Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a14-23067_28k.htm |
Exhibit 99.1
|
|
Efficacy and safety of once-daily umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two replicate randomized 12-week studies Thomas M. Siler Midwest Chest Consultants, PC, 330 First Capitol Drive, Suite 470, St Charles, Missouri, USA |
|
|
Disclosures Dr Siler has: Received research support from: Boehringer-Ingelheim, Elevation, Forest Research Institute, GlaxoSmithKline, Novartis, Pearl Therapeutics and Sunovion Took part in Speaker bureau for: Astra Zeneca, Boehringer-Ingelheim and UCB Acted as a Consultant for: Astra Zeneca and Vapotherm The studies presented here were funded by GlaxoSmithKline (200109, NCT01957163; 200110, NCT02119286) |
|
|
Off-label Discussion Declaration Thomas M. Siler I will be discussing data of the combination use of FF/VI and UMEC at doses of 62.5 mcg and 125 mcg. UMEC 125 mcg is not an approved dose. |
|
|
COPD and unmet medical needs 1. Global Initiative for Chronic Obstructive Lung Disorder Report (2014) www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jan23.pdf COPD is characterized by persistent airflow limitation and contributes substantially to global morbidity and mortality1 Central to the pharmacological management of COPD are bronchodilators (e.g. LAMAs, LABAs) and inhaled anti-inflammatories (e.g. ICS)1 |
|
|
Study rationale Combination therapies can offer improvements in pulmonary function over monotherapies, with a lower risk of side effects compared with increasing the dose of a single agent1 These two replicate studies evaluated the efficacy and safety of UMEC added to FF/VI in patients with moderate-to-very-severe COPD 1. Global Initiative for Chronic Obstructive Lung Disorder Report (2014) www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jan23.pdf; 2. Glaxo Group Limited. RELVAR ELLIPTA® Summary of product characteristics. http://www.ema.europa.eu/docs; 3. GlaxoSmithKline. INCRUSE® Summary of product characteristics. http://www.ema.europa.eu/ema/; 4. GlaxoSmithKline. INCRUSE® Prescribing Information. https://www.gsksource.com/gskprm/ Umeclidinium (UMEC, 62.5 mcg) is a LAMA indicated for long-term, once-daily maintenance treatment of airflow obstruction in COPD3,4 Fluticasone furoate/vilanterol (FF/VI [combined ICS/LABA]) is indicated for long-term, once-daily maintenance treatment of airflow obstruction in COPD2 |
|
|
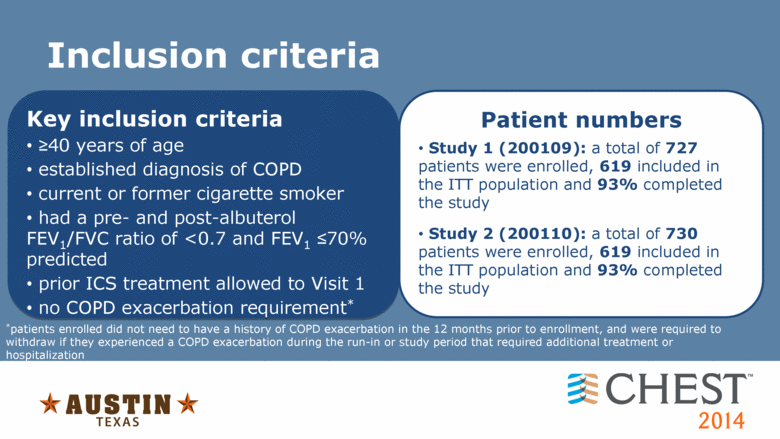
Inclusion criteria Key inclusion criteria >40 years of age established diagnosis of COPD current or former cigarette smoker had a pre- and post-albuterol FEV1/FVC ratio of <0.7 and FEV1 <70% predicted prior ICS treatment allowed to Visit 1 no COPD exacerbation requirement* Patient numbers Study 1 (200109): a total of 727 patients were enrolled, 619 included in the ITT population and 93% completed the study Study 2 (200110): a total of 730 patients were enrolled, 619 included in the ITT population and 93% completed the study *patients enrolled did not need to have a history of COPD exacerbation in the 12 months prior to enrollment, and were required to withdraw if they experienced a COPD exacerbation during the run-in or study period that required additional treatment or hospitalization FEV1, forced expiratory volume in one second; FVC, forced vital capacity |
|
|
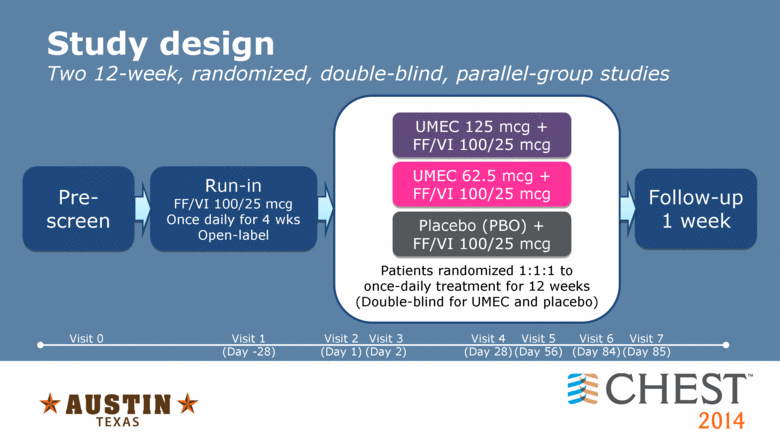
