Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Unilife Corp | d636924d8k.htm |
 Annual General
Meeting of Stockholders November 21, 2013
1
Exhibit 99.1 |
 Chairman’s
Welcome. Mr. Jim Bosnjak.
2 |
 Introducing our
Board of Directors In Attendance
John Lund, CPA
Non-Executive Director
since 2009
Chair of Audit
Committee
Alan Shortall
CEO
Executive Director
since 2002
Mary Kate Wold, J.D.
Non-Executive
Director since 2010
Chair of Strategic
Partnerships
Committee
William Galle
Non-Executive
Director since 2008 |
 Introducing our
Board of Directors Apologies
Jeff Carter MApp. BFin
Non-Executive Director
since 2006
Based in Australia
November 21, 2013 |
 Agenda
1.
Confirmation of Quorum
2.
Resolutions
3.
Presentation from the CEO
4.
Questions
5.
Confirmation of Results
6.
Other Business |
 Resolutions
1.
Election of six directors
2.
Ratify KPMG as independent registered public accounting firm
3.
Ratify compensation paid to certain directors
4. –
8.
Approve grant of securities to non-employee directors
9.
Approve special grant of securities to William Galle
10.
Ratify common stock issue under Controlled Equity Sales
Agreement with Cantor Fitzgerald
November 21, 2013 |
 Confirmation of
Quorum Unilife’s
quorum
requirement
is
one-third
of
outstanding
shares
being
represented
either
in
person
or
by
proxy
At
least
59.72%
of
the
total
outstanding
shares
entitled
to
vote
have
been
received
and
are
represented
by
person
or
proxy |
 Unilife Business
Update Unilife CEO. Mr. Alan Shortall.
8 |
 An Innovative,
Differentiated and Customizable Portfolio of Product Platforms 9
Confidential
–
Unilife
Corporation
Auto-
Injectors
Ocular
Systems
Wearable
Injectors
Reconstitution
Systems
Prefilled
Syringes
Novel
Systems |
 10
Platform of Prefilled Syringes
Unifill®
Unifill Finesse™
Unifill Select™
Unifill Allure™
Unifill Nexus™
Fully Integrated. Highly Differentiated.
|
 Sanofi Supply
Contract for Lovenox® Unilife Product:
Unifill Finesse -
customized product from Unifill platform
Sanofi Drug:
Enoxaparin Sodium (Lovenox®
/ Clexane®)
Drug Area:
Anti-thrombotics (low molecular weight heparin)
Geographic Territory:
Global
Contract Period:
Can extend up to 2024
Exclusivity:
Use of Unifill Finesse with anti-thrombotic drugs
Minimum Volumes:
Minimum of 150MM units per year (after four-year
high-volume ramp period) to maintain exclusivity
Additional Payments:
Up to $15MM ($5MM received, next $5MM during FY14)
Other Terms:
Replaces and supersedes previously signed agreements
Non-exclusive use to Unifill Finesse for biologic drugs
11
“Currently
Sanofi
provides
Clexane
/
Lovenox
with
different
devices
coming
therefore
from
different
facilities.
The
deal
with
Unilife
will
bring
added
value
to
our
customers,
reinforcing
the
safety
aspects
of
a
performing
device
looking
at
reducing
the
risk
of
needlestick
injuries.
Sanofi
has
kept
evolving
with
cutting
edge
technology
for
delivering
its
products”…and
this
deal
demonstrates
“an
evolution
in
safety
device
syringes”
-
Sanofi
spokesman
Frederic
Lemonde*
*
Sep
11,
2013
http://www.in-pharmatechnologist.com/Drug-Delivery/Sanofi-Inks-Deal-for-Prefilled-Syringes-with-Unilife
|
 Market for
Generic Injectables $11 B global market for global generic injectables (13% CAGR to
2017) The U.S. accounts for $7 B (60%) of the global market
1.5 B doses of generic injectable drugs used in the U.S. every year
About 70% of U.S. hospital patients receive a generic injectable
drug
Rapid conversion of generic injectables from vials to prefilled syringes
Benefits include fewer steps of use, time savings, dose accuracy
and less inventory
FDA
cites
patient
safety
concerns
with
prefilled
drugs
used
for
IV
infusion
Spontaneous disconnection, leakage or occlusion of medication and breakage
No universal attachment with any ISO standard needle hub or IV connector
12 |
 The Unifill
Nexus™ 13
Universal Connectivity. No Clogging. No Breakage.
