Attached files
| file | filename |
|---|---|
| 8-K - 8-K - NANOSPHERE INC | d807395d8k.htm |
| EX-99.1 - EX-99.1 - NANOSPHERE INC | d807395dex991.htm |
 COMPANY
OVERVIEW OCTOBER 2014
Exhibit 99.2 |
 NANOSPHERE
This
presentation
contains
forward-looking
statements
about
us
and
our
industry
that
involve
substantial
risks
and
uncertainties.
We
intend
such
forward-looking
statements
to
be
covered
by
the
safe
harbor
provisions
for
forward-looking
statements
contained
in
Section
21E
of
the
Securities
Exchange
Act
of
1934,
as
amended,
and
Section
27A
of
the
Securities
Act
of
1933,
as
amended.
All
statements,
other
than
statements
of
historical
facts,
included
in
this
presentation
regarding
our
strategy,
future
operations,
future
financial
position,
future
net
sales,
projected
expenses,
products’
placements,
performance
and
acceptance,
prospects
and
plans
and
management’s
objectives,
as
well
as
the
growth
of
the
overall
market
for
our
products
in
general
and
certain
products
in
particular
and
the
relative
performance
of
other
market
participants
are
forward-looking
statements.
These
statements
involve
known
and
unknown
risks,
uncertainties
and
other
factors
that
may
cause
our
actual
results,
levels
of
activity,
performance
or
achievement
to
be
materially
different
from
those
expressed
or
implied
by
the
forward-looking
statements.
In
some
cases,
you
can
identify
forward-looking
statements
by
terms
such
as
“anticipate,”
“believe,”
“estimate,”
“expect,”
“intend,”
“may,”
“might,”
“plan,”
“project,”
“will,”
“would,”
“should,”
“could,”
“can,”
“predict,”
“potential,”
“continue,”
“objective,”
or
the
negative
of
these
terms,
and
similar
expressions
intended
to
identify
forward-looking
statements.
However,
not
all
forward-looking
statements
contain
these
identifying
words.
These
forward-looking
statements
reflect
our
current
views
about
future
events
and
are
based
on
assumptions
and
subject
to
risks
and
uncertainties.
Given
these
uncertainties,
you
should
not
place
undue
reliance
on
these
forward-looking
statements.
Actual
events
or
results
could
differ
materially
from
those
expressed
or
implied
by
these
forward-looking
statements
as
a
result
of
various
factors.
These
forward-looking
statements
represent
our
estimates
and
assumptions
only
as
of
the
date
hereof.
Unless
required
by
U.S.
federal
securities
laws,
we
do
not
intend
to
update
any
of
these
forward-looking
statements
to
reflect
circumstances
or
events
that
occur
after
the
statement
is
made
or
to
conform
these
statements
to
actual
results.
The
following
presentation
should
be
viewed
in
conjunction
with
the
consolidated
financial
statements
and
notes
thereto
appearing
in
our
Annual
Report
on
Form
10-K
and
subsequent
quarterly
reports
on
Form
10-Q.
Our
actual
results
may
differ
materially
from
those
anticipated
in
these
forward-looking
statements
as
a
result
of
various
factors,
including
but
not
limited
to:
•
if we do not achieve significant product revenue, we may not be able to meet our cash
requirements without obtaining additional capital from external sources, and if we are
unable to do so, we may have to curtail or cease operations;
•
estimates of the potential market size for
our products (including the hospital lab market in general and the blood stream infection (BSI)
market in particular) or failure of the market for these products to grow as
anticipated, •
the past performance of other companies which we believe to have
been in a market position analogous to where we believe we are now may not be predictive of our
future results in the manner we believe them to be,
•
our analysis of who our competitors have been, who they are now and who they will be in the
future (particularly in the BSI, enteric, extended tuberculosis and meningitis MDx
product markets) and our predictions of relevant future performance may be inaccurate,
•
comparisons of actual financial results for another company to what we predict will be our
future financial results may be inapposite, •
predictions of when “breakeven customer base”
is achieved and its relationship to our cash flow position, needs and “burn”
may prove to be inaccurate,
•
entrance of other competitors or other factors causing us to lose competitive advantage in the
sample-to-result MDx market, •
a lack of commercial acceptance of the Verigene System, its array of tests, and the development
of additional tests, which could negatively affect our financial results,
•
failure of third-party payors to reimburse our customers for the use of our clinical
diagnostic products or reduction of reimbursement levels, which could harm our ability to sell
our products,
•
failure of our products to perform as expected or to obtain certain approvals or the questioning
of the reliability of the technology on which our products are based, which could cause
lost revenue, delayed or reduced market acceptance of our products, increased costs and damage to our reputation,
•
our inability to manage our anticipated growth, constraints or inefficiencies caused by
unanticipated acceleration and deceleration of customer demand, and
•
those set forth under “Risk Factors”
in our Annual Report on Form 10-K, as amended from time to time under “Risk
Factors” in our Quarterly Reports on Form 10-Q.
FORWARD-LOOKING STATEMENTS |
 NANOSPHERE
INVESTMENT THESIS
3
Nanosphere
•
Proprietary high value tests that enable clinicians to rapidly identify some of the most
complex, costly and deadly infectious diseases
•
Installed and growing customer base with leading hospital-based laboratories and academic
research institutions in the U.S.
