Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PROLOR Biotech, Inc. | v309878_8k.htm |

Long Acting Reversible PEGylated Oxyntomodulin - MOD - 6030 Apr 2012 5th Diabetes Drug Discovery & Development Conference

Safe Harbor Statement This presentation contains forward - looking statements, including statements regarding the results of current studies and pre - clinical experiments and the effectiveness of PROLOR’s long - acting protein programs and are made pursuant to the safe harbour provisions of the Private Securities Litigation Reform Act of 1995 . Investors are cautioned that forward - looking statements involve risks and uncertainties that may affect PROLOR’s business and prospects, including the risks that PROLOR Biotech may not succeed in developing any commercial products, and that ongoing studies may not continue to show substantial or any activity ; and other risks and uncertainties that may cause results to differ materially from those set forth in the forward - looking statements . In addition to the risks described above, investors should read and consider the economic, competitive, governmental, technological and other factors discussed in PROLOR Biotech’s filings with the Securities and Exchange Commission .

Indication Product Market Size Preclin. Ph I Ph II Ph III Growth Disorders hGH - CTP $3B Hemophilia Factor VIIa - CTP $1.3B 2 / week injection Factor IX - CTP $0.7B 1 /week injection Diabetes Type II & Obesity GLP1/Glucagon dual receptor agonist $2B 1/week injection PROLOR’s Expected Product Pipeline 1 /week injection 3

4 Reversible PEGylation - Mechanism 4 PEG chain too large → lost activity • PEG chain hydrolyzes at controlled PK profile • Authentic drug released • Unique linkers, patent protected Native peptide - Fully Active + H 2 0 Physiological hydrolysis pH 7; 37 o C PEG chain PEG chain Hydrolyzed Linker Hydrolyzed Linker
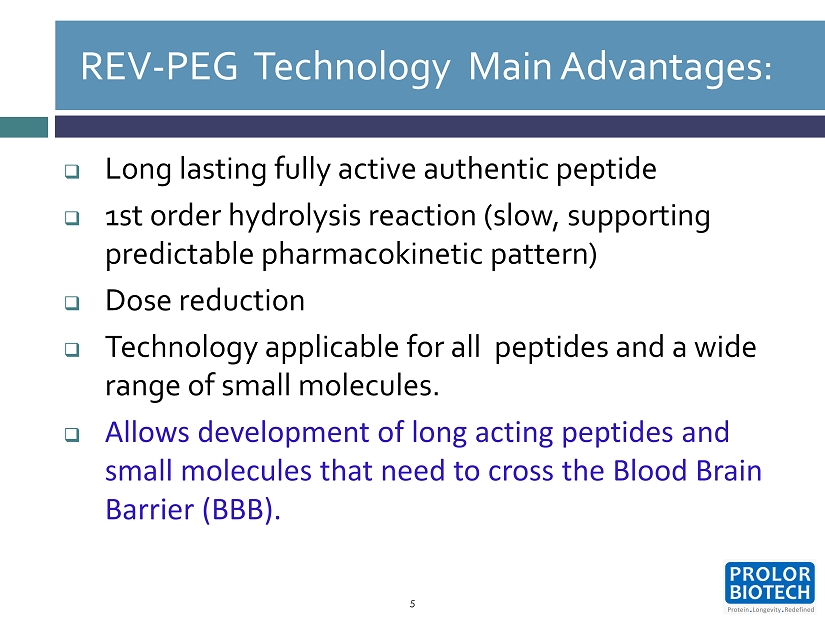
