.5 (435) 16.8 (214) *LONG TERM, FH I, FH II, and HIGH FH were ongoing at time of this analysis, August 2014. †Completed studies include Phase 2 and ODYSSEY COMBO I. ‡Patient discontinued study treatment (did not receive last dose of study drug and did not attend end of treatment visit). |
|
|
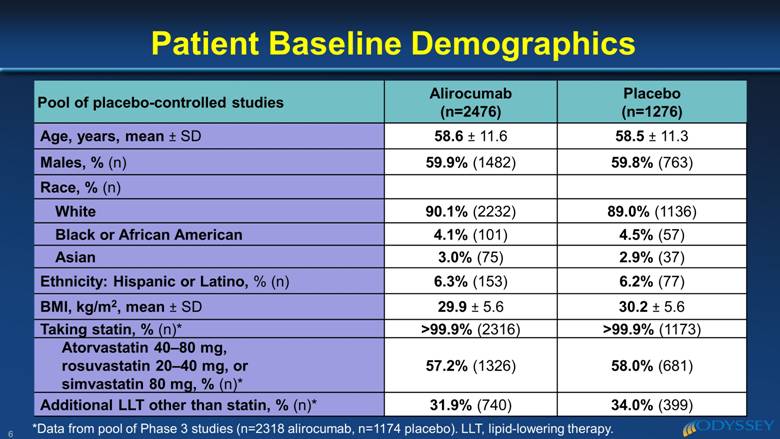
Pool of placebo-controlled studies Alirocumab (n=2476) Placebo (n=1276) Age, years, mean ± SD 58.6 ± 11.6 58.5 ± 11.3 Males, % (n) 59.9% (1482) 59.8% (763) Race, % (n) White 90.1% (2232) 89.0% (1136) Black or African American 4.1% (101) 4.5% (57) Asian 3.0% (75) 2.9% (37) Ethnicity: Hispanic or Latino, % (n) 6.3% (153) 6.2% (77) BMI, kg/m2, mean ± SD 29.9 ± 5.6 30.2 ± 5.6 Taking statin, % (n)* >99.9% (2316) >99.9% (1173) Atorvastatin 40–80 mg, rosuvastatin 20–40 mg, or simvastatin 80 mg, % (n)* 57.2% (1326) 58.0% (681) Additional LLT other than statin, % (n)* 31.9% (740) 34.0% (399) Patient Baseline Demographics 6 *Data from pool of Phase 3 studies (n=2318 alirocumab, n=1174 placebo). LLT, lipid-lowering therapy. |
|
|
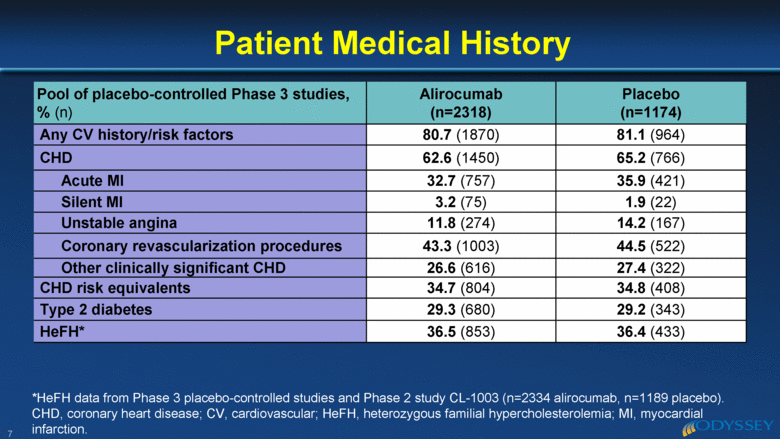
Pool of placebo-controlled Phase 3 studies, % (n) Alirocumab (n=2318) Placebo (n=1174) Any CV history/risk factors 80.7 (1870) 81.1 (964) CHD 62.6 (1450) 65.2 (766) Acute MI 32.7 (757) 35.9 (421) Silent MI 3.2 (75) 1.9 (22) Unstable angina 11.8 (274) 14.2 (167) Coronary revascularization procedures 43.3 (1003) 44.5 (522) Other clinically significant CHD 26.6 (616) 27.4 (322) CHD risk equivalents 34.7 (804) 34.8 (408) Type 2 diabetes 29.3 (680) 29.2 (343) HeFH* 36.5 (853) 36.4 (433) Patient Medical History 7 *HeFH data from Phase 3 placebo-controlled studies and Phase 2 study CL-1003 (n=2334 alirocumab, n=1189 placebo). CHD, coronary heart disease; CV, cardiovascular; HeFH, heterozygous familial hypercholesterolemia; MI, myocardial infarction. |
