Attached files
| file | filename |
|---|---|
| 8-K - 8-K - WEST PHARMACEUTICAL SERVICES INC | wst-20123rdqtrearningsrele.htm |
| EX-99.1 - EXHIBIT 99.1 - WEST PHARMACEUTICAL SERVICES INC | a3q2012erexhibit991.htm |

1 Speakers: Donald E. Morel, Jr. Chairman and Chief Executive Officer William J. Federici Vice President and Chief Financial Officer All trademarks and registered trademarks are the property of West Pharmaceutical Services, Inc., unless noted otherwise. West Pharmaceutical Services, Inc. Third-Quarter 2012 Analyst Conference Call 9 a.m. Eastern Time, November 1, 2012 A webcast of today’s call can be accessed in the “Investors” section of the Company’s web site www.westpharma.com To participate please dial: U.S. toll-free (866)356-4279 or International (617)597-5394 The confirmation number is 67327244 A replay will be available on the web site two hours after the live call and through November 8, 2012. To access the replay by telephone please dial: U.S. toll-free (888)286-8010 or International (617)801-6888. The passcode 20130032. These presentation materials are intended to accompany today’s press release announcing the Company’s results for the quarter and management’s discussion of those results during today’s conference call.

2 Cautionary Statement Under the Private Securities Litigation Reform Act of 1995 This presentation and any accompanying management commentary contain “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to statements about expected financial results for 2012 and future years. Each of these estimates is based on preliminary information, and actual results could differ from these preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in today’s press release, as well as those set forth under the caption "Risk Factors" in our most recent Annual Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially from those estimated or predicted in the forward-looking statements. You should evaluate any statement in light of these important factors. Except as required by law or regulation, we undertake no obligation to publicly update any forward- looking statements, whether as a result of new information, future events, or otherwise. Non-GAAP Financial Measures Certain financial measures included in today’s press release and accompanying tables, in these presentation materials, and which may be referred to in management’s discussion of the Company’s results