Study design Two 12-week, randomized, double-blind, parallel-group studies Patients randomized 1:1:1 to once-daily treatment for 12 weeks (Double-blind for UMEC and placebo) Pre-screen Run-in FF/VI 100/25 mcg Once daily for 4 wks Open-label UMEC 125 mcg + FF/VI 100/25 mcg UMEC 62.5 mcg + FF/VI 100/25 mcg Placebo (PBO) + FF/VI 100/25 mcg Follow-up 1 week Visit 0 Visit 1 (Day -28) Visit 2 (Day 1) Visit 3 (Day 2) Visit 4 (Day 28) Visit 5 (Day 56) Visit 6 (Day 84) Visit 7 (Day 85) |
|
|
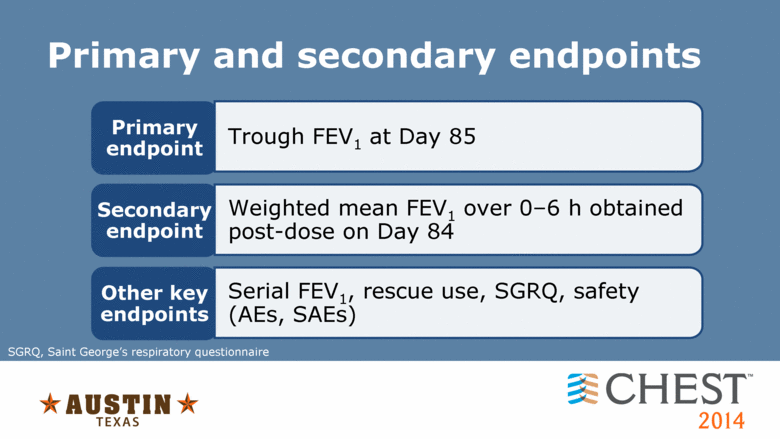
Primary and secondary endpoints Primary endpoint Trough FEV1 at Day 85 Secondary endpoint Weighted mean FEV1 over 0–6 h obtained post-dose on Day 84 SGRQ, Saint George’s respiratory questionnaire Other key endpoints Serial FEV1, rescue use, SGRQ, safety (AEs, SAEs) |
|
|
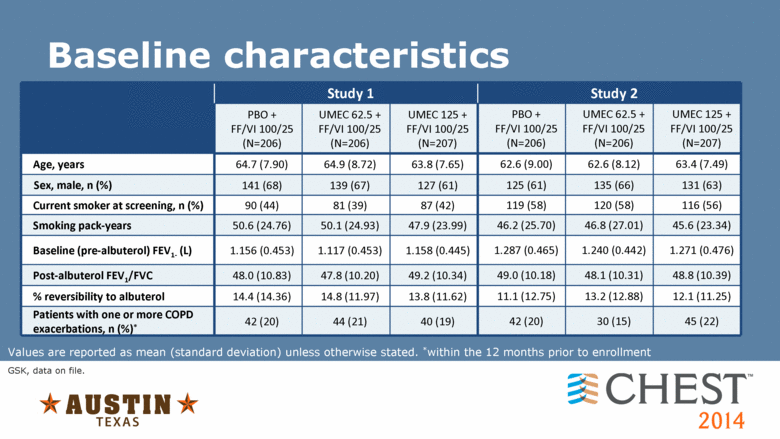
Study 1 Study 2 PBO + FF/VI 100/25 (N=206) UMEC 62.5 + FF/VI 100/25 (N=206) UMEC 125 + FF/VI 100/25 (N=207) PBO + FF/VI 100/25 (N=206) UMEC 62.5 + FF/VI 100/25 (N=206) UMEC 125 + FF/VI 100/25 (N=207) Age, years 64.7 (7.90) 64.9 (8.72) 63.8 (7.65) 62.6 (9.00) 62.6 (8.12) 63.4 (7.49) Sex, male, n (%) 141 (68) 139 (67) 127 (61) 125 (61) 135 (66) 131 (63) Current smoker at screening, n (%) 90 (44) 81 (39) 87 (42) 119 (58) 120 (58) 116 (56) Smoking pack-years 50.6 (24.76) 50.1 (24.93) 47.9 (23.99) 46.2 (25.70) 46.8 (27.01) 45.6 (23.34) Baseline (pre-albuterol) FEV1 (L) 1.156 (0.453) 1.117 (0.453) 1.158 (0.445) 1.287 (0.465) 1.240 (0.442) 1.271 (0.476) Post-albuterol FEV1/FVC 48.0 (10.83) 47.8 (10.20) 49.2 (10.34) 49.0 (10.18) 48.1 (10.31) 48.8 (10.39) % reversibility to albuterol 14.4 (14.36) 14.8 (11.97) 13.8 (11.62) 11.1 (12.75) 13.2 (12.88) 12.1 (11.25) Patients with one or more COPD exacerbations, n (%)* 42 (20) 44 (21) 40 (19) 42 (20) 30 (15) 45 (22) Baseline characteristics Values are reported as mean (standard deviation) unless otherwise stated. *within the 12 months prior to enrollment GSK, data on file. |
|
|
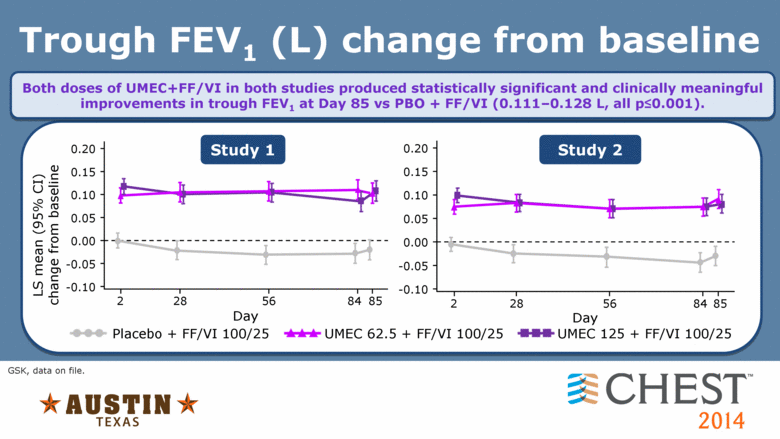
Trough FEV1 (L) change from baseline Study 1 Study 2 Both doses of UMEC+FF/VI in both studies produced statistically significant and clinically meaningful improvements in trough FEV1 at Day 85 vs PBO + FF/VI (0.111–0.128 L, all p<0.001). 2 56 84 85 Day 0.20 0.15 0.10 0.05 0.00 -0.05 -0.10 28 0.20 0.15 0.10 0.05 0.00 -0.05 -0.10 LS mean (95% CI) change from baseline 2 28 56 84 85 Day UMEC 62.5 + FF/VI 100/25 Placebo + FF/VI 100/25 UMEC 125 + FF/VI 100/25 GSK, data on file. |
|
|
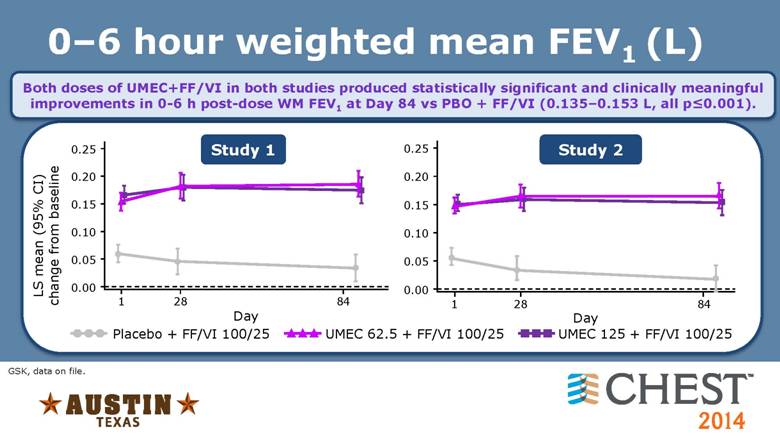
0–6 hour weighted mean FEV1 (L) GSK, data on file. Both doses of UMEC+FF/VI in both studies produced statistically significant and clinically meaningful improvements in 0-6 h post-dose WM FEV1 at Day 84 vs PBO + FF/VI (0.135–0.153 L, all p<0.001). Study 1 Study 2 UMEC 62.5 + FF/VI 100/25 Placebo + FF/VI 100/25 UMEC 125 + FF/VI 100/25 LS mean (95% CI) change from baseline 0.25 0.15 0.10 0.05 0.00 1 28 84 Day 0.20 1 84 Day 0.25 0.20 0.15 0.10 0.05 0.00 28 |
|
|
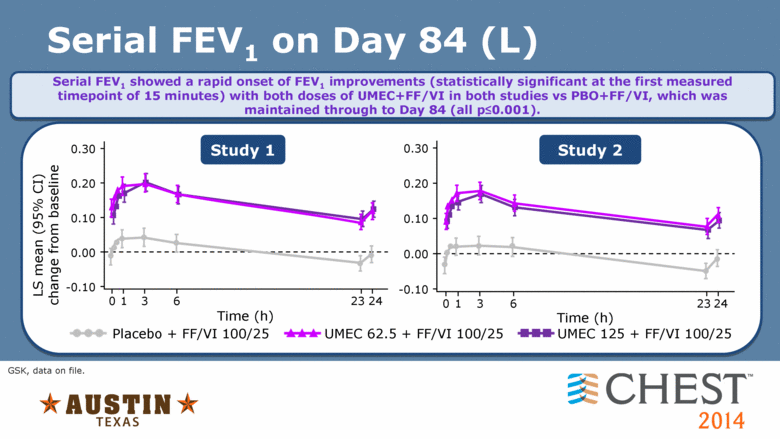
Serial FEV1 on Day 84 (L) GSK, data on file. Serial FEV1 showed a rapid onset of FEV1 improvements (statistically significant at the first measured timepoint of 15 minutes) with both doses of UMEC+FF/VI in both studies vs PBO+FF/VI, which was maintained through to Day 84 (all p<0.001). 0 23 24 1 Study 1 Study 2 Time (h) 0.30 0.20 0.10 0.00 -0.10 3 UMEC 62.5 + FF/VI 100/25 Placebo + FF/VI 100/25 UMEC 125 + FF/VI 100/25 0.30 0.20 0.10 0.00 -0.10 LS mean (95% CI) change from baseline 1 6 23 24 Time (h) 0 3 6 |
|
|
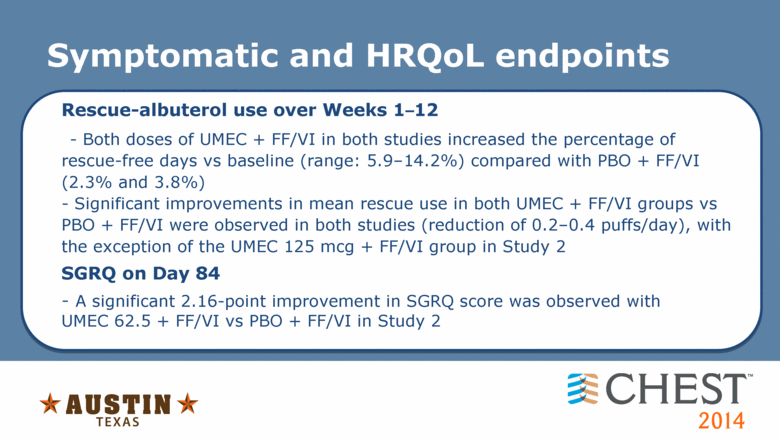