Standard Fill Finish
Luer collar fits any standard
needle or connector
No Clogging or Breakage
Safe, Intuitive, One-Handed |
 Commercial
Supply Contract with Hikma Hikma
is
3
rd
largest
by
volume
in
the
US
generic
injectables
market
15 Year supply agreement to launch 20 generic injectable drugs in
syringes from Unifill platform including Unifill and Unifill Nexus
Hikma to receive exclusivity for use of Unilife devices with target
drugs
Additional drugs may be added to exclusivity list if agreed by both parties
Unilife to receive $40MM in upfront and milestone payments
Minimum unit volumes of 175MM Unifill products a year after high-
volume commercial ramp program
Actual unit volumes expected to reach 250 million units or more per year
Unilife to also customize and supply other products from its portfolio
14 |
 Platform of
Reconstitution Systems 15
One-Step Reconstitution. A World of Differentiation.
AutoMix Presto™
EZMix Genesis™
EZMix Engage™
EZMix Prodigy™ |
 The EZMix
Prodigy™ Compact, integrated single-barrel design for doses between
1-50mL Intuitive single-step reconstitution for liquid or dry drug combinations
Being pursued by multiple pharmaceutical and biotech customers
16 |
 Platform of
Auto-Injectors 17
LISA™
smart reusable
auto-injector
RITA™
disposable
auto-injector
Self-Injection. Simplified.
Multiple programs underway that are
aligned with Unifill syringe products |
 The Market for
Wearable Injectors Around 250 high viscous, large volume
biologics targeted for wearable injectors
Multiple therapy areas targeted including cancer
and auto-immune diseases
List includes 100+ pharma and biotech companies
Estimated 350 million wearable injectors to
be sold annually by 2024
Average selling price of $20 -
$35 a unit
Market size of USD $8.8 billion by 2024
CAGR of 138% from 2015 -
2020
CAGR of 48% from 2020 –
2024
18
Reference
Source
for
Slide
Information:
Bolus
Injectors
Market
2014
-
2024.
2013
Roots
Analysis. |
 Platform of
Wearable Injectors 19
Pre-Filled. Pre-Assembled.
Ready-to-Inject.
Precision-Therapy™
Flex-Therapy™
For long-duration therapies that require
delivery of large dose volumes
For long-duration therapies that require delivery of
large dose volumes with a specific rate profile |
 A Typical
Customer Partnership 20
Average number of drugs being targeted
3 –
8
Average phase of drugs being targeted
Late-stage
Average unit volume range per molecule
2 -
5MM units
Average customization program length
18 -
36 months
Average size of customization program per drug per year
$3 -
6MM*
Average duration of customer commitment
15 -
20 years
* does not include high-cost
device sales during customization
and clinical trial programs |
 Customization
and Supply Contract with MedImmune 21
MedImmune is the global biologics R&D arm of AstraZeneca
One of the richest biologic portfolios in the industry
120 large molecule drugs in R&D (half of AstraZeneca’s total clinical pipeline)
Under this agreement, Unilife will customize and supply devices
from
its platform of wearable injectors for use with target molecules.