and outlook, are Non-GAAP (Generally Accepted Accounting Principles) financial measures. Please refer to the “Non-GAAP Financial Measures” and “Notes to Non-GAAP Financial Measures” at the end of these materials for more information. Non-GAAP financial measures should not be considered in isolation or as an alternative to such measures determined in accordance with GAAP.
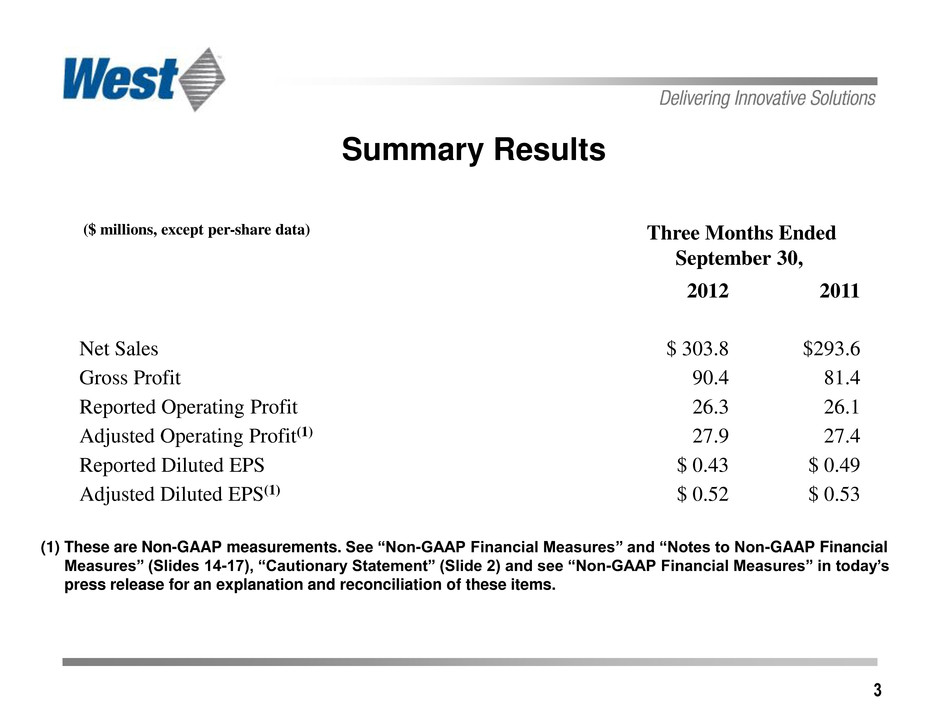
3 Summary Results (1) These are Non-GAAP measurements. See “Non-GAAP Financial Measures” and “Notes to Non-GAAP Financial Measures” (Slides 14-17), “Cautionary Statement” (Slide 2) and see “Non-GAAP Financial Measures” in today’s press release for an explanation and reconciliation of these items. ($ millions, except per-share data) Three Months Ended September 30, 2012 2011 Net Sales $ 303.8 $293.6 Gross Profit 90.4 81.4 Reported Operating Profit 26.3 26.1 Adjusted Operating Profit(1) 27.9 27.4 Reported Diluted EPS $ 0.43 $ 0.49 Adjusted Diluted EPS(1) $ 0.52 $ 0.53

4 Third-Quarter Operating Results • Sales Grew 3.5% (9.2% at constant currency) – Pharmaceutical Packaging Systems 3.3% (10.4% at constant currency) •High-value products grew 17.0% at constant currency • Backlog continues to grow – Pharmaceutical Delivery Systems up 4.4% (6.7% at constant currency) • Strength in U.S. healthcare manufacturing • Proprietary systems grew 17.1%, now 21% of segment sales • Gross Profit Margin increased 2.1 percentage points to 29.8% – PPS sharply higher on high-value product growth, pricing – PDS gains 2.8 margin points on contract healthcare efficiency and proprietary product growth
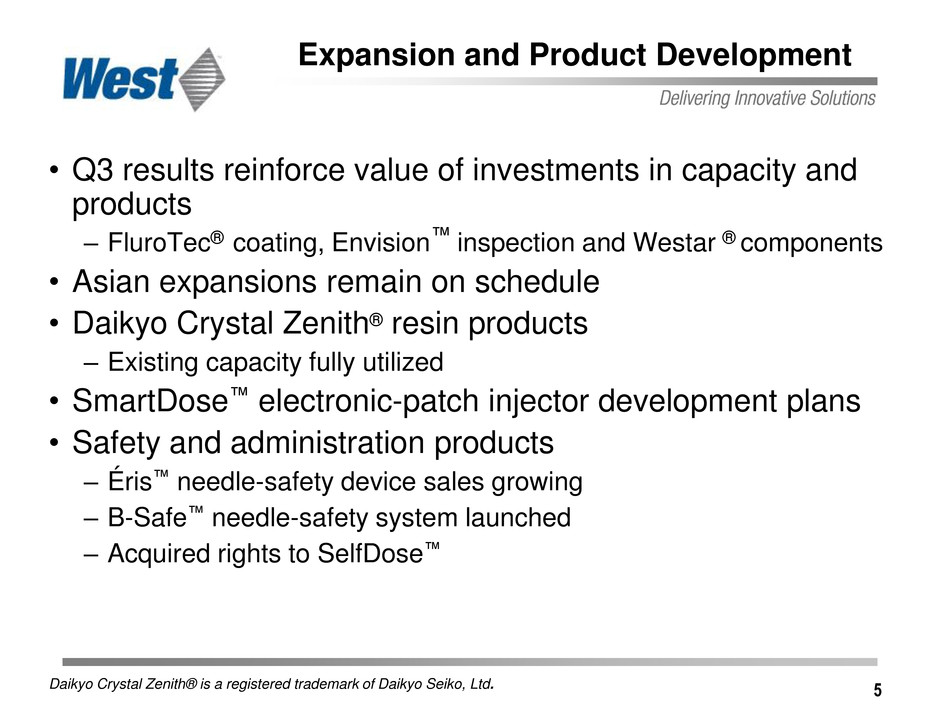
5 Expansion and Product Development • Q3 results reinforce value of investments in capacity and products – FluroTec® coating, Envision™ inspection and Westar ® components • Asian expansions remain on schedule • Daikyo Crystal Zenith® resin products – Existing capacity fully utilized • SmartDose™ electronic-patch injector development plans • Safety and administration products – Éris™ needle-safety device sales growing – B-Safe™ needle-safety system launched – Acquired rights to SelfDose™ Daikyo Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd.

6 Long-term Planning Goals • No fundamental changes in long-term growth strategies and drivers. • 2017 revenue target range: $1.8 billion to $2.0 billion. • Operating profit margin % in the upper teens is achievable.
