Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - LA JOLLA PHARMACEUTICAL CO | d539927dex992.htm |
| 8-K - FORM 8-K - LA JOLLA PHARMACEUTICAL CO | d539927d8k.htm |
 Pioneering Innovative Therapies to Treat
Chronic Organ Failure
George F. Tidmarsh, MD, PhD
Chief Executive Officer
May 2013
Exhibit 99.1 |
 Forward-Looking Statements
These slides contain "forward-looking" statements within the meaning
of the Private Securities Litigation Reform Act of 1995. These
statements may be identified by the use of forward looking terminology
such as "anticipate", "believe", "continue",
"could", "estimate", "expect", "intend",
"may", "might", "plan", "potential", "predict", "should" or "will"
and include statements regarding La Jolla Pharmaceutical’s product
candidates and clinical trial progress and results. These forward-
looking statements are based on our current expectations, speak only
of the date of this presentation and involve risks and uncertainties,
many of which are outside of our control, that can cause actual results
to differ materially from those in the forward-looking statements.
Potential risks and uncertainties include, but are not limited to, our
ability to complete our anticipated clinical trials, the time and expense
required to conduct such clinical trials, the ability to manufacture clinical
or commercial product, issues arising in the regulatory process and the
results
of
such
clinical
trials
(including
product
safety
issues
and
efficacy results). Further information is included in La Jolla’s periodic
reports filed with the SEC at www.sec.gov. We
disclaim any duty to update any forward-looking statements.
2 |
 An
Introduction to La Jolla (LJPC) The Technology of La Jolla
GCS-100 and Organ Failure
GCS-100’s History in the Clinic
Phase 1/2 Clinical Trial
Patents and Financials
3 |
 Mission
La Jolla Pharmaceutical: Reducing Human Suffering
Novel Therapies
Harnessing the Immune System
To Treat Life-Threatening Diseases
4 |
 Corporate Highlights
Corporate Highlights
5
Technology Background
Pipeline
Experienced Management
•
George F. Tidmarsh, MD, PhD, Chief Executive Officer
Milestones
•
Near-term, cost-efficient Phase 2 clinical milestones to drive value
Stanford University MD/PhD, Associate Professor, Pediatrics and Neonatology
3 FDA approved drugs
Founder Horizon Pharma (HZNP: NASDQ:GM), Threshold Pharmaceuticals (THLD:
NASDAQ: GM)
•
Immune therapy platform targeting Galectin-3, an innate protein with a
demonstrated role in organ failure via immune regulation
•
Product candidate GCS-100: the leading, clinical stage galectin-3
antagonist May prevent or reverse organ failure by reducing fibrosis via
neutralization of galectin-3 Phase 1 and 2 clinical safety data with
evidence for activity improving kidney function |
 Robust Product Pipeline
Robust Product Pipeline
6 |
 An
Introduction to La Jolla The Technology of La Jolla
GCS-100 and Organ Failure
GCS-100’s History in the Clinic
Phase 1/2 Clinical Trial
Patents and Financials
7 |
 Galectins and Galectin-3
Galectins and Galectin-3
3
8
•
Galectins are proteins that can
bind to specific sugars on other proteins
(receptors) to modulate cellular function
and communication.
•
Galectin-3 is unique in that it can self-associate allowing it to bind
to several receptors at once.
