Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a13-20084_18k.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a13-20084_1ex99d2.htm |
Exhibit 99.1
Poster No. P3640
Efficacy and safety of umeclidinium/vilanterol compared with umeclidinium or tiotropium in COPD
Marc Decramer,(1) Antonio Anzueto,(2) Edward Kerwin,(3) Nathalie Richard,(4) Glenn Crater,(4) Maggie Tabberer,(5) Stephanie Harris,(4) Alison Church(4)
(1)University Hospital, Leuven, Belgium; (2)University of Texas Health, Houston, Texas, USA; (3)Clinical Research Institute of Southern Oregon, PC, Oregon, USA; (4)GlaxoSmithKline, Respiratory, Research Triangle Park, North Carolina, USA; (5)GlaxoSmithKline, Stockley Park, Uxbridge, UK
INTRODUCTION
· Current guidelines recommend treatment with one or more long-acting bronchodilators for patients with moderate-to-very severe chronic obstructive pulmonary disease (COPD).(1),(2)
· Umeclidinium (UMEC)/vilanterol (VI) is a combined long-acting muscarinic antagonist/long-acting β2-agonist bronchodilator in development for the maintenance treatment of COPD.
AIMS
· To evaluate the efficacy and safety of two once-daily doses of UMEC/VI (125/25 mcg [delivering 113/22 mcg] and 62.5/25 mcg [delivering 55/22 mcg]) compared with UMEC 125 mcg (delivering 113 mcg) or tiotropium (TIO) 18 mcg monotherapies in patients with COPD.
METHODS
Study design and treatments
· This was a 24-week, multicentre, randomised, blinded, double-dummy, parallel-group study (DB2113374; NCT01316913).
· Key eligibility criteria: age >40 years; clinically established history of COPD; current or former cigarette smokers with a smoking history of >10 pack-years; post-salbutamol forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio <0.7; post-salbutamol FEV1 <70% of predicted normal values; and a modified Medical Research Council Dyspnoea Scale(3) score >2. Concurrent use of inhaled corticosteroids (ICS) and rescue use of salbutamol was allowed.
· Following a 7-10-day run-in period, patients were randomised 1:1:1:1 to 24 weeks of treatment with UMEC/VI 125/25, UMEC/VI 62.5/25, UMEC 125 or TIO 18.
· UMEC/VI, UMEC and matching placebo were administered via ELLIPTA™* dry powder inhaler (DPI); TIO and corresponding placebo capsules were administered via HandiHaler® DPI. Each patient took one dose from the HandiHaler® DPI and one dose from the ELLIPTA™ DPI each morning. TIO capsules had trade markings but placebo did not. With a parallel group design, the capsule type was consistent for each patient for the duration of the study. Blister packages were covered with opaque over-labels to shield information appearing on the blister packaging of TIO. Dosing in the clinic was administered without the presence of staff involved with safety and efficacy assessments.
· All patients provided written, informed consent before study participation. The study was conducted in accordance with the declaration of Helsinki and Good Clinical Practice guidelines. Institutional Review Board approval was obtained.
Endpoints
· Primary efficacy: trough FEV1 at Day 169, defined as the mean of FEV1 values obtained 23 and 24 h after dosing on Day 168.
· Secondary efficacy: weighted mean (WM) FEV1 over 0-6 h post-dose at Day 168.
· Additional efficacy: mean transition dyspnoea index (TDI) focal score; St George’s Respiratory Questionnaire (SGRQ) score; rescue salbutamol use and time to first COPD exacerbation.
· Safety: adverse events (AEs); vital signs; 12-lead electrocardiography (ECG); and clinical chemistry/haematology measurements.
RESULTS
Patient demographics and baseline characteristics
· A total of 1191 patients were enrolled; 869 were included in the intention-to-treat (ITT) population (i.e., randomised and received at least one dose of study medication).
· Patient demographics and baseline characteristics were similar across treatment groups (Table 1).
