Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - LifeMD, Inc. | v339577_ex32-1.htm |
| EX-31.1 - EXHIBIT 31.1 - LifeMD, Inc. | v339577_ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2012
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-34246
IMMUDYNE, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 76-0238453 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
50 Spring Meadow Rd.
Mount Kisco, NY 10549
(Address of principal executive offices)
Registrant’s telephone number, including area code:
(914) 244-1777
Securities registered pursuant to Section 12(b) of the Act:
None.
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ¨ No þ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended (“Exchange Act”) during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company þ | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ¨ No þ
The aggregate market value of voting common stock held by non-affiliates computed by reference to the price at which the common stock was last sold on June 30, 2012, was $3,669,254. Accordingly, effective as of June 30, 2012, the registrant’s aggregate market value was less than $50 million and the registrant qualifies for “smaller reporting company” status under Rule 12b-2 of the Exchange Act and is subject to the disclosure requirements and filing deadlines for smaller reporting companies.
As of March 28, 2013, there were 28,909,973 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
None.
IMMUDYNE INC.
Table of Contents
| Page | ||
| PART I | ||
| Item 1. | Business | 1 |
| Item 1A. | Risk Factors | 5 |
| Item 1B. | Unresolved Staff Comments | 15 |
| Item 2. | Properties | 15 |
| Item 3. | Legal Proceedings | 15 |
| Item 4. | Mine Safety Disclosures | 15 |
| PART II | ||
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 16 |
| Item 6. | Selected Financial Data | 16 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 16 |
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk | 20 |
| Item 8. | Financial Statements and Supplementary Data | 21 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 21 |
| Item 9A. | Controls and Procedures | 21 |
| Item 9B. | Other Information | 22 |
| PART III | ||
| Item 10. | Directors, Executive Officers and Corporate Governance | 22 |
| Item 11. | Executive Compensation | 24 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 26 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 27 |
| Item 14. | Principal Accounting Fees and Services | 30 |
| PART IV | ||
| Item 15. | Exhibits, Financial Statement Schedules | 30 |
| Financial Statements | F-1 | |
| Signatures | 32 | |
NOTE ABOUT FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act regarding our company that include, but are not limited to, any projections of earnings, revenue or other financial items; any statements of the plans, strategies and objectives of management for future operations; any statements concerning proposed new products, services or developments; any statements regarding future economic conditions or performance; any statements of belief; and any statements of assumptions underlying any of the foregoing. These forward-looking statements are based on our current expectations, estimates and projections about our industry, management’s beliefs and certain assumptions made by us. Words such as “anticipates,” “expects,” “intends,” “plans,” “predicts,” “potential,” “believes,” “seeks,” “hopes,” “estimates,” “should,” “may,” “will,” “with a view to” and variations of these words or similar expressions are intended to identify forward-looking statements. These forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and assumptions that are difficult to predict. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our actual results could be materially different from our expectations. Important risks and factors that could cause our actual results to be materially different from our expectations are generally set forth in “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Our Business” and other sections in this report. Other sections of this report include additional factors that could adversely impact our business and financial performance.
Unless otherwise indicated, information in this report concerning economic conditions and our industry is based on information from independent industry analysts and publications, as well as our estimates. Except where otherwise noted, our estimates are derived from publicly available information released by third party sources, as well as data from our internal research, and are based on such data and our knowledge of our industry, which we believe to be reasonable. Unless otherwise indicated, none of the independent industry publication market data cited in this report was prepared on our or our affiliates’ behalf.
The forward-looking statements made in this report relate only to events or information as of the date on which the statements are made in this report. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this report and the documents we refer to in this report and have filed as exhibits to this annual report completely and with the understanding that our actual future results may be materially different from what we expect.
Additional information on the various risks and uncertainties potentially affecting our operating results are discussed in this report and other documents we file with the Securities and Exchange Commission, or the SEC, or upon written request to Immdyne, Inc., 50 Spring Meadow Road, Mount Kisco, NY 10549. We undertake no obligation to revise or update publicly any forward-looking statements for any reason, except as required by law. Given these risks and uncertainties, readers are cautioned not to place undue reliance on these forward-looking statements.
As used in this report, “Immudyne,” “Company,” “we,” “our” and similar terms refer to Immudyne Inc., unless the context indicates otherwise.
PART I
Item 1. Business
Our Company
We manufacture, distribute and sell natural immune support products. We believe, based on testing and analysis conducted on our behalf, that our beta glucans derived from yeast are superior to other beta glucans. Beta glucans are a natural extract that has been shown through testing and analysis and scientific research to support the immune system.
Our core nutraceutical and cosmetic product lines are the focal points of our business. Our beta glucan products and manufacturing processes are protected by patents and trade secrets, and are compliant with current good manufacturing practices (“GMPs”). Further, yeast beta glucans are classified as generally recognized as safe (“GRAS”) by the Food and Drug Administration (“FDA”). None of the testing and analysis or scientific research mentioned in this annual report, however, has been subject to the oversight of the FDA or any comparable regulatory body, and no regulatory body has attested to the efficacy of beta glucans in supporting the immune system or otherwise treating disease. Further, the marketing of beta glucans are not subject to FDA approval, and we are prohibited by Federal Trade Commission (“FTC”) and FDA regulations from suggesting in advertisements and product labels that our products mitigate, treat, cure or prevent a specific disease or class of disease.
Historically, we have sold our products primarily on a word-of-mouth basis through distributors and our website as standalone product lines, as well as business-to-business as a cosmetic product, dietary supplement or feed additive for animal use. We believe that we are well positioned to capitalize on our development of proprietary and patented natural immune support products and can now focus on commercializing sales of these products on a more meaningful, global basis.
We originally incorporated under the laws of British Columbia, Canada, in 1987 under the name Anina Resources, Inc. and subsequently changed our name to Immudyne, Inc. and our jurisdiction to the State of Wyoming by continuance in September 1987. On June 30, 1994, we changed our jurisdiction to Delaware by merger with and into Immudyne, Inc., a Delaware corporation formed on June 21, 1994.
Our Products
We have developed a proprietary approach to produce, what we believe to be, superior beta glucans derived from yeast. Our yeast beta glucan is odorless and tasteless, making it suitable for use in a wide variety of oral and topical applications. Yeast beta glucans are classified as GRAS by the FDA. As the U.S. and international markets become more aware of the value of our proprietary products, we believe demand for our beta glucans will increase. Our nutraceutical and cosmetic product lines consist of beta glucan products for oral and topical applications. Historically, we produced other grades of beta glucan products for the animal feeds industry as a substitute for antibiotics. As of 2011, we began exiting this lower-margin market for feed-additive products. Our sales and marketing efforts going forward are concentrated on our oral and topical-use products for healthcare professionals, distributors and direct-to-consumer sales.
Beta Glucans
Beta glucans, or β-Glucans, are a natural extract shown through scientific research to be “biological response modifiers” that support the immune system. The potential benefits of beta glucans to human health continue to emerge. Independent scientific research has demonstrated that beta glucans provide defense against bacteria by activating innate immune cells, which fight off infection. In addition, a growing body of scientific literature suggests that beta glucans have a substantial effect on cancer regression, though such literature has not been attested to by the FDA or any comparable regulatory authority. The most common sources of beta glucans are derived from the cell walls of baker’s yeast, the cellulose in plants, the bran of cereal grains and certain fungi and bacteria. The differences between beta glucan chemical structures are significant in regards to solubility and overall biological activity. Beta glucans derived from mushrooms and cereals do not appear to have the same effects on human health as beta glucans derived from yeast.
We derive our high-grade beta glucan from yeast cell walls using proprietary processes in our manufacturing facilities. Our beta glucan is free of yeast by-product and endotoxins, and demonstrates reliability in terms of both stability and biological response. In fact, we commissioned an analytical side-by-side comparison by V-Labs Inc., a laboratory which conducts testing and analysis of nutraceutical compounds, between our beta glucan and each of the beta glucans manufactured by our two main competitors, which demonstrated the superiority of our beta glucan. This side-by-side comparison found that our beta glucan was far less impure and more uniform in composition than those of our competitors and thus had a more predictable immune support potential.
Healthcare professionals, including licensed physicians, alternative medicine practitioners, scientists and researchers have taken an interest in our immune-support products as a means of offering alternative or complementary approaches for maintaining a healthy and active lifestyle. These expressions of interests have often resulted in proposals for research studies and recommendations of our products. We plan to build upon this interest and hope to grow our contacts with licensed physicians who utilize our product in a clinical setting and with researchers who have the resources to conduct testing and analysis on our products.
| 1 |
The health benefits of yeast beta glucans have been demonstrated through extensive testing and analysis and scientific research on yeast beta glucans generally, and we are committed to supporting evidence-based studies that demonstrate the health benefits of our products. General scientific research on beta glucan derived from yeast cell walls has been conducted in recent years by renowned medical laboratories, including Baylor College of Medicine, U.S. Armed Forces Radiobiology Institute, Stanford University, Southwest Research Institute, Case Western Reserve University, University of Arkansas, North Carolina State University, University of Bern, Switzerland, and the China Agricultural University, China.
We also have relationships with medical doctors who in the past have conducted self-funded studies in which we supplied our product free of charge. We do not have any formal clinical development or research agreements with these institutions and doctors. Dr. Joseph DiTrolio of the Roseland, New Jersey surgery center and St. George’s University School of Medicine and Dr. Allan Whitberg, previously of Roger Williams Medical Center in Providence, Rhode Island and currently affiliated with Boston University School of Medicine, are among the medical doctors with whom we have relationships. We also have established a relationship with National Jewish Health in Denver, Colorado and are exploring possible opportunities to have our products tested at the Boston University Medical Center. Additionally, in two studies reviewed in an article published in the Japanese Journal Society Terminal Systemic Diseases in 2000, participants ingesting our yeast beta glucan showed a decreased reoccurrence of cancer in cancer remission patients and increased survival rates in terminally-ill cancer patients. In addition, female study participants testing the effect of our yeast beta glucan in topical applications saw a significant reduction in the number and intensity of skin wrinkles over the eight-week study. We presented the results of these and other studies supporting the efficacy of our products at the BIO-Europe global biotechnology conference in November 2010 and World Immune Regulator Meeting in Davos, Switzerland, in March 2012.
None of the testing and analysis or scientific research mentioned in this annual report has been subject to oversight of the FDA or any comparable regulatory body, and no regulatory body has attested to the efficacy of beta glucans in supporting the immune system or otherwise treating disease. Further, the marketing of beta glucans are not subject to FDA approval, and we are prohibited by FTC and FDA regulations from suggesting in advertisements and product labels that our products mitigate, treat, cure or prevent a specific disease or class of disease.
Yeast Beta Glucan Product Lines
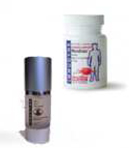 |
|
| Our MacroForce Plus once-daily oral capsules and Skin Care Essentials line of rejuvenating serums and creams. |
Our nutraceutical and cosmetic product lines consist of our all-natural, premium yeast beta glucans in oral and topical applications shown through testing and analysis and scientific research to support the immune system. Neutraceuticals are plant-derived products, such as our yeast beta glucans, with pharmaceutical-like properties that have biologically-therapeutic effects in humans and animals in addition to the basic nutritional value found in foods. Further, yeast beta glucans are classified as GRAS by the FDA and our products are designed to help support the body’s immune system response and defense. We offer our yeast beta glucans as all-natural raw material ingredients in bulk quantities and finished, consumer products packaged under our brands and private label brands.
Our principal, branded nutraceutical and cosmetic products for our yeast beta glucans are the MacroForce line of once-daily oral capsules and Skin Care Essentials line of rejuvenating serums and creams. Our MacroForce line of once-daily oral capsules are dietary supplements containing proprietary combinations of our yeast beta glucan to support immune system function. Our Skin Care Essentials Rejuvenating Serum and Rejuvenating Cream topical applications consist of our patented yeast-derived beta glucan and other natural ingredients to support the skin’s immune system response and defense, skin renewal and repair of sun and environmental damage.
Sales and Marketing
Our sales and marketing strategy is to build brand recognition of our products as superior beta glucans derived from yeast. Historically, we have sold our products primarily on a word-of-mouth basis through distributors and our website as standalone product lines, as well as business-to-business as a cosmetic enhancement, dietary supplement or feed additive for animal use. We have shifted our sales focus to concentrate on our nutraceutical and cosmetic product lines. We believe that we are well positioned to capitalize on our development of proprietary and patented products and can now focus on commercializing sales of these products on a more meaningful, global basis. Our principal products are consumables that can generate a stream of repeat sales with the same end customers over an extended period, providing significant lifetime value for each customer gained. To reach these customers, we anticipate that our marketing strategy will include online sales promotions through our website, commencing an affiliate sales program, trade advertising, consumer research and search engine and digital advertising. In addition, we intend to build our brand recognition with healthcare professionals through testing and analysis and providing practitioners and clinics with education and support. We believe that the recommendation of our products by healthcare professionals to their patients provides the best possible endorsement.
We have appointed Karen Kingston, a distinguished and experienced marketing consultant with over 15 years of experience building brands and sales strategies within men's health, eye care, skin care, immunology and wellness industries, as our Chief Marketing Officer to lead our sales and marketing efforts.
| 2 |
We also market our products by presenting at international biotechnology and alternative medicine conferences and through nutraceutical industry associations and tradeshows. In March 2012, we presented the outcome of our cancer studies at the World Immune Regulator Meeting in Davos, Switzerland. The special focus of the meeting was on innate and adaptive immune response and the role of tissues in immune regulation. In November 2010, we presented the results of the studies demonstrating the efficacy of our yeast beta glucan in terminally ill cancer patients and cancer remission patients at the BIO-Europe biotechnology partnership conference in Munich, Germany.
Manufacturing and Sourcing
Over the past 20 years, we have focused on the production of immune system support compounds, including, what we believe to be, are superior beta glucans derived from yeast. Our beta glucan products and manufacturing processes are protected by registered and pending patents and trade secrets. Our highly trained and experienced staff produces consistently high-grade, particulate and reliable beta glucan, including the raw materials for our nutraceutical and cosmetic product lines, in our Kentucky-based production plant. Our manufacturing facilities and practices are compliant with published current GMPs established by the FDA for dietary supplements. For certain of our packaged consumer goods, such as the MacroForce product line, we use third party contractors for encapsulation, bottling and labeling. These contractors are subject to regular government inspections, comply with current GMPs and hold the necessary drug manufacturing licenses and processed food registrations required by their respective state regulators. Such packaging services are readily available from multiple sources.
The raw materials necessary to manufacture beta glucans in our Kentucky plant, principally consisting of baker’s yeast, are common and readily available. Our principal suppliers are the Lesaffre Group and Brenntag Group. We hold our suppliers to strict quality and delivery specifications as part of our GMP compliance and quality control procedures, including quality assurance of raw materials used in the production of our products.
Customers
We sell our products direct to consumers and to pharmaceutical, nutraceutical and consumer product companies in the U.S. market, and plan to expand our sales to the international market. Sales through distributors and business-to-business typically are made pursuant to supplier agreements executed in the ordinary course of business with individual orders made on standard purchase orders. We focus on establishing and growing long-term relationships with our customers, and we believe that the majority of our customers view us as a strategic long-term supplier and value the quality of our beta glucan products.
As we seek to expand our U.S. and global sales, our marketing concentrates on our nutraceutical and cosmetic product lines as a raw material ingredient available in bulk quantities and finished, consumer products packaged under our brands and private label brands. We anticipate that our customer mix will change accordingly to include more sales through distributors, and our planned affiliate program, and direct sales to consumers. We encourage nutriceutical and nutricosmetic manufacturers and formulators purchasing our beta glucans as all-natural raw material ingredients to identify and promote our brand in their products. As our principal products are consumables that typically generate repeat sales streams with the same end customers over an extended period, we historically have not experienced seasonality of sales.
Sales of our oral and topical-use products consisted of 99% of our sales in 2012, 80% in 2011, and 38% in 2010. Sales of our animal feed additive products consisted of 1% of our sales in 2012 20% in 2011, and 62% in 2010. We anticipate further decreased sales of our feed-additive products for animals as a percentage of our overall sales as we change our product focus to our oral and topical-use products. Our largest customer, Michel Mercier Products, Inc. (d/b/a M.M.P, Inc.), accounted for 73% our sales in 2012, 53% in 2011, and 23% in 2010.
Competition
The markets for nutritional supplements and skin care products are highly competitive, consisting of a large number of manufacturers, distributors and retailers, none of which dominates the fragmented and diverse markets. We compete for sales direct to consumers, through distributors and business-to-business.
Although we believe that our yeast beta glucan is superior, we compete with other companies manufacturing beta glucans from yeast and other sources, as well as companies producing other food ingredients and nutritional supplements for human use and as feed additives. Many end consumers may consider such products to be a replacement for the products we manufacture and distribute. Many of our competitors have greater marketing, research and capital resources than us, and may be able to offer their products at lower costs because of their greater purchasing power or lower cost of raw materials and manufacturing.
| 3 |
We anticipate expanding our sales globally on a meaningful basis as part of our new marketing strategy focusing on our nutraceutical and cosmetic product lines. We believe that we are well positioned to capitalize on our development of proprietary and patented products to compete in the immune support markets. We anticipate competing in these markets on the basis of quality, our proprietary manufacturing processes, research data and effective marketing campaigns promoting the benefits of our natural immune support products. There are no assurances that our products will be able to compete in these markets, however, or that our new marketing strategy will be successful.
Intellectual Property
We rely on the patent and trademark protection laws in the U.S. to protect our intellectual property and maintain our competitive position in the marketplace. We have six registered U.S. patents, consisting of both use and process patents, with expiration dates ranging from 2013 to 2028. Additionally, we currently have two patent applications filed in the U.S. pending formal review and a second provisional patent application pending, and we intend to apply for additional patents in the future as new products, uses and manufacturing processes are developed. We maintain trademarks registered in the U.S. for our business name and related to our product brands. In addition, we have registered and maintain internet domain names related to our business, including “immudyne.com.” Collectively, the patents, trademarks and domain names that we hold are of material importance to us.
Research and Development
Our expertise for many years has been in the enhancement of efficient, stable and cost-effective production systems for beta glucan products derived from yeast, though we have not incurred any research and development expenses to date. We may increase future investments to establish the scientific basis for health claims of our existing products and to develop new products and applications based on our growth and available capital.
Governmental and Environmental Regulation
Our business and the manufacturing, distribution and sale of our beta glucan products are regulated in the U.S. primarily by the FDA and the FTC.