REV - PEG Technology Main Advantages: □ Long lasting fully active authentic peptide □ 1 st order hydrolysis reaction (slow, supporting predictable pharmacokinetic pattern) □ Dose reduction □ Technology applicable for all peptides and a wide range of small molecules. □ Allows development of long acting peptides and small molecules that need to cross the Blood Brain Barrier (BBB ). 5
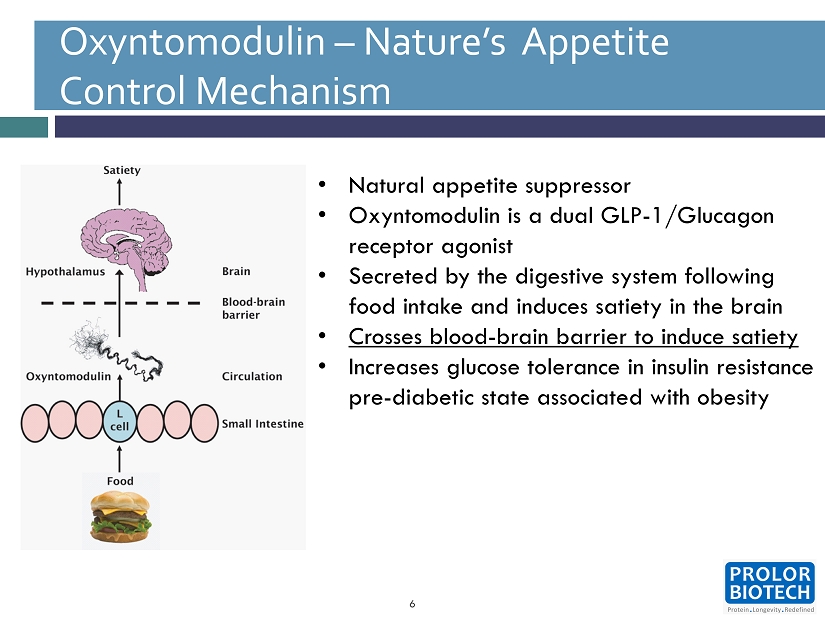
REV - PEG Technology main advantages: 6 Oxyntomodulin – Nature’s Appetite Control Mechanism • Natural appetite suppressor • Oxyntomodulin is a dual GLP - 1 /Glucagon receptor agonist • Secreted by the digestive system following food intake and induces satiety in the brain • Crosses blood - brain barrier to induce satiety • Increases glucose tolerance in insulin resistance pre - diabetic state associated with obesity

MOD - 6030 — Long - Acting GLP - 1/Glucagon Dual Receptor Agonist versus Native Oxyntomodulin: Previous 3 rd party oxyntomodulin studies: Animals (mice, rats): 2 injections per day Glycemic control Food intake reduced Weight loss observed Humans: 3 injections per day Weight Loss of 0.5 kg per week , no special diet or exercise 7 PROLOR’s studies Objectivies : Can we obtain SAME weight loss & reduction of food intake in obesity mice model , with a single weekly injection Can conventionally PEGylated oxyntomodulin cross the BBB and achieve similar results?
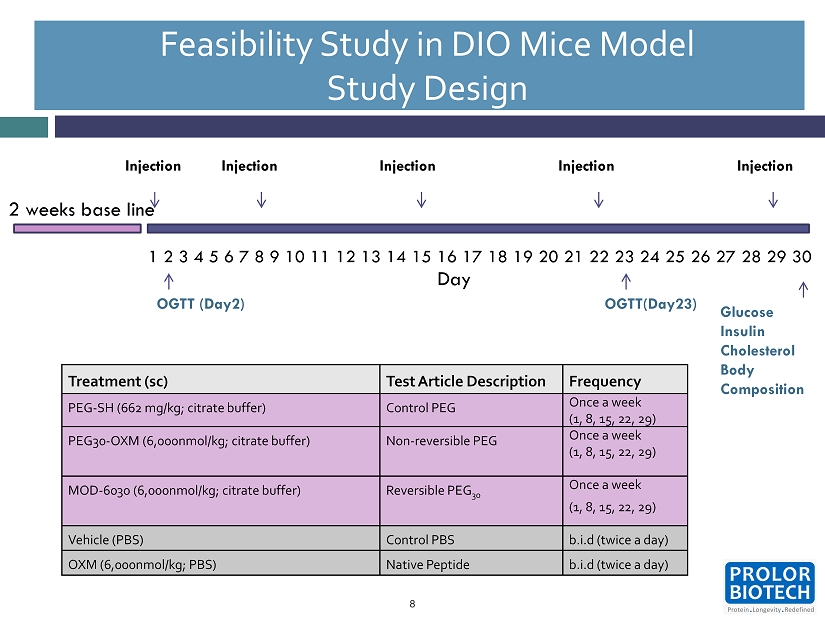
Feasibility Study in DIO Mice Model Study Design 8 Day 2 weeks base line Injection 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Injection Injection Injection Injection OGTT (Day2) OGTT(Day 23 ) Glucose Insulin Cholesterol Body Composition Treatment (sc) Test Article Description Frequency PEG - SH ( 662 mg/kg ; citrate buffer) Control PEG Once a week (1, 8, 15, 22, 29) PEG 30 - OXM ( 6 , 000 nmol/kg ; citrate buffer) Non - reversible PEG Once a week (1, 8, 15, 22, 29) MOD - 6030 ( 6 , 000 nmol/kg ; citrate buffer) Reversible PEG 30 Once a week ( 1 , 8 , 15 , 22 , 29 ) Vehicle (PBS) Control PBS b . i . d (twice a day) OXM ( 6 , 000 nmol/kg ; PBS) Native Peptide b . i . d (twice a day)

