Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a13-22211_18k.htm |
Exhibit 99.1
|
|
INNOVATIVE THERAPIES NOVEL PRODUCTS OCT-2013 ©2013 VIVUS Inc. All rights reserved | www.vivus.com |
|
|
FORWARD LOOKING STATEMENT This presentation contains “forward looking” statements that involve risks and uncertainties. These statements typically may be identified by the use of forward looking words or phrases such as “may,” “believe,” “expect,” “forecast,” “intend,” “anticipate,” “predict,” “should,” “planned,” “likely,” “opportunity,” “estimated,” and “potential,” the negative use of these words or other similar words. All forward looking statements included in this document are based on our current expectations, and we assume no obligation to update any such forward looking statements. The Private Securities Litigation Reform Act of 1995 provides a “safe harbor” for such forward looking statements. In order to comply with the terms of the safe harbor, we note that a variety of factors could cause actual results and experiences to differ materially from the anticipated results or other expectations expressed in such forward looking statements. The risks and uncertainties that may affect the operations, performance, development, and results of our business include but are not limited to: (1) our limited commercial experience with Qsymia® in the United States, or U.S.; (2) the timing of initiation and completion of the clinical studies required as part of the approval of Qsymia by the U.S. Food and Drug Administration, or FDA; (3) the response from the FDA to the data that VIVUS will submit relating to post-approval clinical studies; (4) the impact of the indicated uses and contraindications contained in the Qsymia label and the Risk Evaluation and Mitigation Strategy, or REMS, requirements; (5) our ability to continue to certify and add to the Qsymia retail pharmacy network and sell Qsymia through this network; (6) whether the Qsymia retail pharmacy network will simplify and reduce the prescribing burden for physicians, improve access and reduce waiting times for patients seeking to initiate therapy with Qsymia; (7) that we may be required to provide further analysis of previously submitted clinical trial data; (8) the negative opinion of the European Medicines Agency’s, or EMA, Committee for Medicinal Products for Human Use, or CHMP, for the Marketing Authorization Application, or MAA, for Qsymia; (9) our ability to successfully seek approval for Qsymia in other territories outside the U.S. and European Union, or EU, (10) whether healthcare providers, payors and public policy makers will recognize the significance of the American Medical Association, or AMA, officially recognizing obesity as a disease, or the new American Association of Clinical Endocrinologists, or AACE, guidelines; (11) our ability to successfully commercialize Qsymia including risks and uncertainties related to expansion to retail distribution, the broadening of payor reimbursement, the expansion of Qsymia’s primary care presence, and the outcomes of our discussions with pharmaceutical companies and our strategic and franchise-specific pathways for Qsymia; (12) our ability to focus our promotional efforts on health care providers and on patient education that, along with increased access to Qsymia and ongoing improvements in reimbursement, will result in the accelerated adoption of Qsymia; (13) our ability to eliminate expenses that are not essential to expanding the use of Qsymia; (14) our ability to ensure that the entire supply chain for