•
Over 250 U.S. cumulative placements to date
•
3-year revenue CAGR 85.7%
•
Backlog poised to convert to accelerated revenue ramp
•
Menu and platform strategy defined with compelling data
•
Demonstrated proprietary IP advantage in protein capabilities
•
Positioned to be low cost provider |
 NANOSPHERE
COMPANY BACKGROUND
State of the Art Molecular Diagnostic Testing
4
•
Rapid viral and bacteria disease detection
•
Expanding menu of tests
•
Proprietary Verigene®
System platform installed in over 250 U.S. hospitals and labs
•
Licensing relationship with the International Institute for Nanotechnology at
Northwestern •
Over 180+ issued patents, 6 pending applications and over 30+ non-exclusive licensed
patents •
Founded 1999
•
Headquartered in Northbrook, IL
•
165 full-time employees, as of December 31, 2013
o
56 in R&D
o
42 in manufacturing
o
50 in sales and marketing
o
17 in general and administrative |
 NANOSPHERE
OUR MISSION
5
•
Improve Patient Care
•
Lower Costs to the Healthcare System
•
Deliver Exceptional Customer Service
o
Save lives
o
Decrease morbidity
o
Reduce spread of antibiotic resistance
o
Shorter length of stay
o
Antibiotic savings
o
Demonstrated economic outcomes
o
Value to lab / clinician / administration
o
Customer-based development
Enhance Medicine Through Targeted Diagnostics |
 NANOSPHERE
MANAGEMENT TEAM
Officer / Title
Background
Michael McGarrity
President and
Chief Executive Officer
Joined Nanosphere in 2005
13 years with Stryker Corporation
Most recently Vice President, Stryker Instruments, a $700 million division of Stryker
Corporation
Graduate of the University of Notre Dame
Roger Moody
Vice President of Finance,
Treasurer and Chief
Financial Officer
Joined Nanosphere in 2007
Chief Financial Officer and Chief Operating Officer of Medsn
Strategic advisory services to technology and healthcare companies for Volpe Brown
Whelan & Company
B.S. from Syracuse University and M.B.A. from the University of Chicago, Graduate School
of Business
Ken Bahk, Ph.D.
Chief Strategy Officer
Joined Nanosphere in 2013
Director of Investments at Lurie Investments
Served in leadership positions for the premier MDx professional organizations (most
recently Chair of Strategic Opportunities, Association for Molecular Pathology)
Ph.D. in biochemistry and molecular biology, M.S. in neurobiology and physiology from
Northwestern University, and M.B.A. from the Kellogg School of Management
6 |
 NANOSPHERE
OUR STATE-OF-THE-ART SOLUTION
The Verigene System utilizes advanced automation, microfluidics and proprietary chemistry to
enable rapid direct detection of nucleic acids and high-sensitivity protein detection
on the same platform High multiplexed
