Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-14957_18k.htm |
Exhibit 99.1
Below is a reproduction of the contents of the poster entitled “A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter Study of the Safety and Efficacy of Avanafil in the Treatment of Erectile Dysfunction Following Bilateral, Nerve-Sparing Radical Prostatectomy”:

Below is a reproduction of the contents of the poster entitled “A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter Study of the Safety and Efficacy of Avanafil in the Treatment of Erectile Dysfunction Following Bilateral, Nerve-Sparing Radical Prostatectomy”:
John P. Mulhall, MD(a); Judd W. Moul, MD(b); Run Wang, MD(c); David Shin, MD(d); Jason D. Engel, MD(e); Wesley W. Day, PhD(f); Karen DiDonato, RN, MSN(f); Winnie Shih, CCRA(f); Charles H. Bowden, MD(f); and the Avanafil Post-Prostatectomy Study Group
(a)Memorial Sloan-Kettering Cancer Center, New York, NY; (b)Duke University Medical Center, Durham, NC; (c)University of Texas Medical School, Houston, TX; (d)Hackensack University Medical Center, Hackensack, NJ; (e)George Washington University Hospital, Washington, DC; (f)VIVUS, Inc., Mountain View, CA
· Introduction
· Following radical prostatectomy, a significant proportion of men experience erectile dysfunction (ED).(1),(2) More than one half of men with bilateral, nerve-sparing radical prostatectomy report ED 18 months after surgery.(3)
· Currently, first-line treatments for ED include oral therapy with phosphodiesterase type 5 (PDE5) inhibitors that work by increasing blood flow to the penis. Most of these must be administered 60-120 minutes prior to sexual activity.(4),(5)
· Avanafil is a highly selective PDE5 isoenzyme inhibitor with distinct pharmacokinetic properties, including rapid absorption and a short plasma half-life, under investigation for the treatment of ED.
· Objectives
· To evaluate the safety and efficacy of avanafil for treatment of mild to severe ED following bilateral, nerve-sparing radical prostatectomy.
· Methods
Study Design
· This double-blind, placebo-controlled Phase 3 trial evaluated avanafil in men with mild to severe ED following bilateral, nerve-sparing radical prostatectomy.
· Men with ED following radical prostatectomy, performed by an experienced surgeon, were recruited from approximately 50 study sites in the United States and were randomized to receive placebo, avanafil 100 mg, or avanafil 200 mg for 12 weeks after a 4-week, nontreatment, run-in period.
· Key criteria for study entry included:
· Aged 18-70 years.
· History of mild to severe ED of >6 months’ duration following bilateral nerve-sparing retropubic radical prostatectomy. Qualification of surgeons had been previously reviewed by the Sponsor.
· History of functional erections without medication assistance prior to diagnosis of prostate cancer.
· Prostate cancer staging <pT2 and Gleason score <7 [4+3].
· Having undergone prostatectomy for localized carcinoma >6 months prior to screening.
· Subjects were permitted to enter the 12-week treatment period if, during the run-in period, they met the following criteria:
· >4 documented attempts at sexual intercourse.
· >50% failure rate in maintaining an erection for successful intercourse.
· International Index of Erectile Function-Erectile Function (IIEF-EF) domain score between 5 and 25, inclusive.
· During the 12-week treatment period, subjects were instructed to take 1 dose of avanafil 30 minutes prior to initiation of sexual activity and agreed to make at least 4 intercourse attempts per month.
Assessments
· Coprimary efficacy endpoints were evaluated at baseline (defined at Visit 2, after the 4-week run-in period) and at follow-up exams using patient diaries with questions from the Sexual Encounter Profile (SEP) and the IIEF questionnaire. These endpoints included:
· Change in percentage of sexual attempts between run-in and end of treatment in which subjects were able to insert the penis into the partner’s vagina (SEP 2).
· Change in percentage of sexual attempts between run-in and end of treatment in which subjects were able to maintain an erection of sufficient duration to have successful intercourse (SEP 3).
· Change from baseline to end of treatment in the IIEF-EF domain score with last observation carried forward (LOCF).
· An exploratory endpoint included the change in SEP 3 by time intervals post dose.
· An analysis of covariance (ANCOVA) model evaluated each coprimary endpoint, with factors of treatment and baseline ED severity category, and baseline values as the covariate.
· Results
Baseline Demographics
· Out of the subjects randomized (n=298), 84.6% (n=252) completed the study.
· A greater proportion of subjects discontinued or withdrew consent in the placebo arm compared with either of the avanafil treatment arms.
· Baseline demographics were similar in each treatment group (Table 1).
· Seventy-two percent of the subjects had severe ED at baseline.
Table 1. Patient Baseline Demographics (Intent to Treat [ITT]).
|
|
|
Placebo |
|
Avanafil 100 mg |
|
Avanafil 200 mg |
|
Patient Characteristic |
|
(n=96) |
|
(n=94) |
|
(n=96) |
|
|
|
|
|
|
|
|
|
Mean age, years (SD) |
|
58.7 (6.0) |
|
58.8 (5.9) |
|
57.7 (6.6) |
|
White, n (%) |
|
81 (84.4) |
|
79 (84.0) |
|
73 (76.0) |
|
ED severity, n (%) |
|
|
|
|
|
|
|
Mild |
|
7 (7.3) |
|
7 (7.4) |
|
11 (11.5) |
|
Moderate |
|
19 (19.8) |
|
16 (17.0) |
|
19 (19.8) |
|
Severe |
|
70 (72.9) |
|
71 (75.5) |
|
66 (68.8) |
|
Mean time elapsed postsurgery, months (SD) |
|
18.2 (10.8) |
|
20.4 (15.4) |
|
19.0 (13.2) |
|
Mean postsurgery ED duration, months (SD) |
|
18.2 (10.9) |
|
20.4 (15.7) |
|
19.1 (13.1) |
|
Type of surgery technique, n (%) |
|
|
|
|
|
|
|
Open |
|
14 (14.6) |
|
19 (20.2) |
|
9 (9.4) |
|
Robotic |
|
79 (82.3) |
|
71 (75.5) |
|
78 (81.3) |
|
Laparoscopic |
|
3 (3.1) |
|
4 (4.3) |
|
9 (9.4) |
Efficacy Endpoints
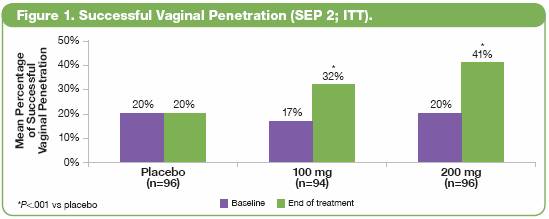
· Treatment with both doses of avanafil was associated with significant improvements compared with placebo in all 3 coprimary endpoints (P<.001), including:
· More successful vaginal penetration attempts (SEP 2; Figure 1).
· Improvements in the percentage of successful intercourse attempts (SEP 3; Figure 2).
· Improvements in the IIEF-EF domain mean score (Figure 3).
· IIEF-EF domain mean changes from baseline were 3.6 (40%) and 5.2 (55%) for 100 mg and 200 mg, respectively, compared to 0.1 (1%) for placebo.


Successful Intercourse by Postdose Time Interval
· In total, 82.4% of sexual attempts studywide were made within 60 minutes or less.
· One-third to one-half of all intercourse attempted at <15 minutes and <30 minutes was successful across both avanafil treatment groups (Figure 4).

Safety Summary
· Discontinuations or interruptions of study drug due to adverse events (AEs) were 3%, 3%, and 2% for placebo, 100 mg, and 200 mg, respectively (Table 2).
· There were no serious AEs and no deaths during the study
Table 2. Summary of Adverse Events (Safety Population).
|
Subjects With Any AE, |
|
Placebo |
|
Avanafil 100 mg |
|
Avanafil 200 mg |
|
n (%) |
|
(n=100) |
|
(n=99) |
|
(n=99) |
|
|
|
|
|
|
|
|
|
Discontinuation or interruptions in study drug due to AEs |
|
3 (3.0) |
|
3 (3.0) |
|
2 (2.0) |
|
Subjects with any serious AE |
|
0 (0) |
|
0 (0) |
|
0 (0) |
|
Subjects with any AE leading to death |
|
0 (0) |
|
0 (0) |
|
0 (0) |
|
· |
The incidence of treatment-emergent AEs (TEAEs) was higher with avanafil vs placebo (23.0%, 38.4%, and 45.5% for placebo, 100 mg, and 200 mg, respectively; Table 3). |
|
|
|
|
· |
The most common TEAEs were headache, flushing, and nasal congestion. |
Table 3. Treatment-Emergent Adverse Events (Safety Population).
|
Subjects With Any TEAE, |
|
Placebo |
|
Avanafil 100 mg |
|
Avanafil 200 mg |
|
n (%) |
|
(n=100) |
|
(n=99) |
|
(n=99) |
|
|
|
|
|
|
|
|
|
Total TEAEs |
|
23 (23.0) |
|
38 (38.4) |
|
45 (45.5) |
|
Most common TEAEs (>3% in any group) |
|
|
|
|
|
|
|
Headache |
|
1 (1.0) |
|
8 (8.1) |
|
12 (12.1) |
|
Flushing |
|
0 (0) |
|
5 (5.1) |
|
10 (10.1) |
|
Nasopharyngitis |
|
0 (0) |
|
3 (3.0) |
|
5 (5.1) |
|
Bronchitis |
|
4 (4.0) |
|
0 (0) |
|
0 (0) |
|
Back pain |
|
1 (1.0) |
|
3 (3.0) |
|
2 (2.0) |
|
Upper respiratory tract infection |
|
0 (0) |
|
2 (2.0) |
|
3 (3.0) |
|
Abnormal electrocardiogram |
|
0 (0) |
|
1 (1.0) |
|
3 (3.0) |
|
Nasal congestion |
|
1 (1.0) |
|
3 (3.0) |
|
1 (1.0) |
· Conclusions
· Avanafil 100 mg and 200 mg were effective and well tolerated in a post-prostatectomy population with mild to severe ED.
· Avanafil showed a rapid onset of action, as early as 15 minutes post dose.
· This study demonstrated that avanafil was an effective and well-tolerated treatment for ED in a post-prostatectomy population, with a rapid onset of action.
This trial is registered at ClinicalTrials.gov, number NCT00895011.
Acknowledgements: We thank the Avanafil Post-Prostatectomy Study Group investigators and study coordinators, the Quintiles team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
References: (1) Carson CC 3rd, et al. Curr Urol Rep. 2005;6(6):461-469. (2) Siegel T, et al. J Urol. 2001;165(2):430-435. (3) Stanford JL, et al. JAMA. 2000;283(3):354-360. (4) Corbin JD. Int J Impot Res. 2004;16(suppl 1):S4-S7. (5) Rosen RC, et al. Am J Cardiol. 2003;92(9A):9M-18M.