The FDA enforces the FDCA and Dietary Supplement Health and Education Act (“DSHEA”) as they pertain to foods, food ingredients, cosmetics and dietary supplement production and marketing. Dietary supplements and nutraceuticals are regulated as a category of food, not as drugs. The FDA classifies “Yeast extract (Bakers)” as GRAS, which substances by definition are not food additives. Most GRAS substances have no quantitative restrictions as to use, although their use must conform to current GMPs. The FDA promulgates GMP guidelines to ensure that dietary supplements are produced in a quality manner, do not contain contaminants or impurities and are accurately labeled. GMPs include requirements for establishing quality control procedures, designing and constructing manufacturing plants, testing ingredients and finished products and record keeping and handling of consumer product complaints. The FDA has broad authority to enforce the provisions of federal law applicable to dietary supplements and cosmetics, including the power to monitor claims made in product labeling, to seize adulterated or misbranded products or unapproved new drugs, to request product recall, to enjoin further manufacture or sale of a product, to issue warning letters and to institute criminal proceedings.
Advertising and product claims regarding the efficacy of products are also regulated by the FTC. The FTC regulates the advertising of dietary supplements and other health-related products to ensure that any advertising is truthful and not misleading, and that an advertiser maintains adequate substantiation for all product claims. FTC enforcement actions may result in consent decrees, cease and desist orders, judicial injunctions and the payment of fines with respect to advertising claims that are found to be unsubstantiated.
Yeast beta glucans are classified as GRAS by the FDA and our oral and topical-use product lines containing our yeast beta glucan are marketed as dietary supplements and cosmetics, respectively. Under current U.S. regulations, our products must comply with certain labeling requirements enforced by the FDA and FTC, but otherwise generally are not required to receive regulatory approval prior to introduction into the U.S. market. We believe we are in compliance with all material government regulations applicable to our products.
In the EU markets, the European Food Safety Authority (“EFSA”), an advisory panel to the European Commission, performs all scientific assessments of health claims on food and supplement labels. The European Commission will consider the opinions of EFSA in determining whether to include a health claim on a list of permissible claims. Once published, only health claims for ingredients and products included on the list may be used in promotional materials for products marketed and sold in the European Union. The marketability of our products may be limited as we look to expand our sales in the EU if the health claims of our products are not included on the list.
| 4 |
In addition to the foregoing, our operations are subject to federal, foreign, state and local government laws and regulations, including those relating to zoning, workplace safety and accommodations for the disabled, and our relationship with our employees is subject to regulations, including minimum wage requirements, anti-discrimination laws, overtime, working conditions and citizenship requirements. We currently do not incur any material costs in connection with our compliance with applicable environmental laws as our manufacturing processes generate minimal discharge. Furthermore, the cost of maintaining compliance with applicable environmental laws has not, and we believe, in the future, will not, have a material adverse effect on our business, results of operations and financial condition. We believe we are in substantial compliance with all material governmental regulations applicable to our operations.
Employees
As of December 31, 2012, we had 3 full-time employees and 11 part-time employees and consultants worldwide. We outsource many of our research, product development and marketing activities to consultants who provide services to us on a project basis. We believe that relations with our employees are satisfactory. We have no collective bargaining agreements with our employees. All full-time employees and our officers and directors are eligible to participate in our group health and dental insurance plans.
Item 1A. Risk Factors
Our business and an investment in our securities are subject to a variety of risks. The following risk factors describe the most significant events, facts or circumstances that could have a material adverse effect upon our business, financial condition, results of operations, ability to implement our business plan, and the market price for our securities. Many of these events are outside of our control. If any of these risks actually occurs, our business, financial condition or results of operation may be materially adversely affected. In such case, the trading price of our common stock could decline and investors in our common stock could lose all or part of their investment.
Risks Related to Our Business
We have generated losses and not yet achieved positive cash flows, which may adversely affect our liquidity and ability to continue as a going concern.
Our net cash used in operating activities was approximately $230,000 in 2012, and approximately $18,000 in 2011. We cannot assure you that we will be able to achieve revenue growth, profitability or positive cash flow, on either a quarterly or annual basis, or that profitability, if achieved, will be sustained. Our ability to meet our long-term business objectives likely will be dependent upon our ability to raise additional financing through public or private equity financings, establish increasing cash flow from operations or securing other sources of financing. We will need to reduce operating expenses and increase cash flow to fund current operations if we are not able to fund these operations by raising capital. We have implemented a new sales and marketing strategy to focus on higher-margin products with what we believe to be greater opportunities for growth in the U.S. and international markets. In addition, management has instituted cost-cutting measures; including terminating certain employees that were not contributing to the business and ceasing our operations in the low-margin feed additive product line, which we believe should result in improved efficiencies of our operations going forward. If our losses continue, however, our liquidity may be severely impaired, our stock price may fall and our shareholders may lose all or a significant portion of their investment.
We may not be able to implement our growth and marketing strategy successfully on a timely basis or at all.
Our future success depends, in large part, on our ability to implement our growth strategy of expanding distribution and sales of our beta glucan oral and topical application products, attracting new consumers to our brand and introducing new product lines and product extensions. Our ability to implement this growth strategy depends, among other things, on our ability to:
| · | enter into distribution and other strategic arrangements with other potential distributors of our all-natural raw material products; |
| · | increase our brand recognition; |
| · | expand and maintain brand loyalty; and |
| · | research new applications for existing products and develop new product lines and extensions. |
Our sales and operating results will be adversely affected if we fail to implement our growth strategy or if we invest resources in a growth strategy that ultimately proves unsuccessful.
If we fail to develop and maintain our brand, our business could suffer.
We believe that developing and maintaining our brand of what we believe to be superior beta glucans derived from yeast is critical to our success. The importance of our brand recognition may become greater as competitors offer more products similar to ours. Our brand-building activities involve increasing awareness of our brand, creating and maintaining brand loyalty and increasing the availability of our products. If our brand-building activities are unsuccessful, we may never recover the expenses incurred in connection with these efforts, and we may be unable to implement our business strategy and increase our future sales.
| 5 |
We are subject to government regulation of the processing, formulation, packaging, labeling and advertising of our consumer products, and any failure to comply with such regulations could require us to repackage, recall or undergo regulatory approval of our products, which would have a material adverse effect on our business.
Under the FDCA and DSHEA companies that manufacture and distribute foods, food ingredients, cosmetics and dietary supplements in the U.S., such as our yeast beta glucan products, are limited in the claims that they are permitted to make about nutritional support on the product label without the approval of the FDA. Any failure by us to adhere to the labeling requirements could lead to the FDA requiring that our products be repackaged and relabeled, which would have a material adverse effect on our business. In addition, advertising and product claims regarding the efficacy of products are also regulated by the FTC. Companies are responsible for the accuracy and truthfulness of, and must have substantiation for, any such statements. These claims must be truthful and not misleading. Statements must not claim to diagnose, mitigate, treat, cure or prevent a specific disease or class of disease. We are able to market our oral and topical application products in reliance on the self-affirmed GRAS status of our active ingredient, yeast beta glucan. No governmental agency or other third party has made a determination as to whether or not our products have achieved GRAS status. We make this determination based on independent scientific opinions that yeast beta glucan is not harmful under its intended conditions of use. If the FDA, another regulatory authority or other third party denied our self-affirmed GRAS status for our yeast beta glucan products, we could face significant penalties or be required to undergo the regulatory approval process in order to market our products. In such event, our business, financial condition and results of operations would be adversely affected. We cannot assure you that in such a situation our yeast beta glucan products would be approved.
The FDA’s current GMPs describe policies and procedures designed to ensure that dietary supplements are produced in a quality manner, do not contain contaminants or impurities, and are accurately labeled and cover the manufacturing, packaging, labeling and storing of supplements, with requirements for quality control, design and construction of manufacturing plants, testing of ingredients and final products, record keeping, and complaints processes. Those who manufacture, package or store dietary supplements must comply with current GMPs. If we or our suppliers fail to comply with current GMP procedures, the FDA may take enforcement action against us or our suppliers.
The processing, formulation, packaging, labeling and advertising of our yeast beta glucan products in the U.S. are subject to regulation by the FDA, FTC and other federal agencies, and our activities are also subject to regulation by various agencies of the states and localities in which our yeast beta glucan products are sold. Any changes in the current regulatory environment could impose requirements that would limit our ability to market our yeast beta glucan products and make bringing new products to market more expensive. In addition, the adoption of new regulations or changes in the interpretation of existing regulations may result in significant compliance costs or discontinuation of product sales and may adversely affect our business, financial condition and results of operations. While our yeast beta glucan products currently are categorized as foods, it is possible that the FDA or a state regulatory agency could classify these products as a cosmetic or a drug. If the products are classified as cosmetics rather than a food, we would be limited to making claims that the products cleanse and beautify, but we could not make structure or function claims. If our yeast beta glucan products are classified as drugs, we would not be able to market our products without going through the drug approval process. Either of these events would limit our ability to market our products effectively and cost-efficiently, and would adversely affect our financial condition and results of operations. If the FDA or a state regulatory agency viewed the products as cosmetics or drugs, they could claim that the products are misbranded and require that we repackage and relabel the products and impose civil and criminal penalties on us. Either or both of these situations could adversely affect our business and operations.
In the European Union, or the EU, markets, the European Food Safety Authority, or EFSA, an advisory panel to the European Commission, performs all scientific assessments of health claims on food and supplement labels. The European Commission will consider the opinions of EFSA in determining whether to include a health claim on the list of permissible claims. Once published, only health claims for ingredients and products included on the list may be used in promotional materials for products marketed and sold in the European Union. The marketability of our products may be limited as we look to expand our sales in the EU if the health claims of our products are not included on the list.
We have and will continue to subject our products to testing and analysis to ensure we are able to continue to deliver a superior product. If the findings of these trials are challenged or found insufficient to support our health claims, we may need to perform additional testing and analysis before we are able to successfully market such products.
Although our yeast beta glucan products are dietary supplements and cosmetics, as opposed to drugs, we have, and will continue to, subject our products to testing and analysis to ensure that we are able to continue to deliver a superior product so that we may successfully market such products, though no such trials are currently required for marketing approval by the FDA or any comparable regulatory body. Testing and analysis for new product uses can require a significant amount of resources and there is no assurance that the results will be favorable to the claims we make for our products, or that they will be sufficient to support our claims. If the findings of our testing and analysis are challenged or found to be insufficient to support our claims, additional testing and analysis may be required before we are able to successfully market our products. No such testing and analysis has been, nor will it be when conducted, subject to the approval by the FDA or any comparable regulatory body.
| 6 |
If we undertake product recalls or incur liability claims with respect to our yeast beta glucan products, such recalls or claims could increase our costs and adversely affect our reputation, business and results of operations.
Our yeast beta glucan products are designed for human and animal consumption and we may face product recalls or liability claims if the use of our products is alleged to have resulted in injury or death. However, to date, we have not (i) conducted any product recalls, (ii) received any product liability claims from third parties, or (iii) received any reports from an end consumer of any adverse effect resulting from our products. Yeast beta glucan is classified as a food ingredient and is not subject to pre-market regulatory approval in the U.S. Our yeast beta glucan products contain ingredients that do not have long histories of human or animal consumption. Previously unknown adverse reactions resulting from consumption of these ingredients could occur. We may have to undertake various product recalls or be subject to liability claims, including, among others, that our yeast beta glucan products include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. A product recall or liability claim against us could result in increased costs and could adversely affect our reputation with our customers, which, in turn, could have an adverse effect on our business, financial condition and results of operations.
We currently do not maintain product liability insurance coverage. Product liability insurance is expensive, is subject to deductibles and coverage limitations, and may not be available to us in the future. In addition, we cannot be sure that we will be able to obtain or maintain insurance coverage at acceptable costs or in a sufficient amount, that our insurer will not disclaim coverage as to a future claim or that a product liability claim would not otherwise adversely affect our business, financial condition and results of operations. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. Uncertainties resulting from the initiation and continuation of product liability litigation or other proceedings could have an adverse effect on our ability to compete in the marketplace. Product liability litigation and other related proceedings may also require significant management attention.
We derive a substantial part of our sales from one major customer. If we lose this customer or it reduces the amount of business it does with us, or if it fails to meet its obligations to us, our sales, financial condition and results of operations would be adversely affected.
Our largest customer, Michel Mercier Products, Inc. (d/b/a M.M.P, Inc.) (“MMP”), accounted for 73% of our sales in 2012, 53% in 2011, and 23% in 2010. Our relationship with MMP is governed by a written contract, which is subject to a confidentiality agreement. The initial term of our contract with MMP will expire on December 19, 2016. Pursuant to its terms, the written contract will be automatically renewed for continuous one-year periods unless either party gives notice of its intent to terminate at least 90 days prior to the expiration of any renewal term. If we lose this customer or they reduce the amount of business they do with us, our sales and profitability would be adversely affected. In addition, we are subject to credit risk due to concentration of our trade accounts receivables, and the inability of MMP to meet its obligations to us would adversely affect our financial results. At December 31, 2012, accounts receivable from MMP amounted to 32% of total accounts receivable, and at December 31, 2011 this customer accounted for 85% of accounts receivable. Although we are making efforts to reduce our dependency on a limited number of customers, we believe this concentration of sales to one customer will continue in the near future.
Many of our other customers buy from us under purchase orders, and we generally do not have agreements with or commitments from these customers for the purchase of our products. We cannot provide assurance that our smaller customers will maintain or increase their sales volumes or orders for the products supplied by us or that we will be able to maintain or add to our existing customer base. Decreases in our customers’ sales volumes or orders for products supplied by us may have an adverse effect on our business, financial condition or results of operations.
Our yeast beta glucan products face various forms of competition from other products in the marketplace, which could adversely affect our market share and result in a decrease in our future sales and earnings.
The pharmaceutical and biotechnology industries are characterized by intense competition, rapid product development and technological change. Most of the competition that our yeast beta glucan products face comes from companies that are larger, well established with greater financial, marketing, sales and technological resources than we have. Our products compete with a range of consumer and nutraceutical products. Our commercial success will depend on our ability to compete effectively in marketing and product development areas including, but not limited to, sales and branding, product safety, efficacy, ease-of-use, customer compliance, price, marketing and distribution. There can be no assurance that competitors will not succeed in developing and marketing products that are more desirable or effective than our products or that would render our products obsolete and non-competitive.
| 7 |
We may, in the future, be subject to risks of doing business internationally as we attempt to expand our sales through international consulting and distributor relationships.
We anticipate entering into international consulting and distributor agreements for our yeast beta glucan products. As a result, we expect to increase our revenues from international sales. A number of factors can prevent international sales, or substantially increase the cost of international sales, and we may encounter certain risks of doing business internationally including the following:
| · | increased government regulation of the processing, formulation, packaging, labeling and advertising of our consumer products for international markets; |
| · | reduced protection and enforcement for our intellectual property rights; |
| · | unexpected changes in, or impositions of, legislative or regulatory requirements that may limit our ability to sell our products and repatriate funds to the U.S.; |
| · | political and economic instability; |
| · | fluctuations in foreign currency exchange rates; |
| · | difficulties in developing and maintaining distributor relationships in foreign countries; |
| · | difficulties in negotiating acceptable contractual terms and enforcing contractual obligations; |
| · | exposure to liabilities under the U.S. Foreign Corrupt Practices Act; |
| · | potential trade restrictions and exchange controls; |
| · | creditworthiness of foreign distributors, customer uncertainty and difficulty in foreign accounts receivable collection; and |
| · | the burden of complying with foreign laws. |
As we attempt to expand our sales internationally, our exposure to these risks could result in our inability to attain the anticipated benefits and our business could be adversely impacted. Our success will depend, in large part, on our ability to anticipate and effectively manage these and other risks associated with our international operations. However, any of these factors could adversely affect our international operations and, consequently, our operating results.
A material disruption at our manufacturing facilities in Kentucky could result in material delays, quality control issues, increased costs and loss of business opportunities, which may negatively impact our sales and financial results.
We rely on our manufacturing facilities in Kentucky to operate our business and produce our yeast beta glucan products. Our manufacturing facilities, or any of our machines within our otherwise operational facilities, could cease operations or no longer comply with current GMP guidelines unexpectedly due to a number of events, including prolonged power or equipment failures, disruptions in the transportation infrastructure, fires, floods or other catastrophes. If our manufacturing facilities no longer comply with GMP, our products may be deemed adulterated under U.S. regulations and subject to recall. Furthermore, a significant majority of our raw material product inventory is located in our Kentucky facility. If any material amount of our inventory were damaged as a result of a material disruption, we would be unable to meet our contractual obligations. While we seek to operate our manufacturing facilities in compliance with applicable rules and regulations and take measures to minimize the risks of disruption at our facilities, any such material disruption at our facilities could prevent us from meeting customer demand, reduce our sales and negatively impact our financial results.
If we lose our key personnel, or are unable to attract and retain additional qualified personnel, the quality of our services may decline and our business may be adversely affected.
We rely heavily on the expertise, experience and continued services of our senior management, including our President, Mark McLaughlin. Loss of his services could adversely affect our ability to achieve our business objectives, if we are unable to find a suitable replacement. Mr. McLaughlin is an integral factor in establishing relationships and the continued development of our business depends upon his continued employment. If he were to resign or retire, we would have to find a suitable replacement who shared his expertise and relationships. Any delay in finding a suitable replacement, would adversely affect the pace at which we are able to successfully grow our business and could harm our existing business, resulting in a decrease in sales and revenue. We have entered into an employment agreement with Mr. McLaughlin that includes provisions for non-competition and confidentiality.
We believe our future success will depend upon our ability to retain key employees and our ability to attract and retain other skilled personnel and consultants. While we have been able to find a sufficient number of skilled personnel consistent with our growth to date, we cannot guarantee that any employee will remain employed by us for any period of time or that we will be able to attract, train or retain qualified personnel in the future consistent with our growth. Such loss of personnel could have a material adverse effect on our business and company. Furthermore, we may need to employ additional personnel to expand our business. Qualified employees and consultants in the dietary supplement industry are in great demand and may be unavailable in the time frame required to satisfy our customers’ requirements. There is no assurance we will be able to attract and retain sufficient numbers of highly skilled employees in the future. The loss of personnel or our inability to hire or retain sufficient personnel at competitive rates could impair the growth of our business.
| 8 |
Current and future economic and market conditions could adversely affect demand for our products.
The U.S. economy and the global economy are recovering from a severe recession. Factors such as uncertainties in consumer spending, a sustained regional and global economic downturn or slow recovery may reduce the demand for our yeast beta glucan products. Furthermore, challenging economic conditions also may impair the ability of our customers to pay for our commercial, direct-to-consumer products. Consumer spending for our yeast beta glucan products generally is considered a discretionary purchase because they are non-prescription nutriceutical supplements and nutricosmetics, and we may experience a more negative impact on our business due to these conditions than other companies that do not depend on discretionary spending. If demand for our products declines or our customers are otherwise unable to pay for our products, we may be required to offer extensive discounts or spend more on marketing than budgeted and our revenues, expense levels and profitability will be adversely affected.
We may need additional capital to execute our business plan and fund operations and may not be able to obtain such capital on acceptable terms or at all.