*ELLIPTATM is a trademark of the GlaxoSmithKline group of companies
TABLE 1. PATIENT DEMOGRAPHICS AND BASELINE CHARACTERISTICS (ITT POPULATION)
|
|
|
UMEC |
|
UMEC/VI |
|
UMEC/VI |
|
TIO |
|
|
Age, years |
|
64.5 (8.33) |
|
65.0 (8.62) |
|
63.8 (8.51) |
|
65.2 (8.30) |
|
|
Male, n (%) |
|
148 (67) |
|
140 (65) |
|
148 (69) |
|
153 (71) |
|
|
Current smoker, n (%)(a) |
|
98 (44) |
|
92 (42) |
|
96 (45) |
|
102 (47) |
|
|
Smoking pack-years |
|
47.6 (27.58) |
|
47.8 (26.13) |
|
46.9 (24.90) |
|
54.0 (31.59) |
|
|
Cardiovascular risk factor, n (%)(b) |
|
127 (57) |
|
131 (60) |
|
121 (56) |
|
123 (57) |
|
|
ICS use, n (%)(c) |
|
124 (56) |
|
103 (47) |
|
113 (53) |
|
115 (53) |
|
|
Pre-bronchodilator FEV1, L |
|
1.140 (0.4479) |
|
1.170 (0.4655) |
|
1.159 (0.4384) |
|
1.175 (0.4287) |
|
|
Post-salbutamol FEV1, L |
|
1.294 (0.4679) |
|
1.322 (0.4899) |
|
1.313 (0.4235) |
|
1.328 (0.4310) |
|
|
Reversibility to salbutamol, % |
|
16.1 (15.25) |
|
14.9 (14.95) |
|
15.8 (15.17) |
|
15.5 (15.55) |
|
|
Post-salbutamol % predicted FEV1 |
|
46.2 (13.03) |
|
47.7 (13.55) |
|
47.1 (12.88) |
|
47.4 (13.10) |
|
|
Post-salbutamol FEV1/FVC |
|
45.29 (11.37) |
|
46.23 (11.86) |
|
45.94 (10.39) |
|
45.80 (11.65) |
|
Values are presented as mean (standard deviation) unless otherwise stated.
(a)Patient was reclassified as a current smoker if he/she smoked within 6 months of screening; (b)Current medical history of angina pectoris, diabetes, hyperlipidaemia, hypertension or myocardial infarction; (c)ICS use was defined as those patients who were currently taking ICS medications at the screening visit.
Primary efficacy: trough FEV1
· Treatment with UMEC/VI 125/25 resulted in a statistically significant improvement in trough FEV1 at Day 169 compared with TIO (p=0.003) but not UMEC 125 (p=0.142) (Figure 1, Table 2).
· An improvement was also observed with UMEC/VI 62.5/25 vs TIO (p=0.018) but not vs UMEC 125 (p=0.377).
FIGURE 1. TROUGH FEV1 (ITT POPULATION)
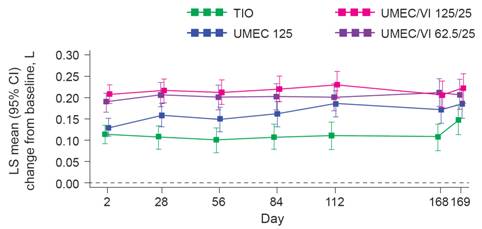
CI, confidence interval; LS, least squares.
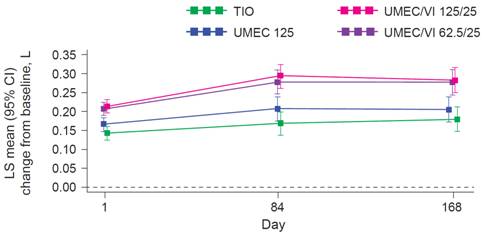
Secondary efficacy: 0-6 h post-dose WM FEV1
· Both UMEC/VI doses showed improvements in 0-6 h post-dose WM FEV1 at Day 168 compared with TIO and UMEC 125 (Figure 2, Table 2).