Several drugs may be selected for use with Unilife's wearable injectors
Unilife selected after an extensive evaluation process
Revenue to begin being generated in the first quarter of fiscal 2014
|
 Ocular Delivery
and Novel Device Platforms 22
Ocu-ject™
Micro-ject™
Beyond
Conventional Delivery Systems
Depot-ject™ |
 Moving
Forward Revenue to grow sequentially each and every quarter in FY 2014
Significant annual revenue growth this fiscal year and beyond
Down the road, as contracts reach peak commercial sales, we will
attain a
position of global leadership for injectable drug delivery
Prefilled Syringes: Targeting production of 400MM units or more a year
Wearable Injectors: Taken leadership position in market worth $8B 2024
Together with revenue generated from other product platforms, Unilife on
way to generating future expected revenue in excess of $1 B a year
23 |
 Annual General
Meeting of Stockholders November 21, 2013
Consider and Vote for Matters set forth in Proxy Statement
24 |
 Election of Directors
Item 1. |
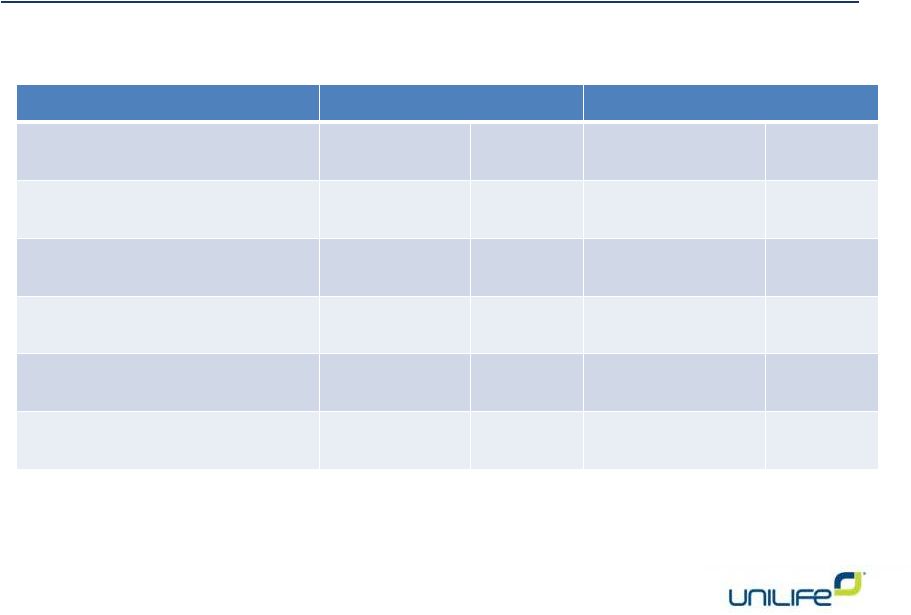 Election of Directors
Item 1.
For
Withheld
Item 1.1 Jim Bosnjak
37,893,553
89.64%
4,381,120
10.36%
Item 1.2 Jeff Carter
38,248,088
90.48%
4,026,585
9.52%
Item 1.3 William Galle
39,975,506
94.56%
2,299,167
5.44%
Item 1.4 John Lund
39,968,156
94.54%
2,306,517
5.46%
Item 1.5 Mary Kate Wold
40,048,663
94.73%
2,226,010
5.27%
Item 1.6 Alan Shortall
39,480,958
93.39%
2,793,715
6.61% |
 Item 2.
Ratification of the appointment of KPMG LLP as the
Corporation’s independent registered public
accounting firm for Fiscal Year 2014 |
 Item 2.
Ratification of the appointment of KPMG LLP as the
Corporation’s independent registered public
accounting firm for Fiscal Year 2014
For
Against
Abstain
Item 2.
58,319,458
904,285
684,984 |
 Item 3.
To consider and act on an advisory vote regarding
the approval and compensation paid to certain
executive officers |
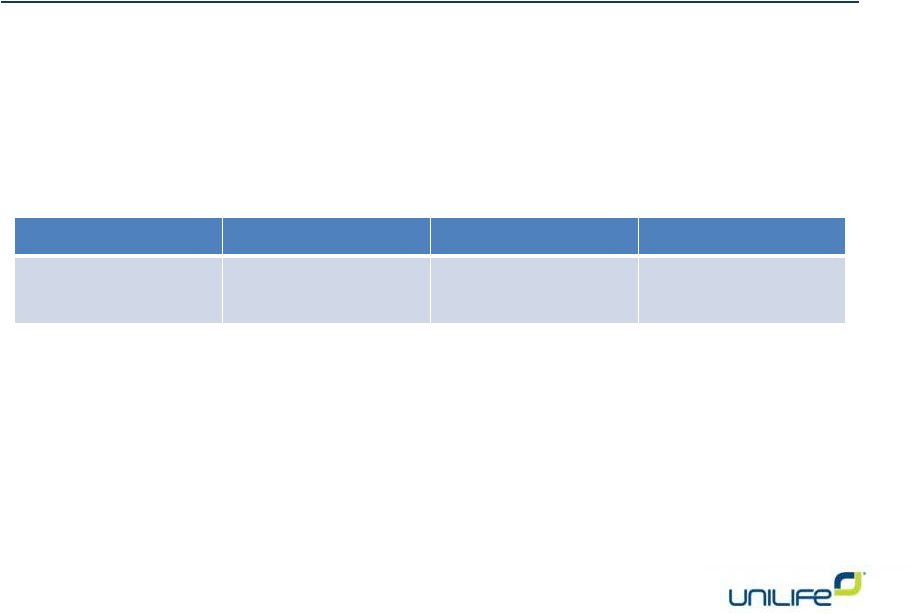 Item 3.
For
Against
Abstain
Item 3.
36,033,719
5,784,484
456,470
To consider and act on an advisory vote regarding
the approval and compensation paid to certain
executive officers |
 Items 4
- 8.
The approval of the grant of 105,000 securities to each
of the five non-employee Directors |
 Items 4
- 8.
For
Against
Abstain
Item 4. Jim Bosnjak
30,270,781
11,676,609
327,283
Item 5. Jeff Carter
30,130,410
11,683,867
460,396
Item 6. William Galle
30,410,105
11,400,255
464,313
Item 7. John Lund
30,410,221
11,400,755
463,697
Item 8. Mary Kate Wold
30,433,812
11,382,098
458,763
The approval of the grant of 105,000 securities to each
of the five non-employee Directors |
 33
Item 9.
To approve a special grant of 52,500 securities to
William Galle in the form of restricted stock units. |
 34
Item 9.
For
Against
Abstain
Item 9.
30,306,626
11,483,493
484,554
To approve a special grant of 52,500 securities to
William Galle in the form of restricted stock units. |
 To ratify the
issuance and sale of 3,512,153 shares of common stock (equivalent to 21,072,918 CHESS
Depositary Interests (“CDIs”)) under the Controlled Equity Offering Sales
Agreement we entered into with Cantor Fitzgerald & Co. dated October 3, 2012,
pursuant to a registration statement filed by us with the U.S. Securities and
Exchange Commission (“SEC”), and the accompanying prospectus
supplement that we filed with the SEC on October 4, 2012.
35
Item 10. |
 To ratify the
issuance and sale of 3,512,153 shares of common stock (equivalent to 21,072,918 CHESS
Depositary Interests (“CDIs”)) under the Controlled Equity Offering Sales
Agreement we entered into with Cantor Fitzgerald & Co. dated October 3, 2012,
pursuant to a registration statement filed by us with the U.S. Securities and
Exchange Commission (“SEC”), and the accompanying prospectus
supplement that we filed with the SEC on October 4, 2012.
36
Item 10.
For
Against
Abstain
Item 10.
39,787,618
1,938,609
548,446 |
 Annual General
Meeting of Stockholders November 21, 2013
Other Business
37 |
 Annual General
Meeting of Stockholders November 21, 2013
Close of Meeting
38 |