Qsymia efficiently and consistently delivers Qsymia to our customers; (15) risks and uncertainties related to the launch and commercialization of STENDRA™ in the United States and Canada by Auxilium Pharmaceuticals, Inc.; (16) risks and uncertainties related to the launch and commercialization of SPEDRA in the EU, plus Australia and New Zealand, by Menarini Group through its subsidiary Berlin-Chemie AG, or Menarini; (17) our ability to successfully complete on acceptable terms and on a timely basis avanafil partnering discussions for territories in which we do not have a commercialization alliance; (18) the timing of the qualification and subsequent approval by regulatory authorities of Sanofi Chimie as a qualified supplier of STENDRA/SPEDRA™ and Sanofi Chimie’s ability to undertake worldwide manufacturing of the avanafil active pharmaceutical ingredient, or API; (19) whether the FDA and/or EMA will approve the amendments we intend to submit to include the recently announced study results showing avanafil (STENDRA/SPEDRA) is effective for sexual activity within 15 minutes in men with ED; (20) the ability of our partners to maintain regulatory approvals to manufacture and adequately supply our products to meet demand; (21) our history of losses and variable quarterly results; (22) substantial competition; (23) risks related to the failure to protect our intellectual property and litigation in which we may become involved; (24) uncertainties of government or third-party payor reimbursement; (25) our reliance on sole source suppliers; (26) our reliance on third parties and our collaborative partners; (27) our failure to continue to develop innovative investigational drug candidates and drugs; (28) risks related to the failure to obtain FDA or foreign authority clearances or approvals and noncompliance with FDA or foreign authority regulations; (29) our ability to demonstrate through clinical testing the safety and effectiveness of our investigational drug candidates; (30) the timing of initiation and completion of clinical trials and submissions to foreign authorities; (31) the results of post-marketing studies are not favorable; (32) compliance with post-marketing regulatory standards is not maintained; (33) the volatility and liquidity of the financial markets; (34) our liquidity and capital resources; (35) our expected future revenues, operations and expenditures (36) potential change in our business strategy to enhance long term stockholder value; and (37) other factors that are described from time to time in our periodic filings with the Securities and Exchange Commission, or the SEC, or the Commission, including our Annual Report on Form 10-K for the year ended December 31, 2012, filed with the SEC on February 26, 2013 (as amended by Form 10-K/A, filed with the SEC on April 30, 2013 and by Form 10-K/A, filed with the SEC on June 12, 2013) and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2013, filed with the SEC on August 8, 2013. ©2013 VIVUS Inc. All rights reserved | www.vivus.com 1 |
|
|
RECENT HIGHLIGHTS – 2H 2013 Qsymia available in certified retail pharmacies July 1 New Board of Directors elected August 14 New CEO, Seth Fischer, announced September 3 Qsiva EU strategy update provided September 20 New Qsymia data in prediabetes/metabolic syndrome published in Diabetes Care October 8 STENDRA deal - U.S. & Canada - announced October 11 ©2013 VIVUS Inc. All rights reserved | www.vivus.com 2 |
|
|