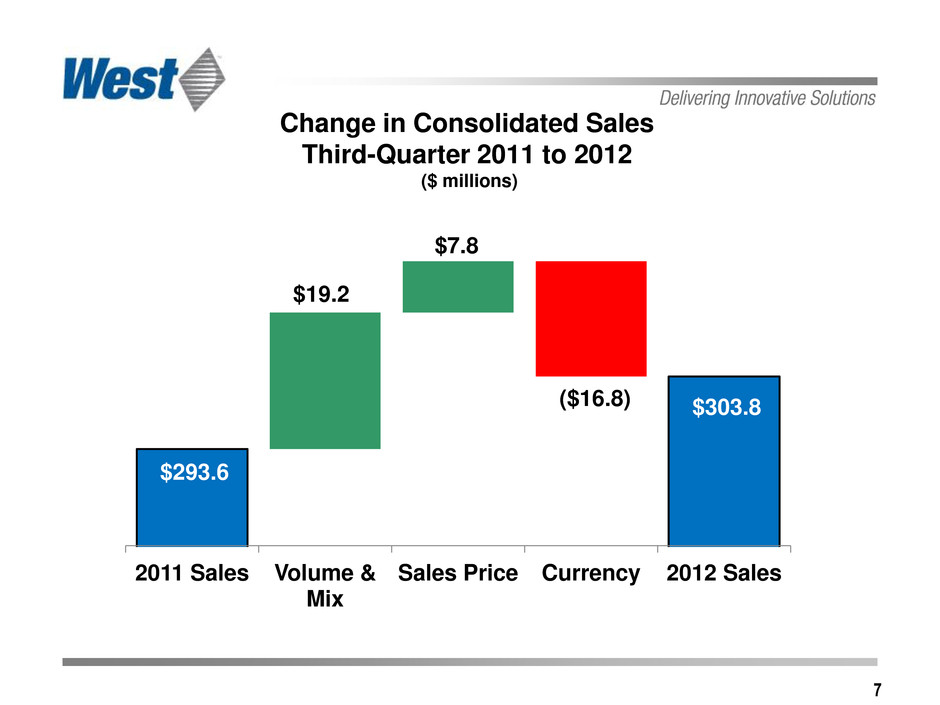
7 Change in Consolidated Sales Third-Quarter 2011 to 2012 ($ millions) $303.8 ($16.8) $19.2 $7.8 $293.6 2011 Sales Volume & Mix Sales Price Currency 2012 Sales
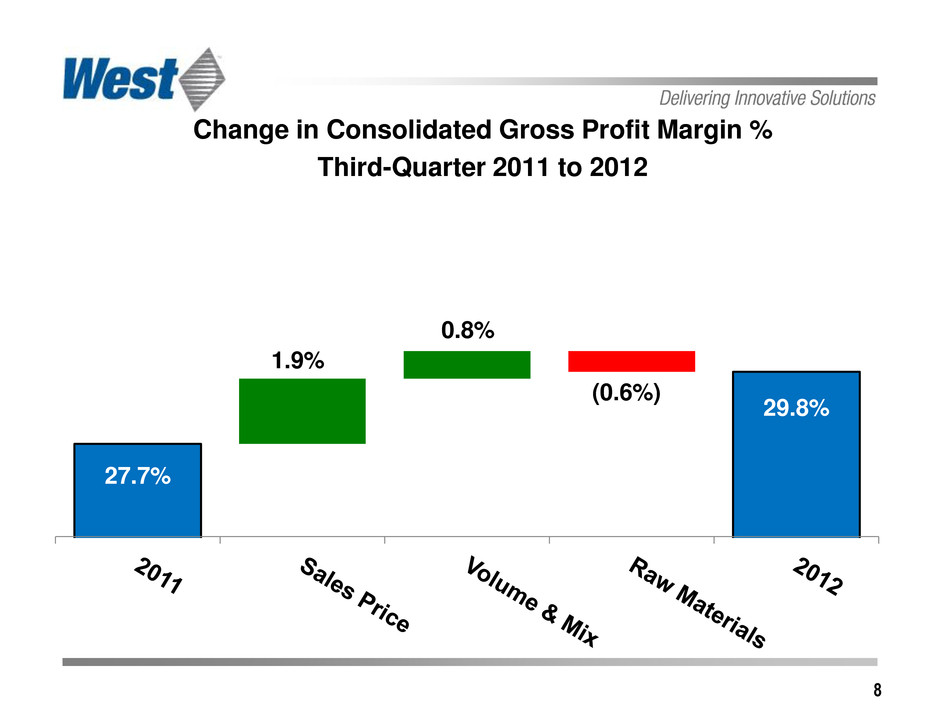
8 Change in Consolidated Gross Profit Margin % Third-Quarter 2011 to 2012 29.8% (0.6%) 1.9% 0.8% 27.7%
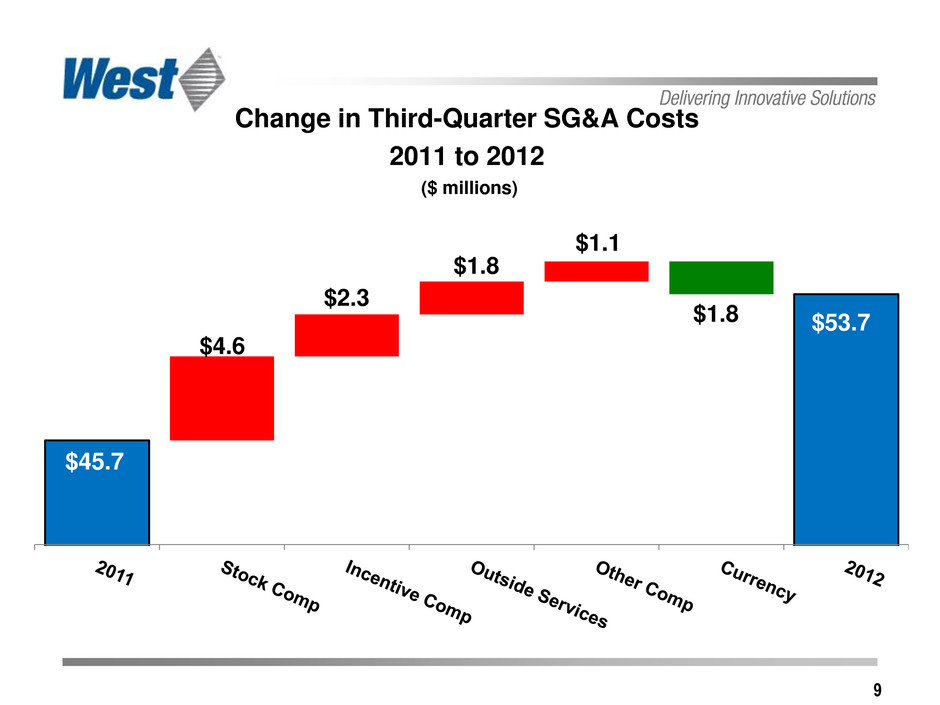
9 Change in Third-Quarter SG&A Costs 2011 to 2012 ($ millions) $53.7 $1.8 $4.6 $2.3 $1.8 $1.1 $45.7
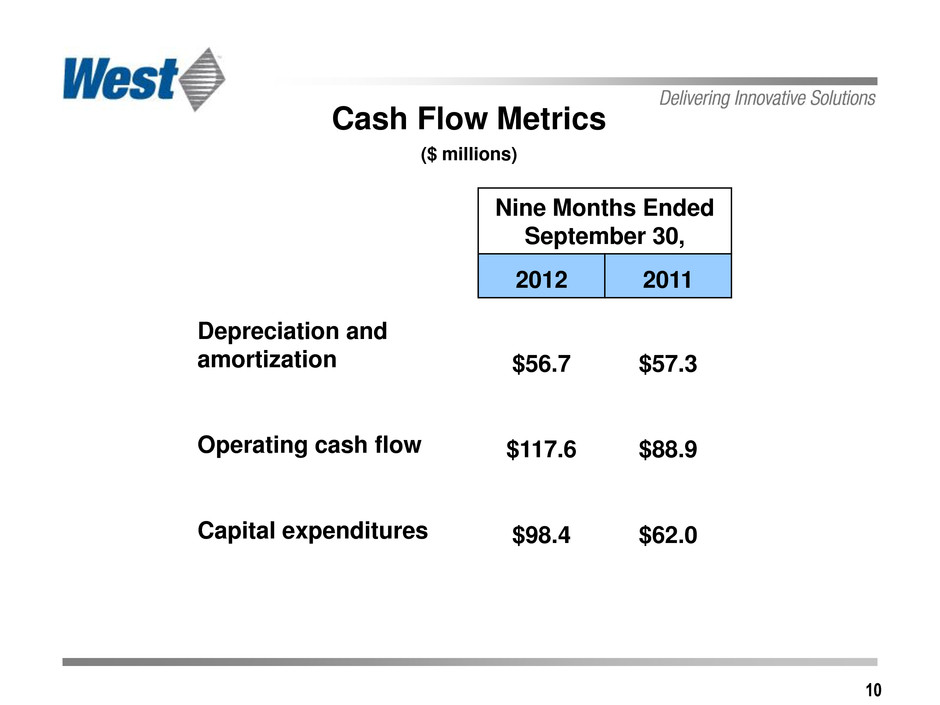
10 Cash Flow Metrics ($ millions) Nine Months Ended September 30, 2012 2011 Depreciation and amortization $56.7 $57.3 Operating cash flow $117.6 $88.9 Capital expenditures $98.4 $62.0