Once Weekly Administration of MOD - 6030 Significantly Reduces Body Weight in DIO Mice 9 30 35 40 45 50 55 60 65 -6 -5 -4 -3 -2 -1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Body weight (g) Day - 1.6% - 16.6% - 27.6 % Effect of MOD - 6030 (Hydrolyzed Linker), OXM - PEG30 (Non - Cleavable Linker) & Native OXM on Body Weight in Male C57BL/6J Mice Exhibiting Diet - Induced Obesity Single weekly injection of MOD - 6030, during 30 - day period, provided 27% weight loss, compared to 16% weight loss for the group injected twice per day with native oxyntomodulin – while the cumulative dosing of net oxyntomodulin injected with MOD - 6030 was only 8.6% for the 30 - day period
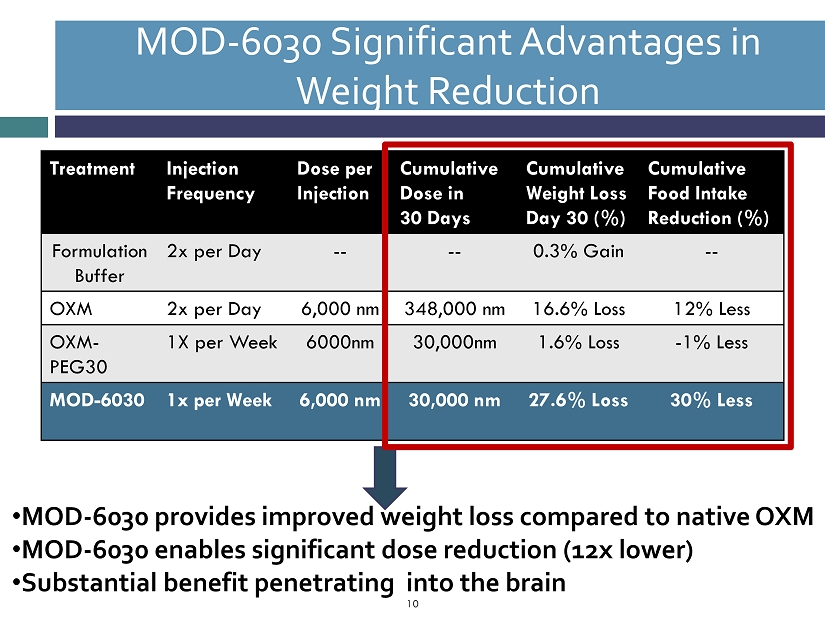
MOD - 6030 Significant Advantages in Weight Reduction 10 Treatment Injection Frequency Dose per Injection Cumulative Dose in 30 Days Cumulative Weight Loss Day 30 (%) Cumulative Food Intake Reduction (%) Formulation Buffer 2x per Day -- -- 0.3% Gain -- OXM 2x per Day 6,000 nm 348,000 nm 16.6% Loss 12% Less OXM - PEG30 1X per Week 6000nm 30,000nm 1.6% Loss - 1% Less MOD - 6030 1x per Week 6,000 nm 30,000 nm 27.6% Loss 30% Less • MOD - 6030 provides improved weight loss compared to native OXM • MOD - 6030 enables significant dose reduction (12x lower) • Substantial benefit penetrating into the brain
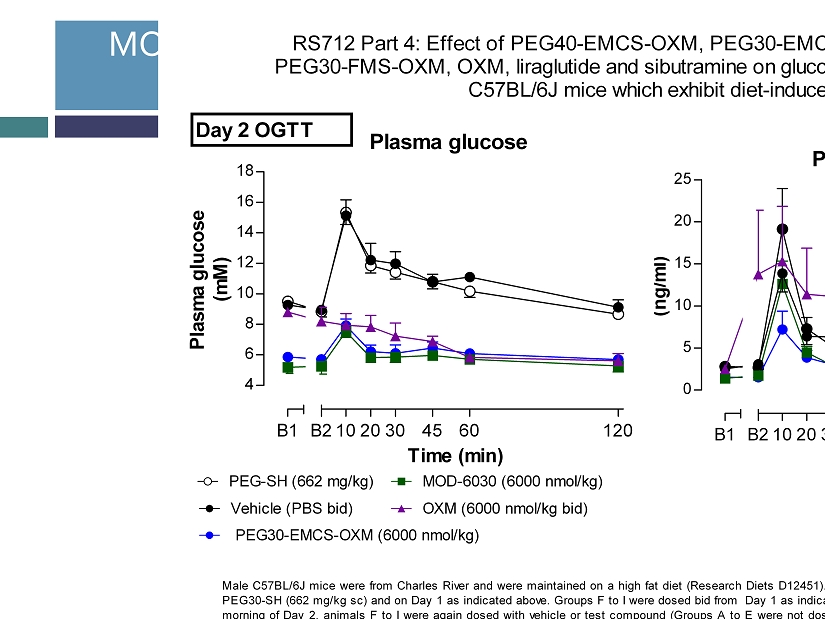
MOD - 6030 Induces Glucose Tolerance in DIO Mice 11 RS712 Part 4: Effect of PEG40-EMCS-OXM, PEG30-EMCS-OXM, PEG40-FMS-OXM, PEG30-FMS-OXM, OXM, liraglutide and sibutramine on glucose tolerance (2g/kg po) in male C57BL/6J mice which exhibit diet-induced obesity Male C57BL/6J mice were from Charles River and were maintained on a high fat diet (Research Diets D12451). Groups A to E were dosed once during baseline on Day -6 with PEG30-SH (662 mg/kg sc) and on Day 