SETH H. Z. FISCHER, CEO Three decades of healthcare experience Pharmaceuticals & Medical Devices, Johnson & Johnson Company Group Chairman and Worldwide Franchise Chairman, Cordis Corporation (2008 to 2012) Company Group Chairman, North America Pharmaceuticals (2004 to 2007 - Ortho-McNeil Pharmaceuticals, Janssen and Scios) President, Ortho-McNeil Pharmaceuticals (2000 to 2004) Operating responsibilities: commercialization of products in multiple therapeutic categories including Topamax® for epilepsy and migraine, and products in the analgesia, anti-infective, cardiovascular, neurologic, psychiatric and women's health areas Member of the Board of Directors of BioSig Technologies, Inc. and advisor to MedHab, LLC ©2013 VIVUS Inc. All rights reserved | www.vivus.com 3 |
|
|
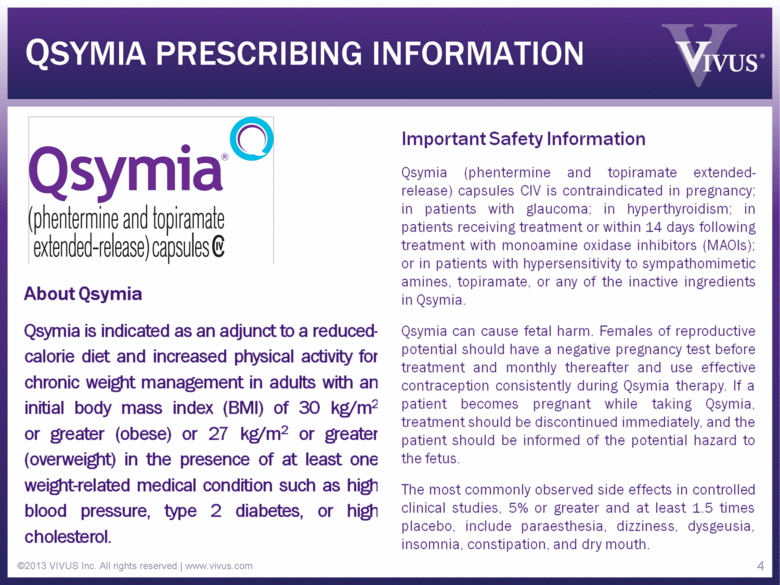
QSYMIA PRESCRIBING INFORMATION ©2013 VIVUS Inc. All rights reserved | www.vivus.com 4 |
|
|
VIVUS CORPORATE PRIORITIES 1) Expand use of Qsymia through targeted HCP and patient education 2) Find the right partner for Qsymia 3) Quickly create a pathway for approval in Europe 4) Eliminate expenses that are not essential to expanding use of Qsymia ©2013 VIVUS Inc. All rights reserved | www.vivus.com 5 |
|
|
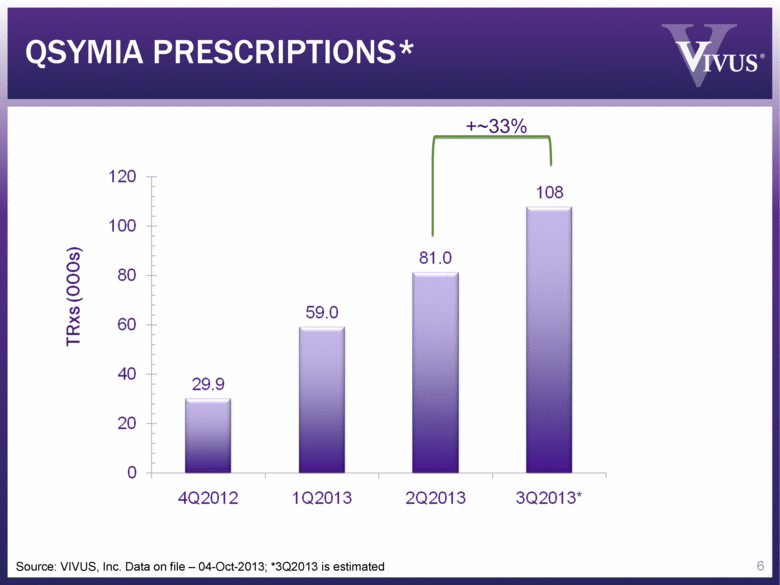
Source: VIVUS, Inc. Data on file – 04-Oct-2013; *3Q2013 is estimated QSYMIA PRESCRIPTIONS* 6 +~33% |
|
|
QSYMIA RETAIL ROLL-OUT SUCCESS Certified Retail Pharmacy Status (14-Oct-2013) >26,000 pharmacies now certified to dispense Qsymia Chains include: A&P, Albertsons, Costco, CVS, Kmart, Kroger, Publix, Rite Aid, Safeway, Walgreens/Duane Reade, Walmart, Wegmans, Winn-Dixie, many others Prescriber and patient experience vastly simplified Independent pharmacies being certified 4Q2013 ©2013 VIVUS Inc. All rights reserved | www.vivus.com 7 |
|
|
QSYMIA REIMBURSEMENT CONTINUES TO IMPROVE As of 30-Sep-2013, ~38% of U.S. Commercial Lives have access to Qsymia at Tier 3 or better YE2013 Goal: 50% 2014 Focus: Improve access & pull-through ©2013 VIVUS Inc. All rights reserved | www.vivus.com 8 |
|
|
RECENT PUBLICATION REPORTS IMPACT OF QSYMIA ON TYPE 2 DIABETES Publication: Diabetes Care October 8, 2013 - doi:10.2337/dc13-1518 - W. Timothy Garvey, MD, et al Title: “Prevention of Type 2 Diabetes in Subjects With Prediabetes and Metabolic Syndrome Treated With Phentermine and Topiramate Extended-Release” Patients on the Recommended (7.5/46) and Top (15/92) doses experienced reduced annualized incidence rates of progression to type 2 diabetes of 70.5% and 78.7%, respectively, compared to placebo, driven by magnitude of weight lost (10.9% and 12.1%, respectively vs. placebo; p<0.0001; ITT-MI) Qsymia therapy was well tolerated by this subgroup over two years Data further support Qsymia reimbursement discussions as part of AMCP dossier ©2013 VIVUS Inc. All rights reserved | www.vivus.com 9 |
|
|
QSYMIA EUROPEAN STRATEGY Goal Satisfy FDA post-approval requirements with a single CVOT that could also support EU registration Strategy Create an early stop in CVOT so that ~two years of data could be used to support an application while the trial continues Tactics Use the new 90-day EU consultation process to gain as much certainty as possible regarding EU regulatory expectations Submission for 90-day consultation to be completed shortly Re-consult with FDA following EU response 10 years of exclusivity via CHMP central process ©2013 VIVUS Inc. All rights reserved | www.vivus.com 10 |
|
|