In connection with the development and expansion of our business, we may incur significant capital and operational expenses. We believe that we can increase our sales and net income by implementing a growth strategy that focuses on (i) diversifying revenues to include greater direct-to-consumer and healthcare professional sales and (ii) expanding our distribution to Europe and Asia. To implement our growth strategy, we anticipate (i) continuing our exit from lower-margin, feed-additive sales, (ii) increasing our marketing to healthcare professionals and end consumers, (iii) entering into additional distribution agreements with manufacturers and formulators in Europe and Asia and (iv) developing our branded product lines.
Management anticipates that our existing capital resources, cash flows from operations and the proceeds from our recent private placement will satisfy the liquidity requirements of our business for the next 12 months. However, if available funds are not sufficient to meet our plans for expansion or current operating expenses, our plans include pursuing alternative financing arrangements, including bank loans, advances from our directors and officers or funds raised through offerings of our equity or debt, if and when we determine such offerings are required. Our ability to obtain additional capital on acceptable terms or at all is subject to a variety of uncertainties, including: investors’ perceptions of, and demand for, companies in our industry; conditions of the U.S. and other capital markets in which we may seek to raise funds; our future results of operations, financial condition and cash flows; and economic, political and other conditions in the U.S.
There is no assurance we will be successful in locating a suitable financing transaction in a timely fashion or at all. In addition, there is no assurance we will obtain the capital we require by any other means or that our directors and officers who have in the past willingly funded operations through personal advances will commit to do so in the future. Future financings through equity investments are likely to be dilutive to our existing shareholders. Also, the rights and preferences of securities we may issue in future capital transactions may be more favorable for our new investors. Newly-issued securities may include preferences or superior voting rights, be combined with the issuance of warrants or other derivative securities, or be the issuances of incentive awards under equity employee incentive plans, which may have additional dilutive effects. Furthermore, we may incur substantial costs in pursuing future capital and financing, including investment banking fees, legal fees, accounting fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we may issue, such as convertible notes and warrants, which will adversely impact our financial condition.
If we cannot raise additional funds on favorable terms or at all, we may not be able to carry out all or parts of our strategy to maintain our growth and competitiveness.
We may not be able to protect our proprietary rights adequately, which could adversely affect our competitive position and reduce the value of our products and brands, and litigation to protect our intellectual property rights may be costly.
We attempt to strengthen and differentiate our products by developing new and innovative yeast beta glucan products and manufacturing processes. As a result, our patents, trademarks and other intellectual property rights are important assets to our business. Our success will depend in part on our ability to obtain and protect our products, methods, processes and other technologies, to preserve our trade secrets, and to operate without infringing on the proprietary rights of third parties in the U.S. and other international markets. Despite our efforts, any of the following may reduce the value of our owned and used intellectual property:
| · | issued patents and trademarks that we own or have the right to use may not provide us with any competitive advantages; |
| · | our efforts to protect our proprietary rights may not be effective in preventing misappropriation of our intellectual property; |
| 9 |
| · | our efforts may not prevent the development and design by others of products or technologies similar to or competitive with, or superior to those we use or develop; or |
| · | another party may obtain a blocking patent and we would need to either obtain a license or design around the patent in order to continue to offer the contested feature in our products or services. |
Policing the unauthorized use of our proprietary technology can be difficult and expensive. Litigation might be necessary to protect our intellectual property rights, which may be costly and may divert our management’s attention away from our core business. Furthermore, there is no guarantee that litigation would result in an outcome favorable to us. To date, we have no knowledge of any infringement of our intellectual property by third parties. If we are unable to protect our proprietary rights adequately, it would have a negative impact on our operations.
We may be subject to claims that we have infringed the proprietary rights of others, which could require us to obtain a license or otherwise change our manufacturing processes or product offerings.
Although we do not believe any of our products or manufacturing processes infringe upon the proprietary rights of others, there is no assurance that infringement or invalidity claims, or claims for indemnification resulting from infringement claims, will not be asserted or prosecuted against us or that any such assertions or prosecutions will not have a material adverse effect on our business. To date, we are not aware of any material infringement nor have we been put on notice by third parties of any material infringement of proprietary rights of others. Regardless of whether any such claims are valid or can be asserted successfully, defending against such claims could cause us to incur significant costs and could divert resources away from our other activities. In addition, assertion of infringement claims could result in injunctions that prevent us from distributing our products. If any claims or actions are asserted against us, we may seek to obtain a license to the intellectual property rights that are in dispute. Such a license may not be available on reasonable terms, or at all, which could force us to change our manufacturing processes or product offerings.
We incur significant costs as a result of our operating as a public reporting company and our management’s requirement to devote substantial time to new compliance initiatives, which may adversely affect our business and results of operations.
While we are a public company quoted on the OTC Markets-OTCQB, our compliance costs prior to the effectiveness of our registration statement were not substantial in light of our limited operations and limited public reporting obligations. As a company subject to public reporting requirements under the Securities Exchange Act of 1934, as amended, or the Exchange Act, we incur increased legal, accounting and other expenses. The costs of preparing and filing annual, quarterly and current reports and other information with the SEC and furnishing audited reports to shareholders is time-consuming and costly, and may adversely affect our business and results of operations.
It will also be time-consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. Our management has limited or no experience operating a company subject to the rules and reporting practices required by the federal securities laws and applicable to a publicly traded company. Our management currently relies in many instances on the professional experience and advice of third parties including our attorneys and accountants. We will need to train our current management and staff and retain additional financial reporting, internal control and other personnel in order to develop and implement appropriate accounting, internal controls and reporting procedures.
If we fail to establish and maintain an effective system of internal controls, we may not be able to report our financial results accurately. Any inability to report and file our financial results accurately and timely could harm our business and adversely affect the trading price of our common stock.
We are required to establish and maintain internal controls over financial reporting and disclosure controls and procedures and to comply with other requirements of the Sarbanes-Oxley Act and the rules promulgated by the SEC. Our management will need to include a report on our internal control over financial reporting and its assessment on whether such internal controls were effective for the prior fiscal year with our annual reports that we file under the Exchange Act with the SEC. Under current federal securities laws, our management may conclude that our internal control over financial reporting is not effective.
However, for as long as we remain an “emerging growth company,” or EGC, as defined in the Jumpstart our Business Startups Act of 2012, or JOBS Act, we may, and we intend to, take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not EGCs, including not being required to comply with the auditor attestation requirements concerning management’s reports on effectiveness of internal controls over financial reporting otherwise required under the Sarbanes-Oxley Act and the rules promulgated by the SEC. We may, and we intend to, take advantage of these reporting exemptions until we are no longer an EGC. We will cease to be an EGC at the earliest of (A) the last day of the fiscal year in which we have total annual gross revenues of $1,000,000,000 (as indexed for inflation in the manner set forth in the JOBS Act) or more; (B) the last day of the fiscal year in which the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act occurs; (C) the date on which we have, during the previous 3-year period, issued more than $1,000,000,000 in non-convertible debt; or (D) the date on which we are deemed to be a “large accelerated filer,” as defined in Rule 12b-2 under the Exchange Act or any successor thereto.
| 10 |
Once we cease to be an EGC, as of each fiscal year end thereafter, our independent registered public accounting firm will be required to evaluate and report on our internal controls over financial reporting in the event we become an accelerated filer or large accelerated filer. To the extent we find material weaknesses or other deficiencies in our internal controls, we may determine that we have ineffective internal controls over financial reporting as of any particular fiscal year end, and we may receive an adverse assessment of our internal controls over financial reporting from our independent registered public accounting firm. Moreover, any material weaknesses or other deficiencies in our internal controls may delay the conclusion of an annual audit or a review of our quarterly financial results.
Our management has limited or no experience operating as a public reporting company under the Exchange Act or establishing the level of internal control over financial reporting required by the Sarbanes-Oxley Act. Our management currently relies in many instances on the professional experience and advice of third parties including our attorneys and accountants. At present, we have started reviewing and instituting internal controls, but it may take time to implement them fully as a newly public reporting company under the Exchange Act.
Our management cannot guarantee that our internal controls and disclosure controls and procedures will prevent all possible errors. Because of the inherent limitations in all control systems, no system of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. These inherent limitations include the possibility that judgments in decision-making can be faulty and subject to simple error or mistake. Furthermore, controls can be circumvented by individual acts of some persons, by collusion of two or more persons, or by management override of the controls. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, a control may become inadequate because of changes in conditions or the degree of compliance with policies or procedures may deteriorate. Because of inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected.
Our accounting personnel who are primarily responsible for the preparation and supervision of the preparation of our financial statements under generally accepted accounting principles in the U.S. have limited relevant education and training in generally accepted accounting procedures in the U.S., or U.S. GAAP, and SEC rules and regulations pertaining to financial reporting, which could impact our ability to prepare our financial statements and convert our books and records to U.S. GAAP.
Our management and accounting personnel have limited experience operating as a public company and establishing the level of internal control and financial reporting expertise required of a public company in the U.S. Our accounting personnel who have the primary responsibilities of preparing and supervising the preparation of financial statements in accordance with U.S. GAAP for our reporting under the Exchange Act have limited relevant education and training in U.S. GAAP and related SEC rules and regulations. As such, they may be unable to identify potential accounting and disclosure issues that may arise upon the conversion of our books and records to U.S. GAAP, which could affect our ability to prepare our financial statements in accordance with U.S. GAAP. We have taken steps to ensure that our financial statements are prepared in accordance with U.S. GAAP, including our hiring of a U.S. GAAP consultant to work with our accounting personnel and management to convert our books and records to U.S. GAAP and prepare our financial statements. In addition, our annual financial statements are audited by an independent auditor for compliance, in all material respects, with U.S. GAAP. Until such time as we hire qualified accounting personnel or train our current accounting personnel with the requisite U.S. GAAP experience, however, the measures we have taken may not be sufficient to mitigate the foregoing risks associated with the limited education and training of our accounting personnel in U.S. GAAP and related SEC rules and regulations.
Risks Related to Our Securities
Our stock price may be volatile or may decline regardless of our operating performance, and you may lose part or all of your investment.
The market price of our common stock may fluctuate widely in response to various factors, some of which are beyond our control, including:
| · | market conditions or trends in the dietary supplement industry or in the economy as a whole; |
| · | actions by competitors; |
| · | actual or anticipated growth rates relative to our competitors; |
| · | the public’s response to press releases or other public announcements by us or third parties, including our filings with the SEC; |
| · | economic, legal and regulatory factors unrelated to our performance; |
| · | any future guidance we may provide to the public, any changes in such guidance or any difference between our guidance and actual results; |
| 11 |
| · | changes in financial estimates or recommendations by any securities analysts who follow our common stock; |
| · | speculation by the press or investment community regarding our business; |
| · | litigation; |
| · | changes in key personnel; and |
| · | future sales of our common stock by our officers, directors and significant shareholders. |
In addition, the stock markets, including the over-the-counter markets where we are quoted, have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These broad market fluctuations may materially affect our stock price, regardless of our operating results. Furthermore, the market for our common stock historically has been limited and we cannot assure you that a larger market will ever be developed or maintained. The price at which investors purchase shares of our common stock may not be indicative of the price that will prevail in the trading market. Market fluctuations and volatility, as well as general economic, market and political conditions, could reduce our market price. As a result, these factors may make it more difficult or impossible for you to sell our common stock for a positive return on your investment. In the past, shareholders have instituted securities class action litigation following periods of market volatility. If we were involved in securities litigation, we could incur substantial costs and our resources and the attention of management could be diverted from our business.
Shares of our common stock lack a significant trading market, which could make it more difficult for an investor to sell our common stock.
Shares of our common stock are not yet eligible for trading on any national securities exchange. Our common stock currently is quoted in the over-the-counter market on the OTC Markets-OTCQB. This market tends to be highly illiquid. There is no assurance that an active trading market in our common stock will develop, or if such a market develops, that it will be sustained. In addition, there is a greater chance for market volatility for securities quoted in the over-the-counter markets as opposed to securities traded on a national exchange. This volatility may be caused by a variety of factors, including the lack of readily available quotations, the absence of consistent administrative supervision of “bid” and “ask” quotations and generally lower trading volume. As a result, an investor may find it more difficult to dispose of, or to obtain accurate quotations as to the market value of, our common stock, or to obtain coverage for significant news events concerning us, and our common stock could become substantially less attractive for investment by financial institutions, as consideration in future capital raising transactions or for other purposes.
Future sales of shares of our common stock, or the perception in the public markets that these sales may occur, may depress our stock price.
The market price of our common stock could decline significantly as a result of sales of a large number of shares of our common stock. In addition, if our significant shareholders sell a large number of shares, or if we issue a large number of shares, the market price of our stock could decline. Any issuance of additional common stock by us in the future, or warrants or options to purchase our common stock, if exercised, would result in dilution to our existing shareholders. Such issuances could be made at a price that reflects a discount or a premium to the then-current trading price of our common stock. Moreover, the perception in the public market that shareholders might sell shares of our stock or that we could make a significant issuance of additional common stock in the future could depress the market for our shares. These sales, or the perception that these sales might occur, could depress the market price of our common stock or make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
We have issued shares of common stock and warrants and options to purchase shares of our common stock in connection with our private placement and certain employment, director and consultant agreements. In addition, we issued shares of our common stock, and options and warrants to purchase shares of our common stock, in financing transactions and pursuant to employment agreements that are deemed to be “restricted securities,” as that term is defined in Rule 144 promulgated under the Securities Act. From time to time, certain of our shareholders may be eligible to sell all or some of their restricted shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, subject to certain limitations. In general, pursuant to Rule 144, after satisfying a six-month holding period: (i) affiliated shareholders, or shareholders whose shares are aggregated, may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then-outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale and (ii) non-affiliated shareholders may sell without such limitations, in each case provided we are current in our public reporting obligations. Rule 144 also permits the sale of securities by non-affiliates that have satisfied a one-year holding period without any limitation or restriction. The resale under this offering or pursuant to Rule 144 of shares acquired from us in private transactions could cause our stock price to decline significantly.
| 12 |
We could issue additional common stock, which might dilute the book value of our common stock.
Our Board of Directors has authority, without action or vote of our shareholders, to issue all or a part of our authorized but unissued shares. Our amended certificate of incorporation authorizes the issuance of up to 50,000,000 shares of common stock, par value $0.01 per share. As of March 28, 2013, there were 7,497,307 authorized and unissued shares of our common stock available for future issuance, based on 28,909,973 shares of our common stock outstanding and our reservation of 3,637,720 shares of our common stock issuable upon exercise of outstanding warrants and 9,955,000 shares of our common stock issuable upon exercise of options outstanding and exercisable. Although we have no commitments as of the date hereof to issue any securities, we may issue a substantial number of additional shares of our common stock or debt securities to complete a business combination or to raise capital. Such stock issuances could be made at a price that reflects a discount or a premium from the then-current trading price of our common stock. In addition, in order to raise capital, we may need to issue securities that are convertible into or exchangeable for a significant amount of our common stock. These issuances would dilute your percentage ownership interest, which would have the effect of reducing your influence on matters on which our shareholders vote, and might dilute the book value of our common stock. You may incur additional dilution if holders of stock options and warrants, whether currently outstanding or subsequently granted, exercise their options or warrants to purchase shares of our common stock.
We are an EGC, and we cannot be certain if the reduced disclosure requirements applicable to EGCs will make our common stock less attractive to investors.
We are an EGC, as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not EGCs. The modified disclosure requirements available to EGCs include reduced disclosure about our executive compensation and omission of a compensation discussion and analysis, which is also available to us as a smaller reporting company, and an exemption from the requirement of holding a nonbinding advisory vote on executive compensation and the requirement that shareholders approve any golden parachute payments not previously approved. In addition, we will not be subject to certain requirements of Section 404 of the Sarbanes-Oxley Act, including the additional testing of our internal control over financial reporting as may occur when outside auditors attest as to our internal controls over financial reporting, which is also not required of smaller reporting companies. We could be an emerging growth company for up to five years, although we could lose that status sooner if our revenues exceed $1 billion, if we issue more than $1 billion in non-convertible debt in a three year period, or if the market value of our common stock exceeds $700 million.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile.
Although the JOBS Act permits an EGC such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies, we are choosing to “opt out” of this provision, and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
The application of the “penny stock” rules could adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
Our common stock may be subject to the “penny stock” rules adopted under Section 15(g) of the Exchange Act. The penny stock rules apply to issuers whose common stock does not trade on a national securities exchange and trades at less than $5.00 per share, or that have a tangible net worth of less than $5,000,000 ($2,000,000 if the company has been operating for three or more years). The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document prepared by the SEC that contains the following information:
| · | a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; |
| · | a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to violation to such duties or other requirements of securities laws; |
| · | a brief, clear, narrative description of a dealer market, including “bid” and “ask” prices for penny stocks and the significance of the spread between the “bid” and “ask” prices; |
| · | a toll-free telephone number for inquiries on disciplinary actions; |
| · | definitions of any significant terms in the disclosure document or in the conduct of trading in penny stocks; and |
| · | such other information and is in such form (including language, type, size and format), as the SEC shall require by rule or regulation. |
Prior to effecting any transaction in a penny stock, the broker-dealer also must provide the customer with the following information:
| · | bid and offer quotations for the penny stock; |
| · | compensation of the broker-dealer and our salesperson in the transaction; |
| 13 |
| · | number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and |
| · | monthly account statements showing the market value of each penny stock held in the customer’s account. |
The penny stock rules further require that, prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks and a signed and dated copy of a written suitability statement.
Due to the requirements of the penny stock rules, many broker-dealers have decided not to trade penny stocks. As a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. Moreover, if our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Our principal shareholder has the ability to exert significant control in matters requiring a shareholder vote and could delay, deter or prevent a change of control in our company.
As of March 28, 2013, Mark McLaughlin, our President and largest shareholder, beneficially owned 31% of our outstanding shares of common stock. Mr. McLaughlin exerts significant influence over us, giving him the ability, among other things, to exercise significant control over the election of all or a majority of the Board of Directors and to approve significant corporate transactions. Such share ownership and control may also have the effect of delaying or preventing a future change in control, impeding a merger, consolidation, takeover or other business combination, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company. This, in turn, could have a negative effect on the market price of our common stock. It could also prevent our shareholders from realizing a premium over the market price for their shares of common stock. Without the consent of Mr. McLaughlin, we could be prevented from entering into potentially beneficial transactions if such transactions conflict with our principal shareholder’s interests.
We do not anticipate paying dividends in the foreseeable future, and, accordingly, any return on investment may be limited to the value of our common stock.
We do not anticipate paying cash dividends on our common stock in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting it at such time as the Board of Directors may consider relevant. We intend to follow a policy of retaining all of our earnings to finance the development and execution of our strategy and the expansion of our business. If we do not pay dividends, our common stock may be less valuable because a return on your investment will occur only if our stock price appreciates.