1 as indicated above. Groups F to I were dosed bid from Day 1 as indicated above. All animals were fasted from 16:00 on Day 1. On the morning of Day 2, animals F to I were again dosed with vehicle or test compound (Groups A to E were not dosed) and then 60 min later all animals were dosed with a glucose challenge (2 g/kg po). Baseline blood samples were taken from the tail vein immediately before dosing (B1; -60 min) and immediately before the glucose challenge (B2; 0 min). Further blood samples were taken from the tail vein at 10, 20, 30, 45, 60, 120 min after the glucose challenge. Samples were analysed for plasma glucose and insulin content. and a log transformation used. Analysis was by robust regression and included treatment and assay day as factors and bleeding order and Day 1 body weight as covariates. Due to the high variance in Group G, the insulin analyses were repeated with this Group excluded and the results of this latter analysis indicated above for the other groups. Data are shown as adjusted means (n=8) and standard errors of the mean (SEM) are calculated from the residuals of the statistical models. Comparisons against the appropriate vehicle were by the multiple t-test and significances are denoted by *p<0.05, **p<0.01 and ***p<0.001. Plasma glucose 4 6 8 10 12 14 16 18 B1 B2102030 45 60 120 Time (min) Plasma glucose (mM) Plasma insulin 0 5 10 15 20 25 B1 B2102030 45 60 120 Time (min) Plasma insulin (ng/ml) Vehicle (PBS bid) OXM (6000 nmol/kg bid) PEG-SH (662 mg/kg) PEG30-EMCS-OXM (6000 nmol/kg) MOD-6030 (6000 nmol/kg) Day 2 OGTT
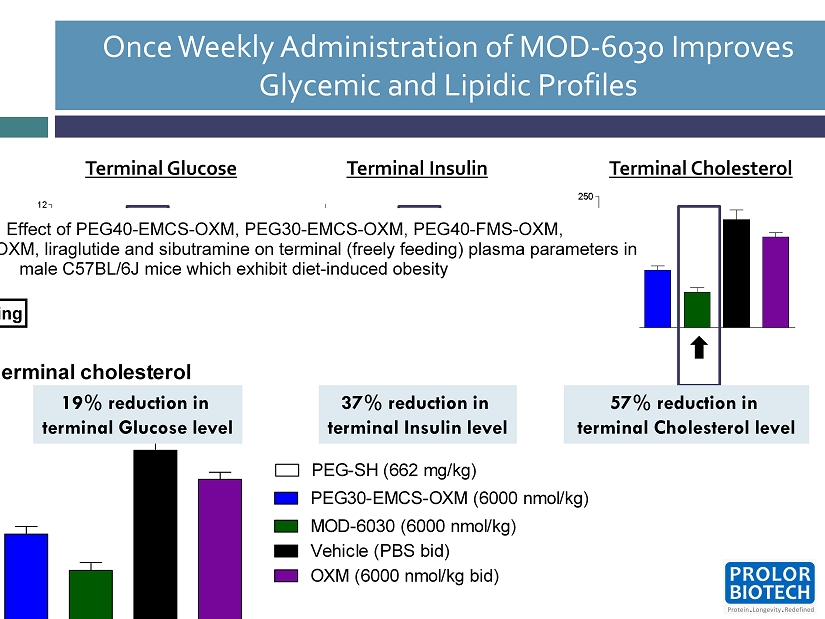
Once Weekly Administration of MOD - 6030 Improves Glycemic and Lipidic Profiles 12 Terminal Glucose Terminal Cholesterol Terminal Insulin Terminal cholesterol A C E F G 0 50 100 150 200 250 PEG-SH (662 mg/kg) PEG30-EMCS-OXM (6000 nmol/kg) MOD-6030 (6000 nmol/kg) Vehicle (PBS bid) OXM (6000 nmol/kg bid) Cholesterol (mg/dl) Male C57BL/6J mice were from