•
Galectin-3 is normally present at low concentration, but is up-regulated in
organ failure and cancer. |
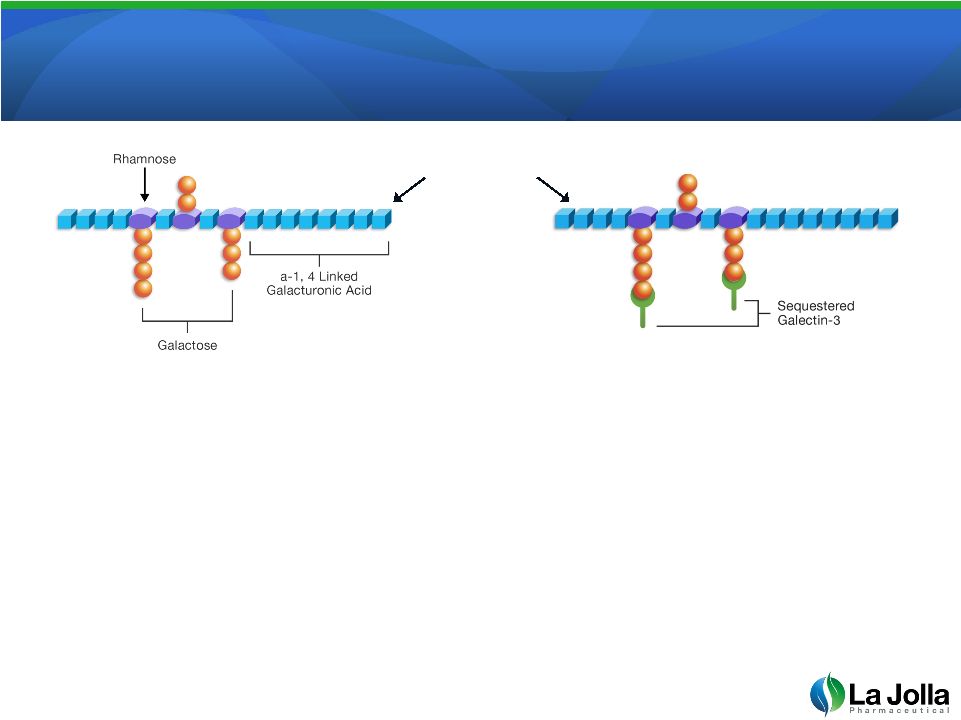 GCS-100: The Leading Galectin-3 Antagonist
GCS-100: The Leading Galectin-3 Antagonist
•
GCS-100 is a well-characterized, complex sugar derived from
pectin
•
GCS-100 binds to and neutralizes galectin-3. Binding activity
is localized to the galactose
containing side-branches
•
Patented manufacturing process required for biologic activity;
unmodified pectin has reduced biologic activity
9
GCS-100 |
 An Introduction to La Jolla
The Technology of La Jolla
GCS-100 and Organ Failure
GCS-100’s History in the Clinic
Phase 1/2 Clinical Trial
Patents and Financials
10 |
 Galectin-3 and Organ Failure
Galectin-3 and Organ Failure
Kidney, Heart, Liver
Kidney, Heart, Liver
•
Mice lacking galectin-3 develop significantly less kidney fibrosis and
failure
after
damage
compared
to
normal
mice
1,2
•
Mice lacking galectin-3 develop significantly less liver fibrosis when
exposed
to
toxin
•
Galectin-3 serum assay is FDA approved to identify patients at risk for
death
due
to
heart
failure
4
•
Serum galectin-3 levels identify patients with end-stage renal disease
who
are
at
highest
risk
for
death
5
•
Higher galectin-3 levels in normal individuals is associated with reduced
survival
6
11
1
The American Journal of Pathology, 2008, Vol. 172, No. 2: 288-298.
2
Transplantation International, 2008, Vol. 21, No. 10: 999-1007.
3
Proceedings
of
the
National
Academy
of
Sciences,
2006,
Vol.
103,
No.
13:
5060-5065.
4
Annals of Medicine, 2011, 43: 60–68.
5
Galectin-3 and Outcomes in Patients with End-Stage Renal Disease: Data from
the German Diabetes and Dialysis Study, presented by Rudolf de Boer, MD, PhD, Associate
Professor of Cardiology at the University of Groningen, the Netherlands; American
Heart Association Scientific Presentation, November 2011
6
Journal of Internal Medicine, 2012, 272; 55-64.