|
|
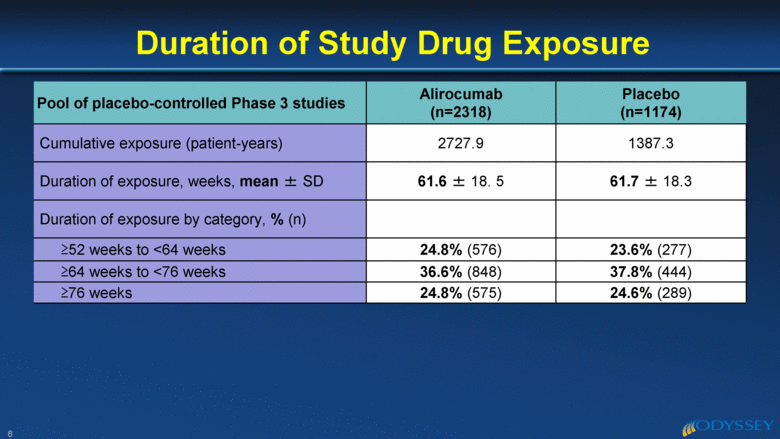
Pool of placebo-controlled Phase 3 studies Alirocumab (n=2318) Placebo (n=1174) Cumulative exposure (patient-years) 2727.9 1387.3 Duration of exposure, weeks, mean ± SD 61.6 ± 18. 5 61.7 ± 18.3 Duration of exposure by category, % (n) >52 weeks to <64 weeks 24.8% (576) 23.6% (277) >64 weeks to <76 weeks 36.6% (848) 37.8% (444) >76 weeks 24.8% (575) 24.6% (289) Duration of Study Drug Exposure 8 |
|
|
Pool of placebo-controlled studies, % (n) Alirocumab (n=2476) Placebo (n=1276) Patients with any TEAE 75.8 (1876) 76.4 (975) Patients with any treatment emergent SAE 13.7 (340) 14.3 (182) Patients with any TEAE leading to death 0.5 (13) 0.9 (11) Patients with any TEAE leading to permanent treatment discontinuation 5.3 (131) 5.1 (65) Adverse Events 9 TEAEs, treatment-emergent adverse events; TEAE period defined as the period between first to last dose of study treatment plus 70 days. |
|
|
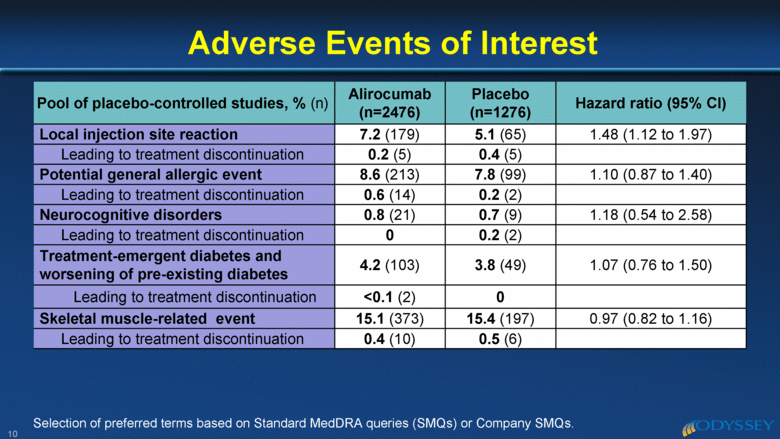
Pool of placebo-controlled studies, % (n) Alirocumab (n=2476) Placebo (n=1276) Hazard ratio (95% CI) Local injection site reaction 7.2 (179) 5.1 (65) 1.48 (1.12 to 1.97) Leading to treatment discontinuation 0.2 (5) 0.4 (5) Potential general allergic event 8.6 (213) 7.8 (99) 1.10 (0.87 to 1.40) Leading to treatment discontinuation 0.6 (14) 0.2 (2) Neurocognitive disorders 0.8 (21) 0.7 (9) 1.18 (0.54 to 2.58) Leading to treatment discontinuation 0 0.2 (2) Treatment-emergent diabetes and worsening of pre-existing diabetes 4.2 (103) 3.8 (49) 1.07 (0.76 to 1.50) Leading to treatment discontinuation <0.1 (2) 0 Skeletal muscle-related event 15.1 (373) 15.4 (197) 0.97 (0.82 to 1.16) Leading to treatment discontinuation 0.4 (10) 0.5 (6) Adverse Events of Interest 10 Selection of preferred terms based on Standard MedDRA queries (SMQs) or Company SMQs. |