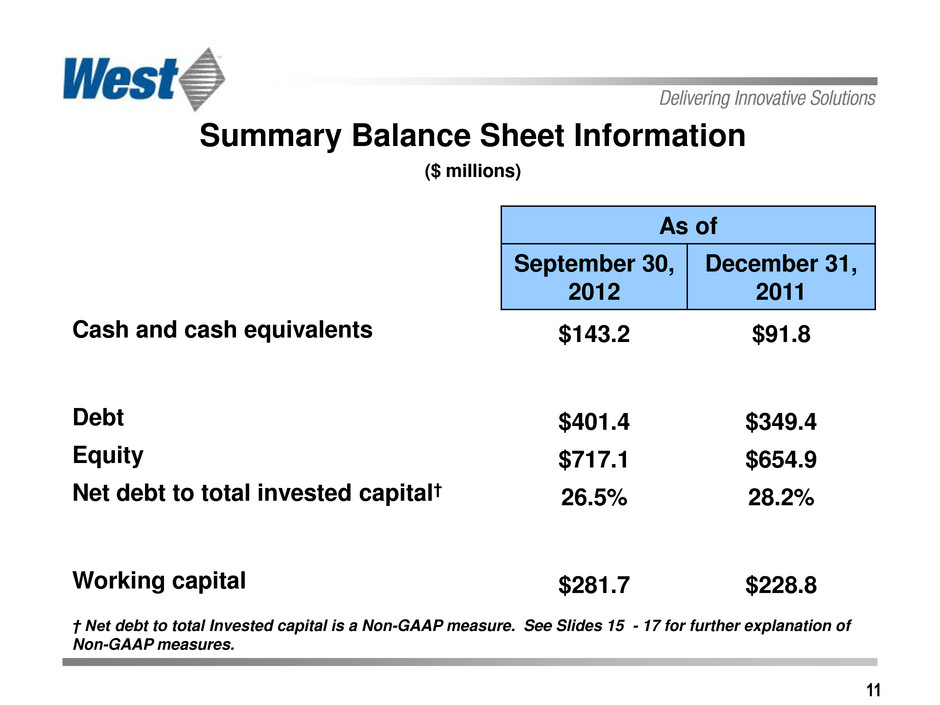
11 Summary Balance Sheet Information ($ millions) As of September 30, 2012 December 31, 2011 Cash and cash equivalents $143.2 $91.8 Debt $401.4 $349.4 Equity $717.1 $654.9 Net debt to total invested capital† 26.5% 28.2% Working capital $281.7 $228.8 † Net debt to total Invested capital is a Non-GAAP measure. See Slides 15 - 17 for further explanation of Non-GAAP measures.
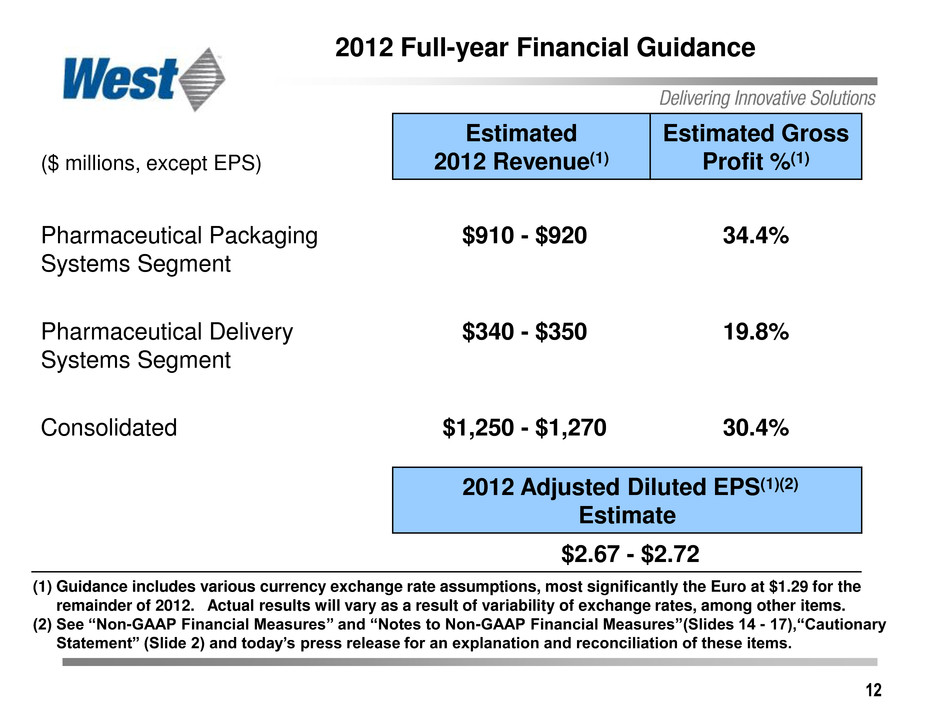
12 2012 Full-year Financial Guidance ($ millions, except EPS) Estimated 2012 Revenue(1) Estimated Gross Profit %(1) Pharmaceutical Packaging Systems Segment $910 - $920 34.4% Pharmaceutical Delivery Systems Segment $340 - $350 19.8% Consolidated $1,250 - $1,270 30.4% 2012 Adjusted Diluted EPS(1)(2) Estimate $2.67 - $2.72 (1) Guidance includes various currency exchange rate assumptions, most significantly the Euro at $1.29 for the remainder of 2012. Actual results will vary as a result of variability of exchange rates, among other items. (2) See “Non-GAAP Financial Measures” and “Notes to Non-GAAP Financial Measures”(Slides 14 - 17),“Cautionary Statement” (Slide 2) and today’s press release for an explanation and reconciliation of these items.

