Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a14-12968_18k.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a14-12968_1ex99d2.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a14-12968_1ex99d1.htm |
Exhibit 99.3
A randomized controlled trial comparing two dry powder inhalers:
more patients with COPD prefer ELLIPTA compared to DISKUS based on inhaler-specific attributes
POSTER #PA145
Suyong Yun Kirby(1), Chang-Qing Zhu(2), Edward Kerwin(3), Richard Stanford(1), George Georges(1)
(1)GlaxoSmithKline, Research Triangle Park, NC, USA; (2)GlaxoSmithKline, Stockley Park, UK;
(3)Clinical Research Institute of Southern Oregon, PC, Medford, Oregon, USA
INTRODUCTION
Virtually all of the maintenance treatments for chronic obstructive pulmonary disease (COPD) are delivered using inhaler technology. Patient preference for an inhaler is an important factor when deciding on maintenance treatment as it may impact compliance with therapy. This study compares patient preference of an existing dry powder inhaler (DISKUSTM) and a novel dry powder inhaler (ELLIPTATM) based on several inhaler specific attributes. It also examines the preference for a once-daily versus a twice-daily dosing regimen.
OBJECTIVES
· The primary objective of this study was to evaluate whether more subjects with COPD prefer the ELLIPTA inhaler to the DISKUS inhaler based on the size of the numbers on the dose counter.
· The secondary objective was to evaluate the subject’s preference for these two inhalers based on the number of steps needed to take the COPD medication and the size of the inhaler.
METHODS
· This is a multicenter (United States), randomized, open-label, crossover study. Patients with COPD who had not used ELLIPTA or DISKUS within 6 months from screening were randomized to use ELLIPTA placebo inhaler once daily followed by DISKUS placebo inhaler twice daily, or vice versa, each for approximately one week (Figure 1). Subjects were allowed to continue their existing prescribed COPD maintenance treatment throughout the study. At the end of the study, patients answered 7 questions to evaluate their preference of inhaler attributes and preferred dosing regimen.

· Subject’s preference was analyzed using Cochran-Mantel-Haenszel test accounting for sequence of inhaler use and order of response options presented (ELLIPTA then DISKUS or vice versa). A step-down testing approach (primary to secondary) and Hochberg (across secondary endpoints) were applied for multiple comparisons. Safety assessments included adverse events (AEs) and COPD exacerbations.
RESULTS
Subject Disposition and Baseline Characteristics
This study was conducted from 28 May to 15 July, 2013. A total of 314 subjects were screened, of which 287 subjects (Intent-to-Treat [ITT] population) were randomized. Two subjects in the ITT Population were excluded from the Per-Protocol (PP) Population as they were unable to complete the preference questions due to AEs that led to withdrawal during Period 1. Two hundred eighty three subjects completed the study. Four subjects withdrew prematurely: 3 due to adverse events (AEs) and one due to a COPD exacerbation.
The demographics and baseline characteristics of the study population were representative of a general COPD population (Table 1).
Table 1. Demographics and Baseline Characteristics
|
Demographics / |
|
ITT Population |
|
|
Male sex, n (%) |
|
153 (53) |
|
|
Age, years |
|
64.7 (9.74) |
|
|
Body mass index, kg/m2 |
|
28.2 (6.34) |
|
|
Duration of COPD, n (%) |
|
|
|
|
>1 to <5 years |
|
94 (33) |
|
|
>5 years to <10 years |
|
97 (34) |
|
|
>10 years |
|
96 (33) |
|
|
Years smoked |
|
41.3 (9.94) |
|
|
Smoking pack years |
|
56.5 (27.02) |
|
Values are mean (SD) unless otherwise stated
Exposure and Inhaler Use Compliance
Table 2. Exposure and Inhaler Use Compliance
|
|
|
ELLIPTA |
|
DISKUS |
|
|
|
|
|
|
|
|
|
Exposure, days |
|
7.2 (0.99) |
|
7.2 (1.17) |
|
|
Compliance rate (%) |
|
105.6 (16.29) |
|
96.1 (18.45) |
|
|
Compliance category, n (%) |
|
|
|
|
|
|
<80% |
|
2 (<1) |
|
24 (8) |
|
|
>80 to <120% |
|
263 (92) |
|
252 (88) |
|
|
>120% |
|
21 (7) |
|
9 (3) |
|
Values are mean (SD) unless otherwise stated
COPD Medications
Concurrent COPD medications used most frequently during the study were salbutamol (58%), tiotropium bromide (39%), budesonide + formoterol fumarate (23%), oxygen (15%), and salbutamol sulphate + ipratropium bromide (11%). Fluticasone propionate and salmeterol + fluticasone propionate were used by 7 and 4 subjects, respectively; they were the hydrofluoroalkane aerosol formulations administered via metered dose inhaler.