Our common stock is not registered under the Exchange Act and, as a result, we will not be subject to the federal proxy rules and our directors, executive officers and 10% beneficial holders will not be subject to Section 16 of the Exchange Act. In addition, our reporting obligations under Section 15(d) of the Exchange Act may be suspended automatically if we have fewer than 300 holders of record on the first day of our fiscal year.
Shares of our common stock are not currently registered under the Exchange Act though we may register our common stock under the Exchange Act in the foreseeable future. We will have to register our common stock under the Exchange Act if we have, after the last day of our fiscal year, holders of record of more than either (1) 2,000 or more persons or (2) 500 or more persons who are not accredited investors, in accordance with Section 12(g) of the Exchange Act, as amended by the JOBS Act. As a result, currently we are only subject solely to the reporting obligations of Section 15(d) of the Exchange Act so long as we do not subsequently register under Section 12(g) of the Exchange Act by filing a Form 8-A or another Exchange Act registration statement. As long as our common stock is not registered under the Exchange Act, we will be required to file only annual, quarterly and current reports pursuant to Section 15(d) of the Exchange Act, and we will not be subject to Section 14 of the Exchange Act, which, among other things, prohibits companies that have securities registered under the Exchange Act from soliciting proxies or consents from shareholders without furnishing to shareholders and filing with the SEC a proxy statement and form of proxy complying with the proxy rules. In addition, so long as our common shares are not registered under the Exchange Act, our directors, executive officers and beneficial holders of 10% or more of our outstanding common stock will not be subject to Section 16 of the Exchange Act. Section 16(a) of the Exchange Act requires directors, executive officers and persons who beneficially own more than 10% of a registered class of equity securities to file with the SEC initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of common stock and other equity securities on Forms 3, 4 and 5, respectively. Such information about our directors, executive officers and 10% beneficial holders will only be available through this and any subsequent registration statement or periodic reports we file pursuant to Section 15(d) of the Exchange Act.
| 14 |
Furthermore, so long as our common stock is not registered under the Exchange Act, our obligation to file reports under Section 15(d) of the Exchange Act will be automatically suspended if, on the first day of any fiscal year, other than a fiscal year in which a registration statement under the Securities Act has gone effective, we have fewer than 300 holders of record. This suspension is automatic and does not require any filing with the SEC. In such an event, we may cease providing periodic reports and current or periodic information, including operational and financial information. As of March 28, 2013, we had 327 holders of record.
Certain provisions of our corporate governance documents and Delaware law could discourage, delay or prevent a merger or acquisition at a premium price.
Our amended certificate of incorporation and bylaws contain provisions that may make the acquisition of our company more difficult without the approval of our Board of Directors. These include provisions that:
| · | provide that our Board of Directors is expressly authorized to adopt, amend or repeal our bylaws; |
| · | provide our Board of Directors with the sole power to set the size of our Board of Directors and fill vacancies; and |
| · | provide that special meetings of shareholders may be called only by our Board of Directors, Chairman of the Board of Directors, upon written notice of demand by our President or upon written notice of demand by the holders of at least 25% of the shares of our common stock outstanding and entitled to vote. |
These and other provisions of our amended certificate of incorporation and bylaws could delay, defer or prevent us from experiencing a change of control or changes in our Board of Directors and management and may adversely affect our shareholders’ voting and other rights.
In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with a shareholder owning 15% or more of such corporation’s outstanding voting stock for a period of three years following the date on which such shareholder became an “interested” shareholder. In order for us to consummate a business combination with an “interested” shareholder within three years of the date on which the shareholder became “interested,” either (1) the business combination or the transaction that resulted in the shareholder becoming “interested” must be approved by our board of directors prior to the date the shareholder became “interested,” (2) the “interested” shareholder must own at least 85% of our outstanding voting stock at the time the transaction commences (excluding voting stock owned by directors who are also officers and certain employee stock plans) or (3) the business combination must be approved by our board of directors and authorized by at least two-thirds of our shareholders (excluding the “interested” shareholder). This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our shareholders. Any delay or prevention of a change of control transaction or changes in our board of directors and management could deter potential acquirers or prevent the completion of a transaction in which our shareholders could receive a substantial premium over the then-current market price for their shares of our common stock. See “Description of Capital Stock—Delaware Anti-Takeover Statute” and “Description of Capital Stock—Anti-Takeover Effects of Certain Provisions of our Amended Certificate of Incorporation and Bylaws.”
Item 1B. Unresolved Staff Comments
Not required.
Item 2. Properties
Our principal executive offices are in office space provided at no cost to us by our President in Mount Kisco, New York. We lease a manufacturing facility with warehouse space consisting of approximately 15,000 square feet in Florence, Kentucky, in the vicinity of the Cincinnati, Ohio, airport. The lease expires in May 2013 and we are currently in negotiations for an extension. We believe that our existing office and manufacturing facilities are adequate for current and presently foreseeable operations. In general, our properties are well maintained and are being utilized for their intended purposes.
Item 3. Legal Proceedings
We may become involved in various lawsuits and legal proceedings arising in the ordinary course of business. Litigation is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may have an adverse effect on our business, financial conditions or operating results. We are currently not aware of any such legal proceedings or claims that will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results.
Item 4. Mine Safety Disclosures
Not applicable.
| 15 |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is qualified for quotation on the OTC Markets-OTCQB under the symbol “IMMD” and has been quoted on the OTCQB since February 8, 2013. Previously, our common stock was quoted on the OTC Markets-OTC Pink Current, also under the symbol “IMMD.” The following table sets forth the range of the high and low bid prices per share of our common stock for each quarter (or portion thereof) as reported in the over-the-counter markets. These quotations represent interdealer prices, without retail markup, markdown or commission, and may not represent actual transactions. There currently is no liquid trading market for our common stock. There can be no assurance that a significant active trading market in our common stock will develop, or if such a market develops, that it will be sustained.
| 2012 | 2011 | 2010 | ||||||||||||||||||||||
| High | Low | High | Low | High | Low | |||||||||||||||||||
| First Quarter (through March 31) | $ | 0.22 | $ | 0.15 | $ | 0.30 | $ | 0.19 | $ | 0.23 | $ | 0.14 | ||||||||||||
| Second Quarter (through June 30) | 0.20 | 0.16 | 0.23 | 0.14 | 0.18 | 0.12 | ||||||||||||||||||
| Third Quarter (through September 30) | 0.20 | 0.16 | 0.28 | 0.15 | 0.40 | 0.16 | ||||||||||||||||||
| Fourth Quarter (through December 31) | 0.20 | 0.11 | 0.22 | 0.13 | 0.33 | 0.18 | ||||||||||||||||||
Holders of Record
On March 28, 2013, there were approximately 327 shareholders of record based on information provided by our transfer agent. Many of our shares of common stock are held in street or nominee name by brokers and other institutions on behalf of shareholders and we are unable to estimate the total number of shareholders represented by these record holders.
Dividend Policy
We have not paid and do not expect to declare or pay any cash dividends on our common stock in the foreseeable future. We currently expect to retain all future earnings for use in the operation and expansion of our business. The declaration and payment of any cash dividends in the future will be determined by our Board of Directors, in its discretion, and will depend on a number of factors, including our earnings, capital requirements, overall financial condition and contractual restrictions, if any.
Item 6. Selected Financial Data
Not required.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Safe Harbor Declaration
The comments made throughout this Annual Report should be read in conjunction with our Financial Statements and the Notes thereto, and other financial information appearing elsewhere in this document. In addition to historical information, the following discussion and other parts of this document contain certain forward-looking information. When used in this discussion, the words, “believes,” “anticipates,” “expects” and similar expressions are intended to identify forward-looking statements. Such statements are subject to certain risks and uncertainties, which could cause actual results to differ materially from projected results, due to a number of factors beyond our control. We do not undertake to publicly update or revise any of its forward-looking statements, even if experience or future changes show that the indicated results or events will not be realized. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Readers are also urged to carefully review and consider our discussions regarding the various factors that affect our business, which are described in this section and elsewhere in this report.
Overview
We manufacture, distribute and sell natural immune support products. We believe, based on testing and analysis conducted on our behalf, that our beta glucans derived from yeast are superior to other beta glucans. Beta glucans are a natural extract that has been shown through testing and analysis and scientific research to support the immune system. Our core nutraceutical and cosmetic product lines consist of yeast beta glucans in oral and topical applications. Our beta glucan products and manufacturing processes are protected by patents and trade secrets and are compliant with current GMPs. Yeast beta glucans are classified as GRAS by the FDA. Historically, we have sold our products primarily on a word-of-mouth basis through distributors and our website as standalone product lines, as well as business-to-business as dietary supplement and a cosmetic enhancement, and as a feed-additive for animal use. As of 2011, we began exiting this lower-margin market for feed-additive products. Our sales and marketing efforts going forward are concentrated on our oral and topical-use products for healthcare professionals, distributors and direct-to-consumer sales.
| 16 |
We originally incorporated under the laws of British Columbia, Canada, in 1987 under the name Anina Resources, Inc. and subsequently changed our name to Immudyne, Inc. and our jurisdiction to the State of Wyoming by continuance in September 1987. On June 30, 1994, we changed our jurisdiction to Delaware by merger with and into Immudyne, Inc., a Delaware corporation formed on June 21, 1994.
Significant factors that we believe could affect our operating results are (i) marketing and advertising expenses; (ii) protection of our intellectual property rights; and (iii) imposition of more stringent government regulations of our products.
Our marketing strategy is to promote sales of our oral and topical-use products, which constitute our core business as we shift from low-margin, feed-additive product sales. We believe that we are well positioned to capitalize on our development of proprietary and patented products and can now focus on commercializing sales on a more meaningful, global basis. We expect that a significant component of our selling, general and administration expenses going forward will consist of marketing and advertising expenses to increase our sales. The primary components of our marketing and advertising expenses may include online sales promotions through our website, trade advertising, direct marketing to nutraceutical companies and industry associations, consumer research and search engine and digital advertising. We expect our selling, general and administrative expenses to increase in absolute dollars as we incur increased costs related to our marketing strategy and growth of our business. These costs, along with the additional costs resulting from our operations as a public reporting company, could impact our future operating profitability.
We historically have expended a significant amount of our funds on obtaining and protecting our patents, trade secrets and proprietary products. We rely on the patent and trademark protection laws in the U.S. to protect our intellectual property and maintain our competitive position in the marketplace. For several years, we were involved in complex litigation regarding patents and licenses critical to our products. In 2010, we prevailed on all major legal matters and reached a favorable settlement. If additional litigation becomes necessary to protect our intellectual property rights, such litigation may be costly, divert our management’s attention away from our core business and have a negative impact on our operations. Furthermore, there is no guarantee that litigation would result in an outcome favorable to us.
Our manufacturing processes are compliant in the U.S. with current cGMPs and our yeast beta glucan products are all natural. Further, yeast beta glucans are designated as GRAS under current FDA regulations. Future government regulations may prevent or delay the introduction or require the reformulation of our products. Some agencies, such as the FDA, could require us to remove a particular product from the market, delay or prevent the import of raw materials for the manufacture of our products or otherwise disrupt the marketing of our products. Any such government actions could result in additional costs to us, including reduced growth prospects, lost sales from products that we are required to remove from the market and potential product liability litigation.
On February 1,2013, we hired a Chief Marketing Officer, the first such position in Company history. We are planning to introduce new products that leverage our proprietary know how and intellectual property. Though there can be no assurance of success, we believe the tools are in place to execute on our marketing plan.
We have limited operating capital and have funded operations in the past through the sales of our products and loans and advances from Mark McLaughlin, our President, and other directors. While we believe that we will begin to generate increased sales, it is likely that the we will require additional operating capital. Until we are in a position to obtain such capital from revenues to meet our normal business obligations, the Company will have to depend on sources other than operating revenues to meet our operating and capital needs. No assurance can be given that such sources will be available and no assurance can be given that Mr. McLaughlin or other directors who have in the past willingly funded operations will commit to do so in the future, or that the Company will be successful in its endeavors to raise additional capital.
| 17 |
Results of Operations
Comparison of Years Ended December 31, 2012 and 2011
The following table sets forth the results of our operations for the years ended December 31, 2012 and 2011:
| 2012 | 2011 | |||||||||||||||
| $ | % of Sales | $ | % of Sales | |||||||||||||
| Sales | 673,778 | 743,828 | ||||||||||||||
| Cost of sales | 125,619 | 19 | % | 241,264 | 32 | % | ||||||||||
| Gross profit | 548,159 | 81 | % | 502,564 | 68 | % | ||||||||||
| Operating expenses | (1,088,184 | ) | (162 | )% | (1,078,931 | ) | (145 | )% | ||||||||
| Loss from operations | (540,025 | ) | (80 | )% | (576,367 | ) | (77 | )% | ||||||||
| Other income (expenses), net | 77,176 | 11 | % | 56,238 | 8 | % | ||||||||||
| Income tax benefit | 17,200 | 3 | % | 17,200 | 2 | % | ||||||||||
| Net loss | (445,649 | ) | (66 | )% | (502,929 | ) | (68 | )% | ||||||||
Sales in 2012 were $0.67 million, a decrease of 9% from $0.74 million in 2011. The decrease in sales resulted primarily from a decline in feed-additive sales and a change in our sales and marketing strategy. We shifted our focus in 2011 to concentrate on our sales and marketing efforts for our oral and topical-use products, which we anticipate will constitute our core business going forward. Sales of our oral and topical-use products increased 12% to $0.67 million in 2012 compared to 2011, and consisted of 99% and 80% of our sales in 2012 and 2011, respectively. Sales of our lower-margin feed-additive products consisted of 1% and 20% of our sales in 2012 and 2011, respectively. We anticipate phasing out our feed-additive products as we continue to change our product focus toward oral and topical-use products.
Cost of sales consists primarily of material costs, labor costs and related overhead directly attributable to the production of our products. Total cost of sales decreased 48% to $0.13 million in 2012 compared to $0.24 million in 2011. The decrease in cost of sales as a percentage of sales in 2012 compared to 2011 resulted primarily from improved operating efficiencies and an increase in the sales of product with a higher profit margin.
Gross profit increased 9% to $0.55 million in 2012 compared to $0.50 million in 2011. The increased gross profit margin from 2011 to 2012 resulted primarily from improved operating efficiencies and an increase in the sales of product with a higher profit margin.
Operating expenses consisted of general and administrative expense, compensation and related expense and professional fees. Operating expenses were essentially unchanged at $1.09 million in 2012 compared to $1.09 million in 2011. General and administration expense increased 43% to $0.45 million due primarily to improvements to our Kentucky facility, the added expense related to market research, as well as the uplisting to the OTCQB. Compensation and related expenses decrease by 46% to $0.38 million due primarily to a reduction in stock based compensation. Professional fees increased 370% to $0.25 million as a result of increase in audit, accounting and legal fees relating primarily to our registration statement filed in 2012.
Other income expense (net) was $77,176 in 2012 compared with other income of $56,248 in 2011, an increase of $20,928. The increase in other income was from proceeds of a litigation settlement, decreased interest expense, and the settlement of an aged payable.
Our net loss for 2012 was $0.45 million compared to net loss of $0.50 million in 2011, a decrease of $0.057 million or 11%. Net loss as a percentage of sales was 66% in 2012 compared to net loss as a percentage of sales of 68% in 2011.
Liquidity and Capital Resources
Our principal demands for liquidity are to increase sales, purchase inventory and for sales distribution and general corporate purposes. We have limited operating capital and intend to meet our liquidity requirements, including purchase of raw materials and the expansion of our business, primarily through cash flow provided by operations. Historically, we also have funded operations through loans and advances from our directors and officers and offerings of equity securities. In 2012 and 2011, we raised $345,883 and $193,500, respectively, through sales of our common stock in private placements, as well as the exercising of stock options. We may seek additional financing in the form of loans from banks or our directors and officers, or funds raised through future offerings of our equity or debt, if and when we determine such offerings are required. Any future issuance of equity securities could cause dilution to our shareholders. Any incurrence of indebtedness could increase our debt service obligations and cause us to be subject to restrictive operating and financial covenants.
Comparison of Years Ended December 31, 2012 and 2011
We had net working capital of $24,902 at December 31, 2012, an increase of $457,756 from net working capital deficit of $432,854 at December 31, 2011. The ratio of current assets to current liabilities was 1.1 -to- 1 at December 31, 2012.
The following is a summary of cash provided by or used in each of the indicated types of activities during the years ended December 31, 2012 and 2011:
| 18 |
| 2012 | 2011 | |||||||
| Cash provided by (used in): | ||||||||
| Operating activities | $ | (229,726 | ) | $ | (17,686 | ) | ||
| Financing activities | 295,154 | 51,188 | ||||||
Net cash flow used in operating activities was $229,726 for the period ending December 31, 2012, compared to net cash flow used in operating activities of $17,686 for the 2011 period. The increase in net cash outflow in operating activities was attributable primarily to our shifting our decision to become a fully reporting public company and our focus to marketing our oral and topical-use products.
Net cash flow provided by financing activities was $295,154 for the period ending December 31, 2012, compared to net cash flow of $51,888 for the 2011 period. In 2012 period, we received $313,383 from private placement sales of our common stock and $32,500 for the exercise of options offset by $50,729 in repayment of notes payable. In the 2011 period, we received $193,500 from private placement sales of our common stock offset by $101,312 in net repayments of notes payable and $41,000 in cash overdraft.
Private Placements
In a series of private placement transactions in the six months ended June 30, 2012, we issued 1,843,428 shares of our common stock and 3-year warrants to purchase 914,106 shares of our common stock at $0.40 per share to accredited investors at $0.17 per unit for $313,383.
In a series of private placement transactions in 2011, we issued (i) 621,053 shares of our common stock at $0.2286 per share for $142,000 and (ii) 302,941 shares of our common stock and 3-year warrants to purchase 151,500 shares of our common stock at $0.40 per share to accredited investors at $0.17 per unit for $51,500.
In a series of private placement transactions in 2010, we issued 200,000 shares of our common stock and 3-year warrants to purchase 100,000 shares of our common stock at $0.40 per share to accredited investors at $0.17 per unit for $34,000.
Indebtedness
From time to time, our directors, officers and other related individuals have made short-term advances to us for our operating needs. During the year ended December 31, 2012, notes payable due to officer and other related individuals totaling $50,729 were repaid and the balance of notes payable and amounts due to officer, aggregating $311,443, were converted to common stock. Interest expense on notes payable amounted to $3,371 for the year ended December 31, 2012.
We are subject to a royalty agreement pursuant to which we are required to pay a monthly royalty of 8% on all sales of certain skin care products up to $227,175. At December 31, 2012, we included $95,938 in accounts payable and accrued expenses relating to this royalty agreement, with the remaining commitment under the royalty agreement at approximately $115,000. Our President, Mr. McLaughlin, has a 60% interest in the royalties paid under the agreement and has voluntarily deferred payments due without interest until we have the financial wherewithal to pay such royalties.