3 |
 Galectin-3 in the General Population
Galectin-3 in the General Population
12 |
 Galectin-3 in the General Population
Galectin-3 in the General Population
•
High galectin-3 is independently associated with lower
survival in the general population
13
Journal of Internal Medicine, 2012, 272; 55-64.
|
 Galectin-3: Promotes Organ Failure
Galectin-3: Promotes Organ Failure
via Scar Formation
via Scar Formation
•
Mice genetically altered to lack
galectin-3 produce much less
scar tissue in the kidney after
injury.
Normal (wild-type, WT) mice or
galectin-3 knockout mice were
either left alone (-) or surgically
injured by obstructing the outflow
of urine from the kidney (UUO).
The amount of collagen and
procollagen
produced is a
measure of scar formation. As
indicated, galectin-3 knockout
mice produced much less
scaring.
14
The American Journal of Pathology, 2008; Vol. 172, No. 2: 288-298.
|
 Chronic Kidney Disease Market
Chronic Kidney Disease Market
•
49 million Americans
suffer with CKD
1
•
$7.1 billion spent on
CKD therapeutics in
2010
2
•
Market growth of 6.4%
CAGR over the next 5
years
2
15
1
The United States Renal Data System, 2012 Annual Data Report
2
$7.1
$11.7
$7.0
$8.0
$9.0
$10.0
$11.0
$12.0
Chronic Kidney Disease (CKD) Therapeutics - Pipeline Assessment and Market Forecasts to 2018,
Globaldata, September 2011 |
 Liver Disease Market
Liver Disease Market
16
1
The National Institute of Diabetes and Digestive and Kidney Diseases
2
Liver
Disease
Treatments:
The
Global
Market,
January
1,
2012
BCC
Research
•
17.5 million
Americans suffer
with CLD
•
$12.4 billion spent on
CLD therapeutics in
2010
•
CLD market growth
of 3.3% CAGR
expected until 2017
1
2
2 |
 GCS-100 is Effective in Liver Fibrosis:
GCS-100 is Effective in Liver Fibrosis:
Stelic NASH Model
Stelic NASH Model
17
1
Boehringer Ingelhiem GmbH & Co. KG
2
Galectin Therapeutics
Effect of GCS-100 in liver fibrosis was studied by an
independent, contract research group
The 61
st
annual meeting of the American Association for the Study of Liver Diseases
(AASLD 2011), “The Dipeptidyl Peptidase-4
Inhibitor Linagliptin is an Effective Therapeutic
for
Metabolic
Liver
Disease
in
Several
Rodent
Models
of
Non-Alcoholic
Fatty
Liver
Disease
(NAFLD)
and
Non-Alcoholic
Steatohepatitis
(NASH)”
1
European
Association
for
the
Study
of
the
Liver
(EASL)
Special
Conference
–
Liver
Transplantation 2011, “Improvement of steatosis, inflammation, and
fibrosis in a mouse model
of
steatohepatitis
following
treatment
with
galectin
inhibitor”
2
EASL The International Liver Congress
TM
2001 –
46
th
Annual Meeting of the European
Association for the Study of the Liver, “Novel FXR agonists with
potent lipid lowering, insulin sensitizing, anti-inflammatory and
anti-fibrotisation effects in mouse models of metabolic syndrome and
NASH” The 84
th
Annual Meeting of the Japanese Pharmacological Society, “Effects of
telmisartan against novel non-alcoholic steatohepatitis model in
mice” |
 Stelic NASH Model
Stelic NASH Model
18
Treatment
Control
GCS-100 1 mg/kg
GCS-100 25 mg/kg
Endpoints
Pathology
Serum Chemistry
NAFLD Score
Fibrosis |
 GCS-100 Treatment Reduced Liver Fibrosis
GCS-100 Treatment Reduced Liver Fibrosis
19
Each circle represents