|
|
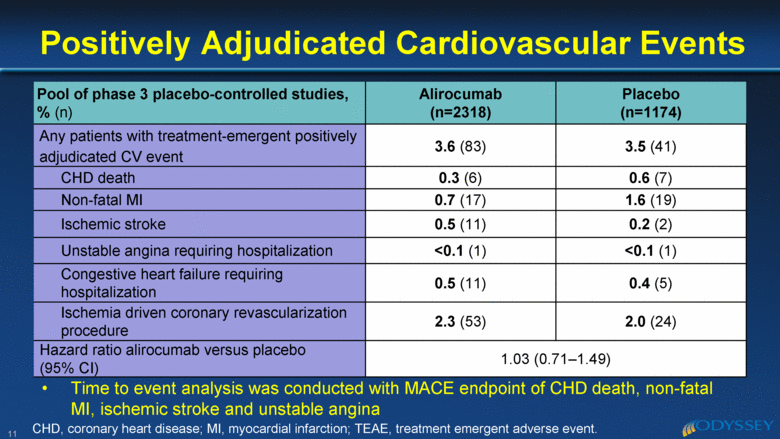
Pool of phase 3 placebo-controlled studies, % (n) Alirocumab (n=2318) Placebo (n=1174) Any patients with treatment-emergent positively adjudicated CV event 3.6 (83) 3.5 (41) CHD death 0.3 (6) 0.6 (7) Non-fatal MI 0.7 ( 17) 1.6 (19) Ischemic stroke 0.5 ( 11) 0.2 (2) Unstable angina requiring hospitalization <0.1 (1) <0.1 (1) Congestive heart failure requiring hospitalization 0.5 ( 11) 0.4 (5) Ischemia driven coronary revascularization procedure 2.3 ( 53) 2.0 (24) Hazard ratio alirocumab versus placebo (95% CI) 1.03 (0.71–1.49) Positively Adjudicated Cardiovascular Events 11 CHD, coronary heart disease; MI, myocardial infarction; TEAE, treatment emergent adverse event. Time to event analysis was conducted with MACE endpoint of CHD death, non-fatal MI, ischemic stroke and unstable angina |
|
|
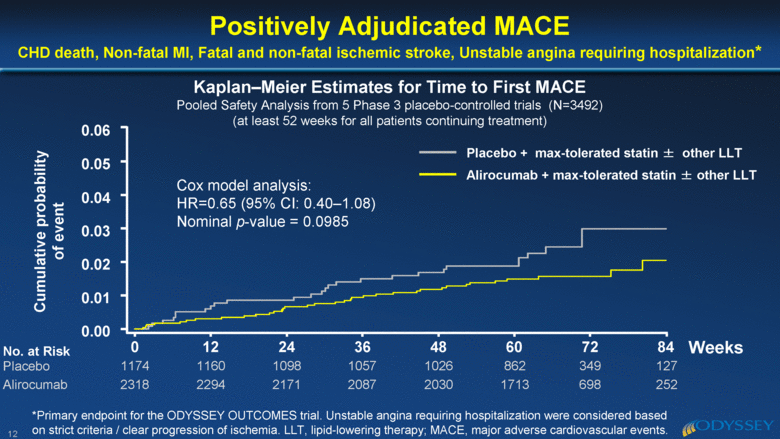
1174 2318 1160 2294 1098 2171 1057 2087 1026 2030 862 1713 349 698 127 252 84 72 60 48 36 24 12 0 0.06 0.05 0.03 0.02 0.01 0.00 0.04 12 Positively Adjudicated MACE CHD death, Non-fatal MI, Fatal and non-fatal ischemic stroke, Unstable angina requiring hospitalization* Kaplan–Meier Estimates for Time to First MACE Pooled Safety Analysis from 5 Phase 3 placebo-controlled trials (N=3492) (at least 52 weeks for all patients continuing treatment) Cumulative probability of event Placebo + max-tolerated statin ± other LLT Alirocumab + max-tolerated statin ± other LLT Cox model analysis: HR=0.65 (95% CI: 0.40–1.08) Nominal p-value = 0.0985 