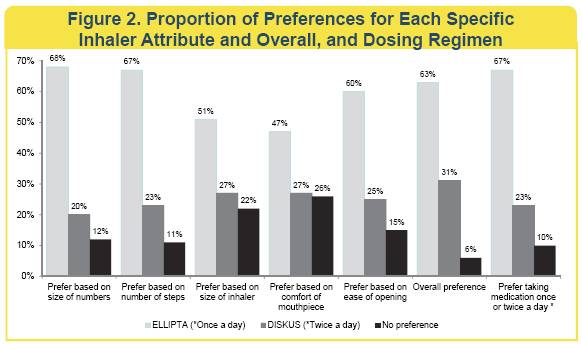
Inhaler and Dosing Regimen Preference
A statistically significant larger proportion of subjects preferred ELLIPTA over DISKUS for each of the 5 specific attributes and overall, and preferred a once-daily over a twice-daily dosing regimen (p<0.001 for each comparison).
Safety
· Overall, AEs were reported for 36 subjects (Table 3).
Table 3. Adverse Events Occurring in a Total of More than One Subject
|
|
|
Number (%) of Subjects |
| ||||
|
|
|
ELLIPTA |
|
DISKUS |
|
Total |
|
|
Adverse event |
|
N=287 |
|
N=285 |
|
N=287 |
|
|
Any AE |
|
23 (8) |
|
14 (5) |
|
36 (13) |
|
|
Headache |
|
2 (<1) |
|
5 (2) |
|
7 (2) |
|
|
Back pain |
|
3 (1) |
|
0 |
|
3 (1) |
|
|
Diarrhea |
|
2 (<1) |
|
0 |
|
2 (<1) |
|
|
Dry mouth |
|
1 (<1) |
|
1 (<1) |
|
2 (<1) |
|
|
Neck pain |
|
1 (<1) |
|
1 (<1) |
|
2 (<1) |
|
|
Conjunctivitis |
|
0 |
|
2 (<1) |
|
2 (<1) |
|
· No deaths were reported during this study.
· Three subjects experienced a total of 5 non-fatal serious AEs (SAEs) [one subject with deep vein thrombosis, esophageal candidiasis, and metastases to liver; one subject with bronchitis; and one subject with vertebrobasilar insufficiency]. The first two subjects were withdrawn due to SAEs. The subject with vertebrobasilar insufficiency remained on study. One additional subject was withdrawn due to a non-serious wrist fracture.
· Two subjects experienced COPD exacerbations and withdrew prematurely from the study (one noted as withdrawn due to an AE).
CONCLUSIONS
· More patients with COPD prefer the ELLIPTA over DISKUS inhaler based on five specific inhaler attributes and overall.
· More patients with COPD prefer to take their COPD medication once daily versus twice daily.
· Safety in subjects with COPD using both placebo inhalers was consistent with health conditions observed in patients with COPD in general.
ACKNOWLEDGMENTS
· The presenting author, S Yun Kirby, is employed by and holds stock in GlaxoSmithKline.
· This research was funded by GlaxoSmithKline (GSK study number RLV116669; ClinicalTrials.gov Identifier, NCT01868009).
· Editorial support (grammatical editing, graphical support) was provided by David Cutler, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GlaxoSmithKline.
|
|
ELLIPTATM and DISKUSTM are trademarks of GlaxoSmithKline |
|
Presented at the American Thoracic Society Annual Congress, San Diego, CA, USA, 16–21 May 2014