13 Selected Factors Expected to Impact Fourth Quarter 2012 and Comparisons See “Cautionary Statement” on slide 2. This is not an exclusive list of risks associated with the forward looking statements included in today’s press release, analyst call and this presentation. • Strong operating performance expected to continue – High-value packaging and proprietary product mix • Currency • Headquarters relocation and related costs • Incentive compensation cost increases vs. 2011 – Stock-price driven – Performance driven • Tax rate – 100% of 2011 R&D Credit reflected in Q4 2011; none this year. – Increase in current estimate of annual effective tax rate based on geographic mix
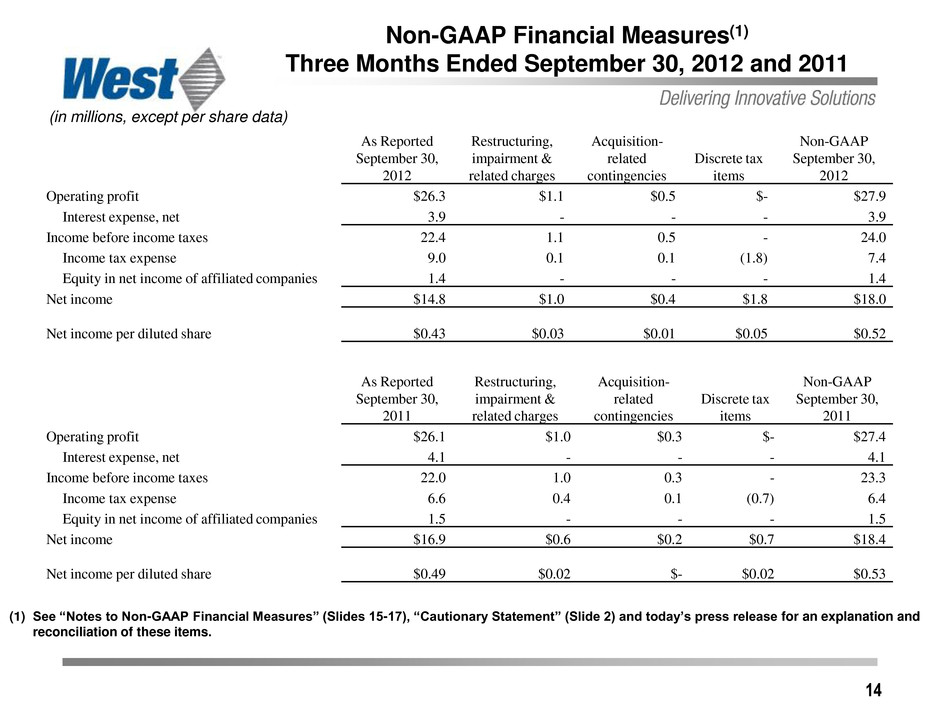
14 Non-GAAP Financial Measures(1) Three Months Ended September 30, 2012 and 2011 (in millions, except per share data) (1) See “Notes to Non-GAAP Financial Measures” (Slides 15-17), “Cautionary Statement” (Slide 2) and today’s press release for an explanation and reconciliation of these items. As Reported September 30, 2012 Restructuring, impairment & related charges Acquisition- related contingencies Discrete tax items Non-GAAP September 30, 2012 Operating profit $26.3 $1.1 $0.5 $- $27.9 Interest expense, net 3.9 - - - 3.9 Income before income taxes 22.4 1.1 0.5 - 24.0 Income tax expense 9.0 0.1 0.1 (1.8) 7.4 Equity in net income of affiliated companies 1.4 - - - 1.4 Net income $14.8 $1.0 $0.4 $1.8 $18.0 Net income per diluted share $0.43 $0.03 $0.01 $0.05 $0.52 As Reported September 30, 2011 Restructuring, impairment & related charges Acquisition- related contingencies Discrete tax items Non-GAAP September 30, 2011 Operating profit $26.1 $1.0 $0.3 $- $27.4 Interest expense, net 4.1 - - - 4.1 Income before income taxes 22.0 1.0 0.3 - 23.3 Income tax expense 6.6 0.4 0.1 (0.7) 6.4 Equity in net income of affiliated companies 1.5 - - - 1.5 Net income $16.9 $0.6 $0.2 $0.7 $18.4 Net income per diluted share $0.49 $0.02 $- $0.02 $0.53

15 NOTES TO NON-GAAP FINANCIAL MEASURES For additional details, please see today’s press release and Safe Harbor Statement. (continued on following slide) These presentation materials and associated presentation use the following financial measures that have not been calculated in accordance with generally accepted accounting principles (GAAP) accepted in the U.S., and therefore are referred to as non-GAAP financial measures: Adjusted operating profit Adjusted net income Adjusted diluted EPS Net debt Net debt to total invested capital West believes that these non-GAAP measures of financial results