Legal Matters
In November 2005, we filed suit in Texas against Biopolymer Engineering, Inc. d/b/a Biothera who was claiming ownership of certain patents we own covering our yeast beta glucan products. Biothera subsequently filed suit against us in Federal Court in Minnesota alleging infringement of the same patents. The state court case proceeded to trial in which it was adjudicated that we were the owner of all of the patents at issue. In November 2009, we entered into a settlement agreement that resolved all litigation aspects of the federal and state court proceedings. The settlement agreement stipulated that we were the owner of all of the patents at issue. As part of the settlement agreement, we received $440,000 as reimbursement for litigation costs. In addition, we were awarded $200,000 in eight installments of $25,000 every six months beginning on January 15, 2011, in return for an exclusive patent license to Biothera for U.S. Patent No. 5,702,719, covering our beta glucan derived from yeast for dermatological and nutritional use. The term of the license agreement is consistent with the term of the $25,000 semi-annual payments. The $25,000 installments are being recorded as revenue only upon receipt of the funds. At December 31, 2012, $100,000 remained to be paid to us under this agreement.
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements between us and any other entity that have, or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to shareholders.
| 19 |
We have not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as stockholders’ equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
Critical Accounting Policies
While our significant accounting policies are described more fully in Note 2 to our financial statements, we believe the following accounting policies are the most critical to aid you in fully understanding and evaluating this management discussion and analysis.
Basis of Presentation and Use of Estimates
The accompanying financial statements have been prepared in conformity with generally accepted accounting principles in the U.S., or U.S. GAAP. In preparing financial statements in conformity with U.S. GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting period. Significant estimates required by management include the valuation of inventory and stockholders’ equity-based transactions. Actual results could differ from those estimates.
Inventory
Inventory is valued at the lower of cost or market value with cost determined on a first-in, first-out basis. Management compares the cost of inventory with the net realizable value and an allowance is made for writing down their inventories to market value, if lower.
Revenue Recognition
The Company’s policy is to record revenue as earned when a firm commitment, indicating sales quantity and price exists, delivery has taken place and collectability is reasonably assured. The Company generally records sales once the product is shipped to the customer. If applicable, provisions for discounts, returns, allowances, customer rebates and other adjustments are netted with gross sales. The Company accounts for such provisions during the same period in which the related revenues are earned. Customer discounts, returns and rebates have not been significant.
Delivery is considered to have occurred when title and risk of loss have transferred to the customer. Sales to international distributors are recognized in the same manner. If title does not pass until the product reaches the customer’s delivery site, then recognition of revenue is deferred until that time. There are no formal sales incentives offered to any of the Company’s customers. Volume discounts may be offered from time to time to customers purchasing large quantities on a per transaction basis. There are no special post shipment obligations or acceptance provisions that exist with any sales arrangements
Stock-based Compensation
The Company follows the provisions of ASC 718, “Share-Based Payment”. Under this guidance compensation cost generally is recognized at fair value on the date of the grant and amortized over the respective vesting periods. The fair value of options at the date of grant is estimated using the Black-Scholes option pricing model. The expected option life is derived from assumed exercise rates based upon historical exercise patterns and represents the period of time that options granted are expected to be outstanding. The expected volatility is based upon historical volatility of our shares using weekly price observations over an observation period that approximates the expected life of the options. The risk-free rate approximates the U.S. Treasury yield curve rate in effect at the time of grant for periods similar to the expected option life. The estimated forfeiture rate included in the option valuation was zero.
Many of the assumptions require significant judgment and any changes could have a material impact in the determination of stock-based compensation expense.
New Accounting Pronouncements
Accounting standards that have been issued or proposed by the FASB that do not require adoption until a future date are not expected to have a material impact on the financial statements upon adoption.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Not required.
| 20 |
Item 8. Financial Statements and Supplementary Data
Our financial statements, together with the report thereon, appear in a separate section of this Annual Report beginning on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our Principal Executive Officer (“PEO”), who is also our Principal Financial Officer (“PFO”), of the design and effectiveness of our “disclosure controls and procedures” (as defined under Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934) as of the end of the period covered by this report. Based on this evaluation, our PEO/PFO concluded that as of the end of the period covered by this report, these disclosure controls and procedures were not effective. The conclusion that our disclosure controls and procedures were not effective was due to the presence of the following material weaknesses in disclosure controls and procedures which are indicative of many small companies with small staff: (i) inadequate segregation of duties and effective risk assessment as the Company had only one officer; (ii) insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of both US GAAP and SEC Guidelines; and (iii) inadequate security and restricted access to computer systems including insufficient disaster recovery plans; and (iv) no written whistleblower policy. Our PEO/PFO plans to implement appropriate disclosure controls and procedures to remediate these material weaknesses, including (i) appointing additional qualified personnel to address inadequate segregation of duties and ineffective risk management; (ii) adopt sufficient written policies and procedures for accounting and financial reporting and a whistle blower policy once funds are available; and (iii) implement sufficient security and restricted access measures regarding our computer systems and implement a disaster recovery plan.
Management’s Annual Report on Internal Control over Financial Reporting
Our PEO/PFO is responsible for establishing and maintaining adequate internal control over financial reporting as defined under Rule 13a-15(f) and Rule 15d-15(f) under the Securities Exchange Act of 1934. As of December 31, 2012 our PEO/PFO assessed the effectiveness of the Company’s internal control over financial reporting based on the criteria for effective internal control set forth in the report entitled “Internal Control — Integrated Framework” published by the Committee of Sponsoring Organizations (“COSO”) of the Treadway Commission. Based on that evaluation, our PEO/PFO concluded that, during the period covered by this report, such internal controls and procedures were not effective to detect the inappropriate application of US GAAP rules as more fully described below. This was due to deficiencies that existed in the design or operation of our internal control over financial reporting that adversely affected our internal controls.
The matters involving internal controls and procedures that the Company’s PEO/PFO considered to be material weaknesses under the standards of the Public Company Accounting Oversight Board were: (1) lack of a functioning audit committee and lack of a majority of outside directors on the Company's board of directors, resulting in ineffective oversight in the establishment and monitoring of required internal controls and procedures; (2) inadequate segregation of duties consistent with control objectives; (3) insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements; and (4) ineffective controls over period end financial disclosure and reporting processes. The aforementioned material weaknesses were identified by the Company's PEO/PFO in connection with his review of our financial statements as of December 31, 2012.
Our PEO/PFO believes that the material weaknesses set forth above did not have an effect on the Company's financial results. However, our PEO/PFO believes that the lack of a functioning audit committee and lack of a majority of outside directors on the Company's board of directors, results in ineffective oversight of the establishment and monitoring of required internal controls and procedures. We recently retained an outside accountant to assist in remediating and improving some of these internal control processes. Additionally, we are looking for outside directors to assist in the oversight of the financial reporting process, and plan to address all of these internal control matters once our business plan is fully implemented and additional funds are available.
We are committed to improving our financial organization. As part of this commitment, we will create a position to segregate duties consistent with control objectives and will increase our personnel resources and technical accounting expertise within the accounting function when funds are available to the Company and to take the following actions: i) Appointing one or more outside directors to our board of directors who shall be appointed to the audit committee of the Company resulting in a fully functioning audit committee who will undertake the oversight in the establishment and monitoring of required internal controls and procedures; and ii) Preparing and implementing sufficient written policies and checklists which will set forth procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements.
| 21 |
Our PEO/PFO believes that the appointment of more outside directors, who shall be appointed to a fully functioning audit committee, will remedy the lack of a functioning audit committee and a lack of a majority of outside directors on the Company's Board. In addition, our PEO/PFO believes that preparing and implementing sufficient written policies and checklists will remedy the following material weaknesses (i) insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of US GAAP and SEC disclosure requirements; and (ii) ineffective controls over period end financial close and reporting processes. Further, our PEO/PFO believes that the hiring of additional personnel who have the technical expertise and knowledge will result proper segregation of duties and provide more checks and balances within the Company. Additional personnel will also provide the cross training needed to support the Company if personnel turn over issues within the Company occur. This coupled with the appointment of additional outside directors will greatly decrease any control and procedure issues the company may encounter in the future.
We will continue to monitor and evaluate the effectiveness of our internal controls and procedures and our internal controls over financial reporting on an ongoing basis and are committed to taking further action and implementing additional enhancements or improvements, as necessary and as funds allow.
There have been no significant changes in our internal controls over financial reporting that occurred during the quarter ended December 31, 2012 that have materially affected or are reasonably likely to materially affect, our internal controls over financial reporting.
This annual report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s independent registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit the Company to provide only its management report in the Annual Report.
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The following table sets forth the names of our directors, executive officer and certain significant employees and their ages, positions and biographical information as of the date of this annual report. Our executive officer is appointed by, and serves at the discretion of, our Board of Directors. Dr. Bruzzese, our Chairman, is the son-in-law of Mr. Agostini, one of our directors. There are no other family relationships among our directors or executive officer.
| Name | Position | Age | ||
| Anthony G. Bruzzese, M.D. | Chairman | 57 | ||
| Mark McLaughlin | President, Chief Executive Officer and Director | 55 | ||
| Dominic J. Agostini | Director | 83 | ||
| John R. Strawn, Jr. | Director | 52 |
Anthony G. Bruzzese, M.D., Chairman
Dr. Bruzzese has served as Chairman of our Board of Directors since April 2004. He is a practicing radiologist in Warwick, Rhode Island, certified by both the American Board of Internal Medicine and the American Board of Radiology. Since 1997, Dr. Bruzzese has served as a principal at Toll Gate Radiology, Inc., providing patients with comprehensive diagnostic imaging services. Dr. Bruzzese also has served on the medical staffs at Roger Williams Medical Center since 2008 and Landmark Medical Center since 2011. He previously served on the medical staff at Kent County Memorial Hospital in Rhode Island from 1997 to 2005. Dr. Bruzzese has served as a Fellow, Councilor and Alternate Councilor to the American College of Radiology on behalf of the Rhode Island Radiology Society. Dr. Bruzzese received his Bachelor of Science and Doctor of Medicine from Brown University. Dr. Bruzzese is the son-in-law of Mr. Agostini, one of our directors. Dr. Bruzzese brings to the Board of Directors over 20 years of experience in medical practice. The Board of Directors believes that Dr. Bruzzese’s knowledge of internal medicine and life sciences will assist us in our future growth and expansion plans.
| 22 |
Mark McLaughlin, President, Chief Executive Officer and Director
Mr. McLaughlin has served as our President and member of the Board of Directors since March 2004 and Chief Executive Officer since April 2011. Mr. McLaughlin brings extensive knowledge about raising capital, marketing, business and corporate development, and of our operations and long-term strategy to the Board of Directors. In addition, Mr. McLaughlin played an integral role in successfully prosecuting several intellectual property violations in our favor. Since 1994, he has served as President of McLaughlin International, Inc., or MII, a management consulting firm controlled by Mr. McLaughlin. Previously, Mr. McLaughlin served as Senior Vice President at Oppenheimer & Co. from 1990 to 1992 and Lehman Brothers from 1981 to 1990. Mr. McLaughlin graduated from the College of the Holy Cross. The Board of Directors believes that Mr. McLaughlin’s leadership and extensive knowledge about us is essential to our future growth.
Dominic J. Agostini, Director
Mr. Agostini has served as a member of our Board of Directors since February 2001. Mr. Agostini brings to the Board of Directors a long and successful business career with extensive experience at the senior management level. Mr. Agostini currently is a director, officer and one-third owner of A. B. Smithfield Associates, which operates a retail shopping center in Rhode Island. Mr. Agostini previously served as Senior Manager of Business Development for the Gilbane Building Company until 1993, and prior to such position, he served as Chief Operating Officer of International Processing Systems and Executive Vice President of ICM Corporation. Mr. Agostini received a Bachelor of Science in Business Administration from Bryant University. Mr. Agostini is the father-in-law of Dr. Bruzzese, our Chairman.
John R. Strawn, Jr., Director
Mr. Strawn has served as a member of our Board of Directors since July 2011. Mr. Strawn brings to the Board of Directors over 25 years of legal experience, including extensive knowledge of our intellectual property portfolio. His practice focuses on complex commercial litigation. Mr. Strawn has successfully represented the company for over 10 years, including in a dispute over the ownership and licensing of multiple patents. After prevailing in a jury trial that was upheld on appeal in 2009, the matter was settled on favorable terms for the company. In 2010, Mr. Strawn became a founding partner of Strawn Pickens LLP in Houston, Texas. Prior to founding Strawn Pickens, Mr. Strawn was the Co-Managing Partner of Cruse Scott Henderson & Allen LLP, a law firm based in Houston, Texas, since 1992. Mr. Strawn received his Juris Doctor from the University of Texas Law School and his bachelor’s degree from Dartmouth College.
Legal Proceedings
During the past ten years, none of our current directors or executive officers has been:
| · | the subject of any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| · | convicted in a criminal proceeding or is subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| · | subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; |
| · | found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, that has not been reversed, suspended, or vacated; |
| · | subject of, or a party to, any order, judgment, decree or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of a federal or state securities or commodities law or regulation, law or regulation respecting financial institutions or insurance companies, law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| · | subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
None of our directors, officers or affiliates, or any beneficial owner of 5% or more of our common stock, or any associate of such persons, is an adverse party in any material proceeding to, or has a material interest adverse to, us or any of our subsidiaries.
Corporate Governance
We currently have no standing audit, compensation or nominating committees or committees performing similar functions, nor do we have written audit, compensation or nominating committee charters. Our Board of Directors believes it unnecessary to have such committees at this time because our Board of Directors can perform the functions of such committees adequately.
| 23 |
We do not have any defined policy or procedural requirements for shareholders to submit recommendations or nominations for directors. The Board of Directors believes that, given the stage of our development, a specific nominating policy would be premature until our business operations develop to a more advanced level. We currently do not have any specific or minimum criteria for the election of nominees to the Board of Directors and we do not have any specific process or procedure for evaluating such nominees. The Board of Directors will assess all candidates, whether submitted by management or shareholders, and make recommendations for election or appointment. A shareholder who wishes to communicate with our Board of Directors may do so by directing a written request addressed to our director at the address on the cover of this report.
Code of Ethics
We have not yet adopted a code of ethics within the definition of Item 406 of Regulation S-K. Currently, we only have a single executive officer and 3 full-time and 11 part-time employees. As our business continues to grow, and we become more experienced as a fully-reporting public company, our Board of Directors plans to implement a code of ethics.
Section 16(a) Beneficial Ownership Reporting Compliance
We are currently not subject to Section 16(a) of the Exchange Act as we do not have a class of equity securities registered pursuant to section 12 of the Exchange Act.
Item 11. Executive Compensation
As a “smaller reporting company,” we have elected to follow the scaled disclosure requirements for smaller reporting companies with respect to the disclosures required by Item 402 of Regulation S-K. Under such scaled disclosure, we are not required to provide a Compensation Discussion and Analysis, Compensation Committee Report and certain other tabular and narrative disclosures relating to executive compensation.
Executive Compensation
The following table sets forth information concerning the compensation of our principal executive officer for the years ended December 31, 2012 and 2011.
| Summary Compensation Table | ||||||||||||||||||||||
| Name and Principal Position | Year | Salary | Option Awards | Non-Equity Incentive Plan Compensation | All Other Compensation | Total | ||||||||||||||||
| ($) | ($)(1) | ($) | ($) | ($) | ||||||||||||||||||
| Mark McLaughlin | 2012 | 123,600 | - | - | (4) | 18,106 | (5) | 141,706 | ||||||||||||||
| President, Chief Executive Officer and Director(2) | 2011 | 120,000 | (3) | 131,900 | - | (4) | 10,682 | (5) | 262,582 | |||||||||||||
| (1) | Amounts shown reflect aggregate grant date fair value and, where applicable, incremental fair value as of modification date, of awards and do not reflect whether the recipient actually has realized a financial benefit from such grant, such as by exercising the options or selling the stock. A discussion of the assumptions used in calculating the award values may be found in Note 2 to our financial statements contained herein. |
| (2) | Mr. McLaughlin receives no compensation for serving as a member of our Board of Directors. |
| (3) | In June 2012, we issued to Mr. McLaughlin 588,236 shares of our common stock and 3-year warrants to purchase 294,118 shares of our common stock at $0.40 per share at $0.17 per unit in satisfaction of $100,000 of deferred salary accrued in 2011. |
| (4) | Under his employment agreement entered into on April 20, 2011, as amended, Mr. McLaughlin earns an annual incentive bonus award consisting of 5% of our pre-tax earnings payable each semi-annual fiscal year. We did not have any pre-tax earnings in 2012 or 2011, and no incentive bonus was earned or awarded. |
| (5) | In June 2011, the Board of Directors authorized a one-year extension of the expiration date for warrants held by Mr. McLaughlin to purchase 1.5 million shares of our common stock at $0.12 per share that were to expire in 2011. These warrants with such one-year extension of the expiration date had an incremental fair value of $10,682. In May 2012, the Board of Directors similarly authorized another one-year extension of the expiration date for these same warrants. The warrants with such one-year extension of the expiration date in 2012 had an incremental fair value of $18,106. |
The following table sets forth information concerning the outstanding equity awards held by our principal executive officer at December 31, 2012.
| 24 |
| Outstanding Equity Awards at Fiscal Year-End for 2012 | ||||||||||||||||||
| Option Awards | ||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Number of Securities Underlying Unexercised Options (#) Unearned | Option Exercise Price ($) | Option Expiration Date | |||||||||||||
| Mark McLaughlin(1) | 1,000,000 | - | - | 0.10 | 03/01/2018 | |||||||||||||
| 1,700,000 | - | - | 0.20 | 04/20/2021 | ||||||||||||||
| 500,000 | - | - | 0.40 | 04/20/2021 | ||||||||||||||
| - | - | 500,000 | (2) | 0.40 | 04/20/2021 | |||||||||||||
| - | - | 500,000 | (3) | 0.80 | 04/20/2021 | |||||||||||||
| (1) | All options held by Mr. McLaughlin are fully vested from grant date and exercisable on a cashless basis. |
| (2) | Options become earned and exercisable upon our achieving $5 million in revenues in any fiscal year prior to the expiration date. |
| (3) | Options become earned and exercisable upon our achieving $10 million in revenues in any fiscal year prior to the expiration date. |
Employment Agreement
On October 12, 2012, we entered into a five-year employment agreement with Mr. McLaughlin, our President and Chief Executive Officer, under which he is to be compensated at $134,000 per annum. As of December 31, 2012, all prior compensation due to Mr. McLaughlin has been satisfied either through cash payments or stock issuances. In addition to his base salary, Mr. McLaughlin will earn an annual incentive bonus award consisting of 5% of our pre-tax earnings payable each semi-annual fiscal year. We also granted to Mr. McLaughlin under his employment agreement, as amended, 10-year, fully-vested options to purchase an aggregate of 3.3 million shares of our common stock, such options consisting of the right to purchase: (i) 1.7 million shares of our common stock at $0.20 per share; (ii) 0.5 million shares of our common stock at $0.40 per share; (iii) 0.5 million shares of our common stock at $0.40 per share upon our achieving $5 million in revenues in any fiscal year prior to the expiration date; and (iv) 0.5 million shares of our common stock at $0.80 per share upon our achieving $10 million in revenues in any fiscal year prior to the expiration date. If at any time prior to the expiration date of the options we merge into or are acquired by another company, any outstanding options granted under Mr. McLaughlin’s employment agreement will become immediately exercisable on the business day immediately preceding the merger or acquisition at $0.40 per share or the preceding average 30-day market price of our common stock prior to the announcement of such merger or acquisition, whichever price is lower.
