Attached files
| file | filename |
|---|---|
| 8-K/A - CYTORI THERAPEUTICS, INC 8-KA 3-4-2015 - PLUS THERAPEUTICS, INC. | form8ka.htm |
Exhibit 99.1

Cytori TherapeuticsNASDAQ: CYTXCorporate UpdateMarch 2015 NASDAQ: CYTX Restoring Lives *

Forward Looking Statements This presentation contains certain ‘forward-looking statements’ about Cytori Therapeutics, Inc. All statements, other than statements of historical fact, that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future are forward-looking statements. Such statements are based upon certain assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate. The forward-looking statements included in this presentation, involve known and unknown risks that relate to future events or our future financial performance and the actual results could differ materially from those discussed in this presentation. Some of those forward-looking statements include: our ability to successfully initiate the planned clinical trials in the United States, Japan and Europe, as well as the financial, clinical and regulatory burdens associated with those trials, and our ability to complete the trials in the time frames referenced, the various medical indications that may be addressed by Cytori Cell Therapy, the potential effectiveness of Cytori Cell Therapy, our ability to maintain a substantially reduced cash burn and increase our percentage of R&D expenditures compared to prior years, Our partners ability to launch products in China and Europe, our ability to refinance our corporate loan, and the anticipated BARDA funding of approximately $8.3 million to cover the costs of the pilot clinical trial for thermal burn. Some risks and uncertainties related to such forward looking statements include: risks in the collection and results of clinical data, final clinical outcomes, regulatory uncertainties, financing uncertainties, dependence on third party performance, future Government funding and procurement priorities, the Government’s sole discretion in determining funding timing and amounts, the Government’s ability to reduce, modify or terminate the BARDA contract if it determines it is in the Government’s best interests to do so, the performance of our products, and other risks and uncertainties described under the "Risk Factors" section in our Securities and Exchange Commission Filings on Form 10-K and Form 10-Q. These risks and uncertainties may cause our actual results to differ materially from those discussed in this presentation. We advise reading our most recent annual report on Form 10-K and quarterly report on Form 10-Q filed with the United States Securities and Exchange Commission for a more detailed description of these risks.The forward-looking statements contained in this presentation represent our estimates and assumptions only as of the date of this presentation and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this presentation as a result of new information, future events or changes in our expectations. NASDAQ: CYTX *
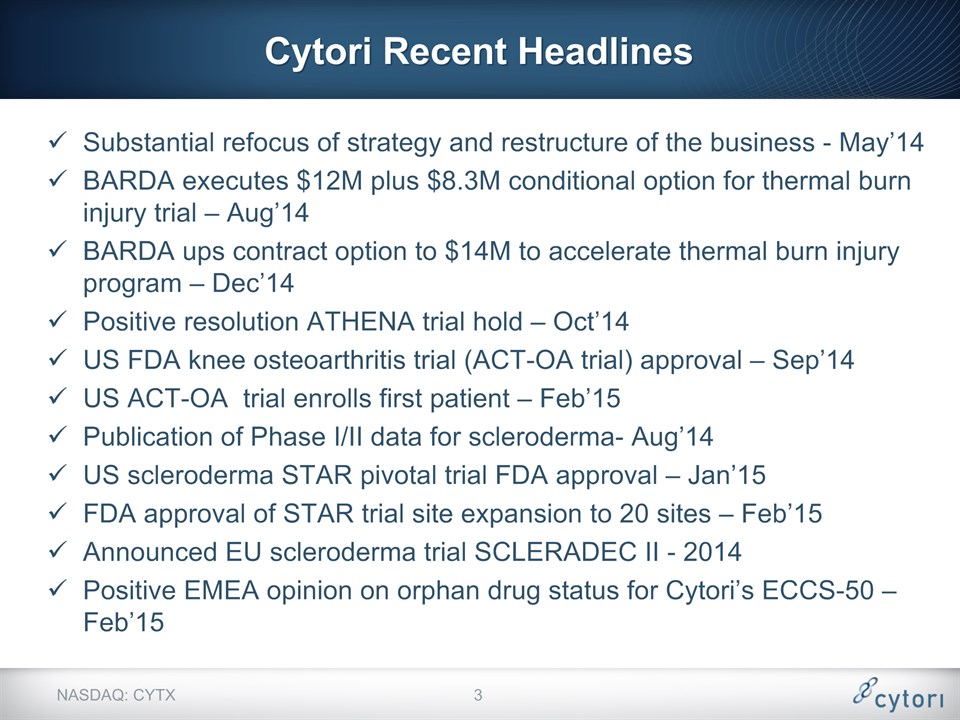
Cytori Recent Headlines Substantial refocus of strategy and restructure of the business - May’14BARDA executes $12M plus $8.3M conditional option for thermal burn injury trial – Aug’14BARDA ups contract option to $14M to accelerate thermal burn injury program – Dec’14Positive resolution ATHENA trial hold – Oct’14US FDA knee osteoarthritis trial (ACT-OA trial) approval – Sep’14US ACT-OA trial enrolls first patient – Feb’15Publication of Phase I/II data for scleroderma- Aug’14US scleroderma STAR pivotal trial FDA approval – Jan’15FDA approval of STAR trial site expansion to 20 sites – Feb’15Announced EU scleroderma trial SCLERADEC II - 2014Positive EMEA opinion on orphan drug status for Cytori’s ECCS-50 – Feb’15 NASDAQ: CYTX *