Charles River and were maintained on a high fat diet (Research Diets D12451). Groups A to E were dosed once during baseline on Day -6 with PEG30-SH (662 mg/kg sc) and on Days 1, 8, 15 and 22 and 29 as indicated above. Groups F to I were dosed bid from Day 1 as indicated above. On Day 30, 24 hours after the final dose on Day 29, freely feeding terminal blood samples were taken by cardiac puncture. Plasma samples were analysed for glucose, insulin, cholesterol, glycerol and true TAG. A log transformation was used except for glucose. Analysis was by General Linear model and included treatment as a factor and bleeding order and Day 1 body weight as covariates. Data are shown as adjusted means (n=7-8) and standard errors of the mean (SEM) are calculated from the residuals of the statistical models. Comparisons against the appropriate vehicle were by the multiple t-test and significances are denoted by *p<0.05 and ***p<0.001. RS712 Part 4: Effect of PEG40-EMCS-OXM, PEG30-EMCS-OXM, PEG40-FMS-OXM, PEG30-FMS-OXM, OXM, liraglutide and sibutramine on terminal (freely feeding) plasma parameters in male C57BL/6J mice which exhibit diet-induced obesity Day 30 freely feeding 37% reduction in terminal Insulin level 57% reduction in terminal Cholesterol level 19 % reduction in terminal Glucose level
Conclusions • RevPEG was shown to be safe and tolerable in different toxicological rodent animal models. • RevPEG enables elongation of peptides’ half lives while maintaining their potential to penetrate target tissues (e.g. penetrate the BBB). • MOD - 6030 demonstrates superior long acting properties, supporting once weekly injection in humans. • MOD - 6030 reduces body weight by specific reduction in fat (body composition assessment). • MOD - 6030 improves glycemic and lipidemic profiles WITH PROVEN GLYCEMIC ACTIVITY AND SUPERIOR FAT LOSS ACTIVITY, MOD - 6030 IS EXPECTED TO PROVIDES LONG - TERM THERAPY FOR OBESITY AND TYPE II DIABETES PATIENTS

14 Thank You