Prior to our entering into this employment agreement, we compensated Mr. McLaughlin for his services as our President at $10,000 per month. From time to time he voluntarily deferred this compensation without interest.
Our employment agreement with Mr. McLaughlin contains provisions prohibiting competition by him following his employment with us. Mr. McLaughlin’s employment agreement specifies the conditions under which the agreement may be terminated and stipulates that he shall not be entitled to severance payments upon termination. Mr. McLaughlin is entitled to retain any options granted under his employment agreement and that remain outstanding at the time his employment agreement is terminated, however. We do not have any other existing arrangements providing for payments or benefits in connection with the resignation, severance, retirement or other termination of Mr. McLaughlin, or a change in control of the company or a change in his responsibilities following a change in control. We currently do not have any defined pension plan for Mr. McLaughlin. We currently do not have any nonqualified defined contribution or other plan that provides for the deferral of compensation for Mr. McLaughlin nor do we currently intend to establish any such plan.
Compensation of Directors
The following table sets forth information concerning the compensation of our directors for the year ended December 31, 2012.
| Director Compensation for 2012 | ||||||||||||||||||||
| Fees Earned or Paid in Cash | Option Awards | Non-Equity Incentive Plan Compensation | All Other Compensation | Total | ||||||||||||||||
| Name | ($) | ($)(1) | ($) | ($) | ($) | |||||||||||||||
| Anthony G. Bruzzese, M.D. | - | - | (2) | - | (3) | 9,288 | (4) | 9,288 | ||||||||||||
| Dominic J. Agostini | - | - | (4) | - | 4,085 | (6) | 4,085 | |||||||||||||
| John R. Strawn, Jr. | - | (7) | - | (8) | - | - | ||||||||||||||
| 25 |
| (1) | Amounts shown reflect aggregate grant date fair value and, where applicable, incremental fair value as of modification date, of awards and do not reflect whether the recipient actually has realized a financial benefit from such grant, such as by exercising the options or selling the stock. A discussion of the assumptions used in calculating the award values may be found in Note 2 to our financial statements contained herein. |
| (2) | As of December 31, 2012, Dr. Bruzzese held fully-vested options to purchase an aggregate of 1,310,000 shares of our common stock, such options consisting of the right to purchase: (i) 500,000 shares of our common stock at $0.20 per share with an expiration date of December 31, 2013; (ii) 560,000 shares of our common stock at $0.20 per share with an expiration date of April 20, 2021; and (iii) 250,000 shares of our common stock at $0.40 per share with an expiration date of April 20, 2021, such options to become exercisable upon our achieving $5 million in revenues in any fiscal year prior to the expiration date. Each such option held by Dr. Bruzzese is exercisable on a cashless basis. |
| (3) | Under his director’s agreement effective as of April 20, 2011, Dr. Bruzzese earns an annual incentive bonus award consisting of 1% of our pre-tax earnings payable each semi-annual fiscal year. We did not have any pre-tax earnings in 2012 and no incentive bonus was earned or awarded. |
| (4) | In May 2012, the Board of Directors authorized a one-year extension of the expiration for fully vested options held by Dr. Bruzzese to purchase 500,000 shares of our common stock at $0.20 per share that were set to expire in 2012. These options, with such one-year extension of the expiration date, had an incremental fair value of $9,288. |
| (5) | As of December 31, 2012, Mr. Agostini held fully-vested options to purchase an aggregate of 875,000 shares of our common stock, such options consisting of the right to purchase: (i) 500,000 shares of our common stock at $0.10 per share with an expiration date of December 31, 2013; (ii) 250,000 shares of our common stock at $0.20 per share with an expiration date of April 20, 2021; and (iii) 125,000 shares of our common stock at $0.40 per share with an expiration date of April 20, 2021, such options to become exercisable upon our achieving $5 million in revenues in any fiscal year prior to the expiration date. Each such option held by Mr. Agostini is exercisable on a cashless basis. |
| (6) | In May 2012, the Board of Directors authorized a one-year extension of the expiration date for fully-vested options held by Mr. Agostini to purchase 500,000 shares of our common stock at $0.10 per share that were to expire in 2012. These options, with such one-year extension of the expiration date, had an incremental fair value of $4,085. |
| (7) | As of December 31, 2012, Mr. Strawn held fully-vested options to purchase an aggregate of 2,000,000 shares of our common stock, such options consisting of the right to purchase: (i) 1,000,000 shares of our common stock at $0.20 per share with an expiration date of July 1, 2021; (ii) 500,000 shares of our common stock at $0.40 per share with an expiration date of July 1, 2021; and (iii) 500,000 shares of our common stock at $0.40 per share with an expiration date of July 1, 2021, such options to become exercisable upon our achieving $5 million in revenues in any fiscal year prior to the expiration date. Each such option held by Mr. Strawn is exercisable on a cashless basis. |
| (8) | Under his director’s agreement effective as of July 1, 2011, Mr. Strawn earns an annual incentive bonus award consisting of 3% of our pre-tax earnings payable each semi-annual fiscal year. We did not have any pre-tax earnings in 2012 and no incentive bonus was earned or awarded. |
The Board of Directors may determine remuneration to be paid to the directors with interested members refraining from voting. Our independent directors each have entered into two-year director’s agreements with us, pursuant to which they will receive annual cash compensation of an amount to be negotiated and agreed upon when we have the financial wherewithal to pay such compensation for their service. In addition to their base compensation, Dr. Bruzzese and Mr. Strawn each will earn an annual incentive bonus award consisting of 1% and 3%, respectively, of our pre-tax earnings payable each semi-annual fiscal year. We also made grants of 10-year, fully-vested options to purchase 810,000, 375,000 and 2,000,000 shares of our common stock as described in the footnotes to the above table to Dr. Bruzzese, Mr. Agostini and Mr. Strawn, respectively, pursuant to their director’s agreements effective as of April 20, 2011, April 20, 2011, and July 1, 2011, respectively. If at any time prior to the expiration date of the options we merge into or are acquired by another company, any outstanding options granted under the directors’ agreements will become immediately exercisable on the business day immediately preceding the merger or acquisition at $0.40 per share or the preceding average 30-day market price of our common stock prior to the announcement of such merger or acquisition, whichever price is lower. We do not compensate our non-independent director, Mr. McLaughlin, for serving as our director. All directors are eligible to receive reimbursement of expenses incurred with respect to attendance at board meetings, which is not included in the above table. We do not maintain a medical, dental or retirement benefits plan specifically for our directors, but all directors are eligible to participate in our employee group health and dental insurance plans.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following sets forth information as of March 28, 2013, regarding the number of shares of our common stock beneficially owned by (i) each person that we know beneficially owns more than 5% of our outstanding common stock, (ii) each of our directors and named executive officer and (iii) all of our directors and named executive officer as a group.
| 26 |
The amounts and percentages of our common stock beneficially owned are reported on the basis of SEC rules governing the determination of beneficial ownership of securities. Under the SEC rules, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or “investment power,” which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has the right to acquire beneficial ownership within 60 days through the exercise of any stock option, warrant or other right. Under these rules, more than one person may be deemed a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest. Unless otherwise indicated, each of the shareholders named in the table below, or his or her family members, has sole voting and investment power with respect to such shares of our common stock. Except as otherwise indicated, the address of each of the shareholders listed below is: c/o Immudyne, Inc., 50 Spring Meadow Rd., Mount Kisco, NY 10549.
As of March 28, 2013, there were 28,909,973 shares of our common stock issued and outstanding.
| Name of beneficial owner | Number of shares | Percent of class | ||||||
| 5% Shareholders | ||||||||
| Lane Deyoe 11997 N. Lake Dr. Boynton Beach, FL 33436 | 2,359,494 | (1) | 8.04 | % | ||||
| Directors and named executive officer | ||||||||
| Mark McLaughlin | 9,112,764 | (2) | 31.56 | % | ||||
| Anthony G. Bruzzese, M.D. | 2,099,999 | (3) | 6.98 | % | ||||
| Dominic J. Agostini | 1,393,500 | (4) | 4.70 | % | ||||
| John R. Strawn, Jr. | 2,110,000 | (5) | 6.90 | % | ||||
| Directors and named executive officer as a group (4 persons) | 12,413,870 | 50.14 | % | |||||
| (1) | Consists of 195,000 shares and presently-exercisable warrants to purchase 474,831 shares held of record by the Deyoe Family Limited Partnership over which Mr. Deyoe has sole voting and dispositive power. |
| (2) | Consists of 588,236 shares held of record by McLaughlin International, Inc., presently-exercisable warrants to purchase 1,500,000 shares, presently-exercisable warrants to purchase 294,118 shares held of record by McLaughlin International, Inc. and presently-exercisable options to purchase 3,300,000 shares. Mr. McLaughlin has sole voting and dispositive power over all shares and warrants held of record by McLaughlin International, Inc. |
| (3) | Consists of 115,000 shares held jointly with Dr. Bruzzese’s spouse, presently-exercisable warrants to purchase 169,666 shares and presently-exercisable options to purchase 1,060,000 shares. |
| (4) | Consists of 143,500 shares held of record by or jointly with Mr. Agostini’s deceased spouse over which he has sole voting and dispositive power and presently-exercisable options to purchase 750,000 shares. |
| (5) | Consists of 400,000 shares and presently-exercisable warrants to purchase 200,000 shares held of record by Strawn Pickens LLP over which Mr. Strawn has shared voting and dispositive power, and presently-exercisable options to purchase 1,500,000 shares. |
We are not aware of any arrangements that could result in a change in control of the Company.
As of December 31, 2012, we have no formal equity compensation plan in effect.
Item 13. Certain Relationships and Related Transactions, and Director Independence
In addition to the executive officer and director compensation arrangements discussed in “Executive Compensation” beginning on page 23, the following describes transactions since January 1, 2009, to which we have been a participant, in which the amount involved in the transaction exceeds the lesser of $120,000 or 1% of the average of our total assets at year end and in which any of our directors, executive officer or holders of more than 5% of our capital stock, or any immediate family member of, or person sharing the household with, any of these individuals, had or will have a direct or indirect material interest.
Royalty Agreement
We are subject to a royalty agreement, pursuant to which we are required to pay a monthly royalty of 8% on all sales of certain skin care products up to $227,175. We entered into the royalty agreement to settle a suit between Mr. McLaughlin and us, over disputed patent and licensing arrangements. Mr. McLaughlin, our President, has a 60% interest in the royalties paid under the agreement, or $136,305, and Akin, Gump, Strauss, Hauer & Feld L.L.P., Mr. McLaughlin’s counsel in the matter, is entitled to the remaining 40% interest. Royalties earned and expensed were $24,000 in 2011 and approximately $40,000 in 2012. We made a royalty payment of approximately $16,061 on August 13, 2012. As of March 28, 2013, the remaining commitment under the royalty agreement is approximately $115,000. Mr. McLaughlin and Akin, Gump customarily defer without interest payments due under the royalty agreement until we have the financial wherewithal to pay such royalties (at December 31, 2012, $95,938 was due under the Royalty Agreement and remains to the be paid).
| 27 |
Indebtedness to our President and Directors
From time to time, Mr. McLaughlin, our President, has made short-term advances to us for our operating needs. These advances bear interest at 5% per annum, are unsecured and have no fixed terms of repayment. Since 2009, the largest aggregate amount of principal outstanding was $222,574. In each of 2009, 2010, 2011 and 2012, principal paid was $30,000, $20,000, $69,000, and $42,074 and interest paid was $2,954, $6,591, 4,883, and $542 respectively.
In April 2011, in satisfaction of $140,000 advance payable, Mr. McLaughlin exercised 1,500,000 options, 1,000,000 options of which were exercised at $0.10 per share, and 500,000 of which were exercised at $0.08 per share. In the first two quarters of 2012, principal paid was $41,531 and interest paid was $543. As of October 17, 2012, no principal and interest remains outstanding on these advances.
In addition, Mr. McLaughlin made an unsecured advance to us in 2005 for our long-term operating needs bearing interest at 10% and payable monthly from date of advance. Since 2009, the largest aggregate amount of principal outstanding on this advance was $123,119. In each of 2009, 2010 and 2011, principal paid was $34,774, $35,066 and $42,381, and interest paid was $10,747, $6,662 and $3,139, respectively. As of the pay-off date in February 2012, principal and interest paid in 2012 was $10,898 and $482, respectively.
In the past, Mr. McLaughlin has voluntarily deferred receipt of his salary as our President until such time as we have the financial wherewithal to pay such salary, but there can be no assurance that Mr. McLaughlin will continue this practice. In months where our cash flow does not permit us to pay his monthly compensation, the amount owed Mr. McLaughlin is accrued without interest. In 2011, the largest amount of accrued salary was $100,000. In June 2012 and in connection with our private placement, we issued to Mr. McLaughlin 588,236 shares of our common stock and 3-year warrants to purchase 294,118 shares of our common stock at $0.40 per share at $0.17 per unit in satisfaction of $100,000 of deferred salary accrued in 2011. As of December 31, 2012, no accrued salary required to be paid remains outstanding.
From time to time, Dr. Bruzzese, our Chairman, has made advances to us for our operating needs. These advances bore interest at 5% per annum, were unsecured and had no fixed terms of repayment. Since 2009, the largest aggregate amount of principal outstanding was $32,500. In each of the uears 2009, 2010 and 2011, no principal was paid and interest paid was $875. In 2012, interest paid was $218. In June 2012 and in connection with our private placement, we issued to Dr. Bruzzese 191,176 shares of our common stock and 3-year warrants to purchase 95,588 shares of our common stock at $0.40 per share at $0.17 per unit in satisfaction of the $32,500 principal outstanding due him.
From time to time, Mr. Agostini, a member of our Board of Directors, has made advances to us for our operating needs. These advances bore interest at 5% per annum, were unsecured and had no fixed terms of repayment. Since 2009, the largest aggregate amount of principal outstanding was $17,500. In each of 2009, 2010 and 2011, no principal was paid and interest paid was $875 for each year. In 2012, interest paid was $292. In April 2012, Mr. Agostini exercised options to purchase 500,000 shares of our common stock at $0.10 per share, with payment consisting of $32,500 in cash and satisfaction of the $17,500 in principal outstanding.
Common Stock Issuances
In April 2011, Mr. McLaughlin, our President, exercised stock options for 1,500,000 shares, in satisfaction of $140,000 due to Mr. McLaughlin, 1,000,000 options of which were exercised at $0.10 per share, and 500,000 of which were exercised at $0.08 per share. Of the options exercised, 500,000 were exercised at $0.08 per share and 1,000,000 were exercised at $0.10 per share.
In July 2011, we issued to Dr. Bruzzese, our Chairman, 72,941 shares of our common stock and 3-year warrants to purchase 36.470 shares of our common stock at $0.40 per share at $0.17 per unit for $12,400 in connection with our private placement.
In June 2012, we issued to Mr. McLaughlin, our President, 588,236 shares of our common stock and 3-year warrants to purchase 294,118 shares of our common stock at $0.40 per share at $0.17 per unit in satisfaction of $100,000 of deferred salary accrued in 2011.
In June 2012, we issued to Dr. Bruzzese, our Chairman, 191,176 shares of our common stock and 3-year warrants to purchase 95,588 shares of our common stock at $0.40 per share at $0.17 per unit in satisfaction of $32,500 due to Dr. Bruzzese. In addition, in July 2012, we issued to Dr. Bruzzese 15,216 shares of our common stock and 3-year warrants to purchase 7,608 shares of our common stock at $0.40 per share at $0.17 per unit for $2,587 in connection with our private placement.
| 28 |
In June 2012, we issued to Mr. Agostini, a member of our Board of Directors, 500,000 shares of our common stock upon his exercise of stock options at $0.10 per share for $50,000, consisting of $32,500 in cash and satisfaction of $17,500 due to him.
In June 2012, in connection with our private placement, we issued 949,663 shares of our common stock and 3-year warrants to purchase 474,831 shares of our common stock at $0.40 per share to Lane Deyoe in satisfaction of a $0.16 million note payable. Following this issuance, Mr. Deyoe beneficially owns more than 5% of our common stock outstanding.
Common Stock Retirement and Option Cancelations
On June 13, 2011, Arun K. Bahl, then a beneficial owner of more than 5% of our common stock, returned options to purchase 3 million shares of our common stock to us for cancelation. The options returned for cancelation consisted of (i) options to purchase 1 million shares of our common stock at $0.07 per share with expiration in January 2015, (ii) options to purchase 1 million shares of our common stock at $0.08 per share with expiration in March 2017, and (iii) options to purchase 1 million shares of our common stock at $0.10 per share with expiration in March 2018.
In September 2011, we retired an additional 1.14 million shares of our common stock that had been issued.
Equity Awards and Employment Agreements
On April 20, 2011, we entered into a five-year employment agreement with Mr. McLaughlin as our President and Chief Executive Officer, which was amended on October 12, 2012, under which he will be compensated at $134,400 per annum. In the past, Mr. McLaughlin has voluntarily deferred receipt of his salary as our President until such time as we have the financial wherewithal to pay such salary, but there can be no assurance that Mr. McLaughlin will continue this practice. As of June 30, 2012, all prior compensation due to Mr. McLaughlin has been satisfied either through cash payments or stock issuances. The employment agreement was entered into between us and McLaughlin International, Inc., a management consulting firm solely controlled by Mr. McLaughlin and through which he had historically provided us with consulting services. Effective as of October 12, 2012, we amended this employment agreement to be between us and Mr. McLaughlin directly as he dedicates all of his business time to his position as our executive officer.
In 2011, we entered into two-year director’s agreements with our non-employee directors pursuant to which we granted stock options to such directors.
In June 2011, the Board of Directors authorized the extension of the expiration date by one year of certain warrants and options held by Dr. Bruzzese and Messrs. McLaughlin and Agostini that were to expire in 2011. The incremental fair value of the extended warrant held by Mr. McLaughlin, our President, as of June 14, 2011, was $10,682. The incremental fair value of the extended options held by Dr. Bruzzese, our Chairman, and Mr. Agostini, a member of our Board of Directors, as of June 14, 2011, were $735 and $7,591, respectively. In May 2012, the Board of Directors again authorized the extension of the expiration date by one year of certain warrants and options held by Dr. Bruzzese and Messrs. McLaughlin and Agostini that were to expire in 2012. The incremental fair value of the extended warrant held by Mr. McLaughlin as of May 22, 2012, was $18,106. The incremental fair value of the extended options held by Dr. Bruzzese and Mr. Agostini as of May 22, 2012, were $9.289 and $4,085, respectively.