TABLE 2. EFFICACY OUTCOMES (ITT POPULATION)
|
|
|
UMEC |
|
TIO |
|
|
Trough FEV1 at Day 169, L |
|
|
|
|
|
|
Difference vs monotherapy (95% CI) |
|
0.037 (–0.012, 0.087) |
|
0.074** (0.025, 0.123) |
|
|
UMEC/VI 62.5/25 |
|
0.022 (–0.027, 0.072) |
|
0.060* (0.010, 0.109) |
|
|
0—6 h WM FEV1 at Day 168, L |
|
|
|
|
|
|
Difference vs monotherapy (95% CI) |
|
0.076*** (0.029, 0.122) |
|
0.101*** (0.055, 0.147) |
|
|
UMEC/VI 62.5/25 |
|
0.070** (0.024, 0.117) |
|
0.096*** (0.050, 0.142) |
|
|
TDI responder at Day 168(a) |
|
|
|
|
|
|
Odds ratio vs monotherapy (95% CI) |
|
1.2 (0.8, 1.8) |
|
1.2 (0.8, 1.8) |
|
|
UMEC/VI 62.5/25 |
|
1.3 (0.9, 2.0) |
|
1.3 (0.9, 1.9) |
|
|
SGRQ responder at Day 168(b) |
|
|
|
|
|
|
Odds ratio vs monotherapy (95% CI) |
|
1.1 (0.8, 1.7) |
|
0.9 (0.6, 1.3) |
|
|
UMEC/VI 62.5/25 |
|
1.3 (0.9, 1.9) |
|
1.0 (0.6, 1.5) |
|
|
Salbutamol use, Weeks 1—24, puffs/day |
|
|
|
|
|
|
Difference vs monotherapy (95% CI) |
|
-1.1*** (-1.7, -0.5) |
|
-1.1*** (-1.7, -0.5) |
|
|
UMEC/VI 62.5/25 |
|
–0.6 (–1.2, 0.0) |
|
–0.6 (–1.2, 0.0) |
|
***p<0.001; **p<0.01; *p<0.05 for combinations vs monotherapy; values in brackets = 95% CIs.
(a)Response defined as an improvement of at least 1 unit in TDI focal score; (b)Response defined as an improvement of at least 4 units in SGRQ score.
FIGURE 2. 0-6 h POST-DOSE WM FEV1 (ITT POPULATION)
Efficacy: additional endpoints
· UMEC/VI 125/25 reduced salbutamol use in comparison with both TIO and UMEC 125 monotherapies (Table 2).
· On-treatment COPD exacerbations were observed in 12% of patients receiving UMEC/VI 62.5/25 or UMEC 125 and 7% of patients receiving UMEC/VI 125/25 or TIO.
Safety
· The incidence of AEs was similar across treatment groups; headache and nasopharyngitis were the most common AEs reported (Table 3).
· The incidence of on-treatment serious AEs (SAEs) ranged from 4% (TIO group) to 10% (UMEC/VI 62.5/25 group). The most common SAE was COPD.
· Four patients died during the study (one in the UMEC/VI 125/25 group, one in the UMEC/VI 62.5/25 group and two in the TIO group); none were judged to be related to study drug.
· No clinically meaningful changes in vital signs, ECG or clinical laboratory parameters were observed for UMEC/VI treatments compared with UMEC 125 or TIO monotherapies.