provide useful information to management and investors regarding business trends, results of operations, and the Company’s overall performance and financial position. Our executive management team uses these financial measures to evaluate the performance of the Company in terms of profitability and efficiency, to compare operating results to prior periods, to evaluate changes in the operating results of each segment, and to measure and allocate financial resources to our segments. The Company believes that the use of these non-GAAP financial measures provides an additional tool for investors to use in evaluating ongoing operating results and trends in comparing its financial measures with other companies. Our executive management does not consider such non-GAAP measures in isolation or as an alternative to such measures determined in accordance with GAAP. The principal limitation of these financial measures is that they exclude significant expenses and income that are required by GAAP to be recorded. In addition, they are subject to inherent limitations as they reflect the exercise of judgment by management about which items are excluded. In order to compensate for these limitations, non-GAAP financial measures are presented in connection with GAAP results. We urge investors and potential investors to review the reconciliations of our non-GAAP financial measures to the comparable GAAP financial measures, and not to rely on any single financial measure to evaluate the Company’s business. In calculating adjusted operating profit, adjusted net income and adjusted diluted EPS, we exclude the impact of items that are not considered representative of ongoing operations. Such items include restructuring and related costs, certain asset impairments, other specifically identified gains or losses, and discrete income tax items. A reconciliation of these adjusted non-GAAP measures to the comparable GAAP financial measures is included in the accompanying tables. (continued on following page)
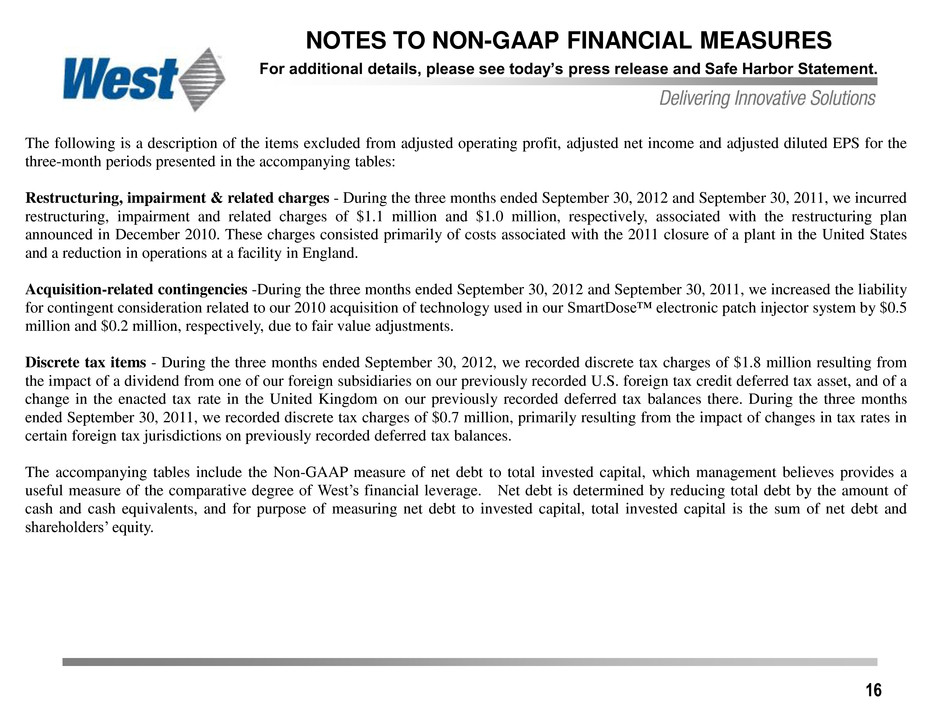
16 NOTES TO NON-GAAP FINANCIAL MEASURES For additional details, please see today’s press release and Safe Harbor Statement. The following is a description of the items excluded from adjusted operating profit, adjusted net income and adjusted diluted EPS for the three-month periods presented in the accompanying tables: Restructuring, impairment & related charges - During the three months ended September 30, 2012 and September 30, 2011, we incurred restructuring, impairment and related charges of $1.1 million and $1.0 