data from one mouse. |
 GCS-100 Treatment Reduced Liver Fibrosis
GCS-100 Treatment Reduced Liver Fibrosis
20 |
 GCS-100 Improved Gross Pathology
GCS-100 Improved Gross Pathology
21
Vehicle
GCS-100 |
 GCS-100 Effect of NASH: Summary
GCS-100 Effect of NASH: Summary
•
Significant reduction in fibrosis as measured by Sirius Red
staining
•
Significant reduction in NAFLD score
•
Significant reduction in plasma ALT
•
No change in:
Body weight
Serum glucose
Liver weight
Liver hydroxyproline
Collagen mRNA
22 |
 An Introduction to La Jolla
The Technology of La Jolla
GCS-100 and Organ Failure
GCS-100’s History in the Clinic
Phase 1/2 Clinical Trial
Patents and Financials
23 |
 GCS-100 Clinical Summary
GCS-100 Clinical Summary
•
179 patients dosed in 10 Phase 1 and Phase 2 clinical trials
•
Pharmacokinetic parameters established
Single-
and
multiple-dose
administration
of
30-160
mg/m
Effective half-life in blood of 36 hours
•
Evidence of positive effect on renal function
24
2 |
 Source of Patients in Retrospective Analysis
Source of Patients in Retrospective Analysis
25
Study No.
Design
Treated Population
Treatments
# Patients with
eGFR <60
PR-CS008
Phase 2
24 CLL
160 mg/m
2
days 1-5
8
GLY-101-01
Phase 1
24 subjects various solid
tumors
30-200 mg/m
2
days 1-5
4
GCS-100-01-001
Phase 1
12 subjects various solid
tumors
30-80 mg/m
2
x2/week
2
GBC-590-II-001
Phase 2
20 subjects pancreatic cancer
20 mg/m
2
x2/week
2
GBC-590-II-002
Phase 2
23 subjects advanced CRC
20 mg/m
2
x2/week
3
C96-002-01
Phase 1
22 various solid tumors
1.9-20 mg/m
2
x2/week
5
C96-001-01
Phase 1
13 various solid tumors
1.9-13 mg/m
2
x2/week
1
Totals
138
25 |
 Low
Dose GCS-100 Increases Renal Low Dose GCS-100 Increases Renal
Function
Function
26
17 patients treated with low dose GCS-100
GCS-100 |
 GCS-100 Clinical Summary
GCS-100 Clinical Summary
•
Well tolerated and tolerable side effects based on 179 patients
treated to date
Principle side effects: mild rash, joint pain, muscle pain
•
Dosing exposure up to 1000 mg/m per week
•
Evidence for improving kidney function
27
2 |
 An Introduction to La Jolla
The Technology of La Jolla
GCS-100 and Organ Failure
GCS-100’s History in the Clinic
Phase 1/2 Clinical Trial
Patents and Financials
28 |
 Clinical Trial Rational
Clinical Trial Rational
•
Galectin-3 is increased and the level correlates to overall
survival in end-stage renal disease patients
•
Galectin-3 knockout mice develop less kidney scar after injury
•
Retrospective analysis shows low dose GCS-100 improves
renal function in patients
•
Kidney disease patients have a high mortality rate and
biomarkers correlate with survival
•
Patients with chronic kidney disease have no existing
therapeutic options to reverse their disease
29 |
 CKD
Phase 1/2 Trial CKD Phase 1/2 Trial
Phase 1 Portion Complete
Phase 1 Portion Complete
30
Part A
Day
-21
Day
-8
Day
0
Day
7
Screening
Visit 1
Screening
Visit 2
Each cohort n=3 patients
(up to 6 cohorts)
GCS-100
1.5-20 mg/m²
IV
D –
-21,-8
Data collected on:
Blood gal-3, Cytokine, Serum
chemistry, Quantitative urine
analysis, and eGFR rate
Data Collection Point
Dosing Point
Day
14
D –
7,14
D –
0,2,5
Evaluation for
dose escalation
Key |
 Proposed Phase 2a CKD Trial