TABLE 3. OVERVIEW OF AEs and SAEs (ITT POPULATION)
|
|
|
UMEC |
|
UMEC/VI |
|
UMEC/VI |
|
TIO |
|
Any on-treatment AEs, n (%) |
|
131 (59) |
|
127 (59) |
|
133 (62) |
|
126 (59) |
|
AEs reported by >3% patients, n (%) |
|
|
|
|
|
|
|
|
|
Headache |
|
25 (11) |
|
21 (10) |
|
20 (9) |
|
15 (7) |
|
Nasopharyngitis |
|
6 (3) |
|
14 (6) |
|
16 (7) |
|
17 (8) |
|
Upper respiratory tract infection |
|
17 (8) |
|
6 (3) |
|
10 (5) |
|
14 (7) |
|
Back pain |
|
10 (5) |
|
8 (4) |
|
6 (3) |
|
11 (5) |
|
Cough |
|
14 (6) |
|
5 (2) |
|
8 (4) |
|
6 (3) |
|
Hypertension |
|
9 (4) |
|
1 (<1) |
|
4 (2) |
|
7 (3) |
|
Oropharyngeal pain |
|
8 (4) |
|
3 (1) |
|
6 (3) |
|
3 (1) |
|
Diarrhoea |
|
8 (4) |
|
4 (2) |
|
1 (<1) |
|
5 (2) |
|
Gastritis |
|
6 (3) |
|
6 (3) |
|
5 (2) |
|
1 (<1) |
|
Pain in extremity |
|
1 (<1) |
|
7 (3) |
|
6 (3) |
|
4 (2) |
|
Urinary tract infection |
|
6 (3) |
|
2 (<1) |
|
5 (2) |
|
4 (2) |
|
COPD |
|
2 (<1) |
|
7 (3) |
|
6 (3) |
|
1 (<1) |
|
Influenza |
|
6 (3) |
|
3 (1) |
|
2 (<1) |
|
5 (2) |
|
Lower respiratory tract infection |
|
1 (<1) |
|
9 (4) |
|
3 (1) |
|
2 (<1) |
|
Dyspnoea |
|
6 (3) |
|
1 (<1) |
|
0 |
|
3 (1) |
|
Any on-treatment SAEs, n (%) |
|
15 (7) |
|
22 (10) |
|
15 (7) |
|
9 (4) |
|
SAEs reported by >1% patients, n (%) |
|
|
|
|
|
|
|
|
|
COPD(a) |
|
2 (<1) |
|
7 (3) |
|
6 (3) |
|
1 (<1) |
|
Pneumonia |
|
2 (<1) |
|
2 (<1) |
|
3 (1) |
|
2 (<1) |
(a) Only serious COPD was recorded as an AE
CONCLUSIONS
· Both doses of UMEC/VI improved lung function compared with TlO.
· All treatments were well tolerated and no notable treatment-related changes were observed in vital signs, ECGs or clinical laboratory parameters.
· This study supports the use of once-daily UMEC/VI as long-term maintenance treatment for COPD.
REFERENCES
(1) GOLD 2013. Available at: http://www.Goldcopd.org/. [Accessed August 2013].
(2) Celli BR, Macnee W. Eur Respir J 2004;23:932—946.
(3) Manali ED, et al. BMC Pulm Med 2010;10:32.
ACKNOWLEDGEMENTS
· M Decramer has been part of advisory boards for Altana, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Takeda/Nycomed and Ventura. He has performed consulting work for AstraZeneca, Boehringer Ingelheim, Pfizer, Dompé, GlaxoSmithKline, Novartis and Takeda/Nycomed. He has also received lecture fees from these companies and a research grant from AstraZeneca, Boehringer Ingelheim and GlaxoSmithKline. AA has received consultancy and advisory board fees from AstraZeneca, Boehringer Ingelheim, Forest and GlaxoSmithKline. EK has served on advisory boards and speaker panels for, and/or received travel reimbursement from AstraZeneca, Forest, Ironwood, Merck, Mylan, Novartis, Pearl, Pfizer, Sanofi Aventis, Sunovion, and Targacept. He has conducted multicentre clinical research trials for approximately seventy pharmaceutical companies. AC, GC, MT, NR and SH are employees of GlaxoSmithKline and hold stocks/shares in GlaxoSmithKline.
· This study was sponsored by GlaxoSmithKline (ClinicalTrials.gov: NCT01316913, protocol number DB2113374). Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Stuart Wakelin, PhD, of FWG Scientific Communications, which was funded by GlaxoSmithKline.
|
|
Placeholder |
|
|
for QR code |

Presented at the European Respiratory Society (ERS) Annual Congress, Barcelona, Spain, 7–11 September, 2013