For a description of these director and employment agreements and equity awards, see “Executive Compensation” beginning on page 24.
Employment Arrangements with an Immediate Family Member of our President
Brunilda McLaughlin, the wife of Mr. McLaughlin, our President, provides bookkeeping services for us. Mrs. McLaughlin was compensated for these services at $2,000 per month during 2009, 2010 and the first four months of 2011. In April 2011, we entered into a two-year employment agreement, as amended, with Mrs. McLaughlin doing business as McLaughlin International, Inc., a management consulting firm solely controlled by Mr. McLaughlin, under which we would compensate her for her services with (a) cash compensation of $2,000 per month; (b) 10-year, fully-vested options with cashless exercise rights to purchase 200,000 shares of our common stock at $0.20 per share; (c) 10-year, fully-vested options with cashless exercise rights to purchase 100,000 shares of our common stock at $0.40 per share, such options to become exercisable upon our achieving $5 million in revenues in any fiscal year prior to the expiration date; and (d) an annual incentive bonus award amounting to 0.5% of our pre-tax earnings.
| 29 |
Legal Services Provided by Director
Strawn Pickens LLP, a law firm co-founded by one of our directors, Mr. Strawn, performs legal services on our behalf on an hourly-fee basis in the ordinary course and has a contingency fee arrangement with us in a suit with former officers of the company and their affiliated entities. In June 2012, we issued Strawn Pickens LLP 402,333 shares of our common stock and 3-year warrants to purchase 200,000 shares of our common stock at $0.40 per share in satisfaction of approximately $68,000 in legal services. In 2011, Cruse Scott Henderson & Allen LLP received $225,000 as payment for accrued legal services, $200,000 of which was paid by an investor in the Company in exchange for warrants to purchase 2,400,000 share of common stock held by Cruse Scott, 1,136,842 shares of which were subsequently retired. Mr. Strawn served as Co-Managing Partner until January 2010 and during the period in which Cruse Scott Henderson & Allen LLP represented the company and accrued legal services.
Office Space Provided by our President
Our principal executive offices are in office space provided at no cost to us by our President, Mr. McLaughlin.
Director Independence
Our Board of Directors currently is comprised of four directors, Dr. Bruzzese and Messrs. McLaughlin, Agostini and Strawn. While we are not subject to any director independence requirements because of our quotation on the OTC Markets-OTQB, we have adopted the NASDAQ listed company standards for the purposes of determining director independence. Under these standards, the Board of Directors has determined that Dr. Bruzzese and Mr. Agostini qualify as independent directors. In determining the independence of our directors, the Board of Directors considered all transactions in which we and any director had any interest, including those discussed under “Certain Relationships and Related Transactions” beginning on page 26 of this Annual report. The Board of Directors currently has no separately designated standing committees.
Item 14. Principal Accounting Fees and Services
Our Board of Directors has selected PKF O’Connor Davies, a division of O’Connor Davies, LLP (“PKF”) as the independent registered public accounting firm to audit our books and accounts for the fiscal years ending December 31, 2012 and 2011. PKF has served as our independent accountant since 2010. The aggregate fees billed, or expected to be billed, for the last two fiscal years ended December 31, 2012 and 2011, for professional services rendered by PKF were as follows:
| 2012 | 2011 | |||||||
| Audit fees | $ | 51,825 | $ | 34,175 | ||||
| Audit-related fees | — | — | ||||||
| Tax fees | 7,500 | 7,925 | ||||||
| All other fees | — | — | ||||||
In the above table, “audit fees” are fees billed for services provided related to the audit of our annual financial statements, quarterly reviews of our interim financial statements, filing of our S-1 Registration Statement and amendments thereto and services normally provided by the independent accountant in connection with statutory and regulatory filings or engagements for those fiscal periods. “Tax fees” are fees billed, or to be billed, by the independent accountant for professional services rendered for tax compliance, tax advice and tax planning.
Our Board of Directors pre-approves all services provided by our independent accountants. Our Board of Directors reviewed and approved all of the above services and fees.
PART IV
Item 15. Exhibits, Financial Statement Schedules
The following documents are filed as part of or are included in this Annual Report:
| 1. | Financial statements listed in the Index to Financial Statements, filed as part of this Annual Report beginning on page F-1; and |
| 2. | Exhibits listed in the Exhibit Index filed as part of this Annual Report. |
| 30 |
IMMUDYNE, INC.
Financial Statements
For The Years Ended
December 31, 2012 and 2011
| 31 |
| Table of Contents | Table | |
| Report of Independent Registered Public Accounting Firm | F-2 | |
| Balance Sheet as of December 31, 2012 and 2011 | F-3 | |
| Statement of Operations for the years ended December 31, 2012 and 2012 |
F-4 | |
| Statement of Stockholders’ Equity (Deficit) for the years ended December 31, 2012 and 2011 |
F-5 | |
| Statement of Cash Flows for the years ended December 31, 2012 and 2011 |
F-6 | |
| Notes to Financial Statements | F-7 |
Report of Independent Registered Public Accounting Firm
Board of Directors
Immudyne, Inc.
We have audited the accompanying balance sheet of Immudyne, Inc. as of December 31, 2012 and 2011, and the related statements of operations, stockholders’ equity (deficit), and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Immudyne, Inc. as of December 31, 2012 and 2011, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has incurred a significant accumulated deficit through December 31, 2012, and has significant negative cash flow from operating activities for the year ended December 31, 2012. The Company may not have adequate readily available resources to fund operations through December 31, 2013. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ PKF O’Connor Davies | |
| A Division of O’Connor Davies, LLP | |
| New York, NY | |
| March 28, 2013 |
| F-2 |
Immudyne, Inc.
Balance Sheet
| December 31 | ||||||||
| 2012 | 2011 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 98,930 | $ | 33,502 | ||||
| Trade accounts receivable | 49,690 | 36,357 | ||||||
| Inventory | 63,378 | 58,800 | ||||||
| Total Current Assets | 211,998 | 128,659 | ||||||
| Furnishings and equipment | 157,697 | 214,671 | ||||||
| Total Assets | $ | 369,695 | $ | 343,330 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Current Liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 187,096 | $ | 199,341 | ||||
| Due to officer | - | 100,000 | ||||||
| Current portion of notes payable | - | 262,172 | ||||||
| Total Current Liabilities | 187,096 | 561,513 | ||||||
| Deferred tax liability | 47,600 | 64,800 | ||||||
| Total Liabilities | 234,696 | 626,313 | ||||||
| Stockholders’ equity (deficit) | ||||||||
| Common stock, $0.01 par value; 50,000,000 shares authorized, 28,909,973 and 24,729,030 shares issued and outstanding in 2012 and 2011, respectively | 289,099 | 247,290 | ||||||
| Additional paid-in capital | 7,641,499 | 6,871,510 | ||||||
| Treasury stock 505,000 shares in 2011 | - | (51,833 | ) | |||||
| Accumulated (deficit) | (7,795,599 | ) | (7,349,950 | ) | ||||
| Total Stockholders’ Equity (Deficit) | 134,999 | (282,983 | ) | |||||
| Total Liabilities and Stockholders’ Equity (Deficit) | $ | 369,695 | $ | 343,330 | ||||
The accompanying notes are an integral part of these financial statements
| F-3 |
Immudyne, Inc.
Statement of Operations
| Year Ended December 31 | ||||||||
| 2012 | 2011 | |||||||
| Sales | $ | 673,778 | $ | 743,828 | ||||
| Cost of sales | 125,619 | 241,264 | ||||||
| Gross Profit | 548,159 | 502,564 | ||||||
| Compensation and related expenses | (381,263 | ) | (707,235 | ) | ||||
| Professional fees | (253,407 | ) | (53,341 | ) | ||||
| General and administrative expenses | (453,514 | ) | (318,355 | ) | ||||
| Operating (Loss) | (540,025 | ) | (576,367 | ) | ||||
| License Fee | 25,000 | 50,000 | ||||||
| Customer signing fee | - | 27,500 | ||||||
| Other income | 55,547 | - | ||||||
| Interest (expense) | (3,371 | ) | (21,262 | ) | ||||
| Net (Loss) Before Taxes | (462,849 | ) | (520,129 | ) | ||||
| Deferred income tax benefit | 17,200 | 17,200 | ||||||
| Net (Loss) | $ | (445,649 | ) | $ | (502,929 | ) | ||
| Basic and diluted (loss) per share | $ | (0.02 | ) | $ | (0.02 | ) | ||
| Average number of common shares outstanding | 27,523,000 | 22,391,000 | ||||||
The accompanying notes are an integral part of these financial statements
| F-4 |
Immudyne, Inc.
Statement of Stockholders’ Equity (Deficit)
| Additional | ||||||||||||||||||||||||
| Common Stock | Paid-in | Accumulated | Treasury | |||||||||||||||||||||
| Shares | Amount | Capital | (Deficit) | Stock | Total | |||||||||||||||||||
| Balance at December 31, 2010 | 20,053,194 | $ | 200,532 | $ | 6,110,768 | $ | (6,847,021 | ) | $ | (51,833 | ) | $ | (587,554 | ) | ||||||||||
| Issuance of common shares | 923,994 | 9,239 | 184,261 | - | - | 193,500 | ||||||||||||||||||
| Common stock issued to advisor and consultant | 375,000 | 3,750 | (3,750 | ) | - | - | - | |||||||||||||||||
| Issuance of stock options | - | - | 400,000 | - | - | 400,000 | ||||||||||||||||||
| Cashless exercise of warrants | 2,273,684 | 22,737 | (22,737 | ) | - | - | - | |||||||||||||||||
| Retirement of common stock | (1,136,842 | ) | (11,368 | ) | 11,368 | - | - | - | ||||||||||||||||
| Warrants exercised with notes payable | 740,000 | 7,400 | 66,600 | - | - | 74,000 | ||||||||||||||||||
| Options exercised with notes payable | 1,500,000 | 15,000 | 125,000 | - | - | 140,000 | ||||||||||||||||||
| Net (loss) | - | - | - | (502,929 | ) | - | (502,929 | ) | ||||||||||||||||
| Balance at December 31, 2011 | 24,729,030 | 247,290 | 6,871,510 | (7,349,950 | ) | (51,833 | ) | (282,983 | ) | |||||||||||||||
| Issuance of common stock | 1,843,428 | 18,434 | 294,949 | - | - | 313,383 | ||||||||||||||||||
| Issuance of common stock for notes and other payables | 1,729,075 | 17,291 | 276,652 | - | - | 293,943 | ||||||||||||||||||
| Issuance of common stock for services | 719,440 | 7,194 | 115,111 | - | - | 122,305 | ||||||||||||||||||
| Issuance of stock options | - | - | 52,500 | - | - | 52,500 | ||||||||||||||||||
| Extension of option and warrant expiration dates | - | - | 31,500 | - | - | 31,500 | ||||||||||||||||||
| Options exercised | 325,000 | 3,250 | 29,250 | - | - | 32,500 | ||||||||||||||||||
| Options exercised with notes payable | 175,000 | 1,750 | 15,750 | - | - | 17,500 | ||||||||||||||||||
| Retirement of common stock | (106,000 | ) | (1,060 | ) | 1,060 | - | - | - | ||||||||||||||||
| Retirement of treasury stock | (505,000 | ) | (5,050 | ) | (46,783 | ) | - | 51,833 | - | |||||||||||||||
| Net (loss) | - | - | - | (445,649 | ) | - | (445,649 | ) | ||||||||||||||||
| Balance at December 31, 2012 | 28,909,973 | $ | 289,099 | $ | 7,641,499 | $ | (7,795,599 | ) | $ | - | $ | 134,999 | ||||||||||||
The accompanying notes are an integral part of these financial statements
| F-5 |
Immudyne, Inc.
Statement of Cash Flows
| Year Ended December 31 | ||||||||
| 2012 | 2011 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net (Loss) | $ | (445,649 | ) | $ | (502,929 | ) | ||
| Adjustments to reconcile net (loss) to net cash (used) by operating activities | ||||||||
| Depreciation | 56,974 | 56,689 | ||||||
| Deferred tax benefit | (17,200 | ) | (17,200 | ) | ||||
| Stock compensation expense | 84,000 | 400,000 | ||||||
| Common stock issued for services | 122,305 | - | ||||||
| Changes in Assets And Liabilities | ||||||||
| Trade accounts receivable | (13,333 | ) | 43,968 | |||||
| Inventory | (4,578 | ) | 46,200 | |||||
| Accounts payable and accrued expenses | (12,245 | ) | (134,414 | ) | ||||
| Due to officer | - | 90,000 | ||||||
| Net Cash (Used) by Operating Activities | (229,726 | ) | (17,686 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Cash overdraft | - | (41,000 | ) | |||||
| Issuance of common stock | 345,883 | 193,500 | ||||||
| Increase in notes payable | - | 75,000 | ||||||
| Repayment of note payable | (50,729 | ) | (176,312 | ) | ||||
| Net Cash Provided by Financing activities | 295,154 | 51,188 | ||||||
| Net increase in cash | 65,428 | 33,502 | ||||||
| Cash at beginning of the period | 33,502 | - | ||||||
| Cash at end of the period | $ | 98,930 | $ | 33,502 | ||||
| Supplemental Schedule of Non-Cash Investing and Financing Activities | ||||||||
| Cash paid during the year for interest | $ | - | $ | 21,262 | ||||
| Notes payable and other payables used to exercise options and warrants | $ | 311,443 | $ | 214,000 | ||||
The accompanying notes are an integral part of these financial statements
| F-6 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 1. | Organization and Going Concern |
Immudyne, Inc. (the “Company”) is a Delaware corporation established to develop, manufacture and sell natural immune support products. The Company has developed a proprietary approach to produce what it believes, based on testing and analysis conducted on its behalf, to be superior particulate and soluble beta glucans derived from yeast. The Company’s core nutraceutical and cosmetic product lines consist of yeast beta glucans in oral and topical applications to support the immune system. The Company concentrates its sales and marketing efforts on healthcare professionals, distributors for its all-natural raw material ingredient products and direct-to-consumer sales.
The accompanying financial statements have been prepared on the basis that the Company will continue as a going concern, which assumes the realization of assets and the satisfaction of liabilities in the normal course of business. At December 31, 2012, the Company has an accumulated deficit approximating $8,000,000 and for the year ended December 31, 2012 incurred negative cash flows from operations of approximately $230,000. These conditions raise substantial doubt about the Company's ability to continue as a going concern. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Based on the Company's cash balance at December 31, 2012 and projected cash needs in 2013, management estimates that it will need to raise additional capital to cover operating and capital requirements for the 2013 year. Management plans on raising the additional needed funds through increased sales volume, issuing additional shares of common stock or other equity securities, or obtaining debt financing. Although management has been successful to date in raising necessary funding, there can be no assurance that required future financing can be successfully completed on a timely basis, or on terms acceptable to the Company.
The Company has funded operations in the past through the sales of its products, issuance of common stock and through loans and advances from officers and directors. The Company’s continued operations are dependent upon obtaining an increase in its sales volume and the continued financial support from officers and directors or the issuance of additional shares of common stock.
| 2. | Summary of Significant Accounting Policies |
Basis of Presentation and Use of Estimates
The Company prepares its financial statements in conformity with accounting principles generally accepted in the United States of America which requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Some of the more significant estimates required to be made by management include the valuation of stockholders’ equity based transactions. Actual results could differ from those estimates.
Reclassification
Certain amounts in the prior year have been reclassified to conform to the current year presentation.
Inventory
At December 31, 2012, inventory consisted of nutraceutical and cosmetic products. At December 31, 2011, inventory also included animal feed product, a product line the Company began exiting in 2011. Inventory is maintained in the Company’s leased Kentucky warehouse.
| F-7 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 2. | Summary of Significant Accounting Policies (continued) |
Inventory (continued)
Inventory is valued at the lower of cost or market with cost determined on a first-in, first-out (“FIFO”) basis. Management compares the cost of inventory with the net realizable value and an allowance is made for writing down inventory to market value, if lower. Inventory consists of the following:
| December 31 | ||||||||
| 2012 | 2011 | |||||||
| Raw materials | $ | 12,800 | $ | 37,720 | ||||
| Finished products | 50,578 | 21,080 | ||||||
| $ | 63,378 | $ | 58,800 | |||||
Included in inventory at December 31, 2012 and 2011 is $0 and $10,661, respectively, for products related to animal feed.
Furnishings and Equipment
Furnishings and equipment are stated at cost. Depreciation is provided using the straight-line method over the estimated useful lives of the assets ranging from three to ten years.
Revenue Recognition
The Company’s policy is to record revenue as earned when a firm commitment, indicating sales quantity and price exists, delivery has taken place and collectability is reasonably assured. The Company generally records sales once the product is shipped to the customer. If applicable, provisions for discounts, returns, allowances, customer rebates and other adjustments are netted with gross sales. The Company accounts for such provisions during the same period in which the related revenues are earned. Customer discounts, returns and rebates have not been significant.
Delivery is considered to have occurred when title and risk of loss have transferred to the customer. Sales to international distributors are recognized in the same manner. If title does not pass until the product reaches the customer’s delivery site, then recognition of revenue is deferred until that time. There are no formal sales incentives offered to any of the Company’s customers. Volume discounts may be offered from time to time to customers purchasing large quantities on a per transaction basis. There are no special post shipment obligations or acceptance provisions that exist with any sales arrangements
| F-8 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 2. | Summary of Significant Accounting Policies (continued) |
Revenue Recognition (continued)
Revenue for the years ended December 31, 2012 and 2011, are summarized as follows:
| 2012 | 2011 | |||||||
| Animal feed | $ | 5,690 | $ | 148,507 | ||||
| Nutraceutical and cosmetic | 668,088 | 595,321 | ||||||
| Total | $ | 673,778 | $ | 743,828 | ||||
Customer signing fees represent fees received from a customer to perform a concept review on a product. These fees are recorded as revenue when the concept review is complete and when no future obligation exists. If the Company has future obligations in regards to a customer signing fee, the revenue is deferred until the future obligation has been satisfied.
Segments
The guidance for disclosures about segments of an enterprise requires that a public business enterprise report financial and descriptive information about its operating segments. Generally, financial information is required to be reported on the basis used internally for evaluating segment performance and resource allocation. The Company manages its operations as a single segment for purposes of assessing performance and making operating decisions. Revenue is generated predominately in the United States, and all significant assets are held in the United States.