Accurate
Flexible
Easy to use
On demand
Moderate complexity
Current Verigene Platform
Next Generation Verigene System
(1)
One step sample-to-result
Lowest cost per test
Consolidates consumables into
one
Room temperature stability
Optimal footprint
Engineering system to be best-in-
class interface
Competitive
Engineering to be Best-in-Class
(1) Commercial release planned for 2016
7 |
 NANOSPHERE
STRONG PATENT PROTECTION
Patents
Proprietary Technology
Our proprietary technology is based on and
was exclusively licensed from the
International Institute for Nanotechnology
at Northwestern in May 2000
Technology generally covers core
technology, including nanotechnology-
based biodiagnostics and biobarcode
technology
As of September 2014, our patent portfolio
is comprised, on a worldwide basis, of over
180+ issued patents and 6 pending patent
applications
The issued patents cover approximately 11
different technological claims and the
pending patent applications cover
approximately three additional
technological claims
In addition, as of September 2014, we hold
non-exclusive licenses for over 30+ U.S.
patents that cover 11 different
technological claims from various
third parties
8 |
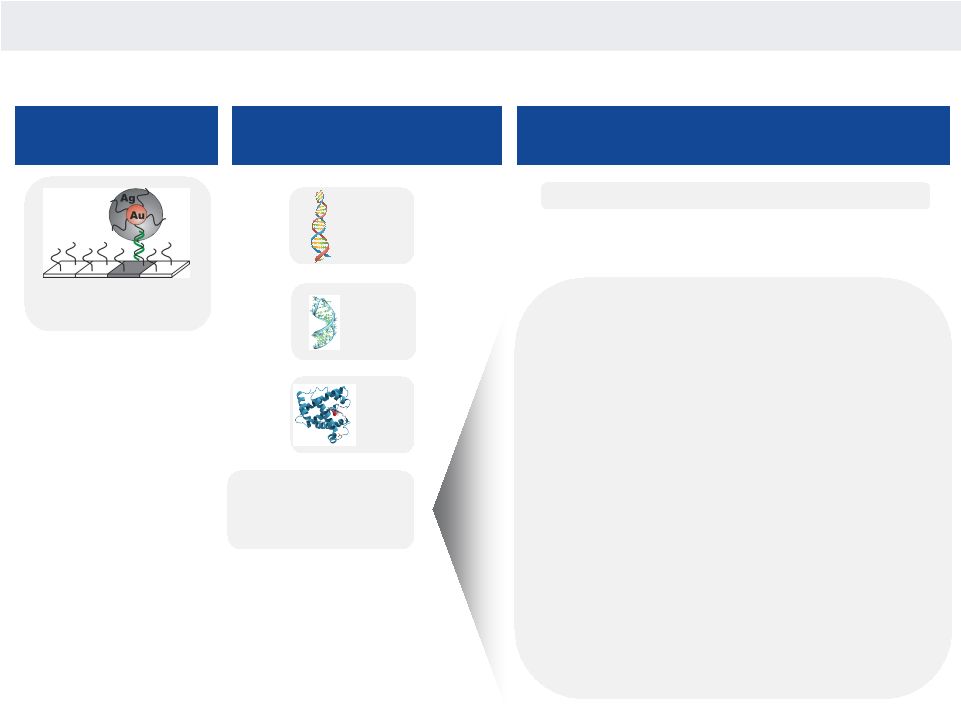 NANOSPHERE
HIGHLY VERSATILE TECHNOLOGY
Diagnostics | Drug Development | Life Sciences
1)
Multiplexed nucleic acid panels
—
Infectious diseases
—
Human genetics, personalized medicine
—
Security/defense
2)
High sensitivity and/or multiplexed protein
—
cTnI, PSA
—
Rheumatoid arthritis
—
Allergies
3)
Next gen diagnostics (combined NA+protein):
—
Infectious diseases (NxGen sepsis, C. diff)
—
Cancer (inflammation and immunity play
critical roles in pathogenesis)
Gold nanoparticle
DNA
RNA
Proteins
One
Technology
To Detect
Multiple Analytes
For Multiple Applications
and Markets
Significant
Potential
9 |
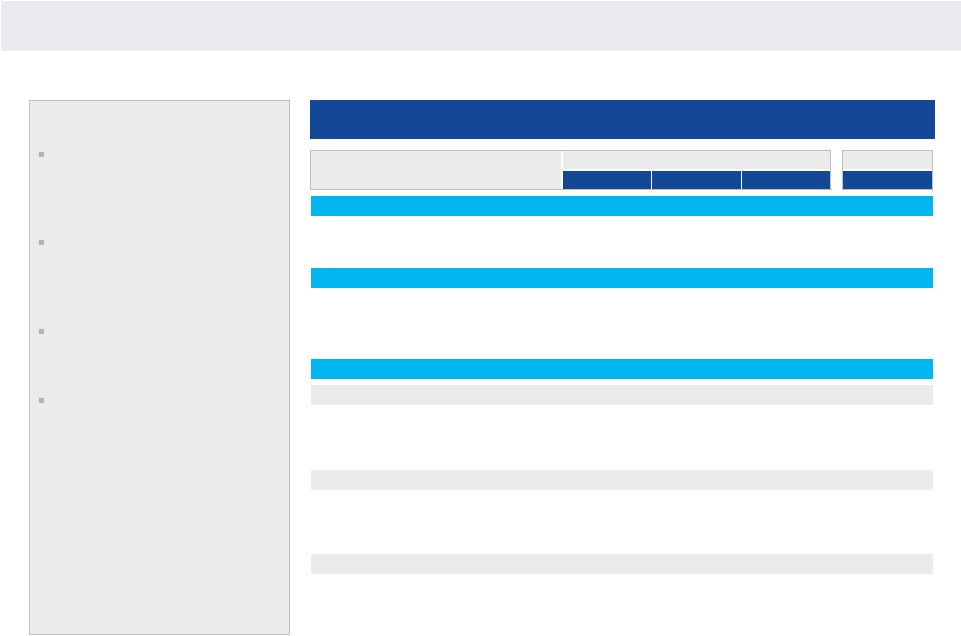 NANOSPHERE
MULTIPLEX MDX MARKET
Total Addressable Market
Key Points
Total global addressable market
for multiplex MDx applications is in
excess of approximately $1.1
billion
Core multiplex menu items include
respiratory, sepsis and
gastrointestinal, with others in
development (within microbiology)
According to Street estimates,
multiplex MDx market is only
7% penetrated
Availability of core tests on
sample-to-result platform is key
to broader penetration
Source: Wall Street research.
(1)
Includes
respiratory,
sepsis
and
GI
panels;
all
assumed
at
$100
U.S.
ASP
and
$80
OUS
ASP.
(2)
Includes
gram
positive,
negative
and
fungal.
(3)
US:
20
million
blood
culture
per
year,
of
which
10%
are
positive.
(4)
US:
20
million
stool
culture
per
year;
C.
difficile
alone
represents
about
5
million
tests.