Cytori Corporate Overview NASDAQ: CYTX * Autologous cell therapeutics from adipose Regulated as PMA device BARDA, Japan MHLW funded clinical trialsSignificant new opportunities possible PHASE III /Pivotal trial for SclerodermaEst. market value > $1B PHASE IIB for knee osteoarthritisEst. Market value >$3B PLATFORMTechnology ORPHANIndication DISCIPLINED Business Development Strategy SIZABLEMarket Indication
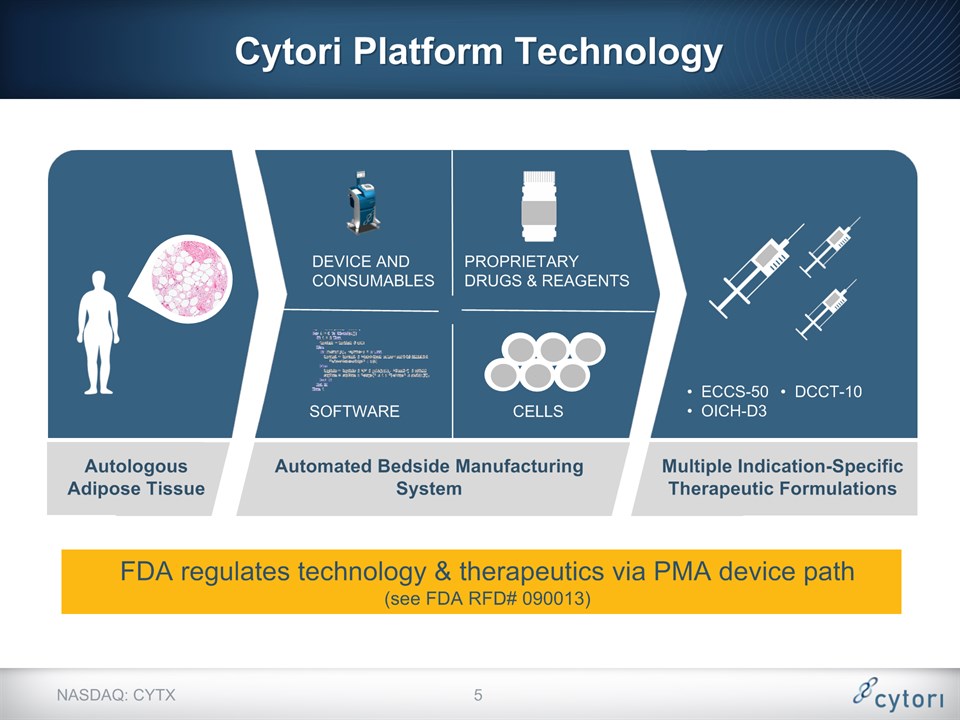
Cytori Platform Technology NASDAQ: CYTX ECCS-50OICH-D3 PROPRIETARY DRUGS & REAGENTS DEVICE AND CONSUMABLES SOFTWARE FDA regulates technology & therapeutics via PMA device path(see FDA RFD# 090013) * Automated Bedside Manufacturing System Autologous Adipose Tissue CELLS DCCT-10 Multiple Indication-Specific Therapeutic Formulations
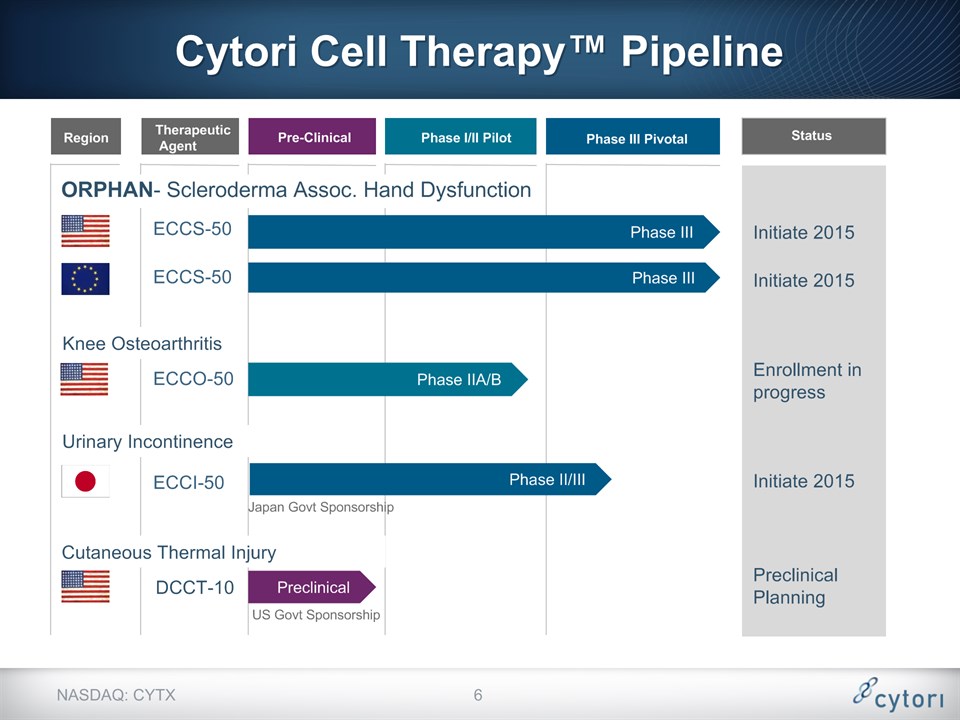
Phase II/III Initiate 2015 Initiate 2015 Cytori Cell Therapy™ Pipeline Urinary Incontinence ECCI-50 Japan Govt Sponsorship US Govt Sponsorship Knee Osteoarthritis Phase IIA/B ECCO-50 Enrollment in progress Cutaneous Thermal Injury Preclinical DCCT-10 Preclinical Planning NASDAQ: CYTX * Initiate 2015 Phase III ECCS-50 Phase III ECCS-50 ORPHAN- Scleroderma Assoc. Hand Dysfunction Region Therapeutic Agent Pre-Clinical Status Phase I/II Pilot Phase III Pivotal