Income Taxes
The Company records current and deferred taxes in accordance with Accounting Standards Codification (ASC) 740, “Accounting for Income Taxes.” This ASC requires recognition of deferred tax assets and liabilities for temporary differences between tax basis of assets and liabilities and the amounts at which they are carried in the financial statements, based upon the enacted rates in effect for the year in which the differences are expected to reverse. The Company establishes a valuation allowance when necessary to reduce deferred tax assets to the amount expected to be realized. The Company periodically assesses the value of its deferred tax asset, a majority of which has been generated by a history of net operating losses and determines the necessity for a valuation allowance.
| F-9 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 2. | Summary of Significant Accounting Policies (continued) |
Income Taxes (continued)
ASC 740 also provides a recognition threshold and measurement attribute for the financial statement recognition of a tax position taken or expected to be taken in a tax return. Using this guidance, a company may recognize the tax benefit from an uncertain tax position in its financial statements only if it is more likely-than-not (i.e., a likelihood of more than 50%) that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position.
The Company’s tax returns for all years since December 31, 2009, remain open to most taxing authorities.
Stock-Based Compensation
The Company follows the provisions of ASC 718, “Share-Based Payment”. Under this guidance compensation cost generally is recognized at fair value on the date of the grant and amortized over the respective vesting periods. The fair value of options at the date of grant is estimated using the Black-Scholes option pricing model. The expected option life is derived from assumed exercise rates based upon historical exercise patterns and represents the period of time that options granted are expected to be outstanding. The expected volatility is based upon historical volatility of the Company’s shares using weekly price observations over an observation period that approximates the expected life of the options. The risk-free rate approximates the U.S. Treasury yield curve rate in effect at the time of grant for periods similar to the expected option life. The estimated forfeiture rate included in the option valuation was zero.
Many of the assumptions require significant judgment and any changes could have a material impact in the determination of stock-based compensation expense.
Earnings (Loss) Per Share
Basic earnings (loss) per common share is based on the weighted average number of shares outstanding during each period presented. Warrants and options to purchase common stock are included as common stock equivalents only when dilutive. Potential common stock equivalents are excluded from dilutive earnings per share when the effects would be antidilutive.
Common stock equivalents comprising 13,592,720 and 11,057,500 shares underlying options and warrants at December 31, 2012 and 2011, respectively, have not been included in the loss per share calculation as the effects are anti-dilutive.
| F-10 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 2. | Summary of Significant Accounting Policies (continued) |
Recent Accounting Pronouncements
Accounting standards that have been issued or proposed by the FASB that do not require adoption until a future date are not expected to have a material impact on the financial statements upon adoption.
Fair Value of Financial Instruments
FASB ASC Topic 820, Fair Value Measurements and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. FASB ASC Topic 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes the following three levels of inputs that may be used to measure fair value:
Level 1 - Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities.
Level 2 - Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The carrying value of the Company’s financial instruments, including cash, trade accounts receivable and accounts payable and accrued expenses approximate fair value for all periods. The fair value of notes payable and due to officer at December 31, 2011, is also an approximation of their fair value..
Concentration of Credit Risk
The Company grants credit in the normal course of business to its customers. The Company periodically performs credit analysis and monitors the financial condition of its customers to reduce credit risk.
The Company monitors its positions with, and the credit quality of, the financial institutions with which it invests. The Company, at times, maintains balances in various operating accounts in excess of federally insured limits.
| F-11 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 2. | Summary of Significant Accounting Policies (continued) |
Concentration of Credit Risk (continued)
One customer accounted for 73% and 53% of sales for the years ended December 31, 2012 and 2011, respectively. At December 31, 2012 and 2011, this customer accounted for 32% and 85% of accounts receivable, respectively.
A second customer accounted for 12% of sales for the year ended December 31, 2012. At December 31, 2012, this customer accounted for 57% of accounts receivable.
| 3. | Furnishings and Equipment |
Furnishings and equipment consisted of the following:
| December 31 | ||||||||
| 2012 | 2011 | |||||||
| Furnishings and equipment, at cost | $ | 679,291 | $ | 679,291 | ||||
| Accumulated depreciation | 521,594 | 464,620 | ||||||
| $ | 157,697 | $ | 214,671 | |||||
Depreciation expense amounted to approximately $56,000 for each of the years ended December 31, 2012 and 2011, respectively.
| 4. | Notes Payable |
Notes payable are due to officers, directors and other related individuals. Certain notes are payable on demand while repayment of others is based on scheduled monthly payments. Interest rates vary from 0% to 10%. A summary of notes payable activity is as follows:
| Balance at December 31, 2010 | $ | 577,484 | ||
| Borrowing | 75,000 | |||
| Repayment | (176,312 | ) | ||
| Notes payable used to exercise options and warrants | (214,000 | ) | ||
| Balance at December 31, 2011 | 262,172 | |||
| Repayment | (50,729 | ) | ||
| Issuance of common stock for notes payable | (193,943 | ) | ||
| Notes payable used to exercise options | (17,500 | ) | ||
| Balance at December 31, 2012 | $ | - |
Interest expense on notes payable amounted to $3,371 and $21,262 for the years ended December 31, 2012 and 2011, respectively.
| F-12 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 5. | Income Taxes |
The Company incurred a loss for the years ended December 31, 2012 and 2011 and accordingly, no provision for federal income tax has been made in the accompanying financial statements. At December 31, 2012, the Company had available net operating loss carryforwards of approximately $2,800,000, expiring during various years through 2032.
A summary of the deferred tax asset using an approximate 34% tax rate is as follows:
| December 31 | ||||||||
| 2012 | 2011 | |||||||
| Net operating loss | $ | 954,000 | $ | 870,000 | ||||
| Valuation allowance | (954,000 | ) | (870,000 | ) | ||||
| Total | $ | - | $ | - | ||||
The net operating loss carryforwards could be subject to limitation in any given year in the event of a change in ownership as defined by IRC Section 382.
The deferred tax liability of $47,600 and $64,800 at December 31, 2012 and 2011, respectively, results from the difference in the carrying amount of furnishings and equipment between financial reporting and income tax reporting.
The deferred tax benefit included in the statement of operations represents the change in the deferred tax liability at each balance sheet date.
The difference between the statutory and the effective tax rate is primarily due to a change in valuation allowance on deferred taxes, as the Company has fully reserved the deferred tax asset resulting from available net operating loss carryforwards.
| 6. | Stockholders’ Equity |
During 2012, the Company raised $313,383 in a common stock offering at $0.17 per share. In addition, $293,943 of notes and other payables were converted into common stock under the same terms as the common stock offering. Each two shares of common stock issued included a three-year warrant for one share exercisable at $0.40. The Company raised an additional $32,500 and satisfied notes payable in the amount of $17,500 through the exercise of 500,000 options. The Company issued 719,440 common shares for services valued at $122,305.
During 2012, the Company extended the expiration date of 1,000,000 options and 1,500,000 warrants one year from 2012 to 2013, and issued an additional 1,400,000 options as discussed below. As a result of this transaction, the Company recorded a charge to stock-based compensation expense of $84,000 during the year ended December 31, 2012.
| F-13 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 6. | Stockholders’ Equity (continued) |
During 2011, the Company raised $193,500 through the issuance of 923,994 shares of common stock, and retired 1,136,842 of common stock. In addition, $214,000 of notes payable were satisfied through the exercise of 740,000 warrants and 1,500,000 options. Also during 2011, the Company issued 375,000 shares to an advisor and consultant and further issued 2,273,684 shares of common stock for the cashless exercise of 2,400,000 warrants with an exercise price of $0.01.
During 2011, the Company extended the expiration date of 1,500,000 options and 1,500,000 warrants one year from 2011 to 2012. The Company computed the fair value of this extension agreement and determined that such amount was not material.
Service-Based Stock Options
During 2012, the Company issued 1,200,000 options to three consultants at an exercise price of $0.20 per share that expire in 10 years. Of these options, 200,000 vest immediately and 1,000,000 vest fully in June 2013. Additionally, during June 2012 the Company also issued 200,000 options to employees with an exercise price of $0.20. These options expire in 10 years and vest semi-annually over 2 years.
During 2011, the Company issued 6,260,000 service-based options to various employees and consultants. The options, which vested immediately, were issued at exercise prices of $0.20 and $0.40 and expire in 10 years.
A summary of the outstanding service-based stock options are as follows:
| Number of Options | ||||
| Balance at December 31, 2010 | 7,822,500 | |||
| Granted | 6,260,000 | |||
| Cancelled | (3,000,000 | ) | ||
| Exercised | (1,500,000 | ) | ||
| Expired | (240,000 | ) | ||
| Balance at December 31, 2011 | 9,342,500 | |||
| Granted | 1,400,000 | |||
| Exercised | (500,000 | ) | ||
| Expired | (287,500 | ) | ||
| Balance at December 31, 2012 | 9,955,000 | |||
Options exercisable at December 31, 2012 and 2011, amounted to 9,305,000 and 9,342,500, respectively.
| F-14 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 6. | Stockholders’ Equity (continued) |
Service-Based Stock Options (continued)
All outstanding options have a cashless exercise provision, and certain options provide for accelerated vesting provisions and modifications, as defined, if the Company is sold or acquired.
The intrinsic value of options outstanding and exercisable amounted to $110,650 and $163,300 at December 31, 2012 and December 31, 2011, respectively. The intrinsic value of options exercised in 2012 and 2011 amounted to $35,000 and $115,000, respectively.
The following is a summary of outstanding service-based options at December 31, 2012:
| Exercise Price | Number of Options | Weighted Average Remaining Contractual Life | ||||
| $0.07 - $0.10 | $ | 1,500,000 | 4 years | |||
| $0.13 - $0.20 | 7,455,000 | 10 years | ||||
| $0.40 | 1,000,000 | 10 years | ||||
| Total | $ | 9,955,000 | ||||
All of the options issued through December 31, 2011, are fully vested. The 1,400,000 options issued in 2012 vest over one and two year periods through June 2014. The fair value of the 1,400,000 options issued in 2012 is $97,000, of which $52,500 has been expensed as of December 31, 2012, and $44,500 remained unearned, and will be expensed over the next 18 months.
Stock based compensation expense amounted to $84,000 and $400,000 for the years ended December 31, 2012 and 2011, respectively. Such amounts are included in compensation and related expenses in the accompanying statement of operations.
Performance-Based Stock Options
During 2011 and 2012 the Company granted performance-based options to purchase 4,625,000 shares of common stock at exercise prices of $0.40 and $0.80. The options expire in 10 years and are exercisable upon the Company achieving annual sales revenue of $5,000,000 and $10,000,000. The fair value of these performance-based options aggregated $147,000 and will be expensed over the implicit service period commencing once management believes the performance criteria will be met. Accordingly, at December 31, 2012, the unearned compensation for performance based options is $147,000.
| F-15 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 6. | Stockholders’ Equity (continued) |
Warrants
The following is a summary of outstanding and exercisable warrants:
| Number of Shares | Weighted Average Exercise Price | Year of Expiration | ||||||||
| Balance at December 31, 2010 | 5,609,823 | 0.12 | 2011 - 2020 | |||||||
| Exercised | (3,140,000 | ) | 0.03 | 2011 | ||||||
| Expired | (754,823 | ) | 0.40 | 2011 | ||||||
| Balance at December 31, 2011 | 1,715,000 | 0.16 | 2012 - 2013 | |||||||
| Granted | 2,037,720 | 0.40 | 2015 | |||||||
| Expired | (115,000 | ) | 0.40 | 2012 | ||||||
| Balance at December 31, 2012 | 3,637,720 | 0.28 | 2013 - 2015 | |||||||
In connection with the issuance of common stock in 2012, the Company granted warrants to acquire 2,037,720 shares of common stock at $0.40 per share. These warrants are immediately exercisable and expire in 2015.
The fair value of options and warrants granted (or extended) during the years ended December 31, 2012 and 2011, was estimated on the date of grant (or extension) using the Black-Scholes option-pricing model with the following weighted-average assumptions:
| 2012 | 2011 | |||||||
| Expected volatility | 50 | % | 50 | % | ||||
| Risk free interest rate | 2 | % | 1 | % | ||||
| Expected dividend yield | - | - | ||||||
| Expected option term (in years) | 1.5 - 5 | 5 | ||||||
| Weighted average grant date fair value | $ | 0.06 | $ | 0.06 | ||||
| 7. | Other Income |
Other income primarily represents prior years accounts payable balances pertaining to legal fees which were written off pursuant to a settlement agreement.
| 8. | Royalties |
The Company is subject to a royalty agreement based upon sales of certain skin care products. The agreement requires the Company to pay a royalty based upon 8% of such sales, up to $227,175. Royalty expense approximated $40,000 and $24,000 for the years ended December 31, 2012 and 2011, respectively. The remaining commitment at December 31, 2012, is approximately $115,000. The Company’s President has a 60% interest in the royalties.
| F-16 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
At December 31, 2012 and 2011, included in accounts payable and accrued expenses was $96,000 and $72,000, respectively, in regards to this agreement.
| 9. | Commitments and Contingencies |
Leases
The Company leases a plant in Kentucky under an operating lease which expires May 31, 2013. Monthly base rental payments approximate $3,300. Rent expense for the years ended December 31, 2012 and 2011, was $59,204 and $61,544, respectively.
Employment and Consulting Agreements
The Company has entered into various agreements with officers, directors, employees and consultants that expire in one to five years. The agreements provide for the issuance of stock options, at exercise prices of $0.40 and $0.80, underlying 4,625,000 shares of common stock issuable upon the Company’s revenue exceeding $5,000,000 and $10,000,000, as defined. The Company’s President annual base salary of $120,000 was amended to $134,000 effective October 12, 2012. Annual compensation agreements for other officers, directors, employees and consultants range from $5,000 per month to amounts to be determined by the Board of Directors. In addition, the agreements provide for bonus compensation to these individuals aggregating 11.5 percent of the Company’s pretax income.
Legal Matters
In the normal course of business operations the Company may become involved in various legal matters. At December 31, 2012, the Company’s management does not believe that there are any potential legal matters that could have an adverse effect on the Company’s financial position.
In November 2009, the Company entered into a settlement agreement to resolve all aspects of litigation relating to a patent suit. As part of that settlement agreement, the Company received $440,000 as reimbursement for litigation costs. The Company also was awarded $200,000 in eight installments of $25,000 every six months beginning on January 15, 2011, in return for an exclusive patent license. The term of the license agreement is consistent with the term of the $25,000 semiannual payments. The $25,000 installments are being recorded as revenue only upon receipt of the funds. As of December 31, 2012, $100,000 remained to be paid to the Company under this agreement.
| 10. | Related Party Transactions |
One of the Company’s directors also provides legal services and was compensated through the issuance common stock. During the year ended December 31, 2012 the Company issued 402,333 shares valued at $68,397 in connection with services provided.
| F-17 |
Immudyne, Inc.
Notes to Financial Statements
December 31, 2012
| 11. | Subsequent Events |
The Company has evaluated subsequent events through the date these financial statements were issued and has determined that there are no subsequent events or transactions requiring recognition or disclosure in the financial statements.
* * * * *
| F-18 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| IMMUDYNE, INC. | ||
| (Registrant) | ||
| Date: March 28, 2013 | By: | /s/ Mark McLaughlin |
|
Mark McLaughlin Chief Executive Officer (Principal Executive Officer) |
Power of Attorney
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Jun Wang his or her attorney-in-fact for him or her in any and all capacities, to sign any amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Anthony Bruzzese | Chairman of the Board | March 28, 2013 | ||
| Anthony G. Bruzzese, M.D. | ||||
| /s/ Mark McLaughlin |
President, Chief Executive Officer and Director (Principal Executive, Financial and Accounting Officer |
March 28, 2013 | ||
| Mark McLaughlin | ||||
| /s/ Dominic J. Agostini | Director | March 28, 2013 | ||
| Dominic J. Agostini | ||||
| /s/ John R. Strawn, Jr. | Director | March 28, 2013 | ||
| John R. Strawn, Jr. |
| *By: | /s/ Mark McLaughlin | |
| Mark McLaughlin | ||
| Attorney-in-fact |
| 32 |
EXHIBIT INDEX
| Exhibit No. | Description | |
| 3.1 | Certificate of Incorporation of Immudyne, Inc. (Incorporated herein by reference to Exhibit 3.1 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 3.2 | Certificate of Amendment of Certificate of Incorporation of Immudyne, Inc. (Incorporated herein by reference to Exhibit 3.2 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 3.3 | Bylaws of Immudyne, Inc. as currently in effect(Incorporated herein by reference to Exhibit 3.3 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 4.1 | Form of Subscription Agreement (Incorporated herein by reference to Exhibit 3.1 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 5.1 | Opinion of Newman & Morrison LLP (Incorporated herein by reference to Exhibit 3.1 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 10.1 | Written Description of Royalty Agreement between Immudyne, Inc. and Mark McLaughlin (Incorporated herein by reference to Exhibit 10.1 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-184487) filed on December 5, 2012) | |
| 10.2# | Employment Agreement, as amended, between Immudyne, Inc. and Mark McLaughlin, effective as of October 12, 2012 Incorporated herein by reference to Exhibit 10.2 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 10.3# | Director Agreement between Immudyne, Inc. and Anthony Bruzzese M.D., dated as of April 20, 2011 (Incorporated herein by reference to Exhibit 10.3 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 10.4# | Director Agreement between Immudyne, Inc. and Dominic Agostini, dated as of April 20, 2011 (Incorporated herein by reference to Exhibit 10.4 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 10.5# | Director and Legal Services Agreement between Immudyne, Inc. and John R. Strawn, dated as of April 20, 2011 (Incorporated herein by reference to Exhibit 10.5 to the Company’s Registration on Form S-1 (File No. 333-184487) filed on October 18, 2012) | |
| 10.6 | Employment Agreement, as amended, between Immudyne, Inc. and Brunilda McLaughlin d/b/a McLaughlin International, dated as of April 20, 2011 (Incorporated herein by reference to Exhibit 10.6 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-184487) filed on December 5, 2012) | |
| 10.7 | Lease Agreement, as amended, between Cabot Industrial Properties L.P. and Immudyne Inc., dated May 15, 2011 (Incorporated herein by reference to Exhibit 10.7 to Amendment No. 1 to the Company’s Registration Statement on Form S-1 (File No. 333-184487) filed on December 5, 2012) | |
| 10.8 | Letter Agreement between Immudyne, Inc. and MMP, dated December 19, 2011(Incorporated herein by reference to Exhibit 10.8 to Amendment No. 3 to the Company’s Registration Statement on Form s-1 (File No. 333-184487) filed on January 23, 2013) | |
| 24.1† | Power of Attorney (Included on the Signature Page of this Annual Report on Form 10K) | |
| 32.1† | Certification of Principal Executive Officer and Principal Financial Officer pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.2† | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, as signed by the Principal Executive Officer and Principal Financial Officer |
| # | Indicates management contract or compensatory plan, contract or arrangement. |
| † | Filed herewith. |
