Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a14-10828_28k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a14-10828_2ex99d1.htm |
Exhibit 99.2
|
|
April 30, 2014 NASDAQ: THRX |
|
|
This presentation contains certain "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Theravance intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. The words “anticipate”, “expect”, “goal,” “intend”, “objective,” “opportunity,” “plan”, “potential”, “target” and similar expressions are intended to identify such forward-looking statements. Examples of such statements include statements relating to: plans for executing the separation of Theravance into two independent companies, the expected timing of the separation, expectations for the amount and estimated duration of the funding of Theravance Biopharma at the time of the separation, the strategies, plans and objectives of the two companies following the separation, the timing, manner and amount of anticipated potential capital returns to stockholders, the status and timing of clinical studies, data analysis and communication of results, the potential benefits and mechanisms of action of product candidates, the enabling capabilities of Theravance’s approach to drug discovery and its proprietary insights, expectations for product candidates through development and commercialization, and the timing of seeking regulatory approval of product candidates. These statements are based on the current estimates and assumptions of the management of Theravance as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to: difficulties in effecting the registration of Theravance Biopharma as a public company, changes in the development or operations of Theravance prior to the separation that could affect the plans for the separation of Theravance into two independent companies or the intended provision of capital returns to stockholders, delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective, Theravance’s dependence on third parties to conduct Theravance’s clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products and risks associated with establishing distribution capabilities for telavancin with appropriate technical expertise and supporting infrastructure. Other risks affecting Theravance are described under the heading "Risk Factors" contained in Theravance’s Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) on March 3, 2014 and the risks discussed in Theravance’s other periodic filings with the SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance assumes no obligation to update its forward-looking statements. 2 |
|
|
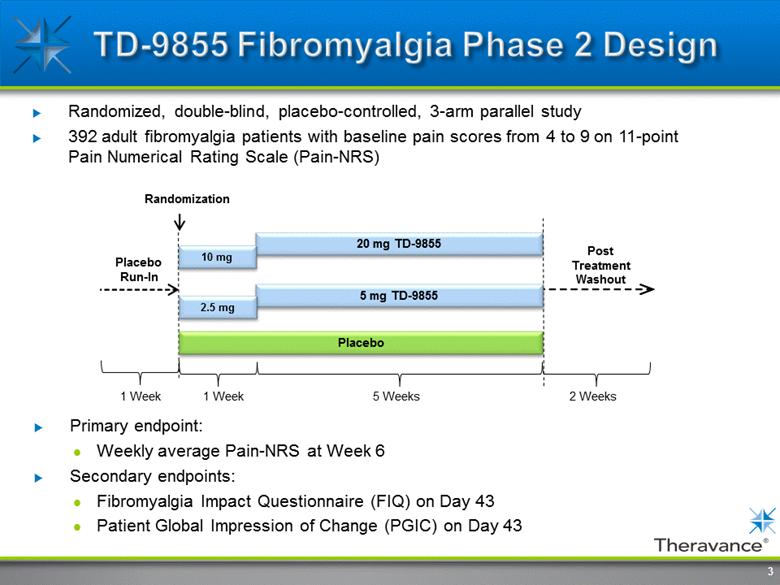
Primary endpoint: Weekly average Pain-NRS at Week 6 Secondary endpoints: Fibromyalgia Impact Questionnaire (FIQ) on Day 43 Patient Global Impression of Change (PGIC) on Day 43 Randomized, double-blind, placebo-controlled, 3-arm parallel study 392 adult fibromyalgia patients with baseline pain scores from 4 to 9 on 11-point Pain Numerical Rating Scale (Pain-NRS) Randomization Post Treatment Washout Placebo Run-In 1 Week 5 Weeks 2 Weeks 20 mg TD-9855 Placebo 5 mg TD-9855 10 mg 2.5 mg 1 Week 3 |
|
|
4 20 mg decreased weekly average pain score from baseline by 1.4 in comparison to 0.9 for placebo at Week 6 (one-sided p=0.022) 5 mg was not significantly different from placebo |
|
|
5 20 mg decreased FIQ from baseline by 16.2 in comparison to 10.4 for placebo on Day 43 (one-sided p=0.010) |
|
|
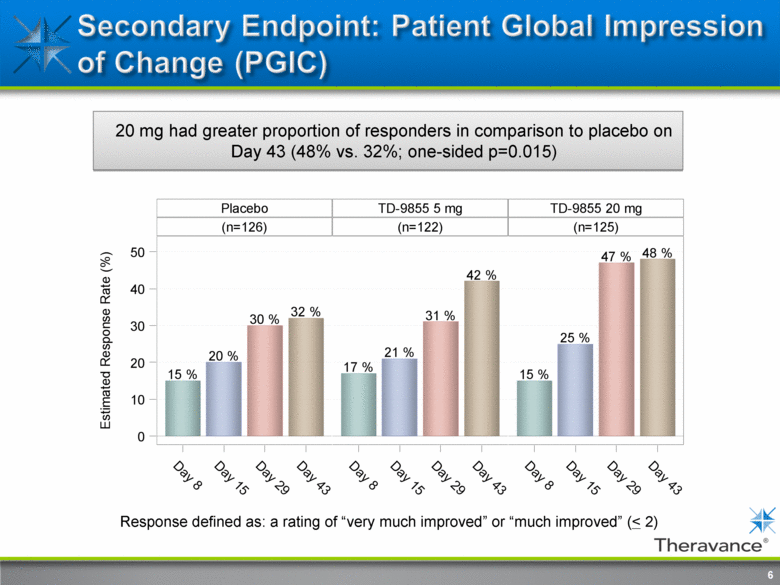
6 20 mg had greater proportion of responders in comparison to placebo on Day 43 (48% vs. 32%; one-sided p=0.015) Response defined as: a rating of “very much improved” or “much improved” (< 2) |
|
|
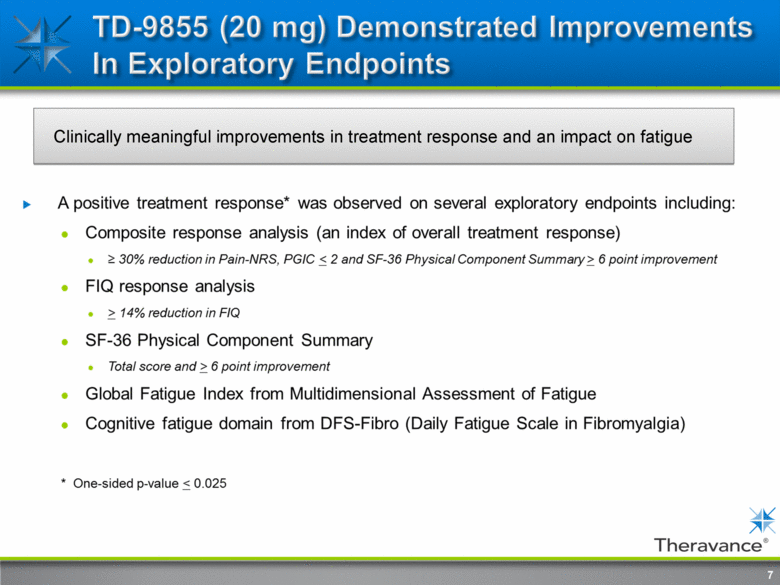
