Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Unilife Corp | d386539d8k.htm |
| EX-99.3 - EX-99.3 - Unilife Corp | d386539dex993.htm |
| EX-99.1 - EX-99.1 - Unilife Corp | d386539dex991.htm |

| Fiscal Fourth Quarter Earnings Call July 30, 2012 NASDAQ (UNIS) and ASX (UNS) Exhibit 99.2 |

| This presentation contains forward looking statements under the safe harbor provisions of the US securities laws. These forward-looking statements are based on management's beliefs and assumptions and on information currently available to our management. Our management believes that these forward-looking statements are reasonable as and when made. However you should not place undue reliance on any such forward looking statements as these are subject to risks and uncertainties. Please refer to our press releases and our SEC filings for more information regarding the use of forward looking statements. Cautionary Note Regarding Forward-Looking Statements |

| Opening Statements July 31, 2012 Delivered on key business milestones in FY2012 Supply of Unifill syringe on schedule and accelerating Significant diversification of product portfolio in response to customer needs Rapidly expanding base of target customers for commercial and development agreements Building an efficient, productive and streamlined enterprise Q4 2011 Earnings Call. August 2011 |

| Financial Data Overview Rich Wieland Chief Financial Officer |
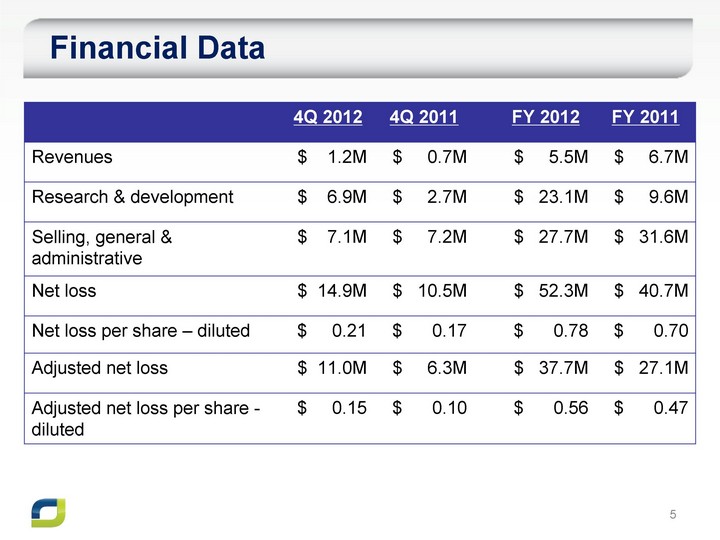
| Financial Data 4Q 2012 4Q 2011 FY 2012 FY 2011 Revenues $ 1.2M $ 0.7M $ 5.5M $ 6.7M Research & development $ 6.9M $ 2.7M $ 23.1M $ 9.6M Selling, general & administrative $ 7.1M $ 7.2M $ 27.7M $ 31.6M Net loss $ 14.9M $ 10.5M $ 52.3M $ 40.7M Net loss per share - diluted $ 0.21 $ 0.17 $ 0.78 $ 0.70 Adjusted net loss $ 11.0M $ 6.3M $ 37.7M $ 27.1M Adjusted net loss per share - diluted $ 0.15 $ 0.10 $ 0.56 $ 0.47 |

| Cash Position Total Cash (including restricted cash) at June 30, 2012 $13.8MM Proceeds from Capital raise received on July 5, 2012 $19.0MM Less: estimated offering expenses ($0.2MM) Pro forma cash balance $32.6MM July 31, 2012 |

| Business Update Alan Shortall |

| Efficient Operating Investment July 31, 2012 R&D Driven by specific customer needs Continued diversification being favorably received by customers Growing IP portfolio building future shareholder value Improving Operating Efficiencies Operating a streamlined business Significant investment in automated assembly, quality and ERP systems Continuing to pursue operating efficiencies to minimize overheads |
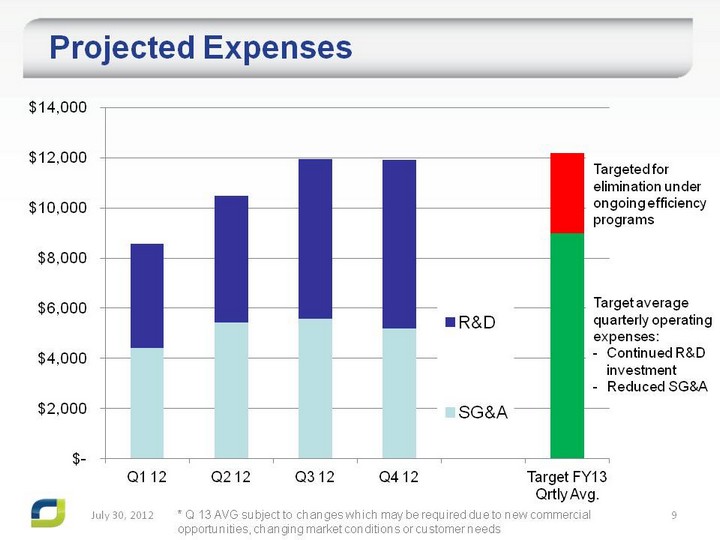
| Projected Expenses July 31, 2012 * Q 13 AVG subject to changes which may be required due to new commercial opportunities, changing market conditions or customer needs Targeted for elimination under ongoing efficiency programs Target average quarterly operating expenses: Continued R&D investment Reduced SG&A |
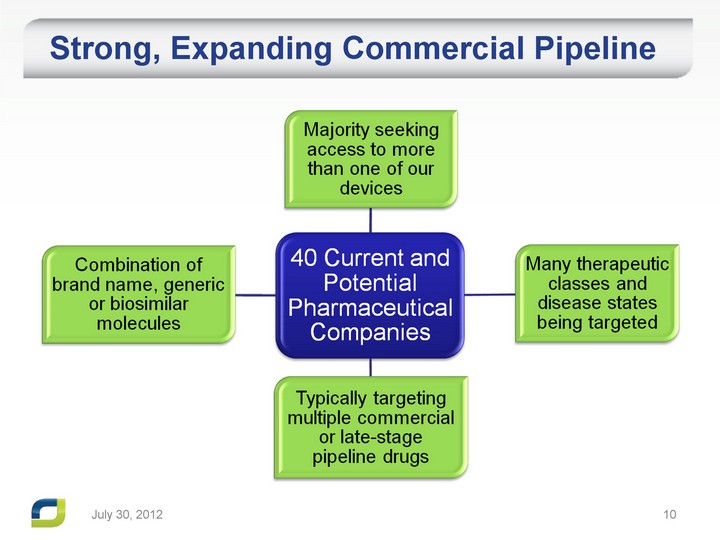
| July 31, 2012 Strong, Expanding Commercial Pipeline |

| The Unifill Syringe July 31, 2012 Transitioned over to next-generation Unifill syringe Reduction in cost of goods and assembly Reduction in activation force for needle retraction Completing transition of first assembly line to produce new edition |