Total Addressable Market
x
Penetrated
($Millions)
US
OUS
WW
x
YE 2013
Installed Base
Hospital, Reference Labs
5,500
5,000
10,500
600
Current Multiplex Penetration
6%
Total Multiplex Menu
(1)
Samples
7.2
5.3
12.5
0.9
Revenue
$720
$421
$1,141
$89
Current Multiplex Penetration
7%
7%
Multiplex Menu
Respiratory Panel
Samples
2.2
1.6
3.8
0.8
Revenue
$220
$124
$344
$80
Current Multiplex Penetration
23%
Sepsis Panel
(2)
Samples
(3)
2.0
1.7
3.7
0.1
Revenue
$200
$134
$334
$6
Current Multiplex Penetration
2%
GI Panel
Samples
(4)
3.0
2.0
5.0
0.0
Revenue
$300
$163
$463
$3
Current Multiplex Penetration
1%
10 |
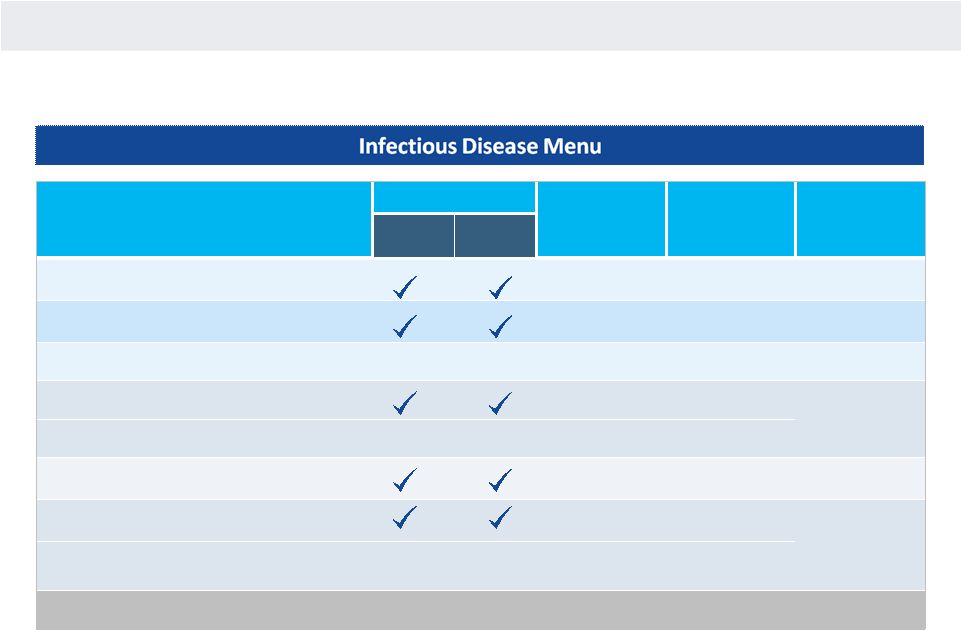 NANOSPHERE
EXPANDING MENU OF HIGH VALUE MICROBIOLOGY TESTS
Menu
Regulatory Status
FDA
Approval
Date
Est. Annual
Revenue Per
Customer
Addressable
Market
($mm)
(2)
FDA
CE IVD
BSI —
Gram Positive (BC-GP)
June 2012
$50,000
$220
BSI —
Gram Negative (BC-GN)
January 2014
$30,000
80
Blood Culture Yeast (BC-Y)
In Development
TBD
TBD
30
Enteric Pathogens (EP)
October 2014
$60,000
350
Expanded Enteric Flex (Expanded EP Flex)
(1)
In Development
TBD
TBD
C. difficile
(C. diff)
December 2012
$30,000
120
Respiratory Virus with Sub-Typing (RV+)
January 2011
$70,000
340
Expanded Respiratory Pathogens Flex (Expanded
RP Flex)
In Development
Q1 2015
(3)
TBD
Total Menu
$1,140
(1)
Includes parasites
(2)
Sourced from Wall Street research
(3)
Management expectations.
11 |
 NANOSPHERE
MAIN DRIVERS OF MDX ADOPTION
Sample-
To-Result
Need for more timely
and cost-effective
results
High Count
Multi-
Plexing
Need to address
costly and complex
disease states
Need
Product
Feature
Quicker
Response
Increased
Capability
12
We believe that conversion of traditional culture-based methods to molecular will
be driven by the need for rapid and clinically actionable results that will either
improve outcomes or improve economics
ACCOUNTABLE CARE |
 NANOSPHERE
VERIGENE CHANGES THE GAME IN SEPSIS
Sepsis is a life-threatening response to bacteria in the
bloodstream:
—
Responsible for 1.6 million hospitalizations and nearly
200,000 deaths per year (one person every two minutes) in
the U.S.
(1)(2)
—
Risk of death increases by 7.6% every hour appropriate
treatment is delayed
(3)
—
Commonly complicated by antimicrobial resistance
Costs U.S. hospitals more than $20 billion annually
(4)
Conventional diagnostic methods require 48 to 96 hours to
deliver bacterial identification and resistance determination
Patients
are
treated
with
broad-based
antibiotics
that
are
often
unnecessary
or
inappropriate
and
frequently
toxic
Large Market With Unmet Need
The Verigene Value Proposition
Rapid and accurate identification of infection-causing bacteria and resistance to commonly
used antibiotics that enable clinicians to prescribe the right therapy at the right
time (1)
Healthcare
Cost
and
Utilization
Project.
Statistical
Brief
#122.
(2)
Angus,
D.C.,
Linde-Zwirble,
W.T.,
Lidicker,
J.,
Clermont,
G.,
Carcillo,
J.,
Pinsky,
M.R.
Epidemiology
of
severe
sepsis
in
the
United
States:
analysis
of
incidence,
outcome,
and
associated
costs
of
care.
Crit
Care
Med.
2001
Jul;29(7):1303-10.
(3)
Kumar
A,
et
al.
Duration
of
hypotension
before
initiation
of
effective
antimicrobial
therapy
is
the
critical
determinant
of
survival
in
human
septic
shock.
Crit
Care
Med
2006,
34:1589-
1596.
(4)
Healthcare
Cost
and
Utilization
Project.
Statistical
Brief
#160.
(5)
Buchan
BW,
Ginocchio
CC,
Manii
R,
Cavagnolo
R,
Pancholi
P,
et
al.
(2013)
Multiplex
Identification
of
Gram-Positive
Bacteria
and
Resistance
Determinants
Directly
from
Positive
Blood
Culture
Broths:
Evaluation
of
an
Automated
Microarray-Based
Nucleic
Acid
Test.
PLoS
Med
10(7):
e1001478.
doi:10.1371/journal.pmed.1001478.
(6)
Performance
of
the
Verigene
gram-positive
blood
culture
assay
for
direct
detection
of
gram-positive
organisms
and
resistance
markers
in
a
pediatric
hospital.
J
Clin
Microbiol.
2014
Jan;52(1):283-7.
doi:
10.1128/JCM.02322-13.
Epub
2013
Oct
16.
Reduces length of stay and enables significant cost
savings via identification and antibiotic resistance
determination directly from positive blood culture
bottles up to 2 days faster than conventional methods
(5)
Enables shift from empiric to targeted antibiotic
treatment
(6)
Identifies contaminants which enables rapid rule out and
avoidance of unnecessary treatment and cost associated
with empiric treatment
13 |
 NANOSPHERE
The traditional blood culture workflow provides identification and resistance information at
>48 hours VERIGENE IMPACT
Blood Culture
and Gram Stain
BC-GP &
BC-GN
Results
Hours
12
15
24
48
Samples Plated for Sub-Culture
Blood Culture
and Gram Stain
Resistance Testing
Culture
Results
Hours
12
15
24
48
Verigene enables patients to be placed on appropriate antibiotics days
faster which
leads to decreased length of stay and significant financial savings
Traditional Blood Culture Workflow
Verigene Workflow
The Verigene Workflow provides identification and resistance information 2-2.5 hours after
the gram stain, or at ~ hour 15
14 |
 NANOSPHERE
Sources:
Sango,
et
al.
A
stewardship
approach
to
optimize
antimicrobial
therapy
through
use
of
a
rapid
microarray
assay
on
blood
cultures
positive
with
Enterococcus
species.
J.
Clinical
Microbiology,
2013
September
23,
01951-13.
Koeneman
et
al.
Rapid
Identification
of
Methicillin-Sensitive
Staphylococcus
aureus
from
Positive
Blood
Cultures
using
the
Verigene
System:
A
System-Wide
Impact
on
Patient
Treatment
and
Physician
Compliance.
Poster
session
presented
at:
American
Society
for
Microbiology:
113
General
Meeting;
2013
May
18-21;
Denver,
CO.
Saves Money
Recent study by UF Shands:
21.7 day reduction in
Length of stay/patient
$61,000 reduction in
cost/patient
Saves Last Line
Therapies
Banner Healthcare:
Appropriate antibiotic
treatment reduced by
40.3 hours and moved
from prophylactic
treatment in 90% vs.
35% of patients
Saves Lives
80% reduction in
I.C.U. mortality
from 47.8%
to 9.5%
Economic
Antibiotic
Stewardship
Treatment
Outcomes
Benefit of Solution:
15
VERIGENE BENEFITS
th |
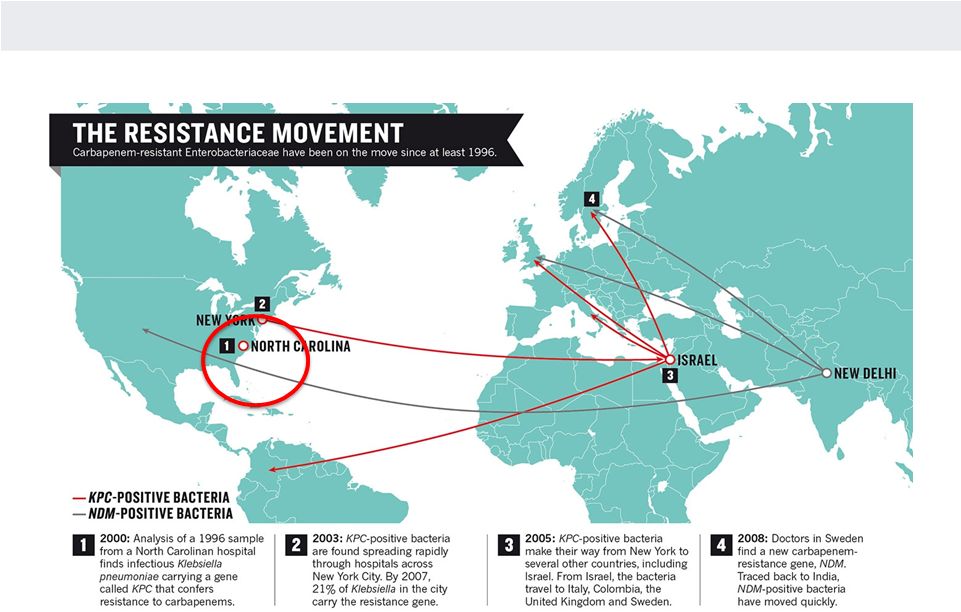 NANOSPHERE
MIGRATION OF ANTIBACTERIAL RESISTANCE
Source: M. McKenna. Nature. Vol 499. 25 July 2013.
16 |
 NANOSPHERE
17
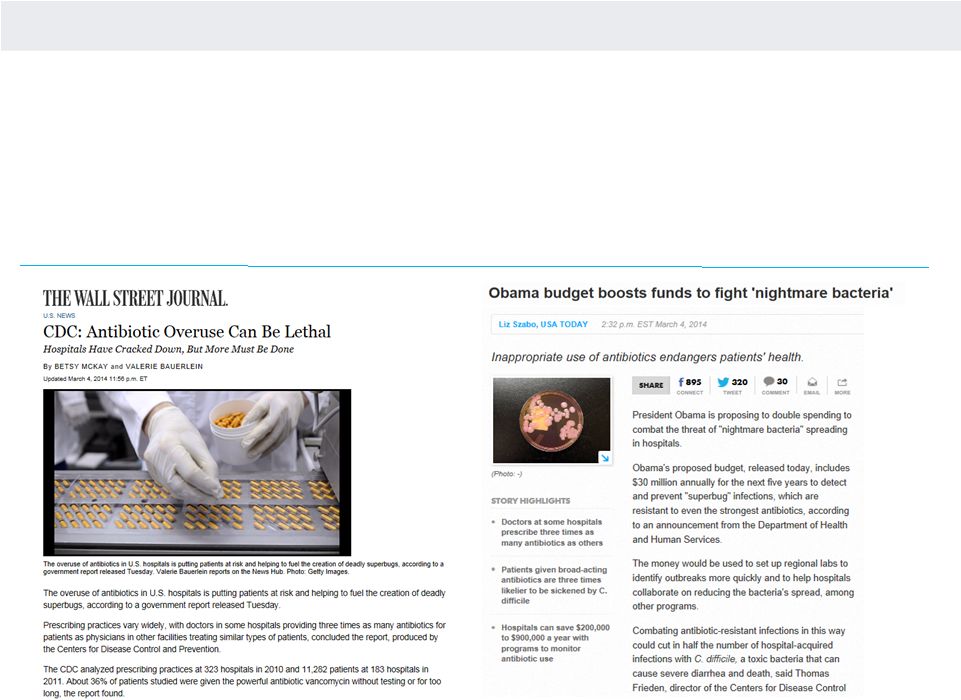
CDC LANDMARK RECOMMENDATIONS
Source: Centers for Disease Control. September 16, 2013
•
Burden and Threat Posed by Antibiotic-Resistant
Infections
o
Drug resistant infections are a threat to human and
economic health
o
Overuse of antibiotics is the single most important factor
o
Urgent action is needed NOW by everyone who uses
antibiotics
•
Four Core Actions Must Be Taken
o
Avoid infections
o
Track infections
o
Improve antibiotic stewardship and use
o
Development and implementation of drugs
and DIAGNOSTIC TESTS |
 NANOSPHERE
COMPETITIVE ADVANTAGES
Reduce Mortality:
Each hour that appropriate antimicrobial
treatment is delayed, a sepsis patient’s mortality rate
increases by 7.6%
(1)
Reduce Hospitalization Costs:
Implementing rapid results
reporting for S. aureus
blood cultures can lead to an
average 6.2-day reduction in length of stay and a $21,387
reduction in costs per S. aureus-infected patient
(2)
Antimicrobial Stewardship:
Rapid mecA
reporting for
patients with S. aureus
bacteremia results in a 25.4-hour
reduction in the time to optimal antimicrobial therapy
(3)
Reduce the Impact of Contaminants:
Patients with false-
positive blood culture results triggered by contaminants
such as S. epidermidis
have hospitalization costs $8,750
higher than true negative blood culture patients
(4)
(1)
Kumar et al. 2006. Crit Care Med, 34:1589-96.
(2)
Bauer et al. 2010. Clin Infect Dis, 51:1074-80.
(3)
Carver et al. 2008. J Clin Microbiol, 46:2381-83.
(4)
Zwang and Albert 2006. J Hosp Med, 1:272-76.
Rapid Blood Culture Testing Benefits
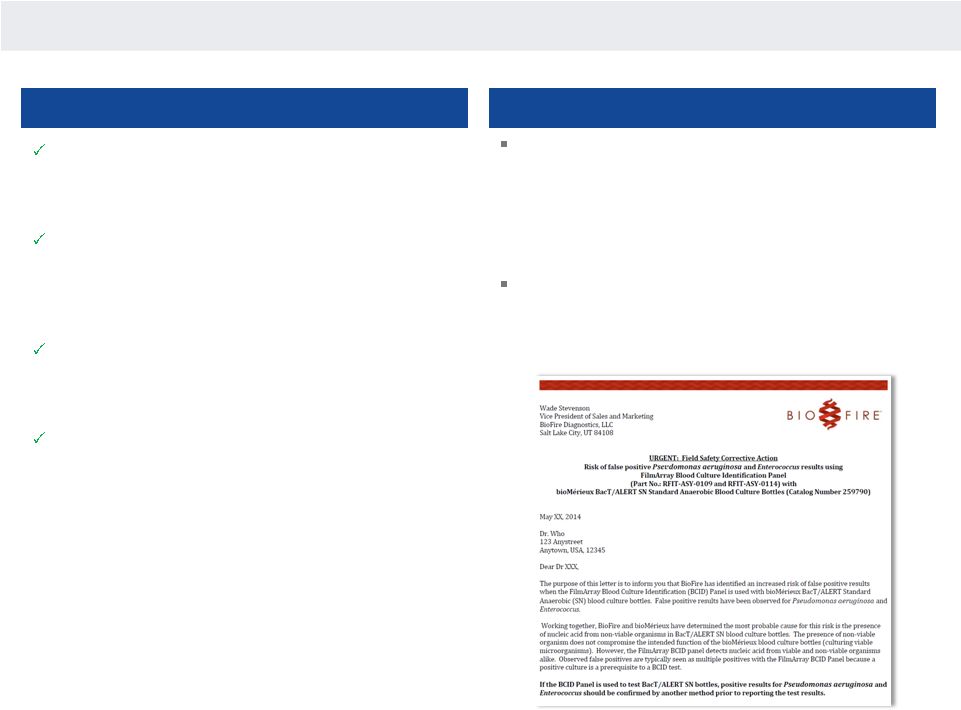
Versus BioFire
Nanosphere direct detection (no PCR) provides for
accuracy in both sensitivity and specificity required for
critical therapeutic change
—
BioFire’s PCR-based approach results in higher risk of
false positives as advised to their customers in the
below Field Safety Corrective Action
Clinically relevant design: assay designed to be based on
gold standard gram-stain
—
BioFire charges for results not indicated by gold
standard results in an additional cost of ~ $47K/year
18 |
 NANOSPHERE
GASTROINTESTINAL INFECTIONS
The Verigene Value Proposition
More sensitive than culture (detects ~3X as many
infections in the same set of samples as stool
culture)
(3)
Replaces a highly labor intensive, slow (3-5 days) process
with a simple 2 hour test
Reduces time on stool bench by ~90% (90% stool
cultures are negative) which frees up tech time
Fast results reduce time to optimal therapy, improve
antimicrobial stewardship, reduce isolation days, and
avoid unnecessary admissions
Community-Acquired Diarrhea (Enteric)
Clinical, economic and workflow benefits to hospitals and laboratories
(1)
Healthcare Cost and Utilization Project. Statistical Brief #150.
(2)
Centers for Disease Control.
(3)
JCM, Khare et al. 2014, 52(10): 3667.
Caused by consumption of contaminated food or water
containing bacterial, viral or parasitic gastrointestinal
pathogens
—
Rotavirus is the most common cause of gastroenteritis in children
—
Norovirus is the leading cause of gastroenteritis among adults in the
U.S., causing greater than 90% of outbreaks
—
Campylobacter is the primary cause of bacterial gastroenteritis,
with
half of these cases associated with exposure to poultry
—
C. difficile
is a common cause of diarrhea in those who are hospitalized
and is frequently associated with antibiotic use
Generally, young children, babies, people with disabilities
and elderly individuals are most at risk for enteric
diseases due to weakened immune systems; travelers to
foreign countries may also be sensitive to bacteria in
food and water abroad
Associated with 3.7 million emergency department visits,
1.3 million inpatient hospitalizations and more than $6
billion in healthcare costs per year
(1)(2)
Most cases are self-resolving and not life-threatening,
yet some can have serious implications
19 |
 NANOSPHERE
CONVERSION OF RESPIRATORY MARKET
Short / Targeted Panel
Expanded / General Panel
Expanded RP Flex
Expanded RP Flex
panel allows customers to select and de-select targets within a
broad panel (sub-panels) as our current respiratory panel already allows, but with
the option of a newly-introduced flexible pricing vehicle
—
Laboratory can now adopt one platform and respond to clinician ordering
patterns for a variety of patient populations and treatment algorithms which will
accelerate adoption beyond current hospital base
Afforded by the low COGS on the Verigene System
Estimated market opportunity of $340 million
(2)
The respiratory market has historically been characterized as crowded and price
sensitive Only a portion of hospitals have adopted molecular respiratory testing
methodologies Many of the molecular-using hospitals have two platforms because of
the way clinicians order tests
based on population and clinical demand
20
(1)
Sourced from Wall Street research |
 NANOSPHERE
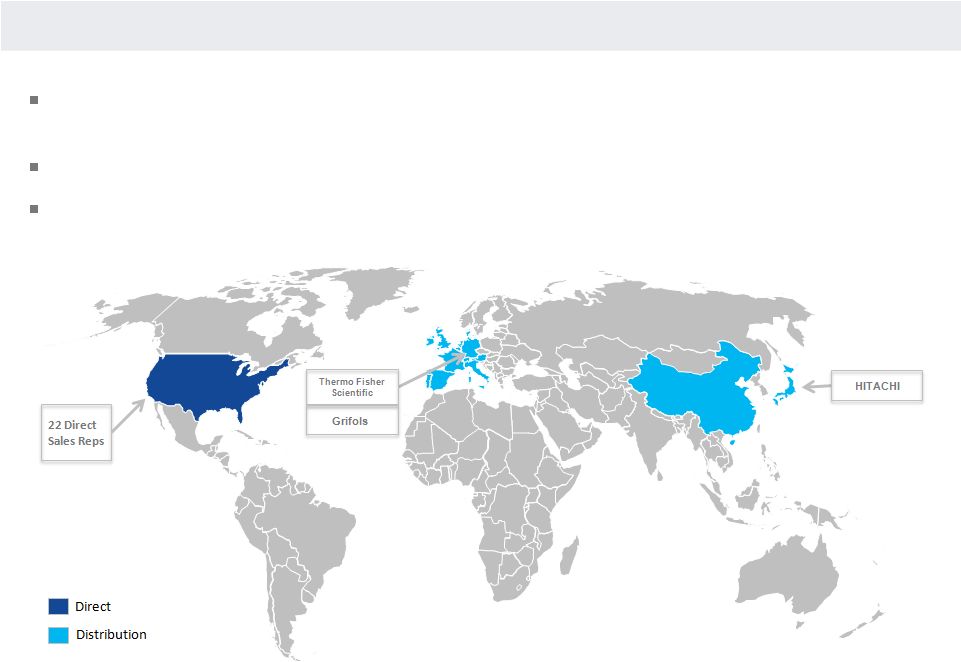
OVERVIEW OF COMMERCIAL PRESENCE
Sales and marketing organization comprises geographically dispersed sales representatives and
clinical support specialists as well as a centralized staff of market and product
managers Direct in the United States
Distribution in Western Europe, Japan and China
21 |
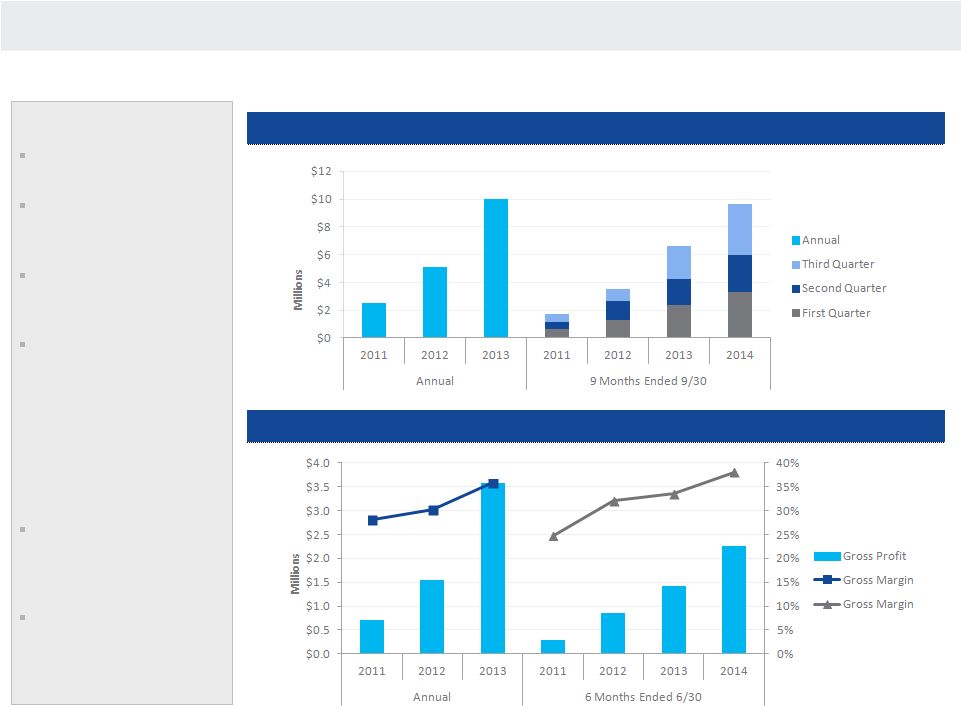 NANOSPHERE
REVENUE AND GROSS PROFIT
Key Points
Consistent revenue growth
driven by test sales
We believed expanded menu
will drive revenue growth
acceleration
Backlog of placements
pending instrument sales is
$7.8 million
Gross margin expansion
primarily driven by recent
consumable manufacturing
investments that lack
scientific risk
—
Increased cavitation
—
New line efficiency
—
Reagent reductions
—
Volume reductions
As consumable margins
expand, consumable
revenue contribution vs.
instruments increases
Cash flow improvements
driven by revenue growth,
margin expansion, expense
and working capital
management
Revenue
Revenue
22
Gross Profit
Gross Profit |
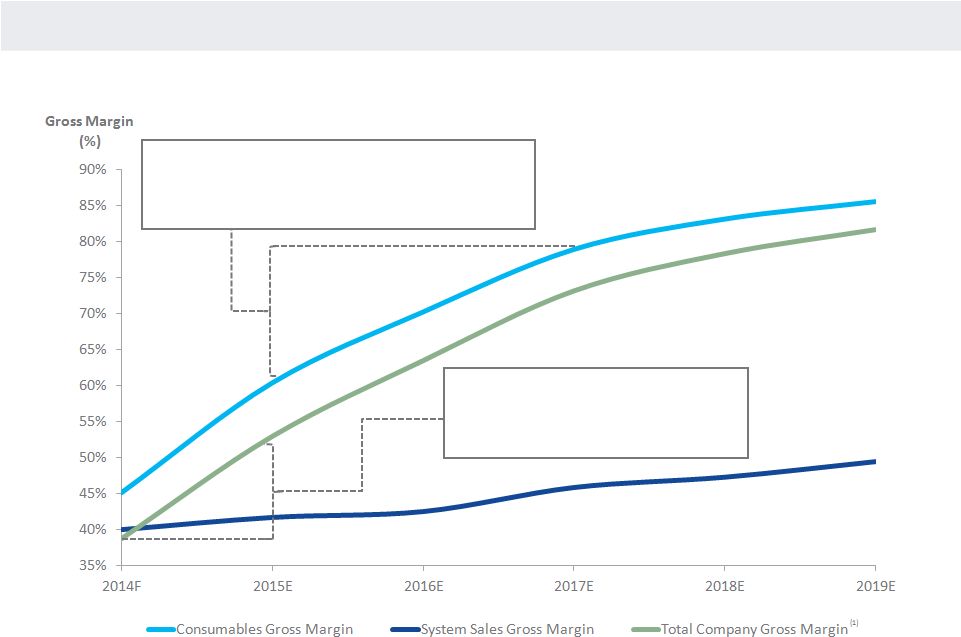 NANOSPHERE
LOW RISK MARGIN EXPANSION
23
2015 Projected Margin Drivers:
Consumable cavitation,
labor and reagents
2016 / 2017 Projected Margin Drivers:
Royalties (patent expiration)
Primarily volume (materials & overhead absorption)
(1)
Includes royalties and license amortization.
(1) |
 NANOSPHERE
SUMMARY INVESTMENT HIGHLIGHTS
Large Addressable Markets With Unmet Needs
Differentiated, High Value Tests in Sepsis and GI Infections
Momentum With Leading Hospitals
Attractive Placement and Assay Pull-Through Trends
Diversified, Customer Base
High Growth and Operating Leverage
Proprietary Multiplexed Molecular Platform
24 |
