Attached files
| file | filename |
|---|---|
| 8-K - PHARMACYCLICS INC | form8k07380_05212012.htm |
| EX-99.3 - PHARMACYCLICS INC | ex993to8k07380_05212012.htm |
| EX-99.1 - PHARMACYCLICS INC | ex991to8k07380_05212012.htm |
Exhibit 99.2
The Bruton's Tyrosine Kinase (BTK) Inhibitor Ibrutinib (PCI-32765) is Highly Active and Tolerable in Relapsed or Refractory (R/R) and Treatment Naïve (TN) Chronic Lymphocytic Leukemia (CLL) Patients, Updated Results of a Phase Ib/II Study
SUSAN O'BRIEN, MD, MD, RICHARD R. FURMAN, MD, STEVEN E. COUTRE, MD, JAN A. BURGER, MD, PHD, KRISTIE A. BLUM, MD, JEFF SHARMAN, MD, IAN W. FLINN, MD, PHD, BARBARA GRANT, MD, NYLA A. HEEREMA, PHD, AMY J. JOHNSON, PHD, TASHEDA NAVARRO, DANELLE JAMES, MD, ERIC HEDRICK, MD, AND JOHN C. BYRD
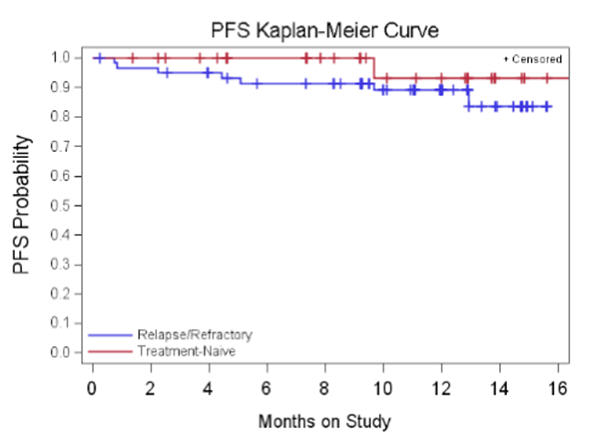
Background: BTK is an essential mediator of B-cell receptor signaling and a critical kinase for lymphoma cell survival. Ibrutinib (PCI-32765), an oral, selective, irreversible inhibitor of BTK, inhibits proliferation, migration and adhesion in CLL cells. The combination of fludarabine, cyclophosphamide and rituximab have markedly improved outcomes of younger-fit patients in the first and second-line setting. However, effective salvage regimens for patients who develop treatment resistance are lacking. Fludarabine-based therapy, while effective is toxic and carries significant risk of morbidity and mortality in elderly pts. Patients who relapse following fludarabine-based regimens and older CLL patients represent a high priority for new therapeutic approaches. A multi-cohort Phase Ib/II trial evaluated 2 doses of single-agent ibrutinib in TN and R/R CLL/SLL patients. Aims: The primary objective was to determine the safety of 2 dose regimens. Secondary objectives were to assess the preliminary efficacy, PK, and long-term safety. Methods: Patients with R/R CLL who had failed at least 2 lines of therapy (including a purine analog) and TN CLL patients >65 years old were treated with oral ibrutinib at doses of 420mg or 840mg administered daily for 28-day cycles until disease progression (PD). Results: The data cut-off for R/R patients was 10/25/11 and 12/10/11 for TN patients. 61 patients were enrolled to the R/R cohorts- 27 patients (420mg) and 34 patients (840mg). 31 patients were enrolled to the TN cohorts- 26 patients (420mg) and 5 patients (840mg). The 840mg TN cohort was terminated after comparable activity and safety between doses was shown in R/R patients. Median age was 64 and 71 years for R/R and TN patients respectively. 59% of R/R and 61% of TN patients had baseline cytopenias consistent with advanced stage disease. Unmutated IgVH was present in 79% of R/R and 43% of TN patients. Del17p was present 36% (R/R) and 6% (TN) of patients. The majority of AEs have been Gr<=2 in severity, most commonly diarrhea, nausea, and fatigue. Hematologic toxicity >= G3 was infrequent. Response (ORR; PR + CR) by IWCLL criteria in the 420mg R/R cohort was 67% with 12.6 months median follow-up. In the 840mg R/R cohort ORR is 68% at 9.3 months median follow-up. An additional 22%, and 24% of patients in these R/R cohorts, respectively, achieved a nodal response with residual lymphocytosis (NodR). With 10.7 months median follow-up the ORR in the 420mg TN cohort is 73% including 8% CRs with morphologically normal marrows. With only 4.6 mo median follow-up, ORR in the 840mg TN cohort is 40%. An additional 12%, and 20% of patients in these TN cohorts, respectively, achieved a NodR. ORR was independent of high-risk factors. Estimated 12 month PFS for those pts treated at 420 mg dose is 88% for R/R patients and 93.3% for TN patients. Conclusions: PCI-32765 is highly active and well tolerated in R/R and elderly TN CLL patients. No differences in activity or toxicity were noted between the two dose levels. The high ORR and very low PD rate indicate that ibrutinib warrants further study in CLL.