| The Unifill Syringe July 31, 2012 Dual-Path Strategy for Unifill Syringe Low-Cost, High Unit Volume Drugs High-Cost, Low Unit Volume Drugs Typical Unit Volumes / Drug 100MM+ No. Target Drugs in our Pipeline Minority Typical Drug Pricing / Dose Tens of dollars Competitive Device Price / Unit Below $1 Target Unit Pricing / Contract Moderate Typical Unit Volumes / Drug 5-50MM Units No. Target Drugs in our Pipeline Majority Typical Drug Pricing / Dose '00s - '000s of dollars Competitive Device Price / Unit Above $1 Target Unit Pricing / Contract High 1 2 |
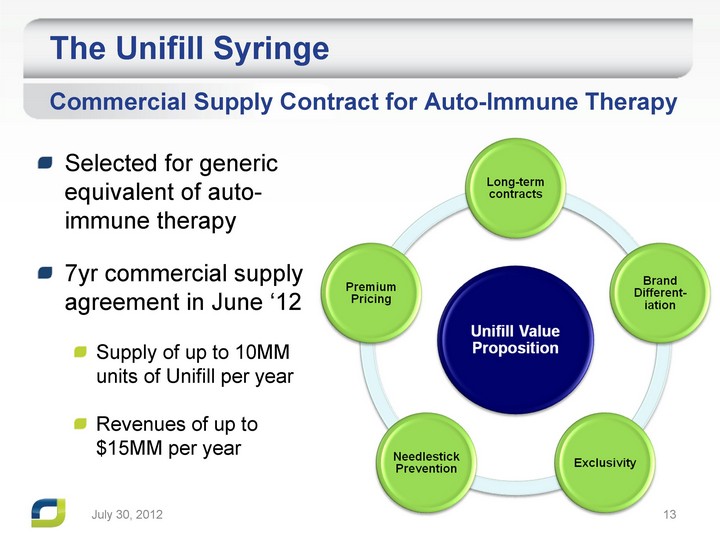
| The Unifill Syringe Selected for generic equivalent of auto- immune therapy 7yr commercial supply agreement in June '12 Supply of up to 10MM units of Unifill per year Revenues of up to $15MM per year Commercial Supply Contract for Auto-Immune Therapy July 31, 2012 |

| The Unifill Syringe July 31, 2012 Commercial Pipeline Current status New / recurring shipments of small batches to multiple customers on regular basis Some customers reported they are in various stages development cycle Confirmed to start initial shipments to other new customers this month Many targeting several approved and pipeline drugs % of Total Unit Volume of Drug Potential |

| July 31, 2012 AutoInfusors A Modular, Flexible Platform Compact, disposable systems for intuitive and comfortable patient self-administration 1mL - 30mL unit dose volumes Preset for specific delivery times and rates Pre-attached or worn on body Standard primary container Standard fill-finish process |
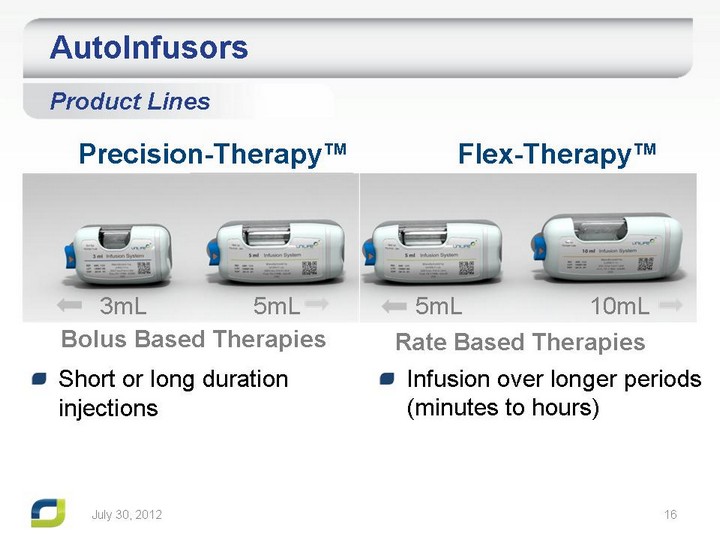
| July 31, 2012 Rate Based Therapies Precision-Therapy(tm) Flex-Therapy(tm) Bolus Based Therapies 3mL 5mL 5mL 10mL AutoInfusors Product Lines Short or long duration injections Infusion over longer periods (minutes to hours) |

| Auto-Injectors July 31, 2012 Unilife RITA(tm) Auto-Injector: Ultra compact and sleek Complete needle hiding at all stages of injection True end-of-dose indicator Easily customizable for target patient population Disposable Reusable Unilife LISA(tm) Reusable Auto- Injector: Completely automated electronic device with a single activation button User selected injection speed LED indicators Only reusable auto-injector safe for user to remove syringe after use |

| Reusable Auto-Injector Steps of Use 1) Open Door & Insert Unifill Syringe 2) Select Speed 3) Press activation button to remove RNS 4) Press activation button to give injection 5) Open door and safely remove Unifill syringe Unilife Confidential 18 |
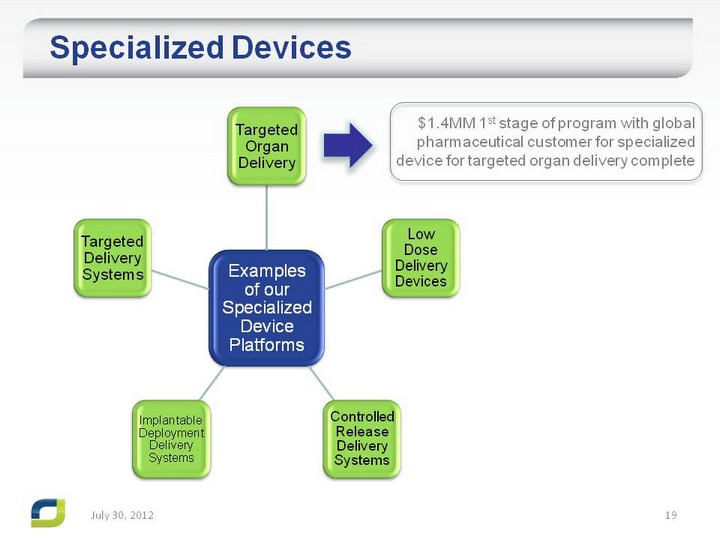
| Specialized Devices Specialized Devices July 31, 2012 $1.4MM 1st stage of program with global pharmaceutical customer for specialized device for targeted organ delivery complete |
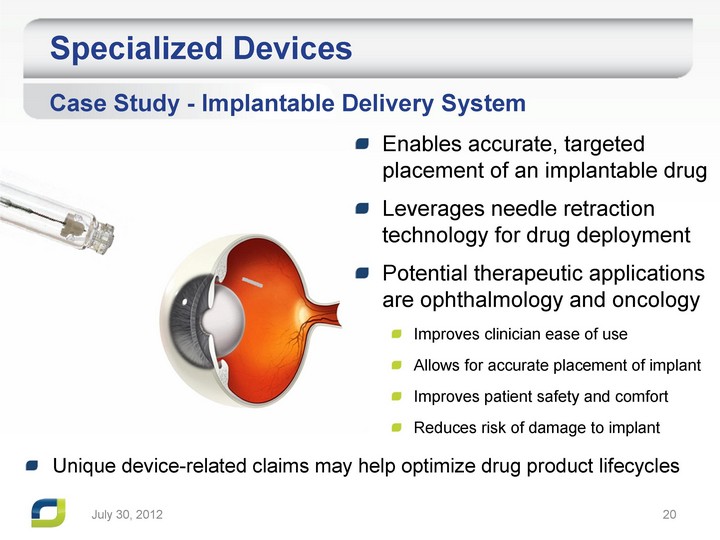
| Specialized Devices Enables accurate, targeted placement of an implantable drug Leverages needle retraction technology for drug deployment Potential therapeutic applications are ophthalmology and oncology Improves clinician ease of use Allows for accurate placement of implant Improves patient safety and comfort Reduces risk of damage to implant Case Study - Implantable Delivery System July 31, 2012 Unique device-related claims may help optimize drug product lifecycles |

| Key Business Milestones - FY 2013 July 31, 2012 Additional Supply Contracts for Unifill Syringe Increasing Unifill production in response to customer demand Development agreements with funding for other devices Supply of devices for use in human clinical drug trials Supply contracts for other proprietary devices Development of additional proprietary devices |

| Questions July 31, 2012 |