million, respectively, associated with the restructuring plan announced in December 2010. These charges consisted primarily of costs associated with the 2011 closure of a plant in the United States and a reduction in operations at a facility in England. Acquisition-related contingencies -During the three months ended September 30, 2012 and September 30, 2011, we increased the liability for contingent consideration related to our 2010 acquisition of technology used in our SmartDose™ electronic patch injector system by $0.5 million and $0.2 million, respectively, due to fair value adjustments. Discrete tax items - During the three months ended September 30, 2012, we recorded discrete tax charges of $1.8 million resulting from the impact of a dividend from one of our foreign subsidiaries on our previously recorded U.S. foreign tax credit deferred tax asset, and of a change in the enacted tax rate in the United Kingdom on our previously recorded deferred tax balances there. During the three months ended September 30, 2011, we recorded discrete tax charges of $0.7 million, primarily resulting from the impact of changes in tax rates in certain foreign tax jurisdictions on previously recorded deferred tax balances. The accompanying tables include the Non-GAAP measure of net debt to total invested capital, which management believes provides a useful measure of the comparative degree of West’s financial leverage. Net debt is determined by reducing total debt by the amount of cash and cash equivalents, and for purpose of measuring net debt to invested capital, total invested capital is the sum of net debt and shareholders’ equity.
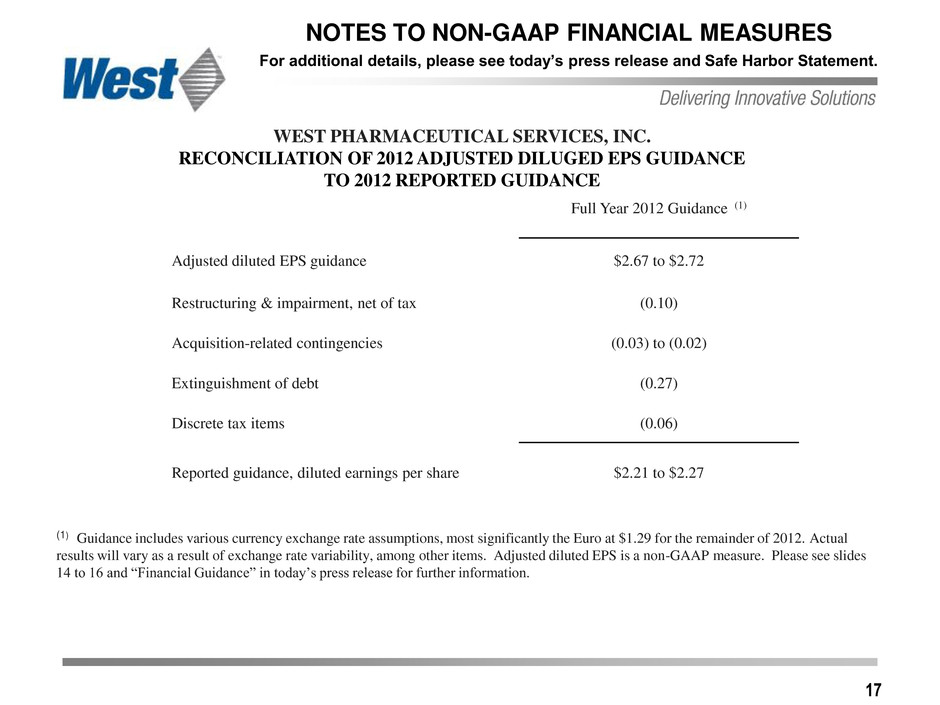
17 NOTES TO NON-GAAP FINANCIAL MEASURES For additional details, please see today’s press release and Safe Harbor Statement. Full Year 2012 Guidance (1) Adjusted diluted EPS guidance $2.67 to $2.72 Restructuring & impairment, net of tax (0.10) Acquisition-related contingencies (0.03) to (0.02) Extinguishment of debt (0.27) Discrete tax items (0.06) Reported guidance, diluted earnings per share $2.21 to $2.27 WEST PHARMACEUTICAL SERVICES, INC. RECONCILIATION OF 2012 ADJUSTED DILUGED EPS GUIDANCE TO 2012 REPORTED GUIDANCE (1) Guidance includes various currency exchange rate assumptions, most significantly the Euro at $1.29 for the remainder of 2012. Actual results will vary as a result of exchange rate variability, among other items. Adjusted diluted EPS is a non-GAAP measure. Please see slides 14 to 16 and “Financial Guidance” in today’s press release for further information.