Proposed Phase 2a CKD Trial
31
Day
0
Day
7
Day
49
Day
56
D –
0,7
GCS-100
PBO, 1.5 & 20 mg/m²
IV
Data collected on:
Blood gal-3, Cytokine, Serum
chemistry, Quantitative urine
analysis, and eGFR rate
Data Collection Point
Dosing Point
Key
Day
14
Day
28
Day
35
Day
21
D –
14,21
D –
28,35
D –
42,49
D –
56
Screening
Visits 1 & 2
n=36 1.5 mg/m
2
/min
n=36 20 mg/m
2
/min
n=36 Placebo
N=108 Randomized
Day
42
Expanded Phase 2/Phase 2a |
 GCS-100 in CKD Status
GCS-100 in CKD Status
•
Phase 1 Complete
4 leading sites in the US
Enrolled 29 patients in 3 months
•
Accelerated path to Phase 2 Data
Randomized, 3 arm study proposed
–
Endpoints:
-
Change in renal function compared to placebo
Protocol currently under review at FDA
32 |
 An Introduction to La Jolla
The Technology of La Jolla
GCS-100 and Organ Failure
GCS-100’s History in the Clinic
Phase 1/2 Clinical Trial
Patents and Financials
33 |
 Title
Status
Expiration
Modified Pectins, Compositions and
Methods Related Thereto
Issued
US 8,128,966
2028
Modified Pectins, Compositions and
Methods Related Thereto
Issued
US 8,187,642
2025
Modified Pectins, Compositions and
Methods Related Thereto
Pending
US 13/588,932
2025
Modified Pectins, Compositions and
Methods Related Thereto
Issued
US 13/588/877
2025
Modified Pectins, Compositions and
Methods Related Thereto
Pending
US 13/400,007
2025
Compositions and Uses of Galectin
Antagonists
Pending
US 11/803,150
2027
Intellectual Property Position
Intellectual Property Position
34 |
 Assets
Assets
35
March
31, 2013
December
31, 2012
Assets
Cash and Cash equivalents
2,700
3,405
Restricted cash
37
–
Prepaids and other current
assets
76
25
Total current assets
2,813
3,430
Total Assets
2,813
3,430 |
 Liabilities, Shareholders’
Liabilities, Shareholders’
Equity
Equity
36
March 31,
2013
December 31,
2012
Current Liabilities:
Accounts payable
69
92
Accrued expenses
176
107
Accrued payroll and related expenses
26
17
Total current liabilities
271
216
Stockholders’
equity:
Common stock
2
1
Preferred stock
10,888
10,907
Additional paid-in capital
443,221
439,672
Accumulated deficit
(451,569)
(447,366)
Total stockholders’
equity (deficit)
2,542
3,214
Total liabilities, preferred stock, and stockholders equity
2,813
3,430 |
 Stock Information
Stock Information
Stock Price
$0.08
Shares Outstanding
30,486,228
Market Cap
$2,438,898
Price Range
(52 Week)
$0.04 –
$0.14
Avg. Volume
215,857
37
33.3% Gain in stock price since 12/31/12
& up 100% gain on intra day trading
$0.050
$0.070
$0.090
54.0%
Outstanding Common Stock
Public Float
Non-Affiliates
Directors, Officers & Management |
 Robust Product Pipeline
Robust Product Pipeline
38 |
 Corporate Highlights
Corporate Highlights
Technology Background
Pipeline
May prevent or reverse organ failure by reducing fibrosis via
neutralization of galectin-3
Phase 1 and 2 clinical safety data with evidence for activity improving
kidney function
Management
Experienced and driven
Milestones
Near-term, cost-efficient Phase 2 clinical milestones to drive value
39
•
Immune therapy platform targeting Galectin-3, an innate protein with a
demonstrated role in organ failure via immune regulation
•
Product candidate GCS-100: the leading, clinical stage galectin-3
antagonist |
 Thank You |