AQCLAIM STUDY (CVOT) A Qsymia® CardiovascuLAr morbIdity and Mortality (AQCLAIM) Study in Subjects with Documented Cardiovascular Disease Primary Efficacy Endpoint: Time to 1st occurrence of nonfatal MI, nonfatal stroke or CV death Number of Subjects: ~11,000 - 16,000 Number of Sites: ~450 (North America, Europe, RoW) Enrollment: 1Q 2014 Seeking input from CHMP in advance of enrollment Length: ~4 years Interim Analysis: ~4Q2016 ©2013 VIVUS Inc. All rights reserved | www.vivus.com 11 |
|
|
STENDRA PRESCRIBING INFORMATION About STENDRA™ STENDRA is a prescription medicine used to treat erectile dysfunction (ED). STENDRA (avanafil) is licensed from Mitsubishi Tanabe Pharma Corporation. VIVUS has development and commercial rights to STENDRA for the treatment of sexual dysfunction worldwide with the exception of certain Asian Pacific Rim countries. Important Safety Information Do not take STENDRA if you take nitrates, often prescribed for chest pain, as this may cause a sudden, unsafe drop in blood pressure. Tell your healthcare provider about all the medicines you take and discuss your general health status to ensure that you are healthy enough to engage in sexual activity. If you experience chest pain, nausea, or any other discomforts during sex, seek immediate medical help. In the rare event of an erection lasting more than 4 hours, seek immediate medical help to avoid long-term injury. STENDRA in combination with other treatments for erectile dysfunction is not recommended. STENDRA does not protect against sexually transmitted diseases, including HIV. The most common side effects of STENDRA are headache, flushing, runny nose and congestion. ©2013 VIVUS Inc. All rights reserved | www.vivus.com 12 |
|
|
STENDRA PARTNERSHIP WITH AUXILIUM October 11, 2013 – Auxilium Pharmaceuticals, Inc. to Launch and Market STENDRA in U.S. and Canada AUXL 2012 Product Revenues: $~303 million Offices: Chesterbrook, PA Employees: 526 U.S. Sales Force: 150 Men’s Health Portfolio: Testim® (testosterone gel) for hypogonadism XIAFLEX® (collagenase clostridium histolyticum (CCH)) for adult Dupuytren’s contracture TESTOPEL® long-acting implantable testosterone replacement therapy Edex® non-oral drug for erectile dysfunction Striant® buccal system for testosterone delivery Osbon ErecAid® device for aiding erectile dysfunction ©2013 VIVUS Inc. All rights reserved | www.vivus.com 13 |
|
|
STENDRA PARTNERSHIP WITH AUXILIUM Territories: U.S. & Canada VIVUS to Receive Up-front Payment Plus Milestones and Royalties Over the Term of the Agreement Total Deal Value: ~$300 million plus royalties Up-front Payment: $30 million Regulatory Milestones: $15 million (expansion of label) Sales Milestones: $255 million Royalties on Sales: Escalating based on sales ranges ©2013 VIVUS Inc. All rights reserved | www.vivus.com 14 |
|
|
SPEDRA PARTNERSHIP WITH MENARINI Menarini Territories: 40 Countries in Europe, plus Australia & New Zealand VIVUS to Receive Up-front Payment Plus Milestones and Royalties Over the Term of the Agreement Total Deal Value: ~$100 million plus royalties Up-front Payments Regulatory Milestones Launch Milestones Royalties on Product Sales Menarini to reimburse VIVUS to cover MTPC obligations First-year cash: ~$51 million, including $21 million up-front ©2013 VIVUS Inc. All rights reserved | www.vivus.com 15 |
|
|
VIVUS SUMMARY Qsymia prescriptions and payor acceptance moving in the right direction Clear inflection in TRxs with retail availability Improving reimbursement Qsiva pathway in EU being finalized; CVOT refinements Avanafil monetization in Europe and U.S. Together, potentially >$95 million in cash through YE2014 $358MM cash and short-term investments at end of 2Q2013 ©2013 VIVUS Inc. All rights reserved | www.vivus.com 16 |