7 Clinically meaningful improvements in treatment response and an impact on fatigue A positive treatment response* was observed on several exploratory endpoints including: Composite response analysis (an index of overall treatment response) > 30% reduction in Pain-NRS, PGIC < 2 and SF-36 Physical Component Summary > 6 point improvement FIQ response analysis > 14% reduction in FIQ SF-36 Physical Component Summary Total score and > 6 point improvement Global Fatigue Index from Multimensional Assessment of Fatigue Congnitive fatigue domain from DFS-Fibro (Daily Fatigue Scale in Fibromyalgia) * One-sided p-value < 0.025 |
|
|
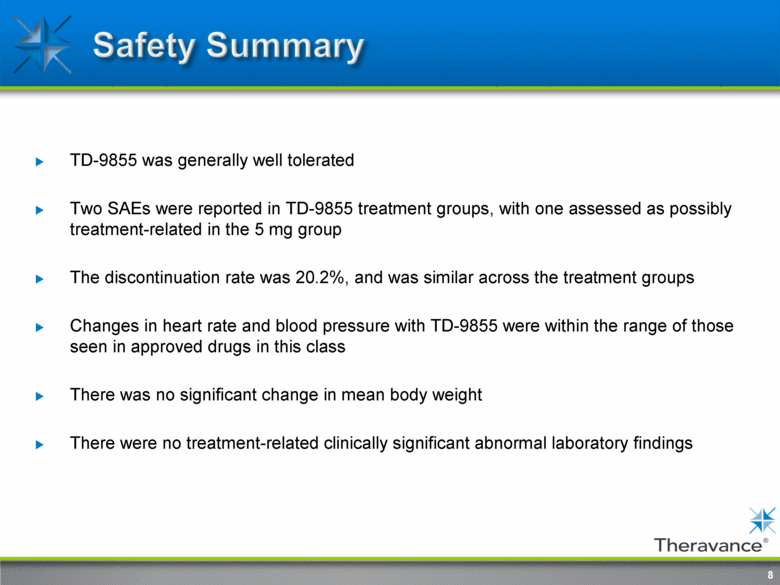
TD-9855 was generally well tolerated Two SAEs were reported in TD-9855 treatment groups, with one assessed as possibly treatment-related in the 5 mg group The discontinuation rate was 20.2%, and was similar across the treatment groups Changes in heart rate and blood pressure with TD-9855 were within the range of those seen in approved drugs in this class There was no significant change in mean body weight There were no treatment-related clinically significant abnormal laboratory findings 8 |
|
|
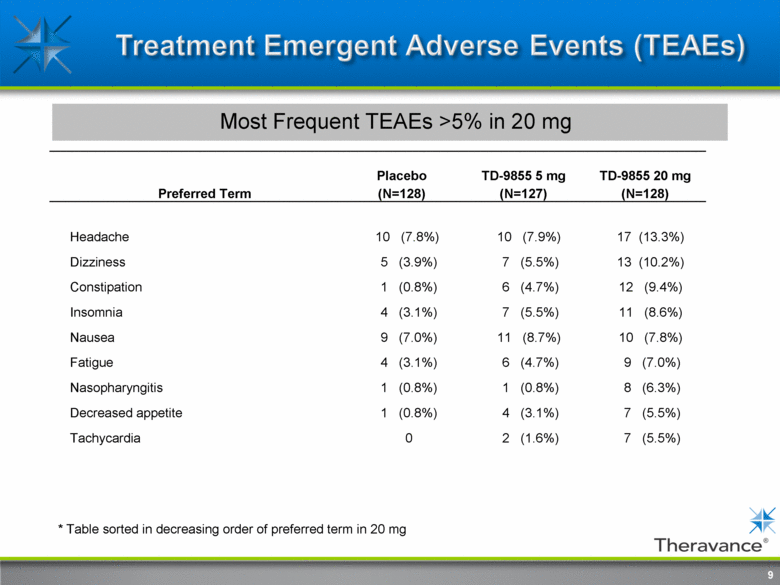
9 Most Frequent TEAEs >5% in 20 mg Preferred Term Placebo (N=128) TD-9855 5 mg (N=127) TD-9855 20 mg (N=128) Headache 10 (7.8%) 10 (7.9%) 17 (13.3%) Dizziness 5 (3.9%) 7 (5.5%) 13 (10.2%) Constipation 1 (0.8%) 6 (4.7%) 12 (9.4%) Insomnia 4 (3.1%) 7 (5.5%) 11 (8.6%) Nausea 9 (7.0%) 11 (8.7%) 10 (7.8%) Fatigue 4 (3.1%) 6 (4.7%) 9 (7.0%) Nasopharyngitis 1 (0.8%) 1 (0.8%) 8 (6.3%) Decreased appetite 1 (0.8%) 4 (3.1%) 7 (5.5%) Tachycardia 0 2 (1.6%) 7 (5.5%) * Table sorted in decreasing order of preferred term in 20 mg |
|
|
20 mg TD-9855 met statistical significance for the primary (Pain-NRS) and secondary (FIQ, PGIC) endpoints 5 mg did not meet statistical significance for the primary endpoint TD-9855 was generally well tolerated Phase 2 results support further development of TD-9855 10 |
|
|
NASDAQ: THRX |