Cytori Cell Therapy forScleroderma Hand Dysfunction NASDAQ: CYTX *
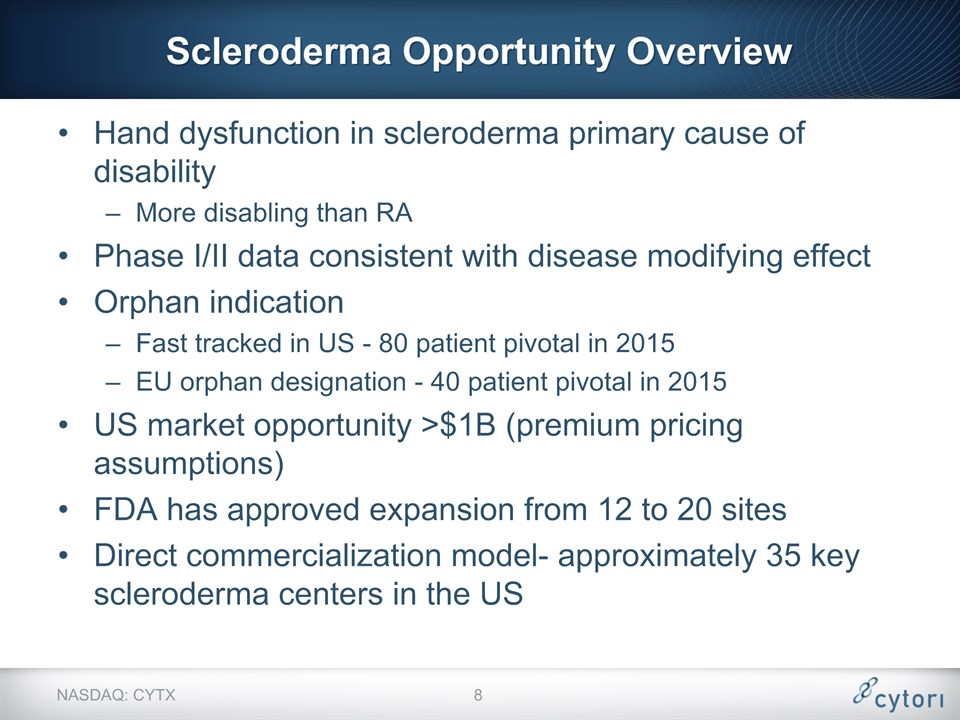
Scleroderma Opportunity Overview Hand dysfunction in scleroderma primary cause of disabilityMore disabling than RA Phase I/II data consistent with disease modifying effectOrphan indication Fast tracked in US - 80 patient pivotal in 2015EU orphan designation - 40 patient pivotal in 2015US market opportunity >$1B (premium pricing assumptions)FDA has approved expansion from 12 to 20 sitesDirect commercialization model- approximately 35 key scleroderma centers in the US NASDAQ: CYTX *

Systemic Scleroderma of the Hand (SSc-H) Systemic sclerosis (SSc) or sclerodermaRare Autoimmune conditionAffects women:men, 4:1Cutaneous and visceral fibrosis Obliteration of the lumen of small vessels >90% patients hand disabilitySSc-H manifestations principal source of functional impairment and reduced quality of lifeFibrosis, pain, and edema result in diminished mobility and hand function even with standard medical care Images reproduced with permission of the nonprofit International Scleroderma Network at sclero.org NASDAQ: CYTX Pathophysiology *

Rare Disease Basis for Scleroderma (SSc-H) & Therapeutic Analogue SclerodermaDefinitionAn autoimmune disorder causing collagen overproduction leading to fibrosis and impaired vasculature. Most commonly effects the hands but often affects multiple organ systems. EpidemiologyPrevalence: 50 – 75,000 (242/million adults)Incidence: 4,400 (18.8/million adults)Predominance in women 20 to 50 years oldTherapeuticsFocus on vasodilation/vasoconstrictionCalcium channel blockersNO pathwayEndothelin-1 receptor antagonistsProstanoidsEstimated US Market Opportunity>$1B Analogous DiseaseRheumatoid Arthritis (RA)DefinitionAn autoimmune disorder causing a systemic inflammation which manifests itself in multiple joints of the body. Primarily affects lining of the joints but can also affect other organs.EpidemiologyPrevalence: 1,500,000 (30x more common than SSc)Incidence: 131,000 (410/million)TherapeuticsNSAIDSDisease modifying drugsMethotrexateBiologicsRA biologics can cost over $30k/year NASDAQ: CYTX *

Comparison of Scleroderma and RA Hand Disability Metric Outcomes Source Work disability (WD) WD was observed in 56% of SSc patients vs. 35% of RA patients Ouimet 2007 Work disability (WD) “…the prevalence of work disability in SSc is substantially higher than other common rheumatic conditions.” Sharif 2011 HAQ-DI “QOL in patients with SSc, as indicated by their level of physical function, was significantly reduced compared to healthy controls, but similar across groups of rheumatology patients… Joint involvement in SSc is more disabling than joint involvement in [psoriatic arthritis]; and patients with SSc experience more severe pain than patients with RA” Johnson 2007 HAQ-DI “…patients with dSSc have more functional impairment than patients with RA or other CTDs [connective tissue diseases]” Morita 2007 Cost (health care utilization) “…indirect comparison with RA in Canada suggests that SSc’s average costs are higher (RA: 10 459; SSc: 12 585 euros/patient/year)” Minier 2010 Cost (health care utilization) “…average annual cost of SSc per patient may be as high as that of RA (the equivalent of $16,141 in 2007 Canadian dollars, based on RA cost estimates from one study [31]), and in diffuse SSc the average annual cost per patient may very well exceed the cost of RA.” Bernatsky 2009 Published studies confirm that SSc disability is similar to or worse than RA NASDAQ: CYTX *
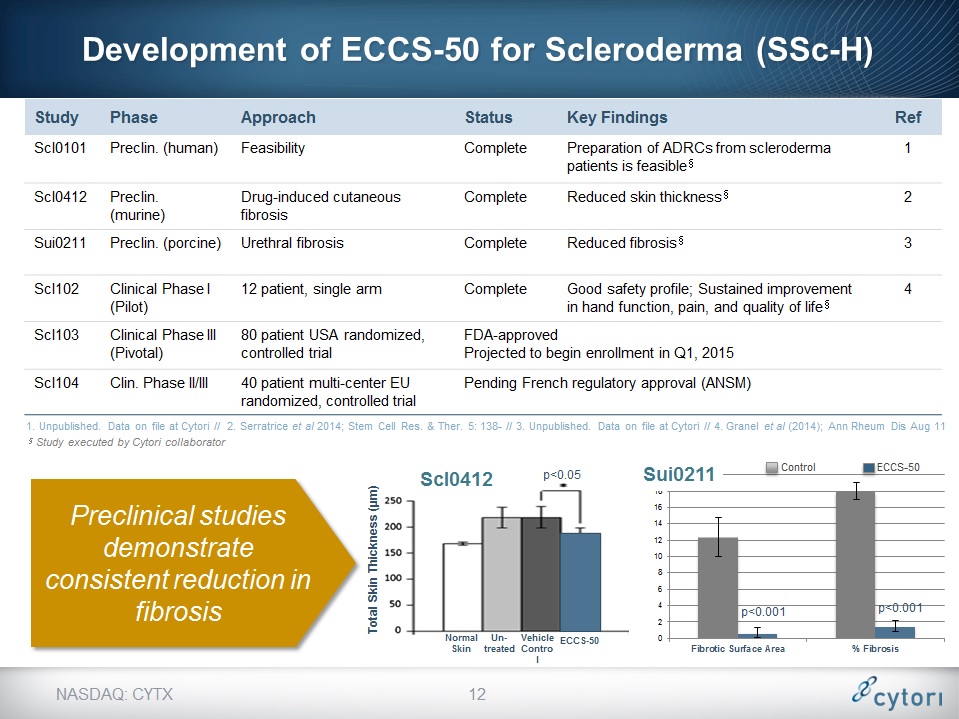
Development of ECCS-50 for Scleroderma (SSc-H) NASDAQ: CYTX Study Phase Approach Status Key Findings Ref Scl0101 Preclin. (human) Feasibility Complete Preparation of ADRCs from scleroderma patients is feasible§ 1 Scl0412 Preclin.(murine) Drug-induced cutaneous fibrosis Complete Reduced skin thickness§ 2 Sui0211 Preclin. (porcine) Urethral fibrosis Complete Reduced fibrosis§ 3 Scl102 Clinical Phase I (Pilot) 12 patient, single arm Complete Good safety profile; Sustained improvement in hand function, pain, and quality of life§ 4 Scl103 Clinical Phase III (Pivotal) 80 patient USA randomized, controlled trial FDA-approvedProjected to begin enrollment in Q1, 2015 FDA-approvedProjected to begin enrollment in Q1, 2015 FDA-approvedProjected to begin enrollment in Q1, 2015 Scl104 Clin. Phase II/III 40 patient multi-center EU randomized, controlled trial Pending French regulatory approval (ANSM) Pending French regulatory approval (ANSM) Pending French regulatory approval (ANSM) 1. Unpublished. Data on file at Cytori // 2. Serratrice et al 2014; Stem Cell Res. & Ther. 5: 138- // 3. Unpublished. Data on file at Cytori // 4. Granel et al (2014); Ann Rheum Dis Aug 11 Preclinical studies demonstrate consistent reduction in fibrosis p<0.001 p<0.001 § Study executed by Cytori collaborator p<0.05 Scl0412 * Control ECCS-50

E.U. SCLERADEC I Pilot Trial for Scleroderma (SSc-H) Study DesignSingle center (Marseille, France), open-label trial of 12 patients (NCT01813279)Funded by Groupe Francophone de Recherche de la SclérodermiePopulationMen and women with diagnosis of limited or diffuse sclerodermaAge ≥ 18 yearsFunctional disability of the handCochin Hand Function Score >20Treatment/DosingECCS-50: 1 mL s.c. into each finger (4 million cells/finger)Study EndpointsPrimary endpoint: CHFSSecondary endpoints:Hand symptoms and function (other than CHFS)Health-related quality of life (S-HAQ questionnaire)Raynaud’s & vasculopathySafety NASDAQ: CYTX *

E.U. SCLERADEC I Pilot Trial Results for Scleroderma (SSc-H) ECCS-50 SafetyNo serious AEs during follow-upFour minor AEs reported by four patientsAll resolved spontaneously within 15dECCS-50 EfficacyHand FunctionAverage 57% improvement in Cochin Hand Function Score at 6 monthsImproved grip and pinch strengthPainAverage 64% improvement in pain at 6 monthsVasculopathy69% reduction in Raynaud’s score (frequency and intensity) at 6 monthsReduced edema (finger size)Ulcer Healing53% reduction in number of ulcers and 90% reduction in average ulcer area at 6 months Scleradec I Results CHFS = Cochin Hand Function ScaleVAS = Visual Acuity Scale (Pain)RCS = Raynaud’s Condition ScoreSHAQ = Scleroderma Health Assessment Questionnairemean ± std err; p values shown for 6 month data NASDAQ: CYTX Granel et al (2014); Ann Rheum Dis Aug 11 *

NASDAQ: CYTX ECCS-50 Treatment led to a progressive decrease in the number of ulcers and average ulcer area mean ± std err …and significant normalization of microvasculature mean ± std err p<0.05 p<0.05 E.U. SCLERADEC I Pilot Trial Results for Scleroderma (SSc-H)- II Granel et al (2014); Ann Rheum Dis Aug 11 *
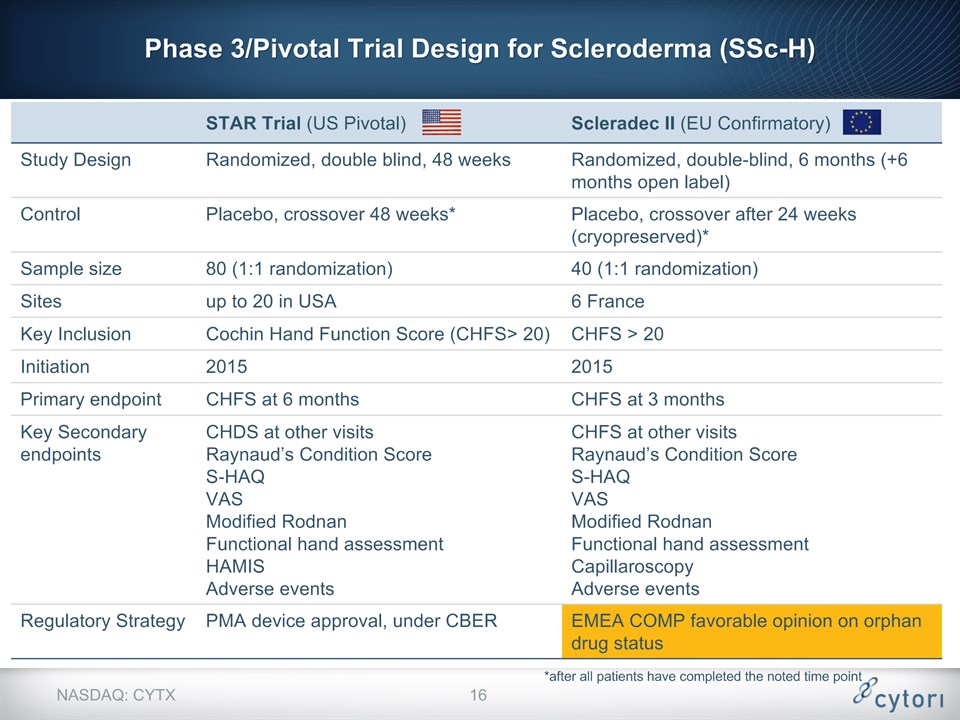
STAR Trial (US Pivotal) Scleradec II (EU Confirmatory) Study Design Randomized, double blind, 48 weeks Randomized, double-blind, 6 months (+6 months open label) Control Placebo, crossover 48 weeks* Placebo, crossover after 24 weeks (cryopreserved)* Sample size 80 (1:1 randomization) 40 (1:1 randomization) Sites up to 20 in USA 6 France Key Inclusion Cochin Hand Function Score (CHFS> 20) CHFS > 20 Initiation 2015 2015 Primary endpoint CHFS at 6 months CHFS at 3 months Key Secondary endpoints CHDS at other visitsRaynaud’s Condition ScoreS-HAQVASModified RodnanFunctional hand assessmentHAMISAdverse events CHFS at other visitsRaynaud’s Condition ScoreS-HAQVASModified RodnanFunctional hand assessmentCapillaroscopyAdverse events Regulatory Strategy PMA device approval, under CBER EMEA COMP favorable opinion on orphan drug status *after all patients have completed the noted time point NASDAQ: CYTX Phase 3/Pivotal Trial Design for Scleroderma (SSc-H) *

Cytori Cell Therapy for Knee Osteoarthritis NASDAQ: CYTX *

Summary of Osteoarthritis Opportunity Knee osteoarthritis has high prevalence and market opportunity >$3BOpportunity for symptom or disease modification- bridge anti-inflammatory drugs and knee replacementSubstantial preclinical and proof of concept clinical dataUS phase IIb trial enrolling- data 2016Seek commercialization partner(s) NASDAQ: CYTX *

ACT-OA (US Phase II) ACT-OA (US Phase II) Study Design Randomized, double blind, 48 weeks duration, dose escalation (low and high dose cell ECCO-50 therapy) Control Placebo, no crossover Sample size 90 (1:1:1 randomization) Sites Up to 15 in USA Key Inclusion OA of Knee, pain > 6 months, pain on walking > moderate, KL score 2-3, Initiation 2015 Primary endpoint KOOS – Pain on Walking at 12 Weeks Key Secondary endpoints Observed Pain Scores on 50-foot Walk TestNumber of Observed OARSI30 Responders Using the 50-Foot Walk TestKnee injury and Osteoarthritis Outcome Score (KOOS) VAS Assessments (0-100 mm scale)Patient global assessmentNumber of tablets of rescue medicationShort-Form (SF)-36 questionnaireMOAKS scoring (MRI Osteoarthritis Knee Score) at Week 48Adverse events Regulatory Strategy Phase III study leading to PMA (under CBER) and approval in EU, Canada and other markets as appropriate NASDAQ: CYTX U.S. Pilot/Phase 2 Trial for Knee Osteoarthritis *

Knee Osteoarthritis NASDAQ: CYTX Osteoarthritis (OA) DefinitionDisease of the entire joint involving the cartilage, joint lining, ligaments, and underlying bone. The breakdown of tissues leads to pain and joint stiffness 2014E 2014E 2014E Treatment Modality # Patients / Treatments ASP Market Size Celebrex/NSAID 3,900,000* $564 $2.2B Knee Viscosupplement Injection 898,000**- $935 $0.8B Total Knee Arthroplasty 780,000-- $4,402 $3.4B * Includes sales of packages for multiple indications: OA, RA, Ankylosing Spondylitis, Acute Pain Management.** Represents a particular course of therapy performed in the U.S. (i.e., one single-injection or multiple-injection treatment). Pathophysiology * Current Therapies EpidemiologyOA is the most common form of arthritis13.9% of adults >25 years33.6% (12.4 million) >65 yearsEstimated ~26.9 million US adults (2005)

Development of ECCO-50 for Knee Osteoarthritis NASDAQ: CYTX Study Phase Approach Status Key Findings Ref OA0103 Preclinical(human) Demonstration of in vitro differentiation towards chondrocytes Complete Expression of multiple markers characteristic of chondrogenesis 1 OA0203 Preclinical(caprine) Injured-induced osteochondral defect Complete Improved healing at 4 months§ 2 OA0205 Preclinical(canine) Injection into injured intervertebral disc Complete Improved disc biochemistry and matrix production 3 OA0501 Veterinary(canine) 21 animal randomized, double-blind trial of OA in the hip Complete Improvement in lameness, pain, and range of motion§ 4 OA0502 Veterinary(canine) Open-label multi-center study of 14 animals with elbow OA Complete Improvement in lameness, pain, and range of motion§ 5 OA104 Clinical Phase I (Pilot) 25 patient, single arm; OUS Complete Improvement in activity and knee function (Lysholm) ¶ 6 OA105 Clinical Phase I (Pilot) 18 single arm; OUS Complete Improvement in pain and knee function (Lysholm and WOMAC) ¶ 7 OA106 Clinical Phase I (Pilot) Higher dose; 25 patient, single arm with 2nd look arthroscopy at 2yrs; OUS Complete Improvement in pain and knee function; 64% positive or very positive on 2nd look; only 12.5% ‘failed’ ¶ 8 OA107 Clinical Phase II (Pilot) Multi-center, USA randomized, double-blind placebo-controlled trial FDA- IDE approvedProjected to begin enrollment in Q1, 2015 FDA- IDE approvedProjected to begin enrollment in Q1, 2015 FDA- IDE approvedProjected to begin enrollment in Q1, 2015 Huang et al 2004; Plast Reconstr Surg. 113(2):585-94Jurgens et al 2013; BioResearch 2 (4) pp. 315-25Ganey et al 2009; 34 (21) 2297-304Black et al 2008; Vet Ther. 8 (4) pp. 272-84Black et al 2008; Vet Ther. 9 (3) pp. 192-200Koh et al 2012; The Knee 19: 902-7Koh et al 2013; Arthroscopy 29 (4) 748-55Koh et al 2013; Knee Surg Sports Traumatol Arthrosc § Study executed by Cytori collaborator¶ Study executed independently of Cytori *

Development of Cytori Cell Therapy for Knee Osteoarthritis NASDAQ: CYTX * Goat Injury ModelTreatment led to greater healing of cartilage 4 months after injury1 Jurgens et al 2013; BioResearch 2 (4) pp. 315-25Black et al 2007; Vet Ther. 8 (4) pp. 272-84Koh et al 2013; Knee Surg Sports Traumatol Arthrosc Study performed with adipose derived cell therapy with PRP Canine veterinary model (randomized, controlled)Treatment led to improvements in lameness, pain, and range of motion2 Clinical StudyTreatment led to reduced pain, increased function, and potential cartilage repair3,4

Cytori Business Development Activity & Financials NASDAQ: CYTX *

Cytori-U.S. Government Collaboration for Thermal Burn Countermeasure GoalDevelop a medical countermeasure for use following mass casualty attack involving thermal burn & radiation injuryContract value: up to $106mGoal - United States Government acquisition contract for Cytori Cell TherapyStatus$4.7m- proof-of-concept phase completed$14m- contract option 1 for additional development activities ongoing$8.3m- contract option 2 to fund US Phase I/II clinical trial pre-reviewed and approvable, subject to FDA IDE approval$69m additional contract options for Phase III clinical trial and for development of countermeasure for combined radiation & thermal injury Other medical countermeasure options possible outside current contract NASDAQ: CYTX * US Government Contract # HHSO100201200008C The BARDA contract provides substantial operating leverage to Cytori’s R&D efforts & potential for acquisition contract
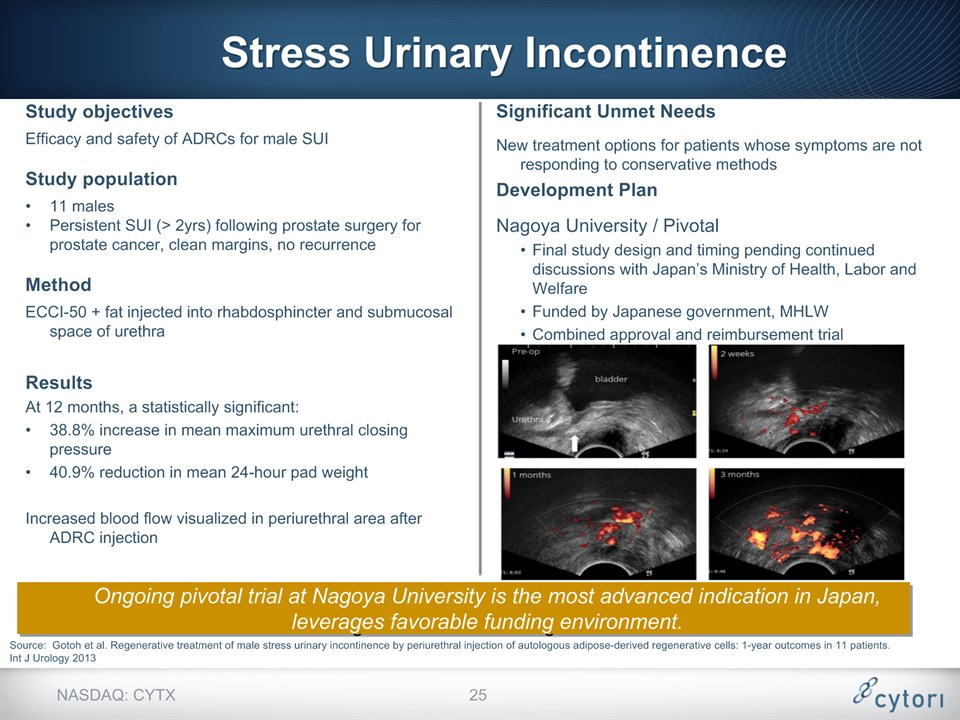
Source: Gotoh et al. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose-derived regenerative cells: 1-year outcomes in 11 patients. Int J Urology 2013 * NASDAQ: CYTX Stress Urinary Incontinence Study objectivesEfficacy and safety of ADRCs for male SUIStudy population11 malesPersistent SUI (> 2yrs) following prostate surgery for prostate cancer, clean margins, no recurrence MethodECCI-50 + fat injected into rhabdosphincter and submucosal space of urethraResultsAt 12 months, a statistically significant:38.8% increase in mean maximum urethral closing pressure40.9% reduction in mean 24-hour pad weightIncreased blood flow visualized in periurethral area after ADRC injection Significant Unmet NeedsNew treatment options for patients whose symptoms are not responding to conservative methodsDevelopment PlanNagoya University / PivotalFinal study design and timing pending continued discussions with Japan’s Ministry of Health, Labor and WelfareFunded by Japanese government, MHLWCombined approval and reimbursement trial Ongoing pivotal trial at Nagoya University is the most advanced indication in Japan, leverages favorable funding environment.

ATHENA TRIAL - Heart Failure * ATHENA Trials SummaryRandomized, DB, PC trial in US of OICH-D3 treatment in patients with chronic heart failure- primary endpoint VO2 max. ATHENA I 28 2:1 active:control lower dose ATHENA II 2:1 active:control higher dose 3 NASDAQ: CYTX Enrollment stopped after 31 patients for safety reviewThorough safety review conducted, permission to proceed with protocol amendmentsCytori decision – truncate enrollment, evaluate 6 and 12 month dataFurther decisions on investment based on analysis of data, optimization of protocol (as per amendments) and incorporation of next generation technology6 month data analysis 1Q201512 month data analysis 4Q2015 STATUS TIMELINES

75 patents issued worldwide; 45 applications pending Cytori’s Global Patent Estate NASDAQ: CYTX Protect Cytori’s proprietary methods and devices for manufacturing Cytori Cell Therapy, as well as methods of using Cytori Cell Therapy in the treatment of scleroderma, osteoarthritis, and several other pipeline indications * 33% 15% 11% 22% 19% OTHER USA EU JAPAN ASIA-PACIFIC

Business Development & Revenue Direct sales- Japan & Europe Approach to Business Development & Revenue NASDAQ: CYTX * Revenue Drivers (*) Excludes share based compensation. Licensing partners US BARDA contract revenue Out licensing activity in non-core geographies and indicationsOver $100MM in non-dilutive or premium capital raised Restructured and focused direct sales forcePositioned in Q4 for positive cash flow that mitigates operating burn

Operating Expense Reductions & Capitalization NASDAQ: CYTX * Capitalization Summary Expense Reductions & Focus: Operating Cash Burn Management FOCUS - 2015 expectation of >50% OPEX in R&D vs. 2014 (~40%)

Forthcoming 2015 Milestones Chinese FDA Class-I clearance, Lorem Vascular POEMEA final opinion on orphan drug status for Cytori’s ECCS-50Initiate enrolment of scleroderma STAR trialPublish SCLERADEC-I 12 month data and initiate enrollment of EU SCLERADEC-II trialComplete enrollment of ACT-OA trial, data expected in 2016 Begin enrollment of MHLW funded Japanese urinary incontinence trial BARDA funded research progress presented at American Burn Association meetingATHENA 6 and 12 month trial data availableComplete development of next generation Celution System NASDAQ: CYTX *
Cytori Corporate OverviewNASDAQ: CYTXThank you! QUESTIONS, please contact ir@cytori.com NASDAQ: CYTX *