Symptomatic and HRQoL endpoints Rescue-albuterol use over Weeks 1–12 - Both doses of UMEC + FF/VI in both studies increased the percentage of rescue-free days vs baseline (range: 5.9–14.2%) compared with PBO + FF/VI (2.3% and 3.8%) - Significant improvements in mean rescue use in both UMEC + FF/VI groups vs PBO + FF/VI were observed in both studies (reduction of 0.2–0.4 puffs/day), with the exception of the UMEC 125 mcg + FF/VI group in Study 2 SGRQ on Day 84 - A significant 2.16-point improvement in SGRQ score was observed with UMEC 62.5 + FF/VI vs PBO + FF/VI in Study 2 |
|
|
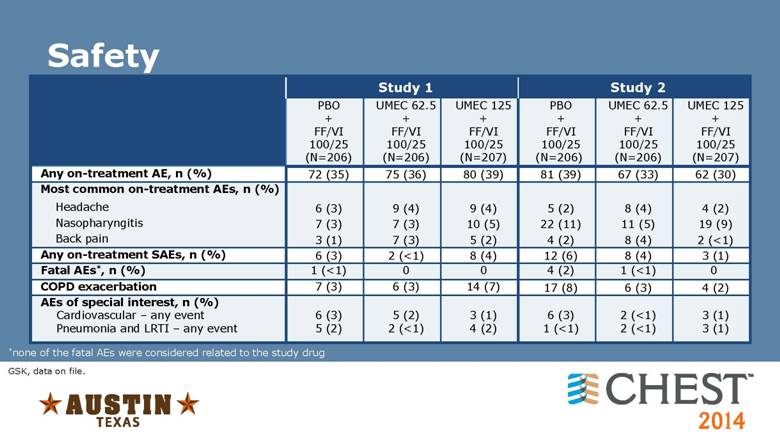
Safety Study 1 Study 2 PBO + FF/VI 100/25 (N=206) UMEC 62.5 + FF/VI 100/25 (N=206) UMEC 125 + FF/VI 100/25 (N=207) PBO + FF/VI 100/25 (N=206) UMEC 62.5 + FF/VI 100/25 (N=206) UMEC 125 + FF/VI 100/25 (N=207) Any on-treatment AE, n (%) 72 (35) 75 (36) 80 (39) 81 (39) 67 (33) 62 (30) Most common on-treatment AEs, n (%) Headache 6 (3) 9 (4) 9 (4) 5 (2) 8 (4) 4 (2) Nasopharyngitis 7 (3) 7 (3) 10 (5) 22 (11) 11 (5) 19 (9) Back pain 3 (1) 7 (3) 5 (2) 4 (2) 8 (4) 2 (<1) Any on-treatment SAEs, n (%) 6 (3) 2 (<1) 8 (4) 12 (6) 8 (4) 3 (1) Fatal AEs*, n (%) 1 (<1) 0 0 4 (2) 1 (<1) 0 COPD exacerbation 7 (3) 6 (3) 14 (7) 17 (8) 6 (3) 4 (2) AEs of special interest, n (%) Cardiovascular – any event Pneumonia and LRTI – any event 6 (3) 5 (2) 5 (2) 2 (<1) 3 (1) 4 (2) 6 (3) 1 (<1) 2 (<1) 2 (<1) 3 (1) 3 (1) *none of the fatal AEs were considered related to the study drug GSK, data on file. |
|
|
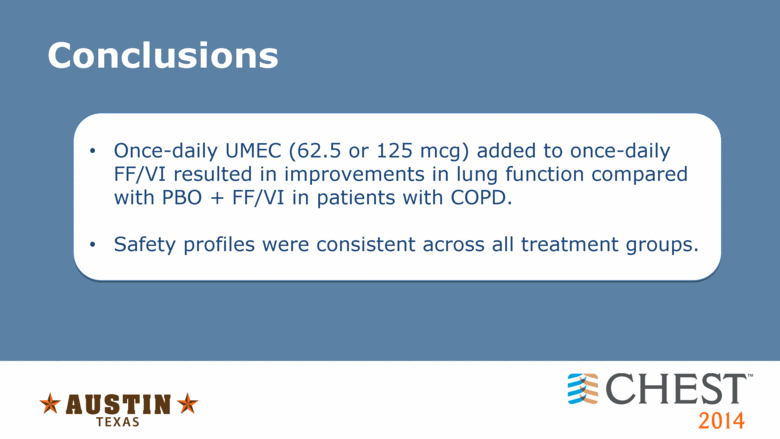
Conclusions Once-daily UMEC (62.5 or 125 mcg) added to once-daily FF/VI resulted in improvements in lung function compared with PBO + FF/VI in patients with COPD. Safety profiles were consistent across all treatment groups. |
|
|
Acknowledgments Any questions? Thank you to all the investigators involved in these studies |