Weeks No. at Risk Placebo Alirocumab *Primary endpoint for the ODYSSEY OUTCOMES trial. Unstable angina requiring hospitalization were considered based on strict criteria / clear progression of ischemia. LLT, lipid-lowering therapy; MACE, major adverse cardiovascular events. |
|
|
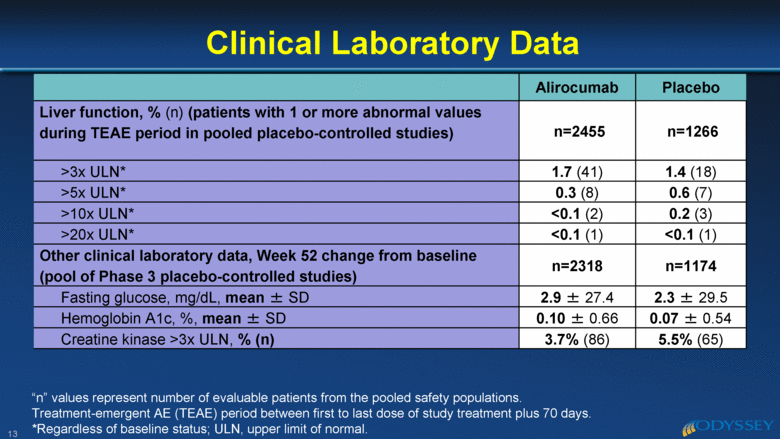
Alirocumab Placebo Liver function, % (n) (patients with 1 or more abnormal values during TEAE period in pooled placebo-controlled studies) n=2455 n=1266 >3x ULN* 1.7 (41) 1.4 (18) >5x ULN* 0.3 (8) 0.6 (7) >10x ULN* <0.1 (2) 0.2 (3) >20x ULN* <0.1 (1) <0.1 (1) Other clinical laboratory data, Week 52 change from baseline (pool of Phase 3 placebo-controlled studies) n=2318 n=1174 Fasting glucose, mg/dL, mean ± SD 2.9 ± 27.4 2.3 ± 29.5 Hemoglobin A1c, %, mean ± SD 0.10 ± 0.66 0.07 ± 0.54 Creatine kinase >3x ULN, % (n) 3.7% (86) 5.5% (65) Clinical Laboratory Data 13 “n” values represent number of evaluable patients from the pooled safety populations. Treatment-emergent AE (TEAE) period between first to last dose of study treatment plus 70 days. *Regardless of baseline status; ULN, upper limit of normal. |
|
|
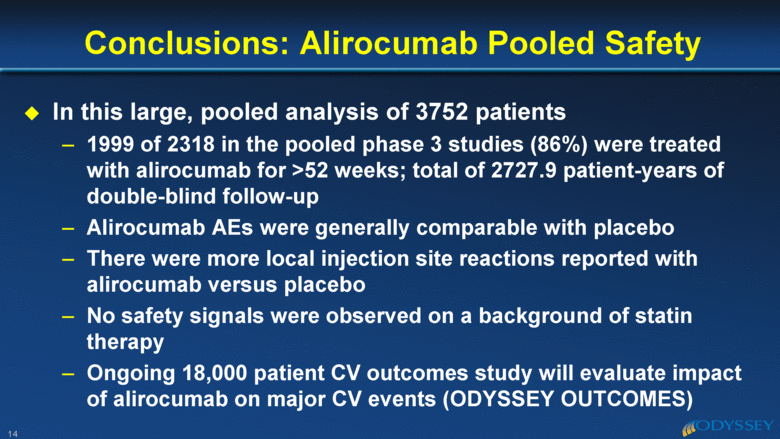
In this large, pooled analysis of 3752 patients 1999 of 2318 in the pooled phase 3 studies (86%) were treated with alirocumab for >52 weeks; total of 2727.9 patient-years of double-blind follow-up Alirocumab AEs were generally comparable with placebo There were more local injection site reactions reported with alirocumab versus placebo No safety signals were observed on a background of statin therapy Ongoing 18,000 patient CV outcomes study will evaluate impact of alirocumab on major CV events (ODYSSEY OUTCOMES) Conclusions: Alirocumab